Significance
Hydrogels are critical components of biological systems; however, how these structures are affected by polymers abundant in their environments (e.g., dietary fiber in the gut and soluble glycoproteins in tissues) remains unknown. Here we find that the colonic mucus hydrogel (a protective barrier and mediator of microbe–host interactions) is compressed by gut polymers. Surprisingly, the predictions of a simple thermodynamic model are able to describe our experiments on this complex biological system, providing insight into the underlying physics. Moreover, we find that gut microbes modulate mucus structure by degrading dietary polymers into smaller, noncompressing fragments. These findings reveal a mechanism of mucus restructuring and illustrate an unexpected interplay between diet, gut microbiota, and the biological structures that protect a host.
Keywords: hydrogel, biophysics, biomaterials, polymers, mucus
Abstract
Colonic mucus is a key biological hydrogel that protects the gut from infection and physical damage and mediates host–microbe interactions and drug delivery. However, little is known about how its structure is influenced by materials it comes into contact with regularly. For example, the gut abounds in polymers such as dietary fibers or administered therapeutics, yet whether such polymers interact with the mucus hydrogel, and if so, how, remains unclear. Although several biological processes have been identified as potential regulators of mucus structure, the polymeric composition of the gut environment has been ignored. Here, we demonstrate that gut polymers do in fact regulate mucus hydrogel structure, and that polymer–mucus interactions can be described using a thermodynamic model based on Flory–Huggins solution theory. We found that both dietary and therapeutic polymers dramatically compressed murine colonic mucus ex vivo and in vivo. This behavior depended strongly on both polymer concentration and molecular weight, in agreement with the predictions of our thermodynamic model. Moreover, exposure to polymer-rich luminal fluid from germ-free mice strongly compressed the mucus hydrogel, whereas exposure to luminal fluid from specific-pathogen-free mice—whose microbiota degrade gut polymers—did not; this suggests that gut microbes modulate mucus structure by degrading polymers. These findings highlight the role of mucus as a responsive biomaterial, and reveal a mechanism of mucus restructuring that must be integrated into the design and interpretation of studies involving therapeutic polymers, dietary fibers, and fiber-degrading gut microbes.
Biological hydrogels (including mucus, blood clots, and the extracellular matrix) provide critical functions, yet little is known about how their structure is influenced by materials they come into contact with regularly. For example, the environments of many hydrogels abound in polymers, such as dietary fibers (1, 2) or administered therapeutics (3–5) in the gut and soluble glycoproteins in tissues. Whether such polymers interact with these hydrogels, and if so, how, remains unclear. An important example is the case of colonic mucus, which protects the gut from infection and physical damage (6–8), mediates drug delivery (9), and mediates host–microbe interactions (10) in a structure-dependent manner; for example, a “tighter” mesh could impede the infiltration of microorganisms from the intestinal lumen (6, 11–13). Mucus restructuring is typically attributed solely to changes in secretion (14–16), or to the activity of specific enzymes (8, 17), detergents (18), or dextran sulfate sodium-induced inflammation (19). However, the physicochemical properties of the gut environment itself—particularly its polymeric composition—have not been considered as a potential regulator of mucus structure. We therefore sought to characterize the structure of the colonic mucus hydrogel in the absence and in the presence of polymers.
Results
In Vivo Thickness of the Colonic Mucus Hydrogel.
To probe the in vivo thickness of murine colonic mucus, we developed a label-free technique that eliminates evaporation and avoids the use of any washing, fixative, labeling, or dehydrating agents that could alter mucus structure (SI Materials and Methods). We used freshly excised colon explants obtained from mice at least 8 wk old—whose mucus hydrogel has been found to be fully developed and stable (20)—and gently removed the luminal contents using FC-40 oil, a fluorocarbon fluid that is immiscible with, and denser than, water. We opened each explant along the intestinal axis and mounted it flat, with its luminal surface facing upward and coated with FC oil. We then used an upright confocal microscope equipped with a dry objective lens to image, in three dimensions, the exposed epithelial surface and the oil overlying the adherent mucus hydrogel (Fig. 1A).
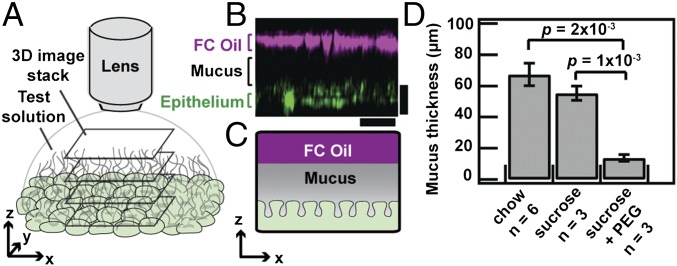
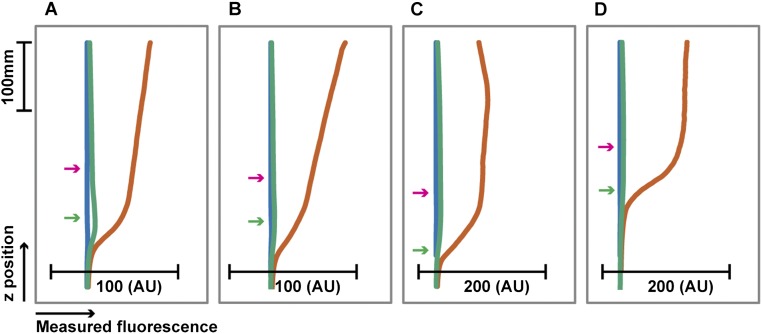
Fig. 1.
Polymers compress colonic mucus hydrogel in vivo. (A) Schematic depicting visualization of adherent colonic mucus hydrogel. (B) Side-view confocal micrograph showing FC oil–mucus interface (magenta) separated from the epithelial surface (green) by the adherent mucus hydrogel (depicted in black). (Scale bars, 30 μm.) (C) Schematic of side view shown in B. (D) FC oil mucus thickness measurements for colonic explants taken from SPF mice given ad libitum access to food on either a standard chow diet, 5% (wt/vol) sucrose in 1× PBS, or 5% (wt/vol) sucrose with 7% (wt/vol) PEG 200k in 1× PBS. Data show means ± SEM.
We first identified both the epithelial surface (Fig. S1 A and B) and the oil–mucus interface using confocal reflectance microscopy (Fig. 1 B and C); the distance between the two provided a measure of the mucus hydrogel thickness. We measured a comparable mucus thickness of 67 ± 7 μm or 55 ± 5 μm (mean ± SEM, n = 6 or 3, P = 0.3) for control mice fed a standard chow diet or a sucrose solution (Fig. 1D), consistent with previous measurements (8). To investigate the role of polymers in altering mucus structure, we then fed mice the same sucrose solution, with added PEG, an uncharged polymer that is well-characterized, is often used as a therapeutic in the gut (3, 4), and has minimal chemical interactions with biomolecules (21). We used PEG of an average molecular weight ∼200 kDa and average radius of gyration Rg,p ∼ 22 nm, denoted as PEG 200k. Unexpectedly, the mucus hydrogel was significantly thinner for these mice, 14 ± 2 μm (mean ± SEM, n = 6, P = 2 × 10−4; Fig. 1D). This finding demonstrates that such polymers can in fact alter the structure of mucus.
Fig. S1.
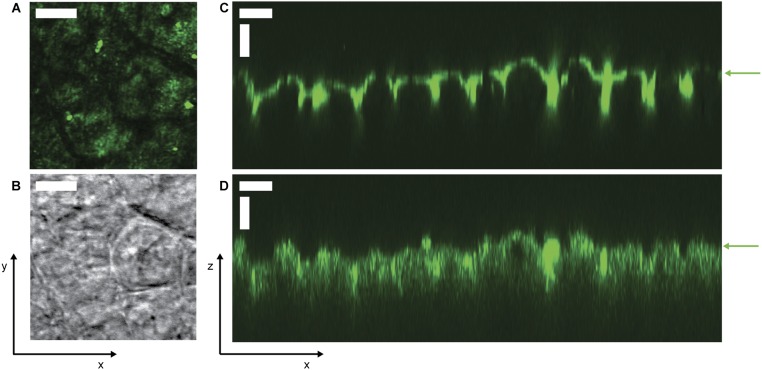
Images of murine epithelium in the xy and xz planes. (A) Two-photon and (B) bright-field micrographs of unwashed epithelium from a mouse fed standard chow, imaged under FC oil. (C and D) Side views of lectin-stained epithelium washed with saline and imaged under aqueous solutions. Staining was performed by incubating a colon explant with 200 μL of a test solution of 2 mg/mL Rhodamine Ulex Europaeus Agglutinin I (Vector Laboratories), which stains α-l-fucose residues on the surface of epithelial cells, in Hepes buffer in a sealed Petri dish for 10 min at 4 °C, then washing the exposed luminal side with several milliliters of ice-cold 1× PBS. We then immediately imaged the explant surface using (C) confocal fluorescence microscopy (543-nm excitation/560-nm long-pass filter) and (D) confocal reflectance microscopy (514-nm excitation/505-nm long-pass filter). Epithelial surface is indicated by green arrows, confirming that the position of the epithelium agrees between the different imaging modalities. The adherent mucus hydrogel overlies the epithelium in the direction of increasing z above the green arrows. (All scale bars, 30 μm.)
Ex Vivo Characterization of Colonic Mucus Hydrogel.
