Abstract
Therapeutic properties of fungal metabolites and silver nanoparticles have been well documented. While fungal metabolites have been used for centuries as medicinal drugs, potential of biogenic silver nanoparticles has recently received attention. We have evaluated the antimicrobial potential of Aspergillus terreus crude extract, silver nanoparticles and an amalgamation of both against four pathogenic bacterial strains. Antimicrobial activity of the following was evaluated – A. terreus extract, biogenic silver nanoparticles, and a mixture containing extract and nanoparticles. Four pathogenic bacteria - Klebsiella pneumoniae, Escherichia coli, Staphylococcus aureus, and Bacillus cereus were used as test organisms. Phenol, flavonoid, and alkaloid content of extract were determined to understand the chemical profile of the fungus. The extract contained significantly high amounts of phenols, flavonoids, and alkaloids. The extract and biogenic silver nanoparticle exhibited significant antibacterial activity at concentrations of 10 μg/ml and 1 μg/ml, respectively. When used in combination, the extract-nanoparticle mixture showed equally potent antibacterial activity at a much lower concentration of 2.5 μg/ml extract + 0.5 μg/ml nanoparticle. Given its high antibacterial potential, the fungal extract can be a promising source of novel drug lead compounds. The extract – silver nanoparticle mixture exhibited synergism in their antibacterial efficacy. This property can be further used to formulate new age drugs.
Keywords: Antibacterial, Aspergillus terreus, biogenic silver nanoparticle, Rann of Kutch
INTRODUCTION
Microorganism-based medicinal drugs have been around for a century. Today, a plethora of medications is available against a wide range of conventional diseases. Most of these trace their origin to a microbial source. Metabolites sourced from microorganisms, particularly fungi and actinomycetes, have been utilized as antibiotics (cephalosporins),[1] immune-suppressants (cyclosporin),[2] antibacterial and antifungal agents (flindersine),[3] and anticancer compounds (carnosol and isouvaretin).[4] Newly emerging diseases such as Zika and Ebola virus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococci and β-lactam-resistant Streptococcus pneumonia, demand a concerted effort toward formulating novel drugs for a postantibiotic era.[5] Nanotechnology-based therapeutics have provided a breakthrough in the field of medical science with wide ranging applications such as drug and gene delivery, detection of proteins and pathogens, and targeted elimination of tumor cells and as antimicrobials, to name a few. Bionanotechnology employs nanoparticles synthesized on biological platforms such as algae, fungi, yeast, bacteria, actinomycetes, and plants.[6,7,8] Biologically synthesized nanoparticles, or bionanoparticles, are as efficient as their chemical counterparts and are more economical to produce.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals were of analytical grade, obtained from Sigma-Aldrich (India) or Merck (India). Culture media were purchased from HiMedia (India). Rifampicin was procured from Sigma-Aldrich (India).
Isolation and identification of Aspergillus terreus
Aspergillus terreus isolated from the salt desert of Little Rann of Kutch, India, was used for the study. Fungal isolation was performed by serial dilution method. Briefly, 1 g soil was dissolved in sterile phosphate-buffered saline and 10−1, 10−3, and 10−5 dilutions were prepared. From each dilution, 100 μl was pipetted out and dispensed onto Czapek Yeast Autolysate agar (CYA) plates.[8] Plates were incubated upside down in dark at 25 ± 1°C for 4 days. Fungal colonies were identified on the basis of macro- and micro-morphology. Colonies bearing resemblance to A. terreus were further subcultured on CYA and observed for colony characteristics such as growth rate, texture, extent of sporulation, mycelial color, and reverse colony color. Culture identification services of Gujarat Genomics Initiative, Gujarat, were utilized for molecular identification based on 18S rDNA sequencing.
Bionanoparticle
Silver bionanoparticles (AgNPs) were synthesized from Chlorella pyrenoidosa (NCIM 2738) procured from National Center for Industrial Microorganism, Pune, India. Batch cultures of C. pyrenoidosa were grown in Bold's basal medium. The culture was used in the mid-exponential growth phase. Nanoparticles were synthesized using cell extract by the methodology outlined by Aziz et al., 2015.[9] Culture was collected in falcon tubes and centrifuged. The pellet was washed thrice with distilled water followed by boiling for 5 min. The suspension was cooled and centrifuged at 10,000 rpm for 10 min. The supernatant was harvested and used as a cell extract. About 10 ml cell extract was mixed with 90 ml 1 mM AgNO3 solution for 24 h. Change in the color indicated the formation of silver nanoparticles. Transmission Electron Microscope (Philips, EM-410 LS) was used to observe the morphology of the biosynthesized Ag NPs. The size of the AgNPs was determined to be 8 ± 2 nm. Energy dispersive X-ray analysis was performed to confirm the presence of elemental silver in the AgNPs.
