Abstract
Introduction
The roll of estrogens in the pathogenesis of breast cancer is well documented and has lead to the development of Selective Estrogene Receptor Modulators and Aromatase Inhibitors for treatment and prevention of breast cancer. However these agents are associated with significant side effects and are therefore not well accepted by healthy women who are at high risk for breast cancer. There has been some evidence from in vitro and in vivo animal studies that grapes have an aromatase inhibiting effect resulting in a decrease in estrogen synthesis and increase in androstenedione and testosterone.
Method
We conducted a randomized, double-blind, dose finding early phase trial. Eligible partici-pants were randomly assigned to one of 4 doses of grape seed extract (200, 400, 600, or 800 mg) to be taken daily for 12 weeks. The primary outcome was the change of plasma hormone levels (estrogen conjugates from baseline to 12 weeks after treatment with grape seed extract).
Results
Forty-six women were enrolled, 39 (84.8%) completed the study. In this pilot study grape-seed extract given in daily doses of 200, 400, 600 or 800 mg for 12 weeks to postmenopausal women did not decrease plasma estrogens (estrone, estradiol, estrone sulfate) and did not increase precursors of androgens (testosterone and androstenedione). There were large variations in pre- and posttreatment estrone, estradiol and estrone sulfate and androgen precursors.
Conclusion
Future research should carefully consider BMI and changes in BMI as well as higher dosing of grape seed extract in their design.
Introduction
The etiologic role of estrogens in the pathogenesis of breast cancer (BC) is widely accepted and well documented1,2. It has been demonstrated repeatedly that estrogens can induce and promote mammary tumors in rodents3. Epidemiologic studies in humans have consistently demonstrated that early menarche, late menopause, nulliparity, late age at first full-term pregnancy and obesity in post-menopausal women are associated with significantly increased risk for BC1. All of these risk factors are thought to arise as a result of length of exposure to estrogen. Evidence in support of an association between estrogens and BC includes studies of endogenous estrogen levels that were performed by Henderson1. Those studies have suggested that, in addition to length of exposure, increased levels of estrogen might also be related to BC risk. Similar observations were reported from a large prospective study in which women with high serum levels of estrogen, particularly free estradiol, the most potent and biologically active estrogen, were at substantially elevated risk for developing BC4,5.
This well documented role of estrogens in the pathogenesis of BC has led to the development of Selective Estrogens Receptor Modulators (SERMs) and Aromatase Inhibitors (AIs) for treatment and prevention of BC.
The main pathway for estrogen biosynthesis is through the conversion of androstenedione to estrone (E1), consisting of the de-methylation of C-19 and the aromaticity of the “A” ring (Figure 1) and a similar conversion of testosterone to estradiol (E2) (Figure 2), catalyzed by aromatase, an enzyme that is present in the ovary as well as in many non-endocrine tissues (fat, muscles, normal and malignant breast tissue). In addition there are also other enzymatic pathways that act to decrease levels of estrogens. Conjugation (addition) of a sulfate group, or sulfation is the major metabolic pathway for estrogen in humans that is involved in removal of active estrogens. These sulfate conjugates can be measured in plasma, and are decreased in patients taking AIs.
Figure 1.
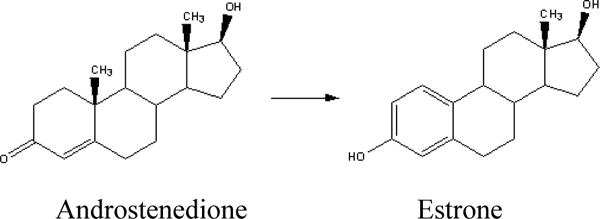
The enzyme aromatase is responsible for the conversion of androstenedione to estrone.
Figure 2.
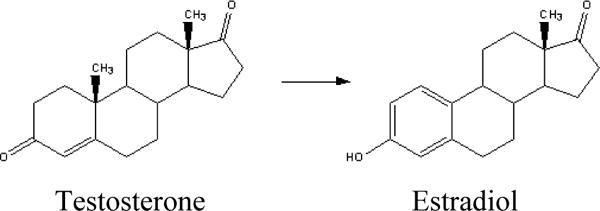
The enzyme aromatase is responsible for the conversion of testosterone to estradiol.
SERMs and AIs are being used for treatment and prevention of BC. Tamoxifen was the first anti-estrogen to be proven effective against estrogen receptor positive BC6. It has further been shown to be effective as a chemopreventive agent7. However, tamoxifen has the potential for serious side-effects that make it ill-advisable for many women6.