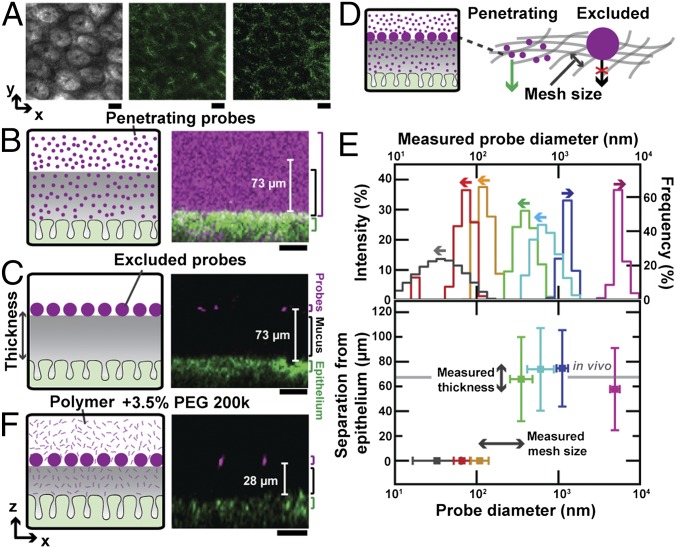
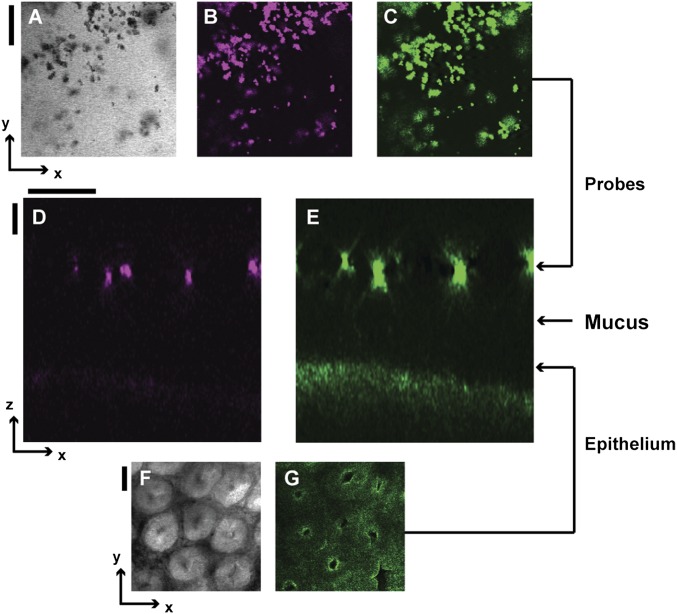
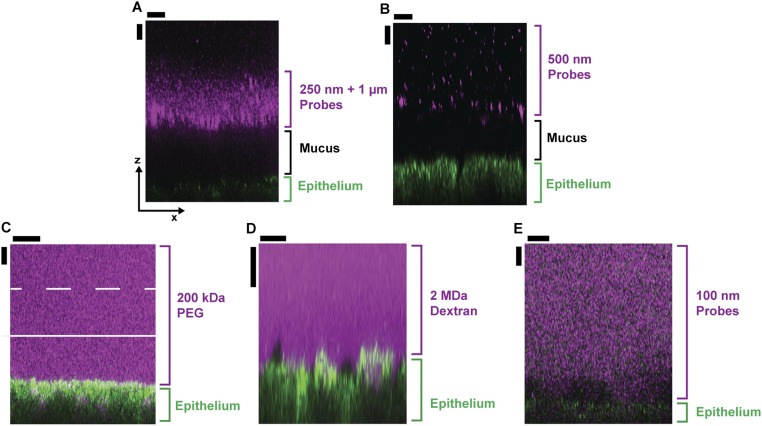
To better understand this phenomenon, we modified our imaging approach so we could directly image the mucus hydrogel ex vivo while simultaneously controlling the physicochemical composition of the aqueous solution to which mucus is exposed (SI Materials and Methods). We again used freshly excised murine colon explants, cut open along the intestinal axis and mounted flat; instead of using FC oil as the test solution, we cleared the luminal contents and coated the luminal surface with cold saline to remove soluble components, including any polymers. We used a water-immersion objective lens to identify the epithelial surface (Fig. 2A) and corroborated this with lectin staining (Fig. S1 C and D). To identify the luminal surface of the mucus hydrogel, we deposited a solution of 1-μm-diameter microparticle probes onto the explant surface. These probes did not penetrate, but instead settled on top of, the mucus hydrogel, indicating that they were larger than its mesh size (Fig. 2C). Previous studies have validated that this region of probe exclusion corresponds to the adherent mucus hydrogel (11, 19, 22, 23); we further confirmed this using lectin staining (Fig. S2). Measuring the distance between the excluded probes (Figs. S3 and S4) and the underlying epithelial surface thus provided a measure of the mucus thickness, 75 ± 30 μm (mean ± SD), consistent with the distance measured when we imaged using FC oil and consistent with other reported measurements (8). Hydrogel thickness did not change appreciably over an observation time of 2.5 h. We found similar results using probes of other sizes (Fig. S5 A and B): All probes 250 nm in diameter or larger were excluded from the mucus and yielded comparable mucus thickness values (Fig. 2E). By contrast, probes 100 nm in diameter or smaller (Fig. S5 C and D) penetrated the mucus and reached the underlying epithelium, indicating that they were smaller than the mesh size (Fig. 2 B and E). We concluded that the mesh size of the adherent mucus hydrogel was between 100–250 nm, in good agreement with measurements of the mesh size of other mucus hydrogels (24, 25).
Fig. 2.
Polymers compress colonic mucus hydrogel ex vivo. (A) Bright-field (Left), confocal reflectance (Middle), and two-photon (Right) micrographs of epithelial surface. Image levels were adjusted for clarity (SI Materials and Methods). (Scale bars, 30 μm.) (B, C, and F) (Left) Schematics. (Right) Side-view confocal micrographs. (Scale bars, 10 μm.) (B) Penetration of mucus by low concentration [0.05% (wt/vol)] of mPEG-FITC 200k. (C) Exclusion from mucus of 1-μm microparticle probes. (D) Schematic depicts mucus mesh structure, with penetrating probes on the left and larger nonpenetrating probe on the right. (E) (Top) Probe size distributions measured using dynamic light scattering (left axis, arrows to the left) or optical microscopy (right axis, arrows to the right). (Bottom) Minimal probe separation from epithelial surface. Horizontal positions and error bars show geometric mean ± geometric SD of lognormal fits to size distributions. Vertical positions and error bars show mean ± SD. Gray bar shows mean of FC oil measurements of in vivo thickness for mice fed chow. Penetration measurements used fluorescently labeled polymers at concentrations below those that cause mucus compression. (F) Compression of colonic mucus by 3.5% (wt/vol) PEG 200k.
Fig. S2.
False-color side view showing wheat germ agglutinin (WGA)-stained adherent mucus hydrogel. We first deposited 1-μm-diameter microparticles onto the explant surface of a freshly excised, washed, and mounted colonic explant. After incubating for 1 h at 4 °C, we then stained the colonic mucus with WGA, a fluorescent lectin that specifically binds to sialic acid sugar residues in the mucins. We prepared 10 μg/mL of WGA-Oregon Green (Invitrogen) in 1× PBS, placed a ∼0.5-mL drop on the exposed surface of the explant, and incubated the sealed Petri dish for 5 min at room temperature. We then washed the exposed surface with several milliliters of ice-cold 1× PBS and immediately imaged the explant surface (lower magenta surface) and the deposited 1-μm microparticles (upper magenta circles) using confocal reflectance microscopy and the stained mucus hydrogel using confocal fluorescence microscopy (488-nm excitation/505-nm long-pass filter). Image is a superimposition of two separate, parallel side views taken at two neighboring positions in the xy plane. We observed that the position of the deposited microparticles agrees with the top of the stained mucus hydrogel. (Scale bars, 30 μm.)
Fig. S3.
Colocalization of signal from microparticle probes and epithelium from different imaging modalities. (A) Bright field, (B) fluorescence excitation, and (C) reflectance images of 1-μm probes of the same xy slice. (D) An xz side view of fluorescence signal from 1-μm probes. (E) The same xz side view as in D but of the reflectance signal from 1-μm probes and epithelial surface. (F) Bright-field and (G) reflectance images of the epithelial surface of the same xy slice. The arrow linking C to E indicates the vertical position of the xy slice shown in A–C. The arrow linking G to E indicates the vertical position of the xy slice shown in F and G. This confirms that the positions of the microparticles given by confocal reflectance and confocal fluorescence microscopy agree. (Scale bars, 30 μm.)
Fig. S4.
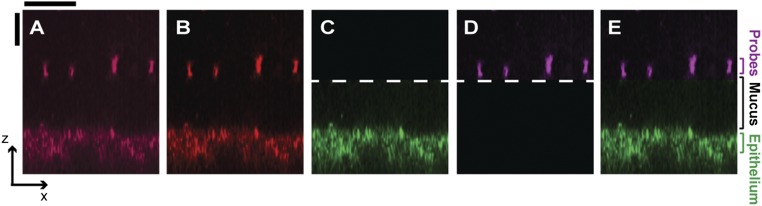
Overview of image processing of confocal side views. To eliminate artifacts associated with staining and accelerate image acquisition, we used label-free confocal reflectance microscopy to simultaneously image the underlying epithelium (lower surface) and the microparticles deposited on the adherent mucus hydrogel (upper bright spots). To obtain the false-color side views, we first thresholded each side view; A shows a representative xz side view before processing and B shows the image after thresholding, with uniform enhancement of brightness and contrast across the entire image. The image was then split into two parts, and the epithelium was false-colorized green (C) and the deposited microparticles or oil–mucus interface (for imaging of unwashed tissues with FC oil) were false-colorized magenta (D). Dashed lines indicate where images (C and D) were split. Merging these two channels produced the side-view images shown, exemplified by E. Unless otherwise noted, all of our experiments mapped z ranges spanning from below the epithelial surface to well above the mucus hydrogel surface. Each of the side-view images presented in this paper was cropped and scaled in xz for clarity (indicated by the x and z scale bars), to focus on the region corresponding to the mucus hydrogel. (Scale bars, 30 μm.)
Fig. S5.
False-color side views (xz plane) of 3D stacks showing probes excluded from (top row) or penetrating (bottom row) the mucus hydrogel. (A) Mixture of both 250-nm and 1-μm microparticles and (B) 500-nm particles were excluded from the adherent mucus hydrogel. The probes (magenta) were unable to diffuse through the mucus and instead deposited on top of the hydrogel. The probes and the epithelium were simultaneously imaged using (A) 514-nm excitation/505-nm long-pass filter and (B) 800-nm excitation/650-nm long-pass filter. (C) Fluorescent PEG 200 kDa, (D) fluorescent dextran 2 MDa, (E) fluorescent 100-nm microparticle probes all penetrate the hydrogel. Note that polymers in A and B were used at concentrations below those that cause mucus compression. The probes (magenta) diffused through the mucus and reached the underlying epithelium (green), except for some isolated regions immediately adjacent to the epithelium observed in some experiments (dark patches). The probes were imaged using confocal fluorescence microscopy (488-nm excitation/505-nm long-pass filter) and the epithelium was imaged using confocal reflectance microscopy. The adherent mucus hydrogel overlies the epithelium in the direction of increasing z above the green arrows; solid and dashed white lines in C indicate the approximate average and maximal positions of the top of the mucus, measured using 1-μm microparticles. In each experiment using probes of different sizes, after placing the test solution onto the exposed luminal surface, we incubated the tissue at 4 °C for 1–2 h before imaging the explant. We estimated the time required for probes 100 nm or smaller to diffuse through the mucus as being <10 min, and the time required for the 250-nm probes to diffuse across the vertical extent of the mucus in free solution as being ∼10 min, both much shorter than the incubation time. We thus deduce that the fluorescent probes smaller than the measured mucus mesh size had sufficient time to diffuse through the mucus to the underlying epithelium, and that the measured exclusion of the larger probes reflects the presence of the adherent mucus hydrogel. (Scale bars, 30 μm.)
Having established a method for characterizing mucus hydrogel structure ex vivo, we next tested the influence of polymers. We placed a solution of the same PEG onto the explant surface, continually monitoring the mucus hydrogel thickness using the deposited microparticles. The PEG penetrated the mucus and reached the underlying epithelium (Fig. S6) and this penetration was reversible, suggesting that strong PEG–mucus chemical interactions—such as complexation, which can play a role under different conditions than those explored here (SI Materials and Methods)—were absent (Fig. S7). Nevertheless, the mucus hydrogel compressed by ∼50–60% of its initial thickness within ∼5–20 min (Fig. 2E), and the level of compression seemed to be stable over an observation time of at least ∼100 min. We verified that any optical effects induced by the polymer solution did not appreciably affect the z measurements (Fig. S8). Interestingly, compression was at least partly reversible; the mucus hydrogel reexpanded to ∼90% of its original thickness after PEG was removed by washing the explant. These findings suggest that the polymer-induced compression observed in the FC oil experiments could be reproduced and investigated further ex vivo.
Fig. S6.
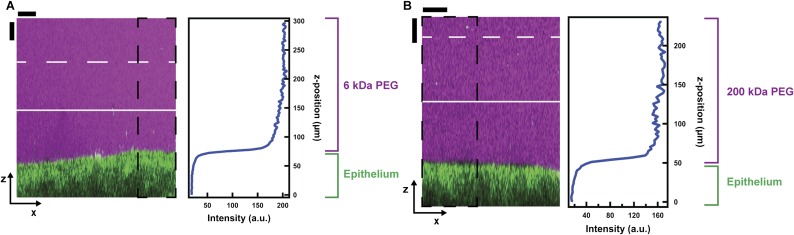
Side view showing penetration of mucus hydrogel by polymers. The polymer self-diffusion coefficient in the free solution outside the mucus, Dfree, is represented by D0 for the dilute polymer solutions and can be estimated as Dfree ≈ D0(c/c*)−7/4 for the polymer solutions that were above their overlap concentration c*. Our experiments spanned D0 ≈ 10−11 to 3 × 10−10 m2/s and c/c* ≈ 0–10, therefore Dfree ≈ 2 × 10−13 to 3 × 10−10 m2/s. The characteristic time taken for the polymers to diffuse through the mucus can thus be estimated as ranging from ∼1 s to 1 h, shorter than the time taken to perform the experiments. We thus assume that the polymer molecules were able to diffuse through the mucus hydrogel before imaging commenced in all of the experiments. To study the steady-state penetration of the PEG into the adherent mucus hydrogel, we imaged two representative test solutions: (A) 13% (wt/vol) PEG 6k spiked with 0.5 mg/mL FITC-PEG 5k and (B) 3% (wt/vol) PEG 200k spiked with 0.6 mg/mL FITC-PEG 200k. Consistent with our expectation, in both cases the polymer penetrated through the adherent mucus hydrogel and reached the underlying epithelium. Traces show the spatial variation of the x-averaged probes fluorescence intensity for the region indicated by the dashed black box. The probes (magenta) diffused through the mucus and reached the underlying epithelium (green). The probes were imaged using confocal fluorescence microscopy and the epithelium was imaged using confocal reflectance microscopy. The adherent mucus hydrogel overlies the epithelium in the direction of increasing z above the epithelium; solid and dashed white lines show the average and maximal positions of the top of the mucus, measured using 1-μm microparticles. (Scale bars, 30 μm.)
