Significance
Collagens are structural extracellular matrix proteins that provide mechanical support to tissues. To gain stability, collagens can form pyridinoline cross-links via enzymatically formed intermediates initiated by lysyl hydroxylase (LH) 2. Individuals with mutations in the gene encoding LH2 share highly overlapping traits with individuals with mutations in the gene encoding the immunophilin FKBP65 that shows no LH activity. We found that FKBP65 is necessary for the dimerization of LH2, which is required for activity of LH2. Collagen cross-linking plays important roles in bone diseases as well as in such pathologies as cancer and fibrosis. Our study has elucidated a mechanism of how to interfere in a specific type of collagen cross-linking and can help advance the design of new treatments toward these pathologies.
Keywords: collagen cross‐linking, lysyl hydroxylase, FKBP65, Bruck syndrome, fibrosis
Abstract
Collagens are subjected to extensive posttranslational modifications, such as lysine hydroxylation. Bruck syndrome (BS) is a connective tissue disorder characterized at the molecular level by a loss of telopeptide lysine hydroxylation, resulting in reduced collagen pyridinoline cross-linking. BS results from mutations in the genes coding for lysyl hydroxylase (LH) 2 or peptidyl-prolyl cis-trans isomerase (PPIase) FKBP65. Given that the immunophilin FKBP65 does not exhibit LH activity, it is likely that LH2 activity is somehow dependent on FKPB65. In this report, we provide insights regarding the interplay between LH2 and FKBP65. We found that FKBP65 forms complexes with LH2 splice variants LH2A and LH2B but not with LH1 and LH3. Ablating the catalytic activity of FKBP65 or LH2 did not affect complex formation. Both depletion of FKBP65 and inhibition of FKBP65 PPIase activity reduced the dimeric (active) form of LH2 but did not affect the binding of monomeric (inactive) LH2 to procollagen Iα1. Furthermore, we show that LH2A and LH2B cannot form heterodimers with each other but are able to form heterodimers with LH1 and LH3. Collectively, our results indicate that FKBP65 is linked to pyridinoline cross-linking by specifically mediating the dimerization of LH2. Moreover, FKBP65 does not interact with LH1 and LH3, explaining why in BS triple-helical hydroxylysines are not affected. Our results provide a mechanistic link between FKBP65 and the loss of pyridinolines and may hold the key to future treatments for diseases related to collagen cross-linking anomalies, such as fibrosis and cancer.
Collagen I is an essential component of the extracellular matrix (ECM) of tissues such as bone and skin, and is involved in a wide variety of biological processes. A deregulated synthesis of collagen type I results in pathologies ranging from severe bone and skin anomalies to fibrosis (1–5). Hydroxylation of specific lysine (Lys) residues into 5-hydroxylysine (Hyl) is performed by lysyl hydroxylases (LHs), also known as the procollagen-lysine, 2-oxoglutarate 5-dioxygenase (PLOD) family. Collagens deposited in the ECM are stabilized by the formation of intermolecular cross-links by members of the lysyl oxidase family, LOX and LOXL (6). Two collagen cross-linking pathways have been identified, the allysine route and the hydroxyallysine route (7). In the allysine route, a telopeptide Lys is oxidized by lysyl oxidases into an aldehyde; in the hydroxyallysine route, this occurs with a telopeptide Hyl. In turn, the reactive aldehydes interact with the Lys or Hyl residues in the helical part of collagen to form difunctional and finally trifunctional cross-links (8–10). The trifunctional cross-links derived from the hydroxyallysine route are referred to as pyridinolines. Collagens cross-linked by means of pyridinolines are difficult to degrade (11–13).
The LH family consists of three individual members, designated LH1, LH2, and LH3. Hydroxylation of Lys present in the Gly-X-Lys sequence in the helical domain of collagen is carried out by LH1 and LH3 (14, 15), whereas LH2 hydroxylates Lys residues in the telopeptides of collagen, where such a sequence motif is not present (16–18). LH2 consists of two splice variants, LH2A and LH2B (19), with LH2B containing an extra exon known as 13A. Although both isoforms contain the same dioxygenase catalytic domain responsible for hydroxylation, only LH2B is thought to be involved in pyridinoline cross-linking by displaying selective hydroxylase activity toward the telopeptide Lys of collagen (18, 20), whereas the biological function and specificity of LH2A is unknown. LHs display activity only when present in a dimer (21–23). Homodimers of LH1, LH2, and LH3 are likely the rule, but notable amounts of the heterodimer LH1/LH3 can be formed as well (24).
Mutations in PLOD2 are linked to the development of Bruck syndrome type 2 (BS2) (MIM 609220) (25–28), a heritable autosomal recessive bone disease characterized by congenital contractures with pterygia, early onset of bone fractures, postnatal short stature, and severe limb deformity and progressive scoliosis. In addition, mutations in FKBP10 result in BS type 1 (MIM 259450) (27, 29–34) and in osteogenesis imperfecta and Kuskokwin syndrome (35–38). As is the case for PLOD2 mutations, FKBP10 mutations result in a dramatic underhydroxylation of collagen telopeptide Lys and a subsequent decrease in the pyridinoline cross-linking of collagen type I in bone (26, 32, 39). FKBP65, encoded by FKBP10, belongs to the family of immunophilins, a class of proteins that exhibit peptidyl-prolyl cis-trans isomerase (PPIase) activity that can be targeted by immunosuppressive drugs, such as FK506 (tacrolimus) and rapamycin (40, 41). Despite the high number of prolyl residues in collagen, the PPIase activity of FKBP65 toward collagen seems rather limited (42, 43). Instead, it is assumed that FKBP65 prevents a premature association between procollagen chains during synthesis and premature aggregation between triple helical procollagen molecules (44).
So far, it is not known how FKBP65 relates to the lysyl hydroxylation process of the telopeptides, and whether there is a molecular interplay between LH2 and FKBP65. In this report, we present compelling evidence showing that there is a physical interaction between FKBP65 and LH2 crucial for LH2 activation and thus collagen telopeptide Hyl formation, but that FKBP65 is not required to direct LH2 binding to procollagen Iα1. Furthermore, we found that LH2A and LH2B are able to form heterodimers with LH1 and LH3, perhaps serving as a toolbox to increase collagen substrate specificities. Our data provide deeper insight into the molecular principles of collagen cross-linking in healthy individuals and patients with Bruck syndrome and fibrotic disorders, and open new avenues for treatments aimed at correcting collagen cross-linking anomalies.
Results
LH2 Splice Variants Form a Complex with FKBP65.
