Abstract
This study investigated the effects of acute and chronic psychological stress on glucose-stimulated insulin secretion from isolated pancreatic islets. Male Wistar rats were divided into two control and stressed groups; each further was allocated into fed and fasted groups. Stress was induced by communication box for one (acute), fifteen and thirty (chronic) days. After islet isolation, their number, size and insulin output were assessed. Plasma corticosterone level was determined. In fasted animals, acute stress increased basal and post stress plasma corticosterone level, while 30 days stress decreased it compared to day 1. In fed rats, acute stress increased only post stress plasma corticosterone concentration, however, after 15 days stress, it was decreased compared to day 1. Acute stress did not change insulin output; however, the insulin output was higher in the fed acutely stressed rats at 8.3 and 16.7 mM glucose than fasted ones. Chronic stress increased insulin output on day 15 in the fasted animals but decreased it on day 30 in the fed animals at 8.3 and 16.7 mM glucose. In the fasted control rats insulin output was lower than fed ones. In the chronic stressed rats insulin output at 8.3 and 16.7 mM glucose was higher in the fasted than fed rats. The number of islets increased in the fasted rats following 15 days stress. This study indicated that the response of the isolated islets from acute and chronically stressed rats are different and depends on the feeding status.
Keywords: psychological stress, insulin secretion, pancreatic islets number, pancreatic islets area, corticosterone
Introduction
Epidemiological studies have indicated that a rising percentage of the population in modern societies are exposed to acute and/or chronic stress which can lead to variety of metabolic related disorders including diabetes mellitus (Cosgrove, 2004[10]; Shiloah et al., 2003[32]). In this regard, studies have shown the effects of acute (Malatinsky et al., 1986[25]; Romeo et al., 2007[30]) and chronic (Armario et al., 1985[2]; Zardooz et al., 2006[43]) stress on blood glucose and insulin levels. Electroconvulsive shock in both the acute or chronic forms and acute immobilization increased plasma glucose level in rats, but did not change plasma insulin concentration (Macho et al., 1999[22]; Thiagarajan et al., 1988[36]). On the other hand chronic immobilization and hypokinesia decreased plasma insulin concentration without changing glucose levels (Macho et al., 1999[22]; Makino et al., 1999[23]). It has been documented that the stress hormones generally antagonize insulin actions on glucose metabolism (Brindley and Rolland, 1989[6]) and also interact with insulin release from pancreatic islets (Billaudel and Shutter, 1982[3]; Smythe et al., 1989[33]). The elevation of blood corticosterone concentration which may occur in response to stressful events increases the plasma glucose concentration (Bratusch-Marrain, 1983[5]; Yamada et al., 1993[39]) and inhibits insulin secretion from the islets (Billaudel and Shutter, 1982[3]). Considering the types of the stresses, it appears that the psychological stress is more prevalent and growing body of evidence show its linkage to many disorders including diseases related to glucose metabolism such as metabolic syndrome (Rosmond, 2005[31]) and diabetes (Littorin et al., 2001[21]). It has been suggested that the effects of psychological stresses on glucose metabolism might be related to the impairment of insulin release from pancreatic islets (De Boer et al., 1990[11]; Nowotny et al., 2010[27]).
Despite studies concerning acute or chronic exposure to stressors, the severities of the impacts of two kinds of stress exposure have not been very well established. Moreover, in the previous studies the changes in the metabolic parameters and also insulin secretion from isolated islets following stress exposure was evaluated either in fed or fasted status and almost no study has compared these two feeding status. The present study has been designed and conducted to clarify the differences between the effects of acute and chronic psychological stress on plasma corticosterone level and insulin secretion from pancreatic isolated islets in fed and fasted states.
Materials and Methods
Animals and housing
Adult male Wistar rats (170-190 g, 2.5-3 months old) obtained from Pasteur Institute, Tehran, Iran were housed 3 per cage in a temperature controlled room (22 ± 2 °C) with 12 h light/dark cycle (light on at 7:00 a.m.). Standard food (Pars Company of animal food producer, Iran) and tap water were provided throughout the experiment ad libitum. In the fasting state, food was removed for 16 h before the experiments. The animals were allowed 1 week to habituate with the environment and all procedures were approved by the Animal Care and Use Committee of the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences.
Experimental procedure
Stress apparatus
A communication box (com-box) (Endo et al., 2001[14]) (48×48×50 cm) was used as the stressor apparatus, in brief, the box consisted of 9 chambers (16×16 cm) divided by transparent Plexiglas sheets, with a grid floor composed of 0.5 cm diameter stainless steel rods placed 1.3 cm apart. The floors of four compartments were covered by Plexiglas plate to prevent animals from receiving electric shock; thus the device provided five chambers with foot shock and four chambers with no foot shock. An electric shock generator (BorjeSanat, Iran) was used to produce foot shock (1 mA, 1 Hz) for 10 s duration every 60 s (Endo et al., 2001[14]). Animals of the psychologically stressed groups (which all were in the fed state while receiving stress) were placed in chambers with no shock and therefore were exposed to only various emotional stimuli (jumping, struggling, vocalizing, defecating and urinating) from the rats in the other five chambers receiving foot shock (these animals were used only in order to induce psychological stress). The daily stress exposure lasted 1 hour and was performed between 10 h to 13 h. The animals of the control group were only placed daily for 1 hour in the com-box without receiving any stimulus. The time between the morning blood sampling and stress exposure was 2 to 5 hours.
