Significance
Excess energy intake and physical inactivity are two major factors causing obesity, but the underlying mechanisms have not been fully understood. Both excess energy intake and physical inactivity increase cellular energy status that is monitored by the energy-sensing AMP-activated protein kinase (AMPK). We demonstrate that the AMPK–tre-2/USP6, BUB2, cdc16 domain family member 1 (the TBC domain is the GTPase activating protein domain) (TBC1D1) signaling nexus regulates insulin-like growth factor 1 (IGF1) secretion. Disruption of this AMPK–TBC1D1 signaling nexus in mice enhances lipogenic gene expression via promoting IGF1 secretion, causes obesity, and eventually leads to development of metabolic syndrome. These findings reveal a novel regulatory mechanism that links energy status to development of obesity via control of IGF1 secretion.
Keywords: AMPK, TBC1D1, phosphorylation, IGF1 secretion, obesity
Abstract
Tre-2/USP6, BUB2, cdc16 domain family member 1 (the TBC domain is the GTPase activating protein domain) (TBC1D1) is a Rab GTPase activating protein that is phosphorylated on Ser231 by the AMP-activated protein kinase (AMPK) in response to intracellular energy stress. However, the in vivo role and importance of this phosphorylation event remains unknown. To address this question, we generated a mouse model harboring a TBC1D1Ser231Ala knockin (KI) mutation and found that the KI mice developed obesity on a normal chow diet. Mechanistically, TBC1D1 is located on insulin-like growth factor 1 (IGF1) storage vesicles, and the KI mutation increases endocrinal and paracrinal/autocrinal IGF1 secretion in an Rab8a-dependent manner. Hypersecretion of IGF1 causes increased expression of lipogenic genes via activating the protein kinase B (PKB; also known as Akt)–mammalian target of rapamycin (mTOR) pathway in adipose tissues, which contributes to the development of obesity, diabetes, and hepatic steatosis as the KI mice age. Collectively, these findings demonstrate that the AMPK–TBC1D1 signaling nexus interacts with the PKB–mTOR pathway via IGF1 secretion, which consequently controls expression of lipogenic genes in the adipose tissue. These findings also have implications for drug discovery to combat obesity.
Due to changes in diet and lifestyle, metabolic syndrome including obesity, type 2 diabetes, and nonalcoholic fatty liver disease is increasing in prevalence worldwide, putting a huge burden on modern society and motivating us to get better understanding of these diseases and how to combat them.
High energy intake and lack of physical activity can result in imbalances of energy metabolism and changed energy status of the body, which is monitored by the energy-sensing kinase AMP-activated protein kinase (AMPK) (1). The AMPK holoenzyme is a heterotrimer, consisting of catalytic α (α1 and α2), regulatory β (β1 and β2), and γ (γ1, γ2 and γ3) subunits (1). AMPK is mainly activated through Thr172 phosphorylation in its catalytic domain by the upstream liver kinase B1 (LKB1) under energy stress conditions that increase the cellular AMP:ATP ratio (2, 3). AMPK can regulate both glucose and lipid metabolism by its phosphorylation of multiple substrates (4). For instance, acetyl-CoA carboxylase (ACC), an enzyme important for fatty acid synthesis and oxidation, is regulated through an inhibitory phosphorylation by AMPK, which is essential for the control of lipid metabolism (5). The LKB1–AMPK pathway can also control glucose uptake into skeletal muscle by promoting translocation of the glucose transporter 4 (GLUT4) from its intracellular storage sites onto plasma membrane in response to a pharmacological AMPK activator, 5-aminoimidazole-4-carboxamide riboside (AICAR), or muscle contraction (6). However, the mechanism by which AMPK promotes translocation of GLUT4 onto plasma membrane is not well understood.
Tre-2/USP6, BUB2, cdc16 domain family member 1 [the TBC domain is the GTPase activating protein (GAP) domain] (TBC1D1) is an Rab GTPase activating protein (RabGAP), and an R125W mutation on this protein has been linked to familial female obesity (7). A truncation or KO of TBC1D1 confers leanness to mice fed on high fat diet with increased fatty acid oxidation in skeletal muscle (8, 9). TBC1D1 has also been implicated in regulating muscle glucose uptake (10), whereas its deficiency causes a decreased expression level of GLUT4 in skeletal muscle (8, 9, 11).
We previously identified TBC1D1 as an AMPK substrate that is phosphorylated on Ser231 by AMPK and interacts with 14-3-3 proteins on Ser231 phosphorylation (12). However, the physiological role of this AMPK–TBC1D1 signal nexus remains elusive. In this study, we generated a TBC1D1Ser231Ala knockin (KI) mouse model to address the functions of TBC1D1 Ser231 phosphorylation in vivo.
Results
Generation and Characterization of the TBC1D1 KI Mice.
