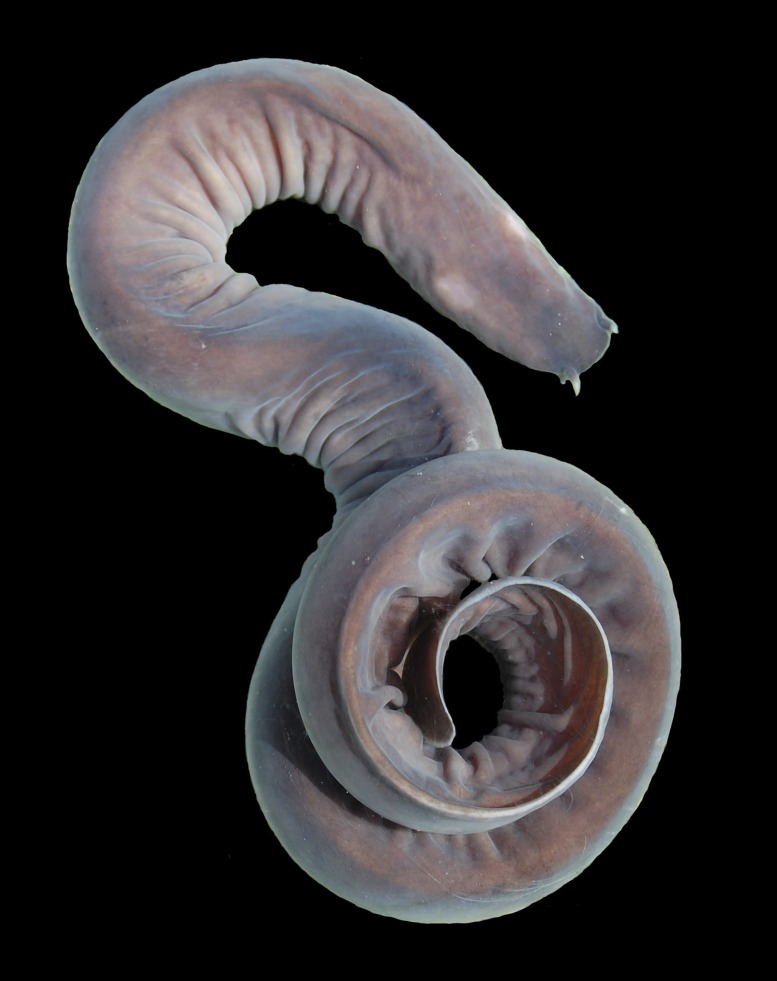
A tankful of wriggling proto-fish could one day offer a novel kind of strong, flexible material for buttressing bulletproof vests and reinforcing lightweight automobile parts. Jawless, spineless hagfish might seem an unlikely source for such advances. But the ancient animals, it turns out, exude a slime with extraordinary properties that just might spawn a new class of earth-friendly materials.
A proto-fish with a knack for exuding an extraordinary slime may one day provide the basis for earth-friendly materials. Image courtesy of Andra Zommers and Douglas Fudge.
At least, that’s the great hope of Douglas Fudge, a biologist at the University of Guelph in Canada. Fudge keeps 50–100 hagfish in chilly seawater tanks to harvest their slime. What makes that slime so extraordinary are its peculiar threads, which are both remarkably strong and bendy. Adapting those threads for human use is one example of biomimetic engineering in which scientists seek to develop materials inspired by nature: gecko foot-inspired glues, for example, or buildings based on heat flow in termite mounds.
Ancient Abilities
Although they are considered vertebrates, hagfish barely make the cut because they lack true bony vertebrae. Instead, they have a rod-like notochord made of cartilage. Although fossil records have not revealed their exact lineage, hagfish might have diverged from other vertebrates early in the evolution of fishes, about 500 million years ago. Regardless, they seem to have retained much of their primordial form, even as they boast some rather impressive biology.
“The slime, in and of itself, is incredibly unique in the animal kingdom,” says Timothy Winegard, a former Master’s student of Fudge’s and now the manager of the Algonquin Wildlife Research Station in Ontario, Canada. When provoked—by a predator or competitor—the hagfish might release just 90 milligrams of milky, concentrated “pre-slime” (what researchers call “exudate”) from glands in its skin. That material combines with seawater to produce nearly a liter of watery slime (1), which scientists believe clogs the gills of any would-be hagfish-eaters. Just how the hagfish makes the slime ingredients—and how it exudes them in less than a second—have been major questions in Fudge’s laboratory. But he is also spinning his own hagfish threads, which he believes might someday replace petroleum-based fibers, such as Kevlar.
In principle, hagfish fibers could be easier to produce on a large scale, compared with another prime candidate for biomimetic fibers, artificial spider silk. Genetically engineering bacteria to make large quantities of big, repetitive spider silk proteins has proven difficult. Plus, the natural silk gets much of its strength as it’s shaped by the spider’s spinnerets, fine for a spider but tricky to replicate. Fudge thinks hagfish have an advantage, and it starts with the makeup of their slimy secretion.
