Abstract
GABAA receptors containing α4 subunits are widely implicated in acute ethanol sensitivity, and their spatial and temporal regulation prominently contributes to ethanol-induced neuroplasticity in hippocampus and cortex. However, it is unknown if α4-containing GABAA receptors in the thalamus, an area of high α4 expression, display similar regulatory patterns following ethanol administration, and if so, by which molecular mechanisms. In the current study, thalamic GABAA receptor α4 subunit levels were increased following a 6-week, but not a 2-week chronic ethanol diet. Following acute high-dose ethanol administration, thalamic GABAA receptor α4 subunit levels were regulated in a temporal fashion, as a decrease was observed at 2 hours followed by a delayed transient increase. PKCγ and PKCδ levels paralleled α4 temporal expression patterns following ethanol exposure. Initial decreases in α4 subunit expression were associated with reduced serine phosphorylation. Delayed increases in expression were not associated with a change in phosphorylation state, but were prevented by inhibiting neuroactive steroid production with the 5α-reductase inhibitor finasteride. Overall, these studies indicate that thalamic GABAA receptor α4 subunit expression following acute and chronic ethanol administration exhibits similar regulatory patterns as other regions and that transient expression patterns following acute exposure in vivo are likely dependent on both subunit phosphorylation state and neuroactive steroids.
Keywords: Ethanol, Thalamus, GABA Receptors, Alpha4 Subunit, PKC, Neuroactive Steroids
INTRODUCTION
Ethanol exposure impacts a number of neurotransmitter systems within the brain, and the plasticity of such systems likely underlies tolerance, dependence, and potentially alcohol addiction. Much evidence supports a prominent role of γ-aminobutyric acid type A (GABAA) receptors in ethanol-induced neuroadaptations (see: Kumar et al., 2009). GABAA receptors are a family of heteropentameric ligand-gated chloride ion channels that mediate the majority of rapid synaptic inhibition within the central nervous system. Although over 19 different subunits exist, most GABAA receptors contain 2α, 2β, and either a γ or δ subunit (Olsen and Sieghart, 2009). Receptors with α1 and γ2 subunits tend to be localized synaptically and contribute to phasic inhibition, whereas receptors containing α4 and δ subunits are extrasynaptic and contribute to tonic inhibition (Farrant and Nusser, 2005). GABA, ethanol, and endogenous neuroactive steroids are more potent at extrasynaptic than synaptic receptor subtypes (Santhakumar et al., 2006; Wei et al., 2004). Although α4-containing receptors are less prevalent than other α subunit types, they exhibit diffuse expression throughout the brain and are particularly enriched in thalamic and hippocampal regions (Chandra et al., 2006).
Multiple studies strongly support the downregulation of ethanol-sensitive extrasynaptic α4-containing receptors in response to acute and chronic ethanol exposure as well as increases in synaptic α4-containing GABAA receptors paired with γ2 subunits (Liang et al., 2007; Liang et al., 2006). Increases in α4- and γ2-containing GABAA receptors result in reduced net postsynaptic inhibitory responses. Much of our understanding of α4-containing receptor regulation following ethanol exposure has been conducted in cortical and hippocampal regions, but little is understood regarding thalamic α4-containing receptors.
Work from our lab and elsewhere strongly implicates protein kinase C (PKC) involvement in α4-containing GABAA receptor trafficking. In particular, not only does PKCγ co-localize with GABAA receptor α4 subunits, but their association is increased following chronic ethanol exposure in vivo (Kumar et al., 2002). Furthermore, our in vitro studies not only demonstrate that PKC activity is necessary for increases in GABAA receptor α4 subunit expression, but knocking down PKCγ prevents increases in α4 subunit expression (Werner et al., 2011). Despite these outcomes, it remains unknown whether rapid changes in response to acute ethanol exposure in vivo involve PKC regulation. PKCδ co-localizes with extrasynaptic receptors and modulates enhanced tonic inhibition by ethanol in thalamic relay neurons (Choi et al., 2008). Furthermore, as GABAA receptor δ subunits do not appear to contain a PKC substrate (Abramian et al., 2010), and endocytosis of extrasynaptic receptors is independent of β3 subunit dephosphorylation (Gonzalez et al., 2012), the phosphorylation state of α4-containing receptors may also be a contributing factor. Conversely, PKC-independent factors such as neuroactive steroids may also be involved (Gulinello et al., 2001; Shen et al., 2005).
In the present study, we explored whether thalamic GABAA receptors are regulated similar to other brain regions following acute and chronic ethanol exposure in vivo. Further, we investigated PKC isoform expression in parallel with rapid reductions in GABAA receptor α4 subunit expression and serine phosphorylation as well as the role of neuroactive steroids in these adaptations.
MATERIALS AND METHODS
Subjects
All experiments were conducted in accordance with guidelines from the National Institutes of Health and Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. Adult male Sprague-Dawley rats (~10–12 weeks of age) were purchased from Harlan (Indianapolis, IN, USA). For acute ethanol exposure experiments, rats were group housed and maintained with ad libitum access to rat chow and water. For chronic ethanol experiments, rats were single-housed and maintained on a liquid diet (see below), but had ad libitum access to water. All rats were maintained on a standard 12-hour light-dark schedule with lights on at 7:00 AM.
Drugs
Ethanol was obtained from Pharmco-AAPER (Brookfield, CT) for use in all experiments. The 5α-reductase inhibitor finasteride (100 mg/kg, Steraloids, Newport, RI) was dissolved in 20% beta-cyclodextrin (2 ml/kg) and administered 1-hour prior to ethanol administration (Khisti et al., 2003; VanDoren et al., 2000).
Ethanol exposure
For acute ethanol experiments, rats were injected with 3.5 g/kg ethanol (20% v/v in saline, intraperitoneally) and tissue was collected at various time points between 1 and 48 hours. Chronic ethanol exposure was conducted similar to prior studies (Boyd et al., 2010; Kumar et al., 2002). Individually housed rats were given free access to a nutritionally complete liquid diet for 3 days (Custom Stanley Diet, MP Biomedicals, Solon, OH, USA), after which they received 6% v/v ethanol for 1 week and 7.5% ethanol for subsequent weeks. Control rats were fed identical diet, but with dextrose as an isocaloric substitute. All dietary consumption was monitored daily. Rats consumed between 6–10 g/kg ethanol per day. Mean body weights for control and ethanol diet rats did not differ following completion of the experiment (p > 0.05). Tissue was collected following either 2- or 6-weeks of chronic ethanol diet. Ethanol-exposed animals had continual access to ethanol diet up until tissue harvesting. All acute ethanol exposures and tissue harvesting were conducted during morning time periods between the beginning of the light cycle (7:00am) and noon.
