Abstract
Purpose of Review
Advanced MRI post-processing techniques are increasingly used to complement visual analysis and elucidate structural epileptogenic lesions. This review summarizes recent developments in MRI post-processing in the context of epilepsy pre-surgical evaluation, with the focus on patients with unremarkable MRI by visual analysis (i.e., “nonlesional” MRI).
Recent Findings
Various methods of MRI post-processing have been reported to show additional clinical values in the following areas: (1) lesion detection on an individual level; (2) lesion confirmation for reducing the risk of over reading the MRI; (3) detection of sulcal/gyral morphologic changes that are particularly difficult for visual analysis; and (4) delineation of cortical abnormalities extending beyond the visible lesion. Future directions to improve performance of MRI post-processing include using higher magnetic field strength for better signal and contrast to noise ratio, adopting a multi-contrast frame work, and integration with other noninvasive modalities.
Summary
MRI post-processing can provide essential value to increase the yield of structural MRI and should be included as part of the presurgical evaluation of nonlesional epilepsies. MRI post-processing allows for more accurate identification/delineation of cortical abnormalities, which should then be more confidently targeted and mapped.
Keywords: Epilepsy, nonlesional, MRI-negative, Post-processing, voxel based morphometry, 7T MRI, focal cortical dysplasia, malformations of cortical development, polymicrogyria, surgery
Introduction
In the presurgical evaluation of drug-refractory epilepsies, the role of MRI is of paramount importance. Accurately detecting lesions and delineating extent of lesions minimizes the amount of brain that needs to be resected and improves the probability of seizure-free outcome [1]. Although visual analysis of high-resolution MRI can already detect a fair number of epileptic lesions, such as hippocampal sclerosis (HS) and Type IIB focal cortical dysplasia (FCD), subtle FCD lesions are missed in up to 30% of surgical candidates [2]. These patients are therefore incorrectly labeled as MR-negative or “non-lesional”. MRI-negative patients tend to have poor surgical outcomes [1,3,4]; consequently they are generally not considered favorable surgical candidates and may not even be referred for potentially “curative” surgery [5].
MRI post-processing carries significant advantages for the detection of subtle lesions: (1) it can be applied without any a priori information on the whole brain; (2) it operates in 3D and allows for simultaneous consideration of information from consecutive slices of the brain, which may not be obvious for visual analysis; (3) Due to the quantitative and objective nature of post-processing analysis, it can be used in a consistent fashion with little operator dependency. Here we provide a review of the additional clinical value provided by the various methods of MRI post-processing in the context of epilepsy pre-surgical evaluation, especially for patients with unremarkable MRI by visual analysis (i.e., “nonlesional” patients).
Epilepsy MRI Protocol and General Image Processing Workflow
Common sequences in a MRI epilepsy protocol include a 3D T1-weighted (T1w) volumetric acquisition, a T2-weighted (T2w) fluid-attenuation inversion recovery (FLAIR) acquisition and other inversion recovery acquisitions. The entire brain should be covered with thin slices, usually oriented perpendicular to the long axis of the hippocampus. Parameters of the 1.5T and 3T epilepsy protocol from our institute are reported in our previous study [6]. The most useful and routinely used sequence for image processing is the T1-weighted volumetric acquisition with contiguous thin slices, which provides high-resolution anatomical details and can be reformatted to any plane. A general first step for the various methods of image processing is correction of MR field inhomogeneity, followed by spatial alignment of the MRI to a 3D common stereotactic space [7]. Subsequently, each voxel is classified into gray matter (GM), white matter (WM), or cerebrospinal fluid. After the tissue classification and segmentation, various flavors of further analyses can be carried out.
MRI Post-processing for Lesion Detection
Subtle FCD is the underlying pathology for a significant number of patients with apparently normal MRI [8]. Typical MRI findings of FCD include blurring of gray-white junction, T2/FLAIR signal abnormality, T1 signal abnormality, abnormally thickened cortex and subcortical T2/FLAIR abnormality, and transmantle sign [9,10]. Histopathologically, these MRI features reflect abnormal neuronal accumulation and positioning, demyelination and gliosis [11-14]. The MRI features of FCD can be very subtle, and easily missed when noninvasive data (e.g. scalp EEG) do not point to a specific area. Under these circumstances, help from a whole-brain MRI post-processing technique directing the reader's attention to suspicious abnormalities may prove to be essential.