Fig. S7.
Fluorescence profiles of test solutions deposited on mucus hydrogel, before and after washing. We expect that the carboxyl groups on the mucin sialic acid residues were negatively charged in our experiments (pH ∼7), and therefore complexation between the added PEG and the mucins is minimal. Moreover, we took care not to expose PEG solutions to light and keep them at low temperatures when not in use, to minimize oxidation. To confirm that labeled PEG molecules were not chemically cross-linked to the mucus hydrogel as they diffused through the hydrogel, we performed four sets of fluorescence measurements, using as test solutions (A) 5 μM fluorescein, (B) 15 μM FITC-PEG 350, (C) 6 μM FITC-PEG 5k, and (D) 15 μM FITC-PEG 350 in 60% (wt/vol) PEG 400. Four different explants were incubated with 1-μm microparticles for >1 h then imaged using confocal reflectance (to identify epithelial surface and microparticles on mucus) and confocal fluorescence (to quantify fluorescence of deposited test solution). Curves show fluorescence profiles of test solutions: horizontal axis shows measured fluorescence, averaged over a 450-μm × 450-μm xy field of view, and the vertical axis shows z position. Green and magenta arrows show average positions of epithelial surface and probes deposited on the mucus hydrogel surface. We first used PBS as the test solution to provide a measure of background fluorescence (blue curves). We then deposited dyed test solution on the mucus (orange curves). We then washed the explant with saline (green curves). Fluorescence profiles returned to background levels after washing, suggesting that strong chemical interactions (such as covalent reactions) between the labeled PEG and the mucus hydrogel do not occur. We used the same gain settings before and after.
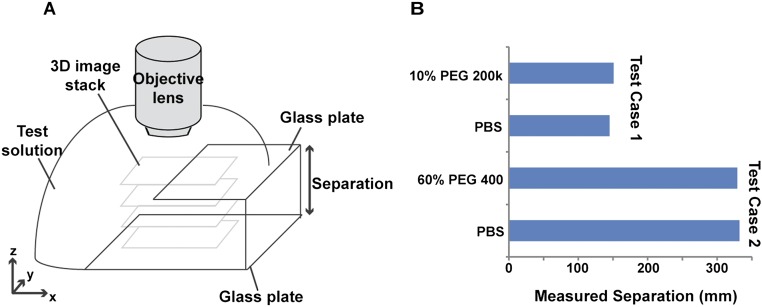
Fig. S8.
Optical properties of polymer solutions do not appreciably affect z measurements. (A) Schematic showing setup of control experiments, measuring separation between two parallel glass plates using the same confocal reflectance microscopy approach. The test solution infiltrated the open gap between the two plates. (B) We first quantified separation using PBS as the test solution filling the space between the two plates, and then used either 10% (wt/vol) PEG 200k (test case 1), or 60% (wt/vol) PEG 400 (test case 2) as the test solution. Introduction of the polymer solution did not change the measured z separation appreciably, indicating that optical effects due to the presence of the polymer solution did not significantly affect the z measurements.
Flory–Huggins Theory of Polymer-Induced Compression.
Large nonpenetrating polymers have been used to osmotically compress synthetic hydrogels (26) and even the periciliary brush after mucus removal in the mammalian lung (27). However, the possibility that even polymers small enough to penetrate a hydrogel could compress it was first recognized by Brochard in 1981 (28) and was subsequently investigated both theoretically and experimentally (29, 30) (further references are provided in SI Materials and Methods). In this case, hydrogel compression arises from a combination of enthalpic and entropic effects. For example, the polymers can reduce the effective solvent quality of the hydrogel environment, due to enthalpic interactions with the hydrogel network strands, forcing the hydrogel to reduce its hydrated volume and compress. Another effect arises from the free energy penalty associated with penetrating the hydrogel mesh: This can lead to an elevated polymer concentration, and therefore an elevated osmotic pressure, outside the hydrogel, which similarly forces the hydrogel to compress. Clarifying the role of these, and other, different effects remains unresolved, even for the case of synthetic hydrogels; however, such effects can be described collectively using the classic Flory–Huggins theory of polymer solutions (29, 30). We therefore asked whether this physical framework could also describe polymer-induced compression of the colonic mucus hydrogel. Indeed, although the predictions of this theory have been experimentally verified using a few model synthetic hydrogels (30, 31), its applicability to the more complex case of biological hydrogels such as colonic mucus is unclear. One signature of this form of compression is its tunability: More concentrated polymer solutions should induce more hydrogel compression (29, 30). Consistent with this prediction, we found that mucus compression was tunable by PEG concentration (green points in Fig. 3B).
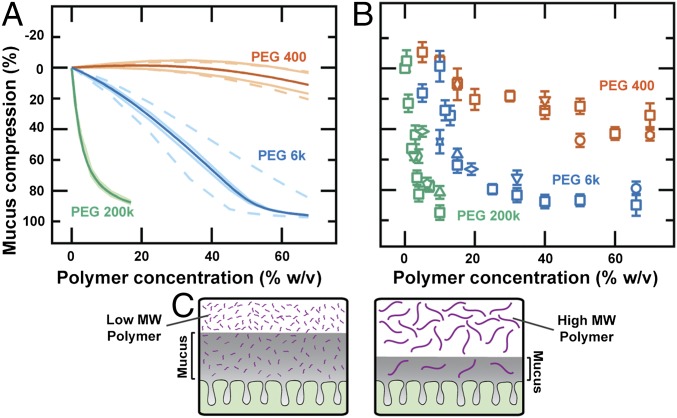
Fig. 3.
Tunable compression of colonic mucus hydrogel can be qualitatively described by Flory–Huggins theory. (A) Theoretically predicted and (B) experimentally measured (using 1-μm microparticles) mucus compression for varying polymer concentrations and molecular weights. Bold curves in A show model results for parameter values (SI Materials and Methods) χSM = 0 and χMP = 0.3; less opaque and dashed curves show sensitivity to variations in these parameters (upper and lower less opaque curves, χSM = 0.1 and −0.1; upper and lower dashed curves, χMP = 0.2 and 0.4). All mice, except for those indicated by upward triangles, were male. Symbols in B indicate different mouse types and experimental conditions: squares, C57BL/6 mice; circles, BALB/c mice; upward triangles, female C57BL/6 mice; vertical diamond, washed explants from GF mice; downward triangles, all solutions have added 2× Roche protease inhibitor mixture; pentagons, all solutions have added 5 mM MgSO4; horizontal diamonds, experiments performed at 37 °C instead of 22 °C using a heated microscope stage; stars, polyacrylic acid of ∼8-kDa average molecular weight instead of PEG; hexagons, Hepes buffer instead of PBS for all solutions. Each data point represents the mean of a series of five measurements on a single explant; error bars represent measurement uncertainty. (C) Schematic showing one effect potentially underlying mucus compression: molecular weight-dependent partitioning of the polymer.
To test the applicability of Flory–Huggins theory, we used the same theoretical framework (30) to describe our experimental system (details and limitations of this theory are described in SI Materials and Methods). We first modeled the mucus as a swollen, cross-linked hydrogel. We then considered how the addition of polymers changes the extent to which the mucus hydrogel is swollen and its equilibrium thickness. We made the simplifying assumption (30, 31) that the mucus behaves as an elastic gel on the timescale of our experiments, even though hydrogels, including colonic mucus, are known to be viscoelastic—they relax stresses over long times. This assumption is supported by our observations that the hydrogel thickness remained stable in either the uncompressed or polymer-induced compressed states (over observation times of at least ∼100 min). It is further supported by the reversibility of the observed compression. We therefore calculated the total free energy of the ternary solvent–mucus–polymer system, G, as the sum of the elastic free energy, which accounts for deformations of the individual mucus network strands, and the free energy of mixing the polymer and the solvent with the mucus hydrogel. We then used this total free energy to calculate the chemical potentials of both the added PEG and the solvent, μP ≡ ∂G/∂nP and μS ≡ ∂G/∂nS, respectively, both inside and outside of the mucus network; nP and nS are the respective numbers of moles:
| [1] |
| [2] |
| [3] |
| [4] |
Here, R is the gas constant, T is the temperature, is the volume fraction of species i, is the mucus hydrogel volume fraction in its initial preparation state, is the volume fraction of the free polymer in external solution, is the average number of segments in a mucus network strand, y is the number of segments in a polymer molecule, and is the Flory–Huggins interaction parameter, which quantifies enthalpic interactions, between species i and j; we denoted solvent, mucus, and free polymers as i = S, M, P, respectively. At thermodynamic equilibrium, and ; these equalities enabled us to numerically calculate the equilibrium mucus thickness for a given PEG concentration (details of calculations, parameters used, and sensitivity to parameters are described in SI Materials and Methods). Consistent with our experimental observations, the Flory–Huggins model predicted that exposure to PEG compresses the adherent mucus hydrogel. Moreover, the model predicted (green curve in Fig. 3A) a similar dependence of mucus compression on PEG concentration as we measured in our experiments using microparticles (green points in Fig. 3B).
Another key prediction of the model is that the extent of mucus compression should depend on the polymer molecular weight: For a given PEG concentration, smaller polymers should compress the mucus hydrogel less (Fig. 3A). One intuitive explanation for this is the free energy penalty paid by PEG to penetrate the mucus, which is smaller for smaller polymers; thus, even though they can exert a larger osmotic pressure, smaller polymers are less likely to be excluded from the mucus hydrogel (Fig. S9E) and are expected to compress it less (Fig. 3C). To test this prediction, we measured the extent of mucus compression induced by two smaller polymers, PEG 6k and PEG 400, characterized by Rg,p ∼ 3 nm and 0.7 nm, respectively. These polymers again compressed the mucus hydrogel within 5 min, and the compression level seemed to be stable over an observation time of up to several hours. Despite the mean-field nature of the Flory–Huggins model, which is not expected to capture the full complexity of the experiments, we observed qualitative similarities between the calculations (Fig. 3A) and the experimental data (Fig. 3B). We also found similar results for varying values of the model parameters (Fig. 3A and Fig. S9 A–D). Moreover, the observed compression was similar for mice of different sexes and strains, for washed explants originating from germ-free or microbe-colonized mice, for different buffers, in the presence and the absence of Mg2+ ions, for buffers also containing protease inhibitor, for polymer solutions prepared using the liquid fraction of specific-pathogen-free (SPF) mice colonic contents instead of buffer, for experiments performed at 22 °C or 37 °C, and for a similar, but charged, polymer, demonstrating that our results were not an artifact of the choice of the animal model or details of experimental conditions. The similarity between the theoretical predictions and the experimental data suggests that Flory–Huggins theory provides a physical description of the concentration and molecular weight dependence of the polymer-induced compression of colonic mucus and provides a foundation for more sophisticated modeling to better characterize the full complexity of this phenomenon (SI Materials and Methods).
