Abstract
Sickle cell disease (SCD) is an inherited blood disorder associated with significant morbidity, which includes severe episodic pain, and, often, chronic pain. Compared to healthy individuals, patients with SCD report enhanced sensitivity to thermal detection and pain thresholds and have altered inflammatory profiles, yet no studies to date have examined biomarker reactivity following laboratory-induced pain. We sought to examine this relationship in SCD patients compared to healthy control participants. We completed quantitative sensory testing (QST) in 83 patients with SCD and sequential blood sampling in 27 of them, whom we matched (sex, age, race, BMI, education) to 27 healthy controls. Surprisingly, few QST differences emerged between groups. Heat pain tolerance, pressure pain threshold at the trapezius, thumb and quadriceps, and thermal temporal summation at 45°C differed between groups in the expected direction, while conditioned pain modulation and pain ratings to hot water hand immersion were counterintuitive, possibly due to tailoring the water temperature to a perceptual level; SCD patients received milder temperatures. In the matched subsample, group differences and group by time interactions were observed in biomarkers including TNFα, IL-1β, IL-4, and NPY. These findings highlight the utility of laboratory pain testing methods for understanding individual differences in inflammatory cytokines. Our findings suggest amplified pain-evoked pro-inflammatory cytokine reactivity among patients with SCD relative to carefully matched controls. Future research is warranted to evaluate the impact of enhanced pain-related cytokine response and whether it is predictive of clinical characteristics and the frequency/severity of pain crises in SCD patients.
Keywords: Sickle cell disease, clinical pain, laboratory pain, QST, central sensitization, cytokines, inflammation
Summary
Sickle cell disease patients showed moderate pain sensitivity and enhanced reactivity to several cytokines, a vasoactive peptide and a neuropeptide compared to healthy controls.
Introduction
Sickle cell anemia is the most common inherited blood disorder and approximately one in twelve African Americans carry the trait (http://www.genome.gov/10001219). Sickle cell disease (SCD) patients suffer a number of acute and chronic health problems, including severe pain from vaso-occlusive crises. A landmark diary study revealed that chronic pain in SCD patients is quite common, with 30% of patients experiencing pain more than 95% of recorded days [65]. Despite tremendous individual differences in clinical outcomes among patients with SCD and the widespread experience of daily pain, very little is known about pain phenotypes in SCD.
Quantitative sensory testing (QST) is the use of standardized, calibrated, consistently applied noxious stimuli to measure individual differences in pain processing. It is increasingly utilized to measure individual patient characteristics that might be associated with long-term pain outcomes [30]. Reviews [1;67] highlight the value of QST, and many studies demonstrate its clinical relevance. In general, increased sensitivity to painful stimuli (e.g., low pain threshold and tolerance, high or increasing ratings of pain perception in response to application of an identically intense stimulus) increases risk for poor outcomes. Greater pain sensitivity, including lower pain tolerance [16;23] and higher levels of temporal summation [17], are associated with more frequent, intense, and disabling episodes of recent pain. The few studies that have examined QST in adults with sickle cell disease generally find that SCD patients are more sensitive to thermal detection and pain thresholds than healthy controls [7;27;28]. SCD patients also show evidence of central and/or peripheral sensitization and even a neuropathic pain component [22].
Several reports also suggest that SCD patients have altered inflammatory biomarker profiles, important due to their association with pain [73]. During non-crisis, “steady state”, SCD patients show high plasma levels of a number of important pain-related cytokines and other biomarkers of inflammation and nociception that are further increased during a painful crisis, including tumor necrosis factor-alpha (TNFα), interleukin 1 β (IL-1 β), IL-6, IL-8, interferon γ (IFN-γ) [58] and Substance P [50]. Thus, SCD patients are known to experience chronic inflammation. No studies to date have examined reactivity of inflammatory biomarkers following noxious stimulation or their association with pain in SCD patients. The current study sought to examine differences between patients with sickle cell disease and healthy controls in a battery of QST measures and evaluate cytokine reactivity over a two-hour period following QST testing in a subgroup of SCD patients compared with race, sex, age, BMI, and education matched healthy controls. These variables were chosen due to their relationship with pain. Of note, education was specifically chosen for multiple reasons. Education is related to health, SES[54;75] and both clinical and laboratory measures of pain[6;11;18;62]. Additionally, SCD patients may experience a number of SCD complications, particularly neurological complications, and recurrent painful crises that may interfere with schooling and result in lower educational attainment. Thus, equating groups on education level is likely important in order to avoid unintentionally creating bias between samples.
Methods
The current case control study is part of a larger ongoing project designed to examine pain and crises in SCD patients. All participants were recruited for participation from the Sickle Cell Center for Adults at Johns Hopkins Hospital or through posted advertisements. The current analyses focused on 110 volunteers (83 with SCD and 27 healthy controls; see Table 1 for demographic data). A subgroup of SCD patients (n=27) were carefully matched with healthy controls and provided blood samples during the QST portion of the study. Major inclusion criteria for SCD patients included age ≥18 years, formal diagnosis of SCD (by genotyping or confirmation by study hematologist), and no changes in dose (if any) of NSAIDs or acetaminophen one month prior to pain testing. Exclusion criteria included use of active alcohol or substance abuse/dependence; significant cognitive impairment; unstable psychiatric illness; HIV infection, viral hepatitis, or other current infection. Additional exclusion criteria for the SCD blood subgroup included long- and short-acting opioids and recent blood transfusion. Each of these SCD patients were matched with a control participant on race, sex, age, BMI, and education. Additional exclusion criteria for healthy controls included any acute or chronic pain, regular use of anti-inflammatory medication, opioids, or antidepressant medication; and smoking greater than 1 pack/day (as SCD patients did not have to meet a nonsmoking requirement). Cognitive impairment and unstable psychiatric illness was evaluated through examination of responses to medical and mental health screening questions and through medical record review. If a question arose regarding either of these issues, the team psychiatrist (CPC) was consulted for a decision regarding appropriateness for inclusion in the study.
Table 1.