When administered to postmenopausal women, AIs effectively inhibit the peripheral synthesis of estrogens, leading to a more than 90% reduction in circulating estrogen levels8. AIs work by selectively inhibiting the enzyme aromatase. The new third-generation AIs are extremely potent and include anastrozole, letrozole, and exemestane. Recent data from randomized BC clinical trials have demonstrated the superiority of AIs over tamoxifen9 in patients with metastatic disease10. Trials evaluating the effectiveness of AIs for BC prevention are presently open for enrollment of high risk postmenopausal women. As a result of the extreme potency of these AIs, estrogen levels are reduced to nearly non-existent levels. Although AIs have a better side-effect profile than tamoxifen, AIs are associated with hot flashes, bone density losses, and other menopausal symptoms. These symptoms are more likely to be tolerated by women with BC who are trying to prevent recurrences than by healthy women who are trying to prevent BC. Additionally, the currently available AIs are quite expensive, especially if they are to be taken long term as preventative agents. For these reasons, it is important to search for preventive agents that are similar in function, but perhaps are less likely to lead to dangerous and uncomfortable side effects, and are more reasonably priced.
For many years epidemiologic studies have suggested that a diet rich in fruits and vegetables is associated with a reduced risk of BC11. Grapes are one of the most commonly consumed fruits in the world and have a high content of flavonoids, known for their strong anti-oxidant properties. Grapes are an abundant source of polyphenols. In 2003 Eng12 reported the ability of grape seed extract (GSE) to act as an AI in vitro as well as in vivo in aromatase-transfected MCF-7 (MCF-7aro) BC xenograft mice. Mice treated with the extract showed a significant reduction in tumor size compared with control mice fed water (p<0.01). To our knowledge no studies on humans have been reported.
The purpose of this study was 1) to evaluate whether GSE taken orally for 12 weeks will decrease plasma estrogen and increase precursor androgens in postmenopausal women and 2) to determine the most effective dose of GSE resulting in a decrease in plasma estrogen levels.
Subjects and Methods
Population studied
This study was approved by the Mayo Clinic Institutional Review Board and registered as NCT00566553 in ClinicalTrials.gov.
This was a dose-finding pilot study. Postmenopausal women presenting for their annual medical evaluation as an outpatient at the regional section of the Division of General Internal Medicine between the dates of 5/2008–9/2009 and 2/2011–9/2011 were invited to participate in this study.
Eligibility Criteria
Postmenopausal (no menstrual period for 1 year or more)
Age 55–75 years
No personal cancer history (except for non-melanoma skin cancer)
No hormone replacement therapy or anti-estrogens within 6 months of baseline
Able to give informed consent
Exclusion Criteria
Known allergy to grapes or grape products
Currently on ACE inhibitors, methotrexate, allopurinol, Coumadin (Warfarin, Jantoven), heparin, clopidogrel (Plavix), or cholesterol lowering medication
Study Design
We conducted a randomized, double-blind, dose finding early phase trial. Eligible participants were randomly assigned to one of 4 doses of GSE (200, 400, 600, or 800 mg) to be taken daily for 12 weeks. A signed consent form was obtained from all participants. The study coordinator called participants once a week to check for compliance and possible side effects.
The primary outcome was the change of plasma hormone level from baseline to 12 weeks after treatment with GSE. Plasma samples were collected at baseline and at 12 weeks and sent as a batch after completion of the study to Taylor Technologies of Princeton, NJ for measurements of plasma E1, E2, estrone sulfate (E1-conjugates), androstenedione and testosterone.
Sample Size
There are no data to suggest the amount of decrease in estrogen conjugates to expect, however, less than 30% decrease in estrogen conjugates would be unlikely to be clinically meaningful. Although this study is a pilot study and does not require a justification of sample size, we attempted to include enough subjects to provide a reasonable chance to test our basic hypothesis that GSE will decrease plasma estrogen levels. Based upon our determination of a clinically relevant minimal decrease of 30%, with 10 subjects in each group we could expect to detect a effect of minimal change of 30%.