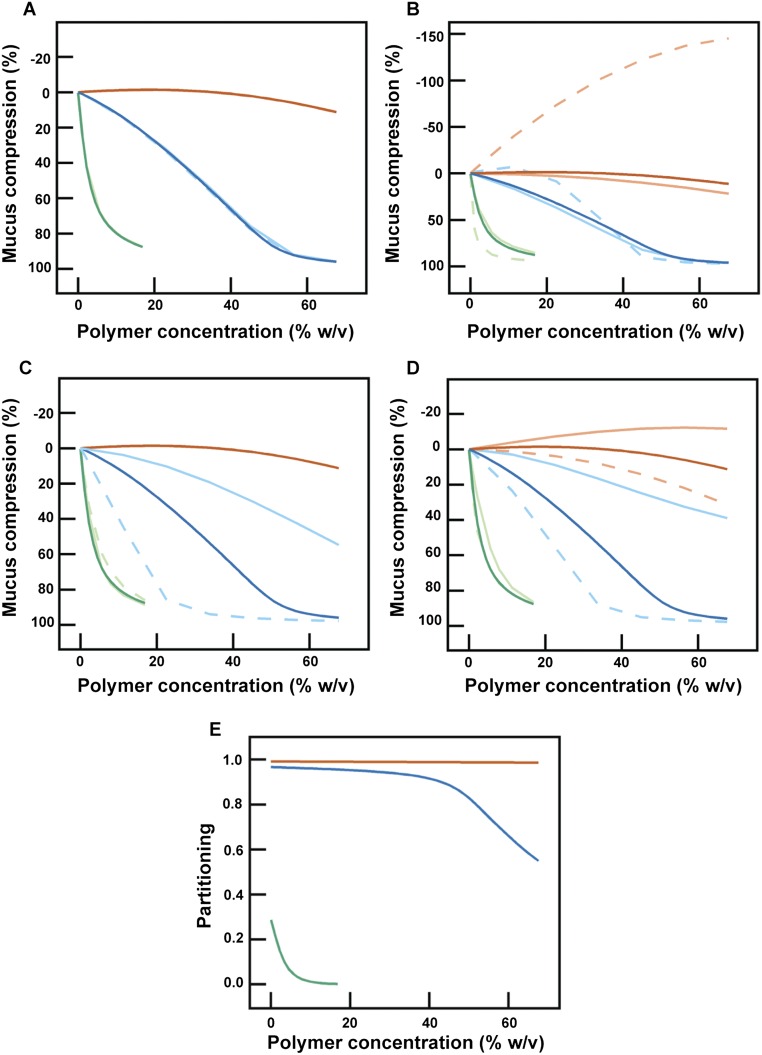
Fig. S9.
Sensitivity of model predictions to variations in numerical parameters. Each panel shows numerical calculations (Materials and Methods) of the mucus hydrogel compression for different concentrations of PEG 400 (orange), 6k (blue), and 200k (green). Note that due to the constraint derived in the initial polymer-free case, some of the parameters are coupled and cannot be varied independently. (A) ν0M values are varied and corresponding values of NM are adjusted to satisfy the initial polymer-free constraint. Light, solid traces correspond to ν0M = 0.07 and NM = 628, and light, dashed traces correspond to ν0M = 0.35 and NM = 2,026. Note the overlap between the solid and dashed traces. (B) χSM values are varied and corresponding values of NM are adjusted to satisfy the initial polymer-free constraint. Light, solid traces correspond to χSM = −0.2 and NM = 715, and light, dashed traces correspond to χSM = 0.45 and NM = 9,425. Upper and lower less opaque curves in Fig. 2A, which correspond to χSM = 0.1 and −0.1, were characterized by NM = 1,247 and NM = 833. (C) The number of Kuhn segments y for each PEG molecule is varied. Light, solid traces correspond to y = 1, 2, and 76, and light, dashed traces correspond to y = 1, 11, and 611 for PEG 400, 6k, and 200k, respectively. (D) χMP is varied. Light, solid traces correspond to χMP = 0 and light, dashed traces correspond to χMP = 0.5. In each panel, the dark solid traces are the simulations presented in Fig. 2A. In all cases, we observed similar trends of compression with polymer concentration and molecular weight as in the experiments. (E) Numerical calculations showing the partitioning between the hydrogel and solution phase for PEG 400 (orange), 6k (blue), and 200k (green). The ratio of PEG inside and outside the hydrogel (, denoted “Partitioning”) is plotted against the PEG concentration outside the hydrogel. Consistent with our expectation, the higher-molecular-weight polymer is more likely to be excluded from the mucus hydrogel.
Microbes Can Modulate Mucus Compression.
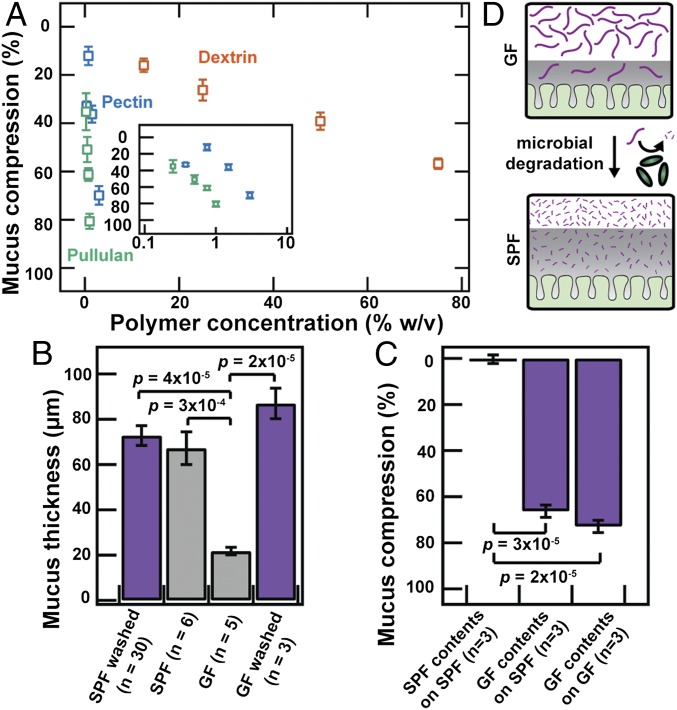
Given the diversity of polymers abundant in fruits, vegetables, and food additives, we next asked whether dietary polymers could also compress colonic mucus. We tested three common dietary polymers: dextrin, pectin, and pullulan. Exposure to each of these polymer solutions caused the colonic mucus hydrogel to compress in a concentration-dependent manner (Fig. 4A). Moreover, as with PEG, for a given polymer concentration, the larger polymers, pectin and pullulan, compressed the mucus more than the smaller polymer, dextrin. These observations demonstrate that, similar to the case of PEG, dietary polymers present in the gut can also induce mucus compression in a manner that depends on the physical properties of the polymers themselves.
Fig. 4.
Gut microbes can modulate mucus compression by modifying the polymeric composition of intestinal contents. (A) Mucus compression induced by dietary polymers, determined using the ex vivo microparticle method. Each data point represents the mean of a series of five measurements on a single explant; error bars represent measurement uncertainty. (Inset) Data for pectin and pullulan with semilogarithmic axes. (B and C) Mucus (B) thickness or (C) compression measurements determined using (purple) ex vivo microparticle method or (gray) FC oil method, for explants from SPF or GF mice. Last bar in B shows measurements for washed GF explants. Data are presented as means ± SEM. We also found using our ex vivo method that SPF contents only compressed mucus on a GF explant by 5 ± 2% (n = 1). (D) Schematic depicting how microbial degradation of polymers alters mucus compression.
Given our results indicating that mucus compression can depend on the polymer molecular weight, we hypothesized that microbial degradation of polymers into smaller fragments (1, 2) may actively modulate compression in vivo. Indeed, we found that whereas pectin strongly compressed the colonic mucus hydrogel (Fig. 4A, blue points), a small molecule, acetate—a typical product of pectin degradation and fermentation by gut microbes—did not (500 mM acetate compressed the mucus only by ∼10%). Moreover, using the wash-free FC oil methodology as in Fig. 1, we found that the adherent mucus of germ-free (GF) mice was only ∼25% as thick as that of SPF mice in vivo (Fig. 4B), consistent with previous observations (8, 32). Thicker SPF mucus was previously attributed solely to altered mucus secretion by the host in response to the presence of microbes, and not to the difference in polymeric composition of the gut fluid. Given our results, however, we hypothesized that mucus compression by intestinal polymers may also contribute to this phenomenon: These polymers remain intact in GF mice, which lack the gut microbiota that normally degrade these polymers into smaller noncompressing fragments. In agreement with this hypothesis, washing the GF explant with excess cold saline, which should dilute out any polymers present in the sample, restored the mucus to the thickness observed in SPF mice (Fig. 4B). This result was surprising, because it could not have been the result of a host response to the presence of microbes. To further test the effect of intestinal polymers on mucus compression, we isolated and analyzed the liquid fractions of the colonic contents of GF and SPF mice. As expected (Fig. S10), the GF contents were enriched in higher-molecular-weight polymers compared with the SPF contents, reflecting polymeric degradation by the SPF gut microbiota. We therefore predicted that the GF contents would compress colonic mucus more than the SPF contents. In agreement with this prediction, whereas SPF contents did not appreciably compress colonic mucus, the GF contents compressed colonic mucus by ∼70% of its initial washed thickness, for washed explants obtained from either SPF or GF mice (Fig. 4C). This finding indicates that gut microbes, by modifying the polymeric composition of intestinal contents, can actively modulate the compression state of the colonic mucus hydrogel (Fig. 4D).
Fig. S10.
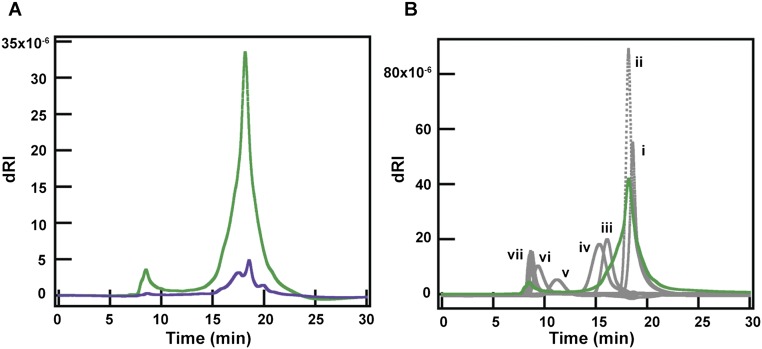
Gel permeation chromatography of luminal contents from SPF and GF mice. We used an Agilent 1100 HPLC with a binary pump and autosampler, which was connected to a Tosoh TSKgel G3000SWxl column equilibrated with 1× PBS, pH 7.4, flow rate 0.7 mL/min. For detection of the polymers, a Wyatt DAWN HELEOS light scattering instrument with a Wyatt Optilab Rex refractive index detector was used. Detected peaks were analyzed using ASTRA V software. For the pullulan standards, the Agilent PL 2090-0101 Pullulan polysaccharide calibration kit (Agilent) was used. An injection volume of 50 μL was used for each. All samples were prepared in 1× PBS and run through a sterile syringe filter (polyvinylidene fluoride, 13-mm diameter, pore size of 0.22 μm; Fisherbrand) before injection. For luminal contents, on the day of the experiment, frozen liquid fractions were warmed to room temperature for 10–20 min then diluted twofold with 1× PBS. Samples were centrifuged at 12,000 × g at 4 °C for 2 h in sterile centrifugal filters (polyvinylidene fluoride, pore size 0.22 μm; EMD Millipore). After centrifugation, samples were allowed to equilibrate to room temperature for 30 min before injection. For all liquid fraction samples, an injection volume of 10 μL was used. If multiple runs were performed on the same sample, the remaining sample volume was stored at 4 °C until prior runs were complete. (A) Chromatograms of luminal contents from four 3-mo-old SPF males (purple) and two male and one female, 4-mo-old GF (green) mice. Differential refractive index (dRI) is plotted against time (minutes). Both runs were run on the same day. (B) Chromatograms of luminal contents of GF mice (green) and pullulan standards (gray). dRI is plotted against time (minutes). Concentrations and peak average molecular weights of the standards used were (i) 5 mg/mL 180 Da, (ii) 8 mg/mL 667 Da, (iii) 4 mg/mL 6,100 Da, (iv) 4 mg/mL 9,600 Da, (v) 1 mg/mL 47,100 Da, (vi) 1 mg/mL 107,000 Da, (vii) 1 mg/mL 194,000 Da, 344,000 Da, and 708,000 Da.
SI Materials and Methods
Details of Animals Used.
