Abstract
Objectives
This longitudinal study examined the influence of Intimate Partner Violence (IPV) experience of pregnant women participating in the Domestic Violence Enhanced Home Visitation Program (DOVE) on the language and neurological and development of infants and toddlers.
Methods
A total of 210 infants and toddlers born to women reporting low, moderate, and high levels of IPV were included in the analysis. Logistic regression analysis was used to determine the bivariate association between maternal IPV and risk of language and neurological delay of infants and toddlers and between covariates and language and neurological delay. Generalized Estimating Equation models with logit link was used to predict the risk for neurological and language delay of infants and toddlers as a result of maternal IPV.
Results
Infants and toddlers born to women exposed to moderate levels of IPV had increased odds of language delay compared to infants and toddlers of women who experienced low levels of violence (OR=5.31, 95% CI=2.94, 9.50, p<0.001). Infants and toddlers born to women who experienced moderate and high levels of IPV were at higher risk of neurological delay respectively, compared to infants and toddlers of women who experienced low levels of IPV (OR=5.42, 95% CI=2.99, 9.82, p<0.001 & OR=2.57, 95% CI=1.11, 5.61, p=0.026).
Conclusions
Maternal IPV is associated with increased risk of language and neurological delay of infants and toddlers. These findings have implications for health care for women and infants exposed to IPV. Clinicians including pediatricians working with pregnant women should screen for IPV throughout pregnancy to identify women and children at risk. Interventions to reduce maternal IPV and early intervention services for infants and toddlers exposed to IPV are necessary for optimal maternal and child health.
Keywords: IPV, Language development, Neurological development, Pregnancy, Infants and Toddlers
Introduction
Intimate partner violence (IPV) is a serious public health problem globally. A multi-country study by the World Health Organization found high prevalence of IPV across countries with the highest lifetime rates (71%) of physical and sexual abuse in Ethiopia with 53% experiencing such violence in the last one year [1]. In the United States, 28.8% of women have experienced rape, physical violence and/or stalking with IPV related impacts [2] and 11% of all homicides committed between 1976 and 2002 were done by an intimate partner [1]. Each year, 2 million injuries and 1300 deaths are attributed to IPV against women [3].
IPV increases during pregnancy, with incidence rates higher than pregnancy complications such as preeclampsia, placenta previa and gestational diabetes, suggesting its relatively high prevalence during pregnancy [4–6]. IPV experience is associated with adverse maternal health outcomes including physical and traumatic brain injuries, Sexually Transmitted Diseases (STDs) including HIV/AIDS, mental health problems, and adverse birth and neonatal outcomes [7–8].
Maternal IPV experience negatively impacts infants and children. Children of abused women experience social, emotional, conduct, cognitive, and behavioral problems compared to children of non-abused women [9–10]. Correlates of IPV such as prenatal stress, maternal anxiety and depression, dysfunctional mother-child interaction, and unhealthy maternal coping strategies have been found to adversely influence child development [11–13]. These studies found increased cognitive deficit, neurological and learning problems, decreased neuromuscular maturation and negative behavioral reactivity to novelty in infants and children exposed to in-utero maternal stress. While these studies focused on prenatal maternal stress and not IPV, as a primary predictor of infant and child development, their findings that prenatal maternal stress has adverse developmental effects on infants and children may hold true for maternal IPV experiences as well.
In addition to the high rates of maternal IPV in the U.S, the prevalence of developmental disabilities among children is also rising, particularly among children under 5 years old. For example, more children are receiving services for speech and language disabilities under the Individuals with Disabilities Education Act [14] and data from the National Health Interview Surveys show an increased prevalence of developmental disabilities including speech and language pathology in the United States [15]. Given this parallel rise in maternal IPV and developmental disabilities, it is important to examine the impact of maternal IPV on children’s developmental outcomes.
The negative impact of IPV on maternal and child health outcomes have been extensively studied among older (school-age) children with most focusing on mental health, behavioral health and school performance as main outcome. However, less is known about how IPV affects younger children, particularly in the first two years of life. The dataset from the evaluation of an IPV prevention intervention – the DOVE study – presents a unique opportunity to examine the impact of maternal IPV on developmental outcomes of infants and toddler. The purpose of this study is to examine the effect of maternal IPV exposure on language and neurological development of infants and toddlers. To the best of our knowledge, this study is one of the first to examine the impact of IPV on these outcomes among children less than 2 years old. Examinations of potential adverse outcomes in pre-school language development are important to provide findings for the development of evidenced based interventions. Early childhood is a very important period in a child’s development, and provides a great opportunity to for early intervention using evidenced-based strategies as well as to achieve national goals related to school readiness.
