Abstract
Objective
The present study investigated the differences in corpus callosum (CC) volumes between women with early stage and late stage bipolar I (BP I) disorder using the criteria previously described in the literature.
Method
We compared women with early and late stage BP I using criteria described in the Staging Systems Task Force Report of the International Society for Bipolar Disorders. We included 20 patients with early stage and 21 patients with late stage BP Iand a group of 25 healthy controls. Patients and controls underwent structural magnetic resonance imaging. Information on the clinical features of bipolar disorder was collected using a standardized questionnaire. Anatomical volumes of 5 regions of CC were compared between the three groups.
Results
Women with late-stage BP I disorder had reduced posterior CC volumes compared to early stage bipolar I patients and controls (F=6.05; P=0.004). The difference was significant after controlling for age, comorbidity with post-traumatic stress disorder, psychotic symptoms during mood episodes, and current use of medication.
Conclusion
The posterior CC was significantly decreased in volume in women with late-stage bipolar disorder. These findings suggest that CC may be an anatomical target of neuroprogression in the course of bipolar disorder in women.
Introduction
Bipolar disorder has been known since early descriptions to be characterized by an accelerating and progressive course (1): later stages are thought to be characterized by reduced inter-episodic intervals (2, 3), lower responsiveness to pharmacological treatments and psychotherapy (4-6), and an augmented rate of hospitalizations (7). Different stages of the disorder might require different approaches (8), therefore a better understanding of the progressive nature of the disorder as it advances through stages may be necessary to gain further insight into its pathophysiology and possible treatment interventions (9). Cognitive deficits also seem to worsen as illness duration and the number of affective episodes increase (10-12), suggesting underlying pathologic changes in the brain. A recent Task-force report proposed a distinction between an early and a late stage bipolar disorder. Accordingly, late-stage would be characterized by low functionality, unremitting illness and higher number of episodes and hospitalizations (8). It is possible that the lower response to treatment observed in late-stage may be related to neuroprogression, described as the functional and anatomical changes that take place in the brain during the course of illness (13). Relatively few neuroimaging studies have investigated the specific ways in which brain networks are affected in different stages of illness (13, 14).
The corpus callosum (CC) is the largest white matter structure in the brain. It is a bundle of white matter fibers that connects the two cerebral hemispheres. It is involved in the integration of processes related to language, attention, arousal, memory, emotion and sensory-motor functions (15-17). The CC seems to be particularly vulnerable to perceived stress (18) and has been shown to be affected by stressful events and trauma (19, 20). Specifically, reductions in CC volume have been demonstrated in both children (21) and adults with posttraumatic stress disorder (PTSD)(22, 23), particularly in the posterior part of the corpus callosum (22).
The repeated episodes that characterize bipolar disorder are stressful experiences that require an adaptive response from physiological systems, and they are associated with cumulative disruptive effects on the brain that could be responsible for the progressive course of bipolar disorder (24). Since the CC is a structure which is vulnerable to stress, it might be progressively damaged by repeated episodes of bipolar disorder.
Indeed, alterations of the integrity of the corpus callosum have been repeatedly reported in bipolar disorder literature (25-28). These findings have been correlated in some studies to specific features of bipolar disorder like presence of psychotic symptoms (29, 14), impulsivity (only in suicidal bipolar patients)(30) and early onset bipolar disorder (31). Thinning of the CC has been found to show an inverse correlation with length of illness in a study by Walterfang and colleagues (27). However, age also showed a significant inverse correlation with the volume of the CC in this study. The co-linearity between the two variables makes the interpretation of these results difficult, since the thinning of the CC could be a result of illness duration or an interaction between illness duration and aging. Furthermore, the lack of information on the number of episodes and inter-episodic remission prevents considerations on the effects of illness severity and cumulative effects of symptoms.
It has been suggested that neuroprogressive changes might take place in the CC in bipolar patients, and that the cumulative damage of illness episodes might determine greater alterations in late stages of the disorder (32, 33), but this hypothesis has not yet been tested.
Aims of the study
The aim of the present study was to compare differences in volumes in five regions of interest in the corpus callosum (CC) in women with early and late stage bipolar I disorder (BP I).
