Abstract
The aim of this study was to investigate the influence of absorption enhancers in the uptake of hydrophilic compounds. The permeation of the two hydrophilic drug models gentamicin and 5 (6)-carboxyfluorescein (CF) across the brush border membrane vesicles and Caco-2 cell lines were evaluated using total saponins of Acanthophyllum squarrosum, Quillaja saponaria, sodium lauryl sulfate, sodium glycocholate, sodium taurodeoxycholate, and Tween 20 as absorption enhancers. Transepithelial electrical resistance (TEER) measurement was utilized to assess the paracellular permeability of cell lines. Confocal laser scanning microscopy (CLSM) was performed to obtain images of the distribution of CF in Caco-2 cells. These compounds were able to loosen tight junctions, thus increasing paracellular permeability. CLSM confirmed the effect of these absorption enhancers on CF transport across Caco-2 lines and increased the Caco-2 permeability via transcellular route. It was also confirmed that the decrease in TEER was transient and reversible after removal of permeation enhancers.
Keywords: Absorption enhancers, brush border membrane vesicles, Caco-2 cell, transepithelial electrical resistance
INTRODUCTION
Hydrophilic drugs usually present low bioavailability after oral administration. One of the causes of this low bioavailability is their poor intestinal permeation through the paracellular pathway due to existence tight junction.[1,2] Saponins are secondary metabolites synthesized by many different plant species, lower marine animals, and some bacteria.[3,4] It has been reported that Quillaja saponin significantly increases cell membranes permeability. The phenomenon is a direct effect on cell membrane and also opening the tight junctions.[2]
In this study, 5(6)-carboxyfluorescein (CF), a hydrophilic anionic dye (log P = 0.14) was also employed as a permeability marker due to its high aqueous solubility.[5,6] Therefore, the objective of this study was to enhance permeation of gentamicin and CF across brush border membrane vesicle (BBMV) and Caco-2 cell models using saponin extracted from Acanthophyllum squarrosum (ATS), Quillaja saponaria (QTS), bile acids (sodium glycocholate [SGC] and sodium taurodeoxycholate [STDC]) and synthetic surfactants (sodium lauryl sulfate [SLS] and Tween 20) as absorption enhancers. ATS is growing wild in different locations of Iran. QTS is a tree native to the Andes region and the commercial saponins are extracted from this plant.
MATERIALS AND METHODS
SLS, SGC, STDC and Tween 20 were purchased from Merck, Germany. Dulbecco's modified Eagle's medium (DMEM) and 5(6)-CF were obtained from Sigma-Aldrich, Germany. Fetal bovine serum (FBS), QTS, and gentamicin sulfate (GM) were purchased from Gibco, USA, Alfa Aesar, Germany and Alborz Daru Pharmaceutical Co., Iran, respectively. Caco-2 cells were kindly donated by the University of Alberta, Canada (ATCC No: CRL-2102). All the solvents were of the analytical grade.
Extraction of Acanthophyllum squarrosum
Saponins were extracted by the method described be Sotan et al.,[7] Moghimipour et al.[8] and Aqel et al.[9] Finally, total saponins were freeze-dried (Operon, Korea) and stored at room temperature.[7,8,9] QTS was commercially available.
Preparation of brush border membrane vesicle
The vesicles were prepared by the method described by Cho et al.[10] and Maenz et al.[11] The purity of the preparation was assayed by alkaline-phosphatase (ALP) using p-nitrophenyl phosphate as a substrate.[12] ALP activity was monitored using a commercially available ALP kit (DarmanKave, Iran).