Theoretical Framework
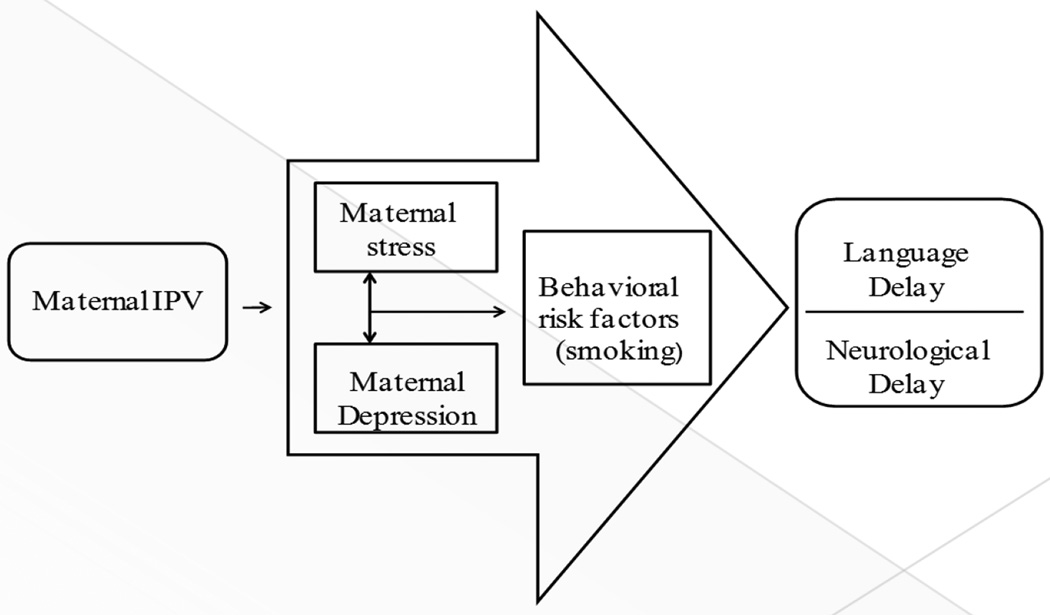
The social cognitive theory and the developmental psychopathology perspective have been used to describe how adverse experiences of children affect their ongoing development mainly through observed learning and maladaptation to adverse environments; however these are among older school age children [16–17]. Our conceptual framework uses Lazarus’ stress and coping theory, developmental psychopathology perspective and Jaffe’s developmental theory to explain the link between maternal IPV and child development [18–19]. Maternal IPV increases maternal stress which is associated with depression and negative coping behaviors (such as substance abuse) that increases risk of adverse health and pregnancy outcomes, negatively influences mother-child attachment and may influence mother’s ability to provide environment that enhances neurological and language development of children (Figure 1).
Figure 1.
Conceptual Model for the Association between Maternal IPV and Language and Neurological Delay of Infants and Toddlers 3–24 Months
Methods
Sample
The present study is a longitudinal secondary analysis of data from the Domestic Violence Enhanced Visitation (DOVE) study (NIH/NINR, R01 NR009093). DOVE was a multisite study that tested the effectiveness of a structured IPV intervention (DOVE intervention), compared to Usual Care (administered by health departments) among women and infants in rural and urban settings in a mid-western and a mid-Atlantic state. The study was conducted over 5 years (2006–2011) as a collaborative effort between the participating universities and health departments.
Data were collected at baseline, delivery, 3, 6, 12, 18, and 24 months post-partum. The unit of analysis for the current study was infants and toddlers 3–24 months; each woman’s data were linked to her child’s.
Measures
Dependent Variables
Language development of infant and toddlers was assessed at 3, 12 and 24 months using the Preschool language scale fourth edition (PLS-4). The scale is an individually administered test used to detect infants and toddlers ages 0 – 6 years, 11 months, with language delay or impairment [20]. Examples of items on the PLS-4 include “glances momentarily at a person who talks to him or her” (birth–2 months), “responds to speaker by smiling” (birth –2 months), various pitch, “length and volume of cries (3–5 months)”. A score of 0 or 1 is given based on whether or not the child meets the criterion for a given item. A child scores 1 when the evaluator elicits the appropriate response, or produces the appropriate response spontaneously at any time during the session or the caregiver reports that child can carry out the given task. The PLS-4 was standardized with diverse population of children. For this study, PLS-4 scores were coded as a dichotomous variable – 1 for “no language delay” and 0 for “language delay”.
Neurological development of infants and toddlers was measured at 3, 12 and 24 months using the Bayley Infant Neurodevelopmental Screener (BINS), a validated screening tool that identifies infants and toddlers between the ages of 3 months-24 months that are at risk for developmental or neurological delay [21]. Typical items on the BINS include “eyes follow ring”; “responds to spoken words” and “transfers object from hand to hand”. The standardized chart categorizes infants as having a high, moderate, and low risk of neurological delay based on the child’s age. We created three levels of risk: 1 for “low risk,” 2 for “moderate risk,” and 3 for “high risk” of neurological delay.