Material and methods
Participants
Subjects were recruited at the outpatient service of the mood disorders Centre of the University of Texas Health Science Center at San Antonio (TX). Although a recent study has not found any significant sex-by-diagnosis interaction in bipolar disorder (34), differences in the corpus callosum between men and women have been found by several studies (35-39). In order to maximize the power of the study we chose to reduce the amount of variance due to factors unrelated to the tested hypothesis by recruiting only women. We enrolled adult females from 19 to 65 years of age in three groups: two groups of patients with bipolar I disorder (early stage and late stage), and one group of controls. Clinicians diagnosed patients according to DSM-IV-TR criteria (40) and the Structured Clinical Interview for DSM-IV axis I disorders (SCID-I)(41). Investigators also administered the Hamilton Depression Rating Scale (HDRS) and the Young Mania Rating Scale Score (YMRS) to all subjects (42, 43). We defined as “late stage” patients that fulfilled all these three staging criteria that have been shown in the literature to predict poorer outcome: they had more than 10 episodes (44); incomplete remission (45) and at least one hospitalization (46). Patients who did not fulfil these criteria were considered “early stage”. We recruited controls in the same age range through advertisement in the community. Subjects with history of neurological disorders, head trauma, current major medical illness or any contraindication for magnetic resonance imaging (MRI) were excluded. Socio-demographic information was collected at baseline; all participants provided written informed consent after being given a complete description of the study. The institutional Review Board of the University of Texas Health Science Center at San Antonio approved the study.
MRI data acquisition
We acquired structural T1-weighted scans using a 1.5 Tesla MRI Philips scanner (Philips Medical System, Andover, MA) using a three-dimensional axial T1-weighted fast field echo sequence with the following parameters: repetition time (TR) = 24 ms, echo time (TE) = 5 ms, flip angle = 40°, field of view (FOW) = 256 mm, slice thickness = 1 mm, matrix size = 256X256 and 150 slices.
MRI data pre-processing
All scans were visually inspected to rule out gross artifacts. The Freesurfer software library (http://surfer.nmr.mgh.harvard.edu/) Version 5.3.0 (47) was used segment the CC through an automated process which is described elsewhere (48, 49). The automated CC segmentation resulted into five CC parcellations, namely: anterior, mid-anterior, central, mid-posterior and posterior. We tested these regions separately to better define anatomically the site of possible alterations in bipolar patients, since alterations in different parts of the corpus callosum are thought to affect connections between specific cortical regions (20). CC volumes were normalized with the intracranial volume (ICV) for each subject before group-level statistical analyses were undertaken.
Statistical analysis
We conducted all statistical analyses using SPSS 20.0 (SPSS Inc., Chicago, Illinois, USA). To compare demographic continuous variable differences between groups, analysis of variance (ANOVA) was used. For each CC measurement, one-way ANOVA was used to compare CC volumes between patients with early stage bipolar disorder, patients with late stage bipolar disorder and healthy controls. Subsequently, for each CC measurement for which a significant group difference was found in the ANOVA a general linear model (GLM) was fitted with Group as fixed factor, CC measurement as dependent variable, age as covariate, history of psychotic features during mood episodes, comorbid post-traumatic stress disorder (PTSD) diagnosis and current medication status as categorical variables of no interest. The GLM analysis was performed to control for these factors, which have been shown to be linked to alterations in the CC (22, 29). P values < than 0.05 were considered significant. Since we performed 5 tests (one for each CC sub-region), correction for multiple comparison (Bonferroni) was used. Therefore, the corrected P value for significance was considered to be 0.01.
Results
Demographics
The socio-demographic characteristics of the subjects at the time of enrollment are reported in Table 1. Mean age and years of education were as follows: healthy controls=40±13, 15±3; early stage bipolar I patients=39±11, 14±1; late stage bipolar I patients=39±11, 14±6. The three groups did not differ significantly regarding these variables (ANOVA for age: F=0.83, P=0.44; ANOVA for years of education: F=0.04; P=0.95). In the early stage bipolar group 11 patients were treated with medication, in the late stage group 16 patients were treated at the time of assessment. Psychotropic drugs administered to early stage bipolar patients included: anticonvulsants (5 patients), antidepressants (3 patients), atypical antipsychotics (4 patients) and benzodiazepines (2 patients); late stage patients were treated with lithium (1 patient), anticonvulsants (6 patients), antidepressants (4 patients), atypical antipsychotics (5 patients), benzodiazepines (6 patients). All the bipolar patients had been previously treated pharmacologically.
