Significance
We present the first evidence, to our knowledge, that the autonomic nervous system (ANS) plays a role in associative memory consolidation during sleep. Compared with a Quiet Wake control condition, performance improvement was associated with vagally mediated ANS activity [as measured by high-frequency (HF) heart rate variability (HRV)] during rapid eye movement (REM) sleep. In particular, up to 73% of the proportion of improvement in associative memory performance could be accounted for by considering both traditionally reported sleep features (i.e., minutes spent in sleep stages and sleep spindles) and HF HRV. We hypothesize that central nervous system processes that favor peripheral vagal activity during REM sleep may lead to increases in plasticity that promote associative processing.
Keywords: sleep, heart rate variability, memory, consolidation, vagal activity
Abstract
Throughout history, psychologists and philosophers have proposed that good sleep benefits memory, yet current studies focusing on the relationship between traditionally reported sleep features (e.g., minutes in sleep stages) and changes in memory performance show contradictory findings. This discrepancy suggests that there are events occurring during sleep that have not yet been considered. The autonomic nervous system (ANS) shows strong variation across sleep stages. Also, increases in ANS activity during waking, as measured by heart rate variability (HRV), have been correlated with memory improvement. However, the role of ANS in sleep-dependent memory consolidation has never been examined. Here, we examined whether changes in cardiac ANS activity (HRV) during a daytime nap were related to performance on two memory conditions (Primed and Repeated) and a nonmemory control condition on the Remote Associates Test. In line with prior studies, we found sleep-dependent improvement in the Primed condition compared with the Quiet Wake control condition. Using regression analyses, we compared the proportion of variance in performance associated with traditionally reported sleep features (model 1) vs. sleep features and HRV during sleep (model 2). For both the Primed and Repeated conditions, model 2 (sleep + HRV) predicted performance significantly better (73% and 58% of variance explained, respectively) compared with model 1 (sleep only, 46% and 26% of variance explained, respectively). These findings present the first evidence, to our knowledge, that ANS activity may be one potential mechanism driving sleep-dependent plasticity.
Sleep has been shown to facilitate the transformation of recent experiences into stable, long-term memories (i.e., consolidation), and specific electrophysiological sleep features have been implicated in this process (1). For example, minutes spent in slow wave sleep (SWS) and the number of sleep spindles [transient neural events in nonrapid eye movement (NREM) sleep, 12–15 Hz] in a posttraining sleep period correlate with the magnitude of explicit memory improvement [e.g., conscious, episodic memories (2, 3)]. Minutes in rapid eye movement (REM) sleep, on the other hand, are associated with improvements in implicit memory [e.g., unconscious, priming, procedural skills (4, 5)]. However, recent reviews and meta-analyses of the literature report inconsistencies in these findings (6, 7), suggesting that there may be unexplored factors critical to sleep-dependent memory consolidation.
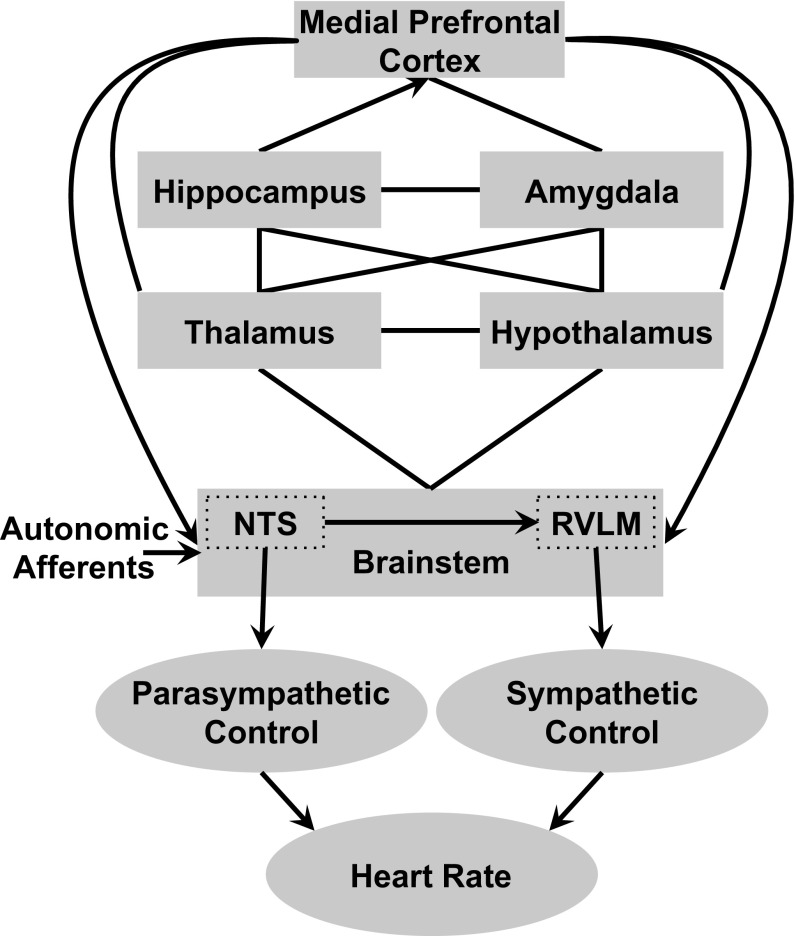
One possible influence that has received little attention in the sleep and memory literature is the autonomic nervous system (ANS). This lack of attention is somewhat surprising, considering that the literature describing ANS modulation of memory during wake is well established (8). Studies have found that memory storage of new information can be enhanced or impaired by directly modifying the activity of peripheral hormones following acquisition (9, 10). Peripheral activity has been purported to affect memory consolidation via vagal afferent nerve fibers, which communicate information about ANS excitation and arousal via projections to the brainstem, which then project to memory-related areas, including the amygdala complex, hippocampus, and prefrontal cortex (PFC) (11). Descending projections from the PFC to autonomic/visceral sites of the hypothalamus and brainstem create a feedback loop allowing for bidirectional communication between central memory areas and peripheral sites (12–14) (Fig. 1).
Fig. 1.
Central nervous system memory-related areas and autonomic control of the heart. ANS fibers from major organs in the periphery, including the heart, innervate the brainstem at the nucleus of the solitary tract (NTS). The brainstem connects with the thalamus, hypothalamus, and other memory-related areas, including the hippocampus, amygdala, and PFC. Descending projections from the PFC to the hypothalamus and brainstem create a feedback loop facilitating the modulation of peripheral activity by the central nervous system, including HRV. Lines denote bidirectional connections, and arrows denote monodirectional projections. Note that, for clarity, we only show a partial representation of the central autonomic network in the current figure. RVLM, rostral ventrolateral medulla. Adapted from ref. 12.
Accordingly, vagus nerve lesions have been shown to impair memory (15, 16). In rats, pairing vagal nerve stimulation with auditory stimuli has been shown to reorganize neural circuits (17) and strengthen neural response to speech sounds in the auditory cortex (18). Also, vagal stimulation has been shown to boost neural representations of paired movements in the motor cortex (19) and to enhance extinction learning of fear memories (20). In humans, vagal nerve stimulation during verbal memory consolidation enhances recognition memory (21, 22). Together, these findings highlight vagal activity as a possible factor influencing plasticity during waking memory consolidation, yet the role of autonomic activity for sleep-dependent memory consolidation has not been examined.