Blood sampling
To obtain the blood samples, animals were briefly anesthetized with isoflurane (1-chloro-2,2,2-trifluoroethyl difluoromethyl ether, Nicholas Pirmal, UK), and the samples were collected by orbital sinus puncture with a heparinized capillary micro tube (Zardooz et al., 2010[41]; Zaringhalam et al., 2010[44]), blood was collected into an Eppendorf tube containing 25 IU/5 µl heparin (Chalkley et al., 2002[8]), immediately centrifuged at 3000×g, plasma was separated and kept at -70 °C for measuring the corticosterone concentration.
Evaluation of the plasma corticosterone levels
The animals were divided into twelve groups (n=6/group) as follows: one category did not receive stress and served as control [(fed day 1), (fed day 15), (fed day 30), (fast day 1), (fast day 15), (fast day 30)]. The second category received psychological stress and served as stressed [(fed day 1), (fed day 15), (fed day 30), (fast day 1), (fast day 15), (fast day 30)]. On the 1st day of the experiments, while the fasted animals were in fasting state from 16:00 p.m. of the day before, blood samples were collected (8-8:30 a.m.) to evaluate the basal plasma corticosterone level (Basal-Before Stress: B-BS). Then all animals (in the fasted or fed groups) allowed having free access to food for 3 to 5 h. Subsequently, they were placed in the com-box for 1 h without any stress application (in the control groups) or with stress application (in the stressed groups) and the second blood sampling were performed immediately after removing the rats from the com-box. One hour after blood sampling they were transferred to the colony room and remained free access to food except for the rats of the fast day 1 group whose food was removed from 16:00 p.m. to 8 a.m. of the next day (i.e., day 2 of the experiments), then blood sampling were performed in the rats of both fed day 1 and fast day 1 groups to evaluate the possible changes of basal plasma corticosterone levels due to acute exposure to stress (Basal-After Stress: B-AS).
This procedure was performed for the groups of days 15 and 30. It means that after 15 and 30 days of placing the animals in the com-box (1 h/day) (while all the animals were in fed state), the blood samples were taken on the 15th and 30th days of the experiment immediately after removing the rats from the com-box and in the morning of the next days (i.e. 16th and 31st days) of the experiments similar to the procedure mentioned above for the groups of day 1.
Islet isolation procedure
The islet isolation was performed by the collagenase technique of Lacy and Kostianovsky (1967[20]) with slight modification. One day after the last stress exposure, following 16 h fasting (in fasted groups), animals were anesthetized lightly with isoflurane, decapitated and bled (n=4/group) and the abdomen was opened. The entrance of common bile duct to duodenum was clamped, the duct was cannulated with a polyethylene catheter (Portex Intravenous Cannula2.5 F, 0.75 mm OD) and 10 ml cold Hank's buffer [containing in mM: NaCl, 137; KCl, 5.4; CaCl2, 1.2; MgSO4 7H2O, 0.8; Na2HPO4 2H2O, 0.3; KH2PO4, 0.4; NaHCO3, 4.2 (Merck, Germany)] (Zardooz et al., 2006[42]), in which collagenase P (Roche, Cat. # 11 213 865 001, Germany, 0.45 mg/ml) was diluted, was gently injected into the duct. The inflated pancreas was removed and placed into a Petri dish and cleaned from non-pancreatic tissue. Then the pancreas was placed into a 50 ml falcon tube and incubated in a 37 °C water bath for 17 min. Digestion was terminated by adding cold Hank's solution up to 40 ml. The tube was shaken for 1 min and the suspension was dispensed into a glass container (7.5 cm diameter and 4.5 cm height). Cold Hank's solution was added and aspirated after precipitation. The supernatant was removed, a process which was repeated three times. After the last aspiration islets were hand picked (Blue Light stereomicroscope, USA) (first-picking).
Glucose-stimulated insulin secretion study
Glucose-stimulated insulin secretion was assessed at different glucose concentrations (5.6, 8.3, 16.7 mM) (Lacy and Kostianovsky, 1967[20]). From the isolated islets of each animal, five groups of ten islets for each glucose concentrations were picked randomly (second-picking) and placed in the plastic cups (20 cups into total for each condition). All procedures for islets separation were carried out on the ice tray. After removing the excess hank's solution, 1 ml of Krebs Ringer Solution (pH 7.4) [containing in mM: NaCl, 111; KCl, 5; MgCl2 6H2O, 1; CaCl2, 1; NaHCO3, 24 (Merck, Germany); Hepes, 10 (Sigma, USA) and BSA, 0.5 g/dl (Sigma, USA)] (Zardooz et al., 2006[42]) containing 5.6, 8.3 or 16.7 mM glucose was added to the cups and incubated for 90 min (at the beginning the cups were gassed with 95 % O2/5 % CO2 for 5 min) at 37 °C. Then the supernatant was removed and stored at -70 °C to insulin assays.
Islets' area and number
Three rats of each group were anesthetized with isoflurane and decapitated. Their pancreas was totally removed and immersed in 10 % buffered formalin fixative for 24 h. Then each pancreas was divided into 3 parts (from head to tail, namely intestine, middle and spleen regions), embedded in paraffin, sectioned into 5-µm-thick sections and Hematoxylin-Eosin staining performed (Rao et al., 1998[29]; Bock et al., 2005[4]). Nine consecutive slices in the middle area of each region were examined for the number of islets, and 10 different fields were viewed randomly in each section. Furthermore the area of the islets was measured by MOTIC software (Nikon, Japan, 2001).
