Summary
Ligation of the CD28 receptor on T cells provides a critical second signal alongside T cell receptor (TCR) ligation for naive T cell activation. Here we discuss the expression, structure, and biochemistry of CD28 and its ligands. CD28 signals play a key role in many T cell processes including cytoskeletal remodeling, production of cytokines, survival, and differentiation. CD28 ligation leads to unique epigenetic, transcriptional, and post-translational changes in T cells that cannot be recapitulated by TCR ligation alone. We discuss the function of CD28 and its ligands in both effector and regulatory T cells. CD28 is critical for regulatory T cell survival and the maintenance of immune homeostasis. We outline the roles that CD28 and its family members play in human disease and we review the clinical efficacy of drugs that block CD28 ligands. Despite the centrality of CD28 and its family members and ligands to immune function, many aspects of CD28 biology remain unclear. Translation of a basic understanding of CD28 function into immunomodulatory therapeutics has been uneven with both successes and failures. Such real-world results may stem from multiple factors including complex receptor-ligand interactions among CD28 family members, differences between the mouse and human CD28 families, and cell-type specific roles of CD28 family members.
The CD28 family of receptors and ligands
The discovery of the T cell receptor (TCR) in the early 1980s prompted efforts to dissect how antigen recognition results in T cell activation. It was soon discovered that TCR engagement was not sufficient for the complete activation of T cells but there was a requirement for a second signal. In fact, early work by Jenkins, Schwartz, and others showed that TCR ligation alone induces T cell anergy or unresponsiveness, and that the necessary “costimulatory signal” that prevents T cell unresponsiveness after TCR ligation was present on B cells and monocytes (Jenkins et al., 1988; Mueller et al., 1989).
These efforts led to the discovery in 1986 that a monoclonal antibody (mAb) against CD28, then called Tp44, could substitute for non-T cells in providing a second signal, when combined with immobilized TCR stimuli, to induce primary human T cell and Jurkat cell activation (Jenkins et al., 1991; Martin et al., 1986; Weiss et al., 1986). CD28 drives critical intracellular biochemical events including unique phosphorylation and transcriptional signaling, metabolism, and the production of key cytokines, chemokines, and survival signals that are essential for long-term expansion and differentiation of T cells (Bluestone et al., 2006; Bour-Jordan et al., 2011; Martin et al., 1986; Weiss et al., 1986). Most importantly, treatment of mice with a soluble CD28 antagonist induced antigen-specific tolerance that prevented the progression of autoimmune diseases and organ graft rejection (Lenschow et al., 1992). This insight led to the development of Abatacept and Belatacept, which are used clinically to treat rheumatoid arthritis and organ transplant rejection, respectively (Vignali, 2016, this issue; Ford, 2016, this issue) (Abrams et al., 1999; Bluestone et al., 2006). Conversely, the advent of CD28 agonists, which can rescue T cells from the tolerant state, may pave the way for a new class of immune activators for the treatment of infectious diseases (Wherry, 2016, this issue) and cancer (Wolcholk, 2016, this issue).
It has become increasing clear that CD28 functions not simply as an amplifier of TCR signals but delivers unique signals that control intracellular biochemical events from post-translational protein modification (e.g. phosphorylation) to epigenetic changes that alter the gene expression program of T cells. Moreover, over the past two decades, there has been an increasing number of cell surface molecules identified that share significant homology with CD28 and its ligands. Thus, there is an increasingly complex set of interactions wherein the single receptor, CD28, binds to multiple ligands and the ligands, B7-1 (CD80), and B7-2 (CD86), which in turn can bind multiple receptors (including CTLA4) (Sharpe, 2016, this issue). In this review, we summarize the current understanding of these complex costimulatory pathways including the individual roles of the CD28, B7-1 (CD80), and B7-2 (CD86) molecules. Here we summarize current biochemical and functional pathways controlled by CD28 co-stimulation, and we also discuss CD28 family members ICOS and CTLA-4 where appropriate. We review evidence that suggests that multiple mechanisms contribute to the biochemical and functional effects of CD28-mediated T cell costimulation. The implications of these complexities and the use of therapies that modulate these signals in patients are discussed.
Expression of CD28 family members
CD28 is the founding member of a subfamily of costimulatory molecules characterized by an extracellular variable immunoglobulin-like domain. Other members of the subfamily include ICOS, CTLA4, PD1, PD1H, and BTLA (Chen and Flies, 2013). CD28 is expressed constitutively on mouse T cells, whereas the expression of other family members ICOS and CTLA4 is induced by T cell receptor stimulation and in response to cytokines such as interleukin 2 (IL-2). CD28 is expressed on roughly 80% of human CD4+ T cells and 50% CD8+ T cells. The proportion of CD28 positive T cells in humans declines with age. Although CD28 expression has been identified on other cell lineages, including bone marrow stromal cells, plasma cells, neutrophils, and eosinophils, the functional importance of CD28 on these cells is not completely understood (Gray Parkin et al., 2002; Rozanski et al., 2011; Venuprasad et al., 2001; Woerly et al., 2004).
The CD28 ligands CD80 and CD86 diverge in their expression patterns, multimeric states, and functionality, adding another layer of complexity to the regulation of CD28 signaling. CD80 is present in predominantly dimeric form on the cell surface whereas CD86 is monomeric (Bhatia et al., 2005). CD86 is expressed constitutively on antigen presenting cells (APCs) and is rapidly upregulated by innate stimuli of APCs (Lenschow et al., 1994), whereas the other CD28 ligand, CD80, is upregulated at later time points (Sharpe and Freeman, 2002). CD86 may therefore be more important in the initiation of immune responses. CD80 and CD86 are induced by different stimuli in different cell types and they are not interchangeable in function. For example, CD86-deficient mice cannot undergo antibody class switching and germinal center formation in response to immunization without adjuvant. By contrast, CD80-deficient mice do not show this defect (Borriello et al., 1997).
CD28 and CTLA4 are highly homologous and compete for the same ligands (B7-1 (CD80) and B7-2 (CD86))(Linsley et al., 1990). CTLA4 binds these ligands with a higher affinity than CD28, which allows CTLA4 to compete with CD28 for ligand and suppress effector T cells responses (Engelhardt et al., 2006). Although CTLA4 binding to CD80 or CD86 is always stronger than CD28 binding, when in competition, CD86 has a relative preference for CD28 compared to CD80, which binds very strongly to CTLA4. Thus, the sequential expression CD86 followed by CD80 on APCs may function to increase the suppressive function of CTLA4 once an immune response has started, since the CTLA4-CD80 interaction later in an immune response is particularly strong (van der Merwe and Davis, 2003)
Now-classic experiments showed that CD28 and CTLA4 have opposing effects on T cell stimulation. CD28 provides an activating signal and CTLA4 provides an inhibitory signal, which is now considered a prototypical immune checkpoint (Krummel and Allison, 1995; Walunas et al., 1994). ICOS, which also contributes to activation, binds to its ligand B7H2 (ICOSL), which also serves as a ligand for human CD28 and CTLA4 (Chen and Flies, 2013; Yao et al., 2011). Thus, this family of receptors and ligands has considerable complexity in both binding pattern and biological effect. Overall, the opposing roles of CD28 and ICOS compared with CTLA4 allow this family of receptors and ligands to serve as a rheostat for the immune response through competing pro- and anti-inflammatory effects.
Complex biological effects mirror the complex binding characteristics of this family. ICOS and CD28 are closely related genes that arose from a duplication event. Nevertheless, these receptors cannot substitute from one another in function. This functional compartmentalization is enforced in part by the E3 ubiquitin ligase Roquin. This functional difference is critical since the ICOS ligand is widely and constitutively expressed whereas the principle ligands for CD28 are induced by exposure to inflammatory environments or microbial patterns (Linterman et al., 2009). Interestingly, basal CD80 and CD86 expression is necessary to prevent autoimmunity by sustaining regulatory T cell populations (Lohr et al., 2003). Activated human T cells can express CD80 and mouse T cells have been shown to acquire CD80 from APCs (Sabzevari et al., 2001). Thus, T cells themselves may be able to serve as ligand-expressing cells for CD28 and CTLA4 (Azuma et al., 1993; Sabzevari et al., 2001; Wyss-Coray et al., 1993). Antibody-mediated crosslinking of CD80 on the surface of T cells augments Ca2+ flux and production of interferon gamma (Podojil and Miller, 2009).
CD80 and CD86 may also act as signal transducing receptors themselves, since ligation with CTLA4Ig has been shown to regulate tryptophan metabolism in APCs (Grohmann et al., 2002). In addition to T cells, plasma cells also express CD28. CD28 signals may regulate antibody production by plasma cells or plasma cell survival although the precise role that CD28 plays in plasma cell biology is still unclear (Njau and Jacob, 2013).
CD28 structure and ligand binding
The CD28 gene is composed of four exons encoding a protein of 220 amino acids that is expressed on the cell surface as a glycosylated, disulfide-linked homodimer of 44 kDa. Members of the CD28 family share a number of common features. These receptors consist of paired V-set immunoglobulin superfamily (IgSF) domains attached to single transmembrane domains and cytoplasmic domains that contain critical signaling motifs (Carreno and Collins, 2002). The CD28 and CTLA4 ligands, CD80 and CD86, consist of single V-set and C1-set IgSF domains. The interaction of these costimulatory receptors with ligand is mediated through the MYPPPY motif within the receptor V-set domains (Evans et al., 2005; Metzler et al., 1997).
Earlier work on the crystal structure of CTLA4 itself and in complex with ligand provided initial clues on the structure and binding orientation of CD28, as both CTLA4 and CD28 share highly similar CDR3-analogous loops, as well as interface residues in the C and F strands (Schwartz et al., 2002; Zhang et al., 2003). Important insights into the distinct binding specificities and stoichiometric properties of CD28 resulted from analyses of the crystal structure of the monomeric form of the extracellular region of CD28 complexed with the Fab fragment of an anti-CD28 antibody (Evans et al., 2005). Docking of CD80 onto the putative CD28 homodimer revealed important differences in the relative orientations of CD28-CD80 versus the known CTLA4-CD80 complex. Within the CD28-CD80 complex, the two CD80 molecules converge so that their membrane proximal domains sterically clash, despite the accessibility of both ligand-binding sites on CD28. Conversely, in the CTLA4 dimer interface, a larger angle between the ligand binding sites eliminates steric interference and allows for bivalent binding (Evans et al., 2005; Stamper et al., 2001). These observations suggest a higher order multimeric interaction is more likely feasible between CTLA4 and CD80 than CD28. Indeed, the crystal structure of CTLA4 bound to CD80 shows that CTLA4 homodimers bind to CD80 homodimers in a “zipper-like” arrangement (Ostrov et al., 2000; Stamper et al., 2001).
Protein multimerization has been implicated as a critical regulatory feature in the counter-receptor interactions on T cells as well as in T cell activation (Bhatia et al., 2005). CD80 primarily exists as a dimer in a mixed dimer and monomer population at the cell surface, whereas CD86 exists solely as a monomer (Bhatia et al., 2005; Girard et al., 2014). Recent characterization of the quaternary structures of CD80 and CD86 has revealed a critical function for the IgC domains of these molecules in preventing higher order multimer formation and maintaining optimal binding to CD28 and IL-2 production in T cells (Girard et al., 2014). As CD28 favors the binding of monomeric ligands, whereas CTLA4 favors that of dimeric ligands, the ratio of CD80 and CD86 in monomeric versus multimeric forms may play a critical role in modulating T cell signaling by influencing the avidity of receptor-ligand interactions.