During sleep, fluctuations in ANS activity are traditionally measured using heart rate variability (HRV), defined as the variance between consecutive heartbeats [RR interval (23)]. Prior studies have established that parasympathetic/vagal activity is associated with the high-frequency component of HRV [HF HRV; 0.15–0.40 Hz (23)]. During SWS sleep, studies have reported a reduced heart rate (i.e., a lengthening of RR intervals) coupled with a reduction in overall cardiac ANS activity and a dominance of parasympathetic/vagal activity (HF HRV) compared with wake and REM (24). In addition, REM sleep shows both greater total ANS activity and higher parasympathetic/vagal activity compared with wake and SWS sleep (25).
Waking HF HRV has been associated with cognitive performance, particularly for tasks that rely on PFC activity (26). Higher resting HRV is associated with better working memory (27, 28), sustained attention (29), and efficient attentional control (30). Using functional magnetic resonance imaging, Lane et al. (31) measured HF HRV during retrieval of emotional memories and reported a positive correlation with activity of the medial PFC. In contrast, decreased resting HF HRV is associated with poor cognitive function and maladaptive emotional processing (14, 26). Until now, no studies have investigated the role of HRV during sleep for memory consolidation.
Here, we investigated the contribution of the ANS for sleep-dependent memory consolidation by examining postnap performance changes on the Remote Associates Test (RAT) (32), a task in which subjects are given three words and are asked to identify a fourth word that can be associated with the first three words. The RAT measures creative processing and is associated with PFC activity (33). Furthermore, distinct memory manipulations of the RAT have been shown to rely on REM sleep (4). We tested two different types of memory conditions adopted from Cai et al. (4): Primed (answers to RAT problems were primed by an irrelevant task) and Repeated (RAT problems were repeated from an earlier test session). In the Novel condition, neither problems nor answers were seen before test. Cai et al. (4) tested three groups, Quiet Wake, NREM nap, and NREM + REM nap, on three RAT conditions. Performance in all groups did not change in the Novel condition compared with baseline, whereas it improved in the Repeated condition. However, only the group with REM sleep improved in the Primed condition. In the current study, we repeated this experimental design to investigate the relative contributions of sleep and HRV during sleep to these memory benefits. We specifically examined sleep features previously associated with memory consolidation, including minutes in REM, minutes in SWS, and sleep spindles. For HRV, we focused on the HF HRV component, which has been associated with PFC-related cognition (34, 35). Given the prior literature on autonomic influences on memory consolidation, and the role of REM sleep for performance improvement on this task, we hypothesized that HF HRV activity during REM sleep would be associated with improved RAT performance in the Primed condition, compared with the Repeated and Novel control conditions.
Results
HRV During a Daytime Nap Is Similar to Nocturnal Sleep.
Sleep and HRV parameters during the nap are reported in Tables S1 and S2. HRV fluctuations across the nap resembled prior reports of cardiac ANS activity across nocturnal sleep stages (25, 36). Repeated measures ANOVAs indicated significant differences in total HRV spectral power across sleep stages [F(3,63) = 7.78, P < 0.01, partial eta squared () = 0.27]. Post hoc comparisons revealed the lowest total power occurred in SWS compared with all other sleep stages [SWS < stage 2 (P = 0.001), < REM (P = 0.001)]. REM sleep showed the highest total power, which was significantly greater than SWS (P < 0.001). Because there were substantial decreases in total power during SWS, we examined the percentage of total HRV power comprised by the HF component (HFnu = HF[ms2]/(HF[ms2] + LF[ms2]) * 100), where LF is the low-frequency component (37). Here, we discovered that substantial changes also occurred in the normalized HF HRV component [F(3,63) = 12.04, P < 0.001, = 0.36], with greatest normalized HF HRV during SWS compared with stage 2 (P < 0.003), REM (P < 0.001), and prenap wake (P = 0.05). Additionally, there was higher normalized HF HRV during stage 2 compared with REM (P = 0.045), but not compared with prenap wake (P = 0.10). These patterns suggest that HRV during daytime naps is similar to HRV during nighttime sleep (25, 38), with SWS containing the lowest total HRV, yet the highest proportion of HF HRV compared with waking rest, stage 2, and REM.
Table S1.
Sleep summary
| Sleep summary | Mean | SD | Minimum | Maximum |
| TIB, min | 103.7 | 10.54 | 80 | 129.5 |
| TST, min | 83.19 | 18.10 | 11.5 | 104.0 |
| Stage 1, min | 7.60 | 4.32 | 2.0 | 23.5 |
| Stage 2, min | 39.72 | 12.74 | 8.0 | 66.5 |
| SWS, min | 22.71 | 14.83 | 0.0 | 52.5 |
| REM, min | 13.15 | 10.58 | 0.0 | 38.5 |
| WASO, min | 12.01 | 15.38 | 0.5 | 54.5 |
| SE, % | 81.47 | 18.95 | 14.4 | 98.4 |
SE, sleep efficiency; TIB, time in bed; TST, total sleep time; WASO, wake after sleep onset.
Table S2.
HRV summary
| HRV parameters | Quiet Wake | Prenap | Stage 2 | SWS | REM |
| HF, ms2 | 1,519 ± 2,040 | 1,265 ± 767 | 2,031 ± 1,367 | 1,581 ± 902 | 2,202 ± 2,040 |
| Total power, ms2 | 7,203 ± 6,411 | 4,910 ± 4,367 | 5,471 ± 3,392 | 3,108 ± 2,709 | 7,268 ± 4,015 |
| HFnu | 42.37 ± 12.11 | 58.10 ± 15.68 | 58.57 ± 14.74 | 68.95 ± 12.25 | 48.87 ± 13.41 |
HF, high frequency HRV; HFnu, normalized HF HRV component. HRV parameters are reported as mean ± SD.
RAT Performance.
Baseline performance.
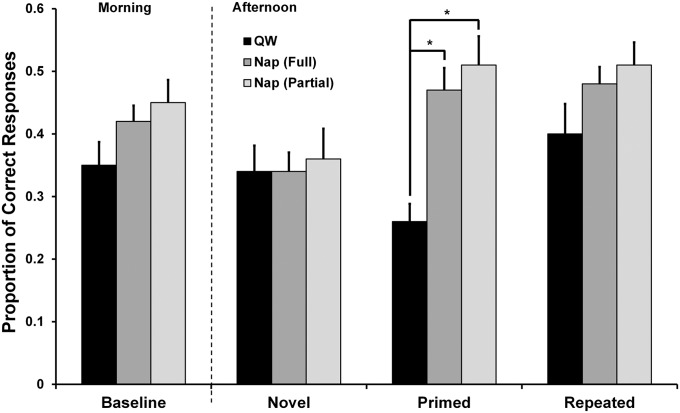
We first compared baseline scores between groups, but found a nonsignificant difference [t(54) = 1.76, P = 0.08]. Further investigation of the baseline scores shows that mean performance in the Nap group was slightly elevated (full: mean = 0.42, partial: mean = 0.45; Table 1). This elevated performance in nappers was likely due to random error, because the mean performance scores at baseline in the Quiet Wake (QW) group and the Novel condition for both nappers and QW subjects were similar (mean scores ∼ 0.35) (Fig. 2).
Table 1.
