Abstract
Purpose
To assess hemodynamic differences between aneurysms that occlude rapidly versus those occluding in delayed fashion after flow diversion in rabbits.
Methods
Thirty six elastase induced aneurysms in rabbits were treated with flow diverting devices. Aneurysm occlusion was assessed angiographically immediately prior to sacrifice at one (n=6), two (n=4), four (n=8) or eight (n=18) weeks after treatment. Aneurysms were classified into a fast occlusion group if they were completely or near completely occluded at 4 weeks or earlier, and into a slow occlusion group if they remained incompletely occluded at 8 weeks. The immediate post-treatment flow conditions in aneurysms of each group were quantified using subject-specific computational fluid dynamics and statistically compared.
Results
Nine aneurysms were classified into the fast occlusion group and six into the slow occlusion group. Aneurysms in the fast occlusion group were on average significantly smaller (fast=0.9 cm, slow=1.393 cm, p=0.024), and had smaller ostia (fast=0.144 cm2, slow=0.365 cm2, p=0.015) than aneurysms in the slow occlusion group. They also had lower mean post-treatment inflow rate (fast=0.047 mL/s, slow=0.155 mL/s, p=0.0239), kinetic energy (fast=0.519 erg, slow=1.283 erg, p=0.0468), and velocity (fast=0.221 cm/s, slow=0.506 cm/s, p=0.0582). However, the differences of the latter two variables were only marginally significant.
Conclusions
Hemodynamic conditions after flow diversion treatment of cerebral aneurysms in rabbits are associated to the subsequent aneurysm occlusion time. Specifically, smaller inflow rate, kinetic energy, and velocity seem to promote faster occlusions, especially in smaller and small-necked aneurysms. These results are consistent with previous studies based on clinical series.
Keywords: cerebral aneurysm, flow diversion, hemodynamics, rabbit model, occlusion time
INTRODUCTION
Flow diversion is increasingly being used to treat wide necked intracranial aneurysms that are difficult to treat with coils alone1–3. However, flow diversion does not immediately exclude the aneurysm from the circulation. Therefore until the aneurysm thromboses and occludes it remains exposed to mechanical loads and biological processes that could potentially cause its rupture4. Presumably reducing the time it takes to completely occlude an aneurysm could prevent complications such as delayed ruptures4,5. Predicting or controlling the occlusion time after flow diversion is therefore important to improve the outcomes of these procedures and avoid complications. The purpose of this study was to assess the differences in the hemodynamic environment generated immediately after placement of flow diverting devices between aneurysms that occluded quickly and aneurysms that remained patent in a series of experimentally created aneurysms in rabbits.
METHODS
Animal Models
A total of 36 elastase-induced aneurysms were created in New Zealand white rabbits, following the approach described by Altes et al.6. Four weeks after their creation the aneurysms were imaged with 3D rotational angiography (3DRA) and flow velocities in the surrounding vessels were measured with Doppler Ultrasound (DUS). Subsequently, the aneurysms were treated with a FD device (Pipeline Embolization Device, Covidien). Two days before embolization, the subjects were premedicated with aspirin (10 mg/Kg PO) and clopidogrel (10 mg/kg PO). This medication regime was continued for one month after embolization. Some of the rabbits employed in this work were used as part of other studies7,8 entirely unrelated to the current study. This animal research was conducted with appropriate institutional approvals.
Angiographic Evaluation
The animals were sacrificed within one week (n=6), at 2 weeks (n=4), at 4 weeks (n=8), and at 8 weeks (n=18) after treatment. Immediately prior to sacrifice angiographic imaging including 3DRA was repeated and the degree of aneurysm occlusion was categorized as: a) complete occlusion – no remnant, b) near complete occlusion – small remnant, c) incomplete occlusion – substantial filling of the aneurysm.
Hemodynamics Modeling
Computational fluid dynamics (CFD) models of the aneurysms were constructed using subject-specific vascular geometries derived from the pre-treatment 3DRA images and pulsatile flow conditions from the DUS measurements7. Models of the implanted FD devices were created and virtually deployed into the vascular models9. Vessel walls were assumed rigid, blood density was set to ρ=1.0 g/cm3 and the Newtonian blood viscosity to μ=0.04 Poise. The unsteady incompressible Navier-Stokes equations were numerically solved using finite elements and adaptive immersed unstructured grids10.