Main Predictor Variable
IPV was measured using the Conflicts Tactics Scale-2 (CTS-2). The scale is a 78-item scale with 5 subscales measuring physical violence, psychological violence, injury, sexual violence, and negotiation and it has been used for decades to assess violence within families and intimate relationships. The CTS-2 measures the frequency of abuse in both partners in an intimate relationship by assessing each partner’s use of a variety of violent behaviors to handle conflicts [22]. Responses on the scale ranged from 0 (never) to 7 (not in the past year, but it happened before); scores between 2 and 6 on the scale represented IPV frequencies from 2 to 20 times in the past year. For this study, only 42 items that described IPV victimization against the woman were used. Scores from items 0, 1 and 7 were re-coded as 0 while scores from items 2 through 6 were re-coded as 1; 0 representing no violence and 1, presence of violence. Total violence scores were then determined for each woman by adding up her responses to all the items from all subscales except negotiation. The Cronbach’s alpha for the recoded scale ranged from 0.74 – 0.90.
Covariates
Participant characteristics adjusted for include mother’s age (14–19 years=0, 20 and up=1), maternal smoking status (0,1), employment status(0,1), maternal education (0-college education or more; 1-high school diploma and 2 – less than high school diploma), race(African/African American/Black, Native Hawaiian/pacific Islander, White, Hispanic and other), child’s gender (0-male, 1-female), marital status (married/partnered=0, single=1) and gestational age (continuous variable).
Other variables adjusted for include maternal depressive symptoms (≤11= 0, >11= 1) measured using Edinburg Depression Scale (EDS) [23] and maternal stress assessed using the Prenatal Psychological Profile (PPP), dichotomized as 0/1, and with a Cronbach’s alpha of 0.70 [24]. The cut-off scores for the EDS range from 9 to 13. While a cut off of 13 has been mostly used as indicative of major depressive disorder, we used a cut-off score of 11 to err on safety’s side. The literature recommends using lower cut-offs particularly for community screening to ensure that all cases of depressive symptoms are identified. A lower cut-off has also been reported to be best for screening for depressive symptoms among pregnant adult women [25–27]. Scale authors also recommend that women who score above 9 be referred for immediate follow-up [23]. Cronbach’s alpha values for our study ranged from 0.82 – 0.89.
Statistical Analysis
Factor analysis was conducted after the CTS-2 scale was administered to participants as a data reduction technique to remove redundancy and duplication in our data. A vertical data file was created for different time points in order to use discrete time logistic regression analysis. IPV factor scores were created for each time points from items on the CTS-2. Factor scores were categorized as low (bottom third), moderate (middle third) and high (top third) exposure and were used to predict language and neurological development of infants and toddlers at the last time point (24 months). Because all the women in the DOVE project had history and /or current experience of IPV at recruitment, there was no comparison group, rather we compared outcomes among the three categories of IPV exposure.
The 12 items that loaded in the factor score included item number 8 (“my partner threw something at me that could hurt me”), 10 (“my partner twisted my arm or hair”), 11(“I had a sprain, bruise, or small cut because of a fight with my partner”), 18 (“My partner pushed or shoved me”), 34 (“My partner choked me”), 38 (“My partner slammed me against a wall”), 44 (“My partner beat me up”), 46 (“My partner grabbed me”), 54 (“My partner slapped me”), 70 (“My partner threatened to hit or throw something at me”), 71(“I felt physical pain that still hurt the next day because of a fight with my partner”) and 74 (“My partner kicked me”). Only scores from these items were included in the final analysis.
All analyses were done with Stata version 11.2. Univariate analysis was conducted by computing descriptive statistics of all independent and outcome variables. Generalized Estimating Equation (GEE) using logit link was used for bivariate and multivariate data analysis to predict language and neurological delay as a result of maternal IPV. For the regression analysis, moderate and high risk were collapsed and recoded as 1 for risk of neurological delay and low risk was re-coded as 0 for no risk neurological delay.
Our full model included neurological development and language development scores as the dependent variable, and the predictor variables were IPV, maternal education, infant’s gender, maternal age, maternal depression, maternal smoking status, marital status, gestational age, maternal stress, support from partner, support from other people, mother’s employment status, maternal race, and DOVE intervention. These covariates were included either because they were significantly associated with our outcome variables in the bivariate model and/or have been shown to be associated with our outcome variables [12, 28–30].
Results
Sample Description
The final sample for this study consisted of 210 children. Table 1 shows descriptive statistics for participants (women & children) at baseline. The mean age of the women was 23.9 years. African American women experienced more moderate to high IPV at baseline than women of other racial background (53% and 50% respectively). Most women had less than high school diploma and half of the women who experienced high IPV had less than high school diplomas. Most women reported smoking before pregnancy. Most of women who experienced high levels of IPV reported smoking before pregnancy and about half of women who experienced IPV smoked during pregnancy. The majority of the women were unmarried. Most of the women who were exposed to moderate violence and half of those exposed to high violence were unmarried.