VBM
Voxel-based morphometry (VBM) is perhaps the most popular post-processing algorithm to date. It is an automated technique that extracts GM and WM maps from individuals to make statistical comparisons to a normal control database [15]. VBM is mostly used to perform group analysis, but its variations can be used to detect cortical abnormalities in individual patients. VBM studies on T1w MRI have demonstrated that in patients with MRI-visible FCD, increase in GM concentration was concordant with the lesion in 63% to 86% of cases [16-21]. Higher sensitivity can be gained through using linear registration algorithms or more complex spatial normalization strategies [21,22], analysis of a combination of GM/WM maps [20], application of a smaller Gaussian kernel [21], or using a lower threshold for statistical testing.
Focke et al. described a voxel-based method that performs spatial and intensity normalization of standard FLAIR images by using parameters derived from a coregistered T1w volume. Their methods demonstrated 88% sensitivity in patients with MRI-visible FCD lesions [23]. The same methodology was applied in a consecutive cohort of 70 patients with normal MRI, and in 8 (11%) the supra threshold clusters were concordant to the electro-clinical findings by video EEG [24]. However, validation with histopathology was not available for both studies.
Computational models of FCD
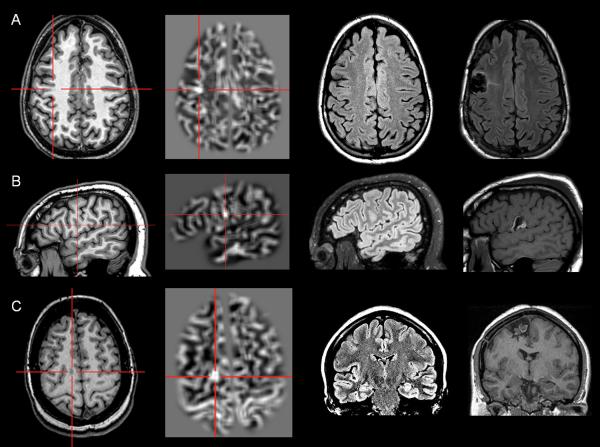
Computer-based models can be generated to search for the distinctive morphologic characteristics of FCD on MRI, such as thickened cortex, blurred gray-white junction and abnormal intensity [25]. Experiences from a number of institutes have demonstrated that gray-white junction blurring can be used as a sensitive and specific feature to detect subtle cortical malformations even on MRI that are considered negative by visual inspection [6,26-31]. Our previous study investigated the use of a Morphometric Analysis Program (MAP) in a consecutive cohort of 150 nonlesional surgical patients, and showed 43% positive rate, sensitivity of 0.9, and specificity of 0.67 [29]. Figure 1 shows examples of three patients with subtle FCD lesions in the frontal lobe, which were not seen by visual analysis but detectable by MAP [27].
Figure 1.
Examples of three patients with MAP+ region; complete resection of the MAP+ region rendered all three patients seizure-free (>12 months). In Figures 1, 2 and 4, the crosshairs pinpoint the location of MAP+ region. First column: T1-weighted MPRAGE images used during pre-surgical evaluation. Second column: gray-white matter junction z-score file, as the output of MAP processing of the T1-weighted image shown in column one. Third column: T2-weighted FLAIR images, chosen to best depict the MAP+ region. Fourth column: post-surgical MRI indicating site and extent of resection. Pathology: A: FCD type IIA; B, FCD type IIB; C: FCD type IIA.
Computational models can also be used to analyze more than one morphological anomalies related to the various forms of FCD [32]. Bernasconi et al. proposed morphologic and first-order texture models creating 3D maps of cortical thickness, gradient (transition between GM and WM), and a relative intensity operator designed to emphasize T1 signal hyperintensity. This model was used on 23 patients with histologically proven FCD and showed that lesions were characterized simultaneously by all three features in 78%, and by two features in 100% [33]. Additionally, these MRI post-processing features occurred not only in large FCD lesions, but also in subtle ones that were previously missed by visual inspection.
Antel et al. reported further development of this approach which consisted of second-order analysis to quantify features not so apparent to the human eye, such as angular second momentum, contrast and difference entropy. These features were used to develop automated FCD recognition using a novel two-stage Bayesian classifier [34]. This fully automated technique detected FCD in 83% of patients (15 of 18), including 4 of 7 lesions that had been missed by conventional MRI analysis.
Recently, Hong et al reported effectiveness of an automated classifier relying on surface-based features of FCD morphology and intensity, taking advantage of their covariance [35]. They reported sensitivity of 74% in a group of 19 patients with extratemporal epilepsy and negative 1.5T/3T MRI. No lesion was falsely detected in controls (24 healthy, 11 temporal love epilepsy). The study showed that the fully automated multivariate approach carries promise to accurately identify FCD among patients initially diagnosed as MRI-negative.