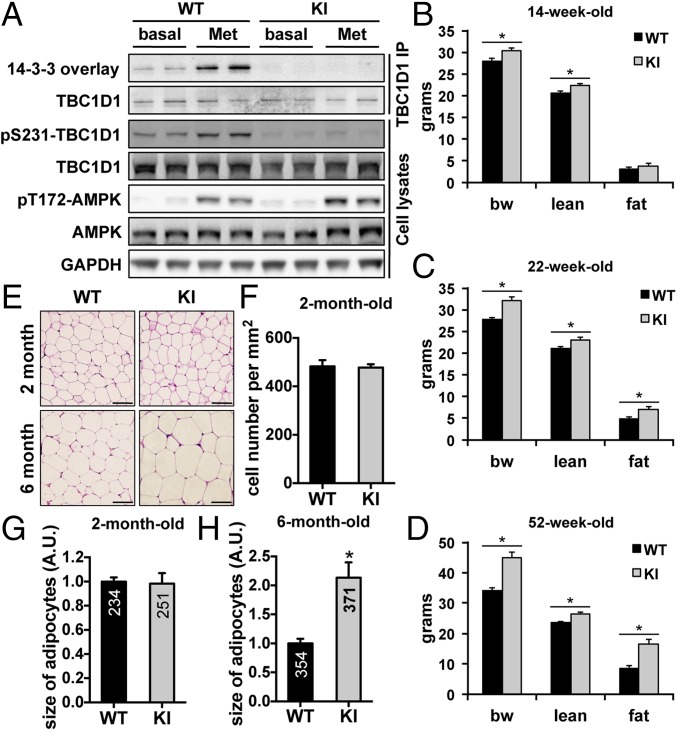
The TBC1D1 KI mice were generated using the gene targeting strategy illustrated in SI Appendix, Fig. S1A. The expression levels of the TBC1D1Ser231Ala mutant proteins were normal, and there was no detectable alteration in the expression of the related RabGAP AS160 (also known as TBC1D4) in various tissues from the KI mice. As expected, the phosphorylation of Ser231 on TBC1D1 was not detectable in the tissues from the KI mice (SI Appendix, Fig. S1B). The pharmacological AMPK activator metformin induced phosphorylation of AMPK in primary hepatocytes from the KI mice to a similar extent as in WT cells. In contrast and as anticipated, metformin-stimulated TBC1D1 Ser231 phosphorylation was only detected in the lysates of WT, but not KI, hepatocytes. The metformin-stimulated 14-3-3–TBC1D1 interaction assessed by 14-3-3 overlay assays was diminished in the TBC1D1 immunoprecipitates from the KI cell lysates compared with the WT controls (Fig. 1A). Taken together, these data validate the suitability of the TBC1D1 KI mice and their derived cells for studying the specific in vivo and in vitro function of TBC1D1 Ser231 phosphorylation.
Fig. 1.
Generation and characterization of the TBC1D1 KI mice. (A) Ser231 phosphorylation and 14-3-3 binding of TBC1D1 isolated from primary hepatocytes that had been treated with metformin (Met) for 1 h. (B–D) Body composition of the male TBC1D1Ser231Ala KI mice measured at the age of 14 wk (B, WT: n = 18; KI: n = 19), 20–22 wk (C, WT: n = 15; KI: n = 15), and 52 wk (D, WT: n = 8; KI: n = 9). (E–H) Histology (E) and cell number (F) and size (G and H) of the adipose from the WT and TBC1D1 KI male mice. Bars indicate 50 μm in length. Six sections per genotype were analyzed to measure cell numbers. The numbers shown in the bar graphs refer to the number of cells measured in the experiments. The data are given as the mean ± SEM, and the asterisk indicates P < 0.05.
The TBC1D1 KI Mice Developed Obesity and Displayed Characteristics of Metabolic Syndrome When They Aged.
We next monitored the growth of the KI mice and found that they became significantly heavier than the WT littermates from 5 wk after birth (SI Appendix, Fig. S1C). The KI mice had longer bodies and tibias than WTs, and their growth plates of tibia were significantly elongated in the zones of proliferation and of hypertrophy (SI Appendix, Fig. S1 D–G). The increased body mass of the young KIs (less than 4 mo old) was accounted for by their significantly increased lean mass. In contrast, the fat mass in the young KI mice was comparable to that of the WT littermates (Fig. 1B and SI Appendix, Fig. S2 A and B). However, in contrast to the normal fat mass and enhanced lean mass of young KI mice, older KI mice became fatter, and had higher lean and fat masses than WTs (Fig. 1 C and D and SI Appendix, Fig. S2 C and D). Around 5 mo of age was a transition period for increased fat mass in the KI mice. Consistent with the increased fat mass, adipocytes were markedly enlarged in the older KI mice (Fig. 1 E–H). Obesity is a high risk factor for development of type II diabetes and hepatic steatosis. The young KI mice (4.5 mo old) had normal blood chemistry parameters including blood glucose, free fatty acid, triglyceride, total cholesterol, and plasma insulin and displayed normal glucose tolerance. However, as these animals aged (∼1 y old), they developed hyperglycemia, hyperinsulinemia, and hypercholesterolemia and became glucose intolerant and insulin resistant (SI Appendix, Fig. S1 H–N). Furthermore, these mice developed hepatic steatosis, and their livers were significantly enlarged and contained larger lipid droplets (SI Appendix, Fig. S1 O–Q).
Lipogenic Gene Expression Was Increased in the Adipose of the TBC1D1 KI Mice.
Although TBC1D1 has been implicated in regulating muscle glucose uptake (10), we found that GLUT4 expression and glucose uptake was normal in multiple tissues/primary cells and insulin-stimulated GLUT4 translocation was not altered in primary adipocytes from the KI mice (SI Appendix, Fig. S3), suggesting that glucose uptake did not account for the obese phenotype of these mice.