Data Analysis
Aneurysms were classified into two groups according to the end-time (time to sacrifice after treatment) and the degree of occlusion. Group 1, or “fast occlusion” group, included aneurysms that were completely or near completely occluded within one week, at 2 weeks or at 4 weeks. Group 2, or “slow occlusion” group, included aneurysms that were incompletely occluded at 8 weeks. In order to keep the fast and slow occlusion groups well defined and with no overlap, subjects not within these two groups were excluded from further analysis. The geometry and post-treatment hemodynamics of each aneurysm were characterized by computing a number of variables from the 3D CFD models. All variables considered were averaged over the aneurysm region and over the cardiac cycle. Exact definitions of the variables can be found in previous reports8,11,12. The geometric and hemodynamic variables computed over the slow and fast occlusion groups were then statistically compared using the non-parametric Wilcoxon rank-sum test. Differences were consider significant if the p-values were smaller than 0.05 (95% confidence). All statistical analyses were performed using the SAS System (version 9.4, Cary, NC).
RESULTS
The end time, degree of occlusion and corresponding occlusion group of each aneurysm are presented in Table 1. A total of 6 aneurysms were classified into the slow occlusion group and 9 into the fast occlusion group. The mean and standard deviation of geometric and hemodynamic variables computed over these two groups are presented in Table 2 along with the corresponding p-values.
Table 1.
End point, occlusion status and grouping of aneurysms.
| Case | End-time | Occlusion | Group |
|---|---|---|---|
| 1 | < 1 week | incomplete | - |
| 2 | < 1 week | incomplete | - |
| 3 | < 1 week | incomplete | - |
| 4 | < 1 week | near complete | fast |
| 5 | < 1 week | near complete | fast |
| 6 | < 1 week | complete | fast |
| 7 | 2 weeks | incomplete | - |
| 8 | 2 weeks | near complete | fast |
| 9 | 2 weeks | incomplete | - |
| 10 | 2 weeks | incomplete | - |
| 11 | 4 weeks | near complete | fast |
| 12 | 4 weeks | near complete | fast |
| 13 | 4 weeks | incomplete | - |
| 14 | 4 weeks | complete | fast |
| 15 | 4 weeks | incomplete | - |
| 16 | 4 weeks | incomplete | - |
| 17 | 4 weeks | near complete | fast |
| 18 | 4 weeks | near complete | fast |
| 19 | 8 weeks | incomplete | slow |
| 20 | 8 weeks | incomplete | slow |
| 21 | 8 weeks | incomplete | slow |
| 22 | 8 weeks | incomplete | slow |
| 23 | 8 weeks | incomplete | slow |
| 24 | 8 weeks | incomplete | slow |
| 25 | 8 weeks | near complete | - |
| 26 | 8 weeks | near complete | - |
| 27 | 8 weeks | near complete | - |
| 28 | 8 weeks | near complete | - |
| 29 | 8 weeks | near complete | - |
| 30 | 8 weeks | complete | - |
| 31 | 8 weeks | complete | - |
| 32 | 8 weeks | complete | - |
| 33 | 8 weeks | complete | - |
| 34 | 8 weeks | complete | - |
| 35 | 8 weeks | complete | - |
| 36 | 8 weeks | complete | - |
Table 2.