Behavioral performance on the RAT: Proportion correct
| Condition | QW, n = 21 | Nap, full, n = 35 | Nap, partial, n = 17 |
| Baseline | 0.35 ± 0.18 | 0.42 ± 0.15 | 0.45 ± 0.15 |
| Novel | 0.34 ± 0.19 | 0.34 ± 0.18 | 0.36 ± 0.20 |
| Primed | 0.26 ± 0.13 | 0.47 ± 0.21 | 0.51 ± 0.19 |
| Repeated | 0.40 ± 0.22 | 0.48 ± 0.16 | 0.51 ± 0.15 |
Nap (full) represents the whole Nap sample. Nap (partial) represents the subset of nap subjects used in the regression analyses. Data are reported as mean ± SD.
Fig. 2.
Sleep boosts performance in the Primed condition on the RAT. Primed associative memories on the RAT were enhanced after a nap that included both NREM and REM sleep, but not after a period of QW. No improvement was seen the Nap group in the Novel or Repeated condition compared with QW, although in the Repeated condition, the Nap group nominally performed better than the QW group. Note that these results are in line with previous research (4). Error bars represent ±1 SEM. Asterisks represent significance at P < 0.05.
Naps with REM and SWS benefit primed memory performance: Full sample.
Next, we used a multivariate ANOVA (MANOVA) with group (Nap and QW) as the independent variable; performance (proportion correct at PM) in Novel, Primed, and Repeated conditions as dependent variables; and baseline performance as a covariate. This analysis revealed a significant effect of group [F(3,51) = 6.57, P = 0.001, = 0.28]. In line with Cai et al. (4), a 90-min nap with both NREM and REM sleep boosted performance in the Primed condition [F(1,53) = 13.63, P = 0.001, = 0.20] above QW. No group differences were found in the Repeated [F(1,53) = 0.01, P = 0.92, < 0.01] or Novel [F(1,53) = 0.87, P = 0.36, = 0.02] condition. Interestingly, we noted that compared with baseline performance, the QW group showed a decrease in performance in the Primed condition [t(20) = −2.63, P = 0.02], an increase in performance in the Repeated condition [t(20) = 2.06, P = 0.05], and no difference in the Novel condition [t(20) = −0.09, P = 0.93]. We hypothesize that the decreased Primed performance may be related to an inability in the QW group to disengage from the associations that were set up by the analogies task, which impaired their ability to generate new associations to the primed words in the RAT task. However, more research is required to tease apart this result. Additionally, the increase in the Repeated condition is consistent with previous findings and supports the notion that incubation of previously exposed problems may be sufficient to facilitate consolidation (4).
Naps with REM and SWS benefit primed memory performance: Partial sample.
Due to the restrictions of HRV calculations (SI Materials and Methods), we could only conduct regressions on a subset of subjects (17 nappers). To ensure similar performance profiles as the full sample, we tested performance differences in this partial sample. First, we found similar nonsignificant, yet trending, baseline differences between nappers and QW in the partial sample [t(36) = 1.85, P = 0.07]. Next, we used a MANOVA on group (Nap and QW) and performance (proportion correct at PM) in Novel, Primed, and Repeated conditions, again controlling for baseline performance. This analysis revealed a significant effect of condition [F(3,33) = 6.66, P = 0.001, = 0.38]. Similar to the full sample, analyses revealed a 90-min nap with both NREM and REM sleep boosted performance in the Primed condition [F(1,35) = 16.08, P < 0.001, = 0.31] above QW. Neither the Repeated condition [F(1,35) = 0.04, P = 0.84, = 0.001] nor the Novel condition [F(1,35) = 0.79, P = 0.38, = 0.02] differed for nappers and QW.
HRV during Quiet Wake is not associated with performance.
No significant correlations were revealed between HF HRV in the Quiet Wake condition and performance [Novel: Pearson correlation coefficient (r) = −0.17, P = 0.48; Primed: r = −0.28, P = 0.23; Repeated: r = 0.006, P = 0.98].
Sleep and HRV do not predict novel performance.
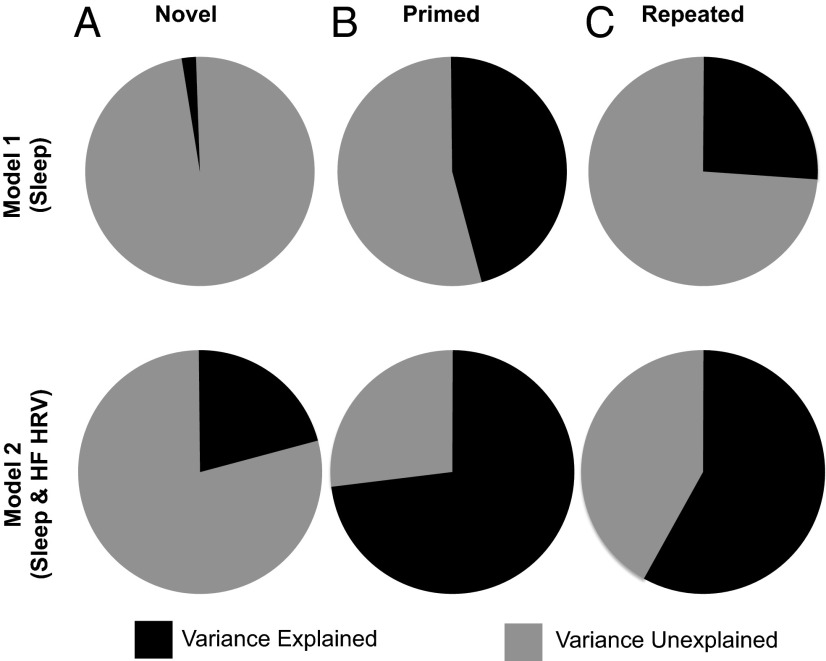
Using a regression framework, we examined the amount of performance change (computed as proportion correct PM − proportion correct AM/proportion correct AM * 100; Table 2 and SI Materials and Methods) in each condition accounted for by the sleep predictors in model 1, as well as the sleep and HRV predictors in model 2. Bivariate relationships between each predictor and the outcomes are reported in Table S3. For the Novel condition, sleep variables did not significantly predict performance in model 1 [F(3,13) = 0.89, P = 0.47]. After adding the HRV variables in model 2, this model was still not significant [F(5,11) = 1.86, P = 0.18; Fig. 3A and Table S4]. These results agree with and extend previous findings demonstrating that native creative abilities are not modulated by sleep (4) or HRV during sleep.
Table 2.
Behavioral performance on the RAT: Percent change
| Condition | QW, n = 21 | Nap, full, n = 35 | Nap, partial, n = 17 |
| Novel | 3.66 ± 56.21 | −13.36 ± 51.66 | −17.91 ± 39.60 |
| Primed | −4.81 ± 46.76 | 20.22 ± 70.39 | 26.71 ± 68.82 |
| Repeated | 11.89 ± 40.73 | 17.61 ± 33.23 | 17.84 ± 31.55 |
Nap (full) represents the whole Nap sample. Nap (partial) represents the subset of nap subjects used in the regression analyses. Data are reported as mean ± SD.
Table S3.