Table 1.
Socio-demographic and clinical characteristics of patients. YMRS = Young Mania Rating Scale; BP I = bipolar I. For all rows mean ± SD are reported, except: N° Psychotic features = N° of patients with Psychotic features during mood episodes (%of the whole group); N° PTSD = N° of patients with comorbid PTSD (%of the whole group).
| BP I early stage (n=20) | BP I late stage (n=21) | |
|---|---|---|
| Age (years) | 39±11 | 39±11 |
| Years of education | 14±1 | 14±6 |
| Age of onset (years) | 21±8 | 24±9 |
| Psychiatric hospitalizations |
0 | 3 |
| YMRS | 8±7 | 8±7 |
| HAMD | 17±6 | 16±9 |
| N° Psychotic features | 7 (35%) | 9 (43%) |
| N° PTSD | 7 (35%) | 4 (19%) |
Group differences in CC measurements
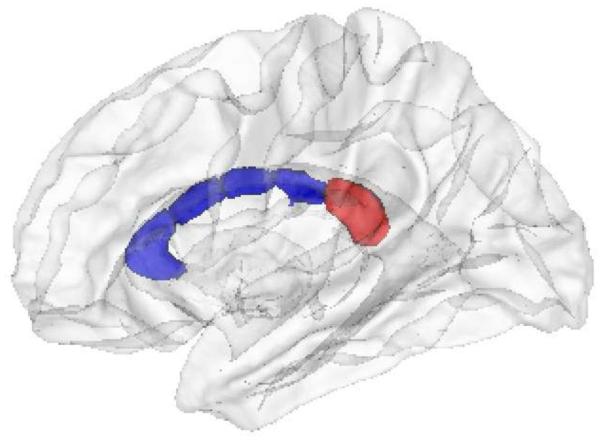
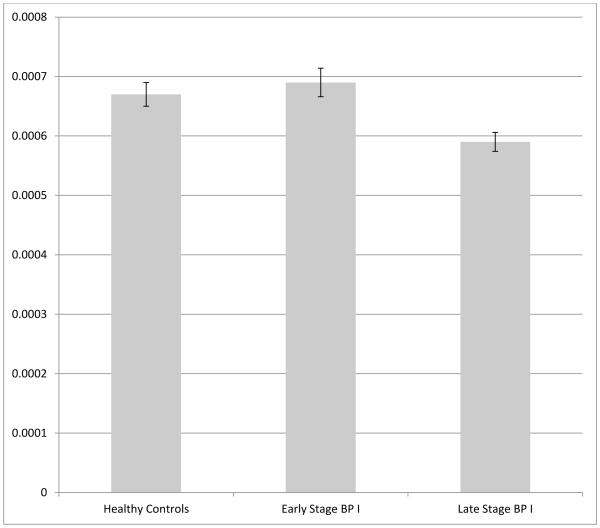
The measurements of the CC volumes for the five CC regions in the three groups are summarized in Table 2. The ANOVA testing the difference between the three groups is significant for comparisons between the three groups in the mid-anterior region (F=3.39; P=0.04) and in the posterior region (F=6.05; P=0.004). After performing a Bonferroni correction for five comparisons (the five CC regions), only the posterior CC remained significant. The posterior CC defined by Freesurfer is represented in Figure 1. In this region, post-hoc analyses revealed a significant difference for the contrast of late-stage BP I< healthy controls (P=0.031) and late-stage BP I < early stage BP I (P=0.005). Group differences in posterior CC volumes are represented in Figure 2. This analysis was performed again after we excluded the patient treated with lithium, in the light of the previously described neurotrophic effects of this drug (33). The significant difference between groups still held.
Table 2.