Summary statistics of geometric and hemodynamic variables over the slow and fast occlusion groups.
| Variable | Slow occlusion | Fast occlusion | P-value | ||
|---|---|---|---|---|---|
| Mean | Stdev | Mean | Stdev | ||
| VOL – aneurysm volume | 0.723 | 0.495 | 0.205 | 0.238 | 0.0299 |
| SIZE – maximum diameter | 1.393 | 0.294 | 0.900 | 0.235 | 0.0239 |
| AREA – aneurysm area | 1.806 | 0.678 | 0.982 | 0.372 | 0.0375 |
| NECK – neck area | 0.365 | 0.082 | 0.144 | 0.078 | 0.0151 |
| DEPTH – maximum distance to neck | 0.920 | 0.255 | 0.687 | 0.197 | 0.0891 |
| AR – aspect ratio | 1.238 | 0.375 | 1.539 | 0.427 | 0.3917 |
| ICI – inflow concentration index | 0.013 | 0.035 | 0.007 | 0.010 | 0.2817 |
| Q – aneurysm inflow rate | 0.155 | 0.095 | 0.047 | 0.053 | 0.0239 |
| KE – kinetic energy | 1.283 | 1.010 | 0.519 | 0.967 | 0.0468 |
| SR – shear rate | 7.160 | 3.326 | 4.009 | 3.514 | 0.0722 |
| VE - velocity | 0.506 | 0.298 | 0.221 | 0.241 | 0.0582 |
| VO - vorticity | 7.714 | 4.220 | 4.294 | 4.221 | 0.0891 |
| VD – viscous dissipation | 2.807 | 1.664 | 1.650 | 2.353 | 0.1968 |
| WSSmax – maximum WSS | 92.635 | 95.690 | 63.854 | 32.524 | 0.7726 |
| WSSmin – minimum WSS | 0.000 | 0.000 | 0.000 | 0.000 | 0.6862 |
| <WSS> - mean WSS | 0.684 | 0.233 | 0.407 | 0.275 | 0.0891 |
| LSA – low WSS area | 59.908 | 28.391 | 69.925 | 29.239 | 0.5274 |
| SCI – WSS concentration index | 10.888 | 15.718 | 11.549 | 9.296 | 0.3917 |
| OSImax – maximum oscillatory shear index | 0.489 | 0.008 | 0.484 | 0.031 | 0.6042 |
| <OSI> - mean oscillatory shear index | 0.319 | 0.119 | 0.371 | 0.109 | 0.2817 |
| CORELEN – vortex coreline length | 1.352 | 0.562 | 1.127 | 0.469 | 0.6862 |
| PODENT – entropy of POD modes | 0.720 | 0.250 | 0.588 | 0.206 | 0.3335 |
| MATT – mean aneurysm transit time | 6.222 | 4.441 | 14.060 | 15.209 | 0.5274 |
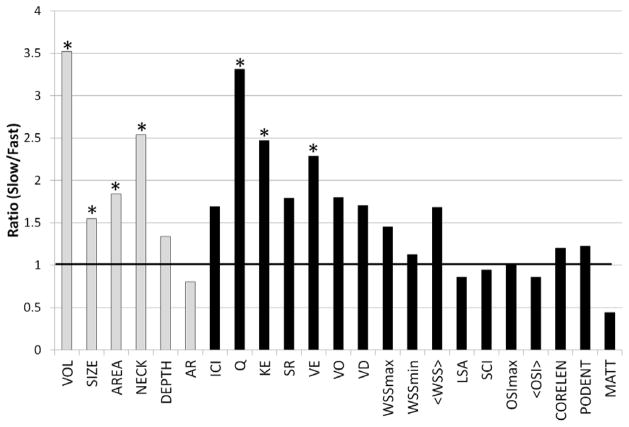
Ratios of mean geometric and hemodynamic variables of the slow over the fast occlusion groups are graphically presented in Figure 1. Bars above 1 indicate that the mean value of the slow occlusion group is larger than the mean value of the fast occlusion group. Statistically significant differences are indicated by a * above the corresponding bar.
Figure 1.
Ratio of average geometric (gray bars) and hemodynamic (black bars) variables of the slow over the fast occlusion groups. Statistically significant differences between the two groups are marked with a *.