Fungal metabolite extraction
Metabolites were extracted from the mycelium.[10] Agar plug from a 7-day-old A. terreus culture was inoculated in Czapek Dox Broth (100 ml) in Erlenmeyer flask and incubated in dark (10 d, 25 ± 1°C). Mycelial mat was harvested, shredded, and suspended in 1:1 chloroform:methanol and kept overnight in a rotary shaker (120 rpm). The mixture was filtered, and equal volumes of chloroform and distilled water were added to the filtrate and shaken in an orbital shaker (120 rpm, 1 h). Bottom organic layer was collected and evaporated to dryness in a Buchi Rotavapor. The dried extract was suspended in 1:1 acetonitrile:methanol (100 ml) and hexane (100 ml) and stirred (120 rpm, 1 h). The bottom organic layer was collected and evaporated to dryness. The extract was suspended in dimethyl sulfoxide at a concentration of 1 mg/ml and stored at −20°C.
Antibacterial assays
Antibacterial assay using fungal extract
Antibacterial activity of A. terreus extract was evaluated against Klebsiella pneumoniae (KJ938546), Escherichia coli (MCC 2412), S. aureus (MCC 2408), and Bacillus cereus (MCC 2243) by agar well diffusion method. K. pneumoniae was obtained from the institute's culture collection facility. E. coli, B. cereus, and S. aureus were acquired from the Microbial Culture Collection at National Centre for Cell Science, Pune, India. Bacteria were cultured on Mueller–Hinton agar. Bacterial inoculants were prepared in nutrient broth at 0.5 McFarland standards. Each test organism (100 μl) was mixed with cooled Mueller–Hinton agar and poured into 80 mm petri dishes. Wells were cut out, and 100 μl crude extract at 5, 10, 15, and 20 µg/ml concentrations were poured. The plates were incubated (37°C, 24 h) and the zones of inhibition were measured. Rifampicin was used as positive control. All experiments were performed in triplicates.
Antibacterial assay using silver bionanoparticles
Antibacterial efficacy of AgNPs was assessed against the bacterial pathogens at four different concentrations (0.5, 1, 1.5, and 2 µg/ml) using the methodology adopted in the previous section.
Antibacterial assay using extract + silver bionanoparticle formulation
Based on the individual antibacterial potency, suspensions containing varying proportions of fungal extract and AgNPs were prepared sequentially and were analyzed for their antibacterial activity.
Determination of phenol, flavonoid, and alkaloid content of fungal extract
Total phenolic content was determined according to the Folin–Ciocalteu colorimetric method.[11] Gallic acid was used as a standard, and a calibration curve was prepared for concentrations ranging from 25 to 300 μg/ml and a calibration curve was obtained using a linear fit.
Total flavonoid content was estimated by the aluminum chloride method.[12] Quercetin concentrations (0–500 μg/ml) were prepared, and a calibration curve was obtained using a linear fit.
Total alkaloid estimation was done by spectrophotometric method of Dragendorff's reagent.[13] Standard curve was prepared using pilocarpine nitrate in HCl solution at different concentrations (750, 500, 400, 250, 200, 150, and 100 mg/l). Alkaloid contents were expressed as pilocarpine nitrate equivalents (µg/mg PNEs). All the samples were analyzed in triplicates.
RESULTS
Isolation and identification
On the basis of colony morphology and microscopic observations, A. terreus was tentatively identified. Sequencing of internal transcribed spacer (ITS) 1-5.8S-ITS 2 region of rDNA confirmed the identity of the fungus as A. terreus.