Except where otherwise noted, all mice were male or female SPF or GF C57BL/6 mice between 2–6 mo of age, fed a standard solid chow diet and given ad libitum access to water. The GF chow was autoclaved and was formulated to have a nutritional profile after autoclaving similar to that of the SPF chow. The mice given only sucrose or only sucrose + PEG were first raised on a standard solid chow diet and given ad libitum access to water then maintained on a restricted diet consisting only of 5% (wt/vol) sucrose or 5% (wt/vol) sucrose + 7% (wt/vol) PEG 200k in 1× PBS (pH 7.4, without calcium and magnesium; Corning) available for ad libitum consumption for the 24-h period before they were euthanized. Four hours after we started administering each of the restricted liquid diets we moved each test mouse to a new, clean cage to minimize the effects of coprophagy.
Details of Microscopy.
All imaging was performed using a Zeiss LSM 510 upright confocal microscope or a Zeiss LSM 880 upright confocal microscope, using either bright-field microscopy, confocal fluorescence microscopy (543-nm excitation/560-nm long-pass filter or 488-nm excitation/505-nm long-pass filter), confocal reflectance microscopy (514-nm excitation/505-nm long-pass filter or 505- to 735-nm detection), or two-photon microscopy (800-nm excitation/650-nm long-pass filter). We collected 3D stacks consisting of multiple xy slices at different z positions.
Imaging of Unwashed Tissue.
We euthanized each mouse, removed the colon, and immediately flushed it gently with Fluorinert FC 40 oil (3M), which is immiscible with the aqueous contents of the colon. We then immediately cut the colon segment open along the longitudinal axis and mounted the opened tissue (luminal surface facing upward) onto a glass slide or a Petri dish using GLUture topical tissue adhesive (Abbott). We then gently deposited ∼0.5–2 mL of additional FC 40 oil onto the exposed luminal surface. The FC 40 is immiscible with water and with the mucus hydrogel; this procedure thus retained the adherent mucus in its in vivo “unwashed” state and prevented it from dehydrating. We imaged the explant with two-photon microscopy. For some mice, we took multiple explant samples and for some explant samples we collected multiple 3D stacks at different fields of view.
We determined the mean mucus thickness (gray bars in Figs. 1 and 4) for each stack obtained from an explant by measuring the distance between the epithelial surface (Fig. S1 A and B) and the FC oil–hydrogel interface at five random positions in xy. In some cases this was repeated for multiple fields of view. When multiple colonic explants were obtained from a single mouse, we calculated the mean mucus thickness of an individual mouse. In Figs. 1 and 4, the thickness values reported are the mean values of the individual mice thicknesses. The error bar on each value reported in Figs. 1 and 4 is the SEM, calculated by taking the SD of mucus thickness for a single mouse and dividing by √n (n, number of different mice).
Imaging of Washed Tissue.
We euthanized each mouse, removed the colon, and immediately flushed it gently with ice-cold 1× PBS and placed ∼1-cm-long segments of the midcolon in ice-cold PBS, ensuring that the ionic composition was homogenized throughout the mucus hydrogel environment. We then cut and mounted the colon segments as described for unwashed tissues, always ensuring the explant surface was covered in PBS to prevent any dehydration or ionic imbalance, and surrounding (but not contacting) the tissue with >10 ∼10-μL drops of water to maintain a humid environment. The measured mucus hydrogel thickness was consistent with the distance measured when we imaged using FC oil and consistent with other reported measurements (8), did not change appreciably over an observation time of 2.5 h, and was similar for probes of other sizes (250 nm in diameter or larger), as discussed in the main text, further confirming the validity of our approach. We then gently deposited an additional ∼10- to 200-μL drop of test solution containing the fluorescent probes onto the explant. We imaged the explant with confocal reflectance or two-photon microscopy. For some mice, we took multiple explant samples and for some explant samples we collected multiple 3D stacks at different fields of view. The levels of the images in Fig. 2A were nonlinearly adjusted in Adobe Illustrator for clarity in print using the following input and output levels: 82, 1, 246/0, 255 (bright field), 34, 0.78, 172/0, 205 (confocal reflectance), and 51, 0.91, 140/0, 255 (two-photon).
Thickness Measurements of Washed Mucus Hydrogel.
In each experiment, after placing a suspension of 1-μm-diameter microparticles onto the exposed luminal surface, we incubated the tissue at 4 °C for 1–2 h, longer than the time required for the microparticles to diffuse across the vertical extent of the mucus in free solution (40 min). This ensured that the microparticles deposited onto the mucus hydrogel surface. We simultaneously imaged both the epithelium and the deposited microparticles using bright-field, confocal reflectance, or two-photon reflectance microscopy.
To determine the mean mucus thickness for tissue obtained from a single mouse (green, light blue, dark blue, and pink points in the bottom graph of Fig. 2D), for each stack on a washed explant we measured the distance between the epithelial surface and the center of the deposited microparticles at five random positions in xy spanning the entire field of view. In some cases this process was repeated for multiple fields of view. If multiple colonic explants were obtained from the same mouse, the thickness was measured in the same way. We then took each of these individual thickness measurements at each xy position from all of the individual mice, explants, and fields of view and calculated the mean and SD. The thickness values reported in Fig. 2D are these mean values, and the error bars are the associated SD. The washed values and error bars reported Fig. 4 (purple bars), were determined as described in Materials and Methods, Imaging of Unwashed Tissue.
Experiments with Probes of Different Sizes.
We used (all in 1× PBS) methoxyl polyethylene glycol-FITC (mPEG-FITC; Nanocs), weight-averaged molecular weight 350, 1.2 × 10−2 mg/mL; mPEG-FITC (Nanocs), weight-averaged molecular weight 5 kDa, 3.3 × 10−2 mg/mL; mPEG-FITC (Nanocs), weight-averaged molecular weight 200 kDa, 0.6 mg/mL; FITC–dextran (Sigma-Aldrich), average molecular weight 2 MDa, 0.1 mg/mL; and fluorescent polystyrene microparticles (micromer; micromod Partikeltechnologie GmbH), coated with PEG 300 to render them chemically inert (41), 0.02–0.2% volume fraction of manufacturer-reported average diameters 100 nm, 250 nm, 500 nm, 1 μm, or 5 μm. Penetration measurements used fluorescently labeled polymers at concentrations below those that cause mucus compression.
We characterized probes or polymers 500 nm or smaller using dynamic light scattering performed on 200–500 μL of each sample with a Wyatt Dynapro NanoStar instrument. The data were collected and analyzed using Wyatt DYNAMICS software 7.1. Hydrodynamic radii were determined by fitting the data using a regularization analysis. The wavelength of the laser was 658 nm and the scattering angle was 90°. The microparticle solutions were unfiltered, whereas we filtered the polymer solutions using either a 0.2-μm Fisherbrand (PEG 400, PEG 6 kDa, PEG 200 kDa, fluorescent PEG 200 kDa, fluorescent dextran 2 MDa, and fluorescent PEG 5 kDa) or a 0.45-μm Puradisc (pullulan and dextrin) syringe filter. All samples were dispersed in 1× PBS, and we used the following concentrations or volume fractions: 3 mg/mL (fluorescent PEG 200 kDa), 1 mg/mL (fluorescent dextran 2 MDa), 0.1% (vol/vol) (100-nm particles), 0.01% (vol/vol) (250-nm particles), 0.02% (vol/vol) (500-nm particles), 100 mg/mL (PEG 400), 10 mg/mL (PEG 6 kDa), 0.5 mg/mL (PEG 200 kDa), 10 mg/mL (pullulan), 10 mg/mL (dextrin), and 0.25 mg/mL (fluorescent PEG 5 kDa). The acquisition time was 5 s, and 10–20 acquisitions were taken for each sample. We characterized the 1-μm and 5-μm microparticles using optical microscopy.
Polymers Used for Compression Measurements.
We used (all in 1× PBS) PEG 400, weight-averaged molecular weight 380–420 Da (Acros Organics); PEG 6k, weight-averaged molecular weight 5.6–6.6 kDa (Acros Organics); PEG 200k, viscosity-averaged molecular weight 200 kDa (Sigma-Aldrich); dextrin, average molecular weight between ∼1–70 kDa (42–45) (Walgreens); pullulan from Aureobasidium pullulans, average molecular weight between ∼50 kDa and 4 MDa (46–50) (Sigma-Aldrich); and pectin from apple, weight-averaged molecular weight ∼100 kDa (51) (Sigma-Aldrich). We estimated the average radius of gyration, Rg,p, of the PEG 400, 6k, and 200k as ∼0.7, 3, and 22 nm, respectively, using published measurements (52) and our own dynamic light scattering measurements.
Quantifying Polymer-Induced Compression of Washed Mucus Hydrogel.
After measuring the initial washed mucus thickness, we gently deposited ∼0.2–2 mL of the test polymer solution onto the exposed luminal surface and then collected the same 3D stacks at the same xy fields of view. To measure the “percent compression,” or the overall percentage change in the thickness, of the colonic mucus after exposure to the polymer solution, we measured the thickness before and after exposure to the solution at the same five xy positions, using the distance between the epithelial surface and the deposited microparticles in the 3D stacks. To calculate the percentage compression, we calculated the percentage change in the thickness measured, as well as the measurement uncertainty (using the optical slice thickness as the experimental uncertainty in the measured thickness), at each of these five xy positions. We then calculated the percentage compression as the mean of these five measured values. The error bars show the uncertainty in the percentage compression measurement, which was calculated using the experimental uncertainty in each of the five strain measurements.
To explore the generality of the observed compression, we tested a number of different conditions and found similar behavior (as shown in Fig. 3B) for mice of different sexes and strains, for washed explants originating from GF or microbe-colonized mice, for different buffers (PBS or Hepes), for buffers also containing protease inhibitor, for polymer solutions prepared using the liquid fraction of SPF mice colonic contents instead of buffer, for experiments performed at 22 °C or 37 °C, for a similar, but charged, polymer, and in the presence and the absence of Mg2+ ions. We note that other multivalent cations (e.g., Ca2+) have been found to induce additional structural changes in mucins, although no measurable changes were reported for Mg2+ (53). We also note that, in vivo, water may be absorbed from the lumen into the epithelium depending on the delivery medium (e.g., as reported in ref. 54). We speculate that this could concentrate the polymer in the lumen, possibly enhancing the mucus compression we measured ex vivo.
Flory–Huggins Model of Compression.
We used the Flory–Huggins theory of polymer solutions to describe polymer interactions with the mucus hydrogel. The adherent mucus is a hydrogel with a network (10, 37, 55) composed of MUC2 proteins having alternating hydrophilic, densely glycosylated regions, which make up the strands of the hydrogel network, and hydrophobic, nonglycosylated regions, which help to cross-link the network, which is also cross-linked via physical entanglements, electrostatic interactions, and chemical cross-links such as disulfide bonds (56, 57). We therefore modeled the mucus as a cross-linked hydrogel swollen in a good solvent. For simplicity, we treated this hydrogel as being structurally isotropic; our model does not incorporate any possible supramolecular structuring of the colonic mucus hydrogel (37). We made the simplifying assumption that the mucus behaves as an elastic gel; although hydrogels, including colonic mucus, are known to be viscoelastic—they relax stresses over long times—the reversibility of the observed polymer-induced compression, and the observed unchanging thickness of the hydrogel after compression, suggest that the colonic mucus is elastic on the timescale of our experiments. This idea is supported by rheological measurements on a scraped porcine colonic mucus hydrogel, which exhibits elastic behavior for timescales of at least ∼100 s (58). Moreover, this assumption has been successfully used to describe the compression of synthetic hydrogels that also contain chemical cross-links (30, 31). However, we note that the exact details of mucus hydrogel rheology remain unknown; we therefore chose to describe the mucus hydrogel as “viscoelastic” in the text for the sake of generality, thereby including any possible elastic or viscous response. Incorporating further details of mucus hydrogel rheology into our theoretical model, such as any possible viscous relaxation at long timescales and the relative importance of the different forms of cross-linking in the network, will be an important direction for future work.