Aneurysms in the fast occlusion group had significantly lower mean size (fast=0.900 cm, slow=1.393 cm, p=0.024), volume (fast=0.205 cm3, slow=0.723 cm3, p=0.030), area (fast=0.982 cm2, slow=1.806 cm2, p=0.038), and neck area (fast=0.144 cm, slow=0.365 cm2, p=0.015), than aneurysms in the slow occlusion group. They also had lower mean post-treatment inflow rate (fast=0.047 mL/s, slow=0.155 mL/s, p=0.0239), kinetic energy (fast=0.519 erg, slow=1.283 erg, p=0.0468), and velocity (fast=0.221 cm/s, slow=0.506 cm/s, p=0.0582). However, the differences of the latter two variables were only marginally significant. The inflow concentration, shear rate, vorticity, viscous dissipation and wall shear stress tended to be larger in the slow occlusion group but there was no statistical significance (p>0.05). The maximum and minimum WSS, OSI, the area under low WSS, the flow complexity (corelen) and stability (podent), and mean aneurysm transit time, as well as the aspect ratio and aneurysm depth were not statistically different between the two groups (p>0.05).
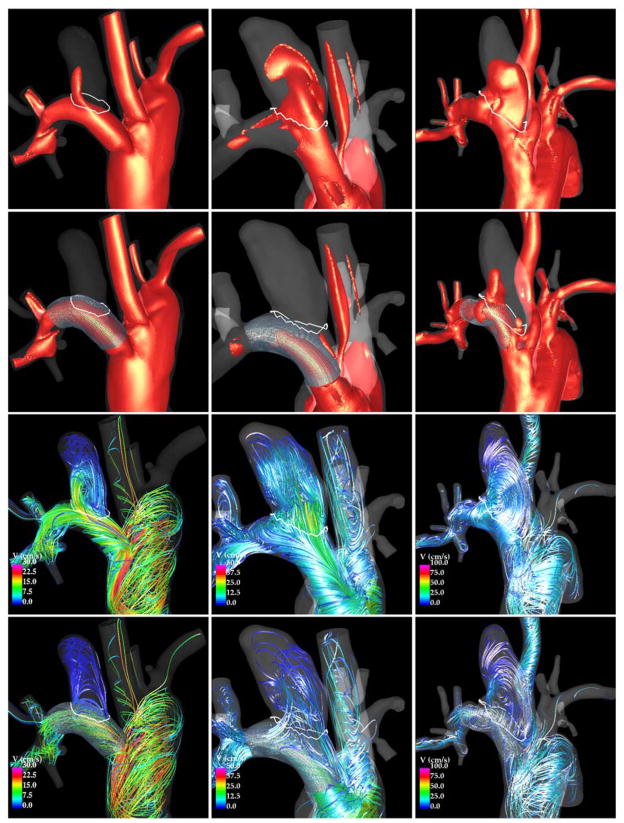
Flow visualizations of three example aneurysms are presented in Figure 2. From top to bottom this figure shows: iso-velocity (v=10 cm/s) before and after treatment and velocity streamlines before and after treatment. The first aneurysm (left column) was completely occluded before 1 week (fast occlusion), the second aneurysm (center column) was completely occluded at 4 weeks (fast occlusion), and the third aneurysm was incompletely occluded at 8 weeks (slow occlusion). It can be seen that immediately after treatment the inflow stream into aneurysms 1 and 2 are substantially reduced while in aneurysm 3 there is still a notable inflow into the aneurysm. The flow pattern in aneurysm 1 changed significantly after treatment. In particular, the inflow shifted to the proximal end of the neck and the intra-saccular streamlines followed a simpler and smoother trajectory. Aneurysm 2 follows the same general trend but with a slightly larger flow activity within the aneurysm sac. The flow structure in aneurysm 3 did not change markedly, although the blood speed along the streamlines was reduced.
Figure 2.
Flow visualizations in aneurysms occluded at 1 week (left column) and 4 weeks (center column), and in an aneurysm incompletely occluded at 8 weeks (right column). Each column shows, from top to bottom: iso-velocity pre-treatment, iso-velocity post-treatment, flow streamlines pre-treatment, and flow streamlines post-treatment.
DISCUSSION
Currently, there is no reliable technique to evaluate flow diversion treatments of intracranial aneurysms and to predict their long term outcomes. Previous studies have focused on the identification of qualitative and/or quantitative characteristics that could be used to prognosticate the outcomes of FD therapies13–18. However, the effects of flow diverting devices and the underlying mechanisms governing the thrombosis and occlusion of cerebral aneurysms are still not well understood.