Antibacterial activity of fungal extract
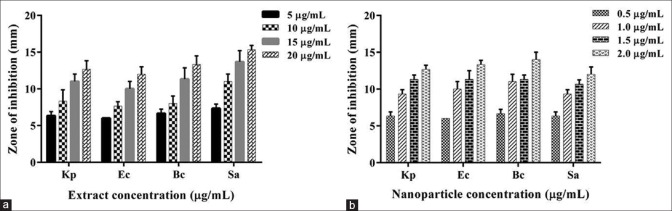
Zone of inhibition was observed against all the test organisms, indicating the broad spectral nature and presence of antimicrobial compounds in the extracts. Figure 1a gives a graphical representation of the zone of inhibition observed against the bacteria at different extract concentrations. Except for S. aureus which was inhibited at 5 µg/ml, the remaining showed significant inhibition only at concentration ≥10 µg/ml. Gram-positive bacteria were more susceptible as compared to the Gram-negative strains.
Figure 1.
Antibacterial activity of fungal extract (a) and silver nanoparticles (b) at different concentrations measured as a zone of inhibition against pathogenic bacteria, Klebsiella pneumoniae, Escherichia coli, Bacillus cereus, and Staphylococcus aureus. Experiment was performed in triplicates, the error bars represents mean ± standard deviation
Antibacterial activity of silver bionanoparticles
Figure 1b represents the antibacterial activity of nanoparticles against the test pathogens. The nanoparticles were found to be extremely potent against all the bacterial strains, with clear zone of inhibition at 1 µg/ml.
Antibacterial activity of extract + silver bionanoparticle formulation
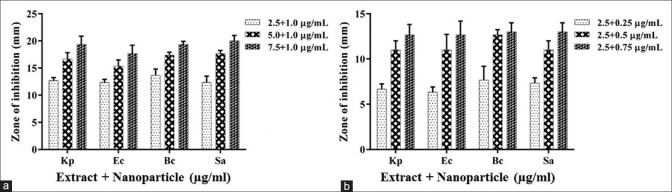
Synergistic effect of AgNPs – metabolite mixture was assessed. Based on the individual antibacterial potency of the extract and AgNPs, concoctions containing different proportions of each were prepared and tested against the bacteria [Figure 2]. Initially, the AgNPs concentration was kept constant at 1 µg/ml, the minimum concentration at which it exhibited bactericidal effect, whereas the concentration of extract was varied [Figure 2a]. About 2.5 µg/ml extract + 1 µg/ml AgNPs showed bactericidal activity higher than either 10 µg/ml extract or 1 µg/ml AgNPs. Further, keeping the extract concentration constant at 2.5 µg/ml, the AgNPs concentration was varied and tested on bacterial pathogens [Figure 2b]. Established on the minimum concentration at which significant clear zones were observed against all four bacteria, 2.5 µg/ml extract + 0.5 µg/ml AgNPs formulation was found to have maximum potency at the lowest concentration.
Figure 2.
Antibacterial activity of extract + silver nanoparticles. Silver nanoparticles concentration kept constant at 1 μg/ml, extract concentration was varied (a). Taking the minimum concentration of extract showing significant activity, 2.5 μg/ml, concentration of silver nanoparticles was varied (b). Experiment was performed in triplicates, the error bars represents mean ± standard deviation
Phenol, flavonoid, and alkaloid content
The extract was found to contain remarkably high amounts of phenol (157.19 ± 1.2 µg/mg gallic acid equivalent [GAE]), flavonoid (86.16 ± 0.80 µg/mg quercetin equivalent [QE]), and alkaloid (68.26 ± 1.5 µg/mg PNEs).
DISCUSSION
Members of the phylum Ascomycota, especially Aspergillus, produce metabolites that have antibacterial, antifungal, insecticidal, and nematicidal activities.[14]A. terreus, a halotolerant soil fungi isolated from the salt desert of Little Rann of Kutch, India, was tested for its antibacterial potential. The fungal extract showed statistically significant bioactivity at a minimum concentration of 10 µg/ml against all four bacterial strains, indicative of its broad spectrum nature. Antibacterial potential of biologically synthesized silver nanoparticles was assessed. AgNPs were found to be extremely potent with inhibition observed at 1 µg/ml concentration against all test organisms. In addition, to investigate if any synergism existed between the metabolites and AgNPs in their antimicrobial action, mixtures containing varying proportions of each were tested. Interestingly, extract + AgNP mixture at 2.5 + 0.5 μg/ml concentration was found to exhibit higher bactericidal activity than either component and at statistically significant lower concentrations, indicating a synergism between the two [Figure 3]. Gram-positive strains were found to be more susceptible as compared to the Gram-negative strains in all three cases. Differences in cell architecture between Gram-negative and Gram-positive bacteria might be the cause of these variations. The Gram-negative bacteria possess an outer membrane of lipopolysaccharide that prevents the invasion of hydrophobic compounds and are therefore more tolerant to antibacterial agents.[15]
Figure 3.