Demographic and clinical characteristics by group [Mean (SD) / % (n)]
| Demographic and Clinical Variables |
SCD Patients N=83 |
Healthy Controls N=27 |
|---|---|---|
| Age | 38.9 (12.1) | 35.0 (10.0) |
| Female | 68.7% (57) | 59.3% (16) |
| Education Level report | ||
| ≤High School/GED | 18.3% (15) | 22.2% (6) |
| Some College/Technical School | 42.6% (35) | 33.3% (9) |
| ≥Bachelor’s degree | 39.0% (32) | 44.4% (12) |
| Occupational Status | ||
| Employed Full-Time | 35.4% (29) | 34.6% (9) |
| Marital Status | ||
| % Single | 56.1% (46) | 76.9% (20) |
| Clinical Variables | ||
| Body Mass Index | 25.5 (5.2) | 25.9 (4.0) |
| Nicotine Use (smoking) | 16.9% (14) | 14.8% (4) |
| Genotype | ||
| SS | 63.4% (52) | 0 |
| S-Beta zero | 7.3% (6) | 0 |
| S-Beta+ | 9.8% (8) | 0 |
| SC | 18.3% (15) | 0 |
| Trait | 0 | 0.04 (1) |
| Unknown | 1.2% (1) | 0.04 (1) |
| Pain Severity (BPI)*** | 2.0 (1.9) | 0.60 (0.9) |
| Pain Interference (BPI Extended)*** | 2.6 (2.7) | 0.30 (0.7) |
| Depression (CES-D)** | 14.5 (10.8) | 8.5 (7.3) |
(p<0.01);
(p<0.001).
Continuous measures are reported as mean (SD), proportional data are reported as percent (count).
Procedures
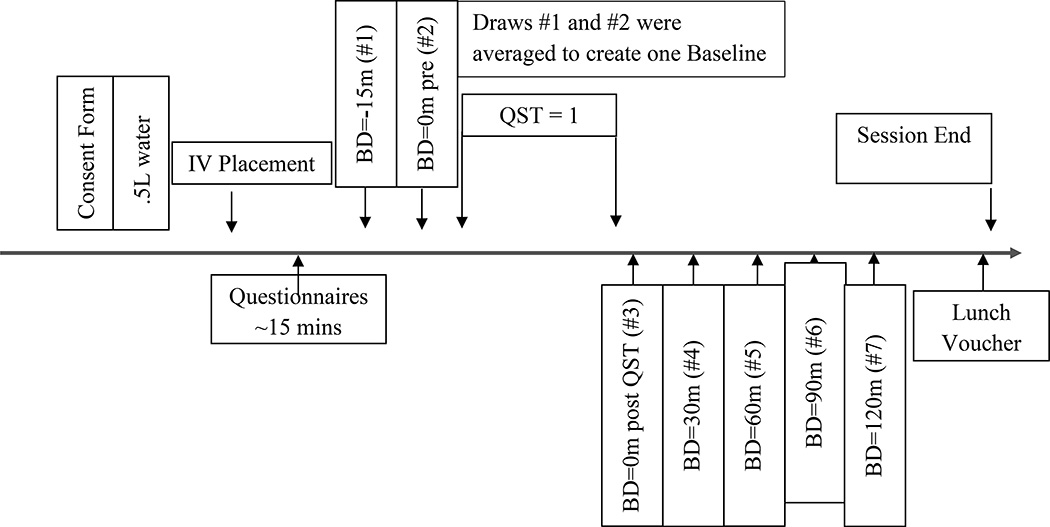
Following initial telephone screening to ensure eligibility criteria were met, participants attended an in-person visit. Participants were asked to attend only when their pain was typical of their SCD pain and at no greater intensity than 5 /10, and they had not experienced a vaso-occlusive crisis in at least the previous 3 weeks. Eligible participants completed a standardized laboratory pain testing protocol, and the SCD subgroup and healthy controls also underwent accompanying blood draws (see Figure 1). Sessions were conducted starting between 9 and 11am to control for circadian variation in cytokine levels [70]. Following informed consent procedures, the SCD subgroup and all healthy controls had an I.V. line placed in the antecubital vein and a 0.45% saline solution infused to maintain catheter patency. Blood samples (10 ml) were drawn 7 times throughout the testing period, twice during rest and prior to the onset of the pain testing procedures (15 minutes apart, the first being 15 minutes after I.V. placement), and 5 times following the pain testing procedures (immediately and then every 30 minutes for 2 hours). Samples were collected with EDTA and heparin Vacutainers®, placed on ice and centrifuged at 4°Celsius within 30 minutes of each blood draw. Aprotinin was mixed with the heparin collected blood, prior to centrifuging, for Substance P assessment. Plasma was aliquoted and stored at −80°C until batch assayed. Participants were allowed to stop or refuse any procedure at any time and all study-related procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board.
Figure 1.
Session Timeline for the SCD subgroup that underwent blood drawing procedures and all healthy controls. SCD participants that did not undergo blood drawing procedures underwent QST only.
Brief Pain Inventory (BPI) items were used to assess self-reported clinical pain severity based on the patients’ current, average, worst and least pain over the preceding week using a 0–10 scale (0 – No Pain to 10 – Pain as bad as it could be). Depression was measured using the Center for Epidemiological Studies Depression scale (CES-D), greater values represent higher levels of depression [59]. Clinical characteristics were additionally queried and/or obtained from participant medical records.
Quantitative Sensory Testing (QST)
Pain threshold/tolerance
Heat pain threshold (HPTh) was assessed via a Peltier element-based stimulator (Medoc, Pathway, Advanced Thermal Stimulator (ATS) thermode), on the dominant ventral forearm, with a 9 cm2 probe, using an ascending method of limits paradigm with a 0.5°C/sec rate of rise. Subjects underwent two trials and indicated when each stimulus first felt painful (HPTh) via button press which recorded the temperature and turned the device off. Heat Pain Tolerance (HPTo) was conducted in a similar manner, with participants indicating when they could no longer tolerate the pain from the thermal device. Pressure pain threshold (PPTh) was assessed via algometer (SBMedic, 1 cm2 hard rubber probe), bilaterally and 2 times each, at the trapezius muscle, interphalangeal joint of the thumb, the proximal third of the brachioradialis muscle (forearm), and middle of the quadriceps insertion point according to standard procedures[8]. Mean HPTh (in °C) and PPTh (in kilopascals) values were averaged across trials, respectively.
Temporal Summation
Thermal Temporal Summation (TTS) was assessed via response to three randomized series (temperatures: HPTh, HPTh+2, 45°C) of 10 heat pulses of each temperature rated on a 0–100 scale (0=no pain; 100=worst pain imaginable), applied to the dominant ventral forearm by the Medoc, Pathway Contact Heat-Evoked Potential Stimulator (CHEPS) [71]. A 2.5 second inter stimulus interval (ISI) and a 70°C/sec rate of rise was employed. A TTS difference score (maximal rated pulse of the series minus first pulse of the series) was created for each temperature. The thermode was moved slightly up the arm between trials to avoid overlapping stimulation skin sites. One additional pain rating was obtained 15 seconds following the final pulse in each series to characterize after sensations. Mechanical Temporal Summation (MTS) was assessed at two weights via response to an initial single stimulus, and then to a sequence of 10 stimuli of weighted punctate probes applied on a flat contact area of 0.2 mm diameter with a force of 128mN and 256mN, to the middle phalanx, dorsal surface of the dominant middle finger. Each series was delivered with an ISI of 1 second, participants were instructed to rate the “peak” pain experienced over the train of 10 stimuli. A MTS difference score was calculated (peak rating minus initial stimulus rating) for each probe weight.