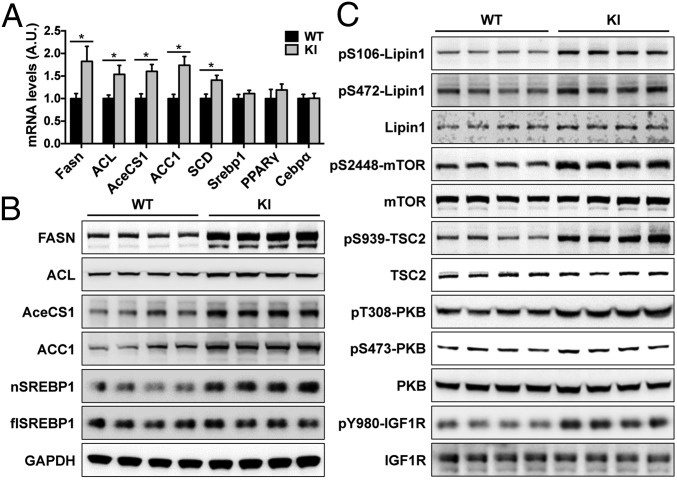
We then investigated whether altered adipogenesis, lipogenesis, or lipolysis in the adipose might account for the obese phenotype of the KI mice. To this end, we analyzed expression of key regulators for these processes in the adipose of young KI mice (4 mo old). Both lipolysis marker [adipose triglyceride lipase (ATGL)] and adipogenesis markers [CAAT/enhancer-binding protein α (CEBPα) and peroxisome proliferator-activated receptor γ (PPARγ)] remained normal in the adipose of the KI mice (Fig. 2A and SI Appendix, Fig. S4A). In contrast, the expression of key enzymes for lipogenesis including fatty acid synthase (FASN), ATP-citrate lyase (ACL), cytoplasmic acetyl-CoA synthetase (AceCS1), acetyl-CoA carboxylase (ACC1), and stearoyl-CoA desaturase (SCD) was significantly up-regulated in the KI adipose tissue, despite the normal mRNA levels of sterol regulatory element-binding protein 1 (SREBP1), which is an upstream transcriptional regulator for these lipogenic genes (Fig. 2 A and B and SI Appendix, Fig. S4B). Full-length SREBP1 (flSREBP1) resides on the endoplasmic reticulum (ER), and its N-terminal fragment (nSREBP1) enters the nucleus after cleavage and regulates lipogenic gene expression (13). Interestingly, the nSREBP1 levels were significantly increased in the adipose of the KI mice (Fig. 2B and SI Appendix, Fig. S4B). The cleavage of SREBP1 on the ER is under control of mammalian target of rapamycin (mTOR) (14). Consistently, phosphorylation of protein kinase B (PKB), tuberous sclerosis 2 (TSC2, also known as tuberin), mTOR, and downstream mTOR targets S6K and 4EBP1 were all increased in the KI adipose tissue (Fig. 2C and SI Appendix, Fig. S2 A–C). Lipin1, a downstream target of mTOR, can down-regulate SREBP1 activity in the nucleus and consequently control the expression of lipogenic genes (15). The entry of lipin1 into the nucleus is under control of its phosphorylation by mTOR, and this phosphorylation promotes lipogenesis by releasing the inhibitory effect of lipin1 on SREBP1 and consequently up-regulates lipogenic gene expression (15). Consistent with mTOR activation, the phosphorylation of lipin1 was significantly up-regulated in the KI adipose tissue (Fig. 2C and SI Appendix, Fig. S4C). Collectively, these data suggest that the activation of PKB–mTOR pathway in the KI mice may up-regulate lipogenic genes via elevation of nSREBP1 levels and relief of the inhibitory effect of lipin1. We further found that the phosphorylation of PKB, TSC2, mTOR, S6K and 4EBP1 was also up-regulated in skeletal muscle and liver of the KI mice (SI Appendix, Fig. S4 D–I). Although the PKB activation in the KI mice expectedly increased the phosphorylation of its downstream targets including TSC2, GSK3, and FOXO1, the phosphorylation of AS160, a key regulator for insulin-stimulated glucose uptake, was not increased in the adipose and skeletal muscle of the KI mice (SI Appendix, Fig. S4).
Fig. 2.
Lipogenenic gene expression in the adipose of the TBC1D1 KI mice. (A) mRNA expression of indicated genes in the epididymal fat of the WT and KI male mice (16 wk old). n = 5. (B) Expression of indicated proteins were measured in the adipose from the WT and KI male mice (16 wk old) using GAPDH as a loading control. (C) Phosphorylated and total lipin1, mTOR, TSC2, PKB, and IGF1R were measured in the adipose from the KI mice and WT littermates (16 wk old). The data are given as the mean ± SEM, and the asterisk indicates P < 0.05.
Plasma Insulin-Like Growth Factor 1 Levels Were Elevated in the TBC1D1 KI Mice.
