Abstract
Objectives
To examine the association of borderline LVEF of 50-55% with cardiovascular morbidity and mortality in a community-based cohort.
Background
Guidelines stipulate left ventricular ejection fraction (LVEF) >55% as normal, but the optimal threshold, if any, remains uncertain. The prognosis of a “borderline” LVEF, 50-55%, is unknown.
Methods
We evaluated Framingham Heart Study participants who underwent echocardiography between 1979 and 2008 (n=10,270 person-observations, mean age 60 years, 57% women). Using pooled data with up to 12 years of follow-up and multivariable Cox regression, we evaluated the associations of borderline LVEF, and continuous LVEF to the risk of developing a composite outcome (heart failure [HF] or death; primary outcome) and incident HF (secondary outcome).
Results
During follow-up (median 7.9 years), 355 participants developed HF and 1070 died. Among participants with LVEF 50-55% (prevalence 3.5%), rates of the composite outcome and HF were 0.24 and 0.13 per 10 years follow-up, respectively, versus 0.16 and 0.05 in those having normal LVEF. In multivariable-adjusted analyses, LVEF 50-55% was associated with increased risk of the composite outcome (Hazards ratio [HR] 1.37, 95% CI 1.05-1.80) and HF (HR 2.15, 95% CI 1.41-3.28). There was a linear inverse relationship of continuous LVEF with the composite outcome (HR per 5 LVEF% decrement: 1.12, 95% CI 1.07-1.16) and HF (HR 1.23 per 5 LVEF% decrement, 95% CI 1.15-1.32).
Conclusions
Individuals with LVEF of 50-55% in the community have greater risk for morbidity and mortality relative to those with LVEF >55%. Additional studies are warranted to elucidate their optimal management.
Keywords: Left ventricular function, heart failure, epidemiology, echocardiography
INTRODUCTION
Clinical heart failure (HF) is associated with substantial morbidity and mortality, despite advances in medical therapy (1), and characterization of at-risk populations is essential to understand its development and to target potentially susceptible individuals for preventive strategies.
European Society of Cardiology and American Society of Echocardiography guidelines report normal LVEF as >50% and >55%, respectively (2,3) and clinical HF trials have defined left ventricular ejection fraction (LVEF) <40-45% to indicate LV systolic dysfunction (4,5). However, groups with asymptomatic LVEF 40-50% show greater risk for HF and mortality compared with those with LVEF >50-55% (6-8), leading investigators to question the optimal cut-point for identifying a ‘normal’ LVEF, or if the association of LVEF with adverse cardiovascular outcomes is continuous (9).
In particular, the prognosis for those individuals with a “borderline” LVEF of 50-55% is unclear. We hypothesized that these individuals are at greater risk for developing cardiovascular events and death relative to individuals with a LVEF>55%. Accordingly, we characterized the clinical correlates and prognosis of individuals with a LVEF 50-55% and the relations of continuous LVEF with adverse outcomes in a large community-based cohort.
METHODS
Participants
The details of the selection criteria and examination of Framingham Heart Study (FHS) Original and Offspring Cohorts have been described (10,11). We included Original Cohort participants who attended examinations 16 (1979-1981) or 20 (1988-1989) and Offspring Cohort participants who attended examinations 4 (1987-1990), 6 (1995-1998), or 8 (2005-2008) (Supplemental Figure 1). Of 14,187 eligible person-observations, we excluded observations with a history of HF (n=270), with inadequate echocardiographic data (n=3,592), and a lack of follow-up data (n=104). Individuals with missing measures were more likely to be obese with greater CVD risk factors (12). After exclusions, we included 10,221 person-observations representing 5,334 unique individuals. The number of observations included at each examination is presented in Supplemental Table 1.
Diabetes was defined as fasting glucose ≥126 mg/dl or the use of hypoglycemic medications. Systolic and diastolic blood pressure were measured as the average of two measurements made on seated participants using a mercury column sphygmomanometer, a appropriately-sized cuff, and a standardized protocol. Use of anti-hypertensive medications and diabetes medications were self-reported and all medications were verified by the Heart Study clinic physician. Between January 1995 and September 1998, plasma brain natriuretic peptide (BNP) levels were collected in n=2,552 of Offspring cohort participants at Examination 6, in the morning after an overnight fast. Samples were stored at −70°C, and analyzed using sensitive noncompetitive immunoradiometric assays (Shionogi, Japan) in June 1999.
Echocardiography and Calculation of LVEF
The following ultrasound machines were used for echocardiography: Original Cohort examination cycles 16 and 20 and Offspring examination cycles 4 and 5: Hewlett Packard (model 77020AC); Offspring examinations 6 and 8: Hewlett-Packard Sonos 1000 and Sonos 5500, respectively.