Table 1.
Descriptive Statistics of Study Sample by Level of IPV
| IPV Level (N= 210) | ||||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| n=71 | % | n=69 | % | n=70 | % | |
| Race | ||||||
| African American | 26 | 36.6 | 37 | 53.6 | 35 | 50.0 |
| Hispanic | 4 | 5.6 | 2 | 2.9 | 4 | 5.6 |
| Others | 3 | 4.2 | 6 | 8.7 | 5 | 7.1 |
| White | 38 | 52.5 | 24 | 34.8 | 26 | 37.1 |
| Maternal Age (years) | ||||||
| 14–19 | 9 | 12.7 | 19 | 27.5 | 22 | 31.4 |
| 20+ | 62 | 87.3 | 50 | 72.5 | 48 | 68.6 |
| Maternal Education | ||||||
| Some college & up | 31 | 44.3 | 23 | 34.3 | 17 | 24.3 |
| High School Diploma | 18 | 25.7 | 13 | 19.4 | 18 | 25.7 |
| <High School | 21 | 30.0 | 31 | 46.3 | 35 | 50.0 |
| Marital Status | ||||||
| Single | 43 | 60.6 | 50 | 73.5 | 39 | 55.7 |
| Married/Partnered | 28 | 39.4 | 18 | 26.5 | 31 | 44.3 |
| Employment | ||||||
| Yes | 51 | 71.8 | 51 | 73.9 | 49 | 70 |
| No | 20 | 28.2 | 18 | 26.1 | 21 | 30 |
| Smoked before pregnancy | ||||||
| Yes | 42 | 60 | 43 | 63.2 | 47 | 68.1 |
| No | 28 | 40 | 25 | 36.8 | 22 | 31.9 |
| Smoked during pregnancy | ||||||
| Yes | 29 | 40.9 | 28 | 41.2 | 36 | 51.4 |
| No | 42 | 59.1 | 40 | 58.8 | 34 | 48.6 |
| Health Insurance | ||||||
| Yes | 70 | 98.6 | 67 | 97.1 | 66 | 94.3 |
| No | 1 | 1.4 | 2 | 2.9 | 4 | 5.7 |
| Depression | ||||||
| Yes | 17 | 27.9 | 22 | 36.1 | 19 | 32.2 |
| No | 44 | 72 | 39 | 64 | 40 | 68 |
| Stress | ||||||
| High | 36 | 59 | 40 | 65.6 | 35 | 59.3 |
| Low | 25 | 41 | 21 | 34.4 | 24 | 40.7 |
| Support from Partner | ||||||
| High | 27 | 44.3 | 19 | 31.2 | 26 | 44.1 |
| Low | 34 | 55.7 | 42 | 68.9 | 33 | 55.9 |
| Support from others | ||||||
| High | 29 | 47.5 | 24 | 39.3 | 28 | 47.5 |
| Low | 32 | 52.5 | 37 | 60.7 | 31 | 52.5 |
| Gestation | ||||||
| Preterm | 10 | 14.1 | 17 | 25.4 | 6 | 8.7 |
| Full-term | 61 | 85.9 | 50 | 74.6 | 63 | 91.3 |
| Infant’s gender | ||||||
| Female | 35 | 49.3 | 35 | 52.2 | 36 | 52.2 |
| Male | 36 | 50.7 | 32 | 47.8 | 33 | 47.8 |
| Birth Weight | ||||||
| Normal | 59 | 85.5 | 51 | 76.1 | 58 | 86.6 |
| LBW | 10 | 14.5 | 16 | 23.9 | 9 | 13.4 |
Bivariate and Multivariate Analyses
The crude and adjusted odds ratios for the association between maternal IPV and risk of language and neurological delay are shown in Table 2. Infants and toddlers of women who experienced moderate and high IPV were at higher risk of language and neurological delay than infants of women who experienced low levels of IPV. Odds of neurological delay were higher than the odds for language delay. Maternal IPV experience was more strongly associated with risk of neurological delay (AOR=5.42; 95% CI=2.99, 9.82 & AOR=2.57; 95% CI=1.11, 5.61 for moderate and high IPV respectively) than it is for the risk of language delay. Odds of language and neurological delay persisted after adjusting for potential confounders for infants and toddlers born to women who experienced high levels of IPV. For infants and toddlers born to women who experienced IPV, risk was attenuated in the adjusted model for language delay but not for neurological delay.
Table 2.