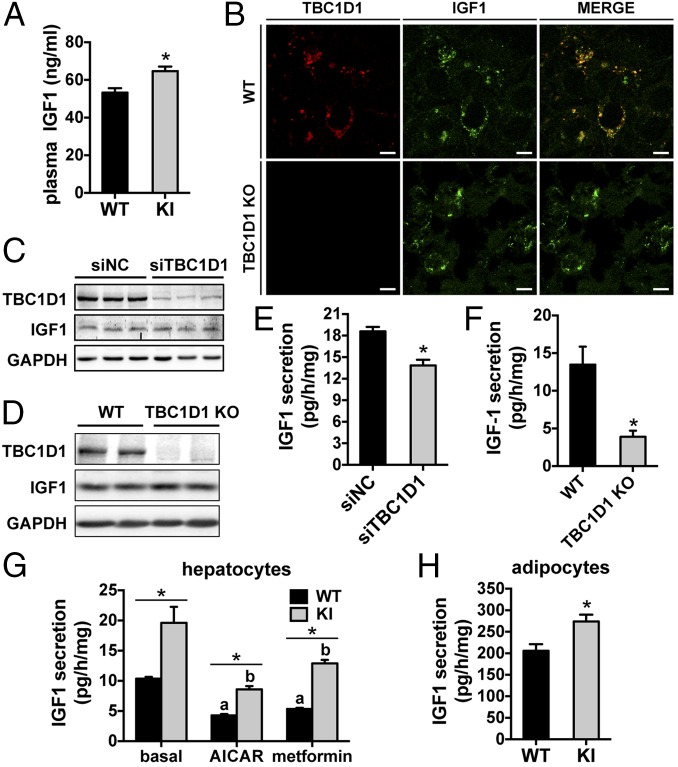
We next sought to find out how the KI mutation causes the activation of the PKB–TSC2–mTOR pathway. Considering the overgrowth phenotype of the KI mice, we surmised that plasma levels of insulin-like growth factor 1 (IGF1), a key growth promoter that activates the PKB–mTOR pathway in multiple tissues, might be elevated. Indeed, plasma IGF1 levels were significantly higher in the KI mice (Fig. 3A and SI Appendix, Fig. S2G). Consistently, activation of the IGF1 receptor (IGF1R) was significantly enhanced in the KI mice (Fig. 2C and SI Appendix, Fig. S4). In contrast to IGF1, plasma IGF2 and IGF1-binding protein IGFBP3 levels were normal in the KI mice (SI Appendix, Figs. S1 R and S, and S2H). Expression of IGF1, IGF2, and IGFBPs were normal in the liver of the KI mice (SI Appendix, Fig. S5). IGF1 expression in the liver is under control of growth hormone (GH), whose levels in the plasma were normal in the KI mice with unaltered hepatic GH signaling (SI Appendix, Figs. S1T, S2I, and S5H). These data show that plasma IGF1 levels were selectively elevated in the KI mice.
Fig. 3.
Plasma IGF1 levels and IGF1 secretion in primary cells from the TBC1D1 KI mice. (A) Plasma levels of IGF1 in the male WT and KI mice (8 wk old). n = 7. (B) Colocalization of TBC1D1 and IGF1 in primary hepatocytes. Bars indicate 10 μm in length. (C and D) Expression of TBC1D1 and IGF1 in TBC1D1-depleted primary hepatocytes via siRNA (C) or in primary hepatocytes from the TBC1D1 KO mice (D). NC, negative control. (E and F) IGF1 secretion in TBC1D1-depleted primary hepatocytes (E) or in primary hepatocytes from the TBC1D1 KO mice (F). NC, negative control. n = 6. (G) IGF1 secretion in primary hepatocytes from the WT and TBC1D1 KI mice. aP < 0.05 (WT metformin or AICAR vs. WT untreated) and bP < 0.05 (KI metformin or AICAR vs. KI untreated). n = 8. (H) IGF1 secretion in primary adipocytes from the KI mice. WT: n = 5; KI: n = 6. The data are given as the mean ± SEM, and the asterisk indicates P < 0.05. Statistical analysis for G was carried out using two-way ANOVA.
TBC1D1 Colocalizes with IGF1 Vesicles and Regulates IGF1 Secretion.
IGF1 is synthesized and temporarily stored within vesicles in hepatocytes and then secreted into the bloodstream. Interestingly, when TBC1D1 and IGF1 were coexpressed in human liver carcinoma HepG2 cells, the two proteins largely colocalized with each other (SI Appendix, Fig. S6A). We further found that endogenous TBC1D1 was localized on the IGF1 storage vesicles in primary hepatocytes (Fig. 3B) and in myotubes (SI Appendix, Fig. S6B). Together, these data suggest that TBC1D1 might be involved in the regulation of IGF1 secretion. Indeed, knockdown of TBC1D1 via small interference RNA (siRNA) significantly decreased IGF1 secretion rates in primary hepatocytes with no alteration in IGF1 expression. Furthermore, in primary hepatocytes from our previously reported TBC1D1 KO mice (16), IGF1 secretion rates were also significantly lower than those of the WT controls, despite normal hepatic IGF1 expression (Fig. 3 C–F).
The TBC1D1Ser231Ala KI Mutation Increases both Endocrinal and Paracrinal/autocrinal IGF1 Secretion.
Consistent with the elevated levels of plasma IGF1 in the KI mice, IGF1 secretion rates of primary hepatocytes from these mice were significantly higher than those of WT cells under unstimulated conditions (Fig. 3G). The widely used AMPK activators, metformin and AICAR, significantly decreased IGF1 secretion rates of WT hepatocytes. Although the rates of IGF1 secretion by TBC1D1 KI hepatocytes decreased in response to metformin or AICAR, these secretion rates still remained significantly higher than those of WT cells treated with these drugs. We confirmed that the activation of AMPK by AICAR and metformin was comparable in the two genotypes of primary hepatocytes, with no effect on IGF1 protein expression (SI Appendix, Fig. S6C). We further found that the KI mutation also enhanced paracrinal/autocrinal IGF1 secretion in primary adipocytes (Fig. 3H), primary chondrocytes (SI Appendix, Fig. S6 C and D), and isolated skeletal muscle ex vivo (SI Appendix, Fig. S6F). Consistent with normal plasma levels of IGFBP3, secretion of IGFBP3 was unaltered in primary hepatocytes from the KI mice (SI Appendix, Fig. S5 L and M). These data show that the KI mutation selectively affects IGF1 secretion. Consistent with the hypersecretion of IGF1, the PKB–TSC2–mTOR pathway was activated in primary adipocytes and hepatocytes isolated from the KI mice (SI Appendix, Figs. S7 A and B, and S8 A–C).