First, we calculated the total free energy of the ternary solvent–mucus–polymer system, G, given by the sum of the elastic free energy, —which accounts for deformations of the individual mucus network strands, thus inhibiting the unphysical case of full mixing of the mucins and solvent—and the free energy of mixing the polymer and the solvent with the mucus hydrogel, . The buffered aqueous solutions are characterized by a high ionic concentration (ionic strength ∼70 mM) and therefore a Debye screening length ∼0.7 nm, over two orders of magnitude smaller than the hydrogel mesh size, suggesting that electrostatic interactions may not play a significant role in our system. Indeed, theoretical predictions for charged semidilute polymer solutions reduce to those for uncharged semidilute polymer solutions when the solvent has a high ionic concentration such as ours (e.g., ref. 59). This idea is also supported by experimental measurements of charged particle diffusion in a mucus hydrogel, which show results similar to the case of uncharged particles at high ionic concentrations similar to ours (60). We therefore did not consider electrostatic effects (59, 61–64) in our work; considering these effects will be an interesting direction for future work.
The total change in free energy can thus be written as
| [S1] |
and is given by the Flory–Huggins (30, 39, 40) free energy of mixing,
| [S2] |
where R is the gas constant, T is the temperature, is the number of moles of species i, is the volume fraction of species i, and is the Flory–Huggins interaction parameter between species i and j; here, we denote solvent, mucus, and free polymers as i = S, M, P, respectively. To describe the free energy of elastic deformation we used rubber elasticity, assuming affine deformation of the network (30, 39):
| [S3] |
where is the mucus hydrogel volume fraction, is the mucus hydrogel volume fraction in its initial preparation state, is the molar volume of the solvent, and is the average number of mucin Kuhn segments, the stiff segments making up each mucin network strand, between cross-links of the network. More sophisticated forms of the elastic free energy would be interesting to explore in future work; we note that the exact choice of the elastic energy may not affect the calculated hydrogel compression trends considerably (29, 30).
At equilibrium, the chemical potentials of both the solvent and the free polymer, μS ≡ ∂G/∂nS and μP ≡ ∂G/∂nP, must be equal inside and outside of the mucus network:
| [S4] |
| [S5] |
By substituting Eqs. S2 and S3 into Eq. S1, and differentiating with respect to the number of moles of solvent and free polymer, we obtained Eqs. 1–4 shown in the main text, which represent the central result of the Flory–Huggins model and have been successfully used to describe polymer-induced compression of synthetic hydrogels (30). These equations are also subject to the constraints and .
We first treated the polymer-free case (), which describes the initial swollen state of the mucus hydrogel. The system is described by Eq. 1 with and ; this provided us with a relationship between , , , and the mucus volume fraction in this initial swollen state, which we denote as . Direct measurements of are lacking; we chose a value of , well within in the range of estimates (65–69) of the volume fraction of swollen mucus, and tested the sensitivity of our results to variations in the numerical parameters used, with the constraint relating , , , and (Fig. S9). As a simplifying assumption, we took to be approximately equal to the mucin volume fraction when initially packed in secretory granules, before being released into the intestinal lumen to form the swollen, cross-linked adherent hydrogel. We found in our sensitivity analysis (Fig. S9) that our results are only weakly sensitive to the choice of the value of . We therefore chose a value = 0.13, within the range of published measurements (70–72) for mucin and other similar secretory granules. However, more work is required to quantitatively determine the exact value of . We expect water to be a good solvent for the mucin network strands, due to the preponderance of hydroxyl, carboxyl, and sulfate groups in the glycosylated domains; we therefore chose . We estimated using published measurements in two different ways. In the first approach, we used measured values (65, 73–76) of the MUC2 radius of gyration, , and Kuhn length, , combined with the relationship for mucus strands swollen in a good solvent (40, 68, 77, 78), . In the second approach, we used our direct measurements of the mucus hydrogel mesh size, combined with the published measurements of bM, to estimate NM. In both cases, we found . The values of , , and , together with Eq. 1, yielded , in this estimated range; we therefore chose . Again, we found qualitatively similar results for different values of (Fig. S9).
We next investigated how added polymer () changed the extent to which the mucus hydrogel is swollen, and therefore its equilibrium thickness. We numerically solved Eqs. S4 and S5 for and , varying ; this yielded the curves presented in Fig. 3A. We focused on the case in which the added polymer is PEG 400, 6k, or 200k, as used in our experiments. We took the number of segments of each PEG, y, to be the number of PEG Kuhn segments, and estimated this (40) using the relationship , where and are the PEG radius of gyration and Kuhn length, respectively, choosing , consistent with the measured range (40, 52, 79–82). Published measurements (83–85) yield nm; we therefore chose nm, in this range. We estimated using our measurements of the PEG 400, 6k, and 200k hydrodynamic radii and converted these to radii of gyration using the Kirkwood–Riseman relationship (86–88). The relationship between , , and y thus yielded y = 1, 4, and 146 for PEG 400, 6k, and 200k, respectively, which we used for the main simulations (Fig. 3A). Based on published measurements for PEG (30, 89), we set . The chemical interactions between PEG and mucins are thought to be slightly attractive or neutral. We therefore estimated to be between 0 and 0.5, and chose .
This Flory–Huggins framework has been successfully applied to qualitatively describe polymer-induced compression of a number of synthetic hydrogels (30, 31, 90–94). However, it is a simple mean-field theory, does not take into account correlations between monomers, and assumes affine deformation of a homogeneous gel. We therefore did not expect strong quantitative agreement between the experiments and numerical calculations. However, we observed similar behavior between the two, using parameters that are consistent with experimentally measured values. In particular, the Flory–Huggins calculations showed that the free polymer does induce compression of the network, even though in the calculations the polymer could penetrate into the mucus hydrogel, and the trends we observed experimentally are qualitatively similar to those predicted by the model. Moreover, we found that polymers of higher molecular weights required a lower monomer volume fraction to compress the network, consistent with our experimental observations. One reason for this is the entropic penalty paid by PEG to penetrate the mucus; because this penalty is larger for larger polymers, they are more likely to be excluded from the mucus hydrogel, and therefore can compress it more by elevating the difference between external and internal osmotic pressure. Consistent with this expectation, we found that the higher-molecular-weight PEG was more likely to be excluded from the mucus hydrogel (Fig. S9E).
More sophisticated modeling could build on the work presented here by incorporating effects such as structuring of the colonic mucus hydrogel (37), viscoelastic relaxation of the mucus network, chemical adhesion (41) or electrostatic interactions, or polymer complex formation. For example, PEG has been observed to form complexes with polycarboxylic acids (30, 95–99), via hydrogen bonding between the ether oxygen of PEG and undissociated carboxylic groups; similar effects could play a role in our experimental system. We note, however, that at the physiological pH explored in our work the carboxyl groups found on the sialic acid residues of mucins are negatively charged (55, 57) and complexation is unlikely (Fig. S7).
Experiments Using Liquid Fraction of Colonic Contents.
Immediately after euthanizing a mouse, we collected its colonic contents in a polypropylene spin column with a 30-μm-pore-size filter (Thermo Scientific Pierce), always kept on ice, and centrifuged at 17,000 × g for 100 min at 4 °C. We then collected the liquid supernatant from the collection tube. We combined the liquid fraction thus obtained from multiple mice, both male and female, 3–4 mo of age, to obtain enough sample for the experiments, and stored aliquots at −20 °C until experimental use.
To test whether luminal contents could affect the polymer-induced mucus compression reported here, we used our ex vivo approach to also test compression induced by polymer solutions prepared in the thawed liquid fraction of SPF mice colonic contents instead of buffer. We tested both 3.1% (wt/vol) PEG 200k and 48.5% (wt/vol) dextrin, using the SPF liquid fraction as the solvent in both cases. In both cases, we verified that addition of the polymer to the SPF liquid fraction did not result in the formation of precipitates. In each case, we incubated a washed explant with 1-μm microparticles and used confocal reflectance microscopy to first measure the initial, washed mucus thickness. We then gently deposited 65–75 μL of the test solution on the exposed luminal explant surface and reimaged to measure the change in mucus thickness. We found that the mucus hydrogel compressed by 55 ± 2% in the case of PEG 200k and 52 ± 2% in the case of dextrin, similar to the compression measured for the same polymers in saline (as shown in Figs. 3B and 4A). This result suggests that additional luminal contents do not mask the effect of polymers in the gut. Further investigations along these lines will be an interesting extension of our work.
For each of the experiments shown in Fig. 4C, we incubated a washed explant with 1-μm microparticles and used two-photon microscopy to first measure the initial, washed mucus thickness. We then thawed the frozen liquid fraction of colonic contents, gently deposited 100 μL of it on the exposed luminal explant surface, and reimaged to measure the change in mucus thickness. We then obtained successive 3D stacks to verify that the thickness did not change in time over a time period of ∼10–30 min. We also collected multiple 3D stacks at different fields of view on the same tissue explant, and for different tissue explants obtained from multiple mice. The difference between the SPF and GF chromatograms in Fig. S10 suggested that, as expected (100–104), the GF contents were enriched in polymers of higher molecular weight compared with the SPF contents, and that these polymers were comparable in size to ∼200- to 700-kDa pullulan standards. As described in the main text, we found that the SPF contents did not appreciably compress colonic mucus, indicating that any residual polymers present in the SPF contents (after microbial degradation) were insufficient to compress the hydrogel; this result is also consistent with our observation that SPF mice and mice maintained on a sucrose diet had colonic mucus hydrogels of comparable thickness (Fig. 1, P = 0.3). By contrast, we found that the GF contents compressed colonic mucus by ∼70% of its initial washed thickness, for washed explants obtained from either SPF or GF mice (Fig. 4C). This finding indicates that gut microbes, by modifying the polymeric composition of intestinal contents, can actively modulate the compression state of the colonic mucus hydrogel (Fig. 4D).
Discussion
This work highlights the role of mucus as a dynamic biomaterial that responds to the polymeric composition of its environment. Our experiments reveal a previously unknown mechanism by which polymers in the gut—including dietary fibers abundant in our diet and therapeutic polymers ingested to relieve intestinal distress—alter the structure of the colonic mucus hydrogel. We speculate that this phenomenon may play a role in studies involving dietary fibers or therapeutic polymers, or their metabolism by microbes, in the gut. This could potentially have considerable physiological consequences (e.g., altering the access of pathogens or endotoxins to the epithelium). Investigating these effects will be a valuable direction for future work.
The work presented here focused on mucus compression induced by PEG, an uncharged polymer that has minimal chemical interactions with biomolecules and is often used as a therapeutic in the gut. We also observed similar behavior for several dietary polymers, suggesting that exploring a wider range of gut polymers will be a useful direction for future experiments. Polymers are also commonly used for the fundamental characterization of mucus itself, with the assumption that they do not alter the hydrogel structure. For example, a polymer solution [e.g., optimal cutting temperature (OCT) compound] is frequently used in cryosection experiments that seek to preserve mucus structure (e.g., refs. 33–36). The polymer-induced compression that we describe in this paper may affect such experiments; indeed, in preliminary experiments, we have found that OCT compound actually alters mucus structure considerably. Our work thus highlights the importance of understanding polymer–mucus interactions, and their resultant biological effects, in experimental design and interpretation.
Our data show that the extent of compression is strongly dependent on polymer concentration and molecular weight; this behavior is remarkably similar to the compression of synthetic hydrogels, which is known to arise from a combination of enthalpic and entropic effects. The role played by these different effects remains to be elucidated, even for the case of simple synthetic hydrogels. However, our data suggest that, similar to the synthetic case, polymer-induced compression of mucus—a complex biological hydrogel—can be described using Flory–Huggins theory. Our results thus motivate further work studying the physics underlying hydrogel compression, and the theoretical description presented here provides a basis for more sophisticated biophysical modeling that could incorporate effects such as nonisotropic structure of the mucus network (37), viscoelastic relaxation of the mucus hydrogel, or electrostatic interactions (further outlined in SI Materials and Methods). This could lead to new strategies for designing polymer-based therapeutics to controllably and predictably alter the morphology of gut mucus. Moreover, this work provides a general biophysical framework for investigating similar, previously overlooked, polymer-induced effects in other biological hydrogels, such as airway mucus, cervico-vaginal mucus, or extracellular matrix in tissues.