MRI-post-processing for Lesion Confirmation
In the management of nonlesional patients, previously unnoted MRI findings are often identified based on convergent multi-modal data that are gathered in the course of non-invasive presurgical evaluation. While many of these findings may indeed point to previously overlooked lesions, others may reflect “over-reading” of MRI studies, which might have led to potentially biased surgical discussion and electrode implantation. This scenario occurred in 80 of the 150 MRI-negative patients included in our previous study [29]. In these 80 patients, resection of the subtle/questionable MRI abnormality that was visually identified was indeed associated with seizure-free outcome (p=0.014). However, in subgroup analysis, we found that this association was significant only in those patients who were MAP-positive (p=0.01), but was no longer significant in the MAP-negative subgroup (p=0.36). This finding suggests that MRI post-processing methods, given their quantitative and less subjective nature, may be used not only as a “search tool” for subtle lesions, but also as a “confirmation tool” as to whether a suspected questionable lesion indeed represents true positive results.
MRI Post-processing for Sulcal Morphology
Sulcal and gyral abnormalities are characterized by a spectrum of changes ranging from clefts of various depth to broad gyri, shallow or deep sulci, or gyral simplification [36-38]. The identification of subtle sulcal morphologic changes can be quite a difficult task as the branching patterns of the sulci are highly variable. However, studies using models of structure and shape have reported success in identification of cortical abnormalities escaping the human eye.
Besson et al. reported that 85% of small FCD lesions that elude visual inspection were found at the bottom of an abnormally deep sulcus [39] and proposed a surface-based approach which preserved cortical topology while including sulcal depth and curvature in the analysis [40]. This approach was able to detect 89% (17 of 19) of small, histologically proven FCD, which had been overlooked by conventional visual analysis [40].
Regis and colleagues utilized a sulcal root/meridian parallel model for automated extraction, identification, and statistical analysis of sulcal morphology out of normal range [41]. In 12 patients with MRI-negative frontal lobe epilepsy, this model detected subtle subclinical abnormal gyration patterns in the epileptogenic zone in 75% of the patients. Additionally, these subtle patterns were frequently located in the depth of the posterior superior and intermediate frontal sulci, perhaps indicating a critical zone in these locations that are more vulnerable during cortical development.
A recent study by Mellerio et al., using a post-processing tool for 3D visualization of brain sulci, assessed central sulcus variants and particularly a sulcal pattern “power button sign” (defined as the interposition of a precentral sulcal segment between the central sulcus and one of its hook-shaped anterior ascending branches) in 37 patients with histologically proven FCD type II of the central region and 44 healthy control subjects [42]. Their data suggest that central sulcus with the FCD type II patient group present with significantly more side branches and connections with the precentral sulcus and sylvian fissure. The power button sign was found in 62% of total patients (including about 46% of the patients with negative MRI), and in only one control subject. Given the high specificity, the data suggest that the power button sign can perhaps be used as a useful qualitative diagnostic feature in the face of suspected central region FCD.
MRI Post-processing for Lesion Extent
Delineating the extent of abnormality surrounding obvious pathology carries significant clinical relevance as it can guide the extent of surgical resection. Studies using VBM methods [18,20,43-45] and computer-based models [31,34,46] have consistently reported GM abnormalities extending beyond the visible FCD, sometimes distant from the suspected epileptogenic area.
Inspection of the feature maps revealed that these abnormalities exhibited patterns similar to FCD; however, visual analysis of these regions on the original structural MRI could not show any perceivable FCD. These regions may indicate dysplastic abnormalities much more widespread across the hemispheres than the changes visible on the MRI, and may provide a measurement of the potentially epileptic/proepileptic lesion extent [47].
In our own series using the MAP methodology to evaluate a consecutive nonlesional cohort of 150 patients, a small percentage (7%) of the patients had multiple MAP+ regions [29]. When faced with multiple lesions, in the absence of direct electro-clinical correlates, it is difficult to infer on the current or potential epileptic nature of the multiple regions. This issue highlights the importance of interpreting MRI processing results with electro-clinical correlation in the management of these patients [6,46].