Assays
Plasma samples were analyzed for corticosterone concentration by the corticosterone Elisa kit (DRG, Germany). The intra- and inter-assay coefficients of variations were 4.08 % and 6.35 %, respectively. Insulin concentration of the supernatant was measured by rat insulin Elisa kit (Mercodia, Sweden). The intra- and inter-assay coefficients of variations were 3.4 % and 2.2 %, respectively.
Statistical analysis
Statistical computations were made by SPSS Version 9.05 program package. All data expressed as Mean ± SEM. A three way ANOVA (using stress × day × feeding state, stress × day × glucose concentration and stress × feeding state × glucose concentration as factors) followed by Bonferroni test was used to analyze the differences between groups. P values less than 0.05 were considered statistically significant.
Results
Effects of acute and chronic psychological stress on plasma corticosterone concentrations
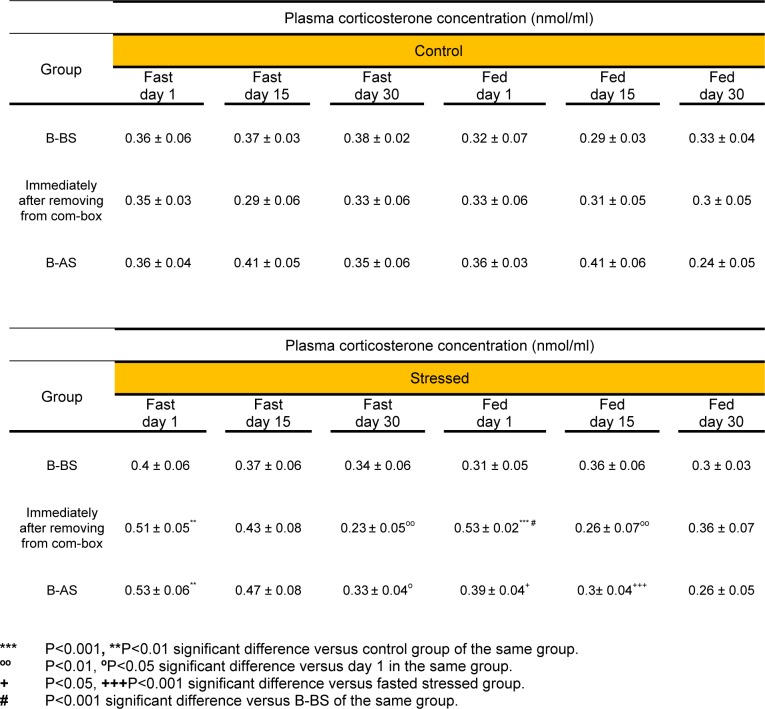
Acute stress (day 1) caused significant increase in B-AS plasma corticosterone of the fasted animals as compared to the controls (P<0.01). Moreover, the B-AS level of corticosterone in the fasted rats under 30 days stress (chronic stress) was significantly less than the B-AS corticosterone level of the rats, who underwent only one day of stress (P<0.05). The B-AS plasma corticosterone concentrations in acute (P<0.05) and chronic (day 15) (P<0.001) fed stressed rats were significantly lower than in the fasted ones (Table 1(Tab. 1)). No significant difference was observed between B-BS and B-AS plasma corticosterone concentrations of any study groups.
Table 1. Basal and immediate post stress plasma corticosterone concentrations following acute (day 1) and chronic (days 15 and 30) exposure to psychological stress. Each value represents Mean ± SEM for 6 rats. B-BS (Basal- Before Stress), B-AS (Basal- After Stress).
Following acute stress exposure, plasma corticosterone values increased immediately after removing the animals from the com-box in the fasted group as compared to the control group (P<0.01) and in the fed group as compared to its control and B-BS groups (P<0.001) (Table 1(Tab. 1)). In addition, immediately after removing from the com-box, the fasted chronic stressed rats (day 30) showed significantly lower plasma corticosterone levels than acutely stressed ones (P<0.01) (Table 1(Tab. 1)). The chronically fed stressed animals (day 15) showed significant reduction in immediately post stress plasma corticosterone levels as compared to the animals of the same group on day 1 (P<0.01) (Table 1(Tab. 1)). However, three way ANOVA showed no significant difference between the plasma corticosterone concentration of the fed and fasted groups, immediately after removing from the com-box, throughout the experiment.