Bivariate correlations between performance, sleep, and HRV for the partial Nap group (n = 17)
| RAT | Sleep features | HRV-nap | |||||
| Variable | Primed, r; p | Repeated, r; p | SWS, r; p | REM, r; p | Spindles, r; p | HF SWS, r; p | HF REM, r; p |
| Novel | 0.21; 0.42 | 0.35; 0.17 | 0.07; 0.80 | 0.31; 0.23 | 0.24; 0.36 | 0.37; 0.15 | 0.41; 0.10 |
| Primed | — | 0.82; 0.001 | −0.02; 0.92 | −0.52; 0.03 | 0.51; 0.03 | 0.68; 0.002 | 0.65; 0.005 |
| Repeated | — | −0.33; 0.20 | −0.22; 0.40 | 0.50; 0.04 | 0.58; 0.01 | 0.70; 0.002 | |
| SWS | — | −0.14; 0.57 | −0.09; 0.74 | 0.01; 0.97 | −0.08; 0.76 | ||
| REM | — | −0.01; 0.99 | −0.44; 0.08 | 0.01; 0.99 | |||
| Spindles | — | 0.49; 0.05 | 0.32; 0.21 | ||||
| HF SWS | — | 0.47; 0.06 | |||||
| HF REM | — | ||||||
Bold text indicates significance after Bonferroni correction (α = 0.05, p = 0.002). r, Pearson correlation coefficient; p, p value.
Fig. 3.
Proportion of variance in associative memory improvement accounted for by sleep and autonomic activity during sleep. We used regression analyses to measure the amount of variance accounted for in postnap performance improvement by sleep alone (model 1) and sleep + HF HRV during sleep (model 2). (A) The Novel condition showed no benefits from a nap (Fig. 2), and neither model accounted for a significant amount of variance in performance (model 1: Adj R2 = −0.02, P = 0.47; model 2: Adj R2 = 0.21, P = 0.18; negative variance indicated by counterclockwise angle meter). (B) In the Primed condition, which showed significant increases in postnap performance, autonomic activity played a significant role in sleep-dependent consolidation. Specifically, sleep alone (SWS minutes, REM minutes, and sleep spindles) significantly predicted improvement (model 1: Adj R2 = 0.46, P = 0.01), but sleep plus HF HRV predicted a greater amount of behavioral change (model 2: Adj R2 = 0.73, P = 0.001). (C) In the Repeated condition, sleep alone predicted a portion of performance change (model 1: Adj R2 = 0.26, P = 0.07), whereas sleep + HF HRV significantly increased the amount of variance accounted for in performance change (model 2: Adjusted R2 = 0.58, P = 0.009).
Table S4.
Regression summary for model 1 and model 2
| Model | Adj R2 | F | df | P | β | t | P | sr2 |
| Novel | ||||||||
| Model I | −0.02 | 0.89 | 3.13 | 0.47 | ||||
| SWS | 0.13 | 0.52 | 0.61 | 0.13 | ||||
| REM | 0.33 | 1.30 | 0.22 | 0.32 | ||||
| Spindles | 0.25 | 0.98 | 0.34 | 0.25 | ||||
| Model II | 0.21 | 1.85 | 5.11 | 0.18 | ||||
| SWS | 0.15 | 0.66 | 0.52 | 0.15 | ||||
| REM | 0.59 | 2.21 | 0.05 | 0.49 | ||||
| Spindles | −0.09 | −0.34 | 0.74 | −0.07 | ||||
| HF SWS | 0.58 | 1.78 | 0.10 | 0.40 | ||||
| HF REM | 0.18 | 0.67 | 0.51 | 0.15 | ||||
| Primed | ||||||||
| Model I | 0.46 | 5.47 | 3.13 | 0.01 | ||||
| SWS | −0.06 | -0.30 | 0.77 | −0.05 | ||||
| REM | −0.55 | −2.93 | 0.01 | −0.54 | ||||
| Spindles | 0.51 | 2.76 | 0.02 | 0.51 | ||||
| Model II | 0.73 | 9.76 | 5.11 | 0.001 | ||||
| SWS | 0.03 | −0.21 | 0.84 | −0.03 | ||||
| REM | −0.51 | −3.32 | 0.007 | −0.43 | ||||
| Spindles | 0.32 | 2.10 | 0.06 | 0.27 | ||||
| HF SWS | 0.06 | 0.33 | 0.75 | 0.04 | ||||
| HF REM | 0.51 | 3.38 | 0.006 | 0.44 | ||||
| Repeated | ||||||||
| Model I | 0.26 | 2.89 | 3.13 | 0.07 | ||||
| SWS | −0.32 | −1.49 | 0.16 | −0.32 | ||||
| REM | −0.26 | −1.21 | 0.25 | −0.26 | ||||
| Spindles | 0.47 | 2.19 | 0.05 | 0.47 | ||||
| Model II | 0.58 | 5.47 | 5.11 | 0.009 | ||||
| SWS | −0.29 | −1.79 | 0.10 | −0.29 | ||||
| REM | −0.20 | −1.03 | 0.32 | −0.17 | ||||
| Spindles | 0.24 | 1.24 | 0.24 | 0.20 | ||||
| HF SWS | 0.13 | 0.55 | 0.59 | 0.09 | ||||
| HF REM | 0.54 | 2.84 | 0.02 | 0.46 |
Bold text indicates significance at P ≤ 0.05. sr2, squared semipartial.
Sleep and HRV predict primed performance.
For the Primed condition, the set of sleep variables in model 1 accounted for 46% of the variance in postnap performance [adjusted (Adj) R2 = 0.46, F(3, 13) = 5.47, P = 0.012; Fig. 3], with REM minutes (β = −0.55, P = 0.01) and sleep spindles (β = 0.51, P = 0.02) emerging as significant predictors, whereas time spent in SWS was not a significant predictor (β = −0.06, P = 0.77; Table S4). When we added the HRV variables in model 2, the variance accounted for by the model significantly increased from 46% to 73% [Adj R2 = 0.73, F(5,11) = 9.76, P = 0.001], with REM minutes (β = −0.51, P = 0.007) remaining a strong predictor, as well as HF HRV during REM (β = 0.51, P = 0.006; Fig. 3B). After accounting for ANS activity, sleep spindles decreased in significance (β = 0.32, P = 0.063). Interestingly, REM minutes were negatively related to performance in both model 1 (sleep variables) and model 2 (sleep and HRV variables). In contrast, higher HF HRV during REM predicted better performance. We further explored this apparent inverse relationship between HRV and REM sleep by testing if these factors are inversely related to each other using a Pearson correlation coefficient. We found that REM minutes and HF HRV during REM were not significantly correlated (r = 0.01, P = 0.99), which suggests independence between these two variables. These findings demonstrate a role for ANS activity during sleep in predicting memory consolidation and suggest separable contributions from sleep and HF HRV during sleep for sleep-dependent memory consolidation.
Sleep and HRV predict repeated performance.
In the Repeated condition, model 1 (sleep variables) accounted for a marginally significant amount (26%) of the variance in performance [Adj R2 = 0.26, F(3, 13) = 2.89, P = 0.08], with sleep spindles as the only significant contributor to this model (β = 0.47, P = 0.05). However, model 2 (sleep and HRV variables) significantly increased the variance explained to 58% [Adj R2 = 0.58, F(5,11) = 5.47, P = 0.009]. Here, sleep spindles were no longer a significant contributor (β = 0.24, P = 0.24). Instead, HF HRV during REM was a predictor (β = 0.54, P = 0.02). Again, similar to the Primed condition, greater HF HRV during REM was associated with better performance. Our model 1 result is consistent with previous literature implicating sleep spindles as a marker of explicit memory consolidation (39). However, model 2 suggests HF HRV may be an even better predictor of explicit memory improvement, implicating previously unidentified avenues of research into how autonomic fluctuations interact with known markers of sleep-dependent plasticity. Interestingly, here and in the study by Cai et al. (4), sleep and quiet wake both contributed to increased performance in the Repeated condition. It is therefore possible that two parallel mechanisms may facilitate performance improvement in the explicit memory condition, one during a quiet wake episode involving rehearsal or rumination on the RAT items, and the other involving memory reactivation processes during sleep. This idea is consistent with the hypothesis that declarative memories consolidate in an opportunistic manner (40), such as in times of low external input (i.e., during a quiet wake period or during sleep), as has been shown in auditory tone sequence learning (41), visual search (42), and pursuit motor tasks (43). In contrast, some nondeclarative information, such as our Primed condition here, mainly rely on sleep processes (5, 44, 45). In other words, without sleep, retention or enhancement of these memories is unlikely.