Tissue and protein preparation
P2 synaptosomal fractions from thalamic and cerebral cortical tissue were prepared as described elsewhere (Werner et al., 2011). Briefly, samples were homogenized in 320 mM sucrose in PBS followed by low-speed centrifugation after which the supernatant was spun at 12,000×g for 20 min. The resulting pellet (P2) was resuspended in phosphate buffered saline with phosphatase inhibitor cocktail (1:100 dilution; a proprietary mixture of microcystin LR, cantharidin, and bromotetramisole; Sigma, St. Louis, MO). For analysis of total lysate, samples were lysed in a homogenization buffer (1% SDS, 1mM EDTA, and 10mM Tris) as noted elsewhere (Grosshans et al., 2002). All protein concentrations were determined through use of a bicinchoninic acid protein assay and stored at -80°C until further use.
Western blot analysis
P2 synaptosomal fractions were denatured and separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene-difluoride membranes (Life Technologies, Carlsbad, CA). Membranes were probed with antibodies for the following proteins: GABAA receptor α4 and α1 subunits (Millipore, Billerica, MA; 1:250 and 1:500, respectively); GABAA receptor δ subunit (Santa Cruz Biotechnology, Santa Cruz, CA, 1:250); GABAA receptor γ2 subunit (kind gift from Jean-Marc Fritschy, University of Zurich, Zurich Switzerland 1:1000); and PKCβ, γ, δ, and ε (BD Biosciences, San Jose, CA, 1:500). Bands were visualized using enhanced chemiluminescence (GE Healthcare, Amersham, UK) under nonsaturating conditions. Blots were then exposed to an actin specific antibody (Millipore, Billerica, MA, 1:1000) for normalization. Densitometric analysis was conducted using NIH Image J. Data were analyzed using Student’s t-test or ANOVA with Newman-Keul’s post hoc where appropriate.
Phosphoserine Immunoprecipitation
P2 membrane fractions (650 µg) were solubilized and denatured in modified radioimmunoprecipitation (RIPA) buffer (Sigma-Aldrich) with PMSF (1 mM), leupeptin (1 µg/µL), sodium fluoride (50 mM), sodium vanadate (200 µM) and EDTA (2 mM) to prevent protein degradation and dephosphorylation as described elsewhere (Kumar et al., 2006). Solubilized protein was centrifuged at 10,000×g for 30 min. The resulting supernatant was incubated overnight with 100 µL of anti-phosphoserine specific antibody (Abcam, Cambridge, MA) linked to magnetized Dynabeads (Life Technologies). The receptor-antibody-bead solution was washed with PBS three times followed by boiling in SDS. Beads were separated from the immunoprecipitate by magnetic exposure. Immunoprecipitated serine phosphorylated protein was analyzed by SDS-PAGE and western blot analysis using the GABAA receptor α4 subunit antibody and normalized to total α4 expression in the P2 fraction. Data were analyzed using Student’s t-test.
3α,5α-THP measurements
3α,5α-THP [(3α,5α)-3-hydroxypregnan-20-one or allopregnanolone] was measured using gas chromatography-mass spectrometry as described elsewhere (Porcu et al., 2009). Serum samples (300 µL) were spiked with 400 pg/ml of deuterated internal standard (d4–17,21,21,21-3α,5α-THP, Cambridge Isotope Laboratories, Inc., Andover, MA, USA) and applied to C18 solid phase extraction columns (RPN1910, 500 mg, GE Healthcare, UK) preconditioned with methanol and distilled water. The samples were washed with distilled water to eliminate polar impurities. 3α,5α-THP was eluted with methanol, and subsequent extracts were dried. Dry residues were resuspended in ethyl acetate/methanol (80/20 v/v) and then filtered through a NH2 column (Supelclean LC-NH2, 500 mg, Supelco, Bellefonte, Pa, USA) previously conditioned with ethyl acetate and ethyl acetate/methanol (80/20 v/v). 3α,5α-THP passed unretained through the sorbent. The percent accuracy for 3α,5α-THP by combining C18 and NH2 column purification is approximately 90% as noted elsewhere (Porcu et al., 2009). Samples were derivatized with heptafluorobutyric anhydride (Pierce, Rockford, IL, USA) then resuspended in 10 µL of heptane of which 2µL were injected in duplicate into an Agilent 6890 gas chromatograph coupled to a 5975 mass selective detector (Agilent Technologies, Inc., Santa Clara, CA, USA) operated in negative chemical ionization mode. Data were analyzed using ANOVA as above.