Laboratory Methods
Hormone level determinations were conducted by Taylor Technologies of Princeton, NJ. A validated bioanalytic method using gas chromatography negative ionization tandem mass spectrometry was used to measure physiologically relevant concentrations of the following steroids from 1.0 mL of human plasma, with lower limits of quantitation (LLQ): E1, 1.56 pg/mL; E2, 0.625 pg/mL; testosterone, 25.0 pg/mL; androstenedione, 25.0 pg/mL; and E1-conjugates, 3.13 pg/mL. Standards and internal standards used were >98% pure and purchased from Steraloids, U.S. Pharmacopeia, Sigma-Aldrich, or CDN Isotopes. For each batch of samples analyzed, two standard curves for each analyte (front and back, eight concentration levels) were prepared in water and qualified with quality control samples (two replicates at low, mid, and high levels) prepared in charcoal-stripped plasma (for the low level) and in unaltered plasma (for mid and high levels). Analytic runs were accepted when >75% of standards had back-calculated concentrations within ±15% of nominal, except at the LLQ, wherein ±20% of nominal concentrations was accepted. In addition, at least 67% of the quality control samples met accuracy requirements of being within ±15% of their nominal concentrations.
Briefly, the analytes and their deuterated internal standards were extracted from 1 mL of plasma using Bond Elut Certify (Varian) solid-phase extraction cartridges. Estrone conjugates were eluted from the cartridges with water/acetonitrile (75:25, v/v), dried down, and hydrolyzed to estrone using Glusulase (β-glucuronidase and sulfatase, NEN Research Products). The unconjugated analytes were then eluted with ethyl acetate. Estrogens were derivatized with pentafluorobenzoyl chloride and N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA); the androgens were derivatized with O-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine and MSTFA. All solvents and reagents were purchased from EMD Science or Sigma-Aldrich. The derivatized analytes were separated on a Varian 3400 gas chromatograph equipped with a DB-17 fused silica capillary column (15 m × 0.025 mm, J&W Scientific) and quantified using an interfaced Finnigan MAT TSQ-700 mass spectrometer operating in single-ion monitoring tandem mass spectrometry negative ion chemical ionization mode.
Products
GSE was obtained from San Joaquin Valley Concentrate (SJVC) Fresno, California. Our maximum dose of 800 mg/day (4 pills per day) is the equivalent of 11.4 mg/kg in a 70 kg woman. We did not believe women would be willing to take more than 4 GSE pills per day. It is unclear from the literature just what dose to propose. Because of the uncertainty of the dose, we included four dose levels.
The safety of standardized GSE in laboratory animals over long periods has been studied extensively13,14. A commercial preparation of GSE is available in the United States as a dietary supplement15. Doses up to 720 mg/day have been used in clinical studies16 for evaluation of the effect of GSE in patients with chronic venous insufficiency. There are little or no side effects reported with GSE, and no drug interactions have been reported either. However, there is some evidence that GSE may cause excessive bleeding in individuals taking blood thinners. There is further some theoretical evidence that grape seed may interact with some prescription drugs, such as angiotensin-converting enzyme inhibitors, methotrexate, allopurinol and cholesterol-lowering drugs by interfering with their metabolism in the liver (http://www.cellhealthmakeover.com/grape-seed-antioxidant.html).
Data collected
Body mass index (BMI) at beginning and end of study
Two menopausal symptom scales
Hormone levels at beginning and end of study
Statistical analysis
Analyses were performed using a per-protocol approach including 39 patients with complete data. Because the magnitude of change in hormone levels was strongly associated with the baseline level, we used percent change from baseline as the primary analysis variable to minimize the impact of this floor effect. The percent change from baseline to 12 weeks was calculated within patient for each hormone level and summarized by dose group with median and interquartile range (IQR; 25th percentile, 75th percentile). Because a change of 30% from baseline was considered to be the minimum clinically meaningful change, we also summarized the number (percent) of patients reaching this threshold. Percent change in hormone levels was compared among dose groups using Kruskal-Wallis tests. Within group comparisons between baseline and 12 weeks were performed using Wilcoxon signed-rank tests. Correlations between continuous variables such as BMI and hormone levels were estimated using Spearman’s rank correlation. P-values less than 0.05 were considered as statistical significant. All statistical analyses were conducted by SAS version 9.2 software (SAS institute Inc., Cary NC).
Results
Forty-six patients were enrolled, 39 (84.8%) completed the study. Four patients asked to be taken off the study due to perceived side effects of GSE (headaches, gas, nausea, vomiting, diarrhea, stomach pain, and cramps during week one and two). Three patients needed to be started on exclusionary medications (Lipitor, Lisinopril, and Coumadin) during the study period and therefore needed to be taken off study (Figure 3). Compliance was satisfactory. Ten patients missed taking their GSE for one day during the 12 week study period, which was mainly related to medical procedures being performed (colonoscopy, EGD) or not feeling well (migraine headache, nauseous feeling) and 2 patients missed 3 days of taking GSE during the 12 week study period.
Figure 3.
