Significance
Electroreception in terrestrial animals is poorly understood. In bumblebees, the mechanical response of filiform hairs in the presence of electric fields provides key evidence for electrosensitivity to ecologically relevant electric fields. Mechanosensory hairs in arthropods have been shown to function as fluid flow or sound particle velocity receivers. The present work provides direct evidence for additional, nonexclusive functionality involving electrical Coulomb-force coupling between distant charged objects and mechanosensory hairs. Thus, the sensory mechanism is proposed to rely on electromechanical coupling, whereby many light thin hairs serve the detection of the electrical field surrounding a bumblebee approaching a flower. This finding prompts the possibility that other terrestrial animals use such sensory hairs to detect and respond to electric fields.
Keywords: electric fields, bees, behavior, sensory biology
Abstract
Bumblebees (Bombus terrestris) use information from surrounding electric fields to make foraging decisions. Electroreception in air, a nonconductive medium, is a recently discovered sensory capacity of insects, yet the sensory mechanisms remain elusive. Here, we investigate two putative electric field sensors: antennae and mechanosensory hairs. Examining their mechanical and neural response, we show that electric fields cause deflections in both antennae and hairs. Hairs respond with a greater median velocity, displacement, and angular displacement than antennae. Extracellular recordings from the antennae do not show any electrophysiological correlates to these mechanical deflections. In contrast, hair deflections in response to an electric field elicited neural activity. Mechanical deflections of both hairs and antennae increase with the electric charge carried by the bumblebee. From this evidence, we conclude that sensory hairs are a site of electroreception in the bumblebee.
Electroreception is common in aquatic animals. First discovered in sharks (1), electroreception has also been found in rays (2), amphibians (3), teleost fish (4, 5), dolphins (6), platypuses (7), and echidnas (8), which use electrosensory organs in their snout to detect prey in wet soil.
The first specialized electrosensory structures discovered were the “ampullae di Lorenzini” (9). Ampullae are small tubular cavities containing an electrolytic jelly (2), which maintains the same electric potential as the water immediately adjacent. In sharks and rays, differences in electric potential between the inside of the animal and the jelly are transduced by epithelial cells (10), where negative deviations in potential are excitatory whereas positive ones are inhibitory (11). Teleost fish have independently evolved electroreceptors that are excited by positive voltages and inhibited by negative voltages (11). This general mechanism for electroreception has evolved independently in several animal lineages (12, 13).
Ampullary electroreception requires the presence of an electrically conductive medium. Even in terrestrial animals such as the platypus and echidna, electroreceptive organs need to be submerged in water or surrounded by damp or humid substrates to function (7, 8). In contrast, bees detect weak electric fields in dry air, an electrically insulating medium. Bumblebees detect the presence of floral static electric fields (14), and honeybees detect oscillating fields associated with their waggle dance (15). In air, ampullary electroreceptors are ineffective because of an absence of conductive medium between the sensory organ and the environment. We thus investigate the possibility that electric fields instead exert forces on charged, mechanosensory structures on the bee: hairs and antennae.
To investigate electroreception in air, we use noncontact laser Doppler vibration measurements and electrophysiology. We test two hypotheses: First, bumblebees use their antennae to detect electric fields. This hypothesis is supported by evidence that honeybee antennae deflect in response to electric fields analogous to those produced by conspecifics performing the waggle dance (15). Second, bumblebees use mechanosensory hairs to detect electric fields. In support of that hypothesis is the rich literature on arthropod sensory hairs detecting small forces associated with fluid flow and sound particle velocity (16). We find that electric fields of ecologically relevant magnitudes cause motion in both the antennae and body hairs, but only hair motion elicits a commensurate neural response. From these data we conclude that hairs are used by bumblebees to detect electric fields.
Results
Bumblebee Hairs and Antennae Mechanically Respond to Electric Fields.
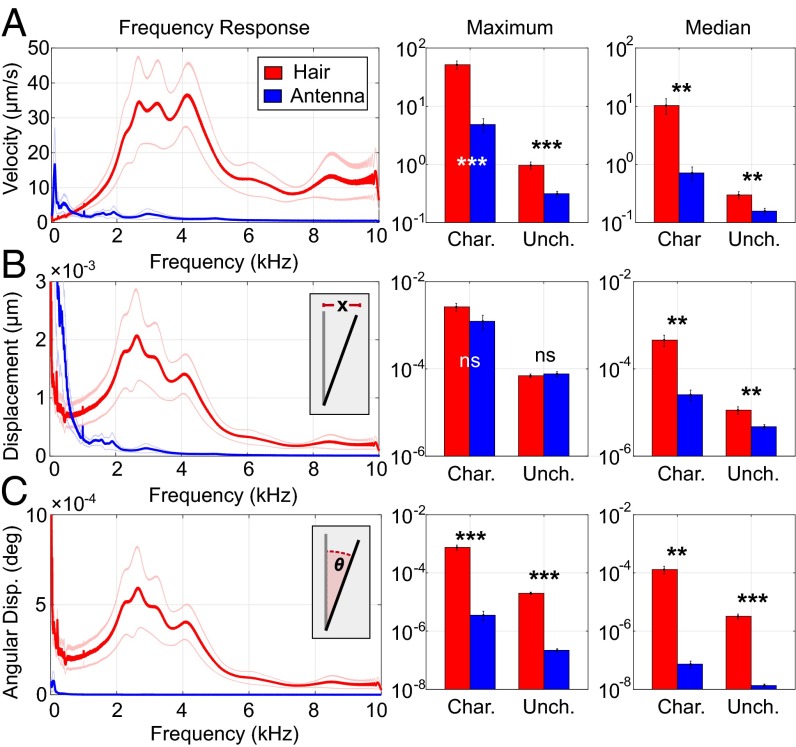
The motion of the antennae and sensory hairs in response to applied electric fields was measured by using a laser Doppler vibrometer (LDV) (Fig. 1). LDV measures the vibrational velocity (v) of structures undergoing oscillations, which was transformed into displacement (x) and angular displacement (θ) (SI Results). “Displacement” is the absolute motion of the structure, whereas “angular displacement” is the motion of the structure in relation to its length. Angular displacement is proportional to the strain on the mechanoreceptors innervating the joint, either at the flagellum-pedicel joint of the antenna (Johnston’s organ) or the base of the hair.