Measurements of the corpus callosum (CC) in the three groups. Raw values normalized for intracranial volume (ICV) are expressed in mm3
| Healthy controls (n=25) Mean ± SD |
Early stage BP I (n=20) Mean ± SD |
Late stage BP I (n=21) Mean ± SD |
F | P (uncorrected for number of regions) |
|
|---|---|---|---|---|---|
| Anterior CC | 0.00062 ± 0.0001 | 0.00061 ± 0.00008 | 0.00056 ± 0.00008 | 2.48 | .09 |
| Mid-anterior CC | 0.00032 ± 0.00005 | 0.00033 ± 0.00007 | 0.00028 ± 0.00005 | 3.39 | .04 |
| Central CC | 0.00031 ± 0.00004 | 0.00032 ± 0.00005 | 0.00029 ± 0.00005 | 1.56 | .21 |
| Mid-posterior CC | 0.0003 ± 0.00007 | 0.0003 ± 0.00005 | 0.00027 ± 0.00005 | 1.65 | .19 |
| Posterior CC | 0.00067 ± 0.0001 | 0.00069 ± 0.0001 | 0.00059 ± 0.00007 | 6.05 | .004* |
significant difference in the ANOVA (Late stage BP I < Healthy controls; Late stage BP I < Early stage BP I; the difference is still significant after Bonferroni correction for multiple comparison, see text for details).
Figure 1.
The corpus callosum (CC). The posterior CC as defined in our analyses is highlighted in red.
Figure 2.
Volumes in the posterior corpus callosum (CC) in the three groups of women: healthy controls, early stage bipolar I disorder, late stage bipolar I disorder. CC volumes of each subject were normalized with the individual intracranial volume (ICV). Bars represent the mean raw volumes for the groups, error bars represent the standard error.
The GLM model was also performed to test for group differences in posterior CC volumes controlling for age, psychotic symptoms during mood episodes, comorbid PTSD diagnosis, current medication status (treated vs not currently treated pharmacologically). The difference between groups was still significant after we controlled for these factors.
Discussion
The present study showed that women with late-stage bipolar I disorder present a decrease in posterior CC volume compared to controls and early stage bipolar I subjects.
The current data confirm other lines of evidence that support a staging model of bipolar disorder. Bipolar disorder has been shown to have a progressive course in some cases (13). Biological processes that involve cytokines, neurotrophins and mediators of oxidative stress are likely to contribute in the brain changes that take place in the course of bipolar disorder (50). Recent analyses suggest that interleukin 6 (IL-6), and inflammatory mediator, could be a useful biomarker for the characterization of the progression of bipolar disorder (51). The glycogen synthase kinase 3 (GSK3) is an enzyme that is involved in maintaining microtubule stability in neurons (52), and the effects of lithium are thought to be mediated by the inhibition of GSK3 and the promotion of myelination (53). Future studies should investigate the presence of changes in GSK3 function in different stages of bipolar disorder and how they correlate with white matter integrity and response to treatment.
In our sample the clinical features that were associated with alterations in the CC in previous studies were carefully controlled for (ICV, co-morbid PTSD, psychotic symptoms during mood episodes, early onsetand pharmacological treatment). In this sense, the changes that we identified seem to be independently associated with late-stage BP I. The corpus callosum is the largest white matter tract in the brain, and it is an area known to be vulnerable to the effects of stress, as the literature on PTSD seems to suggest (22, 23). As expected, the sustained wear and tear that bipolar disorder imposes on the brain with its repeated episodes and the subsequent activation of multiple biochemical mechanisms (24) seems to affect the volume of this brain region.
Several studies have pointed to the role of CC abnormalities in creating deficits of inter-hemispheric connectivity in bipolar disorder (29, 54, 55). Thinning of parts of the corpus callosum, albeit in different portions of it, was found to be correlated with the duration of illness in a previous study (27). While in this study length of illness tended to co-vary with age, in our study the groups were balanced with regards to age and years of education. The methodological approach we used allowed to test the effect of repeated episodes of illness without confounding from age. In this manner, we were able to confirm a specific effect of stage of illness in the volume changes in CC.
A universally accepted definition of “late stage” is not currently available (9), therefore we had to choose between several “proxy” measures of staging available in the literature (8). According to Cosci and Fava the purpose of staging is to find the most effective and less invasive treatment for each stage of illness (56): the issue of how staging can predict prognosis and response to treatment is therefore central to the staging effort. Cosci and Fava emphasize the importance of number of recurrences and residual symptoms in staging models (56). In order to define our staging criteria, we looked for recent longitudinal studies assessing predictors of treatment response in bipolar disorder using large samples. Multiple episodes (>10) of bipolar disorder were found to predict poorer prognosis in an analysis of the STEP-BD database (n=3345)(44). In our sample several patients had more than 10 episodes and incomplete remission, and it was not possible to quantify the precise number of episodes. We are aware that other meaningful definitions of “early” and “late” stage bipolar disorder are possible and defensible. Only with large, prospective studies that investigate which of these classifications show the best correlation with clinical outcomes it will be possible to settle the issue. However, there is an emerging body of evidence supporting the definition of late stage used in the present study. Incomplete remission has emerged as one of the criteria of prognosis together with episode density in a recent study (45). In another recent study in a large sample (n=3762) hospitalizations predicted a negative response to lithium: the rate of non-response was increased by 3% for every psychiatric hospitalization before first being treated with lithium (46). Thus, we selected multiple episodes, incomplete remission and at least one hospitalization to characterize patients as late stage bipolar disorder.