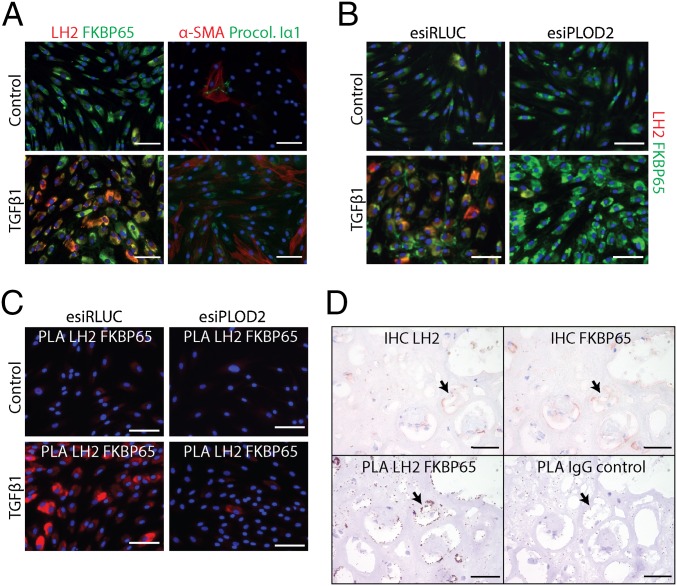
TGFβ1 strongly induces collagen type I and LH2 expression in fibroblasts (45–47). We performed immunocytochemistry to verify the expression of LH2, FKBP65, and procollagen type Iα1 after TGFβ1 stimulation of primary dermal fibroblasts. Indeed, procollagen type Iα1 synthesis, as well as LH2 and FKBP65 expression, were increased by TGFβ1 stimulation (Fig. 1A). Furthermore, LH2 and FKBP65 seemed to colocalize in the same cellular compartments. Interaction between both LH2 and FKBP65 in collagen type I-producing cells was assessed with a proximity ligation assay (PLA), which allowed immunodetection of protein–protein complexes under endogenous circumstances. PLA with antibodies against both LH2 and FKBP65 resulted in a strong fluorescent signal in TGFβ1-stimulated fibroblasts (Fig. 1B), whereas the control (esiRNA knockdown of PLOD2) displayed only a fraction of the signal. This suggests that endogenous LH2 and FKBP65 together form a complex. Moreover, in human fibrotic kidney tissue, PLA confirmed the interaction of LH2 and FKBP65 (Fig. 1C), underscoring the in vivo relevance of the LH2/FKBP65 complex.
Fig. 1.
Endoplasmic reticulum-localized LH2 and FKBP65 exhibit physical interactions. (A) Immunofluorescent detection of LH2, FKBP65, aSMA, and procollagen-Iα1 in NHDFs with or without TGFβ1 treatment for 2 d. (B) Immunofluorescent detection of LH2 and FKBP65 in NHDFs transfected with esiRNA against PLOD2 or control (RLUC), followed by treatment with or without TGFβ1 for 2 d. (C) Fluorescent proximity ligation assay for LH2 and FKBP65 in NHDFs transfected with esiRNA against PLOD2 or control (RLUC), followed by treatment with or without TGFβ1 for 2 d. (D, Upper) Immunohistochemistry for LH2 and FKBP65 on serial sections from fibrotic explanted human renal allograft tissue. (D, Lower) PLA on identical serial sections for LH2 and FKBP65 or IgG isotypes as a control. The arrow indicates colocalization of LH2 and FKBP65 in the serial sections. (Scale bars: white, 100 µm; black, 50 µm.)
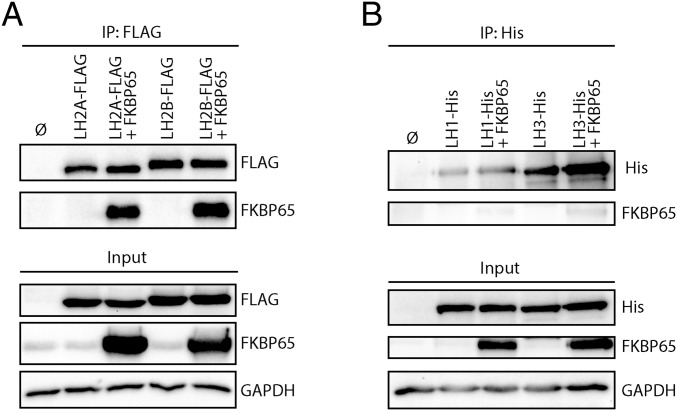
Because when using commercially available LH2 antibodies we could not distinguish whether LH2A, LH2B, or both splice variants can form a complex with FKBP65, we performed coimmunoprecipitation (co-IP) on HEK-293T cells that overexpressed combinations of FLAG-tagged LH2A or LH2B with FKBP65. Pulldown on LH2A-FLAG and LH2B-FLAG both resulted in co-IP of FKBP65, whereas no co-IP was observed under the control conditions (Fig. 2A). These findings indicate that both LH2 splice variants (LH2A and LH2B) can bind FKBP65, and that this interaction is not dependent on the extra exon 13A in LH2B.
Fig. 2.
LH2 splice variants, but not LH1 and LH3, interact with FKBP65. (A) Western blot detection of 3×FLAG-tag co-IP complexes from HEK293T cells overexpressed with combinations of LH2A-FLAG, LH2B-FLAG, and FKBP65. (B) Western blot detection of 6×His-tag co-IP complexes from HEK293T cells overexpressed with combinations of LH1-His, LH2-His, and FKBP65.
LH1 and LH3 Do Not Form a Complex with FKBP65.
Mutations of FKBP65 in Bruck syndrome affects the lysyl hydroxylation only of the telopeptides, not of the triple helix (32, 39). After finding that FKPB65 complexes with LH2, we wondered whether FKBP65 also interacts with LH1 and LH3, the LHs involved in the hydroxylation of Lys in the helical part of collagen. Therefore, we cotransfected HEK-293T cells with LH1-His or LH3-His together with FKBP65 and investigated complex formation by means of co-IP. Even though overexpression of both proteins was achieved, neither LH1 nor LH3 showed significant interaction with FKBP65, with only a very faint band of FKBP65 observed after LH1 and LH3 IP (Fig. 2B).
Mutations in the Catalytic Domain of LH2 and FKBP65 Do Not Reduce Complex Formation.
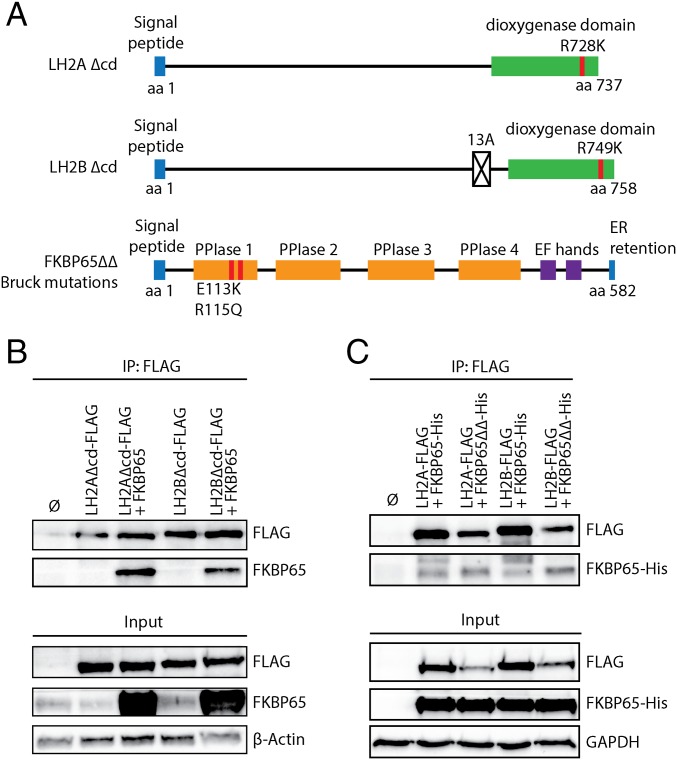
To examine whether the catalytic activity of LH2 affects complex formation with FKBP65, we generated a mutation of the conserved residue of LH2A (R728K) and its corresponding residue in LH2B (R749K) to ablate the catalytic activity of LH2 (Fig. 3A) (23, 48). Co-IPs were performed after overexpression of both FLAG-tagged mutant variants in combination with FKBP65. Ablating the catalytic activity of LH2A and LH2B did not prevent their complex formation with FKBP65 (Fig. 3B), although the IP with the LH2B mutant seemed to be less efficient than that with the LH2A mutant.