Our current study used rabbit models to demonstrate that the flow conditions created immediately after placement of FD devices are quantitatively different between aneurysms that subsequently occluded quickly compared to aneurysms that remained patent for a longer period of time. In particular, the flow diverters more efficiently blocked the inflow stream and the resulting mean aneurysm velocity and flow activity were significantly smaller in fast occluding aneurysms. In the present study no balloons were used to expand the devices, and all devices were reasonably well appositioned against the parent artery wall covering the entire aneurysm orifice, except for two aneurysms of the slow occlusion group. In these two cases, the proximal end of the devices were not completely opposed to the wall which allowed a thin flow stream to slide between the device and the wall and enter the aneurysm (see aneurysm 3 in Figure 2). Imperfect deployments can have a substantial impact on the occlusion time and outcome of FD procedures.
Our results are consistent with previous studies based on clinical series19,20. This not only adds support to the validity of the rabbit aneurysm models but also confirms the idea that post-treatment flow conditions could potentially be used to assess the technical success of FD procedures and to predict the occlusion times and long term outcomes. In particular, our study indicates that the mean aneurysm velocity which can potentially be measured with dynamic angiography20 is a good predictor of occlusion time after FD treatment. However, in clinical situations there may be other factors besides hemodynamics and morphology that could affect the occlusion time after FD, including response to anti-platelet medication, co-morbidities like diabetes known to alter vascular response to implants, habits like smoking that alter platelet function and tissue repair, etc.
This study suffers from a number of limitations associated to assumptions and approximations made in the CFD modeling such as rigid walls, Newtonian rheology, idealized inflow profiles, etc. as well as limitations of the rabbit models such as flow reversal in the parent artery which does not occur in human cerebral arteries, and limited serial imaging that prevents visualization of the progression of aneurysmal occlusion. Nevertheless, these models allowed us to quantitatively compare the subject-specific hemodynamics between fast and slow aneurysm occlusion groups, and to identify target flow characteristics that could be used to accelerate the healing process after flow diversion and ultimately improve outcomes. These findings need to be further investigated and confirmed with larger series.
CONCLUSIONS
Hemodynamic conditions experimentally created immediately after flow diversion treatment of cerebral aneurysms in rabbits are associated to the subsequent aneurysm occlusion time. Specifically, smaller inflow rate, kinetic energy, and intra-saccular velocity seem to promote faster occlusions. These results are consistent with previous studies based on clinical series, and could be used to prognosticate the long term outcomes of flow diversion therapies.
Acknowledgments
We thank Covidien Inc. for generously providing flow diverters.
FUNDING
This work was supported by the National Institutes of Health (grant# NS076491).
Footnotes
COMPETING INTERESTS
None
CONTRIBUTORS
BC and FM: performed CFD simulations, edited the manuscript; RK: performed animal experiments, collected data, edited the manuscript; RL: performed statistical analysis; DK and JC: conceived the work, analyzed and interpreted the results, drafted the manuscript.
DATA SHARING STATEMENT
Computational models are freely available upon request.