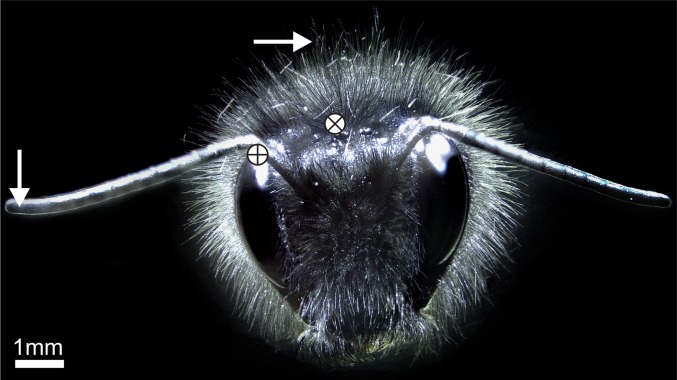
Fig. 1.
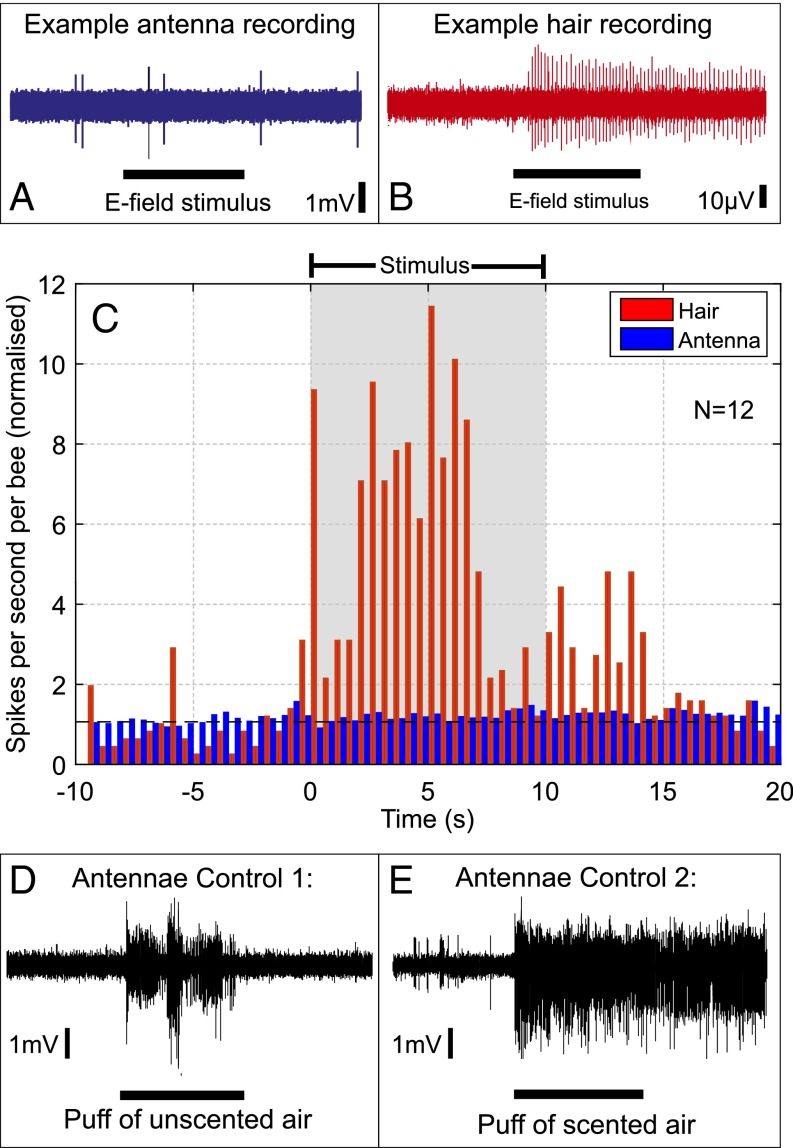
Bumblebee covered in body hairs. The white circle containing a plus (+) denotes electrode insertion points in the antennae. The white circle containing a cross (x) denotes approximate electrode insertion points for hair recordings. White arrows show the laser focal position for hair and antennae LDV recordings.
Electromechanical Responses to Broadband Electric Field Stimulation.
A 400 Vpp sinusoidal frequency sweep from 10 Hz to 10 kHz (sweep duration: 0.64 s) was applied to a steel disk, 1 cm from the hair or antenna. Alternating electric fields were used because they cause steady-state velocity responses suitable for LDV. For each bee (n = 10), the responses of two hairs and both antennae were recorded (Fig. 1), in both a charged and an uncharged preparation (Figs. S1–S3). The resonant frequency was the frequency of maximum response amplitude. The mean resonant frequency for hairs was 3.8 ± 0.2 kHz and 1.1 ± 0.3 kHz for antennae (Table 1).
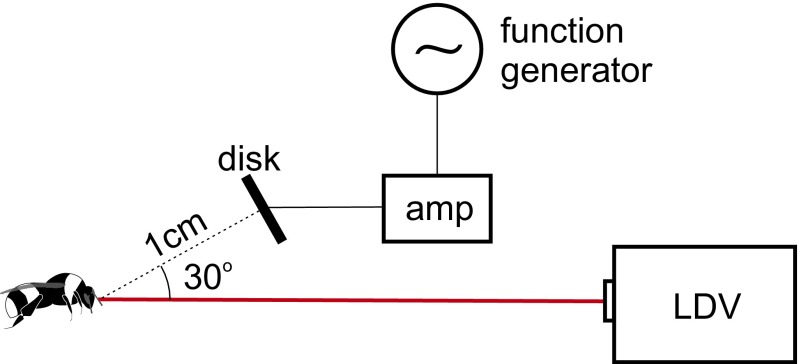
Fig. S1.
Laser vibrometry setup for measuring mechanical response. AC and DC voltages were applied to a brass disk 1 cm away from the bee. An LDV then recorded the motion antennae or hairs in reaction to the voltages on the disk.
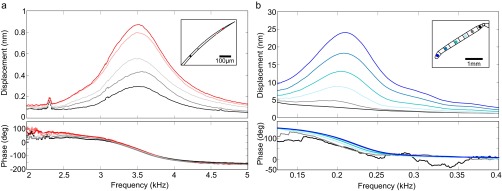
Fig. S3.
Motion of a bumblebee hair (A) and antenna (B) measured by laser Doppler vibrometry in response to an AC voltage sweep (400 V sine wave). (A) Displacement (Top) and phase response (Bottom) of a hair. Colors correspond to measurement points along the antenna (Inset). (B) Displacement (Top) and phase response (Bottom) of an antenna. Colors correspond to measurement points along the antenna (Inset).