Special Ingredients
Hagfish slime is like no other biological ooze. The hagfish makes it with two different ingredients: the threads and mucin vesicles. Mucin-coated threads create a 3D sieve: a network of fibers that doesn’t hold water like a gel does, but simply slows it down. It’s squishy and slimy. But if you stretch it, you feel strong fibers pulling back. And if you hold it up for a few minutes, much of the water drains out, leaving behind a handful of slimy fibers, each one to three microns in diameter.
Those fibers are bundled intermediate filaments, one of the three types of cytoskeleton in cells. The rubber band-like filaments not only support a cell’s architecture, but also combine with other materials to make the hard keratin in hair and fingernails.
The hagfish threads possess another intriguing property: pull them about 33% longer than their original length and they still remain soft and stretchy. But more pulling causes the flexible α-helix–shaped proteins in the filaments to break open and combine with neighboring proteins, creating another shape called a β-sheet. This structure imparts extra strength and stiffness to the thread (2). Once this transition begins, the thread can be stretched farther, to more than triple its original length. It breaks at a stress of around 700 megaPascals, according to Fudge’s measurements. That’s compared with 1,000 megaPascals for spider silk, which also gains strength from β-shaped proteins.
The secret to the hagfish’s quickly deployed, untangled slime fibers lies within its slime gland cells, as Winegard discovered from electron microscopy studies of cells at different stages of maturity. The thread starts out as a thin, disorganized fiber in a cell with a large nucleus in the middle. As the thread grows, it butts up against the cell membrane and naturally starts coiling around the nucleus, like a garden hose wrapping around a ball. The nucleus shrinks, adopts a conical shape, and migrates to one end of the cell. There, it acts as a spindle onto which about 500 neat loops of threads eventually nest together (3). “Tim’s work was a big step forward for us,” says Fudge. “It gave us a glimpse into the precise organization.”
Timothy Winegard, a former student in the laboratory of Douglas Fudge, displays the special, robust threads that make up hagfish slime. Image courtesy of Dean Palmer (photographer).
Hagfish Inspiration
Tough as those hagfish threads are, Fudge has no desire to start a hagfish fiber farm. It would be expensive and not particularly good for the animals. So research associate Atsuko Negishi and then-Master’s student Nicole Pinto, now earning a doctorate at the University of Western Ontario in London, Canada, made the laboratory’s first attempts at artificial fibers. Starting with either hagfish protein from the slime glands or the related intermediate filament vimentin, produced by recombinant bacteria, the researchers freeze-dried the proteins and dissolved them in acid, then dripped the solution on top of a buffer. It readily formed a film, and when the researchers picked it up with forceps it came up as thread bundles (4, 5).
“The fibers were quite terrible compared to native hagfish slime threads,” Fudge admits. The artificial hagfish-protein threads broke around a stress of 150 megaPascals, and the vimentin threads snapped around 170 megaPascals. Nonetheless, the researchers think the initial experiments showed potential.
“I don’t know if I’d go so far as to say it could supplant spider silk in terms of its material properties, but there definitely are some pluses to the hagfish slime,” comments Jan Rainey of Dalhousie University in Halifax, Canada, a structural biologist who works on spider silk. Fudge’s fibers still contain too many α-helices to be really strong, Rainey thinks, and the disorganized protein regions present in spider silk make those threads particularly flexible. But making spider silk requires harsh solvents, such as fluorinated alcohol, whereas the hagfish proteins are soluble in water. This could allow for more environmentally friendly production lines, Rainey suggests.
Fudge speculates that the flexible α-helix threads, incorporated into composites, might prove useful for absorbing big impacts in products, such as safety helmets. And the β-sheet hagfish fibers, if made strong enough, might replace the Kevlar that’s woven into bulletproof vests. Either version, says Rainey, might be useful for imparting flexibility or adding strength to materials, such as car parts, without adding weight. Already, the vimentin fiber’s properties are comparable to human tendons, leading Pinto and Negishi to suggest that they might help replace damaged ones (5, 6).
For now, the peculiar proto-fish offers up more frivolous products. Hagfish leather, often sold as “eel skin,” is already made into wallets, purses, and jackets. Fudge admits to a small collection, but hopes much more important slime-based applications are on the horizon.
References
- 1.Fudge DS, Levy N, Chiu S, Gosline JM. Composition, morphology and mechanics of hagfish slime. J Exp Biol. 2005;208(Pt 24):4613–4625. doi: 10.1242/jeb.01963. [DOI] [PubMed] [Google Scholar]
- 2.Fudge DS, Gardner KH, Forsyth VT, Riekel C, Gosline JM. The mechanical properties of hydrated intermediate filaments: Insights from hagfish slime threads. Biophys J. 2003;85(3):2015–2027. doi: 10.1016/S0006-3495(03)74629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winegard T, et al. Coiling and maturation of a high-performance fibre in hagfish slime gland thread cells. Nat Commun. 2014;5:3534. doi: 10.1038/ncomms4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negishi A, et al. The production of fibers and films from solubilized hagfish slime thread proteins. Biomacromolecules. 2012;13(11):3475–3482. doi: 10.1021/bm3011837. [DOI] [PubMed] [Google Scholar]
- 5.Pinto N, et al. Self-assembly enhances the strength of fibers made from vimentin intermediate filament proteins. Biomacromolecules. 2014;15(2):574–581. doi: 10.1021/bm401600a. [DOI] [PubMed] [Google Scholar]
- 6.Johnson GA, et al. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]