While other white matter alterations in bipolar patients have been found to be present in both patients and their first-degree relatives (e.g. frontolimbic and frontothalamic white matter connectivity) (54), alterations in the corpus callosum seem to be specific to disease expression, differentiating bipolar patients from first degree relatives (54, 27). Our findings suggest that repeated illness episodes and later stages of illness seem to be more correlated with CC alterations than bipolar disorder diagnosis in itself, confirming that CC anomalies could be a marker of illness progression. Alterations in the CC are also present in children with bipolar disorder, and might be linked to different mechanisms like abnormal myelination during development (57).
Some limitations should be considered in interpreting our results. The lack of diffusion tensor imaging data (DTI) which is able to quantify tissue diffusivity prevents us from studying more specific aspects of CC white matter fiber anatomy such as orientation and integrity. Another limitation is related to the lack of data on early traumatic experiences in our sample. Early traumatic events (e.g. maltreatment during childhood) have been found in a recent study by Bücker and colleagues to be correlated to alterations in CC volumes independently of the development of PTSD in a population of patients with bipolar I disorder (32). The study did not include a sample of controls with early trauma, therefore it is at present not possible to establish whether the observed effect was due to a specific interaction of trauma with bipolar disorder or to an independent effect of childhood maltreatment per se. Nevertheless, the authors conclude that there could be two main causes of CC volume reduction in bipolar patients: early trauma, and a further reduction due to the effects of illness, which is compatible to our findings.
Arguably, the main limitation of our study is its cross-sectional design. In order to establish a causal effect of stage of illness on CC volumes a longitudinal design would be needed. The fact that we did not find significant alterations in early stage bipolar patients suggests that the cumulative effects of the illness process on the brain could require many years and repeated illness episodes to become evident. Longitudinal changes in gray and white matter density over a period of 4 years were estimated in a prospective cohort study of subjects with bipolar I disorder by Moorhead and colleagues (58) and no significant changes in white matter density were found. Longitudinal studies covering significant periods of time are probably necessary to definitively establish the effects of disease stage on brain structures (59).
In conclusion, the present study showed a decrease in volume of the posterior CC in late-stage bipolar disorder as compared to healthy controls and to patients in the early stage of illness. Late-stage bipolar patients have a poorer response to treatment. Efforts to gain a better understanding of the biological mechanisms related to illness progression will hopefully lead into more targeted approaches to treat and prevent the progression of BP I (60).
Significant Outcomes.
Late-stage of bipolar disorder seems to be characterized by more pronounced volumetric changes in corpus callosum (CC).
Changes in CC may contribute to features of late-stage bipolar, such as higher rates of refractoriness and poorer prognosis.
Volumetric changes in CC may provide a direct means to assess staging in bipolar disorder ( BP) I.
Limitations.
The study is cross-sectional, therefore inferences on causal mechanisms of disease processes should be considered with caution.
Information on early traumatic events, that have been shown to be associated with alterations in the CC, was not available.
The study design does not allow for considerations as to whether the observed changes are trait markers of more severe presentations of BP I in women or neuroprogressive changes that took place in the course of illness.
Acknowledgements
Supported in part by NIMH grant R01 085667, the Dunn Foundation and the Pat Rutherford, Jr. Endowed Chair in Psychiatry (Jair C. Soares).
Prof. Soares has participated in research funded by Forest, Merck, BMS, GSK and has been a speaker for Pfizer and Abbott.Prof. Kapczinski has received grant/research support from Astra-Zeneca, Eli Lilly, the Janssen-Cilag, Servier, CNPq, CAPES, NARSAD and the Stanley Medical Research Institute; has been a member of the speakers’ boards for Astra-Zeneca, Eli Lilly, Janssen and Servier and has served as a consultant for Servier.Dr. Marsal Sanches has served on the speakers' bureau for AstraZeneca and has received research grants from Janssen.