Hot Water Immersion Tests
Pain ratings were additionally assessed using hot water. This task is similar to the more common cold pressor testing in terms of eliciting a pain response. Hot water was chosen to avoid the possibility of prompting a vaso-occlusive crisis and has the added benefit of avoiding baroreflex activation [68]. Participants immersed the dominant hand in a circulating water bath maintained at a tailored temperature designed to be moderately painful. The tailoring process included a series of up to 5 brief hand immersions starting at 42°C and increasing by 1–2°C until a rating of 60/100 was reached or as high as tolerable for 20 seconds. Following the tailoring procedures, a total of 3 immersions were conducted, two in the context of CPM (as described below) and one hot water tolerance test. Participants were permitted to remove their hand prior to the completion of any trial if the pain became intolerable. Pain ratings on a 0–100 scale were obtained at 30 second intervals for up to two minutes, the (uninformed) time limit during the hot water tolerance test.
Conditioned pain modulation (CPM)
Two PPTh readings were obtained on the non-dominant side trapezius muscle immediately prior to commencing the main hot water immersion tests. At the 20 second point during each of the hand immersion trials, a PPTh reading was obtained on the trapezius muscle. A difference value was created for CPM, such that the 2 PPTh values obtained during each of the CPT trials were averaged and the average of the 2 baseline PpTh readings was subtracted from it (during-baseline to yield a positive number if threshold increased during hand immersion).
Inflammatory Markers
The Human ProInflammatory-9 Ultra-Sensitive multiplex pro-inflammatory panel from MSD (Rockville, MD) was used to quantify IFN γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 and IL-13 on an MS2400 imager (MSD). Commercial enzyme immunoassay kits from R&D Systems (Minneapolis, MN) were used to quantify plasma levels of TNFα, NPY, ET, and SP for all pre- and post-QST time points. The two measures collected before commencing pain testing were averaged to create a baseline level for each marker.
Data Analysis
The initial analyses confirmed adequacy of matching the control and SCD subgroup on all planned criteria (sex, age, race, BMI and education). Chi-square tests and Analysis of Variance (ANOVAs) were used to determine the equivalence of the control group to the patient subgroup. ANOVAs were used to evaluate each QST measure, using all SCD patients (n=83) and the subgroup matched healthy controls (n=27) as the independent variable. Because many of the tests were tailored, we also evaluated group differences in stimulus parameters on which testing was anchored (e.g., temperatures for the TTS and hot water tests).
Cytokine/inflammatory biological markers were examined for subgroup analyses using Mixed Model Repeated Measures (MMRM) analyses, conducted to evaluate group and time main effects as well as group by time interactions for each marker (IFN γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, TNFα, NPY, ET, and SP). One SCD participant’s IL-6 values were greater than 2 standard deviations from the pooled mean at each time point, and were removed from analyses. Advantages of the MMRM approach for this data include its ability to handle missing data and that it accounts for within-person correlations between time points. Additionally, one area under the curve value was calculated for each marker (that included each time point) in order to examine its potential association with clinical pain and a single QST index. All QST measures were z-scored separately, reversed where appropriate, and combined to establish one “sensitivity index,” as previously described [13], where higher values represent greater sensitivity. Pearson bivariate correlation coefficients were analyzed to evaluate these associations.
Results
Participant demographics and clinical information for SCD and control participants are presented in Table 1. All participants self-reported their race to be Black, with three in each group additionally reporting at least one other racial category. A history of acute chest syndrome was observed in 39% of patients with SCD and 31.7% experienced avascular necrosis; 22.9% were prescribed hydroxyurea. Not surprisingly, between group differences were observed in pain severity and pain interference reported on the day of pain testing (ps< 0.001). Additionally, participants in the SCD group endorsed elevated levels of depressive symptomatology compared to controls (p = 0.008).
Quantitative Sensory Testing
Laboratory pain measures are displayed for both groups in Table 2. Significant differences between SCD and healthy control participants were observed in several QST measures including heat pain tolerance, pressure pain threshold at the trapezius, thumb and quadriceps, thermal temporal summation at 45°C, hot water temperature, hot water pain ratings and tolerance and CPM. Heat pain tolerance and pressure pain thresholds were lower (p<0.001, p’s<0.05; respectively), and thermal temporal summation at the fixed 45°C was higher (p=0.03) among SCD patients compared to healthy controls. As expected, the temperature required to elicit a moderately painful response from the hot water testing procedure was lower in SCD patients vs. controls (p<0.001). There were four unexpected findings. In healthy controls, pain intensity ratings of the hot water were significantly higher (p=0.003), tolerance of the hot water was lower (time to withdrawal, p=.04), hot water after-sensations were greater (p=0.009), and conditioned pain modulation scores were lower than in SCD patients (p=0.02). Three of these findings were rendered nonsignificant when controlling for water temperature. After sensation pain ratings remains significantly different between groups when controlling for water temperature.
Table 2.
Quantitative Sensory Testing (QST) measures by group[Mean (SD)]
| QST Measures | SCD (n = 83) |
Healthy Control (n=27) |
|---|---|---|
| Thermal Pain in °Celsius | ||
| Threshold (HPTh) | 40.7 (2.8) | 41.8 (2.9) |
| Tolerance (HPTo) | 44.0 (2.0) | 46.5 (2.2)*** |
| Pressure Pain Threshold in kilopascals (kPa) | ||
| Trapezius | 246.1 (99.1) | 310.9 (139.9)** |
| Thumb | 301.5 (100.0) | 357.0 (112)* |
| Forearm | 239.5 (102.3) | 279.1 (117.2) |
| Quadriceps | 520.7 (230.0) | 625.5 (252.7)* |
| Thermal Temporal Summation difference scores | ||
| At Heat Pain Threshold | 3.6 (7.2) | 2.2 (5.6) |
| At Threshold + 2°C | 3.8 (8.2) | 4.8 (9.7) |
| At 45°C | 8.0 (14.2) | 1.8 (3.5)* |
| After Sensation Ratings (TTS) | 11.8 (17.3) | 6.4 (11.8) |
| Mechanical Temporal Summation difference scores | ||
| 128 mN (Probe 5) | 12.8 (17.0) | 8.3 (13.2) |
| 256 mN (Probe 6) | 16.9 (19.1) | 10.7 (11.3) |
| Hot Water Hand Immersion Tests | ||
| Temperature of Hot Water (in °Celsius) | 45.2 (1.4) | 48.4 (1.1)*** |
| CPM Difference Trapezius (difference score) | 71.4 (64.3) | 37.6 (68.9)* |
| Hot Water Pain Ratings (0–100) | 56.0 (26.3) | 74.4 (19.1)*** |
| Hot Water Tolerance (in seconds) | 47.3(32.4) | 32.3 (28.2)* |
| After Sensation Ratings (hot water; 0–100) | 8.7 (12.8) | 17.5 (19.9)** |
(p<.05),
(p<.01).