References
- 1.Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of Intracranial Aneurysms Using the Pipeline Flow-Diverter Embolization Device: A Single-Center Experience with Long-Term Follow-Up Results. AJNR American journal of neuroradiology. 2012;33(8):1436–46. doi: 10.3174/ajnr.A3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna H, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–42. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR American journal of neuroradiology. 2011;32(1):34–40. doi: 10.3174/ajnr.A2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulcsar Z, Houdart E, Bonafe A, GP, Millar J, Goddard AJ, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR American journal of neuroradiology. 2011;32(1):20–25. doi: 10.3174/ajnr.A2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, et al. Aneurysm Rupture Following Treatment with Flow-Diverting Stents: Computational Hemodynamics Analysis of Treatment. AJNR American journal of neuroradiology. 2011;32(1):27–33. doi: 10.3174/ajnr.A2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altes TA, Cloft HJ, Short JG, DeGast A, Do HM, Helm GA, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. American Journal of Roentgenology. 2000;174(2):349–54. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 7.Cebral JR, Mut F, Raschi M, Ding YH, Kadirvel R, Kallmes DF. Strategy for analysis of flow diverting devices based on multi-modality image-based modeling. Int J Num Meth Biomed Eng. 2014 doi: 10.1002/cnm.2638. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cebral JR, Mut F, Raschi M, Hodis S, Ding YH, Erickson BJ, et al. Analysis of Hemodynamics and Aneurysm Occlusion after Flow-Diverting Treatment in Rabbit Models. AJNR. American journal of neuroradiology. 2014 doi: 10.3174/ajnr.A3913. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mut F, Cebral JR. Effects of Flow-Diverting Device Oversizing on Hemodynamics Alteration in Cerebral Aneurysms. AJNR American journal of neuroradiology. 2012;33(10):2010–16. doi: 10.3174/ajnr.A3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appanaboyina S, Mut F, Löhner R, Putman CM, Cebral JR. Simulation of intracranial aneurysm stenting: techniques and challenges. Computer Methods in Applied Mechanics and Engineering. 2009;198(45–46):3567–82. [Google Scholar]
- 11.Mut F, Löhner R, Chien A, Tateshima S, Viñuela F, Putman CM, et al. Computational hemodynamics framework for the analysis of cerebral aneurysms. Int J Num Meth Biomed Eng. 2011;27(6):822–39. doi: 10.1002/cnm.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrne G, Mut F, Cebral JR. Quantifying the large-scale gemodynamics of intracanial aneurysms. AJNR American journal of neuroradiology. 2014;35(2):333–38. doi: 10.3174/ajnr.A3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting: influence of stent porosity. Annals of Biomedical Engineering. 1997;25(3):460–69. doi: 10.1007/BF02684187. [DOI] [PubMed] [Google Scholar]
- 14.Lieber BB, Livescu V, Hopkins LN, Wakhloo AK. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Annals of Biomedical Engineering. 2002;30:768–77. doi: 10.1114/1.1495867. [DOI] [PubMed] [Google Scholar]
- 15.Seong J, Wakhloo AK, Lieber BB. In Vitro Evaluation of Flow Divertors in an Elastase-Induced Saccular Aneurysm Model in Rabbit. Journal of Biomechanical Engineering. 2007;129(6):863–72. doi: 10.1115/1.2800787. [DOI] [PubMed] [Google Scholar]
- 16.Sadasivan C, Cesar L, Seong J, Wakhloo AK, Lieber BB. Treatment of rabbit elastase-induced aneurysm models by flow diverters: development of quantifiable indexes of device performance using digital subtraction angiography. IEEE Trans Med Imaging. 2009;28(7):1117–25. doi: 10.1109/TMI.2008.2012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadasivan C, Lieber BB, Gounis MJ, Lopes DK, Hopkins LN. Angiographic quantification of contrast medium washout from cerebral aneurysms after stent placement. AJNR American Journal of Neuroradiology. 2002;23:1214–21. [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira VM, Bonnefous O, Ouared R, Brina O, Stawiaski J, Aerts H, et al. A DSA-Based Method Using Contrast-Motion Estimation for the Assessment of the Intra-Aneurysmal Flow Changes Induced by Flow-Diverter Stents. AJNR American journal of neuroradiology. 2013;34(3):805–15. doi: 10.3174/ajnr.A3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mut F, Raschi M, Scrivano E, Bleise C, Chudyk J, Ceratto R, et al. Association between hemodynamic conditions and occlusion times after flow diversion in cerebral aneurysms. Journal of neurointerventional surgery. 2014 doi: 10.1136/neurintsurg-2013-011080. in press. [DOI] [PubMed] [Google Scholar]
- 20.Kulcsar Z, Augsburger L, Reymond P, Pereira VM, Hirsch S, Mallik AS, et al. Flow diversion treatment: intra-aneurismal blood flow velocity and WSS reduction are parameters to predict aneurysm thrombosis. Acta Neurochir (Wien) 2012;154(10):1827–34. doi: 10.1007/s00701-012-1482-2. [DOI] [PubMed] [Google Scholar]