Footnotes
Declaration of interest
All other authors declare that they have no conflicts of interest.
References
- 1.KRAEPELIN E. Manic Depressive Insanity and Paranoia. Thoemmes Press; Bristol: 1921. [Google Scholar]
- 2.KESSING LV, ANDERSEN PK, MORTENSEN PB. Predictors of recurrence in affective disorder: a case register study. J Affect Disord. 1998;49:101–108. doi: 10.1016/s0165-0327(97)00163-8. [DOI] [PubMed] [Google Scholar]
- 3.ROY-BYRNE P, POST RM, UHDE TW, PORCU T, DAVIS D. The longitudinal course of recurrent affective illness: life chart data from research patients at the NIMH. Acta Psychiatr Scand. 1985;317:1–34. doi: 10.1111/j.1600-0447.1985.tb10510.x. [DOI] [PubMed] [Google Scholar]
- 4.KETTER TA, HOUSTON JP, ADAMS DH, et al. Differential efficacy of olanzapine and lithium in preventing manic or mixed recurrence in patients with bipolar I disorder based on number of previous manic or mixed episodes. J Clin Psychiatry. 2006;67:95–101. doi: 10.4088/jcp.v67n0113. [DOI] [PubMed] [Google Scholar]
- 5.SWANN AC, BOWDEN CL, CALABRESE JR, DILSAVER SC, MORRIS DD. Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry. 1999;156:1264–6. doi: 10.1176/ajp.156.8.1264. [DOI] [PubMed] [Google Scholar]
- 6.SCOTT J, PAYKEL E, MORRISS R, et al. Cognitive-behavioural therapy for severe and recurrent bipolar disorders: randomised controlled trial. Br J Psychiatry. 2006;188:313–20. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- 7.GOLDBERG JF, ERNST CL. Features associated with the delayed initiation of mood stabilizers at illness onset in bipolar disorder. J Clin Psychiatry. 2002;63:985–91. doi: 10.4088/jcp.v63n1105. [DOI] [PubMed] [Google Scholar]
- 8.KAPCZINSKI F, MAGALHAES PV, BALANZA’-MARTINEZ V, et al. Staging systems in bipolar disorder: an International Society for Bipolar Disorders Task Force Report. Acta Psychiatr Scand. 2014;130:354–363. doi: 10.1111/acps.12305. [DOI] [PubMed] [Google Scholar]
- 9.GAMA CS, KUNZ M, MAGALHAES PV, KAPCZINSKI F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Rev Bras Psiquiatr. 2013;35:70–4. doi: 10.1016/j.rbp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 10.LEBOWITZ BK, SHEAR PK, STEED MA, STRAKOWSKI, SM. Verbal fluency in mania: relationship to number of manic episodes. Neuropsychiatry Neuropsychol Behavioral Neurol. 2001;14:177–182. [PubMed] [Google Scholar]
- 11.DONALDSON S, GOLDSTEIN LH, LANDAU S, RAYMONT V, FRANGOU S. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry. 2003;64:86–93. [PubMed] [Google Scholar]
- 12.DENICOFF KD, ALI SO, MIRSKY AF, et al. Relationship between prior course of illness and neuropsychological functioning in patients with bipolar disorder. J Affect Disord. 1999;56:67–73. doi: 10.1016/s0165-0327(99)00028-2. [DOI] [PubMed] [Google Scholar]
- 13.FRIES GR, PFAFFENSELLER B, STERTZ L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14:667–75. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 14.SCHNEIDER MR, DELBELLO MP, MCNAMARA RK, STRAKOWSKI SM, ADLER CM. Neuroprogression in bipolar disorder. Bipolar Disord. 2012;14:356–74. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 15.DORON KW, GAZZANIGA MS. Neuroimaging techniques offer new perspectives on callosal transfer and interhemispheric communication. Cortex. 2008;44:1023–9. doi: 10.1016/j.cortex.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 16.HELLIGE JB, TAYLOR KB, LESMES L, PETERSON, S. Relationships between brain morphology and behavioral measures of hemispheric asymmetry and interhemispheric interaction. Brain Cogn. 1998;36:158–192. doi: 10.1006/brcg.1997.0951. [DOI] [PubMed] [Google Scholar]
- 17.BADARUDDIN DH, ANDREWS GL, BOLTE S, et al. Social and behavioral problems of children with agenesis of the corpus callosum. Child Psychiatry Human Develop. 2007;38:287–302. doi: 10.1007/s10578-007-0065-6. [DOI] [PubMed] [Google Scholar]
