Abstract
Griseofulvin is an antifungal drug and is available as oral dosage forms. Development of topical treatment could be advantageous for superficial fungal infections of the skin. In this study, films prepared from the incorporation of griseofulvin-loaded liposomes in chitosan film for topical drug delivery in superficial fungal infections. The properties of the films were characterized regarding mechanical properties, swelling, ability to transmit vapor, drug release, thermal behavior, and antifungal efficacy against Microsporum gypseum and Epidermophyton floccosum. The presence of liposomes led to decreased mechanical properties but lower swelling ratio. Higher amount of drug permeation and rate of flux were obtained by liposomes incorporated in films compared to liposomal formulations. Antifungal efficacy of formulations was confirmed against two species of dermatophytes in vitro. Therefore, two concepts of using vesicular carrier systems and biopolymeric films have been combined and this topical novel composite film has the potential for griseofulvin delivery to superficial fungal infections.
Keywords: Antifungal effect, chitosan film, dermatophytes, griseofulvin, liposomes, skin permeation
INTRODUCTION
Superficial fungal infections are among the most common infections mainly caused by dermatophytes belonging to genera Epidermophyton, Microsporum, and Trichophyton. Griseofulvin is currently available only as oral dosage forms. Since the dermatophytes infect top layers of skin, the topical treatment provides an alternative route to target the drug directly to skin, circumvent the systemic effects associated with oral administration and increase patient compliance.[1]
Phospholipid-based formulations have a high probability of improving the permeation of the active molecules to membranes.[2] In the proper formulations and at the appropriate size, liposomes fuse onto the skin surface and deliver drugs to the skin on the basis of the similarity of the bilayer structure of the lipid vesicles to that of the natural membrane.[3] In general, liposomes have a stability issue with long-term storage.[4]
Chitosan has been extensively evaluated as a drug delivery system in different forms of particles, gels, and films. The film forming property of chitosan has made it interesting for transdermal/dermal drug delivery.[5]
The main goal of this work was to obtain a topical formulation made by liposomes containing griseofulvin embedded in a chitosan film to have a dosage form that solves the instability problem of liposomes and facilitate the usage of a topical drug for nearly long-term.
MATERIALS AND METHODS
Materials
Griseofulvin supplied by Hengo, China, Chitosan (Viscosity 200-600 cp, Deacetylation degree 92%) purchased from Primex, Iceland, soy phosphatidylcholine (soy PC) (Phospholipon 85G®) supplied by Lipoid (Germany). Sabouraud dextrose agar medium supplemented with 0.05% cycloheximide and 0.005% chloramphenicol (SCC) purchased from Merck (Germany). All other materials and reagents were of the highest grade commercially available. Microsporum gypseum and Epidermophyton floccosum were obtained from Iran Zamin Laboratory, Ahvaz, Iran.
Full thickness Balb/c mice dorsal skin was used for the experiments in full compliance with regulatory principles of ethics committee of Ahvaz Jundishapur University of Medical Sciences.
Preparation of griseofulvin liposomes
Griseofulvin-loaded liposomes were prepared using thin film hydration method. Accurately weighed amount of soy PC and cholesterol at a molar ratio of 7: 4 and the drug, was dissolved in 30 mL of chloroform–methanol (2:1) in 250 mL round bottom flasks. The solvent was evaporated at 37 ± 1°C at 120 rpm under reduced pressure using rotary evaporator (EV311, Lab-Tech, Germany). After complete evaporation of solvent, the flask was kept in a refrigerator at 4 ± 1°C to remove the residual solvent. The thin lipid film obtained was hydrated with buffer phosphate pH 7.4 at 45 ± 1°C and then sonicated for 15 min using a bath sonicator. Liposomes of griseofulvin were subsequently stored in the refrigerator overnight before conducting any characterization and evaluation studies.[6]
Calculation of entrapment efficiency
The liposomal suspension was centrifuged at 15,000 rpm at 4°C for 30 min; the supernatant was analyzed for griseofulvin in buffer phosphate (pH = 7.4):methanol (3:1 v/v) at 295 nm by using spectrophotometer (Biochrom WAP Biowave II, England). EE% was calculated as follows:
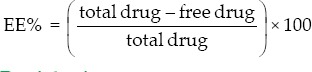
Particle size
The average diameter of liposomes was determined using a DLS particle size analyzer (Qudix, Scatterscope I, South Korea).