Fig. 3.
Catalytic inactive LH2 and Bruck syndrome mutated FKBP65 do not negatively affect complex formation. (A) Illustration of the functional domains of LH2A, LH2B, and FKBP65 (UniProt). Locations of the introduced point mutations are indicated in red. LH2 hydroxylase activity was blocked by mutations (red) in the Fe2-OG dioxygenase domain (green), whereas two known Bruck syndrome (BS) mutations were introduced in the FKBP65 PPIase domain 1. (B) Western blot detection of 3×FLAG-tag co-IP complexes from HEK293T cells overexpressed with combinations of catalytic inactive mutants of LH2A-FLAG and LH2B-FLAG with FKBP65. (C) Western blot detection of 3×FLAG-tag co-IP complexes from HEK293T cells overexpressed with combinations LH2A-FLAG, LH2B-FLAG with 6×His-tag WT (FKBP65-His), or BS mutated FKBP65 (FKBP65ΔΔ-His).
All single amino acid substitutions in any of the PPIase domains of FKBP10 in BS1 patients result in maintenance of FKBP65 protein production, but ablation of pyridinoline cross-links (26, 32, 39). We wished to identify whether BS mutations of the first PPIase domain affect complex formation with both LH2 splice variants. To exclude reduced epitope recognition by our FKBP65 antibody caused by the inserted mutations, we used His-tagged FKBP65 mutant and His-tagged FKBP65 wild type (WT). Both the mutated and the WT FKBP65 were detected on Western blot analyses after IP on FLAG-tagged LH2A and LH2B (Fig. 3C). Interestingly, complex formation seemed to be more efficient for the PPIase-mutated FKBP65 compared with WT FKBP65 for both LH2A and LH2B.
LH2 Splice Variants Can Form Heterodimers with LH1 and LH3.
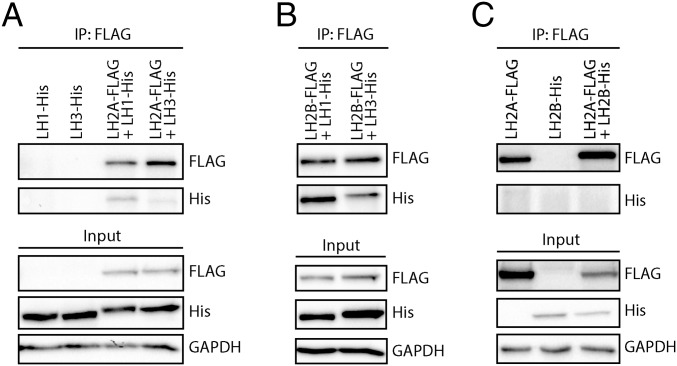
LHs need to form dimers to become catalytically active. It has been reported that heterodimers can be formed between LH1 and LH3 (24). To determine whether LH2A and LH2B also can form heterodimers with LH1 or LH3, we cotransfected HEK-239T cells with FLAG-tagged LH2A or LH2B and with His-tagged LH1 or LH3 and performed co-IP. We found that LH2A-FLAG indeed formed heterodimers with LH1-His as well as with LH3-His, albeit more weakly (Fig. 4A). In addition, LH2B-FLAG showed more heterodimerization with LH1 than with LH3 (Fig. 4B).
Fig. 4.
LH2 splice variants can form heterodimers with LH1 and LH3. (A) Western blot detection of 3×FLAG-tag coimmunoprecipitated heterodimer complexes from HEK293T cells overexpressed with combinations of LH1-His and LH3-His with LH2A-FLAG. (B) Western blot detection of 3×FLAG-tag coimmunoprecipitated heterodimer complexes from HEK293T cells overexpressed with combinations of LH1-His and LH3-His with LH2B-FLAG. (C) Western blot detection of 3×FLAG-tag coimmunoprecipitated heterodimer complexes from HEK293T cells overexpressed with combinations of LH2A-FLAG and LH2B-His.
We next wanted to know whether heterodimer formation between LH2A and LH2B was possible, and thus cotransfected HEK-293T cells with LH2A-FLAG and LH2B-His and assayed complexes by co-IP on FLAG. LH2B-His was not detected, whereas LH2A-FLAG was prominently detected (Fig. 4C). In conclusion, our results indicate that heterodimer formation does not occur between LH2A and LH2B, but that LH2A and LH2B can form heterodimers with LH1 and LH3.
FKBP65 Does Not Affect LH2 Binding to Procollagen Ia1 but Enables Dimerization of LH2.
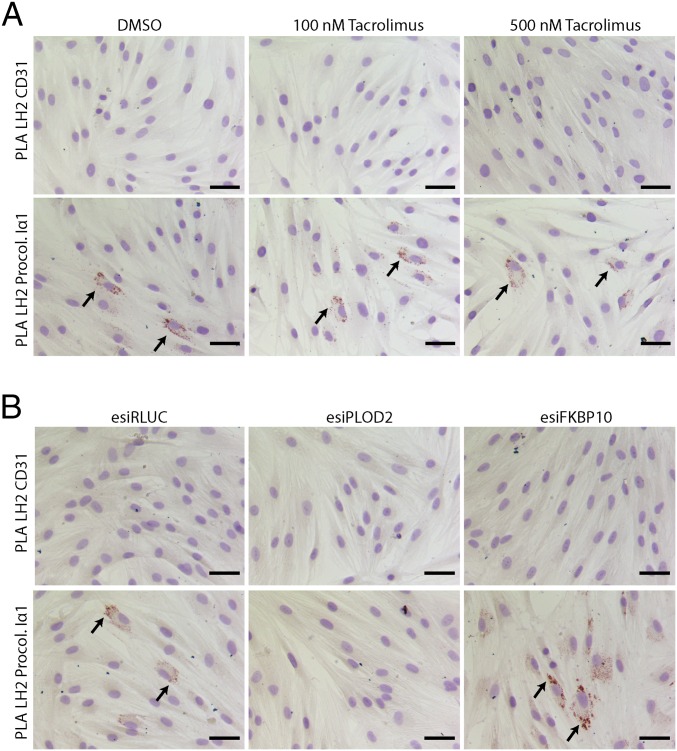
How LH2 specifically hydroxylates Lys residues in the telopeptide is unknown. We wanted to know whether this process could be directed through FKBP65 recruitment of LH2 toward its substrate, procollagen. PLA on endogenous LH2 and procollagen type I in fibroblasts treated with or without tacrolimus revealed that binding of LH2 to procollagen Iα1 was not affected by partial inhibition of the PPIase activity of FKBP65 (Fig. 5A). To examine whether other possible domains of FKBP65 could be responsible for LH2 targeting to procollagen, we performed PLA on fibroblasts transfected with esiRNA against FKBP65 or control esiRLUC. Interestingly, knockdown of FKBP65 resulted in enhanced binding of LH2 to procollagen Iα1 (Fig. 5B). Thus, FKBP65 does not seem to be involved in recruiting LH2 toward the collagen telopeptide area.