Measurements of M-mode LV end-diastolic (LVEDD) and end-systolic dimensions (LVESD) were performed by experienced sonographers using the leading edge technique according to American Society of Echocardiography guidelines (13). LVEF was calculated using these measures using the Z-volume formula by de Simone et al. (14):
This method is based on human (14) and experimental (15) evidence that the epicardial long to short axis ratio is constant through the cardiac cycle and has been widely applied in clinical studies (16-18). We selected this formula to include the longer follow-up of earlier cohorts that did not have routine two-dimensional quantitation of chamber volume.
Additionally, in a subset of participants, both de Simone method and biplane Simpson’s method using two-dimensional echocardiography (available in n=2315 of Offspring cohort at examination 8) were utilized to quantitate LVEF by summation of disks method in four-chamber and two-chamber views (3).
Follow Up
Participant medical records were reviewed and adjudicated for cardiovascular disease (CVD) and death. CVD included history of coronary artery disease, stable and unstable angina, myocardial infarction (MI), cerebrovascular accident (atherothrombotic brain infarct, transient ischemic attack, intracranial or subarachnoid hemorrhage, cerebral embolism) and peripheral arterial disease (intermittent claudication).
The diagnosis of HF was made using the FHS criteria (19) with sensitivity and specificity comparable with other HF criteria (20). The date of onset was noted as the first episode of HF symptoms, physician visit, or hospitalization. HF with a reduced left ventricular ejection fraction (HFREF) and with a preserved ejection fraction (HFPEF) were defined as HF symptoms with LVEF <50% and ≥50%, respectively (21).
Our primary outcome was a composite of new-onset HF and death, because death may be the first adverse event in those with asymptomatic LV systolic dysfunction (6).
Statistical Analysis
We pooled participants of FHS Original cohort examinations 16 and 20 and Offspring cohort examination cycles 4, 6, and 8, retaining participants free of prevalent HF. Participants were grouped by LVEF <50%, 50-55%, and >55%, calculated based on their examination echocardiograms. We evaluated clinical correlates of borderline LVEF relative to LVEF >55% (excluding participants with LVEF <50%), using multivariable logistic regression with the following covariates: age, sex, baseline CVD, diabetes, systolic blood pressure, diastolic blood pressure, and hypertensive treatment.
We followed participants for incident HF or death during a follow-up period of up to 12 years. Ten-year age- and sex-adjusted incidence rates of HF by LVEF category were estimated using the data-augmentation method (22). Cumulative incidence curves describing the occurrence of these outcomes by EF category were presented. The proportions of those who developed HFPEF and HFREF at follow-up were determined for each LVEF group. The hazards ratios (HR) for these outcomes were compared between the categories of LVEF, with LVEF >55% serving as the referent group. We estimated age- and sex-adjusted and multivariable-adjusted proportional hazards models for each outcome, after confirming that the assumption of proportionality of hazards was satisfied for each outcome. We also examined continuous LVEF as a risk factor for these outcomes. Primary models were stratified by cohort type and presence versus absence of prevalent myocardial infarction (MI). To address possible confounding from time (due to changes in echocardiography quality, CVD risk factor prevalence, and CVD treatment during the study) we examined similar models stratifying by examination and prevalent MI.
We also conducted a secondary analysis examining the association of LVEF calculated by biplane Simpson’s method, available in Offspring participants at Examination 8, with our outcomes of interest. The Pearson correlation coefficient, Bland-Altman method (23), and weighted kappa statistic (24) were used to assess the agreement between LVEF calculated by the de Simone and Simpson’s biplane methods. Final models were repeated using robust Lin-Wei covariance estimator to account for clustering of multiple periods of observations within individuals. All multivariable models were adjusted for age, sex, body mass index, baseline CVD, systolic blood pressure, use of antihypertensive treatment, current smoking, and prevalent diabetes; a separate analysis additionally adjusted for LV cavity size. Covariates were selected based upon literature review and clinical judgment of their probable associations with LVEF and the outcomes of heart failure and death, and also upon availability of these covariates in FHS examinations. Furthermore, we conducted several sensitivity analyses to evaluate for consistency with our primary results: using propensity score matching for CVD risk factor variables included in multivariable analysis, excluding individuals with prevalent MI, and accounting for clustered observations among individuals. Restricted penalized cubic-splines were fitted to assess the linearity of the relations between continuous LVEF and the outcomes. Statistical significance was considered at two-tailed p≤0.05. However, in light of multiple significance tests of association, one should interpret modest P values (e.g., 0.005< p <0.05) as denoting modest associations. All analyses were performed using SAS v.9.3 (SAS Institute, Cary, NC).