Acute and chronic psychological stress impact on area and number of the islets
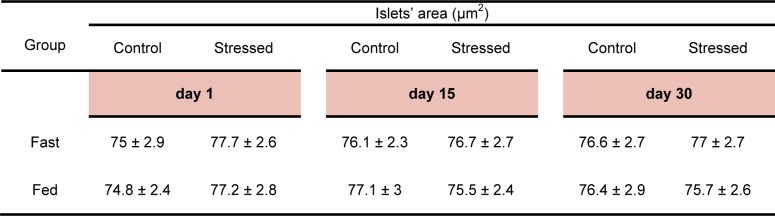
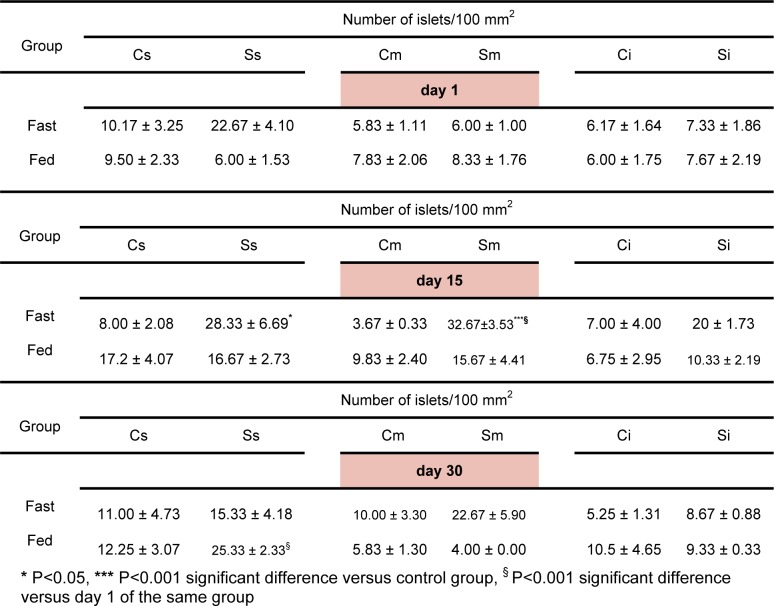
No significant differences were observed in the area of the islets following psychological stress exposure (Table 2(Tab. 2)) (Figure 1(Fig. 1)). However, the number of the islets increased significantly following 15 days stress exposure in the fasted group (in the spleen region P<0.05 as compared to the control group, in the middle region P<0.001 as compared to the control group and also the stressed group on day 1) (Table 3(Tab. 3)). In the fed group, a significant increase in the number of islets was observed following 30 days stress exposure in the spleen region as compared to the stressed group on day 1 (P<0.001) (Table 3(Tab. 3)). Three way ANOVA showed no significant differences between fed and fasted groups in this regard.
Table 2. The pancreatic islets' area (µm2) of the control, acute (day1) and chronic (days 15 and 30) psychologically stressed groups in fed and fasted states. Values are Mean ± SEM of 3 rats.
Figure 1. Pancreatic islets of A) Control (day 1), B) Stressed (day 1), C) Control (day 15), D) Stressed (day 15), E) Control (day 30), F) Stressed (day 30) in fasted rats. H&E, 100 X.
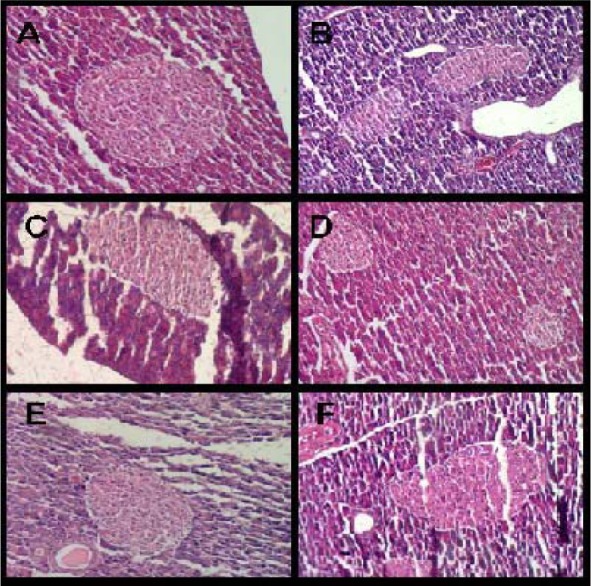
Table 3. Effect of acute (day 1) and chronic (days 15 and 30) psychological stress on the number of islets in fasting and fed states. Each value represents Mean ± SEM for 3 rats. Spleen region of the pancreas of control group (Cs), spleen region of the pancreas of the stressed group (Ss), middle region of the pancreas of the control group (Cm), middle region of the pancreas of the stressed group (Sm), intestine region of the pancreas of the control group (Ci), intestine region of the pancreas of the stressed group (Si).
Effects of acute and chronic psychological stress on insulin release from isolated islets
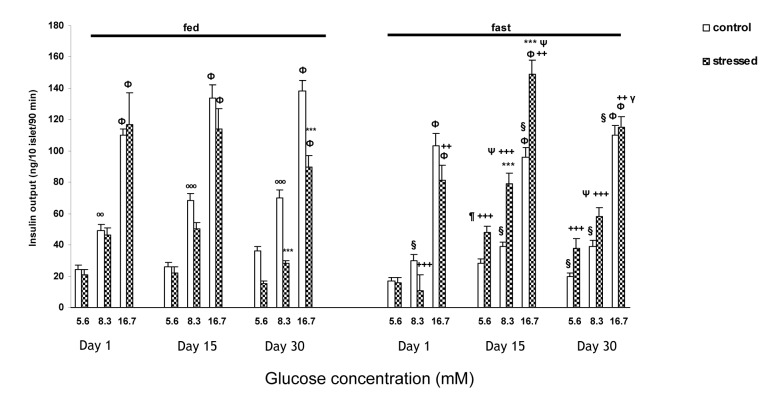
Insulin output from isolated islets of the fasted animals in both control and stressed groups in the presence of 16.7 mM glucose concentration was significantly higher (P<0.001) than both 8.3 mM and 5.6 mM on days 1, 15 and 30 (Figure 2(Fig. 2)). In the fasted rats three way ANOVA showed a significant increase in insulin release from islets of the rats exposed to 15 days psychological stress in response to 8.3 and 16.7 mM glucose concentrations as compared to the controls (P<0.001) (Figure 2(Fig. 2)). Insulin release from isolated islets of fasted chronically stressed rats (days 15 and 30), was significantly elevated in the presence of glucose as compared to acutely stressed rats [on day 15 of stress, in the presence of 5.6 mM (P<0.01) and in the presence of 8.3 and 16.7 mM glucose concentrations (P<0.001); on day 30 of stress, in the presence of 8.3 mM glucose concentration, (P<0.001) (Figure 2(Fig. 2))]. Moreover, fasted stressed rats (day 30) showed lower insulin release in the presence of 16.7 mM glucose concentration than that of day 15 (P<0.001) (Figure 2(Fig. 2)).