Interestingly, the model that CD28 binds its ligands monovalently has been challenged with the observation that TCR signaling induces a rapid reorientation of the cytosolic tail domains within the CD28 homodimer as detected by FRET (Sanchez-Lockhart et al., 2011). Follow up studies suggested that TCR signaling increased the avidity of CD28-CD80 interactions (Sanchez-Lockhart et al., 2014). Specifically, molecular dynamic simulations and site-directed mutagenesis experiments supported a model whereby the TCR signaling induced an increase in CD28 avidity as a consequence of reorientation of the CD28 dimer to engage in bivalent interactions with its ligand (Sanchez-Lockhart et al., 2014). As most studies to date lack insight into the ligand binding affinity of CD28 within the plasma membrane, the valency of CD28 binding warrants more investigation.
CD28 signaling motifs
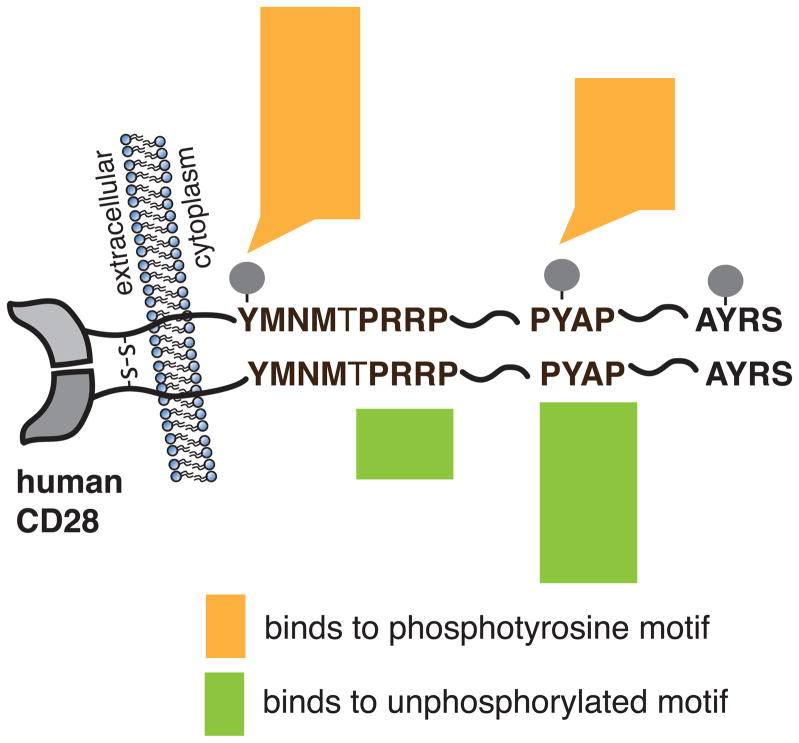
CD28 engagement by ligand initiates signal transduction events that are dependent on specific associations of proteins with the cytoplasmic tail of CD28. Despite having no intrinsic enzymatic activity, the 41 amino acid cytoplasmic tail of human CD28 contains highly conserved tyrosine-based signaling motifs that are phosphorylated in response to TCR or CD28 stimulation, and bind targets with SH2 domains in a phosphotyrosine-dependent manner (Figure 1). Proline rich sequences within the cytoplasmic tail also bind SH3-domain containing proteins. In particular, the membrane proximal YMNM motif, and the distal PYAP motif have been shown to complex with several kinases and adaptor proteins, with some proteins being able to bind to either or both motifs via SH2 and/or SH3 domain interactions (Boomer and Green, 2010). These motifs are important for IL-2 gene expression, which is mediated by the CD28-dependent activation of NFAT, AP-1, and NF-κB family transcription factors (Fraser et al., 1991; June et al., 1987; Thompson et al., 1989) (Figure 2). Additional sites for phosphorylation and ubiquitination are found within the cytoplasmic domain, but the functional output of the posttranslational modification of these sites is unclear.
Figure 1. CD28 cytoplasmic motifs and corresponding interacting molecules.
Engagement of CD28 initiates signal transduction cascades mediated by specific association of proteins with motifs of the CD28 cytoplasmic tail. Proteins that bind specifically to phosphotyrosine motifs or to unphosphorylated motifs are indicated according the legend. Data are adapted from (Tian et al., 2015).
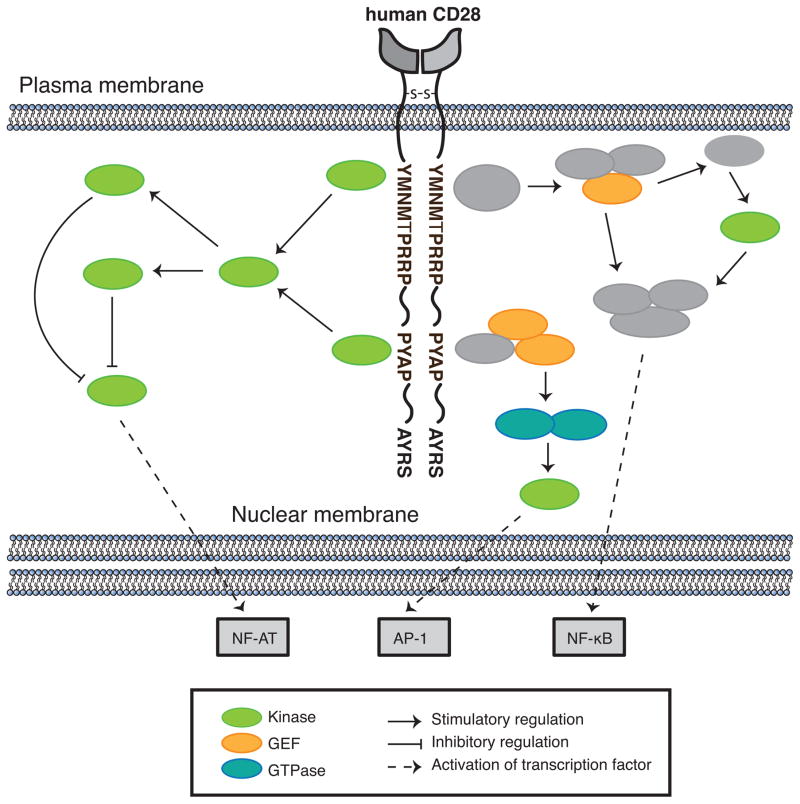
Figure 2. Signaling pathways downstream of CD28.
Lck phosphorylates PDK1, which in turn phosphorylates and activates PKCθ. PKCθ inactivates GSK3β, ultimately leading to enhanced transcription of NFAT-dependent genes. PKCθ also mediates signaling events leading to the activation of the NF-κB, and AP-1 transcription factors. The adaptor proteins GRB2 and GADS bind CD28. GRB2 binds Sos and Vav1 via its SH3 domain. In turn, Sos and Vav1 activate Ras, Rac1, and CDC42, resulting in signaling cascades culminating in JNK activation and formation of the AP-1 transcriptional complex. GRB2 and GADS also mediate the formation of CARMA1-Bcl-10-Malt1 complexes, which contribute to the activation of IKKs that regulate NF-κB activation.
The membrane-proximal YXXM motif is shared between CD28, CTLA4, and ICOS, and is a consensus site for the p85 subunit of the lipid kinase phosphatidylinositol 3-kinase (PI3K) (August and Dupont, 1994; Pages et al., 1994; Prasad et al., 1994; Rudd and Schneider, 2003). In addition to the +3 methionine of the CD28 sequence, YMNM, which confers PI3K specificity, the +2 asparagine confers specificity for the adaptor proteins GRB2 and GADS on CD28 (Cai et al., 1995; Kim et al., 1998; Okkenhaug and Rottapel, 1998; Okkenhaug et al., 2001; Raab et al., 1995; Stein et al., 1994). Both ICOS and CTLA4 can bind to PI3K but lack the ability to bind GRB2, which may account for some of the functional and signaling differences between these costimulatory receptors (Rudd and Schneider, 2003).
The importance of the YMNM motif in mediating proliferation and IL-2 secretion has been controversial, since some groups have reported no effect or a partial defect associated with mutation of the YMNM motif on proliferation and IL-2 secretion despite abrogation of PI3K binding and Akt phosphorylation (Andres et al., 2004; Burr et al., 2001; Dodson et al., 2009; Okkenhaug et al., 2001), whereas others have demonstrated inhibition of proliferation and IL-2 secretion (Harada et al., 2001). In contrast, studies in which the distal proline motif (PYAP) was mutated to AYAA resulted in marked impairment of function, including decreased CD28-dependent proliferation and IL-2 production in vivo, as well as attenuated phosphorylation of GSK3β, and PKCθ (Dodson et al., 2009; Friend et al., 2006). A study in which both the YMNM and PYAP motifs were mutated unmasked the contribution of the YMNM motif in CD28-dependent proliferation, IL-2 secretion, and adaptive immune system defects, highlighting the potential for at least partial compensatory effects of the YMNM motif (Boomer et al., 2014). Importantly, despite mutation of both these signaling motifs, residual CD28-dependent responses were observed in double-mutant cells, suggesting that additional motifs can contribute to CD28 signaling (Boomer et al., 2014; Pagan et al., 2012). A limitation of these mutational studies is that GRB2 is capable of binding to both of these motifs and GRB2 binding to the YMNM motif is preserved even when the tyrosine is mutated (Cai et al., 1995; Cefai et al., 1996; Kim et al., 1998). As such, double mutant cells most likely retain ability to GRB2, which may account for some of the observed CD28-mediated signals in double-mutant cells.
Signaling events downstream of the C-terminal PYAP motif are thought to include the phosphorylation and activation of the kinases PDK1 and PKCθ, and the subsequent inactivation of GSK3β, ultimately leading to enhanced transcription of NFAT-dependent genes, including IL-2. SH3-mediated binding and activation of the Src kinase Lck (Holdorf et al., 1999; King et al., 1997) is proposed as a potential regulator of this pathway. Whether Lck binds to this motif via its SH2 or SH3 domain is still unclear, since Tyr209, the tyrosine residue within this motif, is phosphorylated during CD28 stimulation, and may alternatively promote the binding of Lck via its SH2 domain (King et al., 1997; Sadra et al., 1999). Although the tyrosine kinase Itk has also been reported to phosphorylate Tyr209 in T cells, subsequent studies in Itk-deficient mice demonstrated no defect in the phosphorylation of this site (King et al., 1997; Li and Berg, 2005). Therefore, it is now more generally accepted that Lck mediates the phosphorylation of the tyrosine residues on CD28 (Holdorf et al., 1999; Raab et al., 1995; Sadra et al., 1999) and also binds to the PYAP motif. Consequently, CD28-bound Lck is thought to phosphorylate PDK1 on Tyr9, since the phosphorylation of this site is dependent on the PYAP motif and is augmented in response to CD28 signaling (Dodson et al., 2009; Park et al., 2001). PDK1 in turn phosphorylates and activates PKCθ, a critical downstream effector of CD28, which mediates signaling events leading to the activation of the NF-κB, AP-1, and NF-AT transcription factors (Coudronniere et al., 2000; Dodson et al., 2009; Lin et al., 2000). An additional potentially important interaction that is likely to play a role in PKCθ localization and activation is the CD28-induced interaction of the V3 domain of PKCθ with the SH3 of CD28-bound Lck (Kong et al., 2011). This interaction could also serve to co-localize PDK and PKCθ.