Fig. 5.
FKBP65 does not direct LH2 toward procollagen Iα1. Bright-field PLA for LH2 and procollagen-Iα1 or control PLA for LH2 and CD31 in NHDFs, treated with FKBP65 PPIase inhibitor tacrolimus or DMSO control combined with or without TGFβ1 treatment for 24 h (A), or transfected with esiRNA against FKBP10 or controls (RLUC and PLOD2), followed by TGFβ1 treatment for 2 d (B). Arrows indicate examples of PLA signals from LH2 and procollagen Iα1. (Scale bars: 50 µm.)
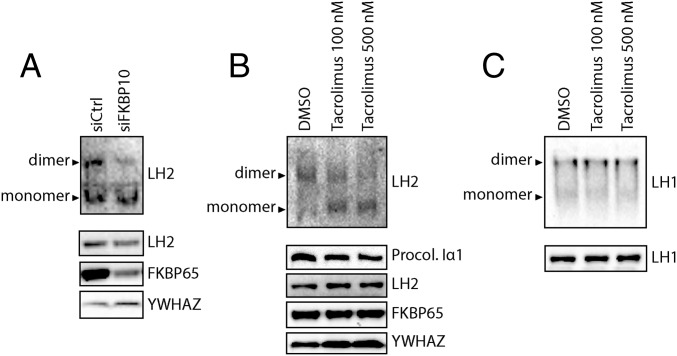
The molecular process facilitating activation of LH by means of dimer formation is unknown. We wondered whether FKBP65, instead of targeting LH2 to collagen, is involved in the dimerization of LH2. Therefore, we performed native-PAGE on TGFβ1-stimulated fibroblasts that were transfected with siRNA against FKBP10 or scrambled siRNA. Western blot analysis of endogenous LH2 showed a reduced dimerization that correlated with a drop in FKBP65 levels (Fig. 6A). This indeed shows that FKBP65 is involved in the dimerization of LH2.
Fig. 6.
PPIase activity of FKBP65 enables LH2 dimerization. (A) Western blot detection of LH2 monomers/dimers by native-PAGE with nondenatured lysates (Upper) and SDS/PAGE with denatured lysates (Lower) from NHDFs transfected with siRNA against FKBP10 and treated with TGFβ1 for 48 h. (B) Western blot detection of LH2 monomers/dimers by native-PAGE with nondenatured lysates (Upper) and SDS/PAGE with denatured lysates (Lower) from NHDFs treated with tacrolimus or DMSO control combined with TGFβ1 for 24 h. (C) Western blot detection of LH1 dimers by native-PAGE (Upper) and SDS/PAGE with denatured lysates (Lower) from NHDFs treated with tacrolimus or DMSO control combined with TGFβ1 for 24 h.
We next wondered whether it is the PPIase activity of FKBP65 that catalyzes the LH2 dimerization. We performed native-PAGE on TGFβ1-stimulated fibroblasts treated with or without tacrolimus to partially inhibit the PPIase activity of FKBP65 (42). Staining for endogenous LH2 showed that dimerization of LH2 was strongly reduced with increasing tacrolimus concentrations, with no effect on total LH2 or FKBP65 protein levels (Fig. 6B). This confirms that FKBP65 PPIase activity is directly responsible for the dimerization of LH2, and that partially blocking the PPIase activity of FKBP65 hampers dimerization of LH2. As a control, we investigated the dimerization of LH1 under the same conditions. Given our finding that FKBP65 does not bind to LH1, we expected to also find that the addition of tacrolimus did not inhibit LH1 dimerization. Indeed, dimerization of LH1 and total protein levels were not affected by the inhibition of FKBP65 PPIase activity by tacrolimus (Fig. 6C).
Discussion
Lysyl hydroxylations of the triple-helical part and telopeptides of collagen are highly regulated processes that contribute to the heterogeneity of collagen. For example, the telopeptides of collagen type I in bone are partially hydroxylated, whereas in skin the same telopeptides are devoid of Hyl (7, 8). Currently, three LHs are known: LH1, LH2 with its splice variants LH2A and LH2B, and LH3. Mutations in LH2 (Bruck syndrome, type 2) results in severe underhydroxylation of the telopeptides of collagen type I in bone, whereas the lysyl hydroxylation level of the triple helix remains unaffected, indicating that LH2 is a telopeptide LH, and that LH1 and LH3 are triple-helical LHs. Indeed, mutations in LH1 (Ehlers–Danlos syndrome, type VIA) result in an underhydroxylation of the triple helix, but the telopeptides remain unaffected, and a knockout model of LH3 has shown unaffected telopeptides in bone (14, 15). Therefore, it was surprising that mutations in FKBP10 (Bruck syndrome, type 1) resulted in a similar phenotype and in the same biochemical defect as seen for mutations in LH2.
Although physical interaction between both LH2 splice variants and FKBP65 has been suggested previously (32, 36), we are the first group, to our knowledge, to actually demonstrate this interaction. The mutations of residue R728K in LH2A and the homologous residue R749K in LH2B (a residue present in the catalytic domain of LH2 and required for LH activity) show that the interaction between FKBP65 and LH2 is not dependent on the catalytic activity of LH2. Furthermore, BS1 double mutation of residues E113K and R115Q in the PPIase domain 1 of FKBP65 shows that interaction between FKBP65 and LH2 is not dependent on full PPIase activity of FKBP65; on the contrary, a slightly increased interaction between mutated FKBP65 and LH2A and LH2B was detected.
Mutations in FKBP65 are known to have no effect on the lysyl hydroxylation level of the triple helix (32, 36, 38, 39). To explain this, we hypothesized that FKBP65 does not interact with LH1 and LH3, but does interact selectively with LH2. Co-IP on LH1 and LH3 with FKBP65 showed that these molecules do not form a complex. Thus, FKBP65 does indeed selectively bind to LH2, explaining why only the telopeptides, and not the triple helix, are affected in Bruck syndrome.