Figure 2. The insulin output (ng/10 islets/90 min) from pancreatic isolated islets in response to different glucose concentrations following acute (one day) and chronic [(15 days) and (30 days)] exposure to psychological stress in fed and fasted states.
Values are Mean ± SEM of 4 rats (5 groups of 10 islets each/glucose concentration/rat).
ФP<0.001, significant difference versus both 5.6 and 8.3 mM glucose concentration of the same group. ºººP<0.001, ººP<0.01, significant difference versus 5.6 mM glucose concentration of the same group. ***P<0.001, significant difference versus control group of the same group. ΨP<0.001, ¶P<0.01, significant difference versus day 1 of the same group. γP<0.001, significant difference versus day 15 of the same group. §P<0.001, significant difference versus fed control group. +++P<0.001, ++P<0.01, significant difference versus fed stressed group
In fed state, the control and stressed groups on days 1, 15 and 30 showed significant increase in insulin release at 16.7 mM glucose concentration in comparison with both 8.3 and 5.6 mM (P<0.001) (Figure 2(Fig. 2)). A significant increase of insulin release was observed in the control rats in the presence of 8.3 mM glucose concentration compared with 5.6 mM (on day 1, P<0.01; on days 15 and 30, P<0.001) (Figure 2(Fig. 2)). A marked reduction was observed in insulin release from islets of the stressed rats at 8.3 and 16.7 mM glucose concentrations only on day 30 as compared to the controls (P<0.001) (Figure 2(Fig. 2)).
Moreover, insulin secretion from the islets of the fasted control rats was lower than the fed ones [significant differences (P<0.001) were observed on day 1 at 8.3 mM glucose, on day 15 at 8.3 and 16.7 mM glucose, and on day 30 at all glucose concentrations (Figure 2(Fig. 2))]. On the other hand, in the fasted stressed rats on days 15 and 30 the insulin output was significantly higher than fed ones at all glucose concentrations (in the presence of 5.6 and 8.3 mM glucose concentrations P<0.001, in the presence of 16.7 mM glucose concentration P<0.01) (Fig 2(Fig. 2)). However, in the fasted acutely stressed group, the insulin secretion from isolated islets was significantly lower in response to 8.3 (P<0.001) and 16.7 (P<0.01) mM glucose concentrations as compared to the fed ones (Figure 2(Fig. 2)).
Discussion
The present study shows, on one hand, the effects of feeding status on the corticosterone response to psychological stress (i.e. basal plasma corticosterone increment in fasted rats and no significant change of the basal plasma corticosterone in fed animals, under acute stress), and, on the other hand, the adaptation of the response to stress (i.e. plasma corticosterone decrement by repeated exposure to stress). But more importantly, regarding the results of glucose stimulated insulin secretion from isolated islets (in fed and fasting states), insulin secretion showed almost no adaptation to stress and depending on the feeding state, the insulin output from isolated islets in response to chronic stress in the fasted and fed rats increased and decreased respectively. Therefore, it is obvious that fasting or fed states in the animals, which were under chronic stress, resulted in diverse changes in islets function. The results of this study may add to data of the current views on stress and its effects on important metabolic hormones, i.e. corticosterone and insulin.
Majority of researches report that acute psychological stress increased plasma corticosterone concentration immediately after stress induction, findings in agreement with those of ours. In a study on male and female Wistar rats, swimming stress caused significant increase in serum corticosterone concentration (Tinnikov, 1999[37]). In addition, acute psychosocial crowding stress caused a significant increase in serum corticosterone immediately and also 48 h after stress exposure in male Wistar rats (Djordjevic et al., 2003[13]). Moreover, acute psychological stresses, such as novelty, handling and water immersion in 35 ºC, also increased plasma corticosterone levels immediately after stress exposure (De Boer et al., 1990[11]). Nevertheless, it must be noticed that the response highly depends on the type, intensity and duration of the stresses; for instance tail snipping as a mild stress (Pitman et al., 1988[28]) did not markedly change the corticosterone level immediately after stress exposure. On the other hand, similar to our results, exposure to repeated stress decreased corticosterone response immediately after the stress exposure as compared to the first stress exposure, reflecting adaptation to stress (Zardooz et al., 2006[43]; De Boer et al., 1990[11]).