SI Materials and Methods
Participants.
Eighty-one healthy, nonsmoking participants (age = 21.79 ± 3.29 y, 31 females) with no personal history of neurological, psychological, or other chronic illness provided informed consent, which was approved by the University of California, Riverside Human Research Review Board. Participants had a regular sleep-wake schedule (reporting a habitual time in bed of 7–9 h per night as assessed by actigraphy), and no presence or history of sleep, psychiatric, cardiovascular, or neurological disorder determined during an in-person, online, or telephone interview. Participants received monetary compensation for participating in the study.
Procedure.
Participants wore actigraphs to monitor sleep-wake activity 1 wk before the experiment to ensure participants were not sleep-deprived and spent at least 6.5 h in bed the night before their visit. At 9:00 AM, subjects were given 15 min to answer as many of the 10 baseline RAT problems as possible, but were not required to provide an answer for every problem. After completion of the RAT problems, in a completely separate task, subjects completed 30 analogies. Subjects were given no time limit for the analogies, and were required to provide one answer for each analogy. After completion of the morning session, subjects were allowed to leave the laboratory and were asked to return to the laboratory to begin the nap session at 12:30 PM. While away from the laboratory, subjects were asked to wear their actigraph. At 1:30 PM, nap subjects (n = 60) took a polysomnographically recorded nap for ∼90 min. They were given a total time of 2 h in bed to sleep. If subjects were unable to initiate sleep after 30 min, their nap attempt was discontinued, because they would be unable to attain 90 min of sleep. Sleep was monitored online by a trained sleep technician. Nap sleep staging is reported in Tables S1 and S2. In the QW group (n = 21), subjects were attached to electroencephalographic (EEG) electrodes and watched a nature videotape lasting 50 min while lying in bed. This session ended at 3:30 PM, and subjects were allowed to leave the laboratory. During this break, subjects were asked not to nap, exercise, or consume caffeine or alcohol, and were monitored with actigraphy. At 5:00 PM, subjects returned to the laboratory and were tested on 30 RAT problems with a time limit of 40 min. Ten problems were exactly the same as the AM baseline RAT problems (Repeated condition), 10 problems had the same answers as 10 (out of 30) items of the AM analogy task (Primed condition), and 10 problems were completely novel (Novel condition).
RAT.
A computerized version of the task was adapted from Mednick (32). Each RAT problem contains a triplet of words presented horizontally along with a blank space. For each problem, the subject has to combine or relate the three words drawn from mutually remote associative clusters (e.g., COOKIES, SIXTEEN, HEART: ______). The subject is required to find a fourth word that serves as an associative link between these three words. The answer to this example problem is SWEET (e.g., cookies are sweet, sweet sixteen, sweetheart). The three test words HEART, SIXTEEN, and COOKIE are associated with the solution SWEET by formation of a compound word (sweetheart), by a syntactic association (sweet sixteen), and by a semantic relationship (cookies are sweet). Subjects were given this problem as an example of the ways in which the fourth word could be linked to the other three, but were informed that the correct answer could be connected to the other three words in many ways. Subjects were read the instructions aloud and were given four practice problems to ensure understanding of the task.
A total of 90 RAT problems were compiled in the current experiment. These 90 items were further parsed into three distinct groups with 30 problems each. Additionally, we varied which set of 10 problems in each group of 30 would be assigned to Novel, Repeated, and Primed conditions. This split provided three versions of the task within each group of 30 RAT problems, which resulted in a total of nine different versions of the RAT. These nine versions were counterbalanced across subjects.
We calculated two performance measures for the task: (i) proportion correct = number of correct RAT items in each subcategory (Baseline, Novel, Repeated, Primed)/total number of items in that subcategory, and (ii) performance change = proportion correct PM − proportion correct AM/AM * 100. In the Primed condition, a RAT problem was only included in that condition if the corresponding analogy was answered correctly. Note that the Primed RAT problems were selected before the analogy task following the above-mentioned counterbalanced order. As a consequence, on average, of the 10 analogy items used as primes, fewer than two were discarded due to this restriction across the subjects (mean = 8.75, SD = 1.1).
Analogies Task.
Thirty analogies (e.g., FAST: SLOW as HARD: E____) were administered at the end of the AM session. The first letter of each answer was given. Subjects were required to provide an answer for all of the analogies and were not given a time limit. Ten of the analogy answers served as primes for the answers to the RAT items administered in the primed condition during the PM session.
Polysomnography.
Polysomnography data were collected using Astro-Med Grass Heritage Model 15 amplifiers with Grass GAMMA software. Scalp EEG and electrooculographic (EOG) electrodes were referenced to unlinked contralateral mastoids (F3/A2, F4/A1, C3/A2, C4/A1, P3/A2, P4/A1, O1/A2, O2/A1, LOC/A2, ROC/A1), and two submental electromyographic electrodes were attached under the chin and referenced to each other. High-pass filters were set at 0.3 Hz, and low-pass filters were set at 35 Hz for EEG and EOG electrodes. Raw data were visually scored in 30-s epochs according to Rechtschaffen and Kales (69). Sleep spindles in NREM sleep were detected with an automated spindle detector that used both MATLAB routines and BrainVision Analyzer software (70). Here, we report data from the parietal EEG channels because prior studies have shown that the greatest spindle power (12–15 Hz) occurs over these sites (71–73).
Electrocardiogram and HRV Analysis.
Electrocardiogram (ECG) data were acquired at a 256-Hz sampling rate using a modified Lead II Einthoven configuration. We analyzed HRV of the R-waves series across the whole sleep/wake period using Kubios HRV Analysis Software 2.0 (MATLAB), according to the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology guidelines (37). Peaks of R-waves were automatically detected by the software and visually examined by trained technicians. Incorrectly detected R-peaks were manually edited. Missing and ectopic beats were corrected via cubic spline interpolation. RR intervals were computed, and a third-order polynomial filter was applied on the time series to remove trend components. Fast Fourier transform quantified the absolute spectral power in the HF (0.15–0.40 Hz) and LF (0.004–0.15 Hz) bands, and total power (a summation of HF power, LF power, and all other spectral components comprising the RR interval; measured in square milliseconds). From these measures, we derived the HF normalized unit as follows: HF[ms2]/(HF[ms2] + LF[ms2]) * 100).
Data Reduction and Statistical Analyses.
For the analysis of RR and frequency-domain HRV measures during different sleep stages, consecutive, artifact-free, 5-min windows of undisturbed sleep (i.e., free from stage transitions) were selected across the nap. Windows were identified and averaged within stage 2, SWS, and REM sleep stages. This method emphasizes consolidated sleep stages and resulted in different percentages of ECG epochs for the HRV analysis. As such, 49% of stage 2, 76% of SWS, and 66% of REM epochs were used for the current analyses. We also analyzed 5 min of prenap wakefulness (Wake). Epochs of stage 1 and wake after sleep onset were not analyzed, because these periods have not been previously reported to contribute to memory consolidation. Because naps have more fragmented sleep due to increased stage transitions, the traditional method of HRV analysis decreased the number of subjects that could be analyzed. Advancement in HRV measurement techniques could allow for more data retention.