Given that both mutated FKBP65 and normal FKBP65 are able to bind to LH2, binding as such does not explain why mutated FKBP65 affects the LH activity of LH2. It is known that monomers of LH do not exhibit LH activity, but that dimers do (21–23). Thus, we investigated whether FKBP65 has a role in the dimerization of LH2B, by adding tacrolimus to TGFβ1-stimulated dermal fibroblasts that express mostly LH2B (28). TGFβ1 increases the endogenous expression of LH2B and FKBP65, and tacrolimus blocks PPIase activity of FKBP65 by ∼25% (42). Native-PAGE on cell lysates showed that most of the LH2B was detected as a dimer form when FKBP65 was present and its PPIase activity not inhibited; however, depletion of FKBP65 or inhibition by tacrolimus resulted in reduced dimer formation. Thus, FKBP65 is involved in the dimerization of LH2B. As a control, we also checked the dimer formation of LH1 with or without the presence of tacrolimus, and observed that dimer formation of LH1 was unaffected. The latter finding was expected, given our earlier finding that FKBP65 did not form a complex with LH1.
The foregoing observations provide a straightforward explanation of why a single mutation in one of the PPIase domains of FKBP65 results in an absence of Hyl in the telopeptides of collagen I: mutant FKBP65 still binds to monomers of LH2 but it can no longer form dimers of LH2. Because only dimers of LH2 have LH activity, mutations in FKPB65 (in BS1, single amino acid mutations have been found in all four PPIase domains) indirectly affect the lysyl hydroxylation process of the telopeptides. The BS1 mutations and our data also suggest that full PPIase activity is required to enable dimer formation of LH2. This is in contrast to another chaperone function of FKBP65, namely the prevention of premature association of procollagen chains during synthesis as well as premature aggregation between triple-helical procollagen molecules. This chaperone function is not inhibited by tacrolimus, indicating that full PPIase activity is not required for FKBP65 to execute this task (44). This also explains why in BS1 procollagen folding rate, trimerization, and secretion are essentially normal, but pyridinoline formation is highly abnormal.
The foregoing explanation is also strengthened by the observation that two reported BS2 mutations in PLOD2 result in impaired folding and oligomerization of LH2 (49), thus highlighting the relevance of correctly folded dimers of LH2 to ensure a proper level of hydroxylation of telopeptide Lys. Remarkably, mutations in FKBP65 do not result in a lack of telopeptide Hyl in collagen type II in cartilage, in contrast to the situation of collagen type I in bone. It is possible that the role of FKBP65 in dimerization of LH2 is taken over by another molecule in cartilage, being absent in bone. It is also possible, but less likely, that monomeric or heterodimers of LH2 display activity toward collagen type II, but not toward collagen type I.
Because LH activity seems to be restricted to dimers, we have investigated this phenomenon in more detail. A previous study reported that LH3 can form a heterodimer with LH1 (24). We have extended that study, and found that LH2A and LH2B can form a heterodimer with LH1. This was also the case with LH3, albeit less efficiently than with LH1. Whether heterodimers also occur in vivo is unknown; however, given that the experiments by the Myllylä group were done in insect cells and our experiments were done in human cells, and we both observed the formation of heterodimers, it seems likely that heterodimers also occur in vivo, potentially bypassing the selectivity of specific LH monomers toward a given collagenous region or tissue. Interestingly, LH2A is not able to form a heterodimer with LH2B. The additional exon 13A present in LH2B apparently excludes its dimerization with LH2A, although this exon does not prohibit the dimerization of LH2B with LH1 and LH3.
Because mutations in the genes coding for LH1 and LH3 do not affect the level of lysyl hydroxylation in the telopeptides (14–16), it seems that this modification is carried out by LH2 only. There are two forms of LH2, the long form (LH2B) with the additional exon 13A and the short form (LH2A) without exon 13A. In fibrotic conditions, an up-regulation of LH2B is seen, and this always results in increased pyridinoline levels. The substrate specificity of LH2A is not known. In a BS2 patient with a mutation affecting the splicing of exon 13A to generate only LH2A expression, underhydroxylated telopeptides were reported (25). Thus, it seems that LH2A has no telopeptide LH activity, in sharp contrast to LH2B. We hypothesize that FKBP65 is not involved in directing LH2 to the telopeptides; FKBP65 forms a complex with LH2A and LH2B, but the LH2A/FKBP65 complex apparently fails to bind and hydroxylate the telopeptides. It is likely that the 20-aa stretch encoded by exon 13A is involved in directing/binding of LH2B to the telopeptides, thereby determining the substrate specificity of LH2B.
In conclusion, we have shown that FKBP65 binds to LH2 but not to LH1 and LH3, and that binding of FKBP65 to LH2 is not hampered by a loss of LH activity of LH2 or a partial loss of FKBP65 PPIase activity, but a partial loss of PPIase activity hampers the dimerization of LH2. Considering that only dimers seem to exhibit LH activity, our data provide a molecular explanation as to how mutations in FKBP10 result in an overall dramatic decrease in lysyl hydroxylation levels of collagen telopeptides, leading to diseases such as Bruck syndrome, type XI osteogenesis imperfecta, and Kuskokwim syndrome. It should be noted that a high level of pyridinoline cross-links has been reported to be causally involved in fibrosis and tumor metastasis (28, 50–54). The increased stiffening of the matrix from accumulating cross-linked collagen is directly linked to the progression of both types of pathologies. Therefore, a better molecular understanding of how pyridinoline cross-linking is regulated, such as aided by the data provided in this paper, is fundamental to the search for new treatments against these pathologies.
Materials and Methods
Cell Culture.
HEK-293T cells (American Type Culture Collection) were cultured in DMEM supplemented with 10% (vol/vol) FBS, 1% l-glutamine, and 1% penicillin/streptomycin. Normal human dermal fibroblasts (NHDFs) (Lonza) were cultured in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS (Thermo Fisher Scientific), 1% l-glutamine, and 1% penicillin/streptomycin. All cells were cultured at 37 °C in 5% (vol/vol) CO2. During all experimental conditions, 50 μg/mL l-ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich) was supplemented to the medium to obtain complete medium, given that l-ascorbic acid is an essential cofactor for LH activity and collagen synthesis (55).
TGFβ1 Stimulation and Tacrolimus Treatment.
Human recombinant TGFβ1 (PeproTech) was reconstituted in PBS with BSA (0.1%). For experimental conditions, NHDFs were serum-starved in complete low-serum medium (0.5% FBS) for 18 h, which was subsequently supplemented with TGFβ1 (5 ng/mL) and refreshed every 24 h. FKBP65 inhibitor tacrolimus (Sigma-Aldrich), also known as FK506, was dissolved in DMSO. For inhibition experiments, NHDFs were preincubated for 1 h with complete low-serum medium supplemented with tacrolimus, after which the medium was replaced with complete low-serum medium containing the same concentration of tacrolimus supplemented with or without TGFβ1, and incubated for 24 h.
Immunofluorescence and in Situ Proximity Ligation Assay.
Immunofluorescence of fixed NHDFs and human renal tissue was used to detect protein expression (for antibodies, see Table S1). In situ complex formation of LH2 and FKBP65 was investigated using PLA kits (Sigma-Aldrich) for fluorescent microscopy or bright-field microscopy. More details are provided in SI Materials and Methods.
Table S1.