Film preparation
The films were prepared using casting and solvent evaporation. Chitosan solution (Cs) (2% w/v) were prepared by dispersing required amount of chitosan in acetic acid solution (1% v/v). Liposomes containing a certain amount of griseofulvin at a final concentration of 0.2% w/v was mixed with Cs at a ratio of 1:0.8 and 1:1 (v/v), vortex and sonicated. About 20 mL of mixture were placed in a glass petri dish (9 cm diameter) and dried at 45°C for 48 h and then peeled off. These formulations were named F1 (1:0.8) and F2 (1:1). Similar film formulations containing blank liposomes (C1 and C2) and a film formulation without liposome containing no drug (Cs) also prepared as a control.[7,8]
Characterization of the films
Film thickness
The thickness of the film was measured at five different spots using a micrometer.
Mechanical properties
Filmstrips (10 mm × 50 mm) were fixed between two clamps of a texture analyzer (WDW-5, Jinan Chenda, China) with a 50 N load cell. The strips were pulled at the rate of 10 mm/min; tensile strength (TS) and elongation at break (EB) were calculated as follows.[9]
TS = Breaking force/cross-sectional area (N/mm2).
EB = Increase in length/original length ×100 (%).
Content uniformity
About 1 cm × 1 cm film was dissolved in 10 mL phosphate buffer (pH = 7.4):methanol 3:1 (v/v) and then filtered through membrane filter 0.45 µm. The concentration of griseofulvin was measured at 295 nm. The test was repeated five times.
Water vapor transmission rate
The films were cut and placed on the top of tubes containing 5 g calcium chloride and held in an oven at 50°C in order to achieve constant weights. Then, tubes were placed in a desiccator containing saturated solution NaCl (75% relative humidity). The vapor penetration was determined by weighing the tubes on day 0, 1, 2, 3, 4, and 5. Linear regression was used to estimate the slope of this line in g/day. Water vapor transmission rate (WVTR) (g/m2/day) was calculated by dividing the slope by the area (m2).[10]
Swelling and erosion
The swelling ratio of the films was measured by gravimetric method. The completely dried films (2 cm × 2 cm) were weighed and immersed in 50 mL phosphate buffer (pH = 7.4) at 37°C. At predetermined intervals (1, 2, 3, 4, 6, 8 and 24 h), the resultant swollen films were removed, and the excess water was omitted carefully with filter paper and weighed immediately.
The swelling ratio (Q) was calculated using the following equation:[11]
Q = (W2 − W1)/W1
W2 is the weight of the film at each time point. W1 is the weight of the initial dry film.
Then, the samples were desiccated in an oven at 60°C for 24 h and weights of dried films W3 were recorded. Erosion (E) was calculated using the following equation:[12]
E = (W1 − W3)/W1
In vitro drug release
Drug release studies were performed in vitro using jacketed Franz cell with a receiver volume of 30 mL at 37°C. Phosphate buffer (pH = 7.4) was used as a receiver medium. The suitable size of the film was mounted in the Franz cell. About 1.5 mL of receiver medium was withdrawn at 0.25, 0.5, 0.75, 1, 2, 4, 6, and 8 h and replaced with the same volume of blank receiver medium solution. Aliquots of the collected sample were analyzed for their griseofulvin content. The derived concentration values were corrected by using below equation:
Mt(n)= Vr × Cn + Vs× ΣCm
Where Mt(n) is the current cumulative mass of drug transported at time t, n is the number (times) of sampling, Cn is the current concentration in the receiver medium, ΣCm is the total of the previously measured concentrations, Vr is the volume of the receiver medium, and Vs corresponds to the volume of the sample removed for analysis.[3]
Ex vivo skin permeation
The studies were performed for F1, F2, and liposomal formulation containing 0.2% griseofulvin using vertical Franz diffusion cell. Phosphate buffer (pH 7.4) was used as receptor media, and the cell contents were maintained at a temperature of 37 ± 1°C. Full thickness Balb/c mice dorsal skin was used for the experiments; the hair of the outer skin surface was removed, and the skin was carefully dissected and rinsed with normal saline. The epidermal side of the mice skin was exposed to ambient conditions while the dermal side was bathed with phosphate buffer (pH 7.4). The suitable size of the film or certain amount of liposomal suspension with same griseofulvin content was mounted on the mice skin. About 1.5 mL aliquot of the receptor fluid was periodically withdrawn at suitable time intervals from the sampling arm of receptor chamber and was replaced with fresh buffer. Aliquots of the collected sample were analyzed for their griseofulvin content.[13]
The steady state flux (Jss) of the formulation was determined by the slope of the linear portion of the plots of the amount of the drug in receiving chamber versus time, divided by the exposed surface area of the film and lag time was estimated from the x-intercept of the linear portion of the graph.[14]
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was carried out on drug alone, liposomes, and film formulations using a DSC-1 Mettler Toledo, Switzerland. The procedure involved heating an accurately weighed sample in an aluminum pan at a scanning rate of 10°C/min, over a temperature range of 20°C–300°C.