RESULTS
GABAA receptor α4 subunit upregulation in the thalamus following chronic ethanol diet
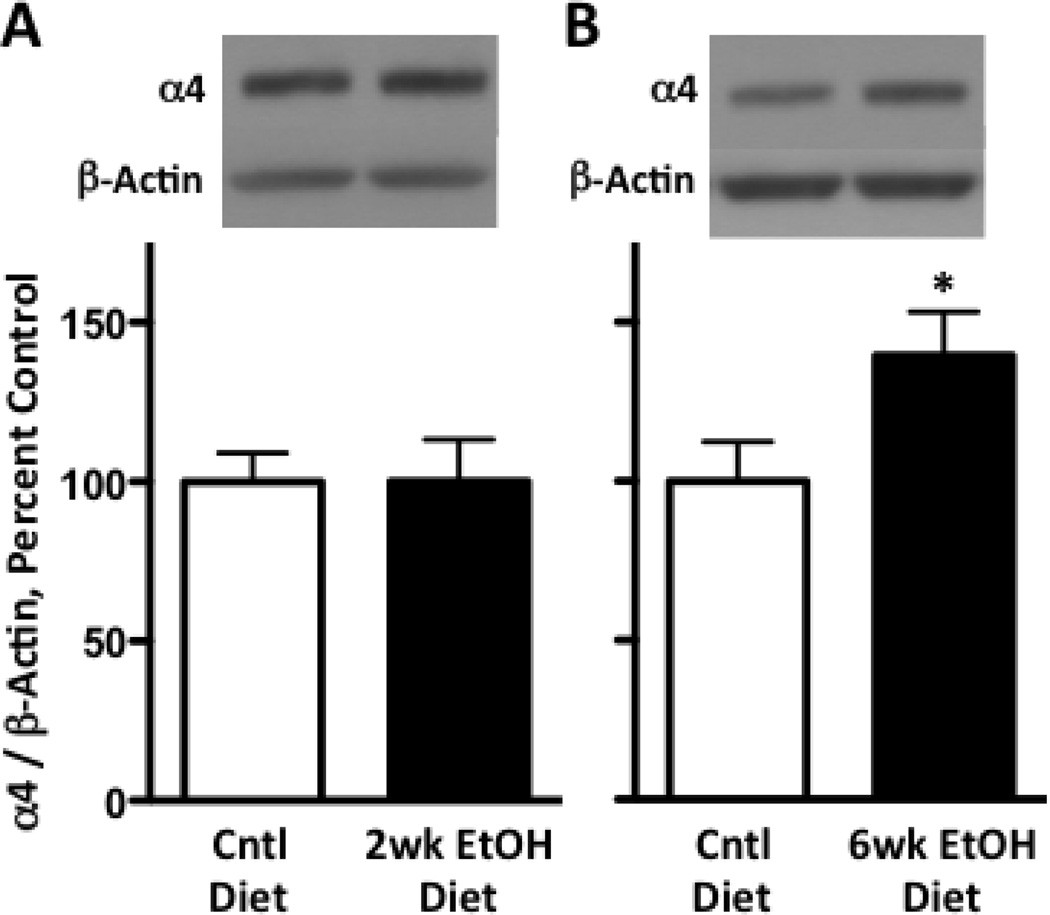
Ethanol exposure is known to regulate GABAA receptor α4 subunit expression, (Grobin et al., 2000; Kumar et al., 2002; Matthews et al., 1998) but it is not known if such effects occur in all regions, such as the thalamus, where α4 expression is abundant. Therefore, we initially investigated whether a 2-week chronic ethanol exposure would alter thalamic GABAA receptor α4 subunit expression. Thalamic GABAA receptor α4 expression in animals exposed to the ethanol diet did not differ from those given a calorically equivalent control diet (Figure 1A). To determine whether the ethanol diet was effective, cortical GABAA receptor α1 and α4 levels were assessed which we previously have shown are changed by 2-week chronic ethanol diet (Grobin et al., 2000; Kumar et al., 2002). Consistent with prior reports, ethanol increased cortical α4 subunit expression by 27.4 ± 10.2% compared to controls (p < 0.05, n = 5–6/group, not shown) whereas α1 subunit expression was decreased by 35.0 ± 5.5% compared to controls (p < 0.05, n =4/group, not shown). We next determined whether a lengthened ethanol exposure period would alter thalamic GABAA receptor α4 subunit expression, as noted for hippocampal tissue (Matthews et al., 1998). Following a 6-week ethanol exposure, α4 subunit expression was increased by 39.5 ± 13.7% as compared to controls (p < 0.05, Figure 1B).
Figure 1. Six weeks, but not two weeks chronic ethanol diet increases thalamic GABAA receptor α4 subunit expression.
Rats were given either a chronic ethanol or isocaloric diet for 2-weeks (A) or 6-weeks (B). Thalamic tissue was isolated followed by preparations of P2 synaptosomal fractions. Western blot analysis revealed that A) GABAA receptor α4 subunit expression was unaffected after 2-weeks, B) GABAA receptor α4 subunit expression was increased by 39.5 ± 13.7% after 6-weeks. Graphs show the mean ± SEM of percent control values normalized to β-actin levels (n=7–8 per group). * p < 0.05 compared to control diet (Student’s t-test).
A single high dose ethanol exposure regulates thalamic GABAA receptor α4 subunit expression
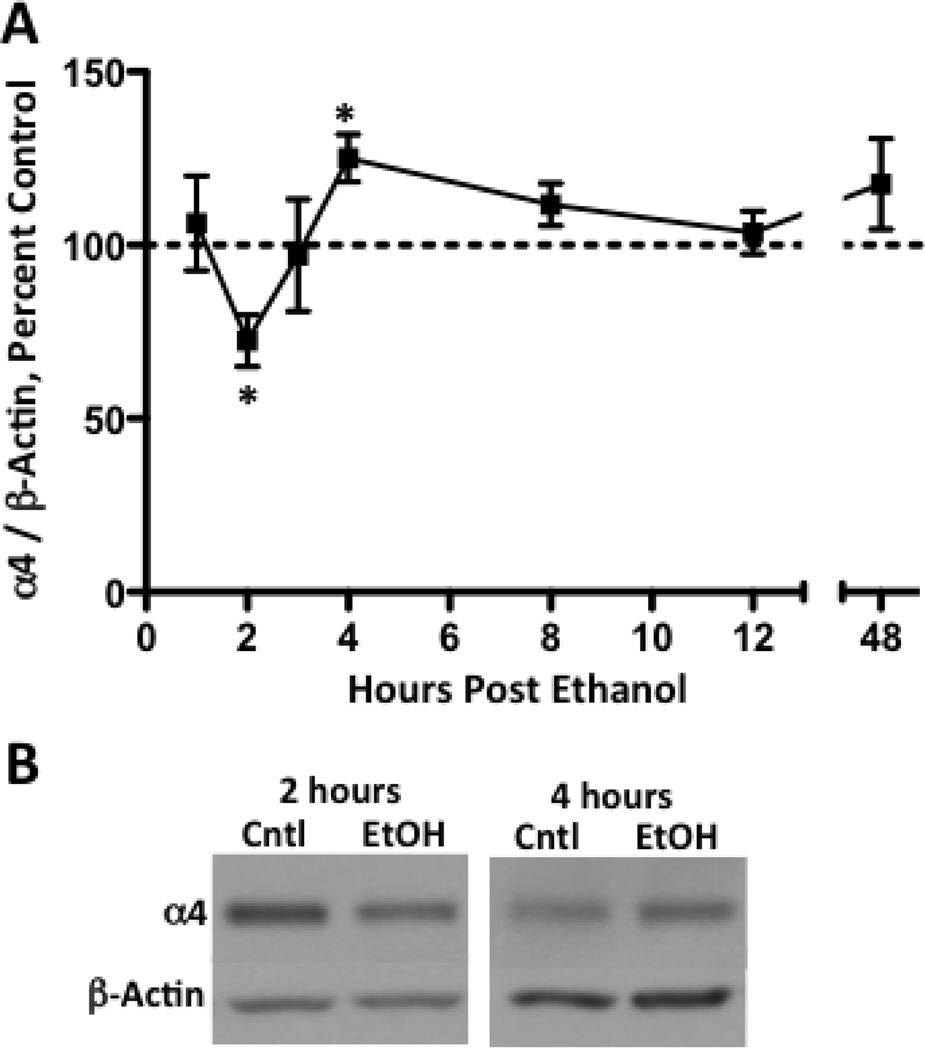
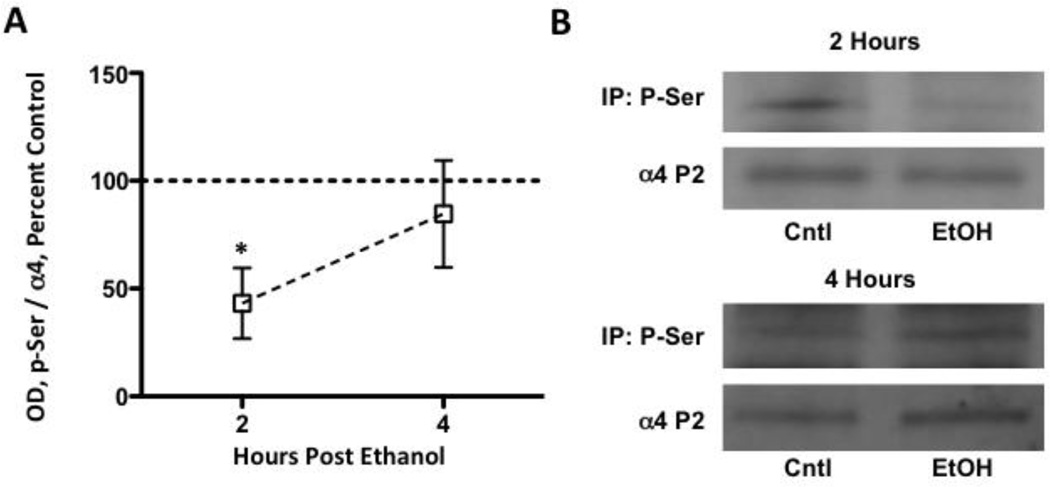