Uptake experiments
Uptake experiments were carried out using ATS, QTS, and Tween 20 as absorption enhancer and GM as drug model. The absorption enhancer was mixed with membrane vesicle suspensions and kept at 25°C for 30 min. After incubation at 37°C, the reactions were stopped by adding 2 ml of ice-cold buffer containing 150 mM NaCl and 20 mM Tris HCl at pH 7.5. Then, the suspension was filtered (0.45 µm pore size, 2.5 cm in diameter, Millipore). The filter was rinsed with 2 ml of the same buffer and concentration of GM was determined using agar diffusion method.[13]
Caco-2 cell culture
Caco-2 cells were grown at 37°C, 5% CO2 and 95% relative humidity using DMEM supplemented with 10% FBS, 1% nonessential amino acids, and 1% penicillin-streptomycin. Media was changed approximately every 48 h. Caco-2 cells were planted on polycarbonate 6-well Transwells® (Costar, 3412, tissue culture treated, 24 mm diameter, 0.4 μm pore size, USA) and used for transport experiments. The transepithelial electrical resistance (TEER) value was measured daily using an volt-ohm-meter device (EVOM, World Percision Instrument, USA). TEER was measured and used as an index of monolayer confluence and integrity in cell culture experiments.[14,15,16,17]
Transport studies
The permeability of CF as drug model was investigated in both the apical to basolateral (AB) and basolateral to apical (BA) directions using ATS, QTS, SLS, SGC, and STDC. TEER was used to check monolayer formation and integrity before the beginning of experiments. When the value of TEER changed after 3 consecutive measurements, 2 ml culture media containing absorption enhancers and 2.5 ml blank media culture was added to the apical and basolateral sides, respectively. At intervals of 5, 10, 15, 20, 25, and 30 min, a 100 µl sample was withdrawn from the basolateral side, replaced with an equal volume of fresh culture media. The amount of CF in the receptor phase was assayed using spectrofluorimetry (Fluoro-Max, SPEX Industries Inc., USA, excitation/emission: 492/515 nm). In the BA direction, the culture media containing above mentioned absorption enhancers was added to the basolateral side.
What of the gentamicin mentioned under materials? Where was it used?
Recovery experiments
For determining the reversibility of the effect of absorption enhancers on intestinal barrier permeability, cell lines were preincubated with each absorption enhancer for 20 min. At the end of the incubation time, the culture medium was removed and Caco-2 cell lines were washed 3 times with PBS buffer and then incubated in fresh medium at 37°C to recover their integrity. TEER was measured at 0, 5, 10, 15, and 20 min after the beginning recovery.
Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) (Molecular Dynamics, USA) was utilized to visualize the effect of permeation enhancers (ATS, QTS, and STDC) on CF transport across Caco-2 lines. A volume of 20 µl of Caco-2 suspensions were incubated with 20 µl culture media containing 8 µg/ml CF and 2 mg/ml absorptions enhancers at 37°C for 1 h. Fluorescence was excited at 488 nm and the emission detected at 510 nm at room temperature.
Statistical analysis
Results are shown as mean ± standard error. ANOVA was performed to compare the results and P < 0.05 was assumed as a significant difference.
RESULTS AND DISCUSSION
According to the results shown in Figure 1, ATS, QTS, and Tween 20 increased the permeation of GM through BBMVs in vitro. Permeability was increased by enhancing the concentration of penetration enhancers. Based on the obtained results, there was no significant difference between total saponins in permeation of drug through vesicles (P > 0.05). Tween 20 was found to exhibit the best enhancing effect in the transport of GM at concentration 3.33 µg/ml. The effect of surfactants on the transport of drugs through BBMVs is not well understood. They may disrupt the lipid arrangements in cell membranes and increase the water content of the membrane proteins. They can interact with the polar head groups of the lipid bilayers, modifying hydrogen bonding, and ionic forces between these groups. Therefore, the lipid membranes become fluidized and promote the diffusion of drug across cell membrane. It is suggested that the saponins and Tween 20 enhance absorption of GM across BBMVs by the mentioned mechanism.[18] Moreover, it was reported that saponins in concentrations more than their critical micelle concentration, can interact with membrane sterols, particularly with cholesterol and form micelles.[19] It has been reported that herbal constituents such as ginseng saponin were identified as potent P-glycoprotein (P-gp) inhibitors.[20] Enhancement in intestinal absorption of GM is probably due to inhibition of P-gp by saponins.
Figure 1.