The adaptor proteins, GRB2 and GADS can bind to CD28 either through their SH3 domains at the distal PYAP motif or via their SH2 domains to the membrane proximal YMNM motif (Figure 2). GRB2 contains an SH2 domain flanked by two SH3 domains and binds the guanine nucleotide exchange factors (GEF) Sos and Vav1 via its SH3 domains (Okkenhaug and Rottapel, 1998). In turn, Sos and Vav1 activate Ras, Rac1, and CDC42, resulting in signaling cascades culminating in JNK and Erk activation and formation of the AP-1 transcriptional complex (Kim et al., 1998). GRB2 association with CD28 has also been reported to activate NFAT (Schneider and Rudd, 2008). GADS shares similar domain features to GRB2, and is predominately expressed in lymphoid tissue and hematopoietic cells (Asada et al., 1999; Law et al., 1999; Liu and McGlade, 1998). GADS appears to play a more dominant role relative to GRB2 in CD28-mediated IL-2 promoter activation through the formation of CARMA1-Bcl-10-Malt1 complexes, which contribute to the activation of IKKs that regulate NF-κB activation (Takeda et al., 2008; Watanabe et al., 2006). Furthermore, GADS binds SLP-76 in T cells and thus, may recruit this critical scaffolding protein, along with additional interacting molecules such as Vav1, to CD28 (Watanabe et al., 2006). The stoichiometry and motif specificity of GRB2, GADS, and PI3K binding to CD28 is still unclear and likely is dependent on many factors. However, evidence suggests that the YMNM motif is sufficient for GRB2 binding, whereas additional interactions between an N-terminal PRRP motif and a GADS SH3 domain are required for optimal GADS binding (Watanabe et al., 2006). However, it is the C-terminal PYAP motif that is thought to play the greater role in NF-κB activation, suggesting that other signaling molecules important for NF-κB activation bind to the C-terminal PYAP motif, such as Lck, as discussed above (Holdorf et al., 1999; Watanabe et al., 2006).
Regulatory T cells and the CD28 family
Although CD28 ligation is critical in promoting proliferation and effector function of conventional T cells, it also promotes the anti-inflammatory function of regulatory T (Treg) cells. Thus, CD28 serves both pro- and anti-inflammatory roles depending on the cell type and context in which it is expressed. CD28 signals are critical for allowing effector T cells to overcome Treg cell-mediated suppression to immunization (Lyddane et al., 2006), but CD28 in another context prevents spontaneous autoimmunity by promoting Treg function (Salomon et al., 2000). This latter role of CD28 and family members in supporting Treg function is an area of very active investigation.
Treg cells are a subset of CD4+ T cells with anti-inflammatory function. Treg cells provide dominant suppression of autoreactive T cells and are necessary to prevent autoimmune disease in mice and humans (Ohkura et al., 2013). Most of the data on the role of CD28 in Treg cell function comes from work in mouse models. In mice, CD28 ligation is required for both thymic development and peripheral homeostasis of Treg cells. Mice lacking CD28 or the ligands CD80 and CD86 have decreased numbers of Treg cells in the thymus and periphery, which predisposes them to autoimmune disease such as diabetes on the non-obese diabetic strain background (Salomon et al., 2000).
CD28 supports T cell homeostasis and function in a variety of ways. CD28 signals support the expression of miR17–92 family members, which are critical for maximal IL-10 production by Treg cells (de Kouchkovsky et al., 2013). Overexpression of Bcl-xL, which is induced by CD28 ligation, does not rescue the numbers of Treg cells in CD28-deficient animals, indicating that CD28 provides additional survival signals (Tang et al., 2003). Mutational analysis of the CD28 cytoplasmic tail showed that the Lck-binding motif but not the Itk kinase-binding motif or the PI3K-binding motif were necessary for efficient CD28-mediated generation of thymic Treg (Tai et al., 2005). Thymocytes require simultaneous TCR and CD28 signals to upregulate Foxp3 and differentiate into Treg cells. Increasing TCR stimulation of thymocytes does not overcome this dependence on CD28 signals. (Tai et al., 2005) Thus, CD28 ligation directly supports Treg cell fate in the thymus and supports Treg survival and proliferation in the periphery.
CD28 is also necessary for the production of peripheral induced Treg cells. CD4+CD25− T cells required CD28 ligation to differentiate into functional Foxp3+ Treg cells when activated with TGF-β. Mutational analysis revealed that iTreg cell generation also requires the Lck-binding motif but not the PI3K or Itk binding motifs of the CD28 intracellular domain (Guo et al., 2008). Importantly, very strong Lck signaling via the CD28 cytoplasmic domain prevents iTreg cell generation, in contrast its role with thymic-derived Treg cell (Semple et al., 2011). Carefully titrated CD28 signals via the anti-human CD28 superagonist TGN1412 (also known as TAB08) support the selective outgrowth of Treg cells in the absence of effector T cell activation, a result that was replicated in a small clinical trial (Tabares et al., 2014). Nevertheless, the balance between promoting Treg cell expansion and effector T cell activation is delicate. Non-specific CD28 agonism caused a cytokine storm and severe morbidity in an initial clinical trial (Suntharalingam et al., 2006).
Much of the early data on the role of CD28 in Treg cell biology used systems in which genetic ablation or biologic treatments (such as CTLA4Ig) affected both thymic production and peripheral homeostasis of Treg cell. More recent data shows the importance of CD28 for the homeostasis and function of peripheral Treg cells was highlighted in mice in which CD28 was ablated in an inducible system using a tamoxifen-induced CRE recombinase. Adult mice showed decreased levels of peripheral Treg cells after tamoxifen-induced deletion of CD28 in bone marrow-derived cells. This result reinforces a cell-intrinsic role for CD28 in Treg cell homeostasis: bone marrow chimeras containing both inducible CD28-deficient cells and CD28-sufficient cells did not prevent a decrease in the CD28-deleted population. Interestingly, CD28 deletion did not decrease CD25 levels on the remaining Treg cells, indicating that other factors such as IL-2 acting in trans support CD25 expression in Treg cell. The decreased number of Treg cells in the inducible CD28 null cells was not due to differences in thymic export of Treg cell. By contrast, the decreases in Treg cell numbers were due to decreased proliferation of Treg cells that lacked CD28. (Gogishvili et al., 2013) Another approach to defining the cell-intrinsic role of CD28 in Treg cells employed Foxp3-CRE-driven deletion of CD28. (Zhang et al., 2013) These mice showed a 25%–30% decrease in the percentage of Treg cells among the CD4+ single positive T cells in the thymus. However by contrast to other reports, Treg percentages in the lymph nodes and spleens of these mice were preserved. Nevertheless, these mice developed systemic autoimmunity with lymphocytic infiltrates in multiple tissues. The CD28-null Treg cells in these mice were found to be hypoproliferative to TCR stimulation in the presence of splenocytes expressing CD80 and CD86. The CD28-null Treg cells failed to suppress colitis in the CD4+CD25−CD45RBhi transfer model. CD28-null Treg cells also failed to effectively suppress experimental autoimmune encephalitis in mice, indicating a defect in function of CD28-null Treg cell. This defect may be due to decreased expansion of CD28-null Treg cells in these systems after adoptive transfer. These CD28-null Treg cells also have decreased expression of CTLA4, PD-1, and CCR6 (Zhang et al., 2013).
Treg cell may function in part by blocking costimulation of effector T cells. CTLA4 contributes to Treg cell function by decreasing CD80 and CD86 expression on dendritic cells (Qureshi et al., 2011; Wing et al., 2008). Treg cells can acquire CD80 and CD86 from APCs as well in a CTLA4-independent manner (Gu et al., 2012). Thus, Treg cells may reduce available ligand for CD28 from effector T cells. Nevertheless, an important study showed that ablation of CTLA4 in adult mice leads to expansion of functional Treg cells, which increases resistance to the development of autoimmunity in mice. This result is especially striking since congenital deletion of CTLA4 in mice leads to lethal lymphoproliferation and autoimmunity. Therefore, under homeostatic conditions in adult animals, CTLA4 is critical in limiting Treg proliferation, possibly through blocking CD28 signals to Treg cells (Paterson et al., 2015).
The functions of CD28 and IL-2 are not identical in promoting Treg cell proliferation and survival. IL-2 is required in to maintain peripheral Treg cell homeostasis, but it is not required for the production of thymic Treg cell (Fontenot et al., 2005). Exogenous IL-2 cannot rescue the proliferation defects of CD28 null Treg cells. This finding may be due to the low expression of CD25 (a component of the IL-2 receptor) on Treg cells deficient for CD28 (Tang et al., 2003). In human Treg cell, IL-2 prevents apoptosis but strong CD28 costimulation is required for Treg cell proliferation. (Hombach et al., 2007)
Treg cell defects in human autoimmune disease such as type 1 diabetes mellitus and multiple sclerosis have been linked to decreased IL-2 production, altered responsiveness to IL-2, and decreased Treg cell proliferation (Carbone et al., 2014; Long et al., 2010; McClymont et al., 2011). These functions are all directly linked to CD28 costimulation, implying that defects in the provision of or response to CD28 signals contributes to the altered Treg cell phenotypes. Treatment with CTLA4-Ig in clinical trials has variable effects on Treg cell numbers and function, as discussed below (“Costimulatory therapeutics). Due to the varied drugs, doses, and diseases in human trials with CTLA4-Ig, it is difficult to draw firm conclusions about the effects of CTLA4-Ig on Treg cells in patients.
A systematic investigation of the roles of CD28, CTLA4, and PD-L1 in human Treg and T effector cell interactions with APCs showed that blockade of CD28 increases Treg cell dwell time on APCs but had no effect on Treg cell motility (Dilek et al., 2013). However, other groups working with a mouse T cell system showed that CD28 blockade increased Treg cell motility (Thauland et al., 2014) or that CD28 deficiency had no effect on Treg cell motility (Lu et al., 2012). Thus, the effects of CD28 on Treg cell behavior with APCs are likely to be both model- and species-dependent.
In primary human cells, increased dwell times with CD28 blockade were dependent on CTLA4 on the Treg, which enhances the interaction between Tregs and APCs. Interestingly, blocking of CD80 and CD86 on APCs did not significantly change Treg cell dwell times compared to control, but it did increase Treg cell motility. Thus, CD28 ligation likely enhances effector T function and blocks Treg cell suppressive function (Dilek et al., 2013) although the mechanisms by which this happens have yet to be fully elucidated.
The CD28 costimulatory pathway regulates T cell activation by multiple processes
CD28 has been reported to augment TCR signaling as well as mediate unique signaling events. (Acuto and Michel, 2003; Boomer and Green, 2010; June et al., 1987; Shapiro et al., 1997). The unique contribution of CD28 signals to T cell activation has been challenging to dissect due to the complexity of numerous protein interactions with CD28 (see above), as well as the unclear stoichiometry and significance of these interactions. Genetic studies have revealed the unique functions of CD28 for which there is no compensation (Dodson et al., 2009). However, a clear picture of the mechanism by which CD28 plays a role in optimizing T cell responses during antigen recognition, either by augmenting TCR signaling or through unique signals, has been difficult to discern by simply studying CD28 interacting proteins or by mutating its cytoplasmic domain.
CD28 co-stimulation has diverse effects on T cell function, including biochemical events at the immunological synapse, downstream phosphorylation and other post-translational modifications, transcriptional changes, and cytoskeletal remodeling. At the most basic level, CD28 signals increase a cell’s glycolytic rate, allowing cells to generate the energy necessary for growth and proliferation (Frauwirth et al., 2002). Below, we discuss the unique mechanisms by which CD28 signals control these aspects of T cell function.