Measures are reported as mean (SD). Difference Scores represent the maximal rated pulse (for Thermal Temporal Summation) or following the train of 10 stimuli (for Mechanical Temporal Summation) of the series minus first pulse of the series. CPM: Conditioned Pain Modulation. CPM Difference represents pressure pain thresholds at the trapezius obtained during water immersion of the hand minus baseline trapezius pressure pain thresholds.
Biomarkers
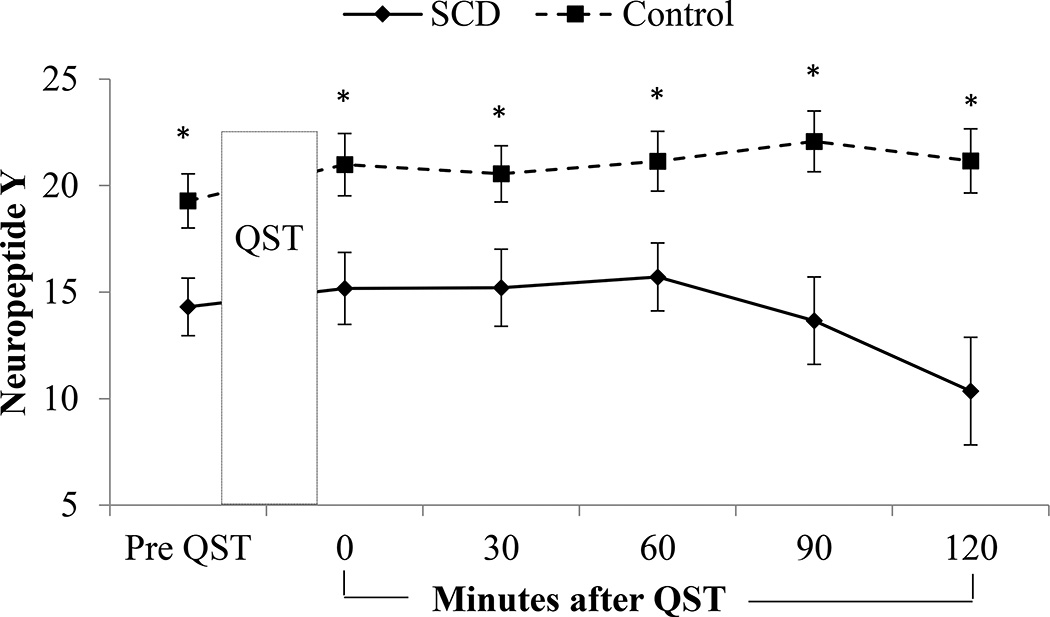
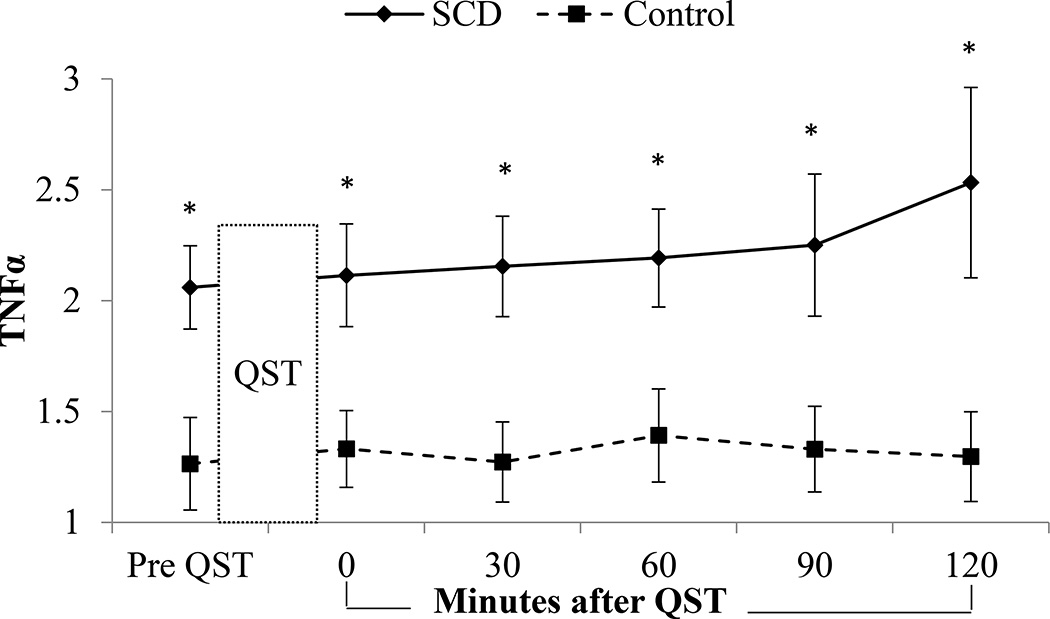
Biomarkers for the SCD subgroup and healthy matched participants at baseline and then following QST are presented in Table 3 and selected assays of interest are displayed graphically in Figures 2–3. Main effects of change over time suggesting biomarker reactivity following QST regardless of group, were observed in IL-6 (p<0.001) and Substance P, with both groups increasing over time (p=0.02). Main effects of group and time were noted for IL-10 (group p<0.001, time p=0.04) and ET (group p=0.02, time p<0.001), with SCD showing greater IL-10 values and both groups declining slightly by the last blood draw; ET was higher in controls and both groups increased over time. A main effect of time (p<0.001) and a group by time interaction (p=0.02) were observed for IL-1β, with an increase over time specifically in the healthy group. A group by time interaction (p=0.04) was observed in IL-4 with an increase over time in SCD patients while the controls decreased in IL4. A main effect of group (p<0.001) and group by time interaction (p=0.04) were observed for TNFα, with SCD patients having higher levels overall and exhibiting an increase over time. A main effect of group (p=0.04) and time (p<0.001) as well as a group by time interaction (p<0.001) were observed for NPY, with healthy controls showing higher levels overall and a slight increase over time, while SCD patients had a five-point reduction in NPY over the final hour of testing. No interaction or group differences were observed in IFN γ, IL-2, IL-6, IL-12, IL-13 and Substance P.