The basal plasma level of corticosterone which can reflect the speed at which the animal recovers from consequences of stress exposure has also been investigated in the present study. Our data showed that B-BS plasma corticosterone levels were not significantly different between the study groups. However, although both fed and fasted animals showed almost the same pattern of B-AS plasma corticosterone level changes in response to stress, only in the fasted status, a significant increase of B-AS plasma corticosterone concentrations were observed in response to acute stress; and by increasing the days of stress exposure, the B-AS plasma corticosterone level decreased markedly compared to the first day which also may reflect adaptation to stress. The difference between fed and fasted groups in B-AS corticosterone response may be due to the effect of fasting on hypothalamic-pituitary-adrenal (HPA) axis (Chang et al., 2002[9]; Woodward et al., 1991[38]). Therefore, it is likely that the psychological stress per se was not able to cause significant increase in plasma corticosterone. Regarding the basal plasma corticosterone level, acute immobilization stress in Sprague-Dawley rats for 2 h (Pitman et al., 1988[28]) and 5 or 30 min exposure to shaker stress (Hashiguchi et al., 1997[18]) in Wistar rats increased basal corticosterone level as compared to controls whereas, in Sprague-Dawley rats acute restraint for 2 h, immobilization for 1 h and acute isotonic saline injection caused no changes (Pitman et al., 1988[28]; García et al., 2000[16]). On the other hand, Armario et al. (1984[1]) showed that 28 days exposure to noise stress for 5 and 60 min/day did not change basal corticosterone levels in male Wistar rats whereas, 2 h/day exposure to restraint for 21 days (Pitman et al., 1988[28]) and 20 min/day immobilization for 8 days (García et al., 2000[16]) increased basal plasma corticosterone concentrations. Moreover, it has been shown that in rats, fasting per se for 1 or 2 days increased basal plasma corticosterone levels as compared to the fed state (Woodward et al., 1991[38]; Chang et al., 2002[9]). At a glance, it seems that type, intensity and duration of stress and also the strain of the animals may contribute in basal corticosterone plasma level changes in response to the stress. In this regard our findings also highlighted the importance of feeding state as well.
In the present investigation no change in the area of the islets was observed in any of the groups but chronic (15 days) exposure to psychological stress did increase the number of islets in the fasted group. This increase may reflect a compensatory response to possibly plasma glucose elevation due to stress exposure (Nichols et al., 2008[26]; Dheen et al., 1997[12]). The study on isolated islets in male Fisher 344 Brown Norway rats under hind limb suspension for 14 days showed that, in spite of serum insulin reduction in young rats, the area and number of islets did not change; however, in old rats the number of islets increased but serum insulin levels did not change (Nichols et al., 2008[26]). In our study, stress was also able to change the number of islets; however, the animals' strain, age, feeding status, duration and types of stress are different.
Our data showed that insulin release is lower in the fasted control animals compared to the fed ones. This result may be due to decrease in the activity of some enzymes such as phospholipase A2 (Tadayyon et al., 1990[34]) and glucokinase (Buchanan et al., 1969[7]), or a decrease in glucagon secretion (Tamarit-Rodriguez et al., 1984[35]), and a high oxidation rate of palmitate (Malaisse et al., 1976[24]) induced by fasting.
In this study, following the application of acute psychological stress, glucose stimulated insulin secretion from isolated islets in fasted and fed groups was not changed significantly. On the other hand, glucose stimulated insulin release from isolated islets of chronic stressed rats (both day 15 and day 30) was higher in the fasted rats than the fed ones. However, this higher insulin output on one hand, is due to the increase of insulin output in the fasted rats on day 15 and on the other hand, the decrease of the insulin output in the fed rats on day 30. This indicated that in the fasted animals a more efficient compensatory mechanism (s) may be activated in response to stress. These mechanisms which are not fully understood may increase the sensitivity of the islets to glucose, as a result higher insulin secretion from isolated islets may occur. In this regard, several studies have shown the effect of stress on islets' insulin secretion. For example three daily foot-shocks caused significant increase of insulin secretion from isolated islets in response to 5.6 mM glucose, whereas the response to higher glucose concentrations remained unchanged (Farias-Silva et al., 2002[15]). In another study, surgical stress decreased the insulin secretion from isolated pancreas 4 days after surgery in response to 16.7 mM glucose, whereas in the presence of 4.2 mM glucose, no change was observed (Hirano et al., 1991[19]). In our previous study, we observed that chronic restraint stress increased isolated islets insulin secretion in response to increased glucose concentrations in the fed rats (Zardooz et al., 2006[42]), which is not in agreement with the results of the present study. The difference may be related to the application of different types of psychological stress. Since in the present study plasma corticosterone level did not increase following chronic stress, it is possible to postulate that another stress activated system, i.e. the sympathoadrenal system may be responsible for the changes in insulin secretion (Smythe et al., 1989[33]; Halter et al., 1984[17]). On the other hand, considering that the area of the islets was not changed, the increase in insulin output in the fasted rats may be due to the increase in sensitivity of the islets to glucose following chronic exposure to stress. In agreement with this hypothesis it has been shown that three days foot-shock increase the sensitivity of the islets to 5.6 mM glucose concentration (Farias-Silva et al., 2002[15]) and also in the other study chronic electrical stress increased sensitivity of isolated islets in response to 16.7 mM glucose concentration (Yamaguchi and Matsuoka, 1982[40]). It seems that in the fasting state the sensitivity of the islets to glucose can be intensified.
This interesting data on the increment of the number of islets and insulin secretion from the isolated islets following chronic exposure to stress in fasting state may indicate that the islets response to glucose could be more pronounced when the animal is fasted. However, when the stress exposure is acute the insulin secretion is higher in fed than fasting state.
In conclusion, this study indicated that the response of the isolated islets from acute and chronically stressed rats are different and depends on the feeding status. This should be considered in any interpretation of the results of the studies on the effect of stress on insulin secretion.