Antibodies used in this study
| Antigen | Species | Company | Catalog no. | Used for |
| Primary antibodies | ||||
| β-Actin | Rabbit polyclonal | Abcam | ab8227 | Western blot |
| CD31 | Goat polyclonal | Santa Cruz Biotechnology | sc-1506 | PLA |
| FKBP65 | Rabbit polyclonal | Proteintech | 12172–1-AP | Western blot, PLA, immunocytochemistry |
| FLAG | Mouse IgG1 | Sigma-Aldrich | F3165 | Western blot, co-IP |
| GAPDH | Rabbit polyclonal | Abcam | ab9482 | Western blot |
| His | Rabbit polyclonal | Abcam | ab9108 | Western blot, co-IP |
| LH1 | Rabbit polyclonal | Sigma-Aldrich | HPA049137 | Western blot |
| LH2 | Mouse IgG2b | R&D Systems | MAB4445 | Western blot |
| LH2 | Mouse polyclonal | Sigma-Aldrich | SAB1400213 | Immunocytochemistry, PLA |
| Procollagen 1α1 | Goat polyclonal | Santa Cruz Biotechnology | sc-8782 | Western blot, PLA, immunocytochemistry |
| αSMA | Mouse IgG2a | DAKO | M0851 | Immunocytochemistry |
| Secondary/tertiary reagents | ||||
| Goat anti-rabbit HRP | DAKO | P0448 | ||
| Rabbit anti-mouse HRP | DAKO | P0260 | ||
| Rabbit anti-goat HRP | DAKO | P0449 | ||
| Donkey anti-rabbit Alexa Fluor 488 | IgG (H+L) | Thermo Fisher Scientific | A21206 | |
| Donkey anti-rabbit Alexa Fluor 555 | IgG (H+L) | Thermo Fisher Scientific | A31572 | |
| Donkey anti-goat Alexa Fluor 488 | IgG (H+L) | Thermo Fisher Scientific | A11055 | |
| Goat anti-mouse biotin | IgG (H+L) | Southern Biotech | 1031–08 | |
| Streptavidin-Cy3 | Thermo Fisher Scientific | 43–8315 | ||
Cloning and Mutagenesis.
FKBP65, LH2A, and LH2B cDNA ORF coding plasmids (Origene) were subcloned either directly into a pcDNA3.1 vector with primers introducing 6×His-tag to their C terminus or via a pCDNA3.1 ligated linker region to obtain in-frame 3×FLAG to the C terminus (Table S2). Catalytic mutants of 3×FLAG-tagged LH2A and LH2B were obtained by site-directed mutagenesis (Table S2). BS1-related FKBP10 missense mutations 337G > A and 344G > A in PPIase domain 1 that result in E112K and R115Q substitutions (31, 32) were derived by site-directed mutagenesis PCR (Table S2). All constructs were validated with Sanger sequencing by BaseClear (Leiden, The Netherlands).
Table S2.
Oligos used to generate affinity tags and site-directed mutagenesis by PCR
| Target | Sequence, 5′–3′ |
| Affinity tags | |
| pCDNA3.1 3×FLAG linker forward | AGCTTTCTAGAATTCGGCGGCCGCGGAAGACTACAAAGACCATGACGGTGATTATAAAGATCATGACATCGACTACAAGGATGACGATGACAAGTGAG |
| pCDNA3.1 3×FLAG linker reverse | GATCCTCACTTGTCATCGTCATCCTTGTAGTCGATGTCATGATCTTTATAATCACCGTCATGGTCTTTGTAGTCTTCCGCGGCCGCCGAATTCTAGAA |
| LH2a/b-3×FLAG forward | TCACGATCTAGAGCCACCATGGGGGGATGC |
| LH2a/b-3×FLAG reverse | GTCCGGCGGCCGCGGGATCTATAAATGACACTGCAATGTA |
| LH2B-6×His forward | CTAGTTAAGCTTCCACCATGGGGGGATGCACGGTGAAGCC |
| LH2B-6×His reverse | GAGGATCCACCGGTTCAGTGGTGATGGTGATGATGTCCTGATCCGAGCTCCTCGTGGACCCGCTCCT |
| FKBP65-6×His forward | CTAGTTAAGCTTCCACCATGTTCCCCGCGGGCCCCCCCAGC |
| FKBP65-6×His reverse | GAGGATCCACCGGTTCAGTGGTGATGGTGATGATGTCCTGATCCGAGCTCCTCGTGGACCCGCTCCT |
| LH1-6×His forward | CTAGTTAAGCTTCCACCATGCGGCCCCTGCTGCTACTG |
| LH1-6×His reverse | GAGGATCCACCGGTTCAGTGGTGATGGTGATGATGTCCTGATCCGGGATCGACGAAGGAGACTGC |
| LH3-6×His forwrad | CTAGTTAAGCTTCCACCATGACCTCCTCGGGGCCT |
| LH3-6×His reverse | GAGGATCCACCGGTTCAGTGGTGATGGTGATGATGTCCTGATCCGGGGTCGACAAAGGACACCATGAT |
| Mutagenesis | |
| LH2a/b ΔCD forward | [Phos]AAATGGAACAGCATACATTGCA |
| LH2a/b ΔCD reverse | [Phos]TTAACAGGAAGTCCTTCA |
| FKBP65 ΔBS 334C > A 344C > T forward | [Phos]TGTGTCAACAAGCGGCAACGCCTCATT |
| FKBP65 ΔBS 334C > A 344C > T reverse | [Phos]CATGCCCATGAGGCCTCGGTCCAT |
Transient Transfection.
On the day after seeding, cells were transfected with plasmids using Lipofectamine LTX PLUS mix (Thermo Fisher Scientific), and esiRNA against RLUC, PLOD2, or FKBP10 (Sigma-Aldrich) and siRNA against FKBP10 or scrambled (Santa Cruz Biotechnology) were transfected with RNAiMAX (Thermo Fisher Scientific). All transfections were performed according to the manufacturer’s guidelines. For HEK-293T, cells were harvested at 2 d after transfection for downstream applications. For NHDFs, medium was changed after transfection to low-serum medium for 18 h and subsequently stimulated with TGFβ1 for 2 d, with daily refreshments of medium and TGFβ1.
Co-IP.
Cell pellets were lysed with RIPA buffer (50 mM Tris⋅HCl pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.25% Na-deoxycholate, and 1 mM EGTA) supplemented with proteinase inhibitor mixture (Sigma-Aldrich) at 4 °C with agitation. Lysates were homogenized by passing through a 23G needle three times, incubated 2 h at 4 °C, and agitated with 750 U/mL of Benzoase (Sigma-Aldrich) to solubilize all proteins. The lysate was then precleared by centrifugation. Equal concentrations of lysates were used in each individual experiment, and were also cleared by preincubating with Protein-G Dynabeads (Thermo Fisher Scientific) at 4 °C with agitation for 30 min. The cleared samples were incubated with 5 μg antibodies against FLAG, 6×His, or FKBP65 (Table S1) for 2 h at 4 °C with agitation. Then Dynabeads were added, followed by incubation for another 2 h at 4 °C with agitation. The beads were washed three times with cold RIPA buffer, and once more with PBS, then resuspended in Laemmli buffer for use in SDS/PAGE and subsequent Western blot analyses to detect coimmunoprecipitated complexes.
SDS/PAGE, Native-PAGE, and Western Blot Analysis.