Table 1.
Key results from laser Doppler vibrometry experiments
| Charged | Uncharged | ||||||
| Quantity | Type | Hair | P | Antenna | Hair | P | Antenna |
| Velocity, µm/s | 95 pctl | 51.8 ± 8.3 | *** | 4.9 ± 1.2 | 0.97 ± 0.1 | *** | 0.31 ± 0.03 |
| Median | 10.3 ± 3.1 | ** | 0.71 ± 0.19 | 0.30 ± 0.04 | ** | 0.16 ± 0.02 | |
| Disp., nm | 95 pctl | 2.6 ± 0.5 | n.s. | 1.2 ± 0.5 | 0.070 ± 0.01 | n.s. | 0.077 ± 0.01 |
| Median | 0.5 ± 0.1 | ** | 0.026 ± 0.01 | 0.011 ± 0.002 | ** | 0.005 ± 0.001 | |
| Ang. disp, deg × 10−9 | 95 pctl | 13,200 ± 270 | *** | 61.7 ± 23 | 345 ± 34 | *** | 3.84 ± 0.49 |
| Median | 2,290 ± 670 | *** | 1.28 ± 0.4 | 56.6 ± 11 | *** | 0.24 ± 0.03 | |
Shown are the 95th percentile (pctl) and median values of velocity, displacement (Disp.), and angular displacement (Ang. disp.) in response to 10 Hz–10 kHz sinusoidal electrical chirps at 400 Vpp amplitude. Results are from both charged and uncharged preparations. Asterisks show significance of difference between hair and antennae response under identical preparation and stimulation [t test (paired): **P ≤ 0.01; ***P ≤ 0.001; n = 10]. n.s., not significant.
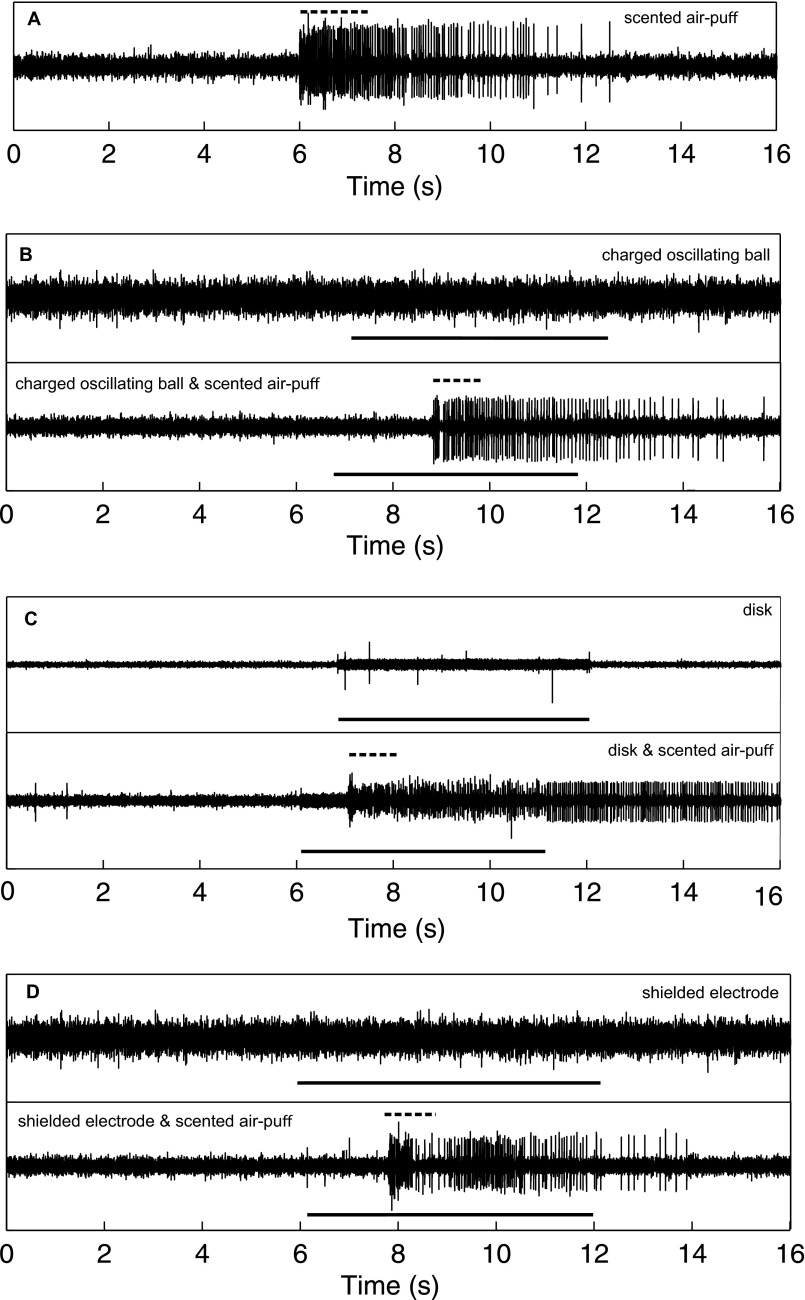
Fig. S2.
Extracellular neurophysiological recordings of the antennae in response to 140 Hz AC electric fields and lavender-scented air puffs. (A) Response to scented air puff. (B) Response to charged oscillating ball (Top). Large spikes are the result of dropped samples (see Inset; dashed black line is the scent stimulus and the solid black line is the electrical stimulus). The large spikes seen in the recording are due to sampling errors and are not neural activity). Response to charged oscillating ball and scented air puff (Bottom). (C) Response to steel disk at 20 V (Top). Response to the disk and a scented air puff (Bottom). (D) Response to a shielded electrode at 400 V (Top). Response to the electrode and a scented air puff (Bottom). Solid black lines indicated the electrical stimulus. Dashed black lines show the scented air-puff stimulus. The antennae reliably responded to scents and mechanical stimuli. In no case did the antenna respond to electric field stimuli.