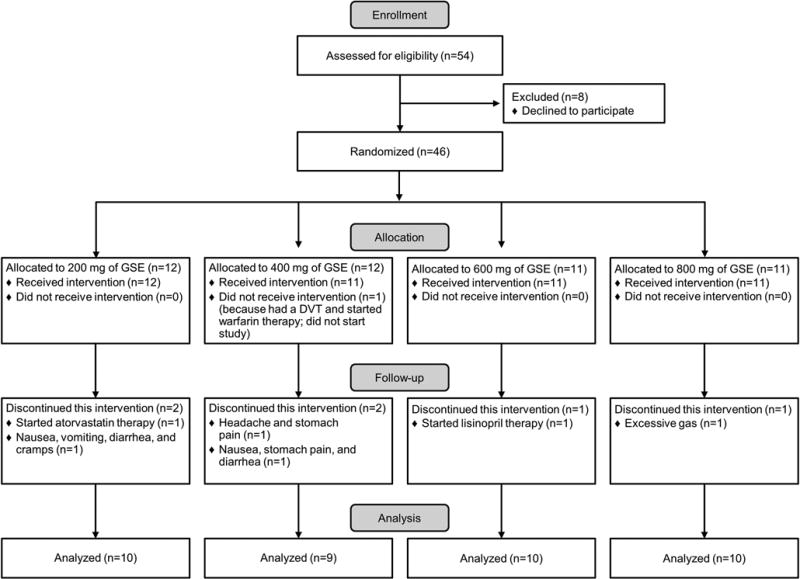
Patient Flow Diagram
Demographic data, BMI and hot flash scores for patients using the 4 different doses of GSE at baseline and at 12 weeks are depicted in Table 1. There was no statistically significant difference among dose groups in age, baseline BMI, or baseline hot flash score. Yet, we note that 50% of the patients in the 800 mg dose group and 44% in the 400 mg dose group were obese (BMI ≥30) compared to only 20% in the 200 mg and 600 mg dose groups. For all dose groups combined, the median BMI change between baseline and 12 weeks was +0.21 (range: −0.95 to +1.28) which was not statistically significant (p=0.09); change in BMI did not differ significantly across the dose groups. The median change in hot flash scores between baseline and 12 weeks as −1 (range −13 to +9) overall (p=0.07) and did not differ significantly across dose groups.
Table 1.
Age, BMI and Climacteric Scale at baseline and at 12 weeks for the 4 patient groups.
| 200 mg | 400 mg | 600 mg | 800 mg | ||
|---|---|---|---|---|---|
| N = 10 | N = 9 | N = 10 | N = 10 | ||
| Age, mean (SD) | 66.7 (5.5) | 66.9 (3.9) | 62.1 (5.2) | 66.3 (5.1) | |
| Baseline BMI ≥30, n (%) | 2 (20) | 4 (44) | 2 (20) | 5 (50) | |
| BMI, mean (SD) | |||||
| Baseline | 27.4 (6.6) | 30.3 (4.8) | 26.5 (4.0) | 29.2 (7.2) | |
| 12 weeks | 27.6 (6.5) | 30.3 (4.9) | 26.8 (4.2) | 29.3 (7.5) | |
| Change | 0.2 (0.5) | −0.02 (0.5) | 0.3 (0.7) | 0.1 (0.6) | |
| Greene Climacteric Scale, mean (SD) | |||||
| Baseline | 5.6 (5.9) | 9.6 (7.0) | 9.4 (6.3) | 6.1 (5.9) | |
| 12 weeks | 3.1 (4.2) | 7.2 (9.1) | 9.0 (8.4) | 4.3 (8.4) | |
| Change | −2.5 (6.1) | −2.3 (6.6) | −0.4 (3.5) | −1.8 (5.4) | |
Hormone levels were correlated with BMI at baseline: E2 (r=0.75), E1 (r=0.63), E1-conjugates (r=0.58), testosterone (r=0.25), androstenedione (r=0.35). The change in BMI through 12 weeks, although small, showed moderate correlations with the percent change in hormone levels: E2 (r=0.41), E1 (r=0.25), E1-conjugates (r=0.44), testosterone (r=0.29), and androstenedione (r=0.30).
Pretreatment hormone plasma levels showed substantial variability. Five of 39 patients had pre-treatment E2 levels of >10 pg/ml, the conventional concentration considered to separate premeno-pausal from postmenopausal women, with a range of 10.2 to 19.9 pg/ml (mean, 13.4 pg/ml). The mean age of these 5 patients was 66.2 years (range 56–72 years), their mean baseline BMI was 35.7 (range 27.4–40.8). The mean age of the remaining 34 patients was similar at 65.4 years (range 56–74) however their mean BMI was lower at 27.2 (range 19.9–40.6) (p=0.006).