Associations between Maternal IPV and Risk of Language and Neurological Delay
| Language Delay | Risk of Neurological Delay | |||
|---|---|---|---|---|
| Crude OR (95% CI) |
Adjusted OR (95% CI) |
Crude OR (95% CI) |
Adjusted OR (95% CI) |
|
| IPV | ||||
| Mild (RC) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 4.47 (2.65, 7.52)*** | 2.31 (2.94, 9.50)*** | 4.92 (2.87, 8.41)*** | 5.42 (2.99, 9.82)*** |
| Severe | 2.19 (1.15, 4.18)* | 1.36 (0.63, 2.98) | 3.31 (1.62, 6.74)*** | 2.57 (1.11, 5.61)* |
| Race | ||||
| African American | 0.84 (0.53, 1.33) | 0.98 (0.54, 1.78) | 0.94 (0.58, 01.53) | 0.96 (0.50, 1.84) |
| Hispanic | 1.87 (0.52, 6.71) | 2.02 (0.46, 8.72) | 1.03 (0.34, 3.01) | 1.23 (0.34, 4.40) |
| Others | 1.05 (0.36, 3.07) | 1.35 (0.35, 5.20) | 0.87 (0.29, 0.60) | 1.16 (0.32, 4.29) |
| White (RC) | 1.00 | 1.00 | 1.00 | - |
| Maternal Age (years) | ||||
| 14–19 (RC) | 1.00 | 1.00 | 1.00 | - |
| 20+ | 0.51 (0.27,0.92)* | 0.45 (0.19, 0.99)* | 1.05 (0.60, 1.84) | 1.16 (0.55, 2.44) |
| Maternal Education | ||||
| Some college & up (RC) | 1.00 | 1.00 | 1.00 | 1.00 |
| High School Diploma | 1.26 (0.71, 2.23) | 1.16 (0.57, 2.34) | 1.12 (0.60, 2.01) | 1.73 (0.80, 3.72) |
| < High School | 1.55 (0.94, 2.56) | 1.49 (0.78, 2.83) | 1.38 (0.82, 2.31) | 2.05 (1.02, 4.10)* |
| Marital Status | ||||
| Single | 0.65 (0.40, 1.04) | 0.68 (0.37, 1.24) | 1.06 (0.66, 1.71) | 0.79 (0.41, 1.42) |
| Married/Partnered (RC) | 1.00 | 1.00 | 1.00 | - |
| Employment | ||||
| Yes | 1.18 (0.74, 1.87) | 1.03 (0.53, 1.85) | 0.69 (0.43, 1.13) | 0.57 (0.31, 1.04) |
| No (RC) | 1.00 | 1.00 | 1.00 | - |
| Smoked during pregnancy | ||||
| Yes | 1.00 (0.64, 1.56) | 1.06 (0.59, 1.88) | 0.78 (0.49, 1.24) | 0.68 (0.37, 1.27) |
| No (RC) | 1.00 | 1.00 | 1.00 | - |
| Depression | ||||
| Yes | 1.14 (0.70, 1.85) | 0.99 (0.52, 1.89) | 0.97 (0.60, 1.55) | 0.85 (0.45, 1.57) |
| No | 1.00 | 1.00 | 1.00 | - |
| Stress | ||||
| High | 1.16 (1.05, 1.20)* | 1.17 (1.03, 1.34)* | 1.04 (0.97, 1.18) | 1.11 (0.97, 1.27) |
| Low (RC) | 1.00 | 1.00 | 1.00 | - |
| Support from Partner | ||||
| High | 0.97 (0.93, 1.02) | 0.94 (0.89, 0.99)* | 0.98 (0.94, 1.03) | 0.98 (0.93, 1.04) |
| Low (RC) | 1.00 | 1.00 | 1.00 | - |
| Support from others | ||||
| High | 1.02 (0.97, 1.09) | 1.03 (0.96, 1.11) | 1.04 (0.98, 1.10) | 1.00 (0.92, 1.08) |
| Low (RC) | 1.00 | 1.00 | 1.00 | - |
| Gestation | ||||
| Preterm | 1.02 (0.94, 1.10) | 0.98 (0.88, 1.09) | 1.02 (0.92, 1.10) | 1.02 (0.91, 1.13) |
| Full-term (RC) | 1.00 | 1.00 | 1.00 | - |
| Infant’s gender | ||||
| Female | 0.57 (0.36, 0.89)* | 0.58 (0.35, 0.98)* | 0.61 (0.98, 0.96)* | 0.63 (0.36, 1.09) |
| Male (RC) | 1.00 | 1.00 | 1.00 | - |
| Received Intervention | ||||
| Yes | 1.16 (0.74, 1.79) | 1.09 (0.64, 1.85) | 0.78 (0.50, 1.25) | 1.09 (0.64, 1.85) |
| No (RC) | 1.00 | 1.00 | 1.00 | - |
IPV: Intimate Partner Violence; OR: Odds Ratio; CI: Confidence Interval; RC: Reference Category
p<0.05;
p<0.01;
p<0.001
Infants and toddlers born to women who were older than 19 years were almost 50% less likely to be at risk for language delay compared to infants and toddlers born to women who were less than 20 years old (AOR=0.45; 95% CI=0.19, 0.99), while infants and toddlers born to women with less than a high school education were 2 times more likely to be at risk for neurological delay than infants and toddlers of women with some college or more education (AOR=2.05; 95% CI=1.02, 4.10). Furthermore, infants and toddlers born to women who reported high levels of stress were slightly (17%) more likely to be at risk of language delay than those born to women who reported low levels of stress. Infant’s gender was independently associated with risk of language delay. Female infants and toddlers were less likely than their male peers to be at risk for language delay (AOR=0.58; 95% CI=0.35, 0.98).