Antifungal assay
M. gypseum and E. floccosum were cultivated for 7 days at 28°C on SCC medium. Inoculums were prepared by purring 9 mL of sterile normal saline containing 0.05% tween 80 on the agar plate surface followed by the gentle scraping and then the suspensions were transferred into sterile tubes. A suspension equal to 0.5 McFarland (106 colony forming units [CFU]/mL) was prepared by dilution in a sterile saline solution and diluted more to 104 CFU/mL. One hundred microliters of these suspensions (104 CFU/mL) was spread on the plates and allowed to dry at room temperature for 15 min. Seven millimeters disk film formulation, blank film, and paper disk containing 0.2% griseofulvin was put on the surface of the plates. After incubation for 9 days at 28°C, inhibition zone diameters formed around the discs were measured.[15]
Statistical analysis
All experiments were carried out in triplicate and expressed as a mean ± standard deviation unless otherwise stated. T-test or one-way ANOVA statistical test following multiple comparison Tukey test was used to assess the significance of the differences among the various groups. P < 0.05 was considered statistically significant.
RESULTS
Liposome characterization
The encapsulation efficiency of griseofulvin in two liposomal formulations prepared for F1 and F2 was determined by the indirect method. The percent encapsulation of both formulations was about 95%, and there was no significant difference between the encapsulation percent of both formulations (P > 0.05) [Table 1]. The particle size of formulations was also presented in Table 1.
Table 1.
Particle size and encapsulation efficiency of liposomes (mean±standard deviation, n=3)

Characterization of the films
Films formed from chitosan and liposomes were easily removed from the Petri dishes and showed acceptable color and appearance. Cs was somehow brittle and it was difficult to peel it off intact.
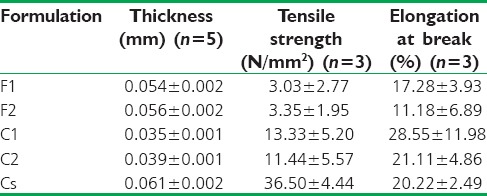
Film thickness and mechanical properties
The thickness, TS, and EB of the films are shown in Table 2. The presence of liposomes led to decreasing film thickness (P < 0.05). The thickness of F1 and F2 was more than C1 and C2 (P < 0.05). There were no statistical significant differences between mechanical properties of F1 and F2 (P > 0.05), but Cs showed higher TS than other formulations (P < 0.05). There were no statistical differences among EB of all formulations (P > 0.05).
Table 2.
Thickness and mechanical properties of film formulations (mean±standard deviation)
Content uniformity
Film formulations containing liposomes were found to be of uniform drug content. Table 3 shows the ratio of the content of griseofulvin per 1 cm2 film to the theoretical amount of loaded drug (drug content) and the variation in the distribution of griseofulvin in different film regions relative to the average (relative standard deviation%).
Table 3.
Drug content and relative standard deviation of film formulations (mean±standard deviation, n=5)

Water vapor transmission rate
WVTR from F1 and F2 was 221.97 ± 5.03 and 214.04 ± 13.88 (g/m2/day), respectively. These values did not show any significant differences (P > 0.05).
Swelling ratio (Q) and erosion
The swelling ratio of films in pH 7.4 phosphate buffer is shown in Figure 1. All films reached maximum swelling at 2 h. F1 and Cs had the lowest and highest swelling ratio, respectively. At the end of the experiment, all formulations showed about 31–45% erosion.
Figure 1.