CD28 and the immunologic synapse
Productive TCR signaling and co-signaling is dependent on the organization and coordination of specific proteins at the contact site of the APC and T cell. Over time, a highly organized and spatially distinct structure is formed, which is referred to as the immunological synapse (IS). The IS is composed of central, peripheral, and distal supramolecular activation complexes (SMACs). CD28 clearly participates in these early interactions and is enriched adjacent to the central SMAC, along with the TCR, CD4, PKCθ, Rltpr and Lck (Liang et al., 2013; Saito et al., 2010; Sanchez-Lockhart et al., 2008). Many more proteins have been found to localize in the plane of the membrane and below it, presumably under the direction of the TCR, CD28, and other membrane receptors that contribute to IS formation. The potential function of CD28 at the interface of the cSMAC has been reviewed elsewhere (Fooksman et al., 2010; Yokosuka and Saito, 2010), and most of our current knowledge is primarily limited to its role in regulating the spatial localization PKCθ (Huang et al., 2002; Yokosuka et al., 2008).
The phosphoproteome
Since tyrosine phosphorylation of CD28 plays a critical role in the early signaling events that characterize CD28 costimulation, many investigations have focused on understanding the role of this posttranslational modification. These investigations have primarily relied on mutagenesis of immortalized cell lines and often used non-physiologic stimuli (such as anti-CD3 and anti-CD28 mAbs) followed by analysis of physiologic events such as IL-2 production. These studies have not clearly delineated the relevant proximal pathways downstream of CD28 engagement. The application of unbiased, wide-scale, and quantitative approaches is a powerful approach to identify and quantify thousands of signaling events and has the potential to yield a more thorough and unbiased analysis of the contribution of CD28 costimulation in T cells. For example, mass spectrometry-based phosphoproteomics has been used as a quantitative investigation of tyrosine signaling events, which revealed both quantitative and qualitative contributions of CD28 costimulation in TCR signaling networks (Kim and White, 2006). A study used a combinatorial proteomic analysis to delineate the pathways mediated by endogenously expressed CD28 receptors during a physiologically relevant intercellular interaction between Jurkat T cells and Raji B cells stimulated with Staphylococcal enterotoxin E. The resultant IL-2 response generated during this cell-cell interaction depended upon signaling by the TCR and, importantly, upon CD28 costimulation (Tian et al., 2015). CTLA4Ig was used to specifically block CD28 receptor activation, leaving signaling by the TCR and other receptors intact, which preserved the complexity of the cell-cell communication. Global phosphorylation analysis (serine and threonine, as well as tyrosine) revealed extensive changes (approximately 4% of the detectable T cell phosphopeptides) that were affected by blocking CD28 across a broad spectrum of different downstream signaling pathways, including those of TCR signaling, as well as other immune signaling pathways. Importantly, the events associated with TCR signaling did not dominate CD28-dependent downregulated signaling events, suggesting that CD28 influences signaling networks independent of those regulated by the TCR. Moreover, the vast majority of CD28-regulated phosphosites were not easily linked to known CD28-regulated signaling pathways (Tian et al., 2015). Further unbiased, wide-scale analyses of CD28 signaling coupled with targeted hypothesis-driven approaches will be critical to revealing mechanistic insights into the role of CD28 in T cell activation.
Mass spectrometry was also used to determine CD28-interacting proteins using long synthesized CD28 cytoplasmic phosphorylated and non-phosphorylated peptides to affinity purify CD28-binding proteins from Jurkat lysates (Tian et al., 2015). From this unbiased screen, 28 CD28-binding proteins were identified, and three families of proteins that bound selectively to either the pYMNM site, the PXXPP motif, or the PpYAPP motif of CD28 were identified. Of the associated signaling proteins identified in this screen, a large subset were involved in regulation of the actin cytoskeleton and the PI3K pathway. Interestingly, a number of interactors were not clearly dependent on one single phosphorylation sites or proline interaction, suggesting potential redundancy between these motifs or, alternatively, the presence of other important binding motifs within CD28. Linking the interactome analysis with the unbiased analysis yielded potential signaling hubs and pathways involved in multiple cellular processes, including a prominent role for the actin cytoskeleton. This analysis has stimulated new hypotheses and directions for studies of CD28 signaling.
CD28 and actin remodeling
CD28 plays a critical role in the remodeling of the actin cytoskeleton independent of the TCR, initiating unique signaling cascades that contribute to the initiation of TCR signaling and to CD28-autonomous signaling functions (Salazar-Fontana et al., 2003). One important function of CD28 has recently been highlighted in thymocytes, in which CD28-mediated actin cytoskeletal changes were required for full downstream TCR signaling (Tan et al., 2014). CD28-mediated signaling events were implicated in remodeling the actin cytoskeleton, which, in turn, was shown to be necessary for phosphorylated phospholipase Cγ1 (PLCγ1) to hydrolyze its substrate PIP2 (Tan et al., 2014). The role of CD28 in modulating actin cytoskeletal dynamics required for regulating PLCγ1 hydrolysis of PIP2 is not clear but could involve PLCγ1 activation, access to PIP2, or PIP2 generation.
A likely candidate linking CD28 to the actin cytoskeleton is Vav1, which can be recruited to stimulate CD28 via GRB2 binding (Kim et al., 1998; Ramos-Morales et al., 1995). The Vav1 GEF activates various Rho GTPases, well-known regulators of the actin cytoskeleton in T cells (Fischer et al., 1998a; Fischer et al., 1998b). A GEF-independent mechanism for Vav1-mediated regulation of the actin cytoskeleton has also been reported through its constitutive binding to talin and vinculin, two proteins that anchor the actin cytoskeleton to the cell membrane (Fischer et al., 1998b). Other recent work has shown that PIP5 kinase α (PIP5Kα) associates with Vav1 and both are recruited to the C-terminal proline-rich motif of CD28 to regulate pathways that promote actin polymerization (Muscolini et al., 2015). PIP5Kα is a lipid kinase that contributes to the generation PIP2, a second messenger that activates several actin-regulating proteins and is a substrate for PLCγ1 (Saarikangas et al., 2010). PIP5Kα activity has been shown to be essential for CD28-mediated actin polymerization (Muscolini et al., 2015). The PYAP motif of CD28 has also been shown to bind with filamin-A, which tethers CD28 to lipid rafts and recruits Rac and Rho GTPases to the vicinity of Vav1 in T cells (Tavano et al., 2006). Recently, the actin-uncapping proteins Rltpr (Liang et al., 2013) and CapZIP (Tian et al., 2015) have been shown to be essential for costimulation via CD28, further highlighting the important role of dynamic regulation of actin in CD28-mediated costimulation.
The transcriptome – IL-2, BclxL and beyond
CD28 ligation in concert with TCR signaling drives a complex transcriptional program in T cells. CD28 signals are critical for IL-2 production and Bcl-xL upregulation. Both of these proteins serve as survival factors for T cells (Boise et al., 2010; Watts, 2010). CD28 ligation also stabilizes mRNA of several cytokines (Lindstein et al., 1989). Recent data has shown that CD28 costimulation leads to broad transcriptional changes (Figure 3). Human T cells activated with and without CD28 costimulation showed that CD28 signals generally amplified the gene expression patterns initiated by T cell receptor ligation (Diehn et al., 2002; Riley et al., 2002). However, these studies were confounded by the use of bulk populations of T cells rather than the most costimulation-dependent naive subsets. CD28 ligation alone had little effect on T cell transcription, although CD28 ligation alone in the absence of TCR signals may increase the transcription of a small set of genes including IL-8 ad Bcl-xL, which is mediated by NF-κB family members, RelA and p52 (Marinari et al., 2004). Subsequent studies on CD28-dependent naive CD4+ T cell subsets showed that CD28 costimulation has a qualitative effect on hundreds of genes after TCR ligation (Butte et al., 2012), and that this effect is amplified over of the first 24 hours after T cell stimulation (Martinez-Llordella et al., 2013). Principle component analysis showed that T cells that receive TCR ligation alone without CD28 signals were more similar to cells that have never been activated by the 24 hour time point than they are to cells that received both signal 1 and signal 2. IL-2 signals cannot substitute for CD28 costimulation and rescue the transcriptional phenotype of cells stimulated via TCR signals alone (Martinez-Llordella et al., 2013). CD28 signals lead to widespread alternative splicing in T cells, an effect which depends in part on the function of splicing factor hnRNPLL. The hnRNPLL transcript itself is up-regulated by CD28 ligation compared to TCR stimulation alone (Butte et al., 2012). CD28 also controls gene expression by inducing epigenetic changes. The H3K27 methyltransferase Ezh2 is induced by CD28 signals and is necessary for the maintenance of Treg cell fate after activation. Ezh2 contributes to Foxp3-induced transcriptional repression (DuPage et al., 2015). CD28 signals lead to histone acetylation and loss of cytosine methylation at the IL-2 promoter, allowing for nucleosome repositioning and transcription factor binding to the IL-2 locus (Attema et al., 2002; Thomas et al., 2005). One caveat is that many of the studies referenced above were performed with human cells in vitro and used antibodies to deliver the CD28 signal. There are limited examples of in vivo confirmation of CD28-dependent transcription, although Ezh2 expression in mouse T cells was confirmed to be B7 and CD28-dependent in vitro and in vivo (DuPage et al., 2015).
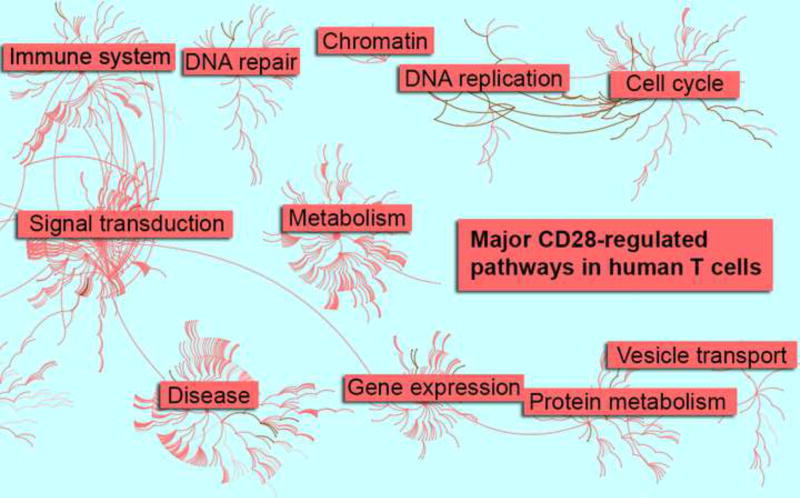
Figure 3. Major CD28 pathways in human T cells.
CD4+CD45RA+ human T cells were stimulated with anti-CD3 antibodies or anti-CD3 and anti-CD28 antibodies for 24 hours before harvest and transcriptome analysis. Differentially regulated genes were mapped to specific pathways (nodes), which are connected to each other based on common function (edges) using the Reactome pathway database (Croft et al., 2014; Milacic et al., 2012). Top-level nodes are categorized by a collection of pathways specific to its category (e.g. Immune System includes CD28 and TCR stimulation and cytokine signaling pathways, among others). The major pathway categories are indicated by pop-out text boxes in the network above. The density of connected nodes indicates the relative enrichment of a given class of pathways in T cells after CD28 stimulation. This figure was created from a re-analysis of data originally published in (Martinez-Llordella et al., 2013).
Several other mechanisms have been described by which CD28 may make a unique contribution to T cell activation signals and further downstream functional consequences. Protein arginine methylation increases within minutes after primary T cell activation. In Jurkat T cells, CD28 ligation alone induces protein arginine-methylation on a rapid time scale for a large number of proteins. This process requires the presence of the CD28 cytoplasmic tail. One of the targets of R-methylation in Jurkat T cells was found to be the guanine nucleotide exchange factor Vav1, which was methylated within minutes and increased for more than 30 minutes after CD28 ligation of Jurkat cells, in the absence of TCR stimulation. The methylation of Vav-1 may serve a kind of “signal memory” that indicates that a T cell has received CD28 costimulation (Blanchet et al., 2005; Blanchet et al., 2006).