Future Directions
Use of Higher Magnetic Field Strength
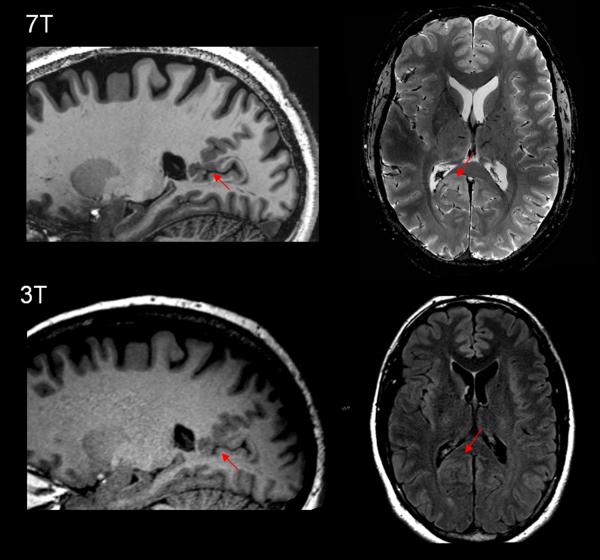
It is conceivable that the increased signal-to-noise and contrast-to-noise ratio and spatial resolution provided by ultra-high-field (7T) MRI could increase the conspicuity of FCD lesions [48,49]. This will also provide the basis for more accurate histopathological correlative studies [50-52]. Figure 2 illustrates a patient with focal epilepsy due to a small FCD/polymicrogyria at the depth of the parietal occipital fissure, which was not previously seen 3T, but became appreciated at 7T. Future studies are needed to confirm the additional diagnostic value of 7T MRI for patients with a negative MRI at lower fields.
Figure 2.
Example of a subtle polymicrogyria/FCD lesion missed by 3T MRI but detected by 7T MRI. Upper row: 7T (MAGNETOM 7T, Siemens) T1w MP2RAGE sequence (left and middle) and T2*w GRE (right). Bottom row: T1w MPRAGE sequence (left and middle) and 3T (SKYRA 3T, Siemens) axial T2w FLAIR (right) from standard epilepsy protocol. The patient is a 37 year-old right-handed male being evaluated for epilepsy surgery, with seizure onset when he was 16. His seizures starts with an aura of feeling hot and dizzy and deja vu, then seizures were complex motor characterized by holding his head, chewing automatism, lip movements, weird hand motions and deep gasping breaths. Postictally he was amnesic and confused. Frequency was cluster of up to 10/day. His 3T MRI did not show any epileptic abnormalities. On 7T MRI T1w MP2RAGE sequence, it was shown that there was likely MCD and possibly PMG at the depth of the parietal occipital fissure, superior to isthmus (first column, arrow). The lesion showed asymmetric signal hyperintensity particularly on axial T2w GRE (second column, arrow). Guided by the 7T finding, we were able to find correlates on the 3T images, but with less conspicuity and only on the exact same slice. This patient is currently undergoing pre-surgical evaluation which will include invasive evaluation to investigate the epileptogenicity of the lesion.
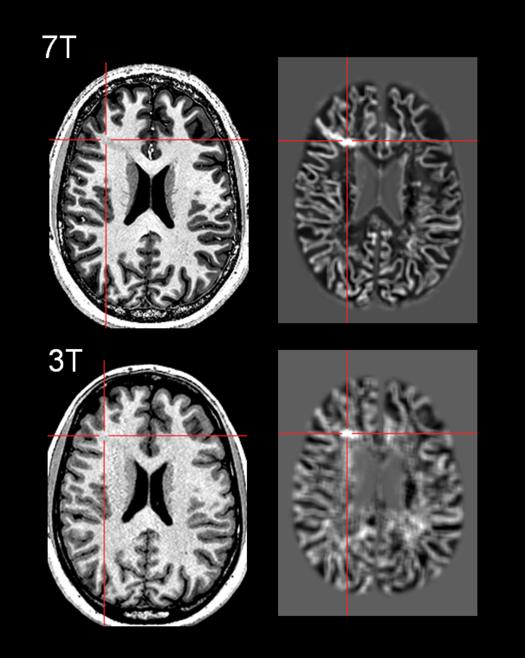
Despite obvious benefits of SNR and in-plane resolution, imaging at 7T exhibits distinct challenges due to B1 field inhomogeneities, causing issues for post-processing. Seiger et al. systematically studied application of VBM on 7T MRI using various pulse sequences, and demonstrated that the MPRAGE sequence needs additional preprocessing pipeline for bias field correction, while the more sophisticated MP2RAGE already accounts for field inhomogeneities and can be used without further conditioning[53]. Their study indicated that the most challenging cortical area for 7T was the inferior cortical regions such as the basal temporal region due to signal dropout. Figure 3 shows an example of our experience using the 7T T1w MP2RAGE sequence as input to MAP post-processing. Both the T1w MP2RAGE and the gray-white junction MAP output at 0.5mm3 voxel resolution showed marked improved conspicuity of the lesion when compared to the 3T images, which in turn resulted in better delineation of the FCD.