Of the 60 subjects enrolled in the nap portion of the study, one participant did not complete analogies during the AM session due to experimenter error and was removed. For 11 nap subjects, we were unable to detect an RR interval and were unable to perform HRV analyses for these subjects, so they were excluded. Because previous data have indicated that REM sleep was important for this task, we further parsed the data into those subjects who received both NREM and REM sleep (n = 35) and those subjects who did not (n = 13). Additionally, because research has suggested that NREM sleep and quiet wake result in similar benefits for the RAT task (4), we focused only on those subjects who received both NREM and REM sleep and compared these subjects with those subjects in a Quiet Wake condition. For the regression analyses, only those subjects who were represented on all five variables (SWS minutes, REM minutes, number of sleep spindles, and HF HRV during SWS and REM) were entered into the models (n = 17).
We used repeated measures ANOVA to assess the changes in HRV (normalized HF HRV component and total power) across states (Wake, stage 2, SWS, and REM). The Huynh–Feldt correction was applied when sphericity assumptions were violated. Additionally, we used a MANOVA to compare performance (proportion correct at test and percent improvement) between Nap and QW groups in all three RAT conditions (Novel, Repeated, and Primed), with baseline performance as a covariate. Tukey’s honest significant difference tests with Bonferroni corrections were used for all post hoc comparisons and η2p is reported for effect size. Pearson correlation coefficients (Bonferroni-corrected) were used to examine the bivariate relationship between HF HRV during sleep, minutes in each sleep stage, sleep spindles, and all performance conditions. Multiple linear regression analyses were used to develop models that could predict performance change on each RAT condition from sleep parameters and HF HRV during sleep. For each model (Novel, Repeated, and Primed), percent improvement was the dependent variable and sleep and/or HRV features during sleep were the independent variables (SWS minutes, REM minutes, number of sleep spindles, and HF HRV during SWS and REM). We used a hierarchical regression approach and entered the sleep variables (SWS minutes, REM minutes, and number of sleep spindles; mean-centered) into the regression first, allowing these three variables to account for variance in performance change before entering in the HRV variables (HF HRV during SWS and REM; mean-centered). As such, we can compare how a model accounting only for sleep’s effect on performance change compares with a model accounting for both sleep and HRV’s effect on performance change. Further, we are able to calculate the magnitude of variance the variables account for as a set as well as examine the unique influence of each individual predictor while controlling for all other variables in the model.
Discussion
Here, we present the first evidence, to our knowledge, that the ANS plays a significant and substantial role in associative memory consolidation during sleep. We showed that vagally mediated ANS activity during sleep (i) is associated with the consolidation of implicit and explicit information and (ii) is stage specific. Additionally, the combination of sleep features and HF HRV during REM sleep accounted for up to 73% of the variance in associative memory enhancement. Although SWS was not a significant predictor of performance, sleep spindles contributed to performance benefits in model 1 in both Primed and Repeated conditions, and this result extends previous research implicating sleep spindles in explicit verbal memory consolidation (3, 46) and suggests a role for spindles in predicting implicit memory performance (47) as well. However, after the addition of HF HRV in model 2, the contribution of sleep spindles was reduced in both Repeated and Primed models. In fact, HF HRV during REM was the only consistent predictor across both models. Specifically, HF HRV during REM sleep was a positive predictor of RAT performance in the Primed and Repeated conditions, whereas time spent in REM sleep was negatively associated with Primed memory performance. We did not find a relationship between ANS activity during wake and any of the memory conditions, or with ANS activity during sleep and the Novel condition, suggesting a specific effect of ANS activity with sleep-related consolidation and not with a general improvement of creative ability. Together, these findings demonstrate a role for ANS activity during sleep in predicting memory-related associative processing and highlight a dissociation between time spent in REM sleep and vagal activity during REM sleep for sleep-dependent, associative memory consolidation.
Indeed, REM sleep was a negative predictor for Primed performance. This result could be related to several factors. Some subjects had atypically long durations of REM sleep. On average, a 90-min nap will typically comprise about 15–20 min of REM sleep (5, 48, 49). REM varied greatly across our subjects (1.5–38.5 min). Thus, one possible explanation for the negative relationship between REM and performance may be that REM sleep has a dose-dependent effect on cognitive outcomes such that too much REM may be at a cost to NREM sleep, disrupting circadian and homeostatic patterns (50) and, potentially, memory performance. One way to test this hypothesis would be to manipulate the timing of the nap to modulate the duration of REM sleep experimentally (e.g., early morning naps vs. late afternoon naps) and compare performance across these different nap conditions. Another explanation that lends itself to the current data is that increased REM sleep was associated with increased forgetting, and therefore worse memory, in the Primed condition. Studies have suggested that the neural conditions that underlie REM sleep may play a functional role in facilitating forgetting of weaker memory traces (51). In the current dataset, associations initiated during the analogy task may have instantiated weaker memories that were downscaled during REM sleep (52), resulting in the inverse relationship between REM sleep and performance indicated in the current study. Other experimental techniques, such as intracranial recordings in humans to assess electrophysiological events like pontine-geniculate-occipital waves during REM sleep (53), may be necessary to reveal relationships between complex performance outcomes and underlying neural mechanisms. Taken together, the negative relationship with performance suggests that there may be several heretofore unconsidered factors responsible for REM-dependent memory consolidation.
Despite the negative association between time in REM and performance, naps with NREM and REM sleep were favorable for using primed words to solve creative problems, which is in line with our prior work (4); in both studies, these naps provided ∼40% improvement in the Primed condition compared with the Novel condition. It is also important to note that the regression analyses revealed that REM sleep accounted for a greater amount of variance compared with all other sleep features. This result suggests that REM sleep offers a unique opportunity for highly associative processing. Creativity and associative thinking have been thought to require the formation of “associative elements into new combinations, which either meet specified requirements or are in some way useful” (32). This leap away from existing associations has been hypothesized to require changes to memory that involve the disintegration of existing schemas to ignite new and useful combinations (54). REM sleep may facilitate schema disintegration due to its highly associative nature. In the current study, REM sleep may have facilitated subjects’ ability to break the schematic relationship between words presented as analogies and enhanced their ability to use the analogy answers in a new and useful way to complete the unrelated RAT problems. In contrast, without sleep, subjects may have had difficulty in breaking with the associations set up by the analogies task, and thus showed decreased ability to use the primed words in the subsequent creativity task.
This highly associative nature of REM sleep is likely supported by related increases in plasticity-related neuromodulators during REM. The neuromodulator profile of REM sleep reveals a powerful boost in acetylcholine, combined with a diminished role of norepinephrine and serotonin (55). In fact, REM sleep has been shown to have the highest levels of cortical acetylcholine compared with active wake, quiet wake, and NREM sleep (56–59). Acetylcholine allows for induction and maintenance of long-term potentiation (60, 61), which is considered a possible mechanism of synaptic plasticity in implicit learning (62). This period of high plasticity and low sensory input (sleep) may situate REM sleep as a brain state optimized for making connections between disparate ideas, which is a principal definition of creativity (32).