Table 3.
Biomarkers at each time point (baseline and following QST) by group (matched SCD patients and healthy matched controls). nMean (SD)
| BL | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|
| Multiplex | ||||||
| IFNγ-SCD | 2710.5 (11.6) | 2311.0 (14.4) | 2312.1 (17.1) | 2311.9 (17.3 | 178.6 (7.4) | 129.2 (10.1) |
| IFNγ-HC | 235.5 (7.1) | 225.4 (6.3) | 225.0 (5.7) | 225.2 (5.9) | 214.5 (5.2) | 214.5 (5.3) |
| IL1B-SCD | 220.03 (0.04) | 180.03 (0.04) | 180.02 (0.04) | 180.03 (0.05) | 130.03 (0.04) | 100.05 (0.07) |
| IL1B-HC | 110.03 (0.03) | 110.03 (0.04) | 100.05 (0.05) | 110.04 (0.04) | 100.17 (0.41) | 90.41 (0.96) |
| IL2-SCD | 260.23 (0.35) | 220.20 (0.33) | 220.24 (0.31) | 220.21 (0.25) | 160.12 (0.13) | 110.12 (0.08) |
| IL2-HC | 230.16 (0.13) | 220.13 (0.14) | 220.13 (0.14) | 220.14 (0.11) | 210.15 (0.13) | 210.13 (0.12) |
| IL4-SCD | 250.02 (0.02) | 200.02 (0.02) | 200.02 (0.02) | 200.03 (0.03) | 140.03 (0.03) | 90.03 (0.02) |
| IL4-HC | 180.02 (0.02) | 160.02 (0.02) | 160.02 (0.02) | 160.03 (0.03) | 160.03 (0.03) | 150.02 (0.02) |
| IL6-SCD | 260.7 (0.74) | 220.8 (0.4) | 221.0 (0.5) | 221.5 (2.1) | 171.3 (0.5) | 121.7 (0.9) |
| IL6-HC | 230.5 (0.3) | 220.6 (0.3) | 220.7 (0.4) | 220.8 (0.4) | 211.1 (0.8) | 211.2 (0.9) |
| IL8-SCd | 278.5 (4.8) | 237.6 (6.4) | 2312.1 (15.2) | 2310.9 (13.0) | 1713.4 (6.9) | 1119.8 (34.8) |
| IL8-HC | 234.0 (2.1) | 224.1 (2.4) | 224.0 (2.3) | 224.3 (2.0) | 216.6 (10.3) | 216.6 (10.9) |
| IL-10-SCD | 270.4 (0.2 | 230.4 (0.3) | 230.4 (0.3) | 230.4 (0.3) | 170.5(0.4) | 120.4 (0.3) |
| IL-10-HC | 230.2 (0.1) | 220.2 (0.1) | 220.2 (0.1) | 220.2 (0.1) | 210.2 (0.1) | 210.2 (0.1) |
| IL-12 | 270.1 (0.1) | 230.2 (0.1) | 230.2 (0.1) | 230.2 (0.1) | 170.2 (0.1) | 110.2 (0.1) |
| IL-12-HC | 230.2 (0.2) | 220.2 (0.2) | 220.2 (0.2) | 220.2 (0.1 | 210.2 (0.1 | 210.2 (0.2 |
| IL-13-SCD | 261.1 (0.8) | 221.1 (0.9) | 221.4 (0.8) | 221.3 (0.8) | 161.3 (0.9) | 121.5 (1.2) |
| IL13-HC | 230.9 (0.9) | 220.8 (1.0) | 220.9 (0.8) | 220.9 (1.0) | 211.0 (1.0) | 210.9 (1.0) |
| Single Assays | ||||||
| TNFα-SCD | 272.1 (1.0) | 222.1 (1.1) | 232.2 (1.1) | 212.2 (1.0) | 172.3 (1.3) | 112.5(1.4) |
| TNFα-HC | 271.3 (1.1) | 251.3 (0.9) | 261.3 (0.9) | 251.4 (1.0) | 251.3 (1.0) | 251.3 (1.0) |
| NPY-SCD | 2714.3 (7.0) | 2315.2 (8.1) | 2315.2 (8.7) | 2215.7 (7.5) | 1913.7 (8.9) | 1610.4 (10.1) |
| NPY-HC | 2319.3 (6.1) | 2221.0 (6.9) | 2220.6 (6.2) | 2221.1 (6.6) | 2122.1 (6.5) | 2121.2 (6.9) |
| ET-SCD | 271.4 (0.4) | 231.6 (0.5) | 231.8 (0.5) | 211.9 (0.6) | 172.0 (0.7) | 112.0 (0.6) |
| ET-HC | 231.8 (0.5) | 222.0 (0.6) | 222.2 (0.8) | 222.2 (0.7) | 212.3 (0.7) | 212.4 (0.8) |
| SP-SCD |
27343.2 (188.3) |
23367.6 (192.5) |
23391.4 (203.8) |
22375.1 (188.5) |
17337.6 (172.5) |
11349.5 (112.0) |
| SP-HC |
23415.0 (754.5) |
22481.2 (874.5) |
22493.8 (859.3) |
22498.6 (859.8) |
21514.7 (902.6) |
21477.0 (738.0) |
IFN-γ: Interferon gamma, IL: Interleukin, TNFα: Tumor necrosis factor alpha, NPY: Neuropeptide Y, ET: Endothelium, SP: Substance P. All measures are reported as sample size n in superscript (n) mean (SD).
Figure 2.
NPY reactivity by group. Main effects of group and time as well as a group by time interaction was observed for NPY (group p=0.04, time p<0.001, interaction p<0.001), with healthy controls having higher levels overall and a slight increase over time, while the SCD patient subgroup had a five-point reduction in NPY over the final hour of testing. Significant differences between groups were also observed at each time point.
Figure 3.
TNFα reactivity by group. A main effect of group (p<0.001) and group by time interaction (p=0.04) were observed for TNFα, with the SCD patient subgroup having higher levels overall and exhibiting an increase over time. Significant differences between groups were also observed at each time point.
Associations between biomarkers and pain
In the SCD subgroup, NPY at baseline and AUC significantly correlated with the QST index (r=−0.55, p=0.003; r=−0.63, p=0.009, respectively) and IL8 AUC, as well as IL12 AUC correlated with BPI severity (r=0.57, p=0.005; r=−0.43, p=.04, respectively). In healthy participants, IL-4 at baseline and AUC was correlated with the QST index (r=0.61, p=0.007; r=0.68, p=0.006, respectively).