Increased α4 subunit expression following chronic ethanol exposure is associated with increased transcription/translation (see: Kumar et al., 2009); however, recent in vitro and in vivo studies demonstrate that acute high dose ethanol exposure rapidly regulates GABAA receptor α4 subunit expression (Liang et al., 2007; Pignataro et al., 2007; Werner et al., 2011). Therefore, we next assessed whether thalamic α4 subunit expression displayed a similar rapid adaptation following a single high dose ethanol exposure. Analysis revealed transient temporal changes. Specifically, P2 synaptosomal GABAA receptor α4 subunit expression was reduced 2-hours post ethanol exposure (Figure 2 A, B: ethanol, 72.4 ± 7.4, control, 100 ± 5.4, p < 0.05). GABAA receptor α4 subunit expression returned to basal levels by 3-hours, but by 4-hours post ethanol, expression was increased (ethanol, 125.1 ± 6.9, control, 100 ± 6.9, p < 0.05). GABAA receptor α4 subunit expression returned to baseline levels by 8-hours and did not differ at later time points.
Figure 2. Acute ethanol administration transiently alters thalamic GABAA receptor α4 subunit expression.
Graph denotes time course for GABAA receptor α4 subunit levels at various time points following ethanol administration (A). α4 subunit expression is decreased by 27.6 ± 7.4% at 2-hours, and is increased by 25.1 ± 6.9% at 4-hours. Representative blots are shown for both the 2- and 4-hour time points (B). Data are presented as mean ± SEM. n = 7–10 per group, in duplicate. * p < 0.05 compared to vehicle controls (Student’s t-test).
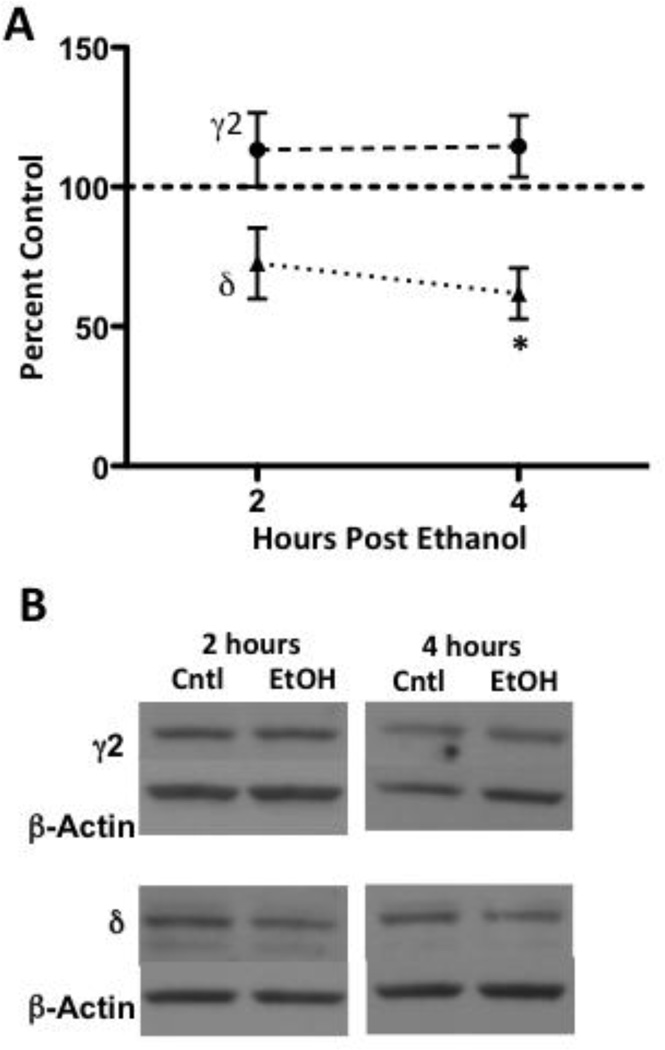
We next examined total thalamic GABAA receptor α4 subunit expression in whole cell lysates to determine whether increased synaptosomal GABAA receptor α4 subunit expression following acute ethanol exposure was due to transcription/translation-related processes or limited to synaptic regions. Total GABAA receptor α4 subunit expression did not differ between controls and ethanol-exposed subjects (100.0 ± 10.3 and 99.4 ± 19.3, respectively, n = 4/group; not shown), thereby suggesting that synaptosomal changes are likely the result of post-translational receptor regulation. To gain some insight as to which GABAA receptor subtypes may be changing at the 2- and 4-hour time points, we next assessed GABAA receptor γ2 and δ subunit expression. GABAA receptor δ subunit expression displayed a trend towards a reduction 2 hours following acute ethanol exposure (Figure 3A, B: 72.6 ± 12.7%, p = 0.055), whereas δ subunit expression was statistically decreased at 4 hours (61.8 ± 9.2, p< 0.05). Conversely, γ2 did not significantly differ at either time point (Figure 3A, B). Taken together, this data suggests that α4/δ-containing GABAA receptor subtypes were initially decreased following acute ethanol exposure, whereas α4-containing receptors independent of δ are likely transiently increased.