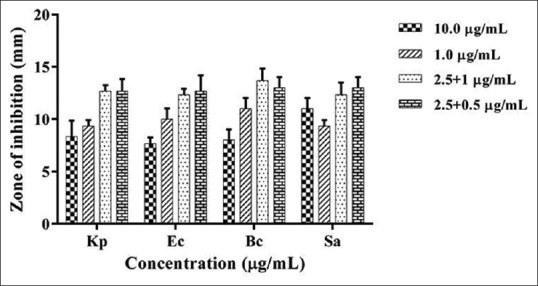
Comparative analysis of the antibacterial efficacy of fungal extract and silver nanoparticles alone and in combination against pathogenic bacteria. Antibacterial activity at (2.5 + 1) μg/ml extract + silver nanoparticles was higher than 10 μg/ml extract and 1 μg/ml silver nanoparticle, respectively (2.5 + 0.5) μg/ml extract + silver nanoparticle found to be as effective as (2.5 + 1) at P value - 0.0001 < 0.01 < 0.05. Experiment performed in triplicates. Error bars represents mean ± standard deviation
Silver has been traditionally used in various topical antimicrobial formulations. Silver is preferred over other metals as it shows higher toxicity to microorganisms and lower toxicity to mammalian tissue. Nanoparticles are more effective in their toxicity because of their large surface area to volume ratio.[16] Although the exact underlying mechanisms are yet to be fully elucidated. Some researchers have proposed that AgNPs (1–10 nm size) act by binding to the cell membrane and altering its permeability. AgNPs disrupt the bacterial respiratory chain leading to generation of reactive oxygen species (e.g., hydrogen peroxide) and resultant oxidative stress and cell damage.[17,18] In addition, the NPs have a high affinity for phosphorus and nitrogen and therefore, post their entry into the cell, these bind with proteins implicated in inhibition of cellular metabolism.[16,19,20,21,22] Biogenic AgNPs have shown to have still higher microbicidal effect than their chemical counterparts.
Phenols and flavonoids exert antimicrobial, antitumoral, antioxidant, antiallergic, and antiviral activities.[23,24] At 157.19 ± 1.2 µg/mg GAE, 86.16 ± 0.80 µg/mg QE, and 68.26 ± 1.5 µg/mg PNE, respectively, the phenol, flavonoid, and alkaloid contents of the fungal extract were found to be significantly high. Antibacterial activity of the extract may be ascribed to the high content of these compounds which have been reported to be involved in inhibition of nucleic acid biosynthesis and other metabolic processes.[25,26] Growth inhibition of bacteria might be brought about by inhibition of hydrolytic enzymes, inactivation of microbial adhesins, and cell envelope transport proteins and nonspecific interactions with carbohydrates.[27]
The findings of the present study have yet again highlighted the significance of the fungal metabolites and AgNPs as potential antimicrobial agents.[28,29,30] The extract needs to be analyzed further for isolation and identification of active principles. Biogenic silver nanoparticles are not only extremely effective antimicrobial agents, as deduced from this study, but also economical. Although the AgNPs and fungal extract displayed significant antibacterial activity, a mixture containing both in much lower concentration was found to be equally potent, thereby displaying a synergistic effect. It would be interesting and vital to understand the underlying mechanism. Further characterization studies are being done on the extract-AgNPs mixture to get clues to this synergism.