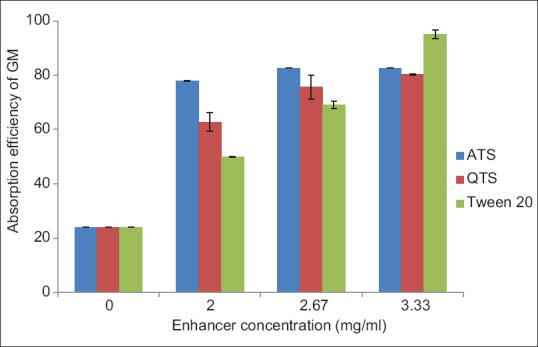
Absorption efficiency of gentamicin sulfate across brush border membrane vesicle in the presence of penetration enhancer (mean ± standard error, n = 3)
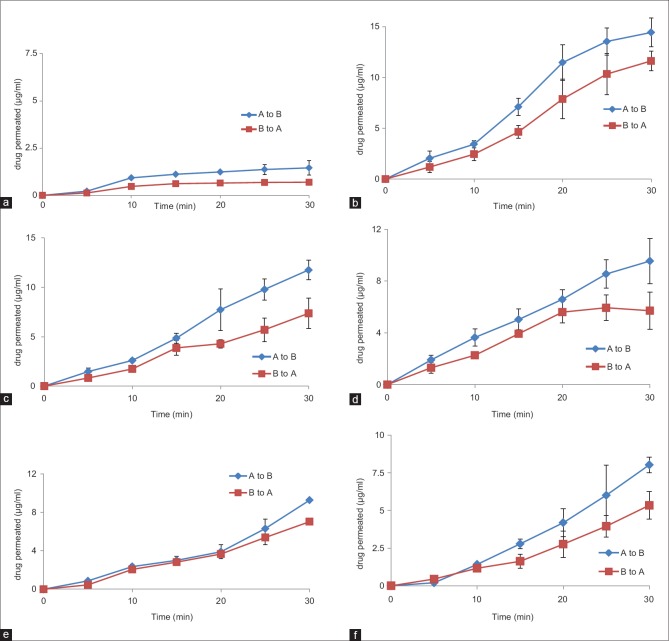
Cell lines were used when TEER reached steady state about 274.30 ± 15.95 Ω cm2 on the 5th day. As shown in Figure 2a, the AB and BA permeability of CF in the absence of absorption enhancers (control) remained constant, that indicated that the cell liner kept its thigh junction integrity. Figure 2b–2e clearly indicates that all of the absorption enhancers could transport CF through AB and BA. SLS significantly increased AB flux of CF across cell lines [Figure 2b]. The ability of SGC in transport of CF was more than STDC, but there was no any significant difference between them (P > 0.05). As illustrated in Figure 2e and f, the amount of CF transport from AB using QTS was more than ATS. However, the difference between QTS and ATS in the absorption of CF through cell lines was not significant (P > 0.05). The reduction in TEER was an indicator for the opening of tight junctions. It has been previously reported that, due to the opening of tight junctions and therefore increasing of ion passage through the paracellular route, the TEER values of Caco-2 cell lines are significantly reduced.[21]
Figure 2.
Permeation of carboxyfluorescein through Caco-2 cell lines due to the presence of different absorption enhancers: (a) control; i.e. no enhancer, (b) sodium lauryl sulfate, (c) sodium glycocholate, (d) sodium taurodeoxycholate, (e) Quillaja saponaria and (f) Acanthophyllum squarrosum
As illustrated in Figure 3, the restoration of TEER value during 20 min after wash-out of the Caco-2 cell monolyers indicates that the paracellular permeation enhancing effects of ATS and QTS are reversible. The findings that are not in accordance with the results of Cho et al. that reported high toxicity of the total saponin at concentration of 0.5 µg/ml resulted in a complete disruption of Caco-2 cells.[22] In the current study, concentration of the saponin was 100 µg/ml. The results shown in Figure 3 indicated a regular recovery of the monolayers after SGC and STDC exposure and the TEER values of Caco-2 cell lines increased gradually in 20 min. However, no significant difference in the mentioned period (P > 0.05) was observed. The rate of enhancement of TEER after removal of culture media containing SLS was constant. These results clearly indicated that the opening of the paracellular pathway with the absorption enhancers was transient and reversible. CLSM photography indicated that bile salt and saponins may also affect transcellular permeation. Images of permeation enhancer treated Caco-2 cell lines showed an increase in accumulation of CF in the inside of Caco-2 cells [Figure 4]. Regarding the results shown in Figure 4, the stimulating effect of STDC on permeability across the Caco-2 lines was higher than saponins.