Notes
The investigation was carried out in the Department of Physiology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Acknowledgements
This work was supported by a grant from Neuroscience Research Center, Shahid Beheshti University of Medical Sciences. The authors would like to express their gratitude to Ms. Nilufar Shiva for her help in preparation of the manuscript.
References
- 1.Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behav Neural Biol. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- 2.Armario A, Castellanos JM, Balasch J. Chronic noise stress and insulin secretion in male rat. Physiol Behav. 1985;34:359–361. doi: 10.1016/0031-9384(85)90196-9. [DOI] [PubMed] [Google Scholar]
- 3.Billaudel B, Shutter BC. Immediate in vivo effect of corticosterone on glucose–induced insulin secretion in the rat. J Endocrinol. 1982;95:315–320. doi: 10.1677/joe.0.0950315. [DOI] [PubMed] [Google Scholar]
- 4.Bock T, Pakkenberg B, Buschard K. Genetic background determines the size and structure of the endocrine pancreas. Diabetes. 2005;54:133–137. doi: 10.2337/diabetes.54.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Bratusch-Marrain PR. Insulin-counteracting hormones: their impact on glucose metabolism. Diabetologia. 1983;24:74–79. doi: 10.1007/BF00297384. [DOI] [PubMed] [Google Scholar]
- 6.Brindley DN, Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin Sci. 1989;77:453–461. doi: 10.1042/cs0770453. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan KD, Vance JE, Williams RH. Effect of starvation on insulin and glucagon release from isolated islets of Langerhans of the rat. Metabolism. 1969;18:155–162. doi: 10.1016/0026-0495(69)90110-3. [DOI] [PubMed] [Google Scholar]
- 8.Chalkley SM, Hettiarachchi M, Chisholm DJ, Kraegen EW. Long-term high-fat feeding leads to severe insulin resistance but not diabetes in Wistar rats. Am J Physiol Endocrinol Metab. 2002;282:1231–1238. doi: 10.1152/ajpendo.00173.2001. [DOI] [PubMed] [Google Scholar]
- 9.Chang LL, Kau MM, Wun WS, Ho LT, Wang PS. Effects of fasting on corticosterone production by zona fasciculata-reticularis cells in ovariectomized rats. J Invest Med. 2002;50:86–94. [PubMed] [Google Scholar]
- 10.Cosgrove M. Do stressful life events cause type 1 diabetes? Occup Med. 2004;54:250–254. doi: 10.1093/occmed/kqh047. [DOI] [PubMed] [Google Scholar]
- 11.De Boer SF, Koopmans SJ, Slangen JL, Van Der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- 12.Dheen ST, Rajkumar K, Murphy LJ. Islet cell proliferation and apoptosis in insulin-like growth factor binding protein-1 in transgenic mice. J Endocrinol. 1997;155:551–558. doi: 10.1677/joe.0.1550551. [DOI] [PubMed] [Google Scholar]
- 13.Djordjevic J, Cvijic G, Davidovic V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol Res. 2003;52:67–72. [PubMed] [Google Scholar]
- 14.Endo Y, Yamauchi K, Fueta Y, Lrie M. Changes of body temperature and plasma corticosterone level in rats during psychological stress induced by the com-box. Med Sci Monitor. 2001;7:1161–1165. [PubMed] [Google Scholar]
- 15.Farias-Silva E, Sampaio-Barros MM, Amaral MEC, Carneiro EM, Boschero AC, Grassi-Kassisse DM, et al. Subsensitivity to insulin in adipocytes from rats submitted to foot-shock stress. Can J Physiol Pharm. 2002;80:783–789. doi: 10.1139/y02-104. [DOI] [PubMed] [Google Scholar]
- 16.García A, Martí O, Vallès A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72:114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- 17.Halter JB, Beard JC, Daniel Porte JR. Islet function and stress hyperglycemia: plasma glucose and epinephrine interaction. Am J Physiol. 1984;247:47–52. doi: 10.1152/ajpendo.1984.247.1.E47. [DOI] [PubMed] [Google Scholar]
- 18.Hashiguchi H, Ye SH, Morris M, Alexander N. Single and repeated environmental stress: effect on plasma oxytocin, corticosterone, catecholamines, and behavior. Physiol Behav. 1997;61:731–736. doi: 10.1016/s0031-9384(96)00527-6. [DOI] [PubMed] [Google Scholar]
- 19.Hirano T, Manabe T, Ando K, Yamaki K. Yoshimura T, Tobe T. Effect of surgical stress on glucose-stimulated insulin release from isolated perfused rat pancreas. Int Surg. 1991;76:250–252. [PubMed] [Google Scholar]
- 20.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Littorin B, Sundkvist G, Nustrom L, Carlson A, Landin–Olsson M, Ostman J, et al. Family characteristics and life events before the onset of autoimmune type 1 diabetes in young adults. Diabetes Care. 2001;24:1033–1037. doi: 10.2337/diacare.24.6.1033. [DOI] [PubMed] [Google Scholar]
- 22.Macho L, Fickova M, Zorad S, Kvetnansky R. Changes of insulin binding in rat tissues after exposure to stress. Physiol Res. 1999;48:51–58. [PubMed] [Google Scholar]