Importantly, animal studies have shown that stimulating the vagus nerve leads to a release of plasticity-related neuromodulators in the brain, including acetylcholine and norepinephrine (63). Release of these neuromodulators has been assumed to underlie the augmentation in learning associated with vagal nerve stimulation during wake (17, 19, 21). Furthermore, central-acting cholinergic antagonists have been shown to block vagus nerve enhancement of neural synchronicity and plasticity effectively (63). However, no vagal nerve stimulation studies in humans or animals have investigated vagal stimulation in brain states outside of wake. Our study suggests that REM sleep, a period of high acetylcholine (but low norepinephrine) and vagal activity, may be a brain state optimally positioned to enhance plasticity. In this view, HRV may be a proxy measure of the level of these ongoing plasticity processes.
The current results complement the neurovisceral integration model (12, 14), which posits that constant dialogue between cardiac and central brain structures (e.g., PFC, amygdala), via vagal afferents, modulates cognitive and emotional processing (14, 35). Indeed, during wake, the PFC integrates sensory, limbic, and autonomic information (64), and HRV is proposed to be an index of this activity (35). We believe that the consideration of sleep within this framework of sleep-dependent consolidation of long-term memories via hippocampal-PFC dialogue could be an important addition to this model (Fig. 1). Specifically, during REM sleep, the PFC shows increased neural activity compared with other sleep stages (65). Additionally, the interactions between medial PFC and the hippocampus during offline consolidation periods seem to play a critical role in the integration and reorganization of new information into preexisting knowledge networks (66). Further, during REM sleep, there is an increase in overall cardiac autonomic activity (24) accompanied by an increase in central control of cardiac regulatory patterns compared with NREM sleep (67). This pattern of activity leads us to speculate that central or peripheral conditions that affect HRV may also influence memory consolidation during sleep, and HF HRV may be an index of these processes.
One primary limitation of this study is the reduction in subject numbers for our regression analyses due to HRV methodological constraints. Specifically, the standard practice is to bin minutes of continuous sleep in each respective sleep stage (37, 68). Previous work from our group has shown that in daytime naps, 5-min bins produce a reliable measure of HRV across nap periods with similar HRV profiles to nighttime sleep (38). Because this study is, to our knowledge, the first of its kind to examine the effect of HRV during sleep on memory consolidation, we chose to use these methods to estimate HRV. Due to the large amount of sleep transitions present in a daytime nap, this approach limits the total amount of data available for the regression analyses. Specifically, subjects may have SWS or REM sleep in their nap, allowing us to include them in the behavioral analyses; however, 5 min of undisturbed sleep in each sleep stage is not as common in daytime naps and confines the total sample considerably. To retain more statistical power, future studies should explore these relationships in a nocturnal sleep paradigm. Another drawback of the current study is the lack of NREM-only naps available for analysis (n = 8). As such, we were unable to examine the added benefit of REM sleep directly and reliably above and beyond NREM sleep only. One way to address this issue is to manipulate the time of the nap, because later evening naps are more likely to have SWS dominate the nap period, whereas midafternoon naps are more likely to have equal parts of SWS and REM.
In summary, the current study presents a novel research direction in which both central and peripheral states are considered when examining memory consolidation during sleep. We propose that during sleep, the central nervous system and ANS communicate bidirectionally and that the neural dynamics of the REM brain combine with increased parasympathetic activity to create an optimized internal environment, specific to REM sleep, that promotes creative associative memory processing.
Materials and Methods
Eighty-one healthy, nonsmoking participants (age = 21.79 ± 3.29 y, 31 females) with no personal history of neurological, psychological, or other chronic illness provided informed consent, which was approved by the University of California, Riverside Human Research Review Board. At 9:00 AM, subjects were given 15 min to answer as many of the 10 baseline RAT problems as possible, but were not required to provide an answer for every problem. For each problem, the subject has to combine or relate the three words drawn from mutually remote associative clusters (e.g., COOKIES, SIXTEEN, HEART: ______). The subject is required to find a fourth word that serves as an associative link between these three words. The answer to this example problem is SWEET (e.g., cookies are sweet, sweet sixteen, sweetheart). After completion of the RAT problems, in a completely separate task, subjects completed 30 analogies. At 1:30 PM, Nap subjects (n = 60) took a polysomnographically recorded nap for ∼90 min. In the QW group (n = 21), subjects were attached to electroencephalographic electrodes and watched a nature videotape lasting 50 min while lying in bed. At 5:00 PM, subjects returned to the laboratory and were tested on 30 RAT problems with a time limit of 40 min. Ten problems were exactly the same as the AM baseline RAT problems (Repeated condition), 10 problems had the same answers as 10 (out of 30) of the AM analogies (Primed condition), and 10 problems were completely novel (Novel condition). We analyzed HRV of the R-waves series across the whole sleep/wake period using Kubios HRV Analysis Software 2.0 (MATLAB), according to the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology guidelines (66). Fast Fourier transformation quantified the absolute spectral power in the HF (0.15–0.40 Hz) and LF bands (0.004–0.15 Hz) and total power (measured in square milliseconds). We used a MANOVA to compare performance (proportion correct at test) between Nap and QW groups in all three RAT conditions: Novel, Primed, and Repeated. Tukey’s honest significant difference (HSD) tests with Bonferroni corrections were used for all post hoc comparisons and η2p is reported for effect size. Pearson correlations were used to examine the bivariate relationship between HF HRV during sleep, minutes in each sleep stage, sleep spindles, and all performance conditions. Multiple linear regression analyses were used to develop models that could predict performance change on each RAT condition from sleep parameters and HF HRV during sleep.
Acknowledgments
In memoriam, Sarnoff A. Mednick, PhD and Dr. Med.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518202113/-/DCSupplemental.
References
- 1.Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens Z, Fabó D, Halász P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Schabus M, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23(7):1738–1746. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 4.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci USA. 2009;106(25):10130–10134. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: A nap is as good as a night. Nat Neurosci. 2003;6(7):697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 6.Genzel L, Spoormaker VI, Konrad BN, Dresler M. The role of rapid eye movement sleep for amygdala-related memory processing. Neurobiol Learn Mem. 2015;122:110–121. doi: 10.1016/j.nlm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Pan SC, Rickard TC. Sleep and motor learning: Is there room for consolidation? Psychol Bull. 2015;141(4):812–834. doi: 10.1037/bul0000009. [DOI] [PubMed] [Google Scholar]
- 8.McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold PE, Van Buskirk R. Enhancement and impairment of memory processes with post-trial injections of adrenocorticotrophic hormone. Behav Biol. 1976;16(4):387–400. doi: 10.1016/s0091-6773(76)91539-x. [DOI] [PubMed] [Google Scholar]
- 10.Introini-Collison IB, McGaugh JL. Naloxone and β-endorphin alter the effects of post-training epinephrine on memory. Psychopharmacology (Berl) 1987;92(2):229–235. doi: 10.1007/BF00177921. [DOI] [PubMed] [Google Scholar]
- 11.Packard MG, Williams CL, Cahill L, McGaugh JL. 1995. The anatomy of a memory modulatory system: From periphery to brain. Neurobehavioral Plasticity: Learning, Development, and Response to Brain Insults, eds Spear NE, Spear LP, Woodruff ML (Lawrence Erlbaum Associates, Inc., Hillsdale, NJ), pp 149–184.