Discussion
When compared to healthy controls, we find group differences on several pain testing parameters, and interesting effects of disease and pain testing on inflammatory markers in the SCD subset. SCD patients were generally assigned a lower temperature for the hot water assessment based on their responses and showed lower heat pain tolerance and pressure pain thresholds as well as greater thermal temporal summation at 45°C. The subset of SCD patients for whom biomarkers were collected, showed elevated resting (baseline) anti-inflammatory (IL-10) and pro-inflammatory activity (TNFα) and both groups showed changes in inflammatory markers in response to pain testing, including reductions in some anti-inflammatory measures (IL-10) and increases in some pro-inflammatory measures (IL-6, TNFα). Group differences in response to pain testing included a differential increase in TNFα in SCD patients, which did not correlate with general sensitivity (QST Index), and a reduction in neuropeptide Y in response to pain testing, which inversely correlated with general sensitivity.
Our findings are inconsistent with the literature showing increased sensitivity to thermal thresholds in SCD compared to healthy controls. Brandow and colleagues [7] reported heat pain thresholds of 42.7 in SCD patients and 45.2 in controls, while our values ranged from 40.7 to 41.8. Brandow’s sample included pediatric patients and a greater percentage of patients on hydroxyurea (42%). While they matched on sex and ethnicity, they did not match on age, BMI or education, factors which may contribute to the discrepancies between these two studies. Our heat pain threshold values are 2 degrees lower than Brandow’s and consistent with a recent study evaluating differences in QST by age and between painful and nonpainful sites [22]. An important matching characteristic for our sample likely is education. Education is a proxy for socioeconomic status (SES) and is often reflective of health status [54]. Lower SES/educational attainment has been associated with enhanced clinical pain [12;39;45] and laboratory-induced pain sensitivity [19], which may explain in part why our healthy control group showed greater pain sensitivity than seen in Brandow’s sample. Neurocognitive testing would have been an interested addition to this study, but was beyond the scope of this project. Future studies may wish to include an evaluation of neurocognitive performance.
Conditioned pain modulation is thought to reflect endogenous opioid function [48], and African Americans show reduced CPM responses when compared to non-Hispanic white participants [10]. Cold water is the most common conditioning stimuli for eliciting CPM effects [48]. Alterations in CPM due to chronic pain are inconsistent across a number of clinical conditions, including chronic low back pain [40] and osteoarthritis [44] among others. In addition to ethnicity, CPM varies according to other demographic and psychosocial factors including sex [24;57], personality [29], and cognitive factors such as expectations [5]. CPM also has been suspected to be altered in individuals with a history of opioid use [60], which is particularly relevant for studies of SCD patients, many of whom have had intermittent opioid exposure since childhood. For example, reductions in opioid receptor binding potential from PET imaging studies seen in chronic pain conditions [31], [37] [38;74] reflecting enhanced “endogenous opioid tone” have been related to a history of opioid use [31]. Harris and colleagues [31] have suggested that alterations in these systems, compared to healthy controls, could be the result of persistent pain as well as exogenous or endogenous opioid agents.
Several biomarkers differed between groups in the resting state (TNFα, IL-10, NPY and endothelin) as well as show general reactivity to pain testing (IL-1Beta, IL-6, IL-10, endothelin and Substance P). The groups differed in pain reactivity on TNFα, NPY, IL-1Beta, and IL-4. A number of reports have documented enhanced steady state levels of inflammation in SCD patients compared to healthy controls [51;55;58;64]. These differences typically include elevated cytokines (e.g., TNFα, IL-6, IFN-γ, and IL-1β) as well as elevations in vasoactive peptides (e.g., endothelin) and neuropeptides (e.g., substance P) implicated in pain transmission [64], although results are not uniform [33;61]. While SCD participants were carefully selected to be outside of a crisis, most reported ongoing, mild pain at the time of testing, a finding that is common in adult SCD patients [65]. Since many of these biomarkers are also known to increase during crisis, we were particularly interested in whether the pain elicited through laboratory testing would provoke changes in these biomarkers. No work to date has evaluated reactivity in these biomarkers following acute, laboratory-induced pain in sickle cell, though we have shown IL-6 reactivity in healthy subjects [20;21;46]. We replicated this effect, finding that both SCD patients and healthy controls show increases in IL-6 in response to laboratory pain testing and extended this effect to substance P and endothelin as well as declines in IL-10, an anti-inflammatory cytokine.
TNFα and NPY were of particular interest in the current study due to their potential role in pain sensitivity [4;9;47;61;63;66]. Pro-inflammatory cytokine levels such as IL-6 and TNF-α correlate with intensity of pain among samples of arthritis, back pain, and fibromyalgia patients [26;41;53;56;69]. TNFα is a key “master” pro-inflammatory cytokine and plays a significant role in VOCs in SCD [2]. TNF inhibitors have been the subject of intensive investigation recently as disease modifying agents for immune-mediated chronic conditions. Specifically, 60–70% of rheumatoid arthritis patients experience a reduction in clinical symptoms with TNF-blocking agents [72]. Biological anti-TNF agents have been approved for a number of systemic inflammatory conditions, many of which include pain as a primary symptom. These include rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Additionally, animal models and preliminary studies in humans suggest that anti-TNF agents have a role in reducing central sensitivity to pain [47]. Our findings indicate a small signal that SCD patients may show central sensitization based on the significant group difference observed in thermal temporal summation (45deg). Given the relatively mild level of clinical pain reported by our SCD sample (average of 1.2/10), this finding will need to be further explored in a larger sample with more severe daily pain and thus greater likelihood of displaying laboratory-assessed central sensitization.
Neuropeptide Y is of interest due to its widespread role in physiological actions related to pain sensitivity, including pain modulation, cortical excitability, emotional regulation and the stress response [4]. Consistent with our findings, NPY has been previously shown to have primarily an antinociceptive effect [4]. Here, we find that healthy participants show higher levels of NPY and only small changes in response to pain testing, whereas SCD patients show lower levels that decrease further with pain testing, such that the larger the reduction in NPY the greater the overall pain sensitivity. NPY has also been shown to have anti-stress properties and laboratory evoked stress is inversely correlated with NPY [32;43]. Taken together, these data suggest that NPY may be a marker of stress-resilience [52]. In our sample, healthy participants were observed to have greater levels of NPY than SCD patients; the stress associated with living with a chronic disease may be partially responsible for this NPY difference and the stress from QST may have further reduced NPY in SCD patients compared to (potentially more resilient) healthy controls. Administering NPY in basic models suggest that it also has antianxiety-actions [32]. Several studies indicate that intrathecally administered NPY in animal models inhibits behavioral and molecular markers of inflammatory and neuropathic pain [36], and it is part of a network of mechanisms that aid in natural recovery from hyperalgesia associated with inflammation or nerve injury [66]. NPY is believed to play a role in the processing of pain from the periphery to the brain stem, including the transmission of pain sensations [9], and has been implicated in the transition from acute to chronic pain [66]. Recent work suggests that it or its receptors may be useful as a therapeutic agent in a number of chronic conditions [9], particularly in treating chronic inflammatory pain [63]. Thus, these agents may deserve consideration as future therapeutic approaches to the management of pain in SCD.