The NF-κB family member c-Rel is known to be necessary for maximal expression of IL-2, a classical CD28-dependent gene. The mechanism of c-Rel activation of CD28-dependent genes has been attributed to a CD28-responsive binding site CD28 REAP, a c-Rel/AP-1 composite binding site, in the IL-2 promoter (Kontgen et al., 1995; Shapiro et al., 1996; Shapiro et al., 1997). A crystal structure of this interaction has been reported that reveal unique features of c-Rel binding to this site over other canonical NF-κB sites (Huang et al., 2001). An alternative explanation for a role for c-Rel has suggested c-Rel is involved in chromatin remodeling of the IL-2 promoter (Rao et al., 2003). c-Rel does appear to contribute to the expression of Foxp3 and the development of Treg cells in the thymus (Isomura et al., 2009; Long et al., 2009; Ruan et al., 2009) but another NF-κB family member RelA may be more important for induced Treg cell production in the periphery (Soligo et al., 2011). A further post-translational protein modification that may contribute to CD28-mediated T cell activation is O-GlcNAc glycosylation. The c-Rel subunit of the NF-κB transcription factor is activated by O-GlcNAcylation on serine 350. A mutation of this residue in c-Rel to alanine (S350A) showed greatly reduced transcription from a CD28-dependent promoter in Jurkat T cells. The S350A mutation was found to impair the binding of c-Rel to a CD28 response element (Ramakrishnan et al., 2013). These findings show that the gene expression program induced by CD28 ligation may depend in part upon glycosylation of c-Rel.
In summary, the CD28 pathway has both quantitative effects at amplifying signals initiated by the T cell receptor and qualitative effects on a variety of processes, including signaling, metabolism, transcription, epigenetic modifications, post-translational modifications, and RNA splicing.
CD28 family members in human disease
There is extensive literature in mouse models that CD28 and CTLA4 are critical regulators autoimmune disease (Salomon et al., 2000; Tivol et al., 1995) and tolerance to solid organ transplants (Lenschow et al., 1992). However, there is less direct data from patients on the role of CD28, CTLA4, ICOS, and their ligands in human disease. Genome-wide association studies have identified single nucleotide polymorphisms (SNPs) in CD28 and CTLA4 that increase risk for autoimmune disease, however there is limited data correlating specific SNPs with functional differences in T cell function (Chu et al., 2011; Maier et al., 2007; Qu et al., 2009; Raychaudhuri et al., 2009).
CD28 levels decrease on human T cells as part of normal aging with CD8+ T cells more prominently affected. These cells represent a population of antigen-experienced cells that may have either pro- or anti-inflammatory characteristics (Boucher et al., 1998; Strioga et al., 2011). In solid organ transplant recipients, CD8+CD28− cells have been found to undergo oligoclonal expansion and may play a suppressive role and promote allograft tolerance (Manavalan et al., 2004; Mou et al., 2014). Indeed, in kidney transplant recipients CD8+CD28− T cells may be protective against organ rejection (Trzonkowski et al., 2008), implying a possible immunosuppressive role for this subset. CD8+CD28− T cells are selectively expanded during viral infections, which may be due in part to the generation of this subset by exposure of T cells to type 1 interferon. These cells are also more susceptible to activation-induced cell death than CD28+ cells (Borthwick et al., 2000).
Increased numbers of circulating oligoclonal CD4+CD28− T cells have been reported in autoinflammatory and autoimmune conditions such as multiple sclerosis and rheumatoid arthritis. These cells can also be found infiltrating into tissue affected by autoimmune processes (Broux et al., 2012). CD4+CD28− cells are also elevated in diverse conditions including acute coronary syndrome, chronic kidney graft rejection, and cytomegalovirus infection. The cells produce interferon gamma and cytotoxic proteins perforin and granzyme B (Maly and Schirmer, 2015). Chronic antigenic stimulation may be associated with the loss of CD28 on CD4+ T cells since CMV responsiveness is 30-fold more common among CD4+CD28− cells than among CD4+CD28+ cells (van Bergen et al., 2009).
It is unclear if changes in the numbers of CD28 negative cells are a cause or a consequence of these infectious and inflammatory conditions. Indeed, CD28 negative cells are heterogeneous and many have pro- or anti-inflammatory effects in different contexts (Mou et al., 2014), making simple quantitation from peripheral blood samples difficult to interpret. Restoring CD28 expression in human T cells slows replicative senescence through increased telomerase activity, increased proliferative potential, and decreased secretion of inflammatory cytokines (Parish et al., 2010). Therefore, CD28 loss is a marker of T cell maturation, but the causes and consequences of its loss have not been fully elucidated.
CTLA4 and ICOS mutations have been linked to human disease. Two notable recent reports of patients with mutations in CTLA4 show its importance for immune homeostasis in humans. Patient with mutations presented with immunodeficiency and autoimmunity, with defects in both effector T cells and Treg cell function (Kuehn et al., 2014; Schubert et al., 2014). ICOS mutations leading to loss of protein expression have been described in multiple families with common variable immunodeficiency (CVID), a heterogeneous disease characterized by decreased immunoglobulin titers. Patients with ICOS deficiency were found to have increased susceptibility to infections, especially respiratory infections, and may also be predisposed to autoimmunity (Grimbacher et al., 2003; Yong et al., 2009).
Costimulatory therapeutics: manipulation of costimulatory pathways in disease
The centrality of CD28 costimulatory signals in T cell function makes it a tempting target for drugs to modulate the function of both effector T cells and Treg cells. Early attempts to make a soluble CD28 protein to block costimulatory signals were ineffective due to a low affinity of CD28 for its ligands (Bluestone et al., 2006). By contrast, a soluble CTLA4 binding domain linked to an Ig constant region was very effective at binding CD80 and CD86 and blocking access to these ligands. In animal models, CTLA4Ig has proven efficacious at inducing tolerance to allografts (Ford, 2016, this issue) and reversing autoimmune disease (Vignali, 2016, this issue) (Finck et al., 1994; Lenschow et al., 1992; Lin et al., 1993). This early success with animal models has translated unevenly into human clinical trials (Ford et al., 2014; Vincenti et al., 2010). Currently, two CTLA4Ig drugs approved by the US Food and Drug Administration are abatacept and its higher affinity version belatacept (Table 1).
Table 1.
Selected clinical trials of CD28 agonist and antagonists
| Drug | Disease | Result | References |
|---|---|---|---|
| Abatacept | Rheumatoid arthritis | efficacious | (Genovese et al., 2005) |
| Abatacept | Juvenile idiopathic arthritis | efficacious | (Ruperto et al., 2010; Ruperto et al., 2008) |
| Belatacept | Renal allograft rejection | efficacious | (Vincenti et al., 2005; Vincenti et al., 2012) |
| Abatacept | Type 1 diabetes mellitus | efficacious | (Orban et al., 2014; Orban et al., 2011) |
| Abatacept | spondyloarthropathies | mixed results | (Lekpa et al., 2012; Mease et al., 2011) |
| Abatacept | Lupus nephritis | mixed results | (Furie et al., 2014; Group, 2014; Merrill et al., 2010; Wofsy et al., 2013) |
| Belatacept | Liver transplant rejection | deleterious | (Klintmalm et al., 2014) |
| Abatacept | Asthma | not efficacious | (Parulekar et al., 2013) |
| Abatacept | Crohn’s disease | not efficacious | (Sandborn et al., 2012) |
| Abatacept | Ulcerative colitis | not efficacious | (Sandborn et al., 2012) |
| TGN1412 / TAB08 | healthy volunteers | Mixed results | (Suntharalingam et al., 2006; Tabares et al., 2014) |
The CTLA4Ig drugs have proven efficacy in ameliorating symptoms in rheumatoid arthritis (Genovese et al., 2005) and juvenile idiopathic arthritis (Ruperto et al., 2010; Ruperto et al., 2008) as well as in prevention of acute rejection of renal transplants (Vincenti et al., 2005; Vincenti et al., 2012). Compared to immunosuppressive regimens with calcineurin inhibitors, belatacept is associated with more episodes of acute rejection and potentially a higher risk of post-transplant lymphoproliferative disorder in renal transplant patients. However, belatacept treatment is associated with better long term renal function compared to calcineurin inhibitors. Therefore, the long term benefits of belatacept in renal transplant may outweight the drawbacks (Ford et al., 2014; Vincenti et al., 2010). Abatacept therapy slows c-peptide decline and lowers HgbA1C1 levels in patients with recent-inset type 1 diabetes (Lekpa et al., 2012; Orban et al., 2014; Orban et al., 2011). CTLA4Ig also showed clinical efficacy in a phase I trial in patients with psoriasis vulgaris (Abrams et al., 1999). Nevertheless, there have also been clinical trial failures and potential drawbacks of CTLA4Ig use: abatacept does not have proven efficacy in the treatment of lupus nephritis, despite positive results in animal studies referenced above and evidence of efficacy in post-hoc analyses of existing clinical trials (Furie et al., 2014; Group, 2014; Merrill et al., 2010; Wofsy et al., 2013). A clinical trial to further investigate abatacept efficacy in lupus nephritis is ongoing. Belatacept treatment was associated with increased graft loss and death in liver transplant patients (Klintmalm et al., 2014). Abatacept does not improve respiratory function in patients with mild asthma (Parulekar et al., 2013). Abatacept is likewise not efficacious in the treatment of Crohn’s disease or ulcerative colitis (Sandborn et al., 2012). There are mixed results in the use of abatacept to treat spondyloarthropathies (Lekpa et al., 2012; Mease et al., 2011). There are more than 50 uncompleted clinical trials currently listed (September 2015) at Clinicaltrials.gov for abatacept or belatacept, most of which are currently recruiting. The diseases being treated in these trials include granulomatosis with polyangiitis, chronic graft versus host disease, Sjögren’s syndrome, primary biliary cirrhosis, uveitis, systemic sclerosis, Beçhet’s syndrome, polymyositis, dermatomyositis, and simultaneous kidney and pancreas transplant.
Despite many clinical successes for CTLA4Ig, the precise mechanism of action of CTLA4Ig is unclear. In vitro assays using naive human CD4+ T cells show that CTLA4Ig blocks activation of transcription factors known to be preferentially activated by CD28 signals including cJUN and NF-κB. CD28 blockade by CTLA4Ig induces hyporesponsiveness but did not induce a regulatory T cell phenotype (Rochman et al., 2015). However, artificial in vitro systems are unlikely to reflect the complex mechanism of action that CTLA4Ig uses to have effects on both effector and regulatory T cells. For example, abatacept therapy in rheumatoid arthritis patients decreases the numbers of peripheral Treg but it increases their suppressive capacity (Alvarez-Quiroga et al., 2011). This result is consistent with in vitro data showing that belatacept decreases Treg generation in mixed lymphocyte reactions with T cells from healthy volunteers (Levitsky et al., 2013). Belatacept may work in part by increasing Treg numbers in target tissues. Patients with acute rejection of renal transplants had greater number of graft-infiltrating Treg cells when treated with abatacept compared with control (Bluestone et al., 2008). This result is unexpected given that CTLA4-Ig treatment of animals leads to decreased Treg cell numbers due to blockade of CD28 signals required for homeostasis. This difference may be due to the sub-saturating dosing of belatacept in the renal transplant patients, so that sufficient B7 ligand is available to maintain Treg cell numbers (Vincenti et al., 2010). Belatacept appears to have less suppressive effect against more mature T cells, especially CD28 negative cells that may have alternative costimulatory pathways (Leitner et al., 2015; Xu et al., 2014). Recent work has shown that CTLA4Ig may be more effective when TCR signals are attenuated in T cells by cyclosporine or vitamin D (Bolling et al., 1994; Gardner et al., 2015).