Materials and Methods
Details of Animals Used.
Except where otherwise noted, all mice were male or female SPF or GF C57BL/6 mice between 2–6 mo of age, fed a standard solid chow diet and given water for ad libitum consumption. The mice given only sucrose or only sucrose + PEG were first raised on a standard solid chow diet and given free access to water then maintained on a restricted diet consisting only of 5% (wt/vol) sucrose or 5% (wt/vol) sucrose + 7% (wt/vol) PEG 200k in 1× PBS (pH 7.4, without calcium and magnesium; Corning) given for ad libitum consumption for the 24-h period preceding euthanasia. All animal experiments were approved by the California Institute of Technology (Caltech) Institutional Animal Care and Use Committee (IACUC). Mice were euthanized via CO2 inhalation as approved by the Caltech IACUC in accordance with the American Veterinary Medical Association Guidelines on Euthanasia (38).
Details of Microscopy.
All imaging was performed using a Zeiss LSM 510 upright confocal microscope or a Zeiss LSM 880 upright confocal microscope, using either bright-field microscopy, confocal fluorescence microscopy (543-nm excitation/560-nm long-pass filter, or 488-nm excitation/505-nm long-pass filter), confocal reflectance microscopy (514-nm excitation/505-nm long-pass filter), or two-photon microscopy (800-nm excitation/650-nm long-pass filter). We collected 3D stacks consisting of multiple xy slices at different z positions.
Imaging of Unwashed Tissue.
We euthanized each mouse, removed the colon, and immediately flushed it gently with Fluorinert FC 40 oil (3M), which is immiscible with the aqueous contents of the colon. We then immediately cut the colon segment open along the longitudinal axis and mounted the opened tissue (luminal surface facing upward) onto a glass slide or a Petri dish using GLUture topical tissue adhesive (Abbott). We then gently deposited ∼0.5–2 mL of additional FC 40 oil onto the exposed luminal surface. The FC 40 is immiscible with water and with the mucus hydrogel; this procedure thus retained the adherent mucus in its in vivo “unwashed” state and prevented it from dehydrating.
Imaging of Washed Tissue.
We euthanized each mouse, removed the colon, and immediately flushed it gently with ice-cold 1× PBS and placed ∼1-cm-long segments of the midcolon in ice-cold PBS. We then cut and mounted the colon segments as described for unwashed tissues, always ensuring the explant surface was covered in PBS to prevent any dehydration. We then gently deposited the test solution onto the explant.
Thickness Measurements of Washed Mucus Hydrogel.
In each experiment, after placing a suspension of 1-μm-diameter microparticles onto the exposed luminal surface, we incubated the tissue at 4 °C for 1–2 h. We simultaneously imaged both the epithelium and the deposited microparticles using confocal or two-photon reflectance microscopy and determined the mucus thickness by measuring the distance between the epithelial surface and the deposited microparticles.
Quantifying Polymer-Induced Compression of Washed Mucus Hydrogel.
After measuring the initial washed mucus thickness, we gently deposited ∼0.2–2 mL of the test polymer solution onto the exposed luminal surface then collected the same 3D stacks at the same xy fields of view and remeasured the distance between the epithelial surface and the deposited microparticles.
Flory–Huggins Model of Compression.
We used the Flory–Huggins theory of polymer solutions to describe polymer interactions with the mucus hydrogel, treating the mucus as a cross-linked hydrogel swollen in a good solvent. First, we calculated the total free energy of the ternary solvent–mucus–polymer system, G, given by the sum of the elastic free energy, , which accounts for deformations of the individual mucus network strands, and the free energy of mixing the polymer and the solvent with the mucus hydrogel, . The total change in free energy is then , where is given by the Flory–Huggins (30, 39, 40) free energy of mixing and is given by rubber elasticity. At equilibrium, the chemical potentials of both the solvent and the free polymer, μS ≡ ∂G/∂nS and μP ≡ ∂G/∂nP, must be equal inside and outside of the mucus network; these equalities provided Eqs. 1–4, which represent the central result of the Flory–Huggins model and have been successfully used to describe polymer-induced compression of synthetic hydrogels (30).
Experiments Using Liquid Fraction of Colonic Contents.
Immediately after a mouse was euthanized, we collected its colonic contents in a polypropylene spin column with a 30-μm-pore-size filter (Thermo Scientific Pierce), always kept on ice, and centrifuged at 17,000 × g for 100 min at 4 °C. We then collected the liquid supernatant from the collection tube. For each of the experiments shown in Fig. 4C, we incubated a washed explant with 1-μm microparticles and used two-photon microscopy to first measure the initial, washed mucus thickness. We then gently deposited 100 μL of the liquid fraction of colonic contents on the exposed luminal explant surface and reimaged to measure the change in mucus thickness.
Detailed contributions from noncorresponding authors:
A.P.S. codesigned all experiments and coanalyzed all experimental results; codeveloped theoretical tools and coperformed the experiments and calculations; performed some of the FC oil measurements and analyzed some of the results in Figs. 1 and S1; performed some of the ex vivo experiments and analyzed some of the results in Figs. 2–4 and S3–S7; codeveloped the theoretical model; cooptimized and coperformed calculations for the theoretical model and coanalyzed results in Fig. 3; performed a sensitivity analysis for the theoretical model shown in Fig. S9; performed dynamic light scattering measurements of polymers and probes; designed and performed GPC measurements in Fig. S10; cowrote the paper.
S.S.D. coplanned the project; codesigned all experiments and coanalyzed all experimental results; codeveloped theoretical tools and coperformed the experiments and calculations; set up polymer-fed animal experiments (Fig. 1); performed some of the FC oil measurements and analyzed some of the results in Figs. 1 and S1; developed the ex vivo experimental approach; performed the ex vivo experiment of WGA-stained mucus (Fig. S2); performed some of the ex vivo experiments and analyzed some of the results in Figs. 2–4 and S3–S7; tested optical properties of test solutions (Fig. S8); codeveloped the theoretical model; developed a computational approach for the theoretical model calculations; cooptimized and coperformed calculations for the theoretical model and coanalyzed results in Fig. 3; developed an approach to extract liquid fraction of murine colonic contents; cowrote the paper.
Acknowledgments
We thank Said Bogatyrev, Andres Collazo, Elaine Hsiao, Julia Kornfield, Octavio Mondragon-Palomino, Ahmad Omar, Alexandre Persat, David Tirrell, Zhen-Gang Wang, and David Weitz for useful discussions; the Beckman Institute Biological Imaging Facility, the Broad Animal Facility, and the Church Animal Facility for experimental resources; the veterinary technicians at the California Institute of Technology for experimental assistance; Jennifer R. Keeffe for assistance with dynamic light scattering measurements; Dorothy Pan for assistance with gel permeation chromatography measurements; and Natasha Shelby for contributions to writing and editing this manuscript. This work was supported in part by Defense Advanced Research Projects Agency Biological Robustness in Complex Settings Contract HR0011-15-C-0093, National Science Foundation (NSF) Emerging Frontiers in Research and Innovation Award Grant 1137089, and NSF Graduate Research Fellowship DGE‐1144469 (to A.P.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1602789113/-/DCSupplemental.
References
- 1.Macfarlane S, Macfarlane GT, Cummings JH. Review article: Prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006;24(5):701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 3.DiPalma JA, DeRidder PH, Orlando RC, Kolts BE, Cleveland MB. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446–450. doi: 10.1111/j.1572-0241.2000.01765.x. [DOI] [PubMed] [Google Scholar]
- 4.DiPalma JA, Brady CE., 3rd Colon cleansing for diagnostic and surgical procedures: Polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol. 1989;84(9):1008–1016. [PubMed] [Google Scholar]
- 5.Wu L, et al. High-molecular-weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology. 2004;126(2):488–498. doi: 10.1053/j.gastro.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61(2):75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 8.Johansson MEV, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanvilkar K, Donovan MD, Flanagan DR. Drug transfer through mucus. Adv Drug Deliv Rev. 2001;48(2-3):173–193. doi: 10.1016/s0169-409x(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MEV, Larsson JMH, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson MEV, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergstrom KSB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6(5):e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmsted SS, et al. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcelo A, et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46(2):218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brownlee IA, Havler ME, Dettmar PW, Allen A, Pearson JP. Colonic mucus: Secretion and turnover in relation to dietary fibre intake. Proc Nutr Soc. 2003;62(1):245–249. doi: 10.1079/pns2003206. [DOI] [PubMed] [Google Scholar]
- 16.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Sulfated polysaccharides, but not cellulose, increase colonic mucus in rats with loperamide-induced constipation. Dig Dis Sci. 2001;46(7):1482–1489. doi: 10.1023/a:1010644021888. [DOI] [PubMed] [Google Scholar]
- 17.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc Natl Acad Sci USA. 2006;103(24):9298–9303. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai SK, Wang YY, Cone R, Wirtz D, Hanes J. Altering mucus rheology to “solidify” human mucus at the nanoscale. PLoS One. 2009;4(1):e4294. doi: 10.1371/journal.pone.0004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson MEV, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5(8):e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson MEV, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18(5):582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentine MT, et al. Colloid surface chemistry critically affects multiple particle tracking measurements of biomaterials. Biophys J. 2004;86(6):4004–4014. doi: 10.1529/biophysj.103.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakobsson HE, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16(2):164–177. doi: 10.15252/embr.201439263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson JK, et al. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2012;302(4):G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui H, et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA. 2006;103(48):18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai SK, et al. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104(5):1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer RF. Deswelling of gels by high polymer solutions. J Chem Phys. 1945;13(9):363–372. [Google Scholar]
- 27.Button B, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337(6097):937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brochard F. Polymer networks swollen by a homopolymer solution. J Phys. 1981;42(3):505–511. [Google Scholar]
- 29.Vasilevskaya VV, Khokhlov AR. Swelling and collapse of polymer gel in polymer solutions and melts. Macromolecules. 1992;25(1):384–390. [Google Scholar]
- 30.Kayaman N, Okay O, Baysal B. Phase transition of polyacrylamide gels in PEG solutions. Polym Gels Netw. 1997;5:167–184. [Google Scholar]
- 31.Saunders BR, Crowther HM, Vincent B. Poly[(methyl methacrylate)-co-(methacrylic acid)] microgel particles: Swelling control using pH, cononsolvency, and osmotic deswelling. Macromolecules. 1997;30(3):482–487. [Google Scholar]
- 32.Li H, et al. The outer mucus layer hosts a distinct intestinal microbial niche. Nat Commun. 2015;6:8292. doi: 10.1038/ncomms9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi K, et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut. 2002;51(2):169–176. doi: 10.1136/gut.51.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zindl CL, et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci USA. 2013;110(31):12768–12773. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5(3):0440–0460. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engevik MA, et al. Human Clostridium difficile infection: Altered mucus production and composition. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G510–G524. doi: 10.1152/ajpgi.00091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambort D, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109(15):5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. American Veterinary Medical Association (2013) AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. www.avma.org/KB/Policies/Documents/euthanasia.pdf. Accessed June 6, 2016.