Figure 3.
Comparison of 7T and 3T images and MAP post-processing in the same patient with surgically confirmed FCD. Top row: 7T T1w MP2RAGE sequence, and the MAP gray-white junction feature map highlighting the subtle FCD with voxel size=0.5mm3. Bottom row: 3T T1w MPRAGE sequence, and the MAP gray-white junction feature map showing the same lesion, with voxel size=1mm3. The patient is a 21 year old right-handed male with intractable focal epilepsy of right frontal onset. Seizure semiology and ictal EEG (maximum evolvement in the right fronto-central region) were both concordant with location of the MRI lesion in the right middle frontal gyrus. The 3T MRI FCD lesion was further confirmed and re-illustrated by 7T MRI. MRI post-processing using MAP markedly enhanced visualization of blurring in the gray-white boundary. The patient underwent resection of the lesion guided by electrocorticography. FCD Type IA was found in surgical pathology.
Adopt a Multi-contrast framework
The T1-weighted volumetric MRI has been most routinely used as input to MRI post-processing, because it is almost always available as part of the routine epilepsy MRI protocol. T2-weighted images by themselves can be extremely important to delineate the epileptogenic lesion [11,54], and can also be used as input to the MRI post-processing algorithms [55,56]. Quantitative MRI contrasts such as T2 relaxometry, double inversion recovery, and magnetization transfer ratio imaging have yielded 87% to 100% sensitivity in patients with MRI visible FCD, although in nonlesional patients, the sensitivity was reported to be less than 30% [43,57-59]. A multi-contrast framework, therefore, may be necessary to optimize detection yield of MRI post-processing [2]. A recently developed novel MR fingerprinting approach, which uses a pseudorandomized acquisition that permits the simultaneous non-invasive quantification of multiple tissue contrasts, may have the potential to improve the separation of subtle FCD lesions from the normal cortex, as compared with single magnetic parameter contrast [60].
Interpret in the Context of other Electro-clinical Data
One needs to note that any MRI post-processing technique is purely structural and not a direct measure of epileptogenicity. Hence, MRI post-processing findings should always be interpreted in the context of the patient's anatomo-electro-clinical presentation (history, semiology, EEG, MEG, PET or SPECT). Our previous study showed the usefulness of combining MRI post-processing with magnetic source imaging (MSI) to increase the yield in patients with nonlesional MRI [6]. Further correlative studies with electrophysiology / pathology and long-term surgical followup will be necessary to determine the clinical significance of abnormalities detected by MRI post-processing, including those distant to the presumed seizure onset zone.
Conclusions
In the presurgical evaluation of refractory epilepsies, noninvasive localization is of paramount importance and can directly affect invasive evaluation and surgical resection. MRI post-processing, with various variations focusing on different features of subtle brain pathology, can serve as a practical and valuable tool to allow for accurate identification of cortical abnormalities, which should then be more confidently targeted and mapped. It is hopeful that future advancements will allow MRI post-processing to reveal the extent of cortical abnormalities invisible to the eye, and help identify some of the more favorable surgical candidates, thereby improve seizure outcomes in “nonlesional” epilepsies.
Keypoints.
MRI post-processing can detect subtle cortical dysplasia lesions on an individual level.
The quantitative nature of MRI post-processing can be utilized to increase confidence of visually noted questionable abnormalities.
MRI post-processing studies have consistently shown abnormalities beyond the visually perceptible lesion and may offer a measure of the extent of cortical disruption.
Results from MRI post-processing should be considered when planning intracranial EEG implantation and surgical resection.
Acknowledgements
The authors would like to acknowledge Dr. Stephen Jones for his continuous gracious support to the MRI post-processing/imaging research at the Cleveland Clinic Epilepsy Center. The authors would also like to acknowledge Dr. Sehong Oh for his technical expertise on 7T MRI.
Financial Support and Sponsorship
This work was supported in part by NIH NINDS grant R01-NS074980 (Z.I.W.) and JoshProvides Epilepsy Assistance Foundation (Z.I.W.). A.V.A received support from UCB, Pfizer, and the American Epilepsy Society.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
Contributor Information
Z. Irene Wang, Desk S51, Epilepsy Center, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH, 44195, USA..
Andreas V. Alexopoulos, Desk S51, Epilepsy Center, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH, 44195, USA. Phone: 216-444-3629. alexopa@ccf.org. Fax: 216-445-4378..