Figure 3. Acute ethanol administration reduces GABAA receptor δ subunit expression.
Graph denotes time course for GABAA receptor γ2 and δ subunit levels at time points related to changes in α4 subunit expression following ethanol administration (A). GABAA receptor δ subunit expression was decreased at 4 hours by 38.2 ± 9.2%, and a trend for a reduction was noted at 2 hours (27.4 ± 12.7%). Representative blots are shown for both subunits (B). Data are presented as mean ± SEM. n = 5–8 per group, in duplicate. * p < 0.05 compared to vehicle controls (Student’s t-test).
Reduced phosphorylation state associates with immediate decreases in thalamic GABAA receptor α4 subunit expression following acute ethanol exposure
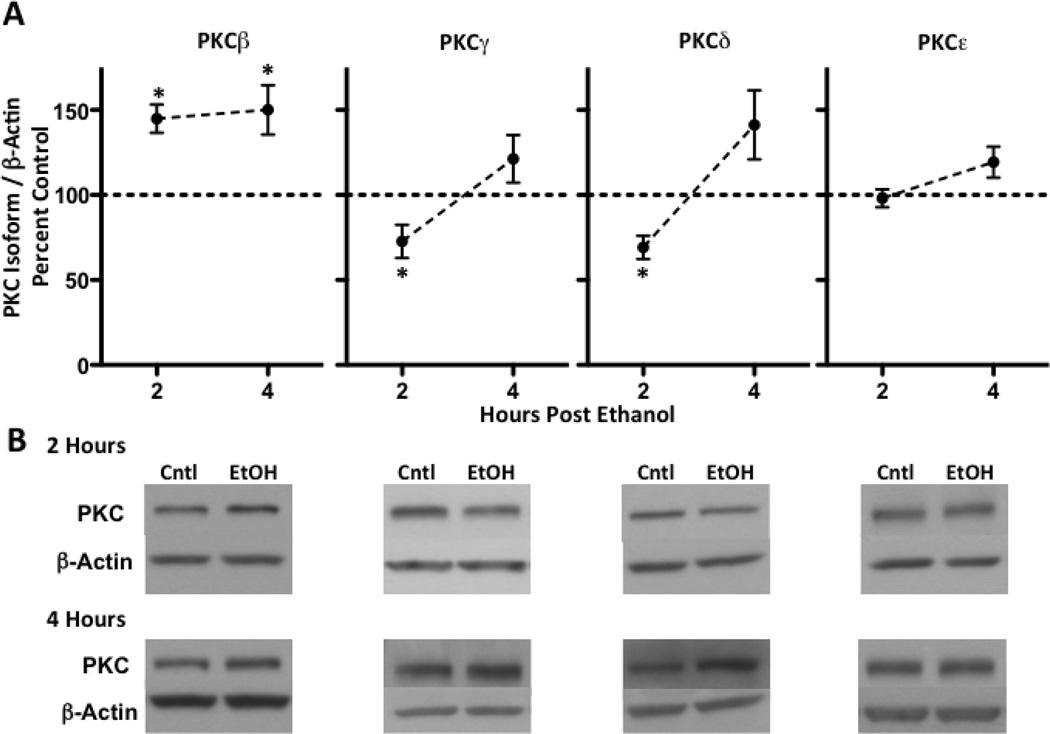
We next determined whether PKC isoform regulation associated with changes in GABAA receptor α4 subunit expression. Similar to GABAA receptor α4 subunits, we noted that both PKCγ and PKCδ expression were decreased at 2-hours post ethanol exposure by 27.3 ± 9.7 and 30.8 ± 6.9%, respectively (Figure 4A, B). At 4-hours, however, both PKCγ and PKCδ had returned to baseline levels. Conversely, PKCβ expression was increased at both time points (44.8 ± 8.3 and 50.0 ± 14.5%, respectively), whereas PKCε was unaffected at either time point (Figure 4A, B). Finally, because of the similar directional changes in P2 synaptosomal GABAA receptor α4 as well as PKCγ and PKCδ expression, we assessed whether GABAA receptor α4 subunit serine residue phosphorylation was impacted. 2-hours post ethanol exposure, serine phosphorylated α4 subunits were reduced by 56.8 ± 16.3% compared to controls (p < 0.05, Figure 5A, B), but no difference was detected between groups at 4-hours.
Figure 4. Acute ethanol administration selectively alters thalamic PKC isoform expression in a temporal specific fashion.
PKCβ, γ, δ and ε isoforms were examined at time points related to changes in GABAA receptor α4 subunit expression (A). Both PKC γ and PKCδ were deceased at 2-hours, but were similar to controls at 4-hours. PKCβ remained elevated at both time points. Representative blots are shown for each PKC isoform at 2- and 4-hours (B). Data are presented as mean ± SEM. n = 8–10 per group, in duplicate. * p < 0.05 compared to vehicle controls (Student’s t-test).
Figure 5. Acute ethanol administration modulates thalamic GABAA receptor α4 subunits serine phosphorylation.
Acute ethanol administration reduced phosphoserine immunoprecipitation of GABAA receptor α4 subunit levels at 2-hours, but not 4-hours, following ethanol exposure (A). Representative blots are shown for each time point (B). Data are presented as mean ± SEM. n = 4–5 per group. * p < 0.05 compared to vehicle controls (Student’s t-test).