Hairs and antennae both move in response to electric fields. Hairs respond with significantly greater maximum and median velocity (vmax, vmedian) than the antennae (Fig. 2A). The difference between maximum displacement (xmax) of hairs and antennae is not statistically significant, although hairs respond with significantly larger median displacement (xmedian) (Fig. 2A). The tips of hairs and antennae move a similar absolute distance in response to electric fields at their respective resonant frequencies, although the hairs move more in response to spectrally broad electrical stimulation (Fig. 2B). Because hairs are much shorter than antennae, the maximum and median angular displacement (θmax, θmedian) of hairs is significantly greater than that of antennae (Table 1 and Fig. 2C). The velocity of the hair is an order of magnitude greater than that of the antennae. Both hairs and antennae move like a stiff rod, pivoting the base where mechanosensory neurons are located. The absence of bending is revealed by the invariant phase response between the stimulus and the displacement at resonance (Fig. S3).
Fig. 2.
Antenna (blue) and hair (red) motion in response to 10 Hz–10 kHz electrical chirps. Rows show the velocity (A), displacement (B), and angular displacement (C). Columns show the response at each frequency (Left), the amplitude of maximum response (Middle), and the median response amplitude across all frequencies (Right). Insets show a representation of the quantity being measured.
How Sensitive Is the Electromechanical Response?
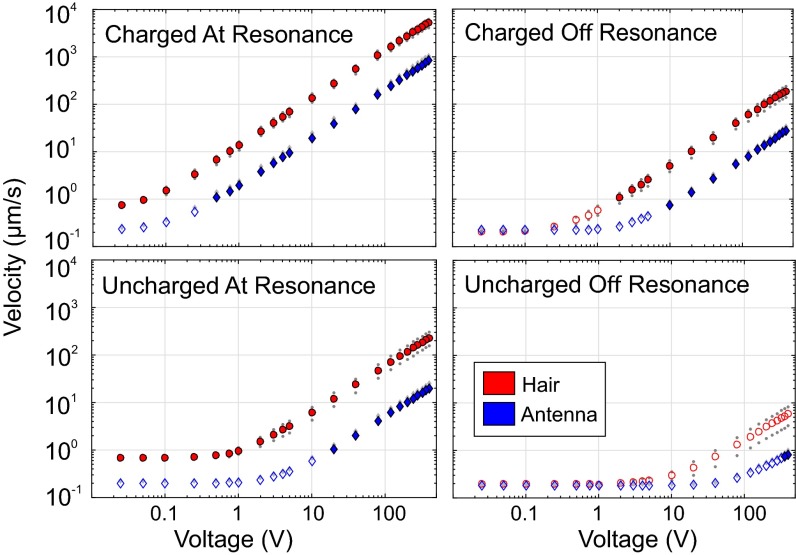
To determine the minimum stimulus voltage generating a measurable mechanical response, spectrally pure sinusoidal voltages are applied to the stimulus delivery disk. To evaluate maximal sensitivity, stimuli were applied at the resonant frequency and at a second nonresonant frequency of the hair or antenna. The voltage at which the measured vibrational velocity is statistically distinguishable from thermal noise (Umin) is recorded for hairs and antennae in both charged and uncharged states.
Throughout the entire range of test conditions and stimulus voltages, the vibrational velocity of the hairs was an order of magnitude greater than that of the antennae (Fig. 3 C and D). Umin was also lower for hairs than for antennae, indicating a higher sensitivity to electric fields (Table 2). Umin for charged hairs was 25 mV for both resonant and nonresonant stimuli. Umin for charged antennae was 500 mV at resonance and 10 V off resonance (between 20 and 400 times greater than the hair). Only when the bee’s charge was deliberately set to zero, did the antennae respond to a lower voltage than the hair. In natural free-flight situation, however, bees are only rarely found with zero charge (15, 17).
Fig. 3.
Antenna (blue) and hair (red) mean velocity in response to oscillating electric fields at the resonant frequency of each structure (Left) and at the frequency of median response (Right) under charged (Top) and uncharged (Bottom) preparations. Gray dots show SEM. Filled shapes denote responses that were significantly larger than thermal noise. Unfilled shapes denote responses statistically indistinguishable from thermal noise.
Table 2.
The minimum voltage on a disk 1 cm away from a bumblebee required to produce a mechanical response (Umin), the electric field corresponding to this voltage (Emin), and the axial distance from a 30-V disk at which electric field strength is equal to this value (Dmax)
| Charged | Uncharged | |||
| Quantity | Hair | Antenna | Hair | Antenna |
| Vmin at resonance, V | 0.025 | 0.5 | 0.025 | 20 |
| Vmin off resonance, V | 2 | 10 | 400 | 360 |
| Emin at resonance, V·m−1 | 0.77 | 15 | 0.77 | 612 |
| Emin off resonance, m−1 | 61 | 306 | 12,249 | 11,024 |
| Dmax at resonance, cm | 55 | 13 | 55 | 1.6 |
| Dmax off resonance, cm | 7.1 | 2.6 | 0.1 | 0.6 |
Minimum Electric Field Strengths Required to Elicit Electromechanical Responses.
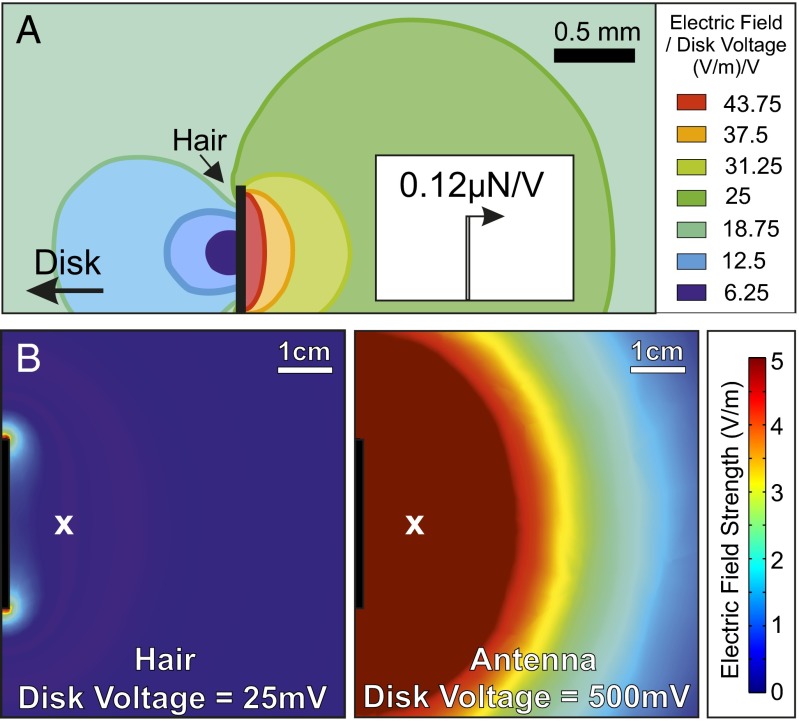
To quantify the electric field associated with our stimuli and to evaluate its distance of action, finite element analysis (FEA) was used to compute field geometry and strength E. The computed field was evaluated at the location of the sensor (1 cm axial distance from the disk) for various disk voltages. The minimum electric field strength (Emin) required to produce mechanical motion in charged hairs is 0.77 V⋅m−1 for resonant stimuli and 61 V⋅m−1 for nonresonant stimuli. For antennae, Emin is much higher (15.3 V⋅m−1 for resonant stimuli and 306 V⋅m−1 for nonresonant stimuli; Table 2).