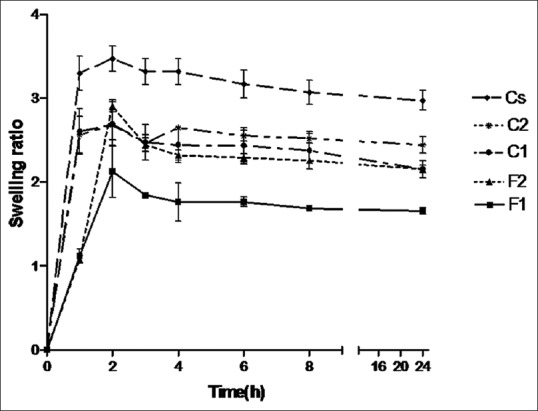
Swelling ratio of the films in phosphate buffer (pH = 7.4) at 37°C (mean ± standard deviation, n = 3)
In vitro drug release
The release profiles of griseofulvin from film formulations are shown in Figure 2. There were no significant differences at any time between two formulations (P > 0.05).
Figure 2.
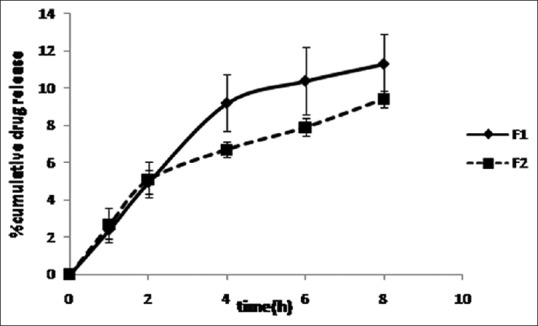
Release profiles of griseofulvin from film formulations in phosphate buffer (pH = 7.4) at 37°C (mean ± standard deviation, n = 3)
Ex vivo skin permeation
As shown in Figure 3, till 1st h F2 showed the highest permeation compared to F1 and liposomal formulation containing 0.2% griseofulvin, at 2nd h the amount of permeated drug was the same for F1 and F2 but more than drug permeated from liposomal formulation and at 6th h, F1 showed the highest permeation than other formulations (P < 0.05).
Figure 3.
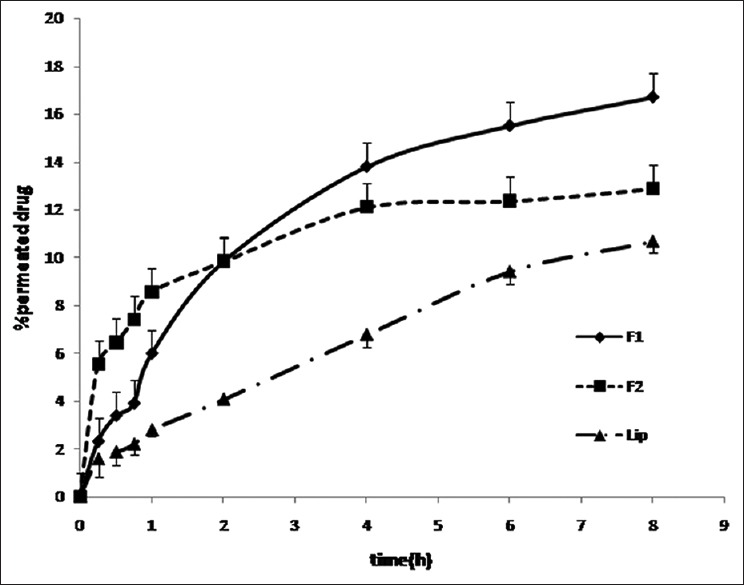
Ex vivo permeation of griseofulvin across mouse skin in phosphate buffer (pH = 7.4) at 37°C (mean ± standard deviation, n = 3)
Jss of F1, F2, and liposomal formulation was 29.38 ± 8.58, 27.59 ± 5.25, and 9.36 ± 3.03 mcg/cm2/h, respectively. F1 and F2 showed higher flux rate than liposomal formulation (P < 0.05). Griseofulvin permeated from all formulations without any lag time.
Differential scanning calorimetry
According to Figure 4, DSC thermogram of griseofulvin showed an endothermic peak at 220°C corresponding to its melting point but it was disappeared in liposomes containing griseofulvin and film formulations. The blank- and griseofulvin-loaded liposomes as well as chitosan, control and film formulations showed peak around 120–145°C in their thermograms, which may be caused by evaporation of bounded water.
Figure 4.