CONCLUSION
The fungal extract of A. terreus and biogenic silver nanoparticles are potent antimicrobial agents. However, their potency is even higher and at much lower concentration, when used in combination. Further characterization studies need to be done to understand the underlying mechanisms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Demain AL. Production of beta-lactam antibiotics and its regulation. Proc Natl Sci Counc Repub China B Life Sci. 1991;15:251–65. [PubMed] [Google Scholar]
- 2.Rüegger A, Kuhn M, Lichti H, Loosli HR, Huguenin R, Quiquerez C, et al. Cyclosporin A, a peptide metabolite from Trichoderma polysporum (Link ex Pers.) Rifai, with a remarkable immunosuppressive activity. Helv Chim Acta. 1976;59:1075–92. doi: 10.1002/hlca.19760590412. [DOI] [PubMed] [Google Scholar]
- 3.Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Flindersine isolated from the traditional medicinal plant, Toddalia asiatica (L.) Lam. J Ethnopharmacol. 2009;123:494–8. doi: 10.1016/j.jep.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Hufford CD, Lasswell WL., Jr Uvaretin and isouvaretin. Two novel cytotoxic c-benzylflavanones from Uvaria chamae L. J Org Chem. 1976;41:1297–8. doi: 10.1021/jo00869a062. [DOI] [PubMed] [Google Scholar]
- 5.Strohl WR, editor. Biotechnology of Antibiotics. New York: Marcel Dekker Publishers; 1997. Industrial antibiotics: Today and the future; pp. 1–47. [Google Scholar]
- 6.Rai M, Yadav A, Gade A. Current trends in phytosynthesis of metal nanoparticles. Crit Rev Biotechnol. 2008;28:277–84. doi: 10.1080/07388550802368903. [DOI] [PubMed] [Google Scholar]
- 7.Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–62. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Pitt JI. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. London: Academic Press Inc.; 1979. [Google Scholar]
- 9.Aziz N, Faraz M, Pandey R, Shakir M, Fatma T, Varma A, et al. Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial, and photocatalytic properties. Langmuir. 2015;31:11605–12. doi: 10.1021/acs.langmuir.5b03081. [DOI] [PubMed] [Google Scholar]
- 10.Vandermolen KM, Raja HA, El-Elimat T, Oberlies NH. Evaluation of culture media for the production of secondary metabolites in a natural products screening program. AMB Express. 2013;3:71. doi: 10.1186/2191-0855-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicco N, Lanorte MT, Paraggio M, Viggiano M, Lattanzio V. A reproducible, rapid and inexpensive Folin – Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem J. 2009;91:107–10. [Google Scholar]
- 12.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 13.Sreevidya N, Mehrotra S. Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff's reagent in plant materials. J AOAC Int. 2003;86:1124–7. [PubMed] [Google Scholar]
- 14.Zain ME, Awaad AS, Al-Othman MR, Alafeefy AM, El-Meligy RM. Biological activity of fungal secondary metabolites. Int J Chem App Biol Sci. 2014;1:14. [Google Scholar]
- 15.Puupponen-Pimiä R, Nohynek L, Meier C, Kähkönen M, Heinonen M, Hopia A, et al. Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 16.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Loo SL, Krantz WB, Fane AG, Hu X, Lim TT. Effect of synthesis routes on the properties and bactericidal activity of cryogels incorporated with silver nanoparticles. RSC Adv. 2015;5:44626–35. [Google Scholar]
- 18.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009;5:452–6. doi: 10.1016/j.nano.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11:371–84. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 22.Aziz N, Fatma T, Varma A, Prasad R. Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. J Nanopart 2014. 2014 doi:10.1155/2014/689419. [Google Scholar]
- 23.Agrawal AD. Pharmaccological activities of flavonoids: A review. Int J Pharm Sci Nanotechnol. 2011;4:1394–8. [Google Scholar]
- 24.Gurovic MS, Lanza AM, Adánez Mdel C, Omaña MC, Gómez IG, Murray AP, et al. Cytotoxic effects induced by combination of heliantriol B2 and dequalinium against human leukemic cell lines. Phytother Res. 2011;25:603–10. doi: 10.1002/ptr.3310. [DOI] [PubMed] [Google Scholar]
- 25.Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343–56. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkada B. Preliminary report on warfarin for the treatmentof herpes 210 simplex. J Ir Coll Phys Surg. 1978;22:56. [Google Scholar]
- 27.Pyla R, Kim TJ, Silva JL, Jung YS. Enhanced antimicrobial activity of starch-based film impregnated with thermally processed tannic acid, a strong antioxidant. Int J Food Microbiol. 2010;137:154–60. doi: 10.1016/j.ijfoodmicro.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Prasad R, Pandey R, Barman I. Engineering tailored nanoparticles with microbes: Quo vadis? Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:316–30. doi: 10.1002/wnan.1363. [DOI] [PubMed] [Google Scholar]
- 29.Prasad R. Synthesis of silver nanoparticles in photosynthetic plants. J Nanopart 2014. 2014 doi:10.1155/2014/963961. [Google Scholar]
- 30.Prasad R, Swamy VS. Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J Nanopart 2013. 2013 doi:10.1155/2013/431218. [Google Scholar]