Hormone Levels by Dose Group
Baseline hormone levels as well as the paired within patient percent changes between baseline and 12 weeks are presented in Table 2.
Table 2.
Hormone levels at baseline and at 12 weeks for the 4 patient groups.
| Median (IQR) | 200 mg | 400 mg | 600 mg | 800 mg | P-value* | |
|---|---|---|---|---|---|---|
|
| ||||||
| N = 10 | N = 9 | N = 10 | N = 10 | |||
|
| ||||||
| E2, pg/mL, mean (SD) | ||||||
| Baseline | 3.1 (2.2, 9.2) | 4.8 (3.9, 10.8) | 2.7 (1.9, 6.0) | 5.4 (2.7, 8.5) | ||
| % change at 12 weeks | −4.7 (−19.6, +18.4) | −4.7 (−19.8, +4.1) | −5.3 (−25.9, +36.4) | −2.5 (−29.1, +21.0) | 0.99 | |
|
| ||||||
| E1, pg/mL, mean (SD) | ||||||
| Baseline | 19.4 (11.8, 37.1) | 30.3 (22, 39.5) | 14.8 (11.5, 25.8) | 21.0 (14.7, 38.1) | ||
| % change at 12 weeks | −17.0 (−27.3, −9.4) | −9.8 (−20.8, −1.1) | −15.1 (−25.5, +8.0) | −10.5 (−23.8, +16.7) | 0.71 | |
|
| ||||||
| E1-conjugates, pg/mL, mean (SD) | ||||||
| Baseline | 209 (56, 377) | 543 (248, 582) | 191 (122, 282) | 286 (194, 497) | ||
| % change at 12 weeks | −0.2 (−45.7, +26.0) | −29.3 (−41.1, −9.5) | −12.4 (−32.5, 4.3) | −11.7 (−28.9, +8.6) | 0.63 | |
|
| ||||||
| Testosterone, pg/mL, mean (SD) | ||||||
| Baseline | 148 (124, 275) | 177 (143, 342) | 96 (83, 171) | 134 (64, 225) | ||
| % change at 12 weeks | −9.4 (−24.6, +11.8) | −6.9 (−15.2, +3.3) | −9.3 (−22.8, +13.8) | −9.5 (−28.1, +9.2) | 0.98 | |
|
| ||||||
| Androstenedione, pg/mL, mean (SD) | ||||||
| Baseline | 399 (316, 624) | 722 (588, 952) | 377 (301, 593) | 456 (266, 626) | ||
| % change at 12 weeks | −19.1 (−44.7, +19.0) | −8.3 (−29.7, +10.0) | −19.3 (−36.7, +9.2) | −21.7 (−41.3, +9.8) | 0.98 | |
E2, estradiol; E1, estrone; E1-conjugates, estrone sulfate;
P-value from Kruskal-Wallis test.
The median percent change was a decrease for E2, E1, and E1-conjugates in each of the 4 dose groups; however the decrease was not statistically significant within any group nor was it dose dependent.
Testosterone and androstenedione levels decreased in all 4 groups; again, the changes were not significant, but importantly they were also not in the hypothesized direction as testosterone and androstenedione were hypothesized to increase.
No significant differences were identified comparing across dose groups. This remained true after adjusting for age and BMI in a multivariate model.
At week 12, 21 (54%) participants maintained stable BMI (± 0.5), 12 (31%) increased BMI by ≥0.5, 6 (15%) decreased BMI by ≥0.5. Table 3 reports hormone changes of 30% or more from baseline after 12 weeks of grape seed consumption by BMI change category. In the stable BMI subgroup, some patients (E2, 24%; E1, 29%; E1-conjugates, 43%) had decreases in estrogen levels ≥30%, but not in a dose-dependent manner nor was the expected corresponding increase in androgens generally observed.
Table 3.