RESULTS
Prevalence and Correlates of Borderline LVEF in the Study Sample
Supplemental Figure 2 displays the distribution of LVEF in our study sample. The prevalence of LVEF categories were as follows: <50%: 1.6%; 50-55%: 3.5%; and >55%: 94.9%. The characteristics of participants with LVEF 50-55% in comparison to groups LVEF <50% and LVEF >55% are presented in Table 1. Individuals in whom LVEF assessment was unavailable had a greater burden of CVD risk factors, but a similar prevalence of myocardial infarction as participants with available echocardiograms (Supplemental Table 2). The proportions of men prevalence of CVD, MI, and diabetes, and BNP level were intermediate in individuals with LVEF 50-55% compared to those with LVEF<50% and LVEF>55% (Table 1). Male sex, prevalent CVD and higher blood pressure were associated with greater odds of having borderline, compared with a normal LVEF, whereas higher mean age and use of antihypertensive medications were inversely associated with borderline LVEF (Table 2).
Table 1.
Demographic and Clinical Characteristics by LVEF category
| Characteristic | LVEF (%) | |||
|---|---|---|---|---|
| <50 (n=164) |
50-55 (n=363) |
>55 (n=9,743) |
p value | |
| Age, y | 61 (13) | 57 (13) | 60 (12) | <0.0001 |
| Male, n (%) | 128 (78) | 216 (60) | 4078 (42) | <0.0001 |
| BMI, kg/m2 | 26.9 (4.7) | 27.6 (4.5) | 27.0 (4.8) | 0.07 |
| Prevalent CVD, n (%) | 74 (45) | 62 (17) | 1086 (11) | <0.0001 |
| Prevalent MI, n (%) | 45 (27) | 23 (6) | 262 (3) | <0.0001 |
| SBP, mm Hg | 132 (19) | 133 (20) | 130 (20) | 0.01 |
| DBP, mm Hg | 77 (11) | 79 (10) | 76 (10) | <0.0001 |
| Hypertension, n (%) | 96 (59) | 174 (48) | 4594 (47) | 0.015 |
| Hypertension treatment, n (%) | 68 (41) | 87 (24) | 3066 (32) | 0.0002 |
| Diuretic use, n (%) | 23 (14) | 49 (14) | 1460 (15) | 0.69 |
| Aspirin use, n (%)* | 64 (40) | 110 (32) | 2537 (30) | 0.026 |
| Lipid-lowering therapy, n (%) | 36 (22) | 40 (11) | 1544 (16) | 0.0042 |
| Diabetes, n (%) | 28 (17) | 42 (12) | 739 (8) | <0.0001 |
| Smoking, n (%) | 34 (21) | 74 (20) | 1588 (16) | 0.048 |
| LVEDD, cm | 5.4 (0.8) | 5.0 (0.5) | 4.8 (0.5) | <0.0001 |
| BNP | 54.3 (39.0) | 33.1 (42.7) | 15.0 (20.0) | <0.0001 |
BMI = body mass index. BNP= Brain natriuretic peptide. CVD = cardiovascular disease (coronary artery disease, myocardial infarction, angina, CVA, or TIA). DBP = Diastolic blood pressure. LVEDD = left ventricular end-diastolic dimension. LVEF = left ventricular ejection fraction. MI = myocardial infarction. SBP = Systolic blood pressure.
Measures are reported as mean (SD) for continuous variables and n (% prevalence) for categorical variables.
Data on aspirin use was not available for Original Cohort examination 16.
Table 2.
Clinical correlates of borderline LVEF
| Characteristic | Odds ratio [OR] (95% CI) |
p value |
|---|---|---|
| Age, per 10 year increment | 0.73 (0.65, 0.82) | <0.0001 |
| Women | 0.57 (0.46, 0.71) | <0.0001 |
| Prevalent CVD | 2.13 (1.56, 2.91) | <0.0001 |
| Diabetes | 1.72 (1.21, 2.45) | 0.003 |
| SBP, per 20 mm Hg increment | 1.22 (1.04, 1.43) | 0.013 |
| DBP, per 10 mm Hg increment | 1.18 (1.03, 1.36) | 0.022 |
| Hypertension treatment | 0.63 (0.48, 0.83) | 0.001 |
CVD = cardiovascular disease. DBP = diastolic blood pressure. LVEF = left ventricular ejection fraction. SBP = Systolic blood pressure.
Odds ratios are presented in relation to LVEF>55% group (referent).