Inhibition of IGF1 Secretion or Action Blunted the Hyperactivation of the PKB Pathway in Primary Cells from the TBC1D1 KI Mice.
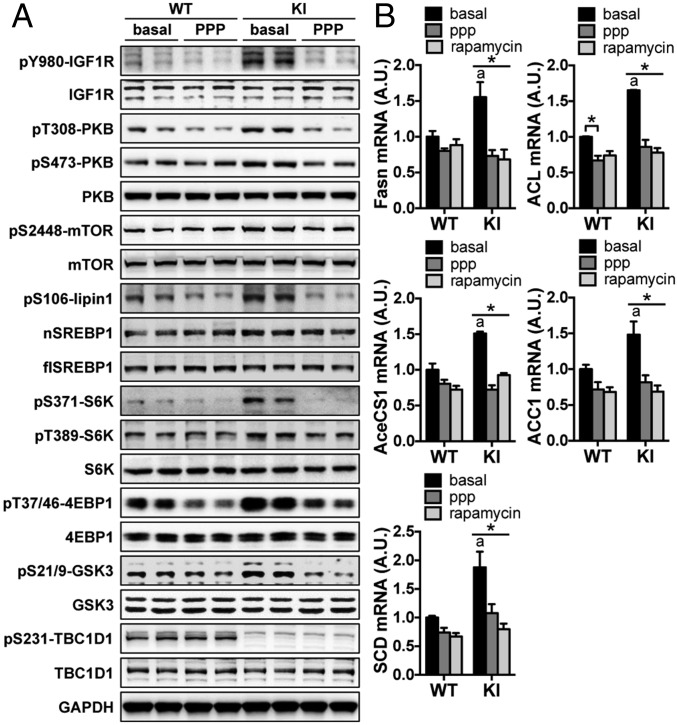
To establish a causal relationship between the hypersecretion of IGF1 and the activation of the PKB–TSC2–mTOR pathway in the KI mice, we used an IGF1R inhibitor, picropodophyllin (PPP) (17), to treat primary adipocytes and hepatocytes from the KI mice. We found that blocking the IGF1 receptor with PPP largely normalized phosphorylation of the signaling proteins in the IGF1R–PKB–mTOR pathway in TBC1D1 KI adipocytes. Moreover, the levels of nSREBP1 were significantly increased in the KI adipocytes under untreated conditions, and PPP treatment diminished this increase (Fig. 4A and SI Appendix, Fig. S7C). Similar effects on PKB activation by PPP were observed in primary hepatocytes (SI Appendix, Fig. S8 D and E). We examined the possibility that WT and TBC1D1 KI cells might differ in their sensitivities to IGF1 or PPP. We found treatment with exogenous IGF1 activated PKB in the two genotypes of cells to similar extents, and PPP treatment could inhibit PKB activation elicited by exogenous IGF1 in cells from both genotypes of mice to similar levels (SI Appendix, Figs. S7D and S8 F and G). These findings suggest that the hyperactivation of PKB in KI adipocytes and hepatocytes was most likely due to hypersecretion of IGF1. It was still possible that the KI mutation affected the IGF1R independent of IGF1 secretion. To examine this possibility, we down-regulated IGF1 via siRNA in primary hepatocytes from the WT and KI mice. IGF1R expression was normal in the KI hepatocytes, whereas its phosphorylation was significantly increased in parallel with PKB phosphorylation in these cells (SI Appendix, Fig. S8 H and I). On knockdown of IGF1, IGF1 secretion rates were decreased in the KI hepatocytes, which largely normalized IGF1R and PKB activation (SI Appendix, Fig. S8 H and I), suggesting that the effects of the KI mutation on the IGF1R–PKB pathway requires IGF1 secretion. Together, these data strongly suggest a causal relationship between hypersecretion of IGF1 and activation of the PKB–TSC2–mTOR pathway in the TBC1D1 KI mice.
Fig. 4.
Effects of IGF1R inhibition on activation of the PKB–TSC2–mTOR pathway and lipogenic gene expression in primary adipocytes from the TBC1D1 KI mice. (A) Phosphorylated and total forms of indicated proteins, and nSREBP1 and flSREBP1 were measured in PPP treated or untreated primary adipocytes from the TBC1D1 KI and WT mice using GAPDH as a loading control. (B) mRNA expression of lipogenic genes in primary adipocytes treated with or without PPP or rapamycin. n = 3. The data are given as the mean ± SEM. Statistical analyses were carried out using two-way ANOVA. The asterisk indicates P < 0.05 (basal vs. PPP or rapamycin). aP < 0.05 (KI basal vs. WT basal).
Inhibition of the IGF1–mTOR Pathway Normalized the Expression of Lipogenic Genes in Primary Adipocytes from the TBC1D1 KI Mice.
IGF1 can stimulate lipogenesis in porcine adipose tissue ex vivo (18), and it can also increase lipogenesis via activation of the PKB pathway in SEB-1 sebocytes (19). Similarly, treatment of WT primary adipocytes with IGF1 activated the PKB–mTOR pathway and strongly increased lipogenic gene expression (SI Appendix, Fig. S9). Again, we used primary adipocytes from the KI mice to investigate the relationship between the hypersecretion of IGF1 and lipogenic gene expression. The mRNA levels of lipogenic genes were significantly increased in primary KI adipocytes under basal conditions, which were significantly inhibited on PPP treatment (Fig. 4B), suggesting that the increased lipogenic gene expression was most likely due to the hypersecretion of IGF1 and consequent hyperactivation of its receptor. Moreover, an mTOR inhibitor rapamycin inhibited the expression of these lipogenic genes in the KI adipocytes (Fig. 4B), which further supports our proposal that hyperactivation of PKB–mTOR pathway downstream of the IGF1R is responsible for the induction of lipogenic genes in the adipose of the KI mice.
