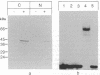
Abstract
Control elements of the tetracycline-resistance operon encoded in Tn10 of Escherichia coli have been utilized to establish a highly efficient regulatory system in mammalian cells. By fusing the tet repressor with the activating domain of virion protein 16 of herpes simplex virus, a tetracycline-controlled transactivator (tTA) was generated that is constitutively expressed in HeLa cells. This transactivator stimulates transcription from a minimal promoter sequence derived from the human cytomegalovirus promoter IE combined with tet operator sequences. Upon integration of a luciferase gene controlled by a tTA-dependent promoter into a tTA-producing HeLa cell line, high levels of luciferase expression were monitored. These activities are sensitive to tetracycline. Depending on the concentration of the antibiotic in the culture medium (0-1 microgram/ml), the luciferase activity can be regulated over up to five orders of magnitude. Thus, the system not only allows differential control of the activity of an individual gene in mammalian cells but also is suitable for creation of "on/off" situations for such genes in a reversible way.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschmied L., Baumeister R., Pfleiderer K., Hillen W. A threonine to alanine exchange at position 40 of Tet repressor alters the recognition of the sixth base pair of tet operator from GC to AT. EMBO J. 1988 Dec 1;7(12):4011–4017. doi: 10.1002/j.1460-2075.1988.tb03290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991 May 11;19(9):2499–2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baim S. B., Labow M. A., Levine A. J., Shenk T. A chimeric mammalian transactivator based on the lac repressor that is regulated by temperature and isopropyl beta-D-thiogalactopyranoside. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5072–5076. doi: 10.1073/pnas.88.12.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Krämmer G., Röwekamp W. G. Construction of a fusion gene that confers resistance against hygromycin B to mammalian cells in culture. Exp Cell Res. 1985 May;158(1):237–243. doi: 10.1016/0014-4827(85)90446-x. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Warren R., Sarthy A., Palmiter R. D. Regulation of metallothionein--thymidine kinase fusion plasmids injected into mouse eggs. Nature. 1982 Mar 4;296(5852):39–42. doi: 10.1038/296039a0. [DOI] [PubMed] [Google Scholar]
- Brown M., Figge J., Hansen U., Wright C., Jeang K. T., Khoury G., Livingston D. M., Roberts T. M. lac repressor can regulate expression from a hybrid SV40 early promoter containing a lac operator in animal cells. Cell. 1987 Jun 5;49(5):603–612. doi: 10.1016/0092-8674(87)90536-8. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Hipskind R. A., Bujard H. RNA polymerase II transcription blocked by Escherichia coli lac repressor. Science. 1990 Apr 27;248(4954):480–483. doi: 10.1126/science.2158670. [DOI] [PubMed] [Google Scholar]
- Deuschle U., Pepperkok R., Wang F. B., Giordano T. J., McAllister W. T., Ansorge W., Bujard H. Regulated expression of foreign genes in mammalian cells under the control of coliphage T3 RNA polymerase and lac repressor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5400–5404. doi: 10.1073/pnas.86.14.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge J., Wright C., Collins C. J., Roberts T. M., Livingston D. M. Stringent regulation of stably integrated chloramphenicol acetyl transferase genes by E. coli lac repressor in monkey cells. Cell. 1988 Mar 11;52(5):713–722. doi: 10.1016/0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Fernandez M. P., Moss B. Transfer of the inducible lac repressor/operator system from Escherichia coli to a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2549–2553. doi: 10.1073/pnas.86.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatz C., Kaiser A., Wendenburg R. Regulation of a modified CaMV 35S promoter by the Tn10-encoded Tet repressor in transgenic tobacco. Mol Gen Genet. 1991 Jun;227(2):229–237. doi: 10.1007/BF00259675. [DOI] [PubMed] [Google Scholar]
- Gatz C., Quail P. H. Tn10-encoded tet repressor can regulate an operator-containing plant promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1394–1397. doi: 10.1073/pnas.85.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Hu M. C., Davidson N. The inducible lac operator-repressor system is functional in mammalian cells. Cell. 1987 Feb 27;48(4):555–566. doi: 10.1016/0092-8674(87)90234-0. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Labow M. A., Baim S. B., Shenk T., Levine A. J. Conversion of the lac repressor into an allosterically regulated transcriptional activator for mammalian cells. Mol Cell Biol. 1990 Jul;10(7):3343–3356. doi: 10.1128/mcb.10.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Nguyen V. T., Morange M., Bensaude O. Protein denaturation during heat shock and related stress. Escherichia coli beta-galactosidase and Photinus pyralis luciferase inactivation in mouse cells. J Biol Chem. 1989 Jun 25;264(18):10487–10492. [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Searle P. F., Stuart G. W., Palmiter R. D. Building a metal-responsive promoter with synthetic regulatory elements. Mol Cell Biol. 1985 Jun;5(6):1480–1489. doi: 10.1128/mcb.5.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Takahashi M., Altschmied L., Hillen W. Kinetic and equilibrium characterization of the Tet repressor-tetracycline complex by fluorescence measurements. Evidence for divalent metal ion requirement and energy transfer. J Mol Biol. 1986 Feb 5;187(3):341–348. doi: 10.1016/0022-2836(86)90437-7. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Hayes L. S., Lloyd D. B. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991 Jul 22;103(2):171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Wyborski D. L., Short J. M. Analysis of inducers of the E.coli lac repressor system in mammalian cells and whole animals. Nucleic Acids Res. 1991 Sep 11;19(17):4647–4653. doi: 10.1093/nar/19.17.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]