For SDS/PAGE, cells were harvested and lysed on ice with RIPA buffer (Thermo Fisher Scientific) supplemented with proteinase inhibitor mixture (Sigma-Aldrich). Proteins were separated on 7% or 10% (vol/vol) polyacrylamide SDS gels and transferred to PVDF membranes with the Trans-Blot Turbo blotting system (Bio-Rad). For native-PAGE, cells were lysed on ice in a nondenaturing buffer [50 mM Tris⋅HCl pH 8.0, 0.8% Nonidet P-40, 150 mM NaCl, and 10% (vol/vol) glycerol]. Equal concentrations of lysates were loaded on 6% (vol/vol) polyacrylamide gels (without SDS) with loading buffer free of denaturing agents, then separated in SDS-free running buffer and transferred to PVDF membranes. The membranes were blocked with 5% (vol/vol) skimmed milk in TBS-T and then incubated with primary antibodies (Table S1) for 2 h and with secondary antibodies (Table S1) for 1 h at room temperature. Protein detection by chemiluminescence (HRP) was achieved with ECL (Thermo Fisher Scientific).
SI Materials and Methods
Complex formation of LH2 and FKBP65 was investigated using an in situ PLA for fluorescent microscopy or bright-field microscopy. Human dermal fibroblasts were fixated in methanol/acetone (1:1) for 10 min at −20 °C, washed with PBS, and permeabilized with 0.5% Triton X-100 for 3 min. To reduce nonspecific staining, cells were incubated with 10% (vol/vol) donkey serum (Jackson ImmunoResearch Laboratories) in PBS for 15 min, followed by incubation with primary antibodies. Next, cells were incubated with PLA Probe Anti-Mouse MINUS and PLA Probe Anti-Rabbit PLUS 1:5 (Sigma-Aldrich) in 2% (vol/vol) BSA/PBS solution in a humidity chamber at 37 °C for 1 h. All subsequent steps were performed according to the manufacturer’s protocol for ligation and amplification. For immunofluorescence, cells were incubated with primary antibodies (Table S1), followed by detection using appropriate combinations of donkey anti-rabbit Alexa Fluor 488, donkey anti-rabbit Alexa Fluor 555, donkey anti-goat Alexa Fluor 488, and goat anti-mouse biotin, followed by streptavidin-Cy3 (Table S1). Secondary antibodies were dissolved in PBS containing DAPI (Sigma-Aldrich). Images were acquired using a Zeiss AxioObserver.Z1 microscope.
Human renal tissue samples were collected from the tissue archive at the Department of Pathology of the University Medical Center Groningen. Frozen sections (3 μm) were cut from an explanted renal allograft with interstitial fibrosis/tubular atrophy and then fixed in acetone for 10 min at room temperature. Endogenous peroxidase was quenched using 0.1% hydrogen peroxide for 10 min at room temperature. To reduce nonspecific staining, sections were incubated with 10% (vol/vol) donkey serum in PBS (Jackson ImmunoResearch Laboratories) for 15 min, followed by incubation with primary antibodies or the appropriate control IgG (Sigma-Aldrich). Sections were then incubated with PLA Probe Anti-Mouse MINUS and PLA Probe Anti-Rabbit PLUS 1:5 in 2% (vol/vol) BSA/PBS solution for 1 h in a humidity chamber at room temperature. Ligation, amplification, hybridization, and visualization were performed according to the manufacturer’s protocol. After dehydration, slides were mounted with VectaMount (Vector Laboratories). For immunohistochemistry, sections were incubated with HRP-labeled secondary and tertiary antibodies (DAKO) (Table S1) and subsequently incubated with 3-amino-9-ethylcarbazole for visualization. Slides were mounted in Aquatex (Merck).
Acknowledgments
This work was supported by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (Grant FES0908) and the Dutch Kidney Foundation. Microscopic imaging was performed at the University Medical Center Groningen Imaging Center, which is supported by the Netherlands Organization for Health Research and Development (Grant 40-00506-98-9021).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600074113/-/DCSupplemental.
References
- 1.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33(1):7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 2.Klingberg F, Hinz B, White ES. The myofibroblast matrix: Implications for tissue repair and fibrosis. J Pathol. 2013;229(2):298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop DJ, Kivirikko KI. Collagens: Molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 4.Byers PH, Murray ML. Ehlers-Danlos syndrome: A showcase of conditions that lead to understanding matrix biology. Matrix Biol. 2014;33:10–15. doi: 10.1016/j.matbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Forlino A, Cabral WA, Barnes AM, Marini JC. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7(9):540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucero HA, Kagan HM. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63(19-20):2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- 9.Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992;6(7):2439–2449. doi: 10.1096/fasebj.6.7.1348714. [DOI] [PubMed] [Google Scholar]
- 10.Robins SP. Analysis of the crosslinking components in collagen and elastin. Methods Biochem Anal. 1982;28:329–379. doi: 10.1002/9780470110485.ch8. [DOI] [PubMed] [Google Scholar]
- 11.Vater CA, Harris ED, Jr, Siegel RC. Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J. 1979;181(3):639–645. doi: 10.1042/bj1810639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnero P, et al. The collagenolytic activity of cathepsin K is unique among mammalian proteinases. J Biol Chem. 1998;273(48):32347–32352. doi: 10.1074/jbc.273.48.32347. [DOI] [PubMed] [Google Scholar]
- 13.van der Slot-Verhoeven AJ, et al. The type of collagen cross-link determines the reversibility of experimental skin fibrosis. Biochim Biophys Acta. 2005;1740(1):60–67. doi: 10.1016/j.bbadis.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Steinmann B, Eyre DR, Shao P. Urinary pyridinoline cross-links in Ehlers-Danlos syndrome type VI. Am J Hum Genet. 1995;57(6):1505–1508. [PMC free article] [PubMed] [Google Scholar]
- 15.Ruotsalainen H, et al. Glycosylation catalyzed by lysyl hydroxylase 3 is essential for basement membranes. J Cell Sci. 2006;119(Pt 4):625–635. doi: 10.1242/jcs.02780. [DOI] [PubMed] [Google Scholar]
- 16.Takaluoma K, Lantto J, Myllyharju J. Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol. 2007;26(5):396–403. doi: 10.1016/j.matbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Uzawa K, et al. Differential expression of human lysyl hydroxylase genes, lysine hydroxylation, and cross-linking of type I collagen during osteoblastic differentiation in vitro. J Bone Miner Res. 1999;14(8):1272–1280. doi: 10.1359/jbmr.1999.14.8.1272. [DOI] [PubMed] [Google Scholar]
- 18.Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J Bone Miner Res. 2004;19(8):1349–1355. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- 19.Yeowell HN, Walker LC. Tissue specificity of a new splice form of the human lysyl hydroxylase 2 gene. Matrix Biol. 1999;18(2):179–187. doi: 10.1016/s0945-053x(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 20.Zuurmond AM, van der Slot-Verhoeven AJ, van Dura EA, De Groot J, Bank RA. Minoxidil exerts different inhibitory effects on gene expression of lysyl hydroxylase 1, 2, and 3: Implications for collagen cross-linking and treatment of fibrosis. Matrix Biol. 2005;24(4):261–270. doi: 10.1016/j.matbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Turpeenniemi-Hujanen TM, Puistola U, Kivirikko KI. Isolation of lysyl hydroxylase, an enzyme of collagen synthesis, from chick embryos as a homogeneous protein. Biochem J. 1980;189(2):247–253. doi: 10.1042/bj1890247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turpeenniemi-Hujanen TM, Puistola U, Kivirikko KI. Human lysyl hydroxylase: Purification to homogeneity, partial characterization and comparison of catalytic properties with those of a mutant enzyme from Ehlers-Danlos syndrome type VI fibroblasts. Coll Relat Res. 1981;1(4):355–366. doi: 10.1016/s0174-173x(81)80012-x. [DOI] [PubMed] [Google Scholar]