Overall, CTLA4Ig has shown a very good safety profile. However, another approach to promoting tolerance with an anti-CD28 superagonist antibody has been plagued by serious toxicities. The experimental drug TGN1412, which had been shown in pre-clinical animal studies to induce Treg cells, caused systemic release of pro-inflammatory cytokines in six volunteers (Hunig, 2012; Suntharalingam et al., 2006). However, this drug is now being tested clinically at much lower doses (Tabares et al., 2014). The precise cause of the severe toxicity of TGN1412 in humans may be multifactorial, including the larger population of CD28+ effector memory T cells in humans compared to laboratory animals (Hunig, 2012) and the decreased expression of PD-1 on the T cell surface after CD28 superagonist binding (Thaventhiran et al., 2014), the ability of CD28 superagonist to stimulate TCR pathways dependent on activation of ZAP-70 (Levin et al., 2008), and the ability of this antibody to cause sustained calcium flux (Waibler et al., 2008), among many other hypotheses (Schraven and Kalinke, 2008).
Another approach to block CD28 signals is the octapeptide AB103 (p2TA), which prevents CD28 homodimerization. This drug has shown efficacy in animal models in reducing radiation-induced inflammation and reducing mortality in endotoxin- and superantigen-mediated models of shock (Mirzoeva et al., 2014; Ramachandran et al., 2015; Ramachandran et al., 2013). Other drugs in pre-clinical development include FR104, a monoclonal anti-CD28 Fab′ antibody that is efficacious in a humanized mouse model of graft versus host disease in mice (Poirier et al., 2012) and agonist CD28 aptamers that have shown immunostimulatory properties in a mouse tumor vaccine model (Pastor et al., 2013).
Conclusion
In the last 30 years, basic insights into the roles of CD28 and its family members in T cell function has led to a variety of breakthroughs in the understanding of human disease and the development of immunomodulatory therapeutics. However, the relatively simple models constructed to understand the functions of CD28 family members have failed to adequately predict the effects of drugs used in clinical trials. There is enormous complexity in the interactions between multiple receptors and ligands, and the cell-type and context-specific functions of receptor ligation. Therefore, a more complete understanding of the biology of the CD28 family will likely require systems biology approaches and large data sets that can adequately capture these complexities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, Menter A, Lowe NJ, Krueger G, Brown MJ, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–1252. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- Alvarez-Quiroga C, Abud-Mendoza C, Doniz-Padilla L, Juarez-Reyes A, Monsivais-Urenda A, Baranda L, Gonzalez-Amaro R. CTLA-4-Ig therapy diminishes the frequency but enhances the function of Treg cells in patients with rheumatoid arthritis. J Clin Immunol. 2011;31:588–595. doi: 10.1007/s10875-011-9527-5. [DOI] [PubMed] [Google Scholar]
- Andres PG, Howland KC, Nirula A, Kane LP, Barron L, Dresnek D, Sadra A, Imboden J, Weiss A, Abbas AK. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat Immunol. 2004;5:435–442. doi: 10.1038/ni1044. [DOI] [PubMed] [Google Scholar]
- Asada H, Ishii N, Sasaki Y, Endo K, Kasai H, Tanaka N, Takeshita T, Tsuchiya S, Konno T, Sugamura K. Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J Exp Med. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attema JL, Reeves R, Murray V, Levichkin I, Temple MD, Tremethick DJ, Shannon MF. The human IL-2 gene promoter can assemble a positioned nucleosome that becomes remodeled upon T cell activation. J Immunol. 2002;169:2466–2476. doi: 10.4049/jimmunol.169.5.2466. [DOI] [PubMed] [Google Scholar]
- August A, Dupont B. CD28 of T lymphocytes associates with phosphatidylinositol 3-kinase. Int Immunol. 1994;6:769–774. doi: 10.1093/intimm/6.5.769. [DOI] [PubMed] [Google Scholar]
- Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated T lymphocytes. J Exp Med. 1993;177:845–850. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S, Edidin M, Almo SC, Nathenson SG. Different cell surface oligomeric states of B7–1 and B7–2: implications for signaling. Proc Natl Acad Sci U S A. 2005;102:15569–15574. doi: 10.1073/pnas.0507257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet F, Cardona A, Letimier FA, Hershfield MS, Acuto O. CD28 costimulatory signal induces protein arginine methylation in T cells. J Exp Med. 2005;202:371–377. doi: 10.1084/jem.20050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet F, Schurter BT, Acuto O. Protein arginine methylation in lymphocyte signaling. Curr Opin Immunol. 2006;18:321–328. doi: 10.1016/j.coi.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Liu W, Yabu JM, Laszik ZG, Putnam A, Belingheri M, Gross DM, Townsend RM, Vincenti F. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 2010;3:87–98. doi: 10.1016/1074-7613(95)90161-2. 1995. [DOI] [PubMed] [Google Scholar]; J Immunol. 185:3788–3799. [Google Scholar]
- Bolling SF, Lin H, Wei RQ, Linsley P, Turka LA. The effect of combination cyclosporine and CTLA4-Ig therapy on cardiac allograft survival. J Surg Res. 1994;57:60–64. doi: 10.1006/jsre.1994.1110. [DOI] [PubMed] [Google Scholar]
- Boomer JS, Deppong CM, Shah DD, Bricker TL, Green JM. Cutting edge: A double-mutant knockin of the CD28 YMNM and PYAP motifs reveals a critical role for the YMNM motif in regulation of T cell proliferation and Bcl-xL expression. J Immunol. 2014;192:3465–3469. doi: 10.4049/jimmunol.1301240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7–1 and B7–2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor gamma chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–1013. doi: 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- Boucher N, Dufeu-Duchesne T, Vicaut E, Farge D, Effros RB, Schachter F. CD28 expression in T cell aging and human longevity. Exp Gerontol. 1998;33:267–282. doi: 10.1016/s0531-5565(97)00132-0. [DOI] [PubMed] [Google Scholar]
- Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broux B, Markovic-Plese S, Stinissen P, Hellings N. Pathogenic features of CD4+CD28− T cells in immune disorders. Trends Mol Med. 2012;18:446–453. doi: 10.1016/j.molmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- Butte MJ, Lee SJ, Jesneck J, Keir ME, Haining WN, Sharpe AH. CD28 costimulation regulates genome-wide effects on alternative splicing. PLoS One. 2012;7:e40032. doi: 10.1371/journal.pone.0040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YC, Cefai D, Schneider H, Raab M, Nabavi N, Rudd CE. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Carbone F, De Rosa V, Carrieri PB, Montella S, Bruzzese D, Porcellini A, Procaccini C, La Cava A, Matarese G. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- Cefai D, Cai YC, Hu H, Rudd C. CD28 co-stimulatory regimes differ in their dependence on phosphatidylinositol 3-kinase: common co-signals induced by CD80 and CD86. Int Immunol. 1996;8:1609–1616. doi: 10.1093/intimm/8.10.1609. [DOI] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu X, Pan CM, Zhao SX, Liang J, Gao GQ, Zhang XM, Yuan GY, Li CG, Xue LQ, Shen M, et al. A genome-wide association study identifies two new risk loci for Graves’ disease. Nat Genet. 2011;43:897–901. doi: 10.1038/ng.898. [DOI] [PubMed] [Google Scholar]
- Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci U S A. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D, Mundo AF, Haw R, Milacic M, Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR, et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472–477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kouchkovsky D, Esensten JH, Rosenthal WL, Morar MM, Bluestone JA, Jeker LT. microRNA-17–92 regulates IL-10 production by regulatory T cells and control of experimental autoimmune encephalomyelitis. J Immunol. 2013;191:1594–1605. doi: 10.4049/jimmunol.1203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehn M, Alizadeh AA, Rando OJ, Liu CL, Stankunas K, Botstein D, Crabtree GR, Brown PO. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci U S A. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilek N, Poirier N, Hulin P, Coulon F, Mary C, Ville S, Vie H, Clemenceau B, Blancho G, Vanhove B. Targeting CD28, CTLA-4 and PD-L1 costimulation differentially controls immune synapses and function of human regulatory and conventional T-cells. PLoS One. 2013;8:e83139. doi: 10.1371/journal.pone.0083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson LF, Boomer JS, Deppong CM, Shah DD, Sim J, Bricker TL, Russell JH, Green JM. Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol Cell Biol. 2009;29:3710–3721. doi: 10.1128/MCB.01869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, Zhang R, Turka L, Marson A, Bluestone JA. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- Evans EJ, Esnouf RM, Manso-Sancho R, Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse B, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I, Sasaki T, Starr M, Chan G, Gardener S, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol. 1998a;8:554–562. doi: 10.1016/s0960-9822(98)70224-6. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Tedford K, Penninger JM. Vav links antigen-receptor signaling to the actin cytoskeleton. Semin Immunol. 1998b;10:317–327. doi: 10.1006/smim.1998.0124. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristan C, Victora GD, Zanin-Zhorov A, Dustin ML. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: transplantation and autoimmunity. Nat Rev Nephrol. 2014;10:14–24. doi: 10.1038/nrneph.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Friend LD, Shah DD, Deppong C, Lin J, Bricker TL, Juehne TI, Rose CM, Green JM. A dose-dependent requirement for the proline motif of CD28 in cellular and humoral immunity revealed by a targeted knockin mutant. J Exp Med. 2006;203:2121–2133. doi: 10.1084/jem.20052230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie R, Nicholls K, Cheng TT, Houssiau F, Burgos-Vargas R, Chen SL, Hillson JL, Meadows-Shropshire S, Kinaszczuk M, Merrill JT. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 2014;66:379–389. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- Gardner DH, Jeffery LE, Soskic B, Briggs Z, Hou TZ, Raza K, Sansom DM. 1,25(OH)2D3 Promotes the Efficacy of CD28 Costimulation Blockade by Abatacept. J Immunol. 2015;195:2657–2665. doi: 10.4049/jimmunol.1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [DOI] [PubMed] [Google Scholar]
- Girard T, Gaucher D, El-Far M, Breton G, Sekaly RP. CD80 and CD86 IgC domains are important for quaternary structure, receptor binding and co-signaling function. Immunol Lett. 2014;161:65–75. doi: 10.1016/j.imlet.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Gogishvili T, Luhder F, Goebbels S, Beer-Hammer S, Pfeffer K, Hunig T. Cell-intrinsic and -extrinsic control of Treg-cell homeostasis and function revealed by induced CD28 deletion. Eur J Immunol. 2013;43:188–193. doi: 10.1002/eji.201242824. [DOI] [PubMed] [Google Scholar]