Incidence of Composite Outcome (HF/All-Cause Mortality) and HF by LVEF Category
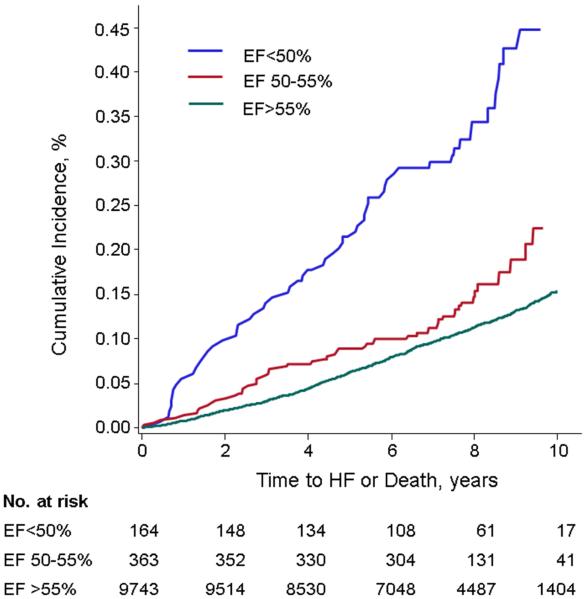
The composite primary outcome (HF or death) occurred in 1,255 (12%) participants (cumulative incidence per LVEF group shown in Table 3 and Figure 1). Individuals with LVEF 50-55% had an age- and sex-adjusted composite event rate of 0.24 per 10-years of follow-up. These event rates were intermediate between corresponding events in those individuals with LVEF <50% and LVEF >55%. The adjusted hazards ratios for the primary outcome for the groups LVEF<50% and LVEF 50-55%, as compared with the referent group LVEF >55%, are shown in Table 4. Participants with a borderline LVEF had a greater risk of the composite outcome in all models compared to those with LVEF >55%. In analyses stratified by examination and prevalent MI, we observed similar, slightly higher hazards for the composite outcome in all LVEF groups compared to the referent (Supplemental Table 3). Individuals with unavailable LVEF had a greater risk of HF/death than those with available LVEF (age- and sex-adjusted HR 1.45, 95% CI 1.33-1.57, p<0.0001, multivariable HR 1.30, 95% CI 1.19-1.42, p<0.0001).
Table 3.
Age- and Sex-adjusted Occurrence of Outcomes
| LVEF (%) | HF or Death (n=1,255) | HF (n=355) | ||
|---|---|---|---|---|
| Events, n | Incidence rate, per 10 years of follow-up |
Events, n | Incidence rate, per 10 years follow-up |
|
| <50 | 62 | 0.37 (0.27, 0.44) | 29 | 0.23 (0.12, 0.32) |
| 50 -55 | 58 | 0.24 (0.16, 0.31) | 24 | 0.13 (0.05, 0.19) |
| >55 | 1,135 | 0.16 (0.15, 0.18) | 302 | 0.05 (0.04, 0.06) |
HF = heart failure. LVEF = left ventricular ejection fraction.
Figure 1.
Cumulative incidence of the composite primary outcome (HF or death) among LVEF groups <50%, 50-55%, and >55%. The incidence among participants with LVEF 50-55% was intermediate between groups with LVEF <50% and LVEF >55%.
Table 4.
Hazards Ratios for Outcomes by Categorical and Continuous LVEF
| LVEF (%) | Model 1 HR (95% CI) | p value | Model 2 HR (95% CI) | p value |
|---|---|---|---|---|
| HF or Death (n=1,255 events) | ||||
|
| ||||
| <50 | 2.35 (1.79, 3.08) | <0.0001 | 2.01(1.53, 2.64) | <0.0001 |
| 50-55 | 1.46 (1.12, 1.91) | 0.0048 | 1.37 (1.05,1.80) | 0.023 |
| >55 | Referent | Referent | ||
| Continuous* | 1.14 (1.10, 1.18) | <0.0001 | 1.12 (1.07, 1.16) | <0.0001 |
|
| ||||
| HF (n=355 events) | ||||
|
| ||||
| <50 | 3.38 (2.23, 5.12) | <0.0001 | 2.84 (1.87, 4.31) | <0.0001 |
| 50-55 | 2.25 (1.48, 3.42) | 0.0002 | 2.15 (1.41, 3.28) | 0.0004 |
| >55 | Referent | Referent | ||
| Continuous* | 1.26 (1.18, 1.35) | <0.0001 | 1.23 (1.15, 1.32) | <0.0001 |
Analyses stratified by Cohort and by prevalent MI. HF = heart failure. HR = hazard ratio. LVEF = left ventricular ejection fraction.