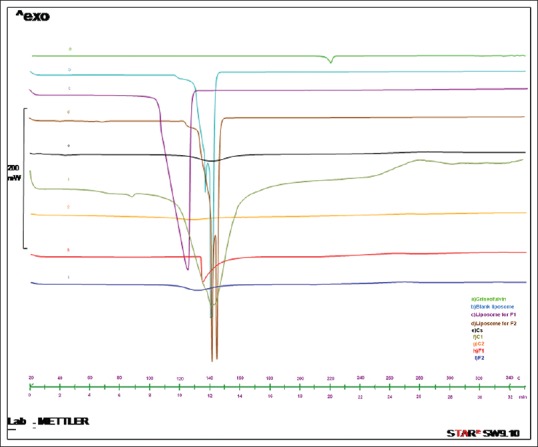
Differential scanning calorimetry thermogram of (a) griseofulvin, (b) blank liposome, (c) liposome for F1, (d) liposome for F2, (e) chitosan film (chitosan solution), (f) control formulation 1 (C1), (g) control formulation 2 (C2), (h) film formulation 1 (F1), and (i) film formulation 2 (F2) over temperature range of 20–300°C
In vitro antifungal effect
The capabilities of film formulations, blank films, and solution form of griseofulvin in inhibiting the growth of M. gypseum and E. floccosum on SCC medium are shown in Table 4. The inhibition zone of the formulations against M. gypseum and E. floccosum in the decreasing order is: griseofulvin solution >F1 and F2 > C1 and C2 (P < 0.05).
Table 4.
Inhibition zones (mm)* of film formulations, blank films, and solution form of griseofulvin against Microsporum gypseum and Epidermophyton floccosum (mean±standard deviation, n=4)

DISCUSSION
Targeting the drug at the site of infection may lead to reduce the duration of treatment and increase patient compliance.[16,17] Topical delivery of griseofulvin using novel drug delivery systems, for example, deformable liposomes,[1] ethosomes,[18] solid lipid nanoparticles,[19] hydrogels,[20] microemulsions,[21] and self-microemulsifying systems[22] have been studied, but a topical formulation of griseofulvin does not still enter the pharmaceutical market. The film forming property of chitosan has made it interesting for transdermal/dermal drug delivery.[5] In this study, two concepts of using vesicular carrier systems and biopolymeric films have been combined to produce effective and easy to use the topical delivery system for griseofulvin.
Liposomal formulations that were prepared for incorporation in film formulations showed suitable size and encapsulation. The mean particle size of empty liposomes was greater than griseofulvin-loaded ones (P < 0.05). The thickness of the films was affected by liposome incorporation, F1, F2, C1, and C2 had less thickness than Cs (P < 0.05). This is probably due to the evaporation of water consisted in liposomal suspension, during film formation. F1 and F2 that contained griseofulvin-loaded liposomes with smaller size were thicker than C1 and C2 (P < 0.05). Less particle size can lead to produce more packed particles in the suspension and less water content.[23] TS of Cs was higher than other formulations. Incorporation of liposomes led to decreasing the strength of the films. Since chitosan provides film forming property, addition of liposomes may disturb the cross-linking between chitosan molecules by a change in the polymer chain interactions.[24] There were no significant differences among strength of F1, F2, C1, and C2, in other words, addition of drug had no impact on mechanical properties of the films as most of the drug was encapsulated in liposomes. Interestingly, in our previous study[25] that composite film was prepared using a molecular dispersion of chitosan and soy PC, TS and EB of chitosan/soy PC film were higher than chitosan film. Ionic and hydrogen bonding interactions of chitosan and PC are possible in molecular form but that was not happened in case of adding soy PC in liposomal form to Cs. The reason could be the engagement of functional groups of soy PC, cholesterol, and water to shape vesicles.
Both film formulations (F1 and F2) were found to be of uniform drug content as seen in the results given in Table 3. There was no significant difference between WVTR of F1 and F2 (P > 0.05), but these formulations showed lower WVTR (about one tenth) compared to that of the formulations in our previous study[25] that soy PC was incorporated to the chitosan film directly. Liposomes may have reduced water diffusivity by increasing the tortuosity of the film matrix and decreasing the water sorption, so improve the water barrier function.[26]
Possession of polymer bearing amine (-NH2) and hydroxyl groups (-OH) increases chitosan affinity to water and hydrogen bond formation with hydrophilic solvents.[8] However, incorporation of liposomes which are somehow hydrophobic resulted in a reduction in swelling ratio.[27] There were no significant differences among the erosion of film formulations (P > 0.05).