Rab8a Plays a Key Role Downstream of the TBC1D1 in Regulating IGF1 Secretion.
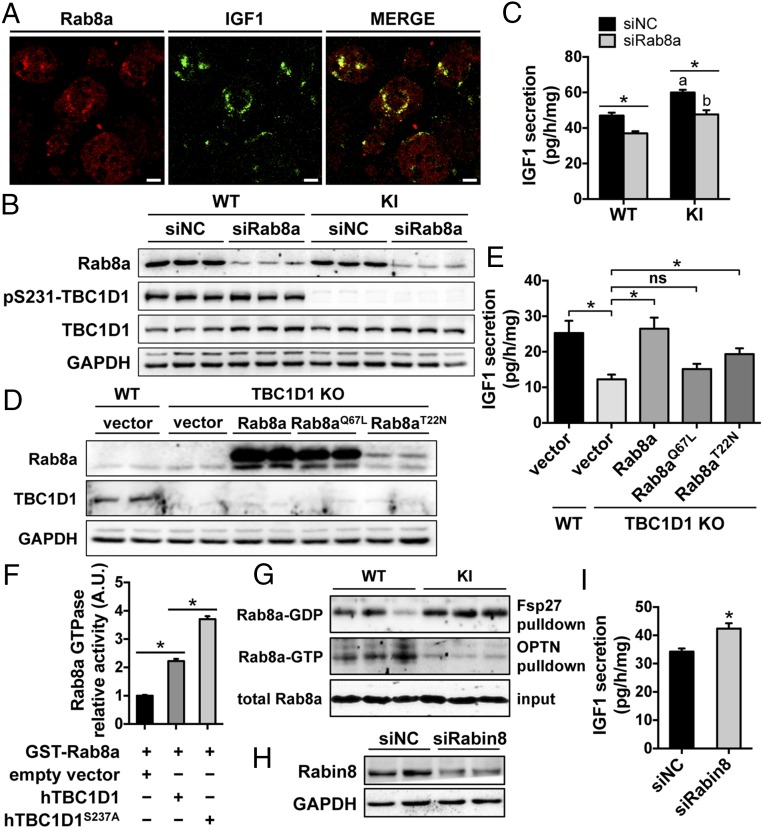
We then sought to find out how TBC1D1 and its Ser231 phosphorylation regulate IGF1 secretion. IGF1 secretion rates were largely restored when a human recombinant TBC1D1 (hTBC1D1) or an hTBC1D1Ser237Ala mutant protein (Ser237 on human TBC1D1 corresponds to Ser231 on the mouse protein) were expressed in the TBC1D1 KO hepatocytes (SI Appendix, Fig. S10). Furthermore, metformin and AICAR could also significantly decrease IGF1 secretion rates in these hepatocytes expressing hTBC1D1 or hTBC1D1Ser237Ala mutant proteins. Interestingly, the expression of a GAP-deficient TBC1D1 mutant (20) could not restore IGF1 secretion rates, suggesting that the GAP activity of TBC1D1 is required for IGF1 secretion. We next investigated which Rab(s) downstream of TBC1D1 mediates IGF1 secretion by downregulating four known in vitro TBC1D1 substrates, namely Rab2a, Rab8a, Rab10, and Rab14 (20). Knockdown of Rab8a caused a significant decrease in IGF1 secretion rates in WT primary hepatocytes, whereas down-regulation of Rab2a, Rab10, and Rab14 had no significant effect (SI Appendix, Fig. S11 A and B). We also found that Rab8a largely colocalized with IGF1 in primary hepatocytes (Fig. 5A). Furthermore, knockdown of Rab8a normalized IGF1 secretion in primary hepatocytes from the TBC1D1 KI mice (Fig. 5 B and C), suggesting that regulation of IGF1 secretion by the TBC1D1 Ser231 phosphorylation requires Rab8a. Overexpression of the WT Rab8a, but not a GTP-bound Rab8aQ67L mutant, could restore IGF1 secretion in the TBC1D1 KO hepatocytes (Fig. 5 D and E). Although a GDP-bound Rab8aT22N mutant was expressed at a much lower level, it could still partially restore IGF1 secretion in the TBC1D1 KO hepatocytes (Fig. 5 D and E). In an in vitro assay, immunoprecipitated recombinant hTBC1D1 displayed significant GAP activity toward Rab8a and enhanced the GTPase activity of Rab8a. The GAP activity of hTBC1D1 toward Rab8a was inhibited by its cellular phosphorylation in response to AMPK activator phenformin (SI Appendix, Fig. S11C) and, in contrast, was enhanced by Ser237Ala mutation (Fig. 5F). Moreover, more GDP-bound Rab8a and less GTP-bound Rab8a were found in the adipose of the TBC1D1 KI mice in pulldown assays (Fig. 5G). These data suggest that the GDP-bound Rab8a promotes IGF1 secretion downstream of TBC1D1. Consistent with this proposal, we found that knockdown of Rabin8, a guanine nucleotide exchange factor (GEF) for Rab8a (21), promoted IGF1 secretion in primary hepatocytes (Fig. 5 H and I). Together, these data show that TBC1D1 and its Ser231 phosphorylation control IGF1 secretion by regulating Rab8a.