HR per 5% decline in LVEF
Model 1: Adjusted for age and sex
Model 2: Adjusted for age, sex, body mass index, baseline cardiovascular disease, smoking, diabetes, systolic blood pressure, history of hypertension treatment
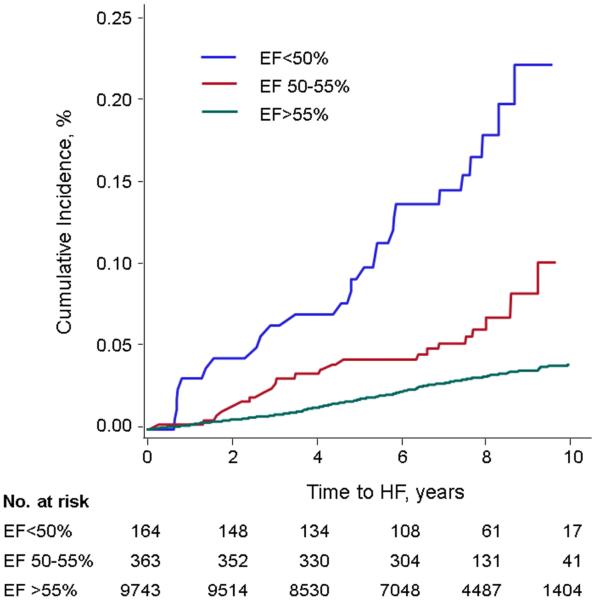
On follow-up, new-onset HF occurred in 355 (3.5%) individuals. Table 3 and Figure 2 show the cumulative incidence of HF according to baseline LVEF category. The HF event rate in individuals with a borderline LVEF was 0.13 per 10 years follow-up, which was intermediate between those with reduced versus normal LVEF. Participants with LVEF 50-55% had a >2-fold increased risk for HF compared to those with LVEF >55% in all models (Table 4). Similar results were seen in analyses stratified by examination and prevalent MI (Supplemental Table 3). The addition of BNP to multivariable models also yielded similar results (Supplemental Table 4).
Figure 2.
Cumulative incidence of HF among LVEF groups <50%, 50-55%, and >55%. The incidence among participants with LVEF 50-55% was intermediate between groups with LVEF <50% and LVEF >55%.
Because LVEDD is associated with CVD, we examined the relations of LVEDD in our models. LVEDD was associated with HF/mortality and HF in multivariable-adjusted models not including LVEF as a covariate (HF/mortality: HR=1.33, 95% CI 1.18-1.50, p<0.0001; HF: HR 2.25, 95% CI 1.81-2.79, p<0.0001), but inclusion in models including LVEF group only modestly attenuated the association of LVEF with the outcomes (Supplemental Table 5).
Among those who developed HF, the prevalence of HFPEF, HFREF, and interim MI are presented in Table 5. Of the participants with baseline LVEF 50-55% who developed HF and in whom LVEF was available, one-third developed HFPEF whereas nearly two-thirds developed HFREF, a prevalence intermediate between those with LVEF <50% and >55%. Interim MI (between echocardiography and follow-up) occurred in 78 (22%) participants who developed HF: n=7 (24%) among baseline LVEF<50%; n=2 (8%) among LVEF 50-55%; and n=69 (23%) among LVEF >55%.
Table 5.
Type of HF Developed and Prevalence of Interim MI among those with HF, by baseline LVEF Category*
| Baseline LVEF (%) |
HFPEF (n=125) | HFREF (n=179) | Uncategorized HF (n=51) |
Interim MI on follow-up (n=78) |
|---|---|---|---|---|
| <50 (n=29) | 2 (7%) | 26 (90%) | 1 (3%) | 7 (24%) |
| 50-55 (n=24) | 8 (33%) | 15 (63%) | 1 (4%) | 2 (8%) |
| >55 (n=302) | 115 (38%) | 138 (46%) | 49 (16%) | 69 (23%) |
HFPEF = heart failure with preserved ejection fraction. HFREF = heart failure with reduced ejection fraction. MI = myocardial infarction.
Some HF cases were not differentiated into HFREF vs. HFPEF because of lack of echocardiographic evaluation proximate to the HF episode.
Risk of Outcomes According to Continuous LVEF
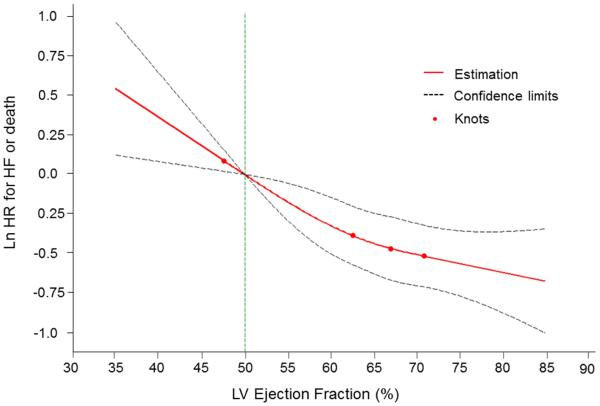
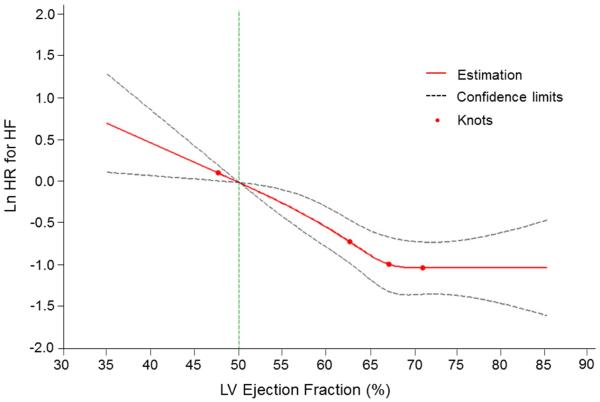
In multivariable-adjusted analyses, every 5% decline in LVEF (modeled as a continuous variable) was associated with a 12% and 23% increase in the risk for the composite outcome and HF, respectively (Table 4). Splines revealed a nearly linear relationship of the risk of both the composite outcome and HF with decreasing LVEF (Figures 3 and 4). Tests for non-linearity of these associations were not statistically significant (p=0.28 for HF or death, p=0.11 for HF).