Discussion
This study showed that moderate and high exposures to maternal IPV were significantly associated with language and neurological delay of infants and toddlers, with neurological development more affected than language development. Although moderate and high levels of maternal IPV were significantly associated with neurological delay of infants and toddlers, infants and toddlers born to women who experienced moderate IPV had higher odds for experiencing neurological delay compared to infants and toddlers born to women experiencing high levels of IPV. A previous study that examined the influence of prenatal maternal stress on general intellectual and language functioning of toddlers reported similar findings. However, contrary to our study, the authors found a dose response relationship between maternal stress and the number of words spoken and understood by toddlers at 2 years [31].
There may be a few reasons for our findings. First, given that we used two independent validated scales to measure language and neurologic development, and observed the same pattern with children of women in the moderate IPV group functioning worse than their peers in the high IPV group, we believe that the high exposure to IPV might have increased help seeking in the “high” IPV exposure group which may have had some protective effect on their children. Also, with the moderate group the frequency of exposure is variable and we speculate the uncertainty of exposure as well as less reluctance of mothers to seek help may mean children were actually getting more exposure and less help from mother or mother less likely to seek help because the exposure was less predictable and may be perceived as less harmful.
Our finding that female infants and toddlers were less affected than their male counterparts, with respect to language development, is consistent with the results from several studies. One study found that the male fetus was more vulnerable to the effects of maternal prenatal stress [29]. Another study found reduced expression and activity of placental 11β-HSD2 (a placental enzyme that protects the fetus from harmful effects of stress) in male fetuses compared to their female counterparts [30, 32]. Finally, many epidemiological studies have shown that fetal morbidity/mortality, neurodevelopmental alterations, and pregnancy complications are more associated with male fetuses than females [10, 33]. The underlying mechanisms in these male-female fetal differences have not been elucidated.
This study found that abused women reported high levels of maternal stress. There was a significant association between maternal stress and language delay in infants and toddlers, but not neurological delay. Even after accounting for other potential confounders such as gestational age, birth weight and infant’s gender, infants and toddlers born to abused women who had high stress scores were more likely to experience language delay than infants and toddlers born to abused women who had lower stress scores. This finding is consistent with what was reported by another study that showed that prenatal maternal stress negatively affected brain development of the fetus reflected in lower intellectual and language abilities of toddlers in their study [34].
In sum, our study found that maternal experience of IPV was significantly associated with language and neurological development of infants and toddlers. For language development, we found that maternal age (>20 years) and infant’s gender (being female) were protective against language delay of infants while abused women’s exposure to high levels of stress was a risk factor for language delay. For neurological development, our study found that maternal educational attainment was an independent predictor of risk of neurological delay of infants and toddlers. Infants and toddlers born to abused women who had less than a high school education were at significantly higher risk of neurological delay than infants born to abused women who had at least a college degree.
This study has strengths and limitations. The results and conclusions are based on a small sample size (N=210 infants/toddlers). Data on smoking status and substance use before and during pregnancy were self–reported, and accuracy for this kind of report is always a concern. The majority of women in this study denied the use of substances during pregnancy. Getting accurate data on the women’s use of substance is crucial as these are important predictors of neurological functioning and may be confounders. All the women in the study had past or current experience of IPV; hence there was no comparison group. By comparing outcomes between women who experienced IPV versus women without history of IPV, adding a no IPV comparison group would have enabled a more in-depth examination of the independent role of maternal IPV experience on language and neurological development of infants and toddlers. This study did not examine the different types of abuse and their unique contributions to the study outcomes nor did it try to understand the specific domain(s) of language development that were most affected—expression or comprehension.
Despite the noted limitations, the findings of the study are very crucial. To the best of our knowledge no study has examined the influence of IPV on this age group (3–24months) specifically targeting language and neurological development as outcomes. A few studies have examined the influence of stress on neurological development, but they did not look at IPV as a potential stressor. We found increased odds of delay (language and neurological) of infants and toddlers using two different validated tools (BINS and PLS). This agreement between measures speaks to the validity of the measures and further supports our results. Further research using larger sample size is recommended. It is also important for future studies to examine the role of the different types of abuse in neurological development of infants and toddlers. Based on the present study’s findings, there is need for continuous assessment and identification of IPV and subsequent reduction of risk factors among women. Healthcare providers and others who work with pregnant women should consistently screen and intervene for intimate partner violence.