- 12.Thayer JF, Lane RD. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142(1):1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 15.Williams C, Jensen R. Vagal afferents: A possible mechanism for the modulation of memory by peripherally acting agents. In: Frederickson R, McGaugh J, Felton D, editors. Peripheral Signaling of the Brain in Neural-Immune and Cognitive Function. Hogrefe and Huber; Toronto: 1991. pp. 467–472. [Google Scholar]
- 16.Williams CL, Jensen RA. Effects of vagotomy on Leu-enkephalin-induced changes in memory storage processes. Physiol Behav. 1993;54(4):659–663. doi: 10.1016/0031-9384(93)90073-o. [DOI] [PubMed] [Google Scholar]
- 17.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470(7332):101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain Stimulat. 2015;8(3):637–644. doi: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter BA, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012;22(10):2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 20.Peña DF, Engineer ND, McIntyre CK. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol Psychiatry. 2013;73(11):1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2(1):94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 22.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cogn Behav Neurol. 2006;19(3):119–122. doi: 10.1097/01.wnn.0000213908.34278.7d. [DOI] [PubMed] [Google Scholar]
- 23.Berntson GG, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 24.Trinder J, Waloszek J, Woods MJ, Jordan AS. Sleep and cardiovascular regulation. Pflugers Arch. 2012;463(1):161–168. doi: 10.1007/s00424-011-1041-3. [DOI] [PubMed] [Google Scholar]
- 25.Bušek P, Vanková J, Opavský J, Salinger J, Nevsímalová S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54(4):369–376. [PubMed] [Google Scholar]
- 26.Thayer JF, Åhs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Cellini N, de Zambotti M, Covassin N, Sarlo M, Stegagno L. Working memory impairment and cardiovascular hyperarousal in young primary insomniacs. Psychophysiology. 2014;51(2):206–214. doi: 10.1111/psyp.12167. [DOI] [PubMed] [Google Scholar]
- 28.Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 29.Luque-Casado A, Zabala M, Morales E, Mateo-March M, Sanabria D. Cognitive performance and heart rate variability: The influence of fitness level. PLoS One. 2013;8(2):e56935. doi: 10.1371/journal.pone.0056935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park G, Vasey MW, Van Bavel JJ, Thayer JF. Cardiac vagal tone is correlated with selective attention to neutral distractors under load. Psychophysiology. 2013;50(4):398–406. doi: 10.1111/psyp.12029. [DOI] [PubMed] [Google Scholar]
- 31.Lane RD, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44(1):213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 32.Mednick SA. The associative basis of the creative process. Psychol Rev. 1962;69(3):220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 33.Razumnikova OM. Creativity related cortex activity in the remote associates task. Brain Res Bull. 2007;73(1-3):96–102. doi: 10.1016/j.brainresbull.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Cellini N, et al. Heart rate variability helps tracking time more accurately. Brain Cogn. 2015;101:57–63. doi: 10.1016/j.bandc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 36.de Zambotti M, et al. Nocturnal cardiac autonomic profile in young primary insomniacs and good sleepers. Int J Psychophysiol. 2014;93(3):332–339. doi: 10.1016/j.ijpsycho.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Camm AJ, et al. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 38.Cellini N, Whitehurst LN, McDevitt EA, Mednick SC. Heart rate variability during daytime naps in healthy adults: Autonomic profile and short-term reliability. Psychophysiology. 2016;53(4):473–481. doi: 10.1111/psyp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schabus M, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 40.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, Wixted JT. An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 2011;34(10):504–514. doi: 10.1016/j.tins.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottselig JM, et al. Sleep and rest facilitate auditory learning. Neuroscience. 2004;127(3):557–561. doi: 10.1016/j.neuroscience.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 42.Mednick SC, Makovski T, Cai DJ, Jiang YV. Sleep and rest facilitate implicit memory in a visual search task. Vision Res. 2009;49(21):2557–2565. doi: 10.1016/j.visres.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rieth CA, Cai DJ, McDevitt EA, Mednick SC. The role of sleep and practice in implicit and explicit motor learning. Behav Brain Res. 2010;214(2):470–474. doi: 10.1016/j.bbr.2010.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 45.McDevitt EA, Duggan KA, Mednick SC. REM sleep rescues learning from interference. Neurobiol Learn Mem. 2015;122:51–62. doi: 10.1016/j.nlm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mednick SC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–4504. doi: 10.1523/JNEUROSCI.3127-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber FD, Wang J-Y, Born J, Inostroza M. Sleep benefits in parallel implicit and explicit measures of episodic memory. Learn Mem. 2014;21(4):190–198. doi: 10.1101/lm.033530.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14(6):557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 49.Carskadon MA, Dement WC. Principles and Practice of Sleep Medicine. 5th Ed. Elsevier Saunders; St. Louis, MO: 2011. Normal human sleep: An overview; pp. 17–26. [Google Scholar]
- 50.Achermann P, Borbély AA. Combining different models of sleep regulation. J Sleep Res. 1992;1(2):144–147. doi: 10.1111/j.1365-2869.1992.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 51.Walker R, Russo V. Memory consolidation and forgetting during sleep: A neural network model. Neural Process Lett. 2004;19(2):147–156. [Google Scholar]
- 52.Born J, Feld GB. Sleep to upscale, sleep to downscale: Balancing homeostasis and plasticity. Neuron. 2012;75(6):933–935. doi: 10.1016/j.neuron.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Datta S, O’Malley MW. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J Neurosci. 2013;33(10):4561–4569. doi: 10.1523/JNEUROSCI.5525-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landmann N, et al. The reorganisation of memory during sleep. Sleep Med Rev. 2014;18(6):531–541. doi: 10.1016/j.smrv.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Stickgold R. Parsing the role of sleep in memory processing. Curr Opin Neurobiol. 2013;23(5):847–853. doi: 10.1016/j.conb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- 57.Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172(3983):601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- 58.Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 1990;47(5):421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- 59.Marrosu F, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671(2):329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 60.Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16(6):218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- 61.Matsukawa M, et al. Serotonin and acetylcholine are crucial to maintain hippocampal synapses and memory acquisition in rats. Neurosci Lett. 1997;230(1):13–16. doi: 10.1016/s0304-3940(97)00460-6. [DOI] [PubMed] [Google Scholar]
- 62.Sale A, et al. Visual perceptual learning induces long-term potentiation in the visual cortex. Neuroscience. 2011;172:219–225. doi: 10.1016/j.neuroscience.2010.10.078. [DOI] [PubMed] [Google Scholar]
- 63.Nichols JA, et al. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience. 2011;189:207–214. doi: 10.1016/j.neuroscience.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 64.Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- 65.Hobson JA. REM sleep and dreaming: Towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10(11):803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 66.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23(17):R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chouchou F, Desseilles M. Heart rate variability: A tool to explore the sleeping brain? Front Neurosci. 2014;8:402. doi: 10.3389/fnins.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trinder J, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–264. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 69.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Natl Inst Neurological Diseases and Blindness; Bethesda: 1968. [Google Scholar]
- 70.Wamsley EJ, et al. Reduced sleep spindles and spindle coherence in schizophrenia: Mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71(2):154–161. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schabus M, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007;104(32):13164–13169. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeitlhofer J, et al. Topographic distribution of sleep spindles in young healthy subjects. J Sleep Res. 1997;6(3):149–155. doi: 10.1046/j.1365-2869.1997.00046.x. [DOI] [PubMed] [Google Scholar]