- 23.Makino S, Asaba K, Nishiyama M, Hashimoto K. Decreased type 2 corticotropin-releasing receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology. 1999;70:160–167. doi: 10.1159/000054472. [DOI] [PubMed] [Google Scholar]
- 24.Malaisse WJ, Sener A, Levy J. The stimulus secretion coupling of glucose-induced insulin release. XXI Fasting-induced adaptation of key glycolytic enzymes in isolated islets. J Biol Chem. 1976;251:1731–1737. [PubMed] [Google Scholar]
- 25.Malatinsky J, Vigas M, Jurcovicova J, Jezova D, Garayova S, Minarikova M. The patterns of endocrine response to surgical stress during different types of anesthesia and surgery in man. Acta Anaesthesiol Belg. 1986;37:23–32. [PubMed] [Google Scholar]
- 26.Nichols KR, Chowdhury P, Dupont-Versteegden EE. Pancreatic response to hind limb suspension in rats is affected by age. Open Clin Chem J. 2008;1:69–74. [Google Scholar]
- 27.Nowotny B, Cavka M, Herder C, Löffler H, Poschen U, Joksimovic L, et al. Effects of acute psychological stress on glucose metabolism and subclinical inflammation in patients with post-traumatic stress disorder. Horm Metab Res. 2010;42:746–754. doi: 10.1055/s-0030-1261924. [DOI] [PubMed] [Google Scholar]
- 28.Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- 29.Rao RM, Salem FA, Gleason-Jordan I. Anti-diabetic effects of dietary supplement "Pancreas Tonic". J Natl Med Assoc. 1998;90:614–618. [PMC free article] [PubMed] [Google Scholar]
- 30.Romeo RD, Karatsoreos IN, Ali FS, McEwen BS. The effects of acute stress and pubertal development on metabolic hormones in the rat. Stress. 2007;10:101–106. doi: 10.1080/10253890701204270. [DOI] [PubMed] [Google Scholar]
- 31.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Shiloah E, Witz S, Abramovitch Y, Cohen O, Buchs A, Ramot Y, et al. Effect of acute psychotic stress in nondiabetic subjects on β-cell function and insulin sensitivity. Diabetes Care. 2003;26:1462–1467. doi: 10.2337/diacare.26.5.1462. [DOI] [PubMed] [Google Scholar]
- 33.Smythe GA, Pascoe WS, Storlien LH. Hypothalamic noradrenergic and sympathoadrenal control of glycemia after stress. Am J Physiol. 1989;256:231–235. doi: 10.1152/ajpendo.1989.256.2.E231. [DOI] [PubMed] [Google Scholar]
- 34.Tadayyon M, Bonney RC, Green IC. Starvation decreases insulin secretion, prostaglandin E2 production and phospholipase A2 activity in rat pancreatic islets. J Endocrinol. 1990;124:455–461. doi: 10.1677/joe.0.1240455. [DOI] [PubMed] [Google Scholar]
- 35.Tamarit-Rodriguez J, Vara E, Tamarit J. Starvation-induced changes of palmitate metabolism and insulin secretion in isolated rat islets stimulated by glucose. Biochem J. 1984;221:317–324. doi: 10.1042/bj2210317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiagarajan AB, Gleiter CH, Nutt DJ. Electroconvulsive shock does not increase plasma insulin in rats. Convulsive Ther. 1988;4:292–296. [PubMed] [Google Scholar]
- 37.Tinnikov AA. Responses of serum corticosterone and corticosteroid-binding globulin to acute and prolonged stress in the rat. Endocrine. 1999;11:145–150. doi: 10.1385/ENDO:11:2:145. [DOI] [PubMed] [Google Scholar]
- 38.Woodward CJH, Hervey GR, Oakey RE, Whitaker EM. The effects of fasting on plasma corticosterone kinetics in rats. Brit J Nutr. 1991;66:117–127. doi: 10.1079/bjn19910015. [DOI] [PubMed] [Google Scholar]
- 39.Yamada F, Inoue S, Saitoh T, Tanaka K, Satoh S, Takamura Y. Glucoregulatory hormones in the immobilization stress induced increase of plasma glucose in fasted and fed rats. Endocrinology. 1993;132:2199–2205. doi: 10.1210/endo.132.5.8477665. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi K, Matsuoka A. Effects of a high fat diet and electric stress on adenylate cyclase activity and insulin release in isolated islets of langerhans. Horm Metab Res. 1982;14:117–121. doi: 10.1055/s-2007-1018943. [DOI] [PubMed] [Google Scholar]
- 41.Zardooz H, Rostamkhani F, Zaringhalam J, Shahrivar FF. Plasma corticosterone, insulin and glucose changes induced by brief exposure to isoflurane, diethyl ether and CO2 in male rats. Physiol Res. 2010;59:973–978. doi: 10.33549/physiolres.931896. [DOI] [PubMed] [Google Scholar]
- 42.Zardooz H, Zahediasl S, GharibNaseri MK. Effect of chronic psychological stress on insulin release from rat isolated pancreatic islets. Life Sci. 2006;79:57–62. doi: 10.1016/j.lfs.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Zardooz H, Zahediasl S, GharibNaseri MK, Hedayati M. Effect of chronic restraint stress on carbohydrate metabolism in rat. Physiol Behav. 2006;89:373–378. doi: 10.1016/j.physbeh.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 44.Zaringhalam J, Akbari A, Tekieh E, Manaheji M, Rezazadeh S. Achillea santolina reduces serum interlukin-6 level and hyperalgesia during complete Freund's adjuvant-induced inflammation in male Wistar rats. J C I M. 2010;8:1180–1189. doi: 10.3736/jcim20101211. [DOI] [PubMed] [Google Scholar]