- Gray Parkin K, Stephan RP, Apilado RG, Lill-Elghanian DA, Lee KP, Saha B, Witte PL. Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J Immunol. 2002;169:2292–2302. doi: 10.4049/jimmunol.169.5.2292. [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, Eibel H, Fischer B, Schaffer AA, Mages HW, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- Group AT. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 2014;66:3096–3104. doi: 10.1002/art.38790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Gao JF, D’Souza CA, Kowalczyk A, Chou KY, Zhang L. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol Immunol. 2012;9:136–146. doi: 10.1038/cmi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 Controls Differentiation of Regulatory T Cells from Naive CD4 T Cells. The Journal of Immunology. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tokushima M, Matsumoto Y, Ogawa S, Otsuka M, Hayashi K, Weiss BD, June CH, Abe R. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- Holdorf AD, Green JM, Levin SD, Denny MF, Straus DB, Link V, Changelian PS, Allen PM, Shaw AS. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach AA, Kofler D, Hombach A, Rappl G, Abken H. Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J Immunol. 2007;179:7924–7931. doi: 10.4049/jimmunol.179.11.7924. [DOI] [PubMed] [Google Scholar]
- Huang DB, Chen YQ, Ruetsche M, Phelps CB, Ghosh G. X-ray crystal structure of proto-oncogene product c-Rel bound to the CD28 response element of IL-2. Structure. 2001;9:669–678. doi: 10.1016/s0969-2126(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci U S A. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol. 2012;12:317–318. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- Kim JE, White FM. Quantitative analysis of phosphotyrosine signaling networks triggered by CD3 and CD28 costimulation in Jurkat cells. J Immunol. 2006;176:2833–2843. doi: 10.4049/jimmunol.176.5.2833. [DOI] [PubMed] [Google Scholar]
- King PD, Sadra A, Teng JM, Xiao-Rong L, Han A, Selvakumar A, August A, Dupont B. Analysis of CD28 cytoplasmic tail tyrosine residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. J Immunol. 1997;158:580–590. [PubMed] [Google Scholar]
- Klintmalm GB, Feng S, Lake JR, Vargas HE, Wekerle T, Agnes S, Brown KA, Nashan B, Rostaing L, Meadows-Shropshire S, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant. 2014;14:1817–1827. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, Schickel JN, Tran DQ, Stoddard J, Zhang Y, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CL, Ewings MK, Chaudhary PM, Solow SA, Yun TJ, Marshall AJ, Hood L, Clark EA. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J Exp Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J, Herndler-Brandstetter D, Zlabinger GJ, Grubeck-Loebenstein B, Steinberger P. CD58/CD2 Is the Primary Costimulatory Pathway in Human CD28-CD8+ T Cells. J Immunol. 2015;195:477–487. doi: 10.4049/jimmunol.1401917. [DOI] [PubMed] [Google Scholar]
- Lekpa FK, Farrenq V, Canoui-Poitrine F, Paul M, Chevalier X, Bruckert R, Bastuji-Garin S, Claudepierre P. Lack of efficacy of abatacept in axial spondylarthropathies refractory to tumor-necrosis-factor inhibition. Joint Bone Spine. 2012;79:47–50. doi: 10.1016/j.jbspin.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodnow CC, Bluestone JA. Differential up-regulation of the B7–1 and B7–2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- Levin SE, Zhang C, Kadlecek TA, Shokat KM, Weiss A. Inhibition of ZAP-70 kinase activity via an analog-sensitive allele blocks T cell receptor and CD28 superagonist signaling. J Biol Chem. 2008;283:15419–15430. doi: 10.1074/jbc.M709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky J, Miller J, Huang X, Chandrasekaran D, Chen L, Mathew JM. Inhibitory effects of belatacept on allospecific regulatory T-cell generation in humans. Transplantation. 2013;96:689–696. doi: 10.1097/TP.0b013e31829f1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CR, Berg LJ. Itk is not essential for CD28 signaling in naive T cells. J Immunol. 2005;174:4475–4479. doi: 10.4049/jimmunol.174.8.4475. [DOI] [PubMed] [Google Scholar]
- Liang Y, Cucchetti M, Roncagalli R, Yokosuka T, Malzac A, Bertosio E, Imbert J, Nijman IJ, Suchanek M, Saito T, et al. The lymphoid lineage-specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat Immunol. 2013;14:858–866. doi: 10.1038/ni.2634. [DOI] [PubMed] [Google Scholar]
- Lin H, Bolling SF, Linsley PS, Wei RQ, Gordon D, Thompson CB, Turka LA. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, O’Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong R, Silva D, Withers D, Anderson G, Verma NK, Brink R, Hutloff A, Goodnow CC, Vinuesa CG. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity. 2009;30:228–241. doi: 10.1016/j.immuni.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Liu SK, McGlade CJ. Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene. 1998;17:3073–3082. doi: 10.1038/sj.onc.1202337. [DOI] [PubMed] [Google Scholar]
- Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–669. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang ZY, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–415. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Schneider H, Rudd CE. Murine regulatory T cells differ from conventional T cells in resisting the CTLA-4 reversal of TCR stop-signal. Blood. 2012;120:4560–4570. doi: 10.1182/blood-2012-04-421420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyddane C, Gajewska BU, Santos E, King PD, Furtado GC, Sadelain M. Cutting Edge: CD28 controls dominant regulatory T cell activity during active immunization. J Immunol. 2006;176:3306–3310. doi: 10.4049/jimmunol.176.6.3306. [DOI] [PubMed] [Google Scholar]
- Maier LM, Anderson DE, De Jager PL, Wicker LS, Hafler DA. Allelic variant in CTLA4 alters T cell phosphorylation patterns. Proc Natl Acad Sci U S A. 2007;104:18607–18612. doi: 10.1073/pnas.0706409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly K, Schirmer M. The story of CD4+ CD28− T cells revisited: solved or still ongoing? J Immunol Res. 2015;2015:348746. doi: 10.1155/2015/348746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, Marboe C, Mancini D, Cortesini R, Suciu-Foca N. Alloantigen specific CD8+CD28− FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- Marinari B, Costanzo A, Marzano V, Piccolella E, Tuosto L. CD28 delivers a unique signal leading to the selective recruitment of RelA and p52 NF-kappaB subunits on IL-8 and Bcl-xL gene promoters. Proc Natl Acad Sci U S A. 2004;101:6098–6103. doi: 10.1073/pnas.0308688101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PJ, Ledbetter JA, Morishita Y, June CH, Beatty PG, Hansen JA. A 44 kilodalton cell surface homodimer regulates interleukin 2 production by activated human T lymphocytes. J Immunol. 1986;136:3282–3287. [PubMed] [Google Scholar]
- Martinez-Llordella M, Esensten JH, Bailey-Bucktrout SL, Lipsky RH, Marini A, Chen J, Mughal M, Mattson MP, Taub DD, Bluestone JA. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J Exp Med. 2013;210:1603–1619. doi: 10.1084/jem.20122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease P, Genovese MC, Gladstein G, Kivitz AJ, Ritchlin C, Tak PP, Wollenhaupt J, Bahary O, Becker JC, Kelly S, et al. Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63:939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace DJ, Bae SC, Sigal L, Becker JC, Kelly S, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- Metzler WJ, Bajorath J, Fenderson W, Shaw SY, Constantine KL, Naemura J, Leytze G, Peach RJ, Lavoie TB, Mueller L, Linsley PS. Solution structure of human CTLA-4 and delineation of a CD80/CD86 binding site conserved in CD28. Nat Struct Biol. 1997;4:527–531. doi: 10.1038/nsb0797-527. [DOI] [PubMed] [Google Scholar]
- Milacic M, Haw R, Rothfels K, Wu G, Croft D, Hermjakob H, D’Eustachio P, Stein L. Annotating cancer variants and anti-cancer therapeutics in reactome. Cancers (Basel) 2012;4:1180–1211. doi: 10.3390/cancers4041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzoeva S, Paunesku T, Wanzer MB, Shirvan A, Kaempfer R, Woloschak GE, Small W., Jr Single administration of p2TA (AB103), a CD28 antagonist peptide, prevents inflammatory and thrombotic reactions and protects against gastrointestinal injury in total-body irradiated mice. PLoS One. 2014;9:e101161. doi: 10.1371/journal.pone.0101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 negative T cells: is their loss our gain? Am J Transplant. 2014;14:2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DL, Jenkins MK, Schwartz RH. An accessory cell-derived costimulatory signal acts independently of protein kinase C activation to allow T cell proliferation and prevent the induction of unresponsiveness. J Immunol. 1989;142:2617–2628. [PubMed] [Google Scholar]
- Muscolini M, Camperio C, Porciello N, Caristi S, Capuano C, Viola A, Galandrini R, Tuosto L. Phosphatidylinositol 4-phosphate 5-kinase alpha and Vav1 mutual cooperation in CD28-mediated actin remodeling and signaling functions. J Immunol. 2015;194:1323–1333. doi: 10.4049/jimmunol.1401643. [DOI] [PubMed] [Google Scholar]
- Njau MN, Jacob J. The CD28/B7 pathway: a novel regulator of plasma cell function. Adv Exp Med Biol. 2013;785:67–75. doi: 10.1007/978-1-4614-6217-0_8. [DOI] [PubMed] [Google Scholar]
- Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J Biol Chem. 1998;273:21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069–1075. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrov DA, Shi W, Schwartz JC, Almo SC, Nathenson SG. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290:816–819. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- Pagan AJ, Pepper M, Chu HH, Green JM, Jenkins MK. CD28 promotes CD4+ T cell clonal expansion during infection independently of its YMNM and PYAP motifs. J Immunol. 2012;189:2909–2917. doi: 10.4049/jimmunol.1103231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Ragueneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 2010;30:798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Hill MM, Hess D, Brazil DP, Hofsteenge J, Hemmings BA. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J Biol Chem. 2001;276:37459–37471. doi: 10.1074/jbc.M105916200. [DOI] [PubMed] [Google Scholar]
- Parulekar AD, Boomer JS, Patterson BM, Yin-Declue H, Deppong CM, Wilson BS, Jarjour NN, Castro M, Green JM. A randomized controlled trial to evaluate inhibition of T-cell costimulation in allergen-induced airway inflammation. Am J Respir Crit Care Med. 2013;187:494–501. doi: 10.1164/rccm.201207-1205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor F, Soldevilla MM, Villanueva H, Kolonias D, Inoges S, de Cerio AL, Kandzia R, Klimyuk V, Gleba Y, Gilboa E, Bendandi M. CD28 aptamers as powerful immune response modulators. Mol Ther Nucleic Acids. 2013;2:e98. doi: 10.1038/mtna.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson AM, Lovitch SB, Sage PT, Juneja VR, Lee Y, Trombley JD, Arancibia-Carcamo CV, Sobel RA, Rudensky AY, Kuchroo VK, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212:1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podojil JR, Miller SD. Cross-linking of CD80 on CD4+ T cells activates a calcium-dependent signaling pathway. J Immunol. 2009;182:766–773. doi: 10.4049/jimmunol.182.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier N, Mary C, Dilek N, Hervouet J, Minault D, Blancho G, Vanhove B. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab′ antibody. Am J Transplant. 2012;12:2630–2640. doi: 10.1111/j.1600-6143.2012.04164.x. [DOI] [PubMed] [Google Scholar]