Hormone changes of 30% or more in hypothesized direction after 12 weeks of GSE consumption by BMI change category
| Change of 30% or more from baseline in hypothesized direction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Change in BMI at 12 weeks versus baseline | Dose (mg) | Estradiol (hypothesized ↓) | Estrone (hypothesized ↓) | Estrone Sulfate (hypothesized ↓) | Testosterone (hypothesized ↑) | Androstenedione (hypothesized ↑) | |||||
| n | % | n | % | n | % | n | % | n | % | ||
| N = 12 BMI ↑ by 0.5 or more |
200 | 0/4 | 0 | 0/4 | 0 | 1/4 | 25 | 1/4 | 25 | 0/4 | 0 |
| 400 | 0/1 | 0 | 0/1 | 0 | 1/1 | 100 | 1/1 | 100 | 0/1 | 0 | |
| 600 | 0/5 | 0 | 0/5 | 0 | 1/5 | 20 | 1/5 | 20 | 1/5 | 20 | |
| 800 | 0/2 | 0 | 0/2 | 0 | 1/2 | 50 | 1/2 | 50 | 1/2 | 50 | |
|
N = 21 No change in BMI (within 0.5 of baseline at 12 weeks) |
200 | 1/5 | 20 | 2/5 | 40 | 3/5 | 60 | 0/5 | 0 | 1/5 | 20 |
| 400 | 1/6 | 17 | 1/6 | 17 | 3/6 | 50 | 0/6 | 0 | 0/6 | 0 | |
| 600 | 1/4 | 25 | 2/4 | 50 | 2/4 | 50 | 0/4 | 0 | 0/4 | 0 | |
| 800 | 2/6 | 33 | 1/6 | 17 | 1/6 | 17 | 0/6 | 0 | 1/6 | 17 | |
| N = 6 BMI ↓ by 0.5 or more |
200 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 |
| 400 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | |
| 600 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 | 0/1 | 0 | |
| 800 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | 0/2 | 0 | |
Table 4 reports hormone changes in patients with a normal BMI at baseline and at end of the study. While E1 and E1-conjugates levels decreased, the change was not statistically significanty. Again a decrease – although not statistically significant – rather than an increase in testosterone and andro-stenedione were noted.
Table 4.
Baseline hormone levels and percent change from baseline to 12 weeks in patients with normal BMI (18.5–24.9).
| Median (IQR) | 200 mg | 600 mg | 800 mg | |
|---|---|---|---|---|
|
| ||||
| N = 4 | N = 4 | N = 4 | ||
|
| ||||
| E2, pg/mL, mean (SD) | ||||
| Baseline | 2.4 (1.0, 3.2) | 2.3 (1.6, 3.2) | 2.7 (1.7, 3.7) | |
| % change at 12 weeks | +5.2 (−7.1, +23.1) | −5.8 (−24.6, +54.6) | −0.4 (−30.4, +19.5) | |
|
| ||||
| E1, pg/mL, mean (SD) | ||||
| Baseline | 11.6 (4.1, 20.2) | 12.6 (9.5, 15.6) | 17.4 (13.1, 21.6) | |
| % change at 12 weeks | −13.6 (−18.8, +16.3) | −11.8 (−31.6, +3.5) | −17.8 (−44.4, +9.0) | |
|
| ||||
| E1-conjugates, pg/mL, mean (SD) | ||||
| Baseline | 129 (37, 325) | 118 (92, 201) | 177 (79, 279) | |
| % change at 12 weeks | −11.4 (−68.5, +24.7) | −10.9 (−57.5, +33.4) | −17.5 (−49.6, +4.0) | |
|
| ||||
| Testosterone, pg/mL, mean (SD) | ||||
| Baseline | 134 (65, 237) | 85 (68, 285) | 97 (60, 291) | |
| % change at 12 weeks | +8.7 (−19.0, +25.5) | +0.7 (−37.8, +16.1) | −16.4 (−50.7, +5.1) | |
|
| ||||
| Androstenedione, pg/mL, mean (SD) | ||||
| Baseline | 380 (116, 588) | 319 (291, 753) | 472 (267, 638) | |
| % change at 12 weeks | −29.5 (−57.9, +9.4) | −8.0 (−42.4, +16.4) | −39.4 (−63.1, +17.8) | |
Note that the 400 mg group is not included in this table because no patients in that dose group met these criteria.
E2, estradiol; E1, estrone; E1-conjugates, estrone sulfate.
Discussion
GSE has been documented in several studies to be an inhibitor of aromatase. In 2001 Eng17 first reported that wine and especially red wine contains phytochemicals that are capable of suppressing wild-type human placenta aromatase, wild-type porcine placenta and blastocyst aromatase in a dose-dependent fashion. The author further examined the aromatase-inhibitory action of red wine extracts with a transgenic mouse model in which aromatase is over-expressed in the mammary tissues and documented that the intake of the active fraction by gavage completely abrogated aromatase-induced hyperplasia and other changes in the mammary tissue.