Figure 3.
Multivariable-adjusted spline modeling the relations of LVEF to the risk of the composite primary outcome (HF or death)
A continuous, nearly linear relationship was seen between LVEF and the combined outcome. Test of non-linearity was non-significant (p=0.28). The spline was adjusted for age, sex, body mass index, baseline CVD, smoking, diabetes, systolic blood pressure, history of antihypertensive therapy. Knots were placed at 1, 25, 50, and 75th percentiles of left ventricular (LV) ejection fraction. HF = heart failure.
Figure 4.
Multivariable-adjusted spline modeling the relations of EF to the risk of new-onset HF. A continuous relationship was seen between LVEF and the outcome of HF. Test of non-linearity was non-significant (p=0.11). The spline was adjusted for age, sex, body mass index, baseline CVD, smoking, diabetes, systolic blood pressure, history of antihypertensive therapy. Knots were placed at 1, 25, 50, and 75th percentiles of left ventricular (LV) ejection fraction. HF = heart failure.
Secondary Analyses using Biplane Simpson’s LVEF
LVEF calculated by the de Simone and biplane Simpson’s methods correlated well (Pearson correlation 0.82, p<0.0001) with good agreement (94% of observations falling within ±5%, Supplemental Figure 3). The weighted kappa statistic for categorical LVEF between de Simone and biplane methods was 0.64, indicating very good agreement between the two methods.
HF or mortality occurred in 225 of 2315 (10%) of FHS Offspring participants. A borderline LVEF was associated with a >2-fold risk of HF/mortality (HR 2.15, 95% CI 1.04-4.45, p=0.04). Each 5% decrement in LVEF was associated with a 17% risk of the composite outcome (p=0.0004). There were 78 HF events. In logistic regression analyses, a trend toward risk of HF was seen, but was not statistically significant (HR 2.61, 95% CI 0.91-7.50, p=0.075). However, every 5% lower LVEF was associated with a 29% greater risk of HF (95% CI 1.13-1.47, p=0.0001).
Examination for Sex-Interaction and Sensitivity Analyses
We did not observe effect modification by sex for either the composite outcome of HF/mortality or HF (p=0.60 and 0.10, respectively). In sensitivity analyses using propensity-score matching, similar results were observed (Supplemental Table 6). In additional sensitivity analyses excluding individuals with prevalent MI and accounting for clustered observations within individuals, results were similar (data not shown).
DISCUSSION
Whereas mildly reduced LVEF is associated with adverse outcomes (6-8,25), the prognosis of those with borderline LVEF is unclear (9). In our large community-based sample, individuals with a borderline LVEF had risks of adverse outcomes intermediate between those with a LVEF <50% and individuals with a LVEF >55%. We observed a graded inverse relationship between continuous LVEF and risk of HF and death. This suggestion of better prognosis with greater LVEF calls into question guidelines defining normal LVEF using thresholds along a continuum, and is an area for future investigation. Our findings suggest that individuals with borderline LVEF have a worse prognosis than those with normal LVEF >55%, and should not be considered ‘normal’. Those with unavailable LVEF had the greatest risks for HF or death even after accounting for their greater prevalence of CVD risk factors, potentially highlighting the role of non-CVD causes of morbidity and mortality.
Characteristics of the Borderline LVEF Group
The prevalence of HFPEF and of HFREF at follow-up among those with baseline LVEF 50-55% was intermediate between individuals with LVEF <50% and LVEF >55%. The etiology of HF in those with borderline LVEF is unclear, but cannot be explained as a consequence of MI, which occurred in a very small proportion of the individuals who developed HF. In contrast, among those with LVEF <50%, development of symptomatic HF may be more attributable to ischemic events. Our findings are consistent with prior reports suggesting that preclinical LV systolic dysfunction may progress to clinical HF along a continuum of incrementally lower LVEF, with possible neurohormonal derangements that may lead to adverse changes in the cellular and extracellular environment (6,26-29).