Mouse models of SCD (HbSS) have been used to evaluate pain responses, and these mice show hyperalgesia, with lower paw withdrawal threshold and latency to thermal and mechanical stimulation as well as grip force compared to control mice [42]. HbSS mice also exhibit allodynia-like behavioral responses to punctate and dynamic light touch stimuli and amplified action potential firing to suprathreshold stimulation [25] as well as peripheral sensitization to noxious mechanical stimulation [34]. Inflammation in these mouse models has also been documented [49] with a CRP-like sequence elevated up to 12-fold and IL-6 nearly two fold compared to control mice [3]. Sickle cell mouse models have also been employed to evaluate drug effects, like hydroxyurea [35] and novel compounds [14;15] on nociception and inflammation.
One limitation of the current analyses is the exclusion of patients on chronic opioid therapy (COT) from the biomarker subgroup, a common treatment for adult SCD. Consequently, the biomarker data presented here may include a “healthier” cohort of SCD patients and may not be generalizable to the larger SCD cohort. Another limitation is the variability in sample size for the biomarker assessment. As displayed in Table 3, sample size varied according to the assay. In particular, a number of samples showed undetectable IL-1β concentrations. Our statistical method used procedures that accommodate missing values, yet some non-significant findings may be due to large variability in the context of a relatively small sample size. Despite these limitations, the current data suggest moderate differences in pain sensitivity between SCD patients and healthy controls, yet within a subgroup of SCD patients- notable differences in cytokines, vasoactive peptides and neuropeptides, both at rest and in response to pain. Variations in resting and pain reactive biomarkers contribute additional evidence that SCD is a condition of chronic and/or heightened inflammation. Future studies may wish to examine potential therapeutic targets related to these cytokines, vasoactive peptides or neuropeptides.
Acknowledgments
This research was supported by National Heart, Lung, and Blood Institute R01HL98110 (JAH, PI) and a Career Development Award from NINDS K23 NS070933 (CMC, PI).
Footnotes
All authors declare that they have no conflicts of interest.
Reference List
- 1.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–572. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, Goncalves RP. Chronic inflammatory state in sickle cell anemia patients is associated with HBB(*)S haplotype. Cytokine. 2014;65:217–221. doi: 10.1016/j.cyto.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 4.Benarroch EE. Neuropeptide Y: its multiple effects in the CNS and potential clinical significance. Neurology. 2009;72:1016–1020. doi: 10.1212/01.wnl.0000345258.18071.54. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkedal E, Flaten MA. Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. J Pain Res. 2012;5:289–300. doi: 10.2147/JPR.S33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 7.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88:37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurement of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 9.Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2:429–439. doi: 10.1002/emmm.201000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic Differences in Diffuse Noxious Inhibitory Controls (DNIC) Journal of Pain. 2008 doi: 10.1016/j.jpain.2008.03.010. In Press, Ref #YJPAI1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croft PR, Rigby AS. Socioeconomic influences on back problems in the community in Britain. J Epidemiol Community Health. 1994;48:166–170. doi: 10.1136/jech.48.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day MA, Thorn BE. The relationship of demographic and psychosocial variables to pain-related outcomes in a rural chronic pain population. Pain. 2010;151:467–474. doi: 10.1016/j.pain.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos JL, Lanaro C, Chelucci RC, Gambero S, Bosquesi PL, Reis JS, Lima LM, Cerecetto H, Gonzalez M, Costa FF, Chung MC. Design, synthesis, and pharmacological evaluation of novel hybrid compounds to treat sickle cell disease symptoms part II: furoxan derivatives. J Med Chem. 2012;55:7583–7592. doi: 10.1021/jm300602n. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos JL, Lanaro C, Lima LM, Gambero S, Franco-Penteado CF, Alexandre-Moreira MS, Wade M, Yerigenahally S, Kutlar A, Meiler SE, Costa FF, Chung M. Design, synthesis, and pharmacological evaluation of novel hybrid compounds to treat sickle cell disease symptoms. J Med Chem. 2011;54:5811–5819. doi: 10.1021/jm200531f. [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Fillingim RB. Effects of age on temporal summation of thermal pain: clinical relevance in healthy older and younger adults. Journal of Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Giles J, Bingham CO, III, Campbell C, Haythornthwaite JA, Bathon J. Moderators of the negative effects of catastrophizing in arthritis. Pain Med. 2010;11:591–599. doi: 10.1111/j.1526-4637.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards RR, Goble L, Kwan A, Kudel I, McGuire L, Heinberg L, Wigley F, Haythornthwaite J. Catastrophizing, pain, and social adjustment in scleroderma: relationships with educational level. Clin J Pain. 2006;22:639–646. doi: 10.1097/01.ajp.0000210918.26159.94. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards RR, Wasan AD, Bingham CO, III, Bathon J, Haythornthwaite JA, Smith MT, Page GG. Enhanced reactivity to pain in patients with rheumatoid arthritis. Arthritis Res Ther. 2009;11:R61. doi: 10.1186/ar2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Schlaeger JM, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain Pract. 2015 doi: 10.1111/papr.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- 24.France CR, Suchowiecki S. A comparison of diffuse noxious inhibitory controls in men and women. Pain. 1999;81:77–84. doi: 10.1016/s0304-3959(98)00272-3. [DOI] [PubMed] [Google Scholar]
- 25.Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain. 2012;8:62. doi: 10.1186/1744-8069-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Gil KM, Phillips G, Webster DA, Martin NJ, Abrams M, Grant M, Clark WC, Janal MN. Experimental pain sensitivity and reports of negative thoughts in adults with sickle cell disease. Behavior Therapy. 1995;26:273–293. [Google Scholar]
- 28.Gil KM, Wilson JJ, Edens JL, Webster DA, Abrams MA, Orringer E, Grant M, Clark WC, Janal MN. Effects of cognitive coping skills training on coping strategies and experimental pain sensitivity in African American adults with sickle cell disease. Health Psychology. 1996;15:3–10. doi: 10.1037//0278-6133.15.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Goodin BR, Kronfli T, King CD, Glover TL, Sibille K, Fillingim RB. Testing the relation between dispositional optimism and conditioned pain modulation: does ethnicity matter? J Behav Med. 2013;36:165–174. doi: 10.1007/s10865-012-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granot M. Can we predict persistent postoperative pain by testing preoperative experimental pain? Curr Opin Anaesthesiol. 2009;22:425–430. doi: 10.1097/ACO.0b013e32832a40e1. [DOI] [PubMed] [Google Scholar]