Neuroactive steroids contribute to delayed increases in thalamic GABAA receptor α4 subunit expression
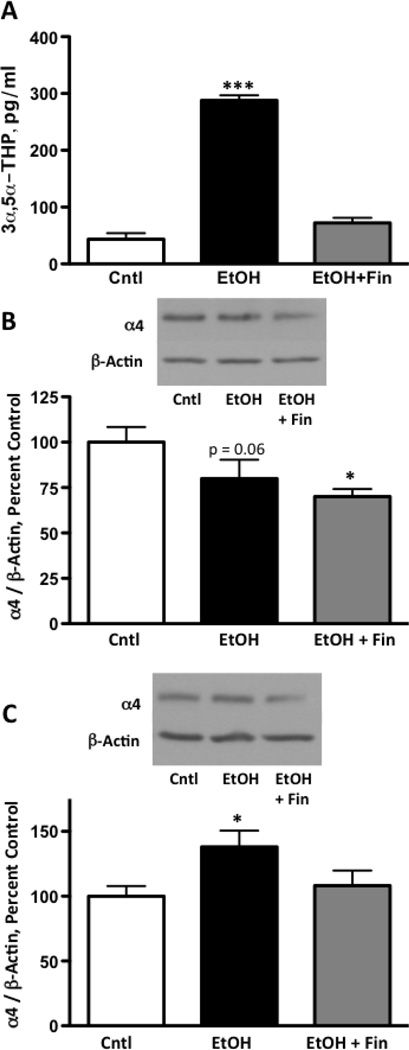
While the pattern of PKCγ and PKCδ expression changes are parallel with GABAA receptor α4 subunit expression, these PKC isoforms may not account for the changes since the level of serine phosphorylation was not increased compared to controls at 4-hours. Higher ethanol doses are known to increase systemic neuroactive steroids, which are potent modulators of α4-containing receptors (Gulinello et al., 2001; Shen et al., 2005); therefore we assessed whether ethanol-induced elevations in neuroactive steroids contributed to GABAA receptor α4 subunit regulation by inhibition of ethanol-induced elevations in these steroids using finasteride. Initial assessment of finasteride treatment indicated an effect of treatment (F2,14 =200.0, p < 0.0001). Further analysis indicated that 3α,5α-THP was higher 2-hours post ethanol exposure compared to controls, but that finasteride pretreated animals exposed to ethanol did not differ from controls, indicating that ethanol-induced increases in 3α,5α-THP were abolished (Figure 6A). Analysis of GABAA receptor α4 expression at 2 hours post ethanol exposure revealed an effect of finasteride treatment (F2,15 = 5.69, p < 0.05). Further analysis indicated a highly suggestive reduction in α4 by ethanol alone (80.0 ± 10.3% compared to controls, p = 0.06), similar to our initial 2-hour results (Figure 6B). Similarly, finasteride pre-treatment also reduced α4 expression (70.1 ± 4.2% compared to controls, p < 0.05). At 4 hours post ethanol exposure, an effect of finasteride treatment was found (F2,33 = 3.39, p < 0.05; Figure 6C). Further analyses indicated that although ethanol alone increased α4 subunit expression by 38.0 ± 12.6%, animals pretreated with finasteride prior to ethanol exposure did not differ from controls. Finasteride exposure alone did not alter basal levels of 3α,5α-THP (vehicle control − 99.9 ± 7.1 pg/mL; finasteride − 97.1 ± 7.4 pg/mL, p > 0.05), nor did it affect basal levels of GABAA receptor α4 subunit expression (vehicle control, 100.0 ± 13.0%; finasteride, 97.1 ±13.9%, p > 0.05). Taken together, these results suggest that the neuroactive steroids such as 3α,5α-THP contribute to increases in GABAA receptor α4 subunit expression.
Figure 6. 3α,5α-THP modulates GABAA receptor α4 subunit expression following acute ethanol administration.
Administration of the 5α-reductase inhibitor finasteride prevents ethanol-induced increases in peripheral 3α,5α-THP (A). Finasteride does not alter ethanol-induced changes in GABAA receptor α4 subunit expression at 2-hours (B), but reverses increases at 4-hours (C). Data are presented as mean ± SEM. Representative blots are shown for 2- and 4-hours. n = 6–12 per group. * p < 0.05, and *** p < 0.001 compared to vehicle controls (one-way ANOVA with Newman-Keuls posthoc).
DISCUSSION
The present study demonstrates that thalamic GABAA receptor α4 subunit expression is regulated following acute and chronic ethanol exposure. Chronic ethanol exposure of 6-weeks, but not 2-weeks, increased P2 synaptosomal GABAA receptor α4 subunit expression. Following acute ethanol exposure, P2 synaptosomal α4 subunit expression was more dynamic, with levels decreased at 2-hours followed by a transient increase at 4-hours before returning to baseline levels by 8-hours. Early reductions in GABAA receptor α4 subunit expression following acute ethanol exposure were paralleled by reductions in both PKCγ and PKCδ isoforms as well as serine phosphorylation of GABAA receptor α4 subunits. In contrast, the delayed increase was associated with a restoration of both PKC isoforms and phosphorylation. Further, the transient increase in GABAA receptor α4 subunit expression at 4-hours was dependent on 5α-reductase derived neuroactive steroids, as it was ablated by finasteride pretreatment.
The finding that GABAA receptor α4 subunit was increased following chronic ethanol exposure is not surprising as this effect has been demonstrated by several other groups in various brain regions (Devaud et al., 1997; Liang et al., 2006). Further, the lack of change in α4 subunit following 2-weeks of chronic ethanol diet is consistent with observations in hippocampus, where 2-week exposure does not alter GABAA receptor α4 subunit levels (Matthews, 1998) but 40 day exposure induces a significant elevation. In agreement, increased hippocampal P2 fraction GABAA receptor α4 subunit expression was also found following chronic intermittent ethanol exposure for 60 days (Cagetti et al., 2004) and this change is driven by elevation of synaptic α4γ receptors that mediate hippocampal mIPSPs (Liang et al., 2006). Similar results are found in cortical neurons where elevations in P2 synaptosomal α4 receptors are driven by elevations in synaptic α4γ receptors that alter mIPSC decay tau (Werner et al., 2011). It is possible that distinct molecular mechanisms account for the temporal delay in GABAA receptor α4 subunit adaptations observed in hippocampus and thalamus as opposed to cortex where increases in α4 are seen much earlier. Our current results indicate that neuroactive steroid levels may contribute to acute increases in α4 subunit expression in the thalamus.; however, neuroactive steroid levels are reduced following chronic ethanol exposure (Janis et al., 1998). Speculatively, such mechanisms may involve the spatial and temporal activation of heat shock factors that alter the transcription of ethanol responsive genes such as α4 (Pignataro et al., 2007). Alternatively, it is possible that neuroactive steroids modify phosphorylation of GABAA receptors and this contributes to the effects on GABAA a4 subunit expression (Adams paper). Further studies are required to explain the regional differences in response to ethanol exposure for 2-weeks. Additionally, as rodents are known to display higher consumption during the beginning of the dark period of their light cycle, we cannot rule out that elevated α4 expression may be due to ethanol withdrawal.