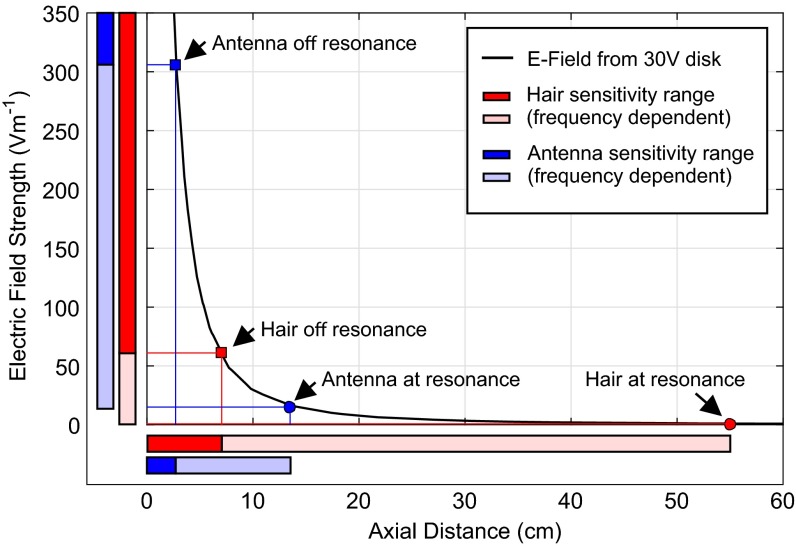
When the disk is held at 30 V, it produces an electric field of comparable magnitude to floral electric fields and can be detected by bees on the wing (14). For a fixed disk voltage, the electric field strength E varies with distance r from disk as E∝r−2 (Fig. 4). The maximum distance at which the disk actuates the hair or antennae can then be used as a proxy for how relatively sensitive the structure is to an electric field. Accordingly, charged hairs can be actuated by a 30-V disk at a distance of 7.1–55 cm depending on stimulus frequency. Antennae are actuated at a maximum distance of 2.6–13 cm depending on stimulus frequency (Table 2 and Figs. 4 and 5). These distances of detection are consistent with the bumblebee’s behavioral abilities reported by Clarke et al. (14).
Fig. 4.
(A) A finite element model of a bumblebee hair under an electric field produced by a steel disk 1 cm away. Electric field values are given per positive volt on the disk. (Inset) The resultant projected force on the hair (0.12 µN/V). (B) The simulated electric field due to the disk at 25 mV (Left) and 500 mV (Right), the minimum voltages which caused observable motion in the hairs and antennae, respectively. The white x shows the position at which the hairs and antennae were located in the LDV experiments.
Fig. 5.
A finite element model of the stimulus delivery system showing electric field strength as a function of axial distance from 30-mm steel disk held at 30 V. The labeled points show the maximum distance of detection calculated for hairs (red) and antennae (blue) for resonant stimuli (circles) and nonresonant stimuli (squares). The bars on each axis represent the range of values of electric field and distance at which these structures show a mechanical response. The lighter area shows the difference between at-resonant and off-resonant stimulation, showing the responses within this range depend on frequency. The dark colored areas of the bars show the range at which the structures respond at all frequencies.
The Effect of Electric Charge on Electromechanical Responses.
Bees accumulate charge during motion through their environment, (14, 17). A similar phenomenology likely applies to other flying or walking insects (18, 19). The bees used in this study, even in their charged state, carried less charge than they do in vivo [in vivo charge: 32 ± 3 pC (15); experimental charge: 4 ± 1 pC, n = 10]. Nevertheless, the effect of this small charge on the mechanical sensitivity of both hairs and antennae was pronounced. Charged bees respond with significantly greater amplitude than uncharged bees (paired t tests between charged and uncharged preparations P < 0.01 throughout). This difference corresponds to a 5- to 53-fold increase in electromechanical sensitivity, across all measurements, between bumblebees carrying no charge and those carrying one-tenth of the charge of a free-flying bumblebee (Table 1).
Electromechanical Responses of Hairs and Antennae to DC Electric Fields.
Hairs and antennae were stimulated with a 400-V square pulse lasting 1 s. The onset of the electric field produces a transient velocity signal measured by the laser that was integrated with respect to time to give the change in position of the structure. This experiment was only performed on charged bees. The average displacement for antennae was 1.2 ± 0.4 μm. This displacement is consistent with observations of the antennae in honeybees, which are displaced ∼1 μm in response to 450 V, 40 Hz electric stimuli (15). The average displacement of the hairs was significantly lower at 0.14 ± 0.05 μm (paired t test: P < 0.005). The corresponding angular displacements were (3.3 ± 1) × 10−3 degrees for the antennae and (3.7 ± 0.01) × 10−2 degrees for the hairs. In response to the same static electric field, the angular deflection of the hair was 11 times greater than the angular deflection of the antenna (paired t test: P < 0.001, n = 10). If 400 V is applied to a needle, which concentrates the electric field near the tip, the hair can be moved hundreds of micrometers, a motion large enough to be visible under the microscope (Movie S1).
Bumblebee Hairs Exhibit Neural Correlates to DC Electric Field Stimulation.
To determine whether the observed mechanical deflection is accompanied by a response from the nervous system, we measured the electrophysiological response of hairs and antennae to a 400-V square pulse applied to a steel disk 1 cm away (Fig. 6 A–C). All electrophysiological recordings were carried out on bees in their uncharged state, due to the necessity of grounding the bee to eliminate noise from the recording.
Fig. 6.