Prevention programs should be expanded for pregnant women to include parenting support programs that assess and intervene for IPV and stress as well as early intervention programs such as home visiting for high risk infants and toddlers.
Significance.
Previous studies have reported adverse impact of maternal experience of IPV on pregnancy outcomes and health outcomes of school age children. However, less is known about how IPV affects younger children, particularly in the first two years of life. Understanding the impact of maternal exposure to IPV on infants and toddlers will help identify infants and toddlers at risk as well as opportunities for early intervention.
Acknowledgments
This research paper is a product of the lead author’s doctoral dissertation. The authors wish to acknowledge the National Institutes of Health – National Institute of Nursing Research for funding the DOVE study (NIH/NINR, R01 NR009093), and the staff and faculty of Morgan State University School of Community Health and Policy for their unwavering support.
Contributor Information
Ifeyinwa E. Udo, Center for Interdisciplinary Research on AIDS; Yale School of Public Health, New Haven CT, USA, Ifeyinwa.udo@yale.edu; Phone: 2037375031; Fax: 203-737-5239.
Phyllis Sharps, Department of Community Public Health, Johns Hopkins University School of Nursing, MD, USA.
Yvonne Bronner, Department of Behavioral Health Science, School of Community Health & Policy, Morgan State University, MD, USA.
Mian B. Hossain, Department of Public Health Analysis, School of Community Health & Policy, Morgan State University, MD, USA.
References
- 1.Ellsberg M, Jansen HA, Heise L, Watts CH, Garcia-Moreno C WHO Multi-country Study on Women's Health and Domestic Violence against Women Study Team. Intimate partner violence and women's physical and mental health in the WHO multi-country study on women's health and domestic violence: An observational study. Lancet. 2008;371(9619):1165–1172. doi: 10.1016/S0140-6736(08)60522-X. [DOI] [PubMed] [Google Scholar]
- 2.Black MC, Basile KC, Breiding MJ, Smith SG, Walters ML, Merrick MT, Chen J, Stevens MR. National intimate partner and sexual violence survey: 2010 summary report. Atlanta GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.Breiding MJ, Ziembroski JS, Black MC. Prevalence of rural intimate partner violence in 16 US states, 2005. The Journal of Rural Health : Official Journal of the American Rural Health Association and the National Rural Health Care Association. 2009;25(3):240–246. doi: 10.1111/j.1748-0361.2009.00225.x. [doi] [DOI] [PubMed] [Google Scholar]
- 4.Abdollahi F, Abhari FR, Delavar MA, Charati JY. Physical violence against pregnant women by an intimate partner, and adverse pregnancy outcomes in mazandaran province, iran. Journal of Family & Community Medicine. 2015;22(1):13–18. doi: 10.4103/2230-8229.149577. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambliss LR. Intimate partner violence and its implication for pregnancy. Clinical Obstetrics and Gynecology. 2008;51(2):385. doi: 10.1097/GRF.0b013e31816f29ce. [DOI] [PubMed] [Google Scholar]
- 6.Flach C, Leese M, Heron J, Evans J, Feder G, Sharp D, Howard LM. Antenatal domestic violence, maternal mental health and subsequent child behaviour: A cohort study. BJOG : An International Journal of Obstetrics and Gynaecology. 2011;118(11):1383. doi: 10.1111/j.1471-0528.2011.03040.x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JC, Glass N, Sharps PW, Laughon K, Bloom T. Intimate partner homicide: Review and implications of research and policy. Trauma, Violence & Abuse. 2007;8(3):246–269. doi: 10.1177/1524838007303505. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JC, Abrahams N, Martin L. Perpetration of violence against intimate partners: Health care implications from global data. CMAJ : Canadian Medical Association Journal = Journal De l'Association Medicale Canadienne. 2008;179(6):511–512. doi: 10.1503/cmaj.081145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredland N, McFarlane J, Symes L, Maddoux J, Pennings J, Paulson R, Gilroy H. Modeling the intergenerational impact of partner abuse on maternal and child function at 24 months post outreach: Implications for practice and policy. Nursing Outlook. 2015 doi: 10.1016/j.outlook.2015.10.005. doi:S0029-6554(15)00279-1 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Maddoux JA, Liu F, Symes L, McFarlane J, Paulson R, Binder BK, Gilroy H. Partner abuse of mothers compromises children's behavioral functioning through maternal mental health dysfunction: Analysis of 300 mother-child pairs. Research in Nursing & Health. 2015 doi: 10.1002/nur.21708. [doi] [DOI] [PubMed] [Google Scholar]