In a later study Eng12 identified one of the active ingredients in the grape extract to be procyanidin B dimers and showed that procyanidin B dimers compete with the binding of the androgen substrate. The authors further evaluated the in vivo efficacy of procyanidin B dimers in an aromatase-transfected MCF-7 (MCF-7aro) BC xenograft model. E2 and estrone sera determinations of mice treated with increasing concentrations of wine extract showed a decreasing trend in the levels of E2 and E1 compared with control. Although average estrogen concentration values for wine extract-treated mice did show a clear difference when compared with mice treated with water the SD values were too large to determine any statistical significance. Investigations reported by Kijima (2006)18 indicated that procyanidin dimers in GSE, grapes and red wine can suppress not only aromatase enzymatic activity but also aromatase expression and promoter activity.
Wang19 reported in 2006 that Resveratrol, a polyphenolic compound isolated from grape peels used in pharmacologic doses on MCF-7 cells transfected with CYP19 (MCF-7aro cells) inhibited aromatase at both the enzyme and mRNA levels.
A recent cross over design study by Shufelt20 reported on 36 women who were assigned to 8 ounces of red wine daily then white wine daily for 1 month each or the reverse. Blood was collected during the menstrual cycles and hormone levels measured. The red wine consuming group was shown to have significantly higher free testosterone levels compared to the white wine consuming group. Total testosterone and androstenedione did not show any statistically significant difference. Free E2 and total E2 levels were lower in the red versus the white wine drinking group, but this was not statistically significant. E1 levels were higher in the red wine group but not statitcially significant.
Based on these publications we expected to see a decrease in plasma estrogen levels and an increase in precursors of androgens. However, in this pilot study GSE given in daily doses of 200, 400, 600 or 800 mg for 12 weeks to postmenopausal women did not decrease plasma estrogens (E1, E2, E1-conjugates) and did not increase precursors of androgens (testosterone and androstenedione). There were large variations in pre- and posttreatment E1, E2 and E1 conjugates and androgen precursors.
There are several possibilities which could explain our result:
-
Possible influence of BMI. Aromatase activity is known to increase proportionately with the degree of obesity in women. Folkerd et al21 have confirmed the significant positive relationship between estrogen levels and BMI in untreated BC patients. This positive relationship was found to also be present during treatment with AIs. The authors postulate that this effect in patients with high BMI might hypothetically be a result of reduced inhibition of aromatase and suppression of plasma estrogen levels and may be overcome by the use of an increased dose of anastrozole or a more potent AI.
In our small study even in the small subgroup of 21 patients (54%) who maintained stable BMI there was no consistent decrease in E1and E2 concentrations.
Ingle et al22 evaluated E1, E2, estrogen conjugates, androstenedione and testosterone levels in 191 women who were treated with anastrozole 1 mg/day as adjuvant therapy for resected early BC. Their results showed large interindividual variation in anastrozole metabolism and its effect on circulating estrogens in postmenopausal patients. The authors suggested that this commonly used agent for the treatment of BC should be evaluated in pharmacogenomics studies aimed at identifying genetic variation in drug metabolism. A similar mechanism could be hypothetically responsible for the findings in our study using GSE as an AI.
The sample size in our dose finding pilot study was not adequate to document the aromatase inhibiting effect of grapes seeds.
The doses of GSE used in this pilot study were not high enough to have an aromatase inhibiting effect.
A modulatory role of diet comsumed while taking GSE. In a study published in 2004 Kim et al23 reported that in rats the admisntration of GSE in AIN-76A diet did not show any protective activity of GSE against DMBA-induced BC. However, administration of GSE in a laboratory dry food diet (Teklad 4% rodent diet) resulted in a 50% reduction in tumor multiplicity. These data suggest the possible need to consider the diet of patients during administration of GSE including wine intake.
Conclusion
In this small pilot study we were unable to identify a promising dose of GSE which could be considered for chemoprevention. Future research should carefully consider BMI and changes in BMI as well as higher dosing of GSE in their design.