The electrophysiological response to an electric field. (A and B) Example response of an antenna (blue) and a hair (red) to an electric field. (C) Plot showing the observed changes in firing rate of 12 antennae (blue) and 12 hairs (red) to an electric field stimulus (applied during the gray box). The value shown is the number of spikes per second, per bee, divided by the mean prestimulus spike rate. A value of 1 (dashed black line) indicates no change in spike rate. (D and E) Two control stimuli applied to the antenna: Puffs of unscented air (D) and a puff of scented air (E) demonstrate the lack of response of the antenna seen in A is not due to damage during the dissection.
Bumblebee hairs (n = 12) showed an increase in neural firing rate in response to the applied DC electric field (Fig. 6 B and C). Some hairs had a background firing rate, whereas others were quiet before stimulus application. During the stimulus, the mean firing frequency of hairs was 5.1 times greater than the prestimulus firing rate (paired t test: P < 10−6, n = 14). In contrast, stimulation failed to increase firing frequency in the antennae (paired t test: P > 0.05, n = 14) (Fig. 6C). Dynamic stimulation at 140 Hz (n = 5) also failed to elicit an electrophysiological response from the antenna (SI Methods and Materials). In control recordings (Fig. 6 E and F and SI Methods and Materials), however, the antenna responded to mechanical (air puffs) and olfactory (lavender oil) stimuli, showing the adequacy of the present electrophysiological preparation. These responses were consistent with previously reported antennal sensitivity to mechanical and olfactory stimulation (20).
SI Results
Neurophysiological Response to AC Electric Fields.
AC stimuli did not elicit a measurable electrophysiological response from any of the three stimulus presentation modes (n = 5) (Fig. S1).
Electrophysiology.
Controls showed that the antenna was responsive to olfactory stimuli as electrophysiological responses were generated on the presentation of lavender scented air puffs both in isolation (n = 5) and paired with AC stimuli (n = 5), but the number of spikes did not increase in response to a 140-Hz stimulus at 30 V using the steel disk, 400 V with the shielded electrode, or the oscillating charged ball.
SI Methods and Materials
Frictional Charging and Charge Removal.
A charged acrylic rod (AR) and a charged nylon ball bearing are used as stimuli in Proboscis Extension Response (PER) trials and the charging of bees in laser Doppler vibrometry, respectively. A nylon ball bearing was also used in electrophysiological recordings under an AC stimulus. Both the AR and ball bearing were charged frictionally by rubbing with fabric. The AR was charged in contact with cotton, whereas the ball bearing was rubbed within a cylinder made from acrylic fabric. To remove electrostatic charge, an antistatic gun (Milty Zerostat 3) was used. Charges, or lack thereof, were verified by measuring the AR or nylon ball bearing in a faraday pail (specifications as in ref. 14).
Stimulus Delivery System.
Electrical stimuli were delivered with a steel disk, 30 mm in diameter, identical to those described in ref. 14. The steel disk forms one end of an open circuit so no current flows in the stimulus delivery system. Instead the electric potential of the disk at an arbitrary time point is equal to that of the function generator output multiplied by the gain of the amplifier. This electric potential creates an electric field in the region of the disk that is proportional to the disk potential (Fig. 3C).
LDV.
For measurements of antennal vibration velocity, the LDV laser beam was focused perpendicular to the length of the antenna on the most distal flagellomere. A target hair on the animal’s head was chosen for hair measurements. To create a clear line of sight for the laser beam, a small number of hairs immediately anterior to the target hair were removed by shaving under a binocular dissecting microscope. The most distal part of the hair that provided strong enough laser backscatter for LDV measurements was targeted, with the laser beam again, perpendicular to the hair’s length.
The vibrometer was placed approximately 10 cm from the bee such that the target hair was within the focal range of the laser. The disk is placed 1.0 cm from the target hair oriented tangentially to the −30° azimuth such that its narrow axis is oriented vertically and its round face is oriented toward the bee. The laser source and disk are at azimuthal angles of 0° (directly anterior to the bee) and −30°, respectively.
Electrophysiology.
DC stimuli.
For recordings made in response to a DC electric field, bees were anesthetized by application of CO2 until unconscious (usually less than 30 s was necessary). For AC recordings, bees were chilled for 10 min at −16 °C. After anesthetization, they were left to recover until fully responsive, which usually occurred in less than 10 min.
Recordings from the both the antenna and the hair were made with 0.1-mm-diameter tungsten wire electrodes. Tungsten electrodes were sharpened electrolytically as in ref. 29 then placed within glass capillary tubes with the sharpened tips protruding and fixed in position by using cyanoacrylate glue. Electrode placement was verified by tapping the antennae lightly to determine whether a mechanosensory response could be recorded. Electrode placement was such that we could not determine whether observed neural activity was from mechanoreceptors within the Johnston’s organ, other mechanoreceptors, chemoreceptors, or other neurons in the antennae. Thus, although the electrodes were able to determine whether there was any activity in the antennae, they were unable to determine exactly which neurons were responding. The only known neurons at the hair base are mechanorceptors; neural responses were therefore mechanoreceptive. For the hair, correct placement of the electrode was tested by puffing air so that it moved the hairs and verifying that a signal from the mechanoreceptors was measured.
AC stimuli.
The antennal response to AC electric fields were recorded extracellularly from the scape, using the same setup as for DC stimuli. Stimuli were delivered by several different means. A 140-Hz signal was produced by using a function generator (Agilent 33120A), amplified where necessary, using a custom-built high voltage amplifier and delivered via a steel disk 30 mm in diameter (the same as used in the stimulus delivery system), a shielded copper electrode, or to drive the oscillation of a charged nylon ball bearing. For the steel disk, 20 V was used to generate the electric field stimulus and the bee was frictionally charged by stroking with a charged acrylic rod. This voltage was limited by cross-talk between the stimulus disk and the electrodes, a factor that is observed for AC and not DC stimuli. Because of the limitation on the range of voltages that could be used with the steel disk, the two other methods of stimulus presentation were also used. Using the shielded electrode 5 mm away from the distal flagellum, the 140-Hz stimulus was delivered at 400 V with low levels of cross-talk observed. The charged nylon ball bearing was attached with modeling clay to an electromagnet oscillating at 140 Hz with a displacement of 132 µm, again with low levels of cross-talk. Control stimuli in the form of scented air puffs were also presented. The scented air puffs were presented both simultaneously with electric field stimuli and on their own, to show that the antenna is responsive and that spikes, when they occur, are visible above any electrical cross-talk. All files were DC and bandpass filtered before spikes were counted by using Matlab (2014a Mathworks).