Fig. 5.
Rab8a and IGF1 secretion in primary hepatocytes. (A) Colocalization of Rab8a and IGF1 in primary hepatocytes. Bars indicate 10 μm in length. (B and C) Down-regulation of Rab8a in primary hepatocytes from the WT and TBC1D1 KI mice via siRNA (B), and IGF1 secretion in Rab8a-depleted primary hepatocytes (C). n = 6. (D and E) Expression of indicated proteins (D) and IGF1 secretion (E) in TBC1D1 KO primary hepatocytes expressing Rab8a WT and mutant proteins. n = 6. (F) The GTPase activity of recombinant GST-Rab8a was measured in the presence of immunoprecipitated WT or hTBC1D1S237A mutant protein. n = 5. (G) Levels of GDP-bound Rab8a and GTP-bound Rab8a in the epididymal fat of the WT and KI male mice (5 mo old) were determined using pulldown assays. (H and I) Down-regulation of Rabin8 in primary hepatocytes via siRNA (H) and IGF1 secretion in Rabin8-depleted primary hepatocytes (I). NC, negative control. n = 6.
Discussion
Our findings shed light on how cellular energy status regulates lipogenesis and are consistent with a model in which the AMPK–TBC1D1 signaling nexus controls lipogenic gene expression by regulating endocrinal and paracrinal/autocrinal IGF1 secretion (SI Appendix, Fig. S11D). Inhibition of TBC1D1 Ser231 phosphorylation increases expression of lipogenic genes in the adipose and consequently causes obesity in mice most likely due to hypersecretion of IGF1 and hyperactivation of the IGF1R–PKB–TSC2–mTOR pathway.
It has long been recognized that the energy and nutrient sensors, AMPK and mTOR, play opposing roles in governing cell growth in response to varying energy status (22, 23). Energy deficiency activates AMPK, which in turn phosphorylates proteins including TSC2 and raptor. Phosphorylation of TSC2 leads to inhibition of the small GTPase Rheb, whereas phosphorylated raptor binds to the dimeric phosphoprotein-binding 14-3-3 proteins, and both of these mechanisms lead to inhibition of mTOR. The AMPK–TSC2–Rheb–mTOR and AMPK–raptor–mTOR pathways restrict cell growth in response to energy shortage (22, 24). Besides this opposing effect on growth, both AMPK and mTOR regulate lipogenesis. AMPK phosphorylates ACC and thereby inhibits fatty acid synthesis (5). In contrast, mTOR phosphorylates lipin1 and the latter promotes lipogenesis by enhancing SREBP1 activity (15).
Our findings reveal a previously unrecognized layer of regulation, involving IGF1 as a systemic signal that links these two signaling pathways to control lipogenesis in response to energy status. In our model, we propose that energy deficiency activates AMPK, which inhibits both endocrinal and paracrinal/autocrinal IGF1 secretion via phosphorylation of TBC1D1 and possibly other regulators of IGF1 secretion. Decreased endocrinal and paracrinal/autocrinal IGF1 secretion results in hypoactivation of the PKB–mTOR pathway, which may contribute to a decline in lipogenesis in adipose tissues. When energy is sufficient, AMPK activity and TBC1D1 phosphorylation decrease in the body, which promotes endocrinal and paracrinal/autocrinal IGF1 secretion. The elevated IGF1 activates PKB–mTOR signaling, which may promote lipogenesis in adipose tissues. Our findings not only shed light on the complexity of the regulatory mechanism linking energy status with lipogenesis but also provide potential targets for drug discovery to combat obesity. These findings may also help to elucidate the therapeutic mechanisms for the anti-diabetic drug metformin, which may regulate lipogenesis in the adipose through modulating IGF1 secretion.
Genetic evidence has suggested that TBC1D1 is linked to obesity although the molecular mechanisms are not fully understood. For instance, the TBC1D1R125W mutation is a candidate for obesity susceptibility in human patients (7), whereas deficiency of TBC1D1 protects mice from diet-induced obesity (8, 9). Our findings provide one mechanism linking TBC1D1 Ser231 phosphorylation to lipogenesis by controlling IGF1 secretion and consequent activation of the IGF1R–PKB–mTOR pathway. Genetic polymorphisms of TBC1D1 have also been associated with growth traits in other animals including pigs (25) and rabbits (26). Hypersecretion of IGF1 activated the IGF1R–PKB–mTOR pathway and phosphorylated classical mTOR substrates S6K and 4EBP1 in the liver, skeletal muscle and possible other tissues, which may regulate protein synthesis and contribute to accelerated body growth. Thus, our data also suggest a link between TBC1D1 and body growth possibly via endocrinal and paracrinal/autocrinal IGF1 secretion. Based on our current data, we propose a possible sequence of the changes in the TBC1D1 KI mice as follows. The KI mutation increases IGF1 secretion, which promotes body growth and enhances lipogenic gene expression via activation of the IGF1R–PKB–mTOR pathway in young mice (less than 4 mo old). The induction of lipogenic genes may increase lipogenesis and possibly lead to fat accumulation over time, which consequently contributes to the development of obesity in older mice (over 5 mo old). Obesity may further lead to the development of type II diabetes and hepatic steatosis as the KI mice age (from 8 mo on). Although expression of lipogenic genes strongly suggests an increase of de novo lipogenesis in the TBC1D1 KI mice, further experimentation is still needed to firmly establish this in vivo.