Implications of Small Decrements in LVEF
Prior findings suggest 3.0-7.3% of the population have asymptomatic LV systolic dysfunction (6,8), among whom the majority have mild LV systolic dysfunction (LVEF 45-54%). We observed a 3.5% prevalence of LVEF 50-55%, which reflects a small but significant proportion of the general population. We noted a significantly increased risk for both HF and death with every 5% decline in LVEF. Whereas a 5% decrement in LVEF may seem to represent a small differential change, the implications at a population level may be large. For example, a 5% difference in LVEF represented a difference between LVEF quartiles in our sample. Thus, our findings may impact a significant proportion of the general population.
Comparison to Prior Studies and Impact of Our Findings
Even mildly decreased LVEF in asymptomatic individuals is associated with HF and mortality (6-8,25,30). To our knowledge, the long-term prognosis of those with borderline LVEF is uncertain. Our finding of a risk for incident HF and death intermediate between LVEF <50% and those >55% is consistent with prior studies, and extends the findings to a large population who currently may not receive attention due to the nearly normal LVEF. In addition, the rising risk of outcome events with progressively decreasing LVEF suggests a continuum of cardiovascular risk across the range of LVEF distribution. Clinically, a borderline LVEF may be referred to as “low-normal”. However, our results suggest that this term for LVEF 50-55% may be misleading, and may not convey the increased adverse risks associated with borderline LV systolic function.
Our findings are supported by consistency between our primary results and several secondary and sensitivity analyses including use of biplane Simpson’s LVEF measurement, exclusion of individuals with MI at baseline, accounting for clustered observations within individuals, additional adjustment for LV cavity size, and propensity score matching. Substantial changes in imaging techniques and CVD risk factor prevalence and treatment have evolved over the three decades encompassed in this study. Nevertheless, our results comparing individuals within the same exam cycle (stratification by exam status and MI) were similar, albeit stronger, than results of our primary analyses, thus strengthening our conclusions. In addition, we examined for and did not observe effect modification by sex in these associations.
The optimal therapy, if any, for individuals with a borderline LVEF 50-55% remains uncertain. Currently, the borderline group represents individuals who, under current guidelines, do not merit medical surveillance or therapy for HF prevention compared to individuals with a LVEF<40%. The absolute event rates in this group are low and would preclude a randomized controlled clinical trial targeting this group to define optimal management strategies, beyond aggressive control of prevalent CVD risk factors.
Strengths and Limitations
The FHS cohorts represent large, community-based samples in which echocardiographic measurements have been well-validated and participants are under regular surveillance for the detection of events. In primary analyses, we used the method of de Simone for the estimation of LVEF, which has been applied in numerous clinical studies (16-18); inter- and intra-correlation measures of the echocardiographic measures were excellent (31). While this method assesses change in LV diameter at the base, it can over- or under-estimate LVEF if regional wall motion abnormalities exist in the apical or basal regions, respectively. However, these misclassification errors would likely have biased our findings towards the null hypothesis of no association of borderline LVEF with the risk of developing outcome events. Furthermore, we observed similar results using biplane Simpson’s LVEF, and our data suggest very good agreement between de Simone and biplane Simpson’s methods of LVEF calculation.
LV diastolic dysfunction is a feature of HF, both with preserved and with reduced ejection fraction. We were unable to evaluate the separate and distinct contribution of diastolic dysfunction to HF as serial diastolic function parameters were unavailable at the baseline examination we chose. However, it is often challenging to differentiate the extent to which clinical HF exacerbations are due to diastolic, as compared with systolic, dysfunction in those with HFREF. We assessed HF outcomes that included the spectrum of LVEF (both preserved and reduced ejection fraction subtypes). Future investigation may elucidate the extent to which diastolic dysfunction contributes to clinical HF and mortality in individuals with a borderline LVEF.
Whereas our observations suggest greater risk for the borderline LVEF group in the population, the LVEF measures are less precise at an individual patient level. Future studies using modern imaging modalities with greater reproducibility may reduce measurement variability, increase the sample size with assessable LVEF, and validate our findings. However, our observed low absolute event rates necessitate a long follow-up period, and thus a similar study may not be feasible with newer echocardiographic methods at this time.
Lastly, additional epidemiologic considerations must be made. Although we included clinical risk factors for HF in our statistical models, we cannot exclude residual confounding from unmeasured variables. Given the observational nature of this study, causal inferences cannot be made. Finally, we highlight that the majority of our sample consisted of middle-aged to older adults of Northern European descent, thus limiting generalizability to individuals of other race and/or ethnicities. It would be of interest to evaluate the prognosis of a borderline LVEF in different population samples, including those identified in the clinical setting and in ethnicially and racially diverse groups.