Discussion
From this evidence, we conclude that bumblebees use mechanosensitive hairs to detect electric fields. In honeybees (Apis mellifera), the antenna has been proposed to detect electric fields, whereby Johnston’s organ transduces mechanical deflections of the flagellum in response to an electric field analogous to that generated during a honeybee’s waggle dance (15). Cockroach antennae have been shown to react to more intense electric fields, in the range of 8–10 kV⋅m−1 (21). Our similar experiments in bumblebees failed to demonstrate that antennae could respond to electric fields.
Mechanosensory hairs are common across the Phylum Arthropoda (16). These sensors typically have mechanical resonances between 100 and 500 Hz and react to vibrations from the wingbeats of approaching predators (22) and air currents (23, 24). In contrast, bumblebee hairs have a resonant frequency of approximately 3.8 kHz, a result of low mass and high stiffness. Their rigid lever-like motion within the socket resembles the acoustic particle velocity induced response of other mechanically sensitive hairs (25) and the feathery antennae of mosquitoes (26). Bumblebee hairs neurally respond to electrically induced deflections of 4 × 10−2 degrees (Table 1), making them less sensitive than cricket filiform hairs, which respond to deflections of 2 × 10−2 degrees (27). Overall, electrosensory bumblebee hairs and mechanosensory hairs reported in other arthropods (16) are mechanically and neurophysiologically similar.
Some substantial differences exist in the biophysics of particle velocity (air movement) and electric field detection. For particle velocity detection, viscous coupling between stimulus and detector transfer energy into momentum of the hair. For electroreception in air, Coulombic interactions couple the hair and the electric field, creating different mechanics. Notably, the boundary layer constraints inherent to particle velocity detection do not apply to electric forces. Particle velocity motion and electric field detection apply a similar magnitude of deflection to hairs, with slow air currents causing cricket cercal hairs to deflect between 5 × 10−3 and 5 × 10−2 degrees (depending on the magnitude of the boundary layer and other effects; ref. 28), and DC electric fields deflecting bumblebee hairs by 4 × 10−2 degrees. The details of momentum transfer between the electric field and a charged hair (the electromechanical transfer function) are unknown, but will depend on the magnitude and distribution of charges along the hair. Forces generated by electric fields constitute a previously unidentified source of mechanical stimuli to arthropod hairs. Interestingly, both particle velocity and electric field stimuli can be generated simultaneously by a single source, such as a charged insect flapping its wings. A priori, both types of stimuli can act simultaneously on a single charged hair. This interaction raises the possibility that particle velocity information and electrical information, and interactions between them, can be encoded by a single hair. The present study enables the formulation of the tantalizing hypothesis that, through the electromechanical sensitivity of hairs, electroreception is widespread in arthropods, fulfilling functions beyond the detection of floral electric fields.
Methods and Materials
Laser Vibrometry.
Bee preparation.
Bees were killed with CO2 and glued ventrally with cyanoacrylate to an electrically isolated piece of wood. They were attached to a mounting pin and placed in front of a laser Doppler vibrometer for measurement of antennal and hair vibration velocity (Fig. 1B). The bee was electrically charged by contact with a frictionally charged nylon ball bearing and left to settle for 10 min. After undergoing charging, bees carried an average of 4 ± 3 pC, where uncharged bees carried 0 ± 0.5 pC. The charge carried by a bumblebee in vivo is 32 ± 3 pC (14), hence in the experiments the charging below that measured in free flight. Charge stimuli used here are thus within the range of naturally occurring electrostatics. After initial measurements, the bee’s charge was neutralized by application of a positive and negative ion beam (SI Methods and Materials). The stimulus regime was then repeated.
Vibrometry.
Measurements of mechanical response of hairs and antennae to electric field stimuli were taken with a microscanning LDV (Polytec PSV300) fitted with a close-up attachment. Data were acquired by using an OFV5000 sensor head, digitized via an on-board data acquisition card (National Instruments PCI-4451) and subsequently analyzed by using PSV software (Polytec version 9.0). The target, laser source, and stimulus delivery disk are placed on the same horizontal plane on an antivibration table (TMC 784-443-12R) in an electrically isolated and sound-proofed booth (SI Methods and Materials and Fig. 1C).
Stimulus regime.
Electrical stimuli were delivered by using an arbitrary function generator (Agilent 33120A) connected in series to a custommade high voltage amplifier. The stimulation electric field was generated by a 30-mm-diameter steel disk connected to the high voltage amplifier by an earthed 50 Ohm BNC cable (as in ref. 14). A 400-V periodic sweep from 10 Hz to 10 kHz was applied to the disk. The frequency response and resonant frequency were recorded. To test for response amplitude relationship, a pure tone sine wave set at the resonant frequency was applied and the hair/antenna response recorded for incrementally decreasing stimulus amplitudes (400-380-360-…-0 V). Stimulus amplitude was then increased back up to 400 V to test each result for linearity. This stimulus was repeated for a second, off-resonant frequency that was chosen by identifying a frequency at which the amplitude of the response was equal to the median response amplitude across all frequencies. The bee was then prepared in its uncharged state, and the whole regime was repeated. This entire procedure was repeated for both hairs and antennae.
Electrophysiology.
Anesthetized bees were ventrally affixed to a post made of modeling clay (SI Methods and Materials). Extracellular recordings were made from both the antenna and the hair using electrolytically sharpened tungsten electrodes (SI Methods and Materials), using a National Instruments data acquisition card (NI 9172/9215) and custom-built amplifier and LabVIEW 2011 to record the signal. For antennal recordings, the experimental electrode was inserted at the proximal end of the scape. The reference electrode was placed in the head, taking care not to place it near an ommatidium. For hair recordings, the experimental electrode was inserted in the basal socket. The reference electrode was placed in nearby cuticle.
Stimuli were delivered with the disk placed 1.0 cm from the bee in an identical arrangement to the LDV experiments. For all trials, there was an initial 10 s of no stimulation, followed by 10 s of electrical stimulation at 400 V, followed by 10 s of no stimulation. For the antennae, additional control stimuli in the form of air puffs, scent, and AC electric fields were applied (SI Methods and Materials).
Supplementary Material
Acknowledgments
This work was funded by Biotechnology and Biological Sciences Research Council Grant BB/M011143/1 and Royal Society Grant UF130537.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7020.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601624113/-/DCSupplemental.