References
- 1.Henderson BE, Bernstein L. Endogenous and exogenous hormone factors. In: Harris JR, Lippman ME, Morrow M, Hellman S, editors. Diseases of the Breast. Lippincott-Raven Publishers; Philadelphia, PA: 1996. pp. 185–200. [Google Scholar]
- 2.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001 Jan 25;344(4):276–85. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- 4.Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, Strax P, Pasternack BS. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst. 1995 Feb 1;87(3):190–7. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- 5.Endogenous Hormones and breast cancer collaborative group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 6.Dunn BK, Wickerham DL, Ford LG. Prevention of hormone-related cancers: breast cancer. J Clin Oncol. 2005 Jan 10;23(2):357–67. doi: 10.1200/JCO.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Maisonneuve P, Rotmensz N, Costa A, Sacchini V, Travaglini R, D’Aiuto G, Lovison F, Gucciardo G, Muraca MG, Pizzichetta MA, Conforti S, Decensi A, Robertson C, Boyle P, Italian Tamoxifen Study Group Italian randomized trial among women with hysterectomy: tamoxifen and hormone-dependent breast cancer in high-risk women. J Natl Cancer Inst. 2003 Jan 15;95(2):160–5. doi: 10.1093/jnci/95.2.160. [DOI] [PubMed] [Google Scholar]
- 8.Demers LM. Effects of Fadrozole (CGS 16949A) and Letrozole (CGS 20267) on the inhibition of aromatase activity in breast cancer patients. Breast Cancer Res Treat. 1994;30(1):95–102. doi: 10.1007/BF00682744. [DOI] [PubMed] [Google Scholar]
- 9.ATAC “(Arimidex, Tamoxifen Alone or in Combination) Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002 Jun 22;359(9324):2131–9. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 10.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003 Jun 1;21(11):2101–9. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 11.Fund WCRL. Food, Nutrition and the Prevention of Cancer: a Global Perspective. Washington, DC: American Institute for Cancer Research; 1997. [Google Scholar]
- 12.Eng ET, Ye J, Williams D, Phung S, Moore RE, Young MK, Gruntmanis U, Braunstein G, Chen S. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res. 2003 Dec 1;63(23):8516–22. [PubMed] [Google Scholar]
- 13.Ray S, Bagchi D, Lim PM, Bagchi M, Gross SM, Kothari SC, Preuss HG, Stohs SJ. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol. 2001 Mar-Apr;109(3–4):165–97. [PubMed] [Google Scholar]
- 14.Dixon RA, Xie DY, Sharma SB. Proanthocyanidins–a final frontier in flavonoid research? New Phytol. 2005 Jan;165(1):9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal C, Sharma Y, Agarwal R. Anticarcinogenic effect of a polyphenolic fraction isolated from grape seeds in human prostate carcinoma DU145 cells: modulation of mitogenic signaling and cell-cycle regulators and induction of G1 arrest and apoptosis. Mol Carcinog. 2000 Jul;28(3):129–38. doi: 10.1002/1098-2744(200007)28:3<129::aid-mc1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Kiesewetter H, Koscielny J, Kalus U, Vix JM, Peil H, Petrini O, van Toor BS, de Mey C. Efficacy of orally administered extract of red vine leaf AS 195 (folia vitis viniferae) in chronic venous insufficiency (stages I–II). A randomized, double-blind, placebo-controlled trial. Arzneimittelforschung. 2000 Feb;50(2):109–17. doi: 10.1055/s-0031-1300174. [DOI] [PubMed] [Google Scholar]
- 17.Eng ET, Williams D, Mandava U, Kirma N, Tekmal RR, Chen S. Suppression of aromatase (estrogen synthetase) by red wine phytochemicals. Breast Cancer Res Treat. 2001 May;67(2):133–46. doi: 10.1023/a:1010641102147. [DOI] [PubMed] [Google Scholar]
- 18.Kijima I, Phung S, Hur G, Kwok SL, Chen S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006;66(11):5960–7. doi: 10.1158/0008-5472.CAN-06-0053. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Lee KW, Chan FL, Chen S, Leung LK. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci. 2006 Jul;92(1):71–7. doi: 10.1093/toxsci/kfj190. [DOI] [PubMed] [Google Scholar]
- 20.Shufelt C, Merz CN, Yang Y, Kirschner J, Polk D, Stanczyk F, Paul-Labrador M, Braunstein GD. Red versus white wine as a nutritional aromatase inhibitor in premenopausal women: a pilot study. J Womens Health (Larchmt) 2012 Mar;21(3):281–4. doi: 10.1089/jwh.2011.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012 Aug 20;30(24):2977–80. doi: 10.1200/JCO.2012.42.0273. [DOI] [PubMed] [Google Scholar]
- 22.Ingle JN, Buzdar AU, Schaid DJ, Goetz MP, Batzler A, Robson ME, Northfelt DW, Olson JE, Perez EA, Desta Z, Weintraub RA, Williard CV, Flockhart DA, Weinshilboum RM. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010 Apr 15;70(8):3278–86. doi: 10.1158/0008-5472.CAN-09-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Hall P, Smith M, Kirk M, Prasain JK, Barnes S, Grubbs C. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004 Dec;134(12 Suppl):3445S–3452S. doi: 10.1093/jn/134.12.3445S. [DOI] [PubMed] [Google Scholar]