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as: * of special interest ** of outstanding interest
- 1.Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi A, Bernasconi N, Bernhardt BC, Schrader D. Advances in MRI for ‘cryptogenic’ epilepsies. Nat Rev Neurol. 2011;7:99–108. doi: 10.1038/nrneurol.2010.199. [DOI] [PubMed] [Google Scholar]
- 3.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009;66:1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- 4.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130:574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 5.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65:912–918. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZI, Alexopoulos AV, Jones SE, et al. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann Neurol. 2014;75:759–770. doi: 10.1002/ana.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 8.Wang ZI, Alexopoulos AV, Jones SE, Jaisani Z, Najm IM, Prayson RA. The pathology of magnetic-resonance-imaging-negative epilepsy. Mod Pathol. 2013;26:1051–1058. doi: 10.1038/modpathol.2013.52. [DOI] [PubMed] [Google Scholar]
- 9**.Kuzniecky R. Epilepsy and malformations of cortical development: new developments. Curr Opin Neurol. 2015;28:151–157. doi: 10.1097/WCO.0000000000000175. [An excellent review summarizing recent developments in the classification of cortical dysplasia, in terms of correlation with syndromes, imaging data, as well as genetic and cellular pathway advances.] [DOI] [PubMed] [Google Scholar]
- 10.Krsek P. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann. Neurol. 2008;63:758–769. doi: 10.1002/ana.21398. [DOI] [PubMed] [Google Scholar]
- 11.Urbach H. Focal cortical dysplasia of Taylor's balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia. 2002;43:33–40. doi: 10.1046/j.1528-1157.2002.38201.x. [DOI] [PubMed] [Google Scholar]
- 12.Sisodiya SM, Fauser S, Cross JH, Thom M. Focal cortical dysplasia type II: biological features and clinical perspectives. Lancet Neurol. 2009;8:830–843. doi: 10.1016/S1474-4422(09)70201-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda K. Neuroradiologic findings in focal cortical dysplasia: histologic correlation with surgically resected specimens. Epilepsia. 2001;42:29–36. [PubMed] [Google Scholar]
- 14.Taylor DC, Falconer MA, Bruton CJ, Corsellis JAN. Focal dysplasia of the cerebral cortex in epilepsy. J. Neurol. Neurosurg. Psychiatry. 1971;34:369–387. doi: 10.1136/jnnp.34.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmond CH. Distributional assumptions in voxel-based morphometry. Neuroimage. 2002;17:1027–1030. [PubMed] [Google Scholar]
- 16.Kassubek J, Huppertz HJ, Spreer J, Schulze-Bonhage A. Detection and localization of focal cortical dysplasia by voxel-based 3-D MRI analysis. Epilepsia. 2002;43:596–602. doi: 10.1046/j.1528-1157.2002.41401.x. [DOI] [PubMed] [Google Scholar]
- 17.Merschhemke M, Mitchell TN, Free SL, et al. Quantitative MRI detects abnormalities in relatives of patients with epilepsy and malformations of cortical development. Neuroimage. 2003;18:642–649. doi: 10.1016/s1053-8119(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 18.Bonilha L, Montenegro MA, Rorden C, et al. Voxel-based morphometry reveals excess gray matter concentration in patients with focal cortical dysplasia. Epilepsia. 2006;47:908–915. doi: 10.1111/j.1528-1167.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 19.Colliot O. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006;29:162–171. doi: 10.1016/j.neuroimage.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Bruggemann JM, Wilke M, Som SS, Bye AM, Bleasel A, Lawson JA. Voxel-based morphometry in the detection of dysplasia and neoplasia in childhood epilepsy: combined grey/white matter analysis augments detection. Epilepsy Res. 2007;77:93–101. doi: 10.1016/j.eplepsyres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Bruggemann JM. Voxel-based morphometry in the detection of dysplasia and neoplasia in childhood epilepsy: limitations of grey matter analysis. J. Clin. Neurosci. 2009;16:780–785. doi: 10.1016/j.jocn.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Wilke M, Kassubek J, Ziyeh S, Schulze-Bonhage A, Huppertz HJ. Automated detection of gray matter malformations using optimized voxel-based morphometry: a systematic approach. Neuroimage. 2003;20:330–343. doi: 10.1016/s1053-8119(03)00296-9. [DOI] [PubMed] [Google Scholar]