- Prasad KV, Cai YC, Raab M, Duckworth B, Cantley L, Shoelson SE, Rudd CE. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci U S A. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HQ, Bradfield JP, Grant SF, Hakonarson H, Polychronakos C, Type IDGC. Remapping the type I diabetes association of the CTLA4 locus. Genes Immun. 2009;10(Suppl 1):S27–32. doi: 10.1038/gene.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab M, Cai YC, Bunnell SC, Heyeck SD, Berg LJ, Rudd CE. p56Lck and p59Fyn regulate CD28 binding to phosphatidylinositol 3-kinase, growth factor receptor-bound protein GRB-2, and T cell-specific protein-tyrosine kinase ITK: implications for T-cell costimulation. Proc Natl Acad Sci U S A. 1995;92:8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran G, Kaempfer R, Chung CS, Shirvan A, Chahin AB, Palardy JE, Parejo NA, Chen Y, Whitford M, Arad G, et al. CD28 homodimer interface mimetic peptide acts as a preventive and therapeutic agent in models of severe bacterial sepsis and gram-negative bacterial peritonitis. J Infect Dis. 2015;211:995–1003. doi: 10.1093/infdis/jiu556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran G, Tulapurkar ME, Harris KM, Arad G, Shirvan A, Shemesh R, Detolla LJ, Benazzi C, Opal SM, Kaempfer R, Cross AS. A peptide antagonist of CD28 signaling attenuates toxic shock and necrotizing soft-tissue infection induced by Streptococcus pyogenes. J Infect Dis. 2013;207:1869–1877. doi: 10.1093/infdis/jit104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci Signal. 2013;6:ra75. doi: 10.1126/scisignal.2004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Morales F, Romero F, Schweighoffer F, Bismuth G, Camonis J, Tortolero M, Fischer S. The proline-rich region of Vav binds to Grb2 and Grb3–3. Oncogene. 1995;11:1665–1669. [PubMed] [Google Scholar]
- Rao S, Gerondakis S, Woltring D, Shannon MF. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J Immunol. 2003;170:3724–3731. doi: 10.4049/jimmunol.170.7.3724. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, Catanese JJ, Xie G, Stahl EA, Chen R, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41:1313–1318. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Yukawa M, Kartashov AV, Barski A. Functional characterization of human T cell hyporesponsiveness induced by CTLA4-Ig. PLoS One. 2015;10:e0122198. doi: 10.1371/journal.pone.0122198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, Chen YH. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. 2010;62:1792–1802. doi: 10.1002/art.27431. [DOI] [PubMed] [Google Scholar]
- Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Perez N, Silva CA, Abud-Mendoza C, Burgos-Vargas R, Gerloni V, Melo-Gomes JA, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2010;90:259–289. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- Sabzevari H, Kantor J, Jaigirdar A, Tagaya Y, Naramura M, Hodge J, Bernon J, Schlom J. Acquisition of CD80 (B7–1) by T cells. J Immunol. 2001;166:2505–2513. doi: 10.4049/jimmunol.166.4.2505. [DOI] [PubMed] [Google Scholar]
- Sadra A, Cinek T, Arellano JL, Shi J, Truitt KE, Imboden JB. Identification of tyrosine phosphorylation sites in the CD28 cytoplasmic domain and their role in the costimulation of Jurkat T cells. J Immunol. 1999;162:1966–1973. [PubMed] [Google Scholar]
- Saito T, Yokosuka T, Hashimoto-Tane A. Dynamic regulation of T cell activation and co-stimulation through TCR-microclusters. FEBS Lett. 2010;584:4865–4871. doi: 10.1016/j.febslet.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Salazar-Fontana LI, Barr V, Samelson LE, Bierer BE. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J Immunol. 2003;171:2225–2232. doi: 10.4049/jimmunol.171.5.2225. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lockhart M, Graf B, Miller J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J Immunol. 2008;181:7639–7648. doi: 10.4049/jimmunol.181.11.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lockhart M, Kim M, Miller J. Cutting edge: A role for inside-out signaling in TCR regulation of CD28 ligand binding. J Immunol. 2011;187:5515–5519. doi: 10.4049/jimmunol.1102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Lockhart M, Rojas AV, Fettis MM, Bauserman R, Higa TR, Miao H, Waugh RE, Miller J. T cell receptor signaling can directly enhance the avidity of CD28 ligand binding. PLoS One. 2014;9:e89263. doi: 10.1371/journal.pone.0089263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn WJ, Colombel JF, Sands BE, Rutgeerts P, Targan SR, Panaccione R, Bressler B, Geboes K, Schreiber S, Aranda R, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62–69. e64. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Schneider H, Rudd CE. CD28 and Grb-2, relative to Gads or Grap, preferentially cooperate with Vav1 in the activation of NFAT/AP-1 transcription. Biochem Biophys Res Commun. 2008;369:616–621. doi: 10.1016/j.bbrc.2008.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraven B, Kalinke U. CD28 superagonists: what makes the difference in humans? Immunity. 2008;28:591–595. doi: 10.1016/j.immuni.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schaffer AA, Gruning BA, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JC, Zhang X, Nathenson SG, Almo SC. Structural mechanisms of costimulation. Nat Immunol. 2002;3:427–434. doi: 10.1038/ni0502-427. [DOI] [PubMed] [Google Scholar]
- Semple K, Nguyen A, Yu Y, Wang H, Anasetti C, Yu XZ. Strong CD28 costimulation suppresses induction of regulatory T cells from naive precursors through Lck signaling. Blood. 2011;117:3096–3103. doi: 10.1182/blood-2010-08-301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro VS, Mollenauer MN, Greene WC, Weiss A. c-rel regulation of IL-2 gene expression may be mediated through activation of AP-1. J Exp Med. 1996;184:1663–1669. doi: 10.1084/jem.184.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro VS, Truitt KE, Imboden JB, Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- Soligo M, Camperio C, Caristi S, Scotta C, Del Porto P, Costanzo A, Mantel PY, Schmidt-Weber CB, Piccolella E. CD28 costimulation regulates FOXP3 in a RelA/NF-kappaB-dependent mechanism. Eur J Immunol. 2011;41:503–513. doi: 10.1002/eji.201040712. [DOI] [PubMed] [Google Scholar]
- Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7–1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- Stein PH, Fraser JD, Weiss A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3′-kinase. Mol Cell Biol. 1994;14:3392–3402. doi: 10.1128/mcb.14.5.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Tabares P, Berr S, Romer PS, Chuvpilo S, Matskevich AA, Tyrsin D, Fedotov Y, Einsele H, Tony HP, Hunig T. Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. Eur J Immunol. 2014;44:1225–1236. doi: 10.1002/eji.201343967. [DOI] [PubMed] [Google Scholar]
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- Takeda K, Harada Y, Watanabe R, Inutake Y, Ogawa S, Onuki K, Kagaya S, Tanabe K, Kishimoto H, Abe R. CD28 stimulation triggers NF-kappaB activation through the CARMA1-PKCtheta-Grb2/Gads axis. Int Immunol. 2008;20:1507–1515. doi: 10.1093/intimm/dxn108. [DOI] [PubMed] [Google Scholar]
- Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15:186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- Tavano R, Contento RL, Baranda SJ, Soligo M, Tuosto L, Manes S, Viola A. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunological synapse. Nat Cell Biol. 2006;8:1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- Thauland TJ, Koguchi Y, Dustin ML, Parker DC. CD28-CD80 interactions control regulatory T cell motility and immunological synapse formation. J Immunol. 2014;193:5894–5903. doi: 10.4049/jimmunol.1401752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaventhiran T, Alhumeed N, Yeang HX, Sethu S, Downey JS, Alghanem AF, Olayanju A, Smith EL, Cross MJ, Webb SD, et al. Failure to upregulate cell surface PD-1 is associated with dysregulated stimulation of T cells by TGN1412-like CD28 superagonist. MAbs. 2014;6:1290–1299. doi: 10.4161/mabs.29758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Gao L, Wells AD. Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol. 2005;174:4639–4646. doi: 10.4049/jimmunol.174.8.4639. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG, Leiden JM, June CH. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci U S A. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Wang H, Gish GD, Petsalaki E, Pasculescu A, Shi Y, Mollenauer M, Bagshaw RD, Yosef N, Hunter T, et al. Combinatorial proteomic analysis of intercellular signaling applied to the CD28 T-cell costimulatory receptor. Proc Natl Acad Sci U S A. 2015;112:E1594–1603. doi: 10.1073/pnas.1503286112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Zilvetti M, Chapman S, Wieckiewicz J, Sutherland A, Friend P, Wood KJ. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8:338–347. doi: 10.1111/j.1600-6143.2007.02078.x. [DOI] [PubMed] [Google Scholar]
- van Bergen J, Kooy-Winkelaar EM, van Dongen H, van Gaalen FA, Thompson A, Huizinga TW, Feltkamp MC, Toes RE, Koning F. Functional killer Ig-like receptors on human memory CD4+ T cells specific for cytomegalovirus. J Immunol. 2009;182:4175–4182. doi: 10.4049/jimmunol.0800455. [DOI] [PubMed] [Google Scholar]
- van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- Venuprasad K, Parab P, Prasad DV, Sharma S, Banerjee PR, Deshpande M, Mitra DK, Pal S, Bhadra R, Mitra D, Saha B. Immunobiology of CD28 expression on human neutrophils. I. CD28 regulates neutrophil migration by modulating CXCR-1 expression. Eur J Immunol. 2001;31:1536–1543. doi: 10.1002/1521-4141(200105)31:5<1536::AID-IMMU1536>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, Klempnauer J, Lang P, Larsen CP, Muhlbacher F, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21:1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- Waibler Z, Sender LY, Merten C, Hartig R, Kliche S, Gunzer M, Reichardt P, Kalinke U, Schraven B. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS One. 2008;3:e1708. doi: 10.1371/journal.pone.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Harada Y, Takeda K, Takahashi J, Ohnuki K, Ogawa S, Ohgai D, Kaibara N, Koiwai O, Tanabe K, et al. Grb2 and Gads exhibit different interactions with CD28 and play distinct roles in CD28-mediated costimulation. J Immunol. 2006;177:1085–1091. doi: 10.4049/jimmunol.177.2.1085. [DOI] [PubMed] [Google Scholar]
- Watts TH. Staying alive: T cell costimulation, CD28, and Bcl-xL. J Immunol. 2010;185:3785–3787. doi: 10.4049/jimmunol.1090085. [DOI] [PubMed] [Google Scholar]
- Weiss A, Manger B, Imboden J. Synergy between the T3/antigen receptor complex and Tp44 in the activation of human T cells. J Immunol. 1986;137:819–825. [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Woerly G, Decot V, Loiseau S, Loyens M, Chihara J, Ono N, Capron M. CD28 and secretory immunoglobulin A-dependent activation of eosinophils: inhibition of mediator release by the anti-allergic drug, suplatast tosilate. Clin Exp Allergy. 2004;34:1379–1387. doi: 10.1111/j.1365-2222.2004.02036.x. [DOI] [PubMed] [Google Scholar]
- Wofsy D, Hillson JL, Diamond B. Comparison of alternative primary outcome measures for use in lupus nephritis clinical trials. Arthritis Rheum. 2013;65:1586–1591. doi: 10.1002/art.37940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mauri-Hellweg D, Baumann K, Bettens F, Grunow R, Pichler WJ. The B7 adhesion molecule is expressed on activated human T cells: functional involvement in T-T cell interactions. Eur J Immunol. 1993;23:2175–2180. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant. 2014;14:319–332. doi: 10.1111/ajt.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Zhu Y, Zhu G, Augustine M, Zheng L, Goode DJ, Broadwater M, Ruff W, Flies S, Xu H, et al. B7-h2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34:729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Saito T. The immunological synapse, TCR microclusters, and T cell activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- Yong PF, Salzer U, Grimbacher B. The role of costimulation in antibody deficiencies: ICOS and common variable immunodeficiency. Immunol Rev. 2009;229:101–113. doi: 10.1111/j.1600-065X.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schwartz JC, Almo SC, Nathenson SG. Crystal structure of the receptor-binding domain of human B7–2: insights into organization and signaling. Proc Natl Acad Sci U S A. 2003;100:2586–2591. doi: 10.1073/pnas.252771499. [DOI] [PMC free article] [PubMed] [Google Scholar]