The release pattern of griseofulvin from F1 and F2 [Figure 2] did not show any significant differences at any time (P > 0.05).
The permeation of griseofulvin from F1, F2, and liposomal formulation showed in Figure 3. Up to 1st h, F2 showed the highest permeation of griseofulvin through mouse skin, up to 4th h the amount of griseofulvin permeation was the same for F1 and F2 but it was more than permeation of griseofulvin from the liposomal formulation. At 6th h, F1 had the highest permeation. That can be contributed to fast initial swelling process of the films. Griseofulvin permeated from all formulations without any lag time, but Jss of F1 and F2 was significantly (P < 0.05) higher than that of liposomal formulation. The presence of chitosan which is hydrophilic in F1 and F2 decreased the lipophilicity of formulations compared to the liposomal formulation and so the tendency of lipophilic griseofulvin to the vehicle has been decreased that led to increasing the rate of flux and amount of drug permeation as well.
DSC thermogram showed that in the case of griseofulvin-loaded liposomes and film formulations containing them, the peak of melting point of griseofulvin was not observed which indicates that it is encapsulated in the liposome.
When the formulations were subjected to agar plate diffusion, it was observed that the disc containing griseofulvin showed larger inhibition zone that could be because of direct availability of the drug.[28] Nevertheless, F1 and F2 showed reasonable inhibition zone. The zones of inhibition for F1 and F2 showed no statistical significant differences (P > 0.05). Extremely low antifungal effect was also seen for blank films (C1 and C2) against E. floccosum that is consistent with other studies that revealed the chitosan has antifungal activity against several fungi species.[29]
The strategy of using liposomes to overcome the barrier nature of the skin and control drug delivery has gained interest. Lipid particles could improve the stability of the drug and released the more amount of drug in more efficient manner to or through the skin.[30] However, instability caused by liposomal aggregation, bilayer fusion, and drug leakage is one of the main problems encountered in any liposomal formulation and could greatly affect the shelf life of liposomes.[31,32]
Aggarwal and Goindi prepared deformable vesicles containing griseofulvin with effective permeability through the skin barrier.[1] They also prepared griseofulvin-loaded ethosomes and solid lipid nanoparticles.[18,19] El-Badry et al. developed microemulsion and liposomal gels containing croconazole and proposed these drug delivery vehicles suitable for the treatment of dermal fungal infections.[33]
Film forming property of chitosan made it interesting for drug delivery, but films composed of chitosan alone showed a lack of stability. Hence, different studies have been done in order to improve stability and physicochemical characteristics of chitosan film by blending it with other materials. Grant et al. used a combination of chitosan and egg PC to prepare composite films for localized drug delivery of paclitaxel and concluded that egg PC produces chitosan-based films with minimal swelling and a high degree of stability as a result of ionic and hydrogen bonding interactions between these two biomaterials.[11]
In this study, we merged two concepts of vesicular drug delivery systems and film formulations to produce stable and feasible topical formulation for delivery of griseofulvin for the treatment of superficial fungal infections. The prepared formulations showed suitable physicochemical characteristics and provided higher drug skin permeation and rate of flux compared to the liposomal formulation.
This strategy has been applied in other studies as well. Li et al. developed composite films by curcumin-loaded polycaprolactane nanoparticles incorporated in polyethylene glycol-grafted chitosan film for wound healing application.[34] Mortazavian et al. prepared thiolated chitosan nanoparticles containing insulin embedded in chitosan with an excellent permeation of insulin nanoparticles from buccal mucosa.[35]
CONCLUSIONS
The aim of the present study was to formulate and characterize chitosan film formulation integrating of griseofulvin-loaded liposomes for dermal delivery.
The presence of liposomes led to lower swelling ratio. Besides, more drug permeation and higher Jss obtained by film formulations containing liposomes rather than liposomes. Different kinds of lipid particles have been studied and recognized as a suitable drug delivery system for topical applications. However, instability of these vesicles is a major drawback. These topical liposomes incorporated films can compensate the instability problem of liposomal formulations and provide easy to use topical formulations, especially for long-term treatment. These formulations have the potential for griseofulvin delivery to superficial fungal infections and in vivo studies examining the efficacy of the formulations on superficial fungal infection are going to be done in future.