- 39.Flory PJ. Principles of Polymer Chemistry. Cornell Univ Press; Ithaca, NY: 1953. [Google Scholar]
- 40.Rubinstein M, Colby RH. Polymer Physics. Oxford Univ Press; New York: 2003. [Google Scholar]
- 41.Wang Y-Y, et al. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chemie-International Ed. 2008;47(50):9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lotfy S. Controlling degradation of low-molecular-weight natural polymer “dextrin” using gamma irradiation. Int J Biol Macromol. 2009;44(1):57–63. doi: 10.1016/j.ijbiomac.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 43.White DR, Jr, Hudson P, Adamson JT. Dextrin characterization by high-performance anion-exchange chromatography--pulsed amperometric detection and size-exclusion chromatography--multi-angle light scattering--refractive index detection. J Chromatogr A. 2003;997(1-2):79–85. doi: 10.1016/s0021-9673(03)00626-5. [DOI] [PubMed] [Google Scholar]
- 44.Pal S, Nasim T, Patra A, Ghosh S, Panda AB. Microwave assisted synthesis of polyacrylamide grafted dextrin (Dxt-g-PAM): Development and application of a novel polymeric flocculant. Int J Biol Macromol. 2010;47(5):623–631. doi: 10.1016/j.ijbiomac.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Zhao R, Zeng J, Li G, Li X. Characterization of dextrins with different dextrose equivalents. Molecules. 2010;15(8):5162–5173. doi: 10.3390/molecules15085162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Kim MR, Lee JH, Lee JW, Kim SK. Production of high molecular weight pullulan by Aureobasidium pullulans using glucosamine. Biotechnol Lett. 2000;22(12):987–990. [Google Scholar]
- 47.Buliga GS, Brant DA. Temperature and molecular weight dependence of the unperturbed dimensions of aqueous pullulan. Int J Biol Macromol. 1987;9(2):71–76. [Google Scholar]
- 48.Kato T, Katsuki T, Takahashi A. Static and dynamic solution properties of pullulan in a dilute solution. Macromolecules. 1984;17(1):1726–1730. [Google Scholar]
- 49.Lazaridou A, Roukas T, Biliaderis CG, Vaikousi H. Characterization of pullulan produced from beet molasses by Aureobasidium pullulans in a stirred tank reactor under varying agitation. Enzyme Microb Technol. 2002;31(1-2):122–132. [Google Scholar]
- 50.Wiley BJ, et al. Control of molecular weight distribution of the biopolymer pullulan produced by Aureobasidium pullulans. J Environ Polym Degrad. 1993;1(1):3–9. [Google Scholar]
- 51.Stephen AM, Phillips GO. Food Polysaccharides and Their Applications. CRC; Boca Raton, FL: 2006. [Google Scholar]
- 52.Kawaguchi S, et al. Aqueous solution properties of oligo- and poly(ethylene oxide) by static light scattering and intrinsic viscosity. Polymer (Guildf) 1997;38(12):2885–2891. [Google Scholar]
- 53.Raynal BDE, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem. 2003;278(31):28703–28710. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- 54.Maisel K, et al. Enema ion compositions for enhancing colorectal drug delivery. J Control Release. 2015;209:280–287. doi: 10.1016/j.jconrel.2015.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mestecky J, et al. Mucosal Immunology. Elsevier; San Diego: 2005. [Google Scholar]
- 56.Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med. 2012;2(11):a009597. doi: 10.1101/cshperspect.a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ambort D, Johansson MEV, Gustafsson JK, Ermund A, Hansson GC. Perspectives on mucus properties and formation--lessons from the biochemical world. Cold Spring Harb Perspect Med. 2012;2(11):a014159. doi: 10.1101/cshperspect.a014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sellers LA, Allen A, Morris ER, Ross-Murphy SB. The rheology of pig small intestinal and colonic mucus: Weakening of gel structure by non-mucin components. BBA - Gen Subj. 1991;1115(2):174–179. doi: 10.1016/0304-4165(91)90027-e. [DOI] [PubMed] [Google Scholar]
- 59.Rubinstein M, Colby RH, Dobrynin AV. Dynamics of semidilute polyelectrolyte solutions. Phys Rev Lett. 1994;73(20):2776–2779. doi: 10.1103/PhysRevLett.73.2776. [DOI] [PubMed] [Google Scholar]
- 60.Lieleg O, Vladescu I, Ribbeck K. Characterization of particle translocation through mucin hydrogels. Biophys J. 2010;98(9):1782–1789. doi: 10.1016/j.bpj.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H. Smart Hydrogel Modelling. Springer; New York: 2010. [Google Scholar]
- 62.Sing CE, Zwanikken JW, de la Cruz MO. Effect of ion-ion correlations on polyelectrolyte gel collapse and reentrant swelling. Macromolecules. 2013;46(12):5053–5065. [Google Scholar]
- 63.Philippova OE, Rumyantsev AM, Kramarenko EY, Khokhlov AR. New type of swelling behavior upon gel ionization: Theory vs experiment. Macromolecules. 2013;46(23):9359–9367. [Google Scholar]
- 64.Dobrynin AV, Rubinstein M. Theory of polyelectrolytes in solutions and at surfaces. Prog Polym Sci. 2005;30(11):1049–1118. [Google Scholar]
- 65.Perez-Vilar J. Mucin granule intraluminal organization. Am J Respir Cell Mol Biol. 2007;36(2):183–190. doi: 10.1165/rcmb.2006-0291TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai SK, Wang Y-Y, Wirtz D, Hanes J. Micro- and macrorheology of mucus. Adv Drug Deliv Rev. 2009;61(2):86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Georgiades P, Pudney PD, Thornton DJ, Waigh TA. Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers. 2014;101(4):366–377. doi: 10.1002/bip.22372. [DOI] [PubMed] [Google Scholar]
- 68.Cai L-H. 2012. Structure and function of airway surface layer of the human lungs and mobility of probe particles in complex fluids. PhD dissertation (Univ of North Carolina, Chapel Hill, NC)
- 69.Szycher M. High Performance Biomaterials: A Complete Guide to Medical and Pharmaceutical Applications. Taylor & Francis; Lancaster, UK: 1991. [Google Scholar]
- 70.Lentle RG, Janssen PWM. The Physical Processes of Digestion. Springer; New York: 2011. Mucins; pp. 232–254. [Google Scholar]
- 71.Sobota JA, Ferraro F, Bäck N, Eipper BA, Mains RE. Not all secretory granules are created equal: Partitioning of soluble content proteins. Mol Biol Cell. 2006;17(12):5038–5052. doi: 10.1091/mbc.E06-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quillin ML, Matthews BW. Accurate calculation of the density of proteins. Acta Crystallogr D Biol Crystallogr. 2000;56(Pt 7):791–794. doi: 10.1107/s090744490000679x. [DOI] [PubMed] [Google Scholar]
- 73.Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: Light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28(13):5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- 74.Aksoy N, Sheehan J. Determination of polydispersity of a human colonic mucus glycoprotein using rate-zonal centrifugation and laser light-scattering. Turk J Med Sci. 2002;32:373–378. [Google Scholar]
- 75.Mikkelsen A, Stokke BT, Christensen BE, Elgsaeter A. Flexibility and length of human bronchial mucin studied using low-shear viscometry, birefringence relaxation analysis, and electron microscopy. Biopolymers. 1985;24(9):1683–1704. doi: 10.1002/bip.360240904. [DOI] [PubMed] [Google Scholar]
- 76.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 77.Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–657. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 78.Sheehan JK, et al. Physical characterization of the MUC5AC mucin: A highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem J. 2000;347(Pt 1):37–44. [PMC free article] [PubMed] [Google Scholar]
- 79.Prasitnok K, Wilson MR. A coarse-grained model for polyethylene glycol in bulk water and at a water/air interface. Phys Chem Chem Phys. 2013;15(40):17093–17104. doi: 10.1039/c3cp52958d. [DOI] [PubMed] [Google Scholar]
- 80.Fischer J, Paschek D, Geiger A, Sadowski G. Modeling of aqueous poly(oxyethylene) solutions. 2. Mesoscale simulations. J Phys Chem B. 2008;112(43):13561–13571. doi: 10.1021/jp805770q. [DOI] [PubMed] [Google Scholar]
- 81.Selser C, et al. Asymptotic behavior and long-range interactions in aqueous solutions of poly(ethylene oxide) Macromolecules. 1991;24:5943–5947. [Google Scholar]
- 82.Ziębacz N, Wieczorek SA, Kalwarczyk T, Fiałkowski M, Hołyst R. Crossover regime for the diffusion of nanoparticles in polyethylene glycol solutions: Influence of the depletion layer. Soft Matter. 2011;7(16):7181–7186. [Google Scholar]
- 83.Kienberger F, et al. Static and dynamical properties of single poly (ethylene glycol) molecules investigated by force spectroscopy. Single Mol. 2000;1(2):123–128. [Google Scholar]
- 84.Poon WCK, Andelman D. Soft Condensed Matter Physics in Molecular and Cell Biology. CRC; Boca Raton, FL: 2006. [Google Scholar]
- 85.Waters DJ, et al. Morphology of photopolymerized end-linked poly (ethylene glycol) hydrogels by small-angle X-ray scattering. Macromolecules. 2010;43(16):6861–6870. doi: 10.1021/ma101070s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dondi F, Guiochon G. Theoretical Advancement in Chromatography and Related Separation Techniques. Springer; Dordrecht, The Netherlands: 2012. [Google Scholar]
- 87.Tanford C. Physical Chemistry of Macromolecules. Wiley; New York: 1961. [Google Scholar]
- 88.Mijnlieff PF, Wiegel FW. Intrinsic viscosity and friction coefficient of polymer molecules in solution: Porous sphere model. J Polym Sci, Polym Phys Ed. 1978;16(2):245–263. [Google Scholar]
- 89.Brandup J, Immergut E. Polymer Handbook. 2nd Ed Wiley; New York: 1975. [Google Scholar]
- 90.Momii T, Nose T. Concentration-dependent collapse of polymer gels in solution of incompatible polymers. Macromolecules. 1989;22(3):1384–1389. [Google Scholar]
- 91.Ishidao T, Akagi M, Sugimoto H, Iwai Y, Arai Y. Swelling behaviors of poly(N-isopropylacrylamide) gel in poly(ethylene glycol)-water mixtures. Macromolecules. 1993;26(26):7361–7362. [Google Scholar]
- 92.Ishidao T, et al. Swelling equilibria of poly(N-isopropylacrylamide) gel in glucose and starch aqueous solution. Fluid Phase Equilib. 1995;104:119–129. [Google Scholar]
- 93.Ito T, Yamazaki M, Ohnishi S. Poly(ethylene glycol)-induced shrinkage of Sephadex gel. A model system for quantitative analysis of osmoelastic coupling. Biophys J. 1989;56(4):707–711. doi: 10.1016/S0006-3495(89)82717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiefer J, Naser M, Kamel A, Carnali J. Osmotic deswelling of microgels by linear polyelectrolytes. Colloid Polym Sci. 1993;271(3):253–261. [Google Scholar]
- 95.Adachi H, Nishi S, Kotaka T. Structure and mechanical properties of sequential interpenetrating polymer networks II. Complex-Forming polyoxyethylene: poly(acrylic acid) systems. Polym J. 1982;14(12):985–992. [Google Scholar]
- 96.Nishi S, Kotaka T. Complex-Forming poly(oxyehtylene):poly(acrylic acid) interpenetrating polymer networks. 1. Preparation, structure and viscoelastic properties. Macromolecules. 1985;18(8):1519–1525. [Google Scholar]
- 97.Chen H-L, Morawetz H. Kinetics of polymer complex interchange in poly(acrylic acid)-poly(oxyethylene) solutions. Macromolecules. 1982;15(5):1445–1447. [Google Scholar]
- 98.Bednar B, Morawetz H, Shafer J. Kinetics of the cooperative complex formation and dissociation of poly(acrylic acid) and poly(oxyethylene) Macromolecules. 1984;17(8):1634–1636. [Google Scholar]
- 99.Philippova OE, Karibyants NS, Starodubtzev SG. Conformational changes of hydrogels of poly(methacrylic acid) induced by interaction with poly(ethylene glycol) Macromolecules. 1994;27(9):2398–2401. [Google Scholar]
- 100.Sonnenburg JL. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307(5717):1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 101.Loesche WJ. Effect of bacterial contamination on cecal size and cecal contents of gnotobiotic rodents. J Bacteriol. 1969;99(2):520–526. doi: 10.1128/jb.99.2.520-526.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Høverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr. 1986;116(9):1772–1776. doi: 10.1093/jn/116.9.1772. [DOI] [PubMed] [Google Scholar]
- 103.Heneghan J. Germfree Research: Biological Effect of Gnotobiotic Environments. Elsevier; New York: 2012. [Google Scholar]
- 104.Spiro RG. Glycoproteins. Annu Rev Biochem. 1970;39(1):599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]