References
- 1.Kalmijn AJ. The electric sense of sharks and rays. J Exp Biol. 1971;55(2):371–383. doi: 10.1242/jeb.55.2.371. [DOI] [PubMed] [Google Scholar]
- 2.Murray RW. The response of the ampullae of Lorenzini of elasmobranchs to electrical stimulation. J Exp Biol. 1962;39:119–128. doi: 10.1242/jeb.39.1.119. [DOI] [PubMed] [Google Scholar]
- 3.Fritzsch B, Wake MH. Electro-reception in amphibians. Am Sci. 1984;72(3):228. [Google Scholar]
- 4.Tong SL, Bullock TH. Electroreceptive representation and its dynamics in the cerebellum of the catfish, Ictalarusnebulosus (Ictaluridae, Siluriformes) J Comput Phys. 1982;145:289–298. [Google Scholar]
- 5.Bullock TH, Northcutt RG. A new electroreceptive teleost – Xenomystusnigri (Osteoglossiformes notopteridae) J Comp Physiol. 1982;3:345–352. [Google Scholar]
- 6.Czech-Damal NU, et al. Electro-reception in the Guiana dolphin (Soltalia guianensis). Proc Roy Soc B-Biol. Sci. 2012;279:663–668. doi: 10.1098/rspb.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory JE, Iggo A, McIntyre AK, Proske U. Electroreceptors in the platypus. Nature. 1987;326(6111):386–387. doi: 10.1038/326386a0. [DOI] [PubMed] [Google Scholar]
- 8.Gregory JE, Iggo A, McIntyre AK, Proske U. Responses of electroreceptors in the snout of the echidna. J Physiol. 1989;414:521–538. doi: 10.1113/jphysiol.1989.sp017701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenzini S. 1678. Osservazioni intorno alle torpedini fatta da Stefano Lorenzini Fiorentino. Firenze: Per l’Onofri’. Available at www.biodiversitylibrary.org/item/29974#page/6/mode/1up. Accessed May 12, 2016.
- 10.Murray RW. Electroreceptor mechanisms: The relation of impulse frequency to stimulus strength and responses to pulsed stimuli in the ampullae of Lorenzini of elasmobranchs. J Physiol. 1965;180(3):592–606. doi: 10.1113/jphysiol.1965.sp007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs MA. Lateral line receptors: Where do they come from developmentally and where is our research going? Brain Behav Evol. 2004;64(3):163–181. doi: 10.1159/000079745. [DOI] [PubMed] [Google Scholar]
- 12.Alves-Gomes JA. The evolution of electro-reception and bioelectrogenesis in teleost fish: A phylogenetic perspective. J Fish Biol. 2001;58(6):1489–1511. [Google Scholar]
- 13.Lavoué S, et al. Comparable ages for the independent origins of electrogenesis in African and South American weakly electric fishes. PLoS One. 2012;7(5):e36287. doi: 10.1371/journal.pone.0036287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke D, Whitney H, Sutton G, Robert D. Detection and learning of floral electric fields by bumblebees. Science. 2013;340(6128):66–69. doi: 10.1126/science.1230883. [DOI] [PubMed] [Google Scholar]
- 15.Greggers U, et al. Reception and learning of electric fields in bees. Proc Roy Soc B-Biol Sci. 2013;280(1759):20130528. doi: 10.1098/rspb.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas J, Dangles O. Physical ecology of fluid flow sensing in arthropods. Annu Rev Entomol. 2010;55:505–520. doi: 10.1146/annurev-ento-112408-085342. [DOI] [PubMed] [Google Scholar]
- 17.Colin ME, Richard D, Chauzy S. Measurement of electric charges carried by bees – evidence of biological variations. J. Bioelectricity. 1991;10(1-2):17–32. [Google Scholar]
- 18.Edwards DK. Electrostatic charges on insects due to contact with different substrates. Can J Zool. 1962;40:259–584. [Google Scholar]
- 19.Jackson C, McGonigle D. Direct monitoring of the electrostatic charge of house-flies (Musca domestica L.) as they walk on a dielectric surface. J Electrost. 2005;63:803–808. [Google Scholar]
- 20.Olsson SB, Hansson BS. Electroantennogram and single sensillum recording in insect antennae. Methods Mol Biol. 2013;1068:157–177. doi: 10.1007/978-1-62703-619-1_11. [DOI] [PubMed] [Google Scholar]
- 21.Newland PL, et al. Static electric field detection and behavioural avoidance in cockroaches. J Exp Biol. 2008;211(Pt 23):3682–3690. doi: 10.1242/jeb.019901. [DOI] [PubMed] [Google Scholar]
- 22.Tautz J, Rostás M. Honeybee buzz attenuates plant damage by caterpillars. Curr Biol. 2008;18(24):R1125–R1126. doi: 10.1016/j.cub.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 23.Barth FG, Wastl U, Humphrey JAC, Devarakonda R. Dynamics of arthropod filiform hairs 2 mechanical properties of spider trichobothria (Cupiennius-Salei Keys) Philos Trans R Soc Lond, B. 1993;340(1294):445–461. [Google Scholar]
- 24.Bathellier B, Steinmann T, Barth FG, Casas J. Air motion sensing hairs of arthropods detect high frequencies at near-maximal mechanical efficiency. J R Soc Interface. 2012;9(71):1131–1143. doi: 10.1098/rsif.2011.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barth FG, Németh SS, Friedrich OC. Arthropod touch reception: Structure and mechanics of the basal part of a spider tactile hair. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190(7):523–530. doi: 10.1007/s00359-004-0497-4. [DOI] [PubMed] [Google Scholar]
- 26.Göpfert MC, Robert D. Active auditory mechanics in mosquitos. Proc Roy Soc B-Biol. Sci. 2001;268(1465):333–339. doi: 10.1098/rspb.2000.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimozawa T, Murakami J, Kumagi T. Cricket wind receptors: Thermal noise for the highest sensitivity known. In: Barth FG, Humphrey JAC, Secomb TW, editors. Sensors and Sensing in Biology and Engineering. Springer; Berlin: 2003. pp. 145–157. [Google Scholar]
- 28.Shimozawa T, Kumagai T, Baba Y. Structural scaling and functional design of the cercal wind-receptor hairs of cricket. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1998;183:171–186. [Google Scholar]
- 29.Brady J. A simple technique for making very fine, durable dissecting needles by sharpening tungsten wire electrolytically. B World Health Organ. 1965;32(1):143–144. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.