- 23.Focke NK, Symms MR, Burdett JL, Duncan JS. Voxel-based analysis of whole brain FLAIR at 3T detects focal cortical dysplasia. Epilepsia. 2008;49:786–793. doi: 10.1111/j.1528-1167.2007.01474.x. [DOI] [PubMed] [Google Scholar]
- 24.Focke NK, Bonelli SB, Yogarajah M, Scott C, Symms MR, Duncan JS. Automated normalized FLAIR imaging in MRI-negative patients with refractory focal epilepsy. Epilepsia. 2009;50:1484–1490. doi: 10.1111/j.1528-1167.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 25.Bernasconi A, Antel SB, Collins DL, et al. Texture analysis and morphological processing of magnetic resonance imaging assist detection of focal cortical dysplasia in extra-temporal partial epilepsy. Ann Neurol. 2001;49:770–775. [PubMed] [Google Scholar]
- 26.Huppertz HJ, Grimm C, Fauser S, et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005;67:35–50. doi: 10.1016/j.eplepsyres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Huppertz HJ, Wellmer J, Staack AM, Altenmuller DM, Urbach H, Kroll J. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia. 2008;49:772–785. doi: 10.1111/j.1528-1167.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 28.Wagner J, Weber B, Urbach H, Elger C, Huppertz H. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134:2844–2854. doi: 10.1093/brain/awr204. [DOI] [PubMed] [Google Scholar]
- 29**.Wang ZI, Jones SE, Jaisani Z, et al. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015;77:1060–1075. doi: 10.1002/ana.24407. [This article highlights the clinical value of using MRI processing of the gray-white junction to improve diagnostic yield/accuracy of MRI in a large cohort of MRI-negative epilepsy patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang ZI, Jones SE, Ristic AJ, et al. Voxel-based morphometric MRI post-processing in MRI-negative focal cortical dysplasia followed by simultaneously recorded MEG and stereo-EEG. Epilepsy Res. 2012;100:188–193. doi: 10.1016/j.eplepsyres.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZI, Ristic AJ, Wong CH, et al. Neuroimaging characteristics of MRI-negative orbitofrontal epilepsy with focus on voxel-based morphometric MRI postprocessing. Epilepsia. 2013;54:2195–2203. doi: 10.1111/epi.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blümcke I, Thom M, Aronica E, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colliot O, Antel SB, Naessens VB, Bernasconi N, Bernasconi A. In vivo profiling of focal cortical dysplasia on high-resolution MRI with computational models. Epilepsia. 2006;47:134–142. doi: 10.1111/j.1528-1167.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- 34.Antel SB. Automated detection of focal cortical dysplasia lesions using computational models of their MRI characteristics and texture analysis. Neuroimage. 2003;19:1748–1759. doi: 10.1016/s1053-8119(03)00226-x. [DOI] [PubMed] [Google Scholar]
- 35*.Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83:48–55. doi: 10.1212/WNL.0000000000000543. [This study demonstrated a promising fully automated multivariate approach relying on surface-based features of FCD morphology and intensity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bronen RA, Spencer DD, Fulbright RK. Cerebrospinal fluid cleft with cortical dimple: MR imaging marker for focal cortical dysgenesis. Radiology. 2000;214:657–663. doi: 10.1148/radiology.214.3.r00mr40657. [DOI] [PubMed] [Google Scholar]
- 37.Yagishita A, Arai N, Maehara T, Shimizu H, Tokumaru AM, Oda M. Focal cortical dysplasia: appearance on MR images. Radiology. 1997;203:553–559. doi: 10.1148/radiology.203.2.9114120. [DOI] [PubMed] [Google Scholar]
- 38.Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995;118(Pt 3):629–660. doi: 10.1093/brain/118.3.629. [DOI] [PubMed] [Google Scholar]
- 39.Besson P, Andermann F, Dubeau F, Bernasconi A. Small focal cortical dysplasia lesions are located at the bottom of a deep sulcus. Brain. 2008;131:3246–3255. doi: 10.1093/brain/awn224. [DOI] [PubMed] [Google Scholar]
- 40.Besson P, Bernasconi N, Colliot O, Evans A, Bernasconi A. Surface-based texture and morphological analysis detects subtle cortical dysplasia. Med. Image Comput. Comput. Assist Interv. 2008;11:645–652. doi: 10.1007/978-3-540-85988-8_77. [DOI] [PubMed] [Google Scholar]