- 18.LI H, L W, WEI D, et al. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage. 2014;92:1–7. doi: 10.1016/j.neuroimage.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 19.RINNE-ALBERS MA, VANDER WEENJ, LAMERS-WINKELMAN F, VERMEIREN RR. Neuroimagingingin children, adolescents and young adults with psychological trauma. Eur Child Adolesc Psychiatry. 2013;22:754–755. doi: 10.1007/s00787-013-0410-1. [DOI] [PubMed] [Google Scholar]
- 20.JACKOWSKI AP, DOUGLAS-PALUMBERI H, JAKOWSKI M, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162:256–61. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DE BELLIS MD, KESHAVAN MS, SHIFFLETT H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a socio-demographically matched study. Biol Psychiatry. 2002;52:1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 22.KITAYAMA N, BRUMMER M, HERTZ L, QUINN S, KIM Y, BREMNER JD. Morphologic alterations in the corpus callosum in abuse-related posttraumatic stress disorder: a preliminary study. J Nerv Ment Dis. 2007;195:1027–9. doi: 10.1097/NMD.0b013e31815c044f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VILLARREAL G, HAMILTON DA, GRAHAM DP, et al. Reduced area of the corpus callosum in posttraumatic stress disorder. Psychiatry Res. 2004;131:227–35. doi: 10.1016/j.pscychresns.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 24.KAPCZINSKI F, VIETA E, ANDREAZZA AC, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–92. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 25.WANG F, KALMAR JH, EDMISTON E, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–3. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATMACA M, OZDEMIR H, YILDIRIM H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. 2007;7:699–704. doi: 10.1017/S0033291706009743. [DOI] [PubMed] [Google Scholar]
- 27.WALTERFANG M, MALHI GS, WOOD AG, et al. Corpus callosum size and shape in established bipolar affective disorder. Aust N Z J Psychiatry. 2009;43:838–45. doi: 10.1080/00048670903107534. [DOI] [PubMed] [Google Scholar]
- 28.LLOYD AJ, ALI HE, NESBITT D, MOORE PB, YOUNG AH, FERRIER IN. Corpus callosum changes in euthymic bipolar affective disorder. Br J Psychiatry. 2014;204:129–36. doi: 10.1192/bjp.bp.112.123687. [DOI] [PubMed] [Google Scholar]
- 29.SARRAZIN S, POUPON C, LINKE J, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA Psychiatry. 2014;71:388–96. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- 30.MATSUO K, NIELSEN N, NICOLETTI MA, et al. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci Lett. 2010;469:75–80. doi: 10.1016/j.neulet.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BARNEA-GORALY N, CHANG KD, KARCHEMSKIY A, HOWE ME, REISS AL. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66:238–44. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 32.BUCKER J, MURALIDHARAN K, TORRES IJ, et al. Childhood maltreatment and corpus callosum volume in recently diagnosed patients with bipolar I disorder: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) J Psychiatr Res. 2014;48:65–72. doi: 10.1016/j.jpsychires.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 33.EMSELL L, LANGAN C, VAN HECKE W, et al. White matter differences in euthymic bipolar Idisorder: a combined magnetic resonance imaging and diffusion tensor imaging voxel-based study. Bipolar Disord. 2013;15:365–76. doi: 10.1111/bdi.12073. [DOI] [PubMed] [Google Scholar]
- 34.JOGIA J, DIMA D, FRANGOU S. Sex differences in bipolar disorder: a review of neuroimaging findings and new evidence. Bipolar Disord. 2012;14:461–71. doi: 10.1111/j.1399-5618.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 35.ALLEN LS, RICHEY MF, CHAI YM, GORSKI RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–42. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LEE BY, SOHN JH, CHOI MH, et al. A volumetric study of the corpus callosum in 20s and 40s Korean people. Brain Struct Funct. 2009:463–7. doi: 10.1007/s00429-009-0209-5. [DOI] [PubMed] [Google Scholar]
- 37.LUDERS E, REX DE, NARR KL, et al. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;10:1084–93. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- 38.SHIN YW, KIM DJ, HA TH, et al. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport. 2005;16:795–8. doi: 10.1097/00001756-200505310-00003. [DOI] [PubMed] [Google Scholar]