Figure 3.
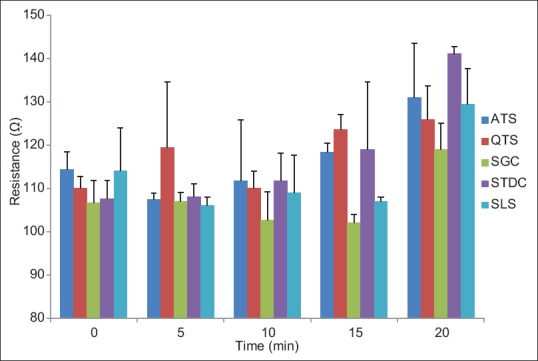
Recovery of transepithelial electrical resistance of Caco-2 cell lines after removal of culture media contaning different absorption enhancesrs
Figure 4.
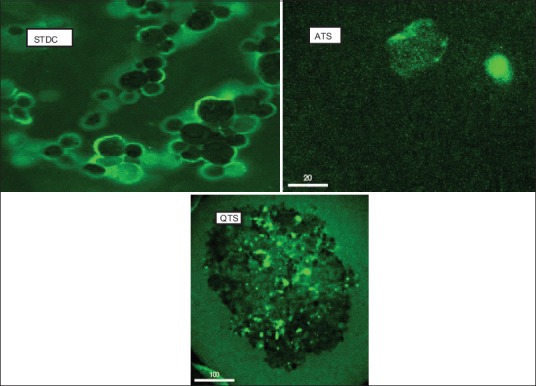
Confocal laser scanning microscopy images of Caco-2 cell liens incubated with sodium taurodeoxycholate (STDC), acanthopyllum squarrusom (ATS) and Quillaja saponaria (QTS)
CONCLUSION
According to our findings, these permeation enhancers can potentially be used to increase the oral bioavailability of therapeutic drug molecules toward intestinal epithelial cells. However, further toxicological and clinical studies are needed to investigate their safety.
Financial support and sponsorship
The authors thank Mashhad University of Medical Sciences for the financial support.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors acknowledge Electron Microscopic Unit of the University of Alberta for their cooperation.
REFERENCES
- 1.Rama Prasad YV, Minamimoto T, Yoshikawa Y, Shibata N, Mori S, Matsuura A, et al. In situ intestinal absorption studies on low molecular weight heparin in rats using labrasol as absorption enhancer. Int J Pharm. 2004;271:225–32. doi: 10.1016/j.ijpharm.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Cho SY, Sim JS, Kang SS, Jeong CS, Linhardt RJ, Kim YS. Enhancement of heparin and heparin disaccharide absorption by the Phytolacca americana saponins. Arch Pharm Res. 2003;26:1102–8. doi: 10.1007/BF02994765. [DOI] [PubMed] [Google Scholar]
- 3.Arif T, Bhosale JD, Kumar N, Mandal TK, Bendre RS, Lavekar GS, et al. Natural products – Antifungal agents derived from plants. J Asian Nat Prod Res. 2009;11:621–38. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 4.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: A review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 5.Murthy SN, Zhao YL, Sen A, Hui SW. Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J Control Release. 2004;99:393–402. doi: 10.1016/j.jconrel.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto A, Tatsumi H, Maruyama M, Uchiyama T, Okada N, Fujita T. Modulation of intestinal permeability by nitric oxide donors: Implications in intestinal delivery of poorly absorbable drugs. J Pharmacol Exp Ther. 2001;296:84–90. [PubMed] [Google Scholar]
- 7.Sotan KO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponins extract of Sorghum Bicolor L. Moench. Afr J Biotechnol. 2006;5:2405–7. [Google Scholar]
- 8.Moghimipour E, Sajadi Tabassi SA, Ramezani M, Lobenberg R. Enhanced permeability of gentamicin sulfate through shed snake skin and liposomal membranes by different enhancers. Iran J Basic Med Sci. 2003;6:9–19. [Google Scholar]
- 9.Aqel H, Al-Charchafchi F, Ghazzawi D. Biochemical, antibacterial and antifungal activity of extracts from Achillea fragrantissima and evaluation of volatile oil composition. Pharm Sin. 2012;3:349–56. [Google Scholar]