Following acute ethanol exposure, our data suggest that P2 synaptosomal GABAA receptor α4 subunits are transiently regulated with a decrease at 2-hours and an increase at 4-hours. Although the regulation of specific GABAA receptor subtypes is not definitive, it is likely that δ-containing receptors are decreased at both time points as there was a suggestive reduction in the δ subunit at 2-hours that persisted at 4-hours. The transient fluctuations in α4 subunits, coupled with γ2 subunit levels not differing following ethanol exposure further supports the idea that perisynaptic trafficking of α4δ receptors occurs in response to ethanol exposure and extends previous reports (Liang et al., 2007). In support of this interpretation, recent work strongly suggests that α4 subunits are necessary for intracellular trafficking of δ subunits and membrane expression (Peng et al., 2013; Sabaliauskas et al., 2012). Potentially, increased pairing of α4-GABAA receptor subunits, potentially with γ2, would lead to extended suppression of δ subunit expression. Future studies will more definitively address this issue. Nonetheless, these results confirm and extend studies examining α4δ subunit-containing GABAA receptors following acute ethanol exposure (Gonzalez et al., 2012; Liang et al., 2007; Suryanarayanan et al., 2011).
The parallel reductions in α4-containing GABAA receptors with PKCγ and PKCδ 2-hours following ethanol exposure suggest potential mechanisms of GABAA α4 receptor regulation. As PKCδ has been shown to co-localize with δ-containing GABAA receptors in the hippocampus and thalamus (Choi et al., 2008), it is likely that the decrement of PKCδ contributes to thalamic α4δ receptor trafficking. Since PKCγ co-immunoprecipitates with α4 receptors (Kumar et al., 2002) and is required for α4 receptor upregulation in cortex following ethanol exposure (Werner et al., 2011), the decrease in PKCγ at 2-hours may prevent up-regulation of α4 receptors at this time point. Decrements in phosphorylation may allow for PKC-independent mechanisms, such as PKA, to more readily internalize α4-containing GABAA receptors (Carlson et al., 2014), as knockdown of PKCγ and blocking PKC activity did not reduce basal levels of α4 expression (Werner et al., 2011). Thalamic PKCγ expression is also highly consistent with GABAA receptor α4 subunit expression across multiple thalamic nuclei, with the exception of the reticular nuclei, which is devoid of both (Ding et al., 2005; Jia et al., 2005). Further work using PKCγ and PKCδ knockouts or thalamic specific knockdown may help elucidate the respective contributions of each PKC isoform.
The present results indicate that the state of GABAA receptor α4 subunit serine phosphorylation is altered in conjunction with GABAA α4 subunit expression. However, it is unknown which serine residue is necessary for GABAA receptor α4 subunit regulation. Serine 443 is the most likely residue involved given that its phosphorylation increases cell surface stability of α4-containing receptors (Abramian et al., 2010). However, the same study also indicated that basal endocytosis of α4 receptors with the serine 443 site mutated did not differ from wildtype receptors. Thus, while our data highly suggest α4 subunit phosphorylation may be a contributing factor following ethanol exposure, we cannot exclude that downregulation of α4δ receptors may also involve phosphorylation of GABAA receptor β subunits (Bright and Smart, 2013); however, interactions between clathrin adaptor proteins and the δ subunit in response to ethanol exposure lessens this possibility (Gonzeles et al., 2012).
It is unclear at present how decrements in α4-containing GABAA receptors and phosphorylation in the thalamic P2 fraction contribute to acute ethanol’s phenotypic responses. It is unlikely that transient effects from a single exposure contribute to withdrawal effects, as synaptosomal changes occurred when blood ethanol are known to be greater than 300 mg/dL as we have reported elsewhere (see: Carter et al., in press). GABAA receptor α4 knockout mice displayed normal behavioral responsivity to acute ethanol exposure (Chandra et al., 2008); however, viral-mediated knockdown of α4 subunit levels in the nucleus accumbens shell reveals that α4 expression impacts ethanol operant responding (Rewal et al., 2012). Given that thalamic extrasynaptic α4-containing GABAA receptors mediate tonic inhibitory currents that are potentiated at socially relevant ethanol concentrations (Chandra et al., 2008; Jia et al., 2008), reducing the number of ethanol sensitive receptors is likely a rapid mechanism to ameliorate ethanol’s increased central nervous system inhibition and behavioral responses. Such rapid adaptation in the thalamus is particularly important given its relevance to cortical circuitry (e.g., Vijayan et al., 2013). Furthermore, it is possible that rapid adaptations may be in response to ethanol-induced elevations in synaptic vesicle proteins and vesicular GABA release (Varodayan and Harrison, 2013; Weiner and Valenzuela, 2006), but further work would need to be done to critically assess such effects.
Neuroactive steroids, including 3α,5α-THP, are potent modulators of extrasynaptic receptor function and expression (Belelli et al., 2002; Brown et al., 2002; Shen et al., 2007; Wohlfarth et al., 2002), particularly related to ovarian cycles (Maguire et al., 2005). We found that initial reductions in GABAA receptor α4 subunit expression in vivo were independent of neuroactive steroids; however, delayed increases following ethanol exposure were dependent on 5α-reductase derived neuroactive steroids. Although acute administration of 3α,5α-THP has been shown to increase α4-containing GABAA receptors (Shen et al., 2005), Neuroactive steroids alone are likely not sufficient for ethanol-induced increases in α4, as 3α,5α-THP and THDOC are rapidly increased in serum following ethanol exposure (VanDoren et al., 2000; Porcu et al., 2010) when α4 levels are initially decreased. More likely, the increases in GABAA receptor α4 subunit expression are the result of multimodal molecular events. Recent evidence indicating that neuroactive steroids may work in concert with PKC activity to regulate α4-containing receptor trafficking and functioning (Abramian et al., 2014; Adams et al., 2015) further supports this idea.