- 39.SULLIVAN EV, ROSENBLOOM MJ, DESMOND JE, PFEFFERBAUM A. Sex differences in corpus callosum size: relationship to age and intracranial size. Neurobiol Aging. 2001;22:603–11. doi: 10.1016/s0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association . Diagnostic and statistical manual for mental disorders. 4th American Psychiatric Press; Arlington: 2000. [Google Scholar]
- 41.FIRST MB, SPITZER RL, GIBBON M, et al. Structured clinical interview for DSM-IV axis I disorders (SCID-I), Clinician version administration booklet. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- 42.WILLIAMS JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 43.YOUNG RC, BIGGS JT, ZIEGLER VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 44.MAGALHAES PV, DODD S, NIERENBERG AA, BERK M. Cumulative morbidity and prognostic staging of illness in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Aust N Z J Psychiatry. 2012;46:1058–1067. doi: 10.1177/0004867412460593. [DOI] [PubMed] [Google Scholar]
- 45.REINARES M, PAPACHRISTOU E, HARVEY P, et al. Towards a clinical staging for bipolar disorder: defining patient subtypes based on functional outcome. J Affect Disord. 2013;144:65–71. doi: 10.1016/j.jad.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 46.KESSING LV, HELLMUND G, ANDERSEN PK. Predictors of excellent response to lithium: results from a nationwide register-based study. Int Clin Psychopharmacol. 2011;26:323–8. doi: 10.1097/YIC.0b013e32834a5cd0. [DOI] [PubMed] [Google Scholar]
- 47.FISCHL B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ROSAS HD, LEE SY, BENDER AC, et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection". Neuroimage. 2010;49:2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DESIKAN RS, SEGONNE F, FISCHL B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 50.BERK M, KAPCZINSKI F, ANDREAZZA AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 51.GRANDE I, MAGALHAES PV, CHENDO I, et al. Staging bipolar disorder: clinical, biochemical, and functional correlates. Acta Psychiatr Scand. 2014;129:437–44. doi: 10.1111/acps.12268. [DOI] [PubMed] [Google Scholar]
- 52.FORDE JE, DALE TC. Glycogen synthase kinase 3: a key regulator of cellular fate. Cell Mol Life Sci. 2007;64:1930–44. doi: 10.1007/s00018-007-7045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MARLINGE E, BELLIVIER F, HOUENOU J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97–112. doi: 10.1111/bdi.12135. [DOI] [PubMed] [Google Scholar]
- 54.LINKE J, KING AV, POUPON C, HENNERICI MG, GASS A, WESSA M. Impaired anatomical connectivity and related executive functions: differentiating vulnerability and disease marker in bipolar disorder. Biol Psychiatry. 2013;74:908–16. doi: 10.1016/j.biopsych.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 55.PETTIGREW JD, MILLER SM. A 'sticky' interhemispheric switch in bipolar disorder? Proc Biol Sci. 1998;265:2141–8. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.COSCI F, FAVA GA. Staging of mental disorders: systematic review. Psychother Psychosom. 2013;82:20–34. doi: 10.1159/000342243. [DOI] [PubMed] [Google Scholar]
- 57.CAETANO SC, SILVEIRA CM, KAUR S, et al. Abnormal corpus callosum myelination in pediatric bipolar patients. J Affect Disord. 2008;108:297–301. doi: 10.1016/j.jad.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MOORHEAD TW, MCKIRDY J, SUSSMANN JE, et al. Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry. 2007;62:894–900. doi: 10.1016/j.biopsych.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 59.LIM CS, BALDESSARINI RJ, VIETA E, et al. Longitudinal neuroimaging and neuropsychological changes in bipolar disorder patients: review of the evidence. Neurosci Biobehav Rev. 2013;37:418–35. doi: 10.1016/j.neubiorev.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 60.VIETA E. The bipolar maze: a roadmap through translational psychopathology. Acta Psychiatr Scand. 2014;129:323–7. doi: 10.1111/acps.12270. [DOI] [PubMed] [Google Scholar]