- 23.Rautavuoma K, et al. Characterization of three fragments that constitute the monomers of the human lysyl hydroxylase isoenzymes 1-3. The 30-kDa N-terminal fragment is not required for lysyl hydroxylase activity. J Biol Chem. 2002;277(25):23084–23091. doi: 10.1074/jbc.M112077200. [DOI] [PubMed] [Google Scholar]
- 24.Heikkinen J, et al. Dimerization of human lysyl hydroxylase 3 (LH3) is mediated by the amino acids 541-547. Matrix Biol. 2011;30(1):27–33. doi: 10.1016/j.matbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Puig-Hervás MT, et al. Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome: osteogenesis imperfecta phenotypic spectrum. Hum Mutat. 2012;33(10):1444–1449. doi: 10.1002/humu.22133. [DOI] [PubMed] [Google Scholar]
- 26.Ha-Vinh R, et al. Phenotypic and molecular characterization of Bruck syndrome (osteogenesis imperfecta with contractures of the large joints) caused by a recessive mutation in PLOD2. Am J Med Genet A. 2004;131(2):115–120. doi: 10.1002/ajmg.a.30231. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P, et al. Novel mutations in FKBP10 and PLOD2 cause rare Bruck syndrome in Chinese patients. PLoS One. 2014;9(9):e107594. doi: 10.1371/journal.pone.0107594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Slot AJ, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278(42):40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 29.Moravej H, et al. Bruck syndrome: a rare syndrome of bone fragility and joint contracture and novel homozygous FKBP10 mutation. Endokrynol Pol. 2015;66(2):170–174. doi: 10.5603/EP.2015.0024. [DOI] [PubMed] [Google Scholar]
- 30.Alanay Y, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86(4):551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley BP, et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res. 2011;26(3):666–672. doi: 10.1002/jbmr.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarze U, et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum Mol Genet. 2013;22(1):1–17. doi: 10.1093/hmg/dds371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaheen R, et al. Mutations in FKBP10 cause both Bruck syndrome and isolated osteogenesis imperfecta in humans. Am J Med Genet A. 2011;155A(6):1448–1452. doi: 10.1002/ajmg.a.34025. [DOI] [PubMed] [Google Scholar]
- 34.Setijowati ED, et al. A novel homozygous 5 bp deletion in FKBP10 causes clinically Bruck syndrome in an Indonesian patient. Eur J Med Genet. 2012;55(1):17–21. doi: 10.1016/j.ejmg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Steinlein OK, Aichinger E, Trucks H, Sander T. Mutations in FKBP10 can cause a severe form of isolated osteogenesis imperfecta. BMC Med Genet. 2011;12:152. doi: 10.1186/1471-2350-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes AM, et al. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum Mutat. 2012;33(11):1589–1598. doi: 10.1002/humu.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venturi G, et al. A novel splicing mutation in FKBP10 causing osteogenesis imperfecta with a possible mineralization defect. Bone. 2012;50(1):343–349. doi: 10.1016/j.bone.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Barnes AM, et al. Kuskokwim syndrome, a recessive congenital contracture disorder, extends the phenotype of FKBP10 mutations. Hum Mutat. 2013;34(9):1279–1288. doi: 10.1002/humu.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bank RA, et al. Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: Indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci USA. 1999;96(3):1054–1058. doi: 10.1073/pnas.96.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coss MC, Winterstein D, Sowder RC, 2nd, Simek SL. Molecular cloning, DNA sequence analysis, and biochemical characterization of a novel 65-kDa FK506-binding protein (FKBP65) J Biol Chem. 1995;270(49):29336–29341. doi: 10.1074/jbc.270.49.29336. [DOI] [PubMed] [Google Scholar]
- 41.Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): Biological diversity--targets--functions. Curr Top Med Chem. 2003;3(12):1315–1347. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 42.Zeng B, et al. Chicken FK506-binding protein, FKBP65, a member of the FKBP family of peptidylprolyl cis-trans isomerases, is only partially inhibited by FK506. Biochem J. 1998;330(Pt 1):109–114. doi: 10.1042/bj3300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bächinger HP, Morris NP, Davis JM. Thermal stability and folding of the collagen triple helix and the effects of mutations in osteogenesis imperfecta on the triple helix of type I collagen. Am J Med Genet. 1993;45(2):152–162. doi: 10.1002/ajmg.1320450204. [DOI] [PubMed] [Google Scholar]
- 44.Ishikawa Y, Vranka J, Wirz J, Nagata K, Bächinger HP. The rough endoplasmic reticulum-resident FK506-binding protein FKBP65 is a molecular chaperone that interacts with collagens. J Biol Chem. 2008;283(46):31584–31590. doi: 10.1074/jbc.M802535200. [DOI] [PubMed] [Google Scholar]
- 45.Gjaltema RA, de Rond S, Rots MG, Bank RA. Procollagen lysyl hydroxylase 2 expression is regulated by an alternative downstream transforming growth factor beta-1 activation mechanism. J Biol Chem. 2015;290(47):28465–28476. doi: 10.1074/jbc.M114.634311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Slot AJ, et al. Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta. 2005;1741(1-2):95–102. doi: 10.1016/j.bbadis.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Remst DF, et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 2014;66(3):647–656. doi: 10.1002/art.38266. [DOI] [PubMed] [Google Scholar]
- 48.Passoja K, Myllyharju J, Pirskanen A, Kivirikko KI. Identification of arginine-700 as the residue that binds the C-5 carboxyl group of 2-oxoglutarate in human lysyl hydroxylase 1. FEBS Lett. 1998;434(1-2):145–148. doi: 10.1016/s0014-5793(98)00966-1. [DOI] [PubMed] [Google Scholar]
- 49.Hyry M, Lantto J, Myllyharju J. Missense mutations that cause Bruck syndrome affect enzymatic activity, folding, and oligomerization of lysyl hydroxylase 2. J Biol Chem. 2009;284(45):30917–30924. doi: 10.1074/jbc.M109.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinckmann J, et al. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: Important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol. 2005;24(7):459–468. doi: 10.1016/j.matbio.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 51.van der Slot AJ, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 2004;23(4):251–257. doi: 10.1016/j.matbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, et al. Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J Clin Invest. 2015;125(3):1147–1162. doi: 10.1172/JCI74725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisinger-Mathason TS, et al. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3(10):1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pinnell SR. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J Biol Med. 1985;58(6):553–559. [PMC free article] [PubMed] [Google Scholar]