Financial support and sponsorship
This paper is issued from Pharm D thesis of Pardis Mohamadipour and financial support was provided by a grant (N-93) from Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Aggarwal N, Goindi S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized guinea pig model of Microsporum canis – Dermatophytosis. Int J Pharm. 2012;437:277–87. doi: 10.1016/j.ijpharm.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Kim YH, Song C, Jung E, Kim DH, Kim DD. A microemulsion-based hydrogel formulation containing voriconazole for topical skin delivery. J Pharm Investig. 2014;44:517–24. [Google Scholar]
- 3.Jaafari MR, Bavarsad N, Bazzaz BS, Samiei A, Soroush D, Ghorbani S, et al. Effect of topical liposomes containing paromomycin sulfate in the course of Leishmania major infection in susceptible BALB/c mice. Antimicrob Agents Chemother. 2009;53:2259–65. doi: 10.1128/AAC.01319-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karn PR, Jin SE, Lee BJ, Sun BK, Kim MS, Sung JH, et al. Preparation and evaluation of cyclosporin A-containing proliposomes: A comparison of the supercritical antisolvent process with the conventional film method. Int J Nanomedicine. 2014;9:5079–91. doi: 10.2147/IJN.S70340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed S, Ibrahim M, Sarhan H, Amin M. Formulation and characterization of biodgradable chitosan films for topical application of terbinafine HCL. Bull Pharm Sci Assiut Univ. 2007;30:111–29. [Google Scholar]
- 6.Ning M, Gu Z, Pan H, Yu H, Xiao K. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antifungal drug clotrimazole. Indian J Exp Biol. 2005;43:150–7. [PubMed] [Google Scholar]
- 7.Srinivasa P, Ramesh M, Tharanathan R. Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocoll. 2007;21:1113–22. [Google Scholar]
- 8.Kouchak M, Ameri A, Naseri B, Kargar Boldaji S. Chitosan and polyvinyl alcohol composite films containing nitrofurazone: Preparation and evaluation. Iran J Basic Med Sci. 2014;17:14–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Nitayaphat W, Jiratumnukul N, Charuchinda S, Kittinaovarat S. Mechanical properties of chitosan/bamboo charcoal composite films made with normal and surface oxidized charcoal. Carbohydr Polym. 2009;78:444–8. [Google Scholar]
- 10.Wittaya-areekul S, Prahsarn C, Sungthongjeen S. Development and in vitro evaluation of Chitosan-Eudragit RS 30D composite wound dressings. AAPS PharmSciTech. 2006;7:E30. doi: 10.1208/pt070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant J, Blicker M, Piquette-Miller M, Allen C. Hybrid films from blends of chitosan and egg phosphatidylcholine for localized delivery of paclitaxel. J Pharm Sci. 2005;94:1512–27. doi: 10.1002/jps.20379. [DOI] [PubMed] [Google Scholar]
- 12.Tang C, Guan YX, Yao SJ, Zhu ZQ. Preparation of ibuprofen-loaded chitosan films for oral mucosal drug delivery using supercritical solution impregnation. Int J Pharm. 2014;473:434–41. doi: 10.1016/j.ijpharm.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Bavarsad N, Fazly Bazzaz BS, Khamesipour A, Jaafari MR. Colloidal, in vitro and in vivo anti-leishmanial properties of transfersomes containing paromomycin sulfate in susceptible BALB/c mice. Acta Trop. 2012;124:33–41. doi: 10.1016/j.actatropica.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Sinko PJ, Singh Y, editors. Martin's Physical Pharmacy and Pharmaceutical Sciences. 6th ed. Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 15.Nweze EI, Mukherjee PK, Ghannoum MA. Agar-based disk diffusion assay for susceptibility testing of dermatophytes. J Clin Microbiol. 2010;48:3750–2. doi: 10.1128/JCM.01357-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassem MA, Esmat S, Bendas ER, El-Komy MH. Efficacy of topical griseofulvin in treatment of tinea corporis. Mycoses. 2006;49:232–5. doi: 10.1111/j.1439-0507.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal N, Goindi S, Mehta SD. Preparation and evaluation of dermal delivery system of griseofulvin containing Vitamin E-TPGS as penetration enhancer. AAPS PharmSciTech. 2012;13:67–74. doi: 10.1208/s12249-011-9722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal N, Goindi S. Dermatopharmacokinetic and pharmacodynamic evaluation of ethosomes of griseofulvin designed for dermal delivery. J Nanopart Res. 2013;15:1–15. [Google Scholar]