- 10.Cho S, Park JH, Yu J, Lee YK, Byun Y, Chung H, et al. Preparation and characterization of reconstructed small intestinal brush border membranes for surface plasmon resonance analysis. Pharm Res. 2004;21:55–60. doi: 10.1023/b:pham.0000012152.86004.aa. [DOI] [PubMed] [Google Scholar]
- 11.Maenz DD, Chenu C, Bellemare F, Berteloot A. Improved stability of rabbit and rat intestinal brush border membrane vesicles using phospholipase inhibitors. Biochim Biophys Acta. 1991;1069:250–8. doi: 10.1016/0005-2736(91)90132-r. [DOI] [PubMed] [Google Scholar]
- 12.Su SF, Amidon GL, Lee HJ. Possible degradative process of cholecystokinin analogs in rabbit jejunum brush-border membrane vesicles. Life Sci. 2002;72:35–47. doi: 10.1016/s0024-3205(02)02198-7. [DOI] [PubMed] [Google Scholar]
- 13.Lourenço FR, Andreoli Pinto TJ. Comparison of three experimental designs employed in gentamicin microbiological assay through agar diffusion. Braz J Pharm Sci. 2009;45:559–66. [Google Scholar]
- 14.Alhamoruni A, Lee AC, Wright KL, Larvin M, O'Sullivan SE. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 15.Mukaizawa F, Taniguchi K, Miyake M, Ogawara K, Odomi M, Higaki K, et al. Novel oral absorption system containing polyamines and bile salts enhances drug transport via both transcellular and paracellular pathways across Caco-2 cell monolayers. Int J Pharm. 2009;367:103–8. doi: 10.1016/j.ijpharm.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, He L, Katsumi H, Sakane T, Fujita T, Yamamoto A. Improvement of intestinal absorption of water-soluble macromolecules by various polyamines: Intestinal mucosal toxicity and absorption-enhancing mechanism of spermine. Int J Pharm. 2008;354:126–34. doi: 10.1016/j.ijpharm.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Lo YL, Huang JD. Effects of sodium deoxycholate and sodium caprate on the transport of epirubicin in human intestinal epithelial Caco-2 cell layers and everted gut sacs of rats. Biochem Pharmacol. 2000;59:665–72. doi: 10.1016/s0006-2952(99)00377-9. [DOI] [PubMed] [Google Scholar]
- 18.Lo YL. Relationships between the hydrophilic-lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Release. 2003;90:37–48. doi: 10.1016/s0168-3659(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 19.Gilabert-Oriol R, Mergel K, Thakur M, von Mallinckrodt B, Melzig MF, Fuchs H, et al. Real-time analysis of membrane permeabilizing effects of oleanane saponins. Bioorg Med Chem. 2013;21:2387–95. doi: 10.1016/j.bmc.2013.01.061. [DOI] [PubMed] [Google Scholar]
- 20.Raghava Srivalli KM, Lakshmi PK. Overview of P-glycoprotein inhibitors: A rational outlook. Braz J Pharm Sci. 2012;48:353–67. [Google Scholar]
- 21.Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm. 2013;83:427–35. doi: 10.1016/j.ejpb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Cho SY, Kim JS, Li H, Shim C, Linhardt RJ, Kim YS. Enhancement of paracellular transport of heparin disaccharide across Caco-2 cell monolayers. Arch Pharm Res. 2002;25:86–92. doi: 10.1007/BF02975268. [DOI] [PubMed] [Google Scholar]