- 41.Regis J, Tamura M, Park MC, et al. Subclinical abnormal gyration pattern, a potential anatomic marker of epileptogenic zone in patients with magnetic resonance imaging-negative frontal lobe epilepsy. Neurosurgery. 2011;69:80–93. doi: 10.1227/NEU.0b013e318212bb1a. discussion 93-84. [DOI] [PubMed] [Google Scholar]
- 42.Mellerio C, Roca P, Chassoux F, et al. The Power Button Sign: A Newly Described Central Sulcal Pattern on Surface Rendering MR Images of Type 2 Focal Cortical Dysplasia. Radiology. 2014;274:500–507. doi: 10.1148/radiol.14140773. [DOI] [PubMed] [Google Scholar]
- 43.Salmenpera TM, Symms MR, Rugg-Gunn FJ, et al. Evaluation of quantitative magnetic resonance imaging contrasts in MRI-negative refractory focal epilepsy. Epilepsia. 2007;48:229–237. doi: 10.1111/j.1528-1167.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda CL, Betting LE, Cendes F. Voxel-based morphometry and epilepsy. Expert Review of Neurotherapeutics. 2010;10:975–984. doi: 10.1586/ern.10.63. [DOI] [PubMed] [Google Scholar]
- 45.Colliot O, Bernasconi N, Khalili N, Antel SB, Naessens V, Bernasconi A. Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage. 2006;29:162–171. doi: 10.1016/j.neuroimage.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Fauser S. Multi-focal occurrence of cortical dysplasia in epilepsy patients. Brain. 2009;132:2079–2090. doi: 10.1093/brain/awp145. [DOI] [PubMed] [Google Scholar]
- 47.Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54:772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- 48.Knake S, Triantafyllou C, Wald LL, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005;65:1026–1031. doi: 10.1212/01.wnl.0000179355.04481.3c. [DOI] [PubMed] [Google Scholar]
- 49.De Ciantis A, Barkovich AJ, Cosottini M, et al. Ultra-high-field MR imaging in polymicrogyria and epilepsy. AJNR Am J Neuroradiol. 2015;36:309–316. doi: 10.3174/ajnr.A4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garbelli R, Zucca I, Milesi G, et al. Combined 7-T MRI and histopathologic study of normal and dysplastic samples from patients with TLE. Neurology. 2011;76:1177–1185. doi: 10.1212/WNL.0b013e318212aae1. [DOI] [PubMed] [Google Scholar]
- 51.Reeves C, Tachrount M, Thomas D, et al. Combined Ex Vivo 9.4T MRI and Quantitative Histopathological Study in Normal and Pathological Neocortical Resections in Focal Epilepsy. Brain Pathol. 2015 doi: 10.1111/bpa.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Zucca I, Milesi G, Medici V, et al. Type II FCD: ex vivo 7 Tesla MRI abnormalities and histopathological comparisons. Ann Neurol 2015. Excellent study comparing ex vivo 7T MRI and histopathology [Google Scholar]
- 53.Seiger R, Hahn A, Hummer A, et al. Voxel-based morphometry at ultra-high fields. a comparison of 7T and 3T MRI data. Neuroimage. 2015;113:207–216. doi: 10.1016/j.neuroimage.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marusic P, Najm IM, Ying Z, et al. Focal cortical dysplasias in eloquent cortex: functional characteristics and correlation with MRI and histopathologic changes. Epilepsia. 2002;43:27–32. doi: 10.1046/j.1528-1157.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 55.House PM, Lanz M, Holst B, Martens T, Stodieck S, Huppertz HJ. Comparison of morphometric analysis based on T1- and T2-weighted MRI data for visualization of focal cortical dysplasia. Epilepsy Res. 2013;106:403–409. doi: 10.1016/j.eplepsyres.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 56.Braga B, Yasuda CL, Cendes F. White Matter Atrophy in Patients with Mesial Temporal Lobe Epilepsy: Voxel-Based Morphometry Analysis of T1- and T2-Weighted MR Images. Radiol Res Pract. 2012;2012:481378. doi: 10.1155/2012/481378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rugg-Gunn FJ, Boulby PA, Symms MR, Barker GJ, Duncan JS. Whole-brain T2 mapping demonstrates occult abnormalities in focal epilepsy. Neurology. 2005;64:318–325. doi: 10.1212/01.WNL.0000149642.93493.F4. [DOI] [PubMed] [Google Scholar]
- 58.Rugg-Gunn FJ. Magnetization transfer imaging in focal epilepsy. Neurology. 2003;60:1638–1645. doi: 10.1212/01.wnl.0000065891.93179.cc. [DOI] [PubMed] [Google Scholar]
- 59.Rugg-Gunn FJ, Boulby PA, Symms MR, Barker GJ, Duncan JS. Imaging the neocortex in epilepsy with double inversion recovery imaging. Neuroimage. 2006;31:39–50. doi: 10.1016/j.neuroimage.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 60.Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495:187–192. doi: 10.1038/nature11971. [DOI] [PMC free article] [PubMed] [Google Scholar]