- 19.Aggarwal N, Goindi S. Preparation and in vivo evaluation of solid lipid nanoparticles of griseofulvin for dermal use. J Biomed Nanotechnol. 2013;9:564–76. doi: 10.1166/jbn.2013.1569. [DOI] [PubMed] [Google Scholar]
- 20.Goindi S, Aggarwal N. Preparation of hydrogels of griseofulvin for dermal application. Int J Pharm. 2006;326:20–4. doi: 10.1016/j.ijpharm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal N, Goindi S, Khurana R. Formulation, characterization and evaluation of an optimized microemulsion formulation of griseofulvin for topical application. Colloids Surf B Biointerfaces. 2013;105:158–66. doi: 10.1016/j.colsurfb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Ofokansi KC, Chukwu KI, Ugwuanyi SI. The use of liquid self-microemulsifying drug delivery systems based on peanut oil/tween 80 in the delivery of griseofulvin. Drug Dev Ind Pharm. 2009;35:185–91. doi: 10.1080/03639040802244292. [DOI] [PubMed] [Google Scholar]
- 23.Kazakov S, Kaholek M, Teraoka I, Levon K. UV-induced gelation on nanometer scale using liposome reactor. Macromolecules. 2002;35:1911–20. [Google Scholar]
- 24.Hu H, Xin JH, Hu H, Chan A, He L. Glutaraldehyde-chitosan and poly (vinyl alcohol) blends, and fluorescence of their nano-silica composite films. Carbohydr Polym. 2013;91:305–13. doi: 10.1016/j.carbpol.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 25.Bavarsad N, Kouchak M, Varmaziar M, Sadeghi-Nejad B. Preparation, characterization and evaluation of antifungal efficacy of chitosan/soy phosphatidylcholine topical films containing griseofulvin. Jundishapur J Nat Pharm Prod. 2015;10:e27562. [Google Scholar]
- 26.Imran M, Revol-Junelles AM, René N, Jamshidian M, Akhtar MJ, Arab-Tehrany E, et al. Microstructure and physico-chemical evaluation of nano-emulsion-based antimicrobial peptides embedded in bioactive packaging films. Food Hydrocoll. 2012;29:407–19. [Google Scholar]
- 27.Binsi PK, Ravishankar CN, Srinivasa Gopal TK. Development and characterization of an edible composite film based on chitosan and virgin coconut oil with improved moisture sorption properties. J Food Sci. 2013;78:E526–34. doi: 10.1111/1750-3841.12084. [DOI] [PubMed] [Google Scholar]
- 28.Chhonker YS, Prasad YD, Chandasana H, Vishvkarma A, Mitra K, Shukla PK, et al. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int J Biol Macromol. 2015;72:1451–8. doi: 10.1016/j.ijbiomac.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Sajomsang W, Gonil P, Saesoo S, Ovatlarnporn C. Antifungal property of quaternized chitosan and its derivatives. Int J Biol Macromol. 2012;50:263–9. doi: 10.1016/j.ijbiomac.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni CV, Moinuddin Z, Patil-Sen Y, Littlefield R, Hood M. Lipid-hydrogel films for sustained drug release. Int J Pharm. 2015;479:416–21. doi: 10.1016/j.ijpharm.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Sipai Altaf Bhai M, Vandana Y, Mamatha Y, Prasanth V. Liposomes: An overview. J Pharm Sci Innov. 2012;1:13–21. [Google Scholar]
- 32.Nounou MI, El-Khordagui LK, Khalafallah NA, Khalil SA. Liposomal formulation for dermal and transdermal drug delivery: Past, present and future. Recent Pat Drug Deliv Formul. 2008;2:9–18. doi: 10.2174/187221108783331375. [DOI] [PubMed] [Google Scholar]
- 33.El-Badry M, Fetih G, Shakeel F. Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv. 2014;21:34–43. doi: 10.3109/10717544.2013.843610. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Nan K, Li L, Zhang Z, Chen H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr Polym. 2012;88:84–90. [Google Scholar]
- 35.Mortazavian E, Dorkoosh FA, Rafiee-Tehrani M. Design, characterization and ex vivo evaluation of chitosan film integrating of insulin nanoparticles composed of thiolated chitosan derivative for buccal delivery of insulin. Drug Dev Ind Pharm. 2014;40:691–8. doi: 10.3109/03639045.2014.886590. [DOI] [PubMed] [Google Scholar]