- 11.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52(2):119. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Developmental Psychobiology. 2008;50(3):232. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grace T, Bulsara M, Robinson M, Hands B. The impact of maternal gestational stress on motor development in late childhood and adolescence: A longitudinal study. Child Development. 2015 doi: 10.1111/cdev.12449. [doi] [DOI] [PubMed] [Google Scholar]
- 14.Berkman ND, Wallace I, Watson L, Coyne-Beasley T, Cullen K, Wood C, Lohr KN. 2015 doi:NBK305674 [bookaccession] [PubMed] [Google Scholar]
- 15.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, Kogan MD. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [doi] [DOI] [PubMed] [Google Scholar]
- 16.Bell KM, Naugle AE. Intimate partner violence theoretical considerations: Moving towards a contextual framework. Clinical Psychology Review. 2008;28(7):1096–1107. doi: 10.1016/j.cpr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg KJ, Baradaran LP, Abbott CB, Lamb ME, Gutermann E. Type of violence, age, and gender differences in the effects of family violence on children’s behavior problems: A mega-analysis. Developmental Review. 2006;26:89-90-112. [Google Scholar]
- 18.Jaffe PG, Wolfe DA, Wilson SK. Children of battered women. CA: sage; 1990. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus RS. Stress, appraisal, and coping. New York: Springer; 1993. [Google Scholar]
- 20.Zimmerman IL, Castilleja NF. The role of a language scale for infant and preschool assessment. Mental Retardation and Developmental Disabilities Research Reviews. 2005;11(3):238–246. doi: 10.1002/mrdd.20078. [DOI] [PubMed] [Google Scholar]
- 21.Aylward GP. Bayley infant neurodevelopmental screener. San Antonio: The Psychological Corporation; 1995. [Google Scholar]
- 22.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The revised conflict tactics scales (CTS2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17(3):283–316. [Google Scholar]
- 23.Cox JL, Chapman G, Murray D, Jones P. Validation of the edinburgh postnatal depression scale (EPDS) in non-postnatal women. Journal of Affective Disorders. 1996;39(3):185–189. doi: 10.1016/0165-0327(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 24.Curry MA, Burton D, Fields J. The prenatal psychosocial profile: A research and clinical tool. Research in Nursing & Health. 1998;21(3):211. doi: 10.1002/(sici)1098-240x(199806)21:3<211::aid-nur4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 25.Alvarado-Esquivel C, Sifuentes-Alvarez A, Salas-Martinez C. Validation of the edinburgh postpartum depression scale in a population of adult pregnant women in mexico. Journal of Clinical Medicine Research. 2014;6(5):374–378. doi: 10.14740/jocmr1883w. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunevicius A, Kusminskas L, Pop VJ, Pedersen CA, Bunevicius R. Screening for antenatal depression with the edinburgh depression scale. Journal of Psychosomatic Obstetrics and Gynaecology. 2009;30(4):238–243. doi: 10.3109/01674820903230708. [doi] [DOI] [PubMed] [Google Scholar]
- 27.Dennis C- Detection, prevention and treatment of postpartum depression. In: Stewart DE, Robertson E, Dennis C-L, Grace SL, Wallington T, editors. Postpartum depression: Literature review of risk factors and interventions. Canada: 2003. p. 72-73-171. [Google Scholar]
- 28.Huang J, Huang H, Chen H, Lin L, Tseng H, Kao T. Inattention and development of toddlers born in preterm and with low birth weight. The Kaohsiung Journal of Medical Sciences. 2012;28(7):390. doi: 10.1016/j.kjms.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(36):9055. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark MJ, Wright IMR, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2009;297(2):R510. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- 31.Burton PJ, Waddell BJ. 11 beta-hydroxysteroid dehydrogenase in the rat placenta: Developmental changes and the effects of altered glucocorticoid exposure. The Journal of Endocrinology. 1994;143(3):505. doi: 10.1677/joe.0.1430505. [DOI] [PubMed] [Google Scholar]
- 32.Kerzner LS, Stonestreet BS, Wu K, Sadowska G, Malee MP. Antenatal dexamethasone: Effect on ovine placental 11beta-hydroxysteroid dehydrogenase type 2 expression and fetal growth. Pediatric Research. 2002;52(5):706. doi: 10.1203/00006450-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Valla L, Wentzel-Larsen T, Hofoss D, Slinning K. Prevalence of suspected developmental delays in early infancy: Results from a regional population-based longitudinal study. BMC Pediatrics. 2015;15(1) doi: 10.1186/s12887-015-0528-z. 215-015-0528-z. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laplante DP, Barr RG, Brunet A, Galbaud du Fort G, Meaney ML, Saucier J, King S. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research. 2004;56(3):400. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]