Clearly, TBC1D1 has other functions, and regulates GLUT4 expression and fatty acid oxidation in skeletal muscle (8, 9, 11). TBC1D1 can be phosphorylated on multiple sites besides Ser231 (12), and electroporation of a TBC1D1-4P mutant (in which Ser231, Thr499, Thr590, and Ser621 are mutated to Ala) into skeletal muscle decreases contraction-stimulated glucose uptake (10). Because our study does not support a role for TBC1D1 Ser231 phosphorylation in regulating GLUT4 expression or trafficking under the conditions tested here, it is possible that other phosphorylation sites on TBC1D1 might be important for contraction-stimulated muscle glucose uptake.
Our knowledge concerning the regulation of IGF1 secretion is surprisingly sparse. Besides the regulatory mechanism we describe here, the only previously known mechanism involves a calcium sensor synaptotagmin-10 that regulates activity-dependent IGF1 secretion in olfactory bulb neurons (27). Given the important functions of IGF1 and potential for modulating its release for therapeutic purposes, we must elucidate the regulation of IGF1 secretion in more detail in future. One open question is whether there are additional kinase(s) besides AMPK that may be able to phosphorylate TBC1D1-Ser231 and regulate IGF1 secretion in vivo, although we established AMPK being the upstream kinase for phosphorylation of Ser231 on TBC1D1 (12). It is also possible that alanine substitution of Ser231 could have gain of function rather than merely mimic a nonphosphorylation state. Therefore, more experimentation is needed in future to further demonstrate that AMPK regulates IGF1 secretion. Another open question stemming from our model is how Rab8a regulates IGF1 secretion in a manner that is dependent on its GDP-bound form. Although Rabs usually regulate their effector proteins in their GTP-bound forms, there are precedents for GDP-bound forms of Rabs having biological functions. For instance, Rab8a has recently been shown to promote fusion of lipid droplets in its GDP-bound form (28). Identification of downstream effector(s) for the GDP-bound form of Rab8a will help to elucidate the mechanism how Rab8a regulates IGF1 secretion and deepen our understanding of this process.
Materials and Methods
Details for reagents, generation of the TBC1D1 KI mice, body composition measurement, cell culture and transfection, primary cell isolation and IGF1 secretion assay, glucose uptake, imaging, tissue/cell lysis, and Western blot are described in SI Appendix, SI Materials and Methods. The animal facility at Nanjing University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal protocols were approved by the Ethics Committees initially at University of Dundee and then at Nanjing University.
Supplementary Material
Acknowledgments
We thank Yanqiu Ji and Gail Fraser for assistance with genotyping of mice and members of the resource units in Nanjing and Dundee for technical assistance. We thank Drs. Rachel Toth and Simon Arthur (University of Dundee) for generating constructs for the TBC1D1 KI mouse. We thank Prof. Thomas C. Südhof (Stanford University) for the IGF1 cDNA and Prof. Peng Li (Tsinghua University) for the Rab8a, Fsp27, and OPTN cDNAs. Funding in support of this work is described in SI Appendix.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600581113/-/DCSupplemental.
References
- 1.Hardie DG, Ross FA, Hawley SA. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62(7):2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fullerton MD, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie DG, Sakamoto K. AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Stone S, et al. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet. 2006;15(18):2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- 8.Chadt A, et al. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet. 2008;40(11):1354–1359. doi: 10.1038/ng.244. [DOI] [PubMed] [Google Scholar]
- 9.Dokas J, et al. Conventional knockout of Tbc1d1 in mice impairs insulin- and AICAR-stimulated glucose uptake in skeletal muscle. Endocrinology. 2013;154(10):3502–3514. doi: 10.1210/en.2012-2147. [DOI] [PubMed] [Google Scholar]
- 10.An D, et al. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59(6):1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stöckli J, et al. The RabGAP TBC1D1 plays a central role in exercise-regulated glucose metabolism in skeletal muscle. Diabetes. 2015;64(6):1914–1922. doi: 10.2337/db13-1489. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, et al. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409(2):449–459. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 14.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson TR, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ducommun S, Wang HY, Sakamoto K, MacKintosh C, Chen S. Thr649Ala-AS160 knock-in mutation does not impair contraction/AICAR-induced glucose transport in mouse muscle. Am J Physiol Endocrinol Metab. 2012;302(9):E1036–E1043. doi: 10.1152/ajpendo.00379.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girnita A, et al. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64(1):236–242. doi: 10.1158/0008-5472.can-03-2522. [DOI] [PubMed] [Google Scholar]
- 18.Walton PE, Gopinath R, Etherton TD. Porcine insulin-like growth factor (IGF) binding protein blocks IGF-I action on porcine adipose tissue. Proc Soc Exp Biol Med. 1989;190(4):315–319. doi: 10.3181/00379727-190-42865. [DOI] [PubMed] [Google Scholar]
- 19.Smith TM, Gilliland K, Clawson GA, Thiboutot D. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128(5):1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roach WG, Chavez JA, Mîinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J. 2007;403(2):353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13(9):3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontanesi L, et al. The porcine TBC1D1 gene: Mapping, SNP identification, and association study with meat, carcass and production traits in Italian heavy pigs. Mol Biol Rep. 2011;38(2):1425–1431. doi: 10.1007/s11033-010-0247-3. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZJ, et al. Identification and association of SNPs in TBC1D1 gene with growth traits in two rabbit breeds. Asian-australas J Anim Sci. 2013;26(11):1529–1535. doi: 10.5713/ajas.2013.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca(2+)-sensor synaptotagmin-10. Cell. 2011;145(2):300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev Cell. 2014;30(4):378–393. doi: 10.1016/j.devcel.2014.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.