- 31.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Hibbert JM, Hsu LL, Bhathena SJ, Irune I, Sarfo B, Creary MS, Gee BE, Mohamed AI, Buchanan ID, Al-Mahmoud A, Stiles JK. Proinflammatory cytokines and the hypermetabolism of children with sickle cell disease. Exp Biol Med (Maywood) 2005;230:68–74. doi: 10.1177/153537020523000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118:3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu LL. Hydroxyurea makes inflammation "just right"? Blood. 2012;119:1796–1798. doi: 10.1182/blood-2011-12-397794. [DOI] [PubMed] [Google Scholar]
- 36.Intondi AB, Dahlgren MN, Eilers MA, Taylor BK. Intrathecal neuropeptide Y reduces behavioral and molecular markers of inflammatory or neuropathic pain. Pain. 2008;137:352–365. doi: 10.1016/j.pain.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, Derbyshire S, Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33:909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- 38.Jones AK, Watabe H, Cunningham VJ, Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur J Pain. 2004;8:479–485. doi: 10.1016/j.ejpain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med. 2013;28:1604–1610. doi: 10.1007/s11606-013-2516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 41.Kaufmann I, Eisner C, Richter P, Huge V, Beyer A, Chouker A, Schelling G, Thiel M. Lymphocyte subsets and the role of TH1/TH2 balance in stressed chronic pain patients. Neuroimmunomodulation. 2007;14:272–280. doi: 10.1159/000115041. [DOI] [PubMed] [Google Scholar]
- 42.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116:456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. 2013;47:401–419. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 45.Koster A, Bosma H, van Lenthe FJ, Kempen GI, Mackenbach JP, van Eijk JT. The role of psychosocial factors in explaining socio-economic differences in mobility decline in a chronically ill population: results from the GLOBE study. Soc Sci Med. 2005;61:123–132. doi: 10.1016/j.socscimed.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 46.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, Edwards RR. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13:936–944. doi: 10.1016/j.jpain.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Makis AC, Hatzimichael EC, Bourantas KL. The role of cytokines in sickle cell disease. Ann Hematol. 2000;79:407–413. doi: 10.1007/s002770000173. [DOI] [PubMed] [Google Scholar]
- 50.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92:3148–3151. [PubMed] [Google Scholar]
- 51.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92:3148–3151. [PubMed] [Google Scholar]
- 52.Morgan CA, III, Wang S, Rasmusson A, Hazlett G, Anderson G, Charney DS. Relationship among plasma cortisol, catecholamines, neuropeptide Y, human performance during exposure to uncontrollable stress. Psychosom Med. 2001;63:412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Mukai E, Nagashima M, Hirano D, Yoshino S. Comparative study of symptoms and neuroendocrine-immune network mediator levels between rheumatoid arthritis patients and healthy subjects. Clin Exp Rheumatol. 2000;18:585–590. [PubMed] [Google Scholar]
- 54.Oakes M. Measuring Socioeconomic Status. In: NIH DoHaHS, editor. Office of Behavioral and Social Sciences Research; 2015. Online Source: http://www.esourceresearch.org/Portals/0/Uploads/Documents/Public/Oakes_FullChapter.pdf. [Google Scholar]
- 55.Pathare A, Al KS, Alnaqdy AA, Daar S, Knox-Macaulay H, Dennison D. Cytokine profile of sickle cell disease in Oman. Am J Hematol. 2004;77:323–328. doi: 10.1002/ajh.20196. [DOI] [PubMed] [Google Scholar]
- 56.Penninx BW, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, Pahor M. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol. 2004;31:2027–2031. [PubMed] [Google Scholar]
- 57.Popescu A, LeResche L, Truelove EL, Drangsholt MT. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150:309–318. doi: 10.1016/j.pain.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 58.Qari MH, Dier U, Mousa SA. Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clin Appl Thromb Hemost. 2012;18:195–200. doi: 10.1177/1076029611420992. [DOI] [PubMed] [Google Scholar]
- 59.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 60.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain. 2008;139:431–438. doi: 10.1016/j.pain.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Sarray S, Saleh LR, Lisa SF, Al-Habboubi HH, Mahdi N, Almawi WY. Serum IL-6, IL-10, and TNFalpha levels in pediatric sickle cell disease patients during vasoocclusive crisis and steady state condition. Cytokine. 2015;72:43–47. doi: 10.1016/j.cyto.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Smith BH, Elliott AM, Chambers WA, Smith WC, Hannaford PC, Penny K. The impact of chronic pain in the community. Fam Pract. 2001;18:292–299. doi: 10.1093/fampra/18.3.292. [DOI] [PubMed] [Google Scholar]
- 63.Smith PA, Moran TD, Abdulla F, Tumber KK, Taylor BK. Spinal mechanisms of NPY analgesia. Peptides. 2007;28:464–474. doi: 10.1016/j.peptides.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 64.Smith TP, Haymond T, Smith SN, Sweitzer SM. Evidence for the endothelin system as an emerging therapeutic target for the treatment of chronic pain. J Pain Res. 2014;7:531–545. doi: 10.2147/JPR.S65923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 66.Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68:322–341. doi: 10.1111/j.1365-2125.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Streff A, Kuehl LK, Michaux G, Anton F. Differential physiological effects during tonic painful hand immersion tests using hot and ice water. Eur J Pain. 2010;14:266–272. doi: 10.1016/j.ejpain.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Sturmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, Brenner H. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. 2005;64:921–925. doi: 10.1136/ard.2004.027045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 71.Vierck CJJ, Cannon RL, Fry G, Maixner W, Whitsel BL. Characteristics of temporal summation of second pain sensations elicited by brief contact of glabrous skin by a preheated thermode. J Neurophysiol. 1997;78:992–1002. doi: 10.1152/jn.1997.78.2.992. [DOI] [PubMed] [Google Scholar]
- 72.Voll RE, Kalden JR. Do we need new treatment that goes beyond tumor necrosis factor blockers for rheumatoid arthritis? Ann N Y Acad Sci. 2005;1051:799–810. doi: 10.1196/annals.1361.123. [DOI] [PubMed] [Google Scholar]
- 73.Watkins LR, Maier SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 74.Willoch F, Schindler F, Wester HJ, Empl M, Straube A, Schwaiger M, Conrad B, Tolle TR. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–220. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 75.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82:816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]