CONCLUSIONS
In our large community-based sample, borderline LVEF of 50-55% was associated with significantly increased risks of death and HF compared with those with normal LVEF >55%. The risk of these outcomes increased linearly and incrementally with even small decrements in LVEF. Those with LVEF 50-55% have an increased cardiovascular risk profile cross-sectionally and elevated risk longitudinally, thereby suggesting that the term “low-normal” for this group may be potentially misleading. Further studies are necessary to confirm our findings, and to determine the optimal surveillance strategy and treatment, if any, for individuals in the community who have an LVEF 50-55%.
Supplementary Material
PERSPECTIVES.
Clinical Competencies
Competency in Medical Knowledge
Risk of heart failure and mortality increase linearly and continuously with decreasing left ventricular systolic function. Thus, individuals with borderline left ventricular systolic function should not be considered to have the same prognosis as those with normal systolic function.
Translational Outlook
Future studies using more contemporary imaging modalities should be considered to determine the optimal management strategy for individuals with borderline left ventricular systolic function.
Acknowledgments
Funding:
This work was supported by the National Heart, Lung and Blood Institute (contract NO1-HC-25195); grants from the American Heart Association 13SDG14250015 (CWT), NIH K23HL118529 (CWT), K99HL107642 (SC), R01HL093328 (RSV), 6R01-NS17950 (RSV) and R01HL080124 (RSV); Harvard Medical School Fellowship (CWT); and Ellison Foundation (SC).
List of Abbreviations
- CVD
Cardiovascular disease
- LVEF
Left ventricular ejection fraction
- HF
heart failure
- HFPEF
Heart failure with preserved ejection fraction
- HFREF
Heart failure with reduced ejection fraction
- HR
Hazard ratio
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors have no relationships relevant to the contents of this manuscript to disclose.
REFERENCES
- 1.Jhund PS, Macintyre K, Simpson CR, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–23. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 7.Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 8.Pandhi J, Gottdiener JS, Bartz TM, Kop WJ, Mehra MR. Comparison of characteristics and outcomes of asymptomatic versus symptomatic left ventricular dysfunction in subjects 65 years old or older (from the Cardiovascular Health Study) Am J Cardiol. 2011;107:1667–74. doi: 10.1016/j.amjcard.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadevan G, Davis RC, Frenneaux MP, et al. Left ventricular ejection fraction: are the revised cut-off points for defining systolic dysfunction sufficiently evidence based? Heart. 2008;94:426–8. doi: 10.1136/hrt.2007.123877. [DOI] [PubMed] [Google Scholar]
- 10.Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 12.Savage DD, Garrison RJ, Kannel WB, Anderson SJ, Feinleib M, Castelli WP. Considerations in the use of echocardiography in epidemiology. The Framingham Study. Hypertension. 1987;9:II40–41. doi: 10.1161/01.hyp.9.2_pt_2.ii40. [DOI] [PubMed] [Google Scholar]
- 13.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.de Simone G, Devereux RB, Ganau A, et al. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–7. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 15.Zile MR, Tanaka R, Lindroth JR, Spinale F, Carabello BA, Mirsky I. Left ventricular volume determined echocardiographically by assuming a constant left ventricular epicardial long-axis/short-axis dimension ratio throughout the cardiac cycle. J Am Coll Cardiol. 1992;20:986–93. doi: 10.1016/0735-1097(92)90202-x. [DOI] [PubMed] [Google Scholar]
- 16.Chinali M, de Simone G, Roman MJ, et al. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005;18:1570–6. doi: 10.1016/j.amjhyper.2005.05.036. discussion 1577. [DOI] [PubMed] [Google Scholar]
- 17.Kozakova M, Paterni M, Bartolomucci F, et al. Epicardial coronary artery size in hypertensive and physiologic left ventricular hypertrophy. Am J Hypertens. 2007;20:279–84. doi: 10.1016/j.amjhyper.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–81. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Mosterd A, Deckers JW, Hoes AW, et al. Classification of heart failure in population based research: an assessment of six heart failure scores. Eur J Epidemiol. 1997;13:491–502. doi: 10.1023/a:1007383914444. [DOI] [PubMed] [Google Scholar]
- 21.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–21. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 22.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–74. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 24.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 25.Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES) Eur Heart J. 2007;28:1128–34. doi: 10.1093/eurheartj/ehm102. [DOI] [PubMed] [Google Scholar]
- 26.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 30.Cheng RK, Cox M, Neely ML, et al. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. American heart journal. 2014;168:721–30. doi: 10.1016/j.ahj.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Sundstrom J, Sullivan L, Selhub J, et al. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J. 2004;25:523–30. doi: 10.1016/j.ehj.2004.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.