In summary, the present results suggest that initial decreases in thalamic GABAA receptor α4 subunit following acute ethanol exposure are associated with decreases in serine phosphorylation and correlate with decreases in PKCγ and PKCδ isoforms, whereas delayed increases are dependent on 5α-derived neuroactive steroids and restoration of α4 phosphorylation in vivo. Further work is required to determine the subtypes of α4 receptors that are affected by these changes.
Highlights.
Thalamic GABAA receptor alpha4 subunit expression is increased following chronic ethanol exposure
Thalamic GABAA receptor alpha4 subunit expression is regulated following a single high dose ethanol exposure.
Decreases in GABAA receptor alpha4 subunit expression is accompanied by reduced phosphorylation states.
Neuroactive steroids contribute to delayed increases in thalamic GABAA receptor alpha4 subunit expression.
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism grants: AA011605, AA015409, AA007573. The authors would also like to thank Justine Landin for reviewing and editing drafts of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- Abramian AM, Comenencia-Ortiz E, Modgil A, Vien TN, Nakamura Y, Moore YE, Maguire JL, Terunuma M, Davies PA, Moss SJ. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. The Journal of biological chemistry. 2010;285:41795–41805. doi: 10.1074/jbc.M110.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Thomas P, Smart TG. Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type GABAA receptors. Neuropharmacology. 2015;88:63–73. doi: 10.1016/j.neuropharm.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Boyd KN, Kumar S, O'Buckley TK, Morrow AL. Chronic ethanol exposure produces tolerance to elevations in neuroactive steroids: mechanisms and reversal by exogenous ACTH. Journal of neurochemistry. 2010;115:142–152. doi: 10.1111/j.1471-4159.2010.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Smart TG. Protein kinase C regulates tonic GABA(A) receptormediated inhibition in the hippocampus and thalamus. Eur J Neurosci. 2013;38:3408–3423. doi: 10.1111/ejn.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. British journal of pharmacology. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Carlson SL, O'Buckley TK, Thomas R, Thiele TE, Morrow AL. Altered GABAA Receptor Expression and Seizure Threshold Following Acute Ethanol Challenge in Mice Lacking the RIIbeta Subunit of PKA. Neurochemical research. 2014;39:1079–1087. doi: 10.1007/s11064-013-1167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM, Landin JD, Gigante ED, Rieger SP, Diaz MR, Werner DF. Inhibitors of calcium activated anion channels modulate hypnotic ethanol responses in adult Sprague-Dawley rats. Alcoholism Clin Exp Res. 2016 doi: 10.1111/acer.12957. In press. [DOI] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Werner DF, Liang J, Suryanarayanan A, Harrison NL, Spigelman I, Olsen RW, Homanics GE. Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor alpha 4 subunit knockout mice. Alcoholism, clinical and experimental research. 2008;32:10–18. doi: 10.1111/j.1530-0277.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. Journal of neurochemistry. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Xiang CX, Chen ZF. Generation and characterization of the PKC gamma-Cre mouse line. Genesis. 2005;43:28–33. doi: 10.1002/gene.20151. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of delta-containing GABAA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17874–17881. doi: 10.1523/JNEUROSCI.2535-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Fritschy JM, Morrow AL. Chronic ethanol administration alters immunoreactivity for GABA(A) receptor subunits in rat cortex in a region-specific manner. Alcoholism, clinical and experimental research. 2000;24:1137–1144. [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 2002;2002:pl8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA(A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22(9):2055–2061. [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley T, Morrow AL. Neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–265. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lane BM, Morrow AL. Differential effects of systemic ethanol administration on protein kinase cepsilon, gamma, and beta isoform expression, membrane translocation, and target phosphorylation: reversal by chronic ethanol exposure. J Pharmacol Exp Ther. 2006;319:1366–1375. doi: 10.1124/jpet.106.110890. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. Association of protein kinase C with GABA(A) receptors containing alpha1 and alpha4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. Journal of neurochemistry. 2002;82:110–117. doi: 10.1046/j.1471-4159.2002.00943.x. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12367–12377. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature neuroscience. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABA(A) receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. Journal of neurochemistry. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Zhang N, Chandra D, Homanics GE, Olsen RW, Houser CR. Altered Localization of the delta Subunit of the GABA Receptor in the Thalamus of alpha4 Subunit Knockout Mice. Neurochem Res. 2013 doi: 10.1007/s11064-013-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3alpha,5alpha/3alpha,5beta neuroactive steroids in human and rat serum. Steroids. 2009;74:463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O'Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3alpha,5alpha/3alpha,5beta neuroactiver steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34(3):432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Donahue R, Gill TM, Nie H, Ron D, Janak PH. Alpha4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of alcohol. Addict Biol. 2012;17(2):309–321. doi: 10.1111/j.1369-1600.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaliauskas N, Shen H, Homanics GE, Smith SS, Aoki C. Knockout of the γ-aminobutyric acid receptor subunit α4 reduces functional δ-containing extrasynaptic receptors in hippocampal pyramidal cells at the onset of puberty. Brain Res. 2012;1450:11–23. doi: 10.1016/j.brainres.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor alpha6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the alpha6 gene. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nature neuroscience. 2007;10:469–477. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayanan A, Liang J, Meyer EM, Lindemeyer AK, Chandra D, Homanics GE, Sieghart W, Olsen RW, Spigelman I. Subunit Compensation and Plasticity of Synaptic GABA(A) Receptors Induced by Ethanol in alpha4 Subunit Knockout Mice. Frontiers in neuroscience. 2011;5:110. doi: 10.3389/fnins.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Harrison NL. HSF1 transcriptional activity mediates alcohol induction of Vamp2 expression and GABA release. Front Integr Neurosci. 2013;7:89. doi: 10.3389/fnint.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan S, Ching S, Purdon PL, Brown EN, Kopell NJ. Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:11070–11075. doi: 10.1523/JNEUROSCI.5670-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacol Ther. 2006 doi: 10.1016/j.pharmthera.2005.11.002. In Press. [DOI] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. PKCgamma is required for ethanol-induced increases in GABA(A) receptor alpha4 subunit expression in cultured cerebral cortical neurons. Journal of neurochemistry. 2011;116:554–563. doi: 10.1111/j.1471-4159.2010.07140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]