Significance
Second-language learning in adulthood is a topic of wide interest given globalization, and levels of proficiency are highly variable among individuals. Here we demonstrate a significant correlation between individuals’ white matter fiber-tract properties in language areas and participation in an English language immersion program. Moreover, we found that this relationship was influenced by genetic variation (catechol-O-methyltransferase gene). Individuals with the Methionine (Met)/Valine (Val) or Val/Val genotype, but not the Met/Met genotype, showed higher fractional anisotropy and lower radial diffusivity during immersion. Values of brain measurements reversed after the immersion ended. These results suggest that second-language learning is influenced by an interaction between brain white matter structure and genetic factors.
Keywords: genetic variation, short-term plasticity, dopamine
Abstract
Adult human brains retain the capacity to undergo tissue reorganization during second-language learning. Brain-imaging studies show a relationship between neuroanatomical properties and learning for adults exposed to a second language. However, the role of genetic factors in this relationship has not been investigated. The goal of the current study was twofold: (i) to characterize the relationship between brain white matter fiber-tract properties and second-language immersion using diffusion tensor imaging, and (ii) to determine whether polymorphisms in the catechol-O-methyltransferase (COMT) gene affect the relationship. We recruited incoming Chinese students enrolled in the University of Washington and scanned their brains one time. We measured the diffusion properties of the white matter fiber tracts and correlated them with the number of days each student had been in the immersion program at the time of the brain scan. We found that higher numbers of days in the English immersion program correlated with higher fractional anisotropy and lower radial diffusivity in the right superior longitudinal fasciculus. We show that fractional anisotropy declined once the subjects finished the immersion program. The relationship between brain white matter fiber-tract properties and immersion varied in subjects with different COMT genotypes. Subjects with the Methionine (Met)/Valine (Val) and Val/Val genotypes showed higher fractional anisotropy and lower radial diffusivity during immersion, which reversed immediately after immersion ended, whereas those with the Met/Met genotype did not show these relationships. Statistical modeling revealed that subjects’ grades in the language immersion program were best predicted by fractional anisotropy and COMT genotype.
Second-language learning in adulthood is becoming increasingly prevalent as globalization advances. Previous studies show that gray matter volume and density are related to foreign language speech learning (1, 2), and that the degree of volumetric change in an individual predicts the level of foreign language proficiency achieved by that person (3, 4). Recent studies using diffusion tensor imaging (DTI) techniques further show that diffusion properties of brain white matter structure are correlated with foreign language learning (5–7). One of these studies demonstrated that the changes in diffusion properties predicted students’ second-language proficiency at the end of a language immersion program (7). These findings suggest that the properties of brain structure change with the acquisition of a new language, and that the adult human brain is capable of tissue reorganization in response to intense use of a new language after the putative “critical period.”
What remains unknown is whether and how genetic factors are related to brain white matter fiber-tract properties as learning ensues. Cumulative evidence using DTI analysis has suggested that brain white matter fiber-tract properties are related to skill learning (for a review, see ref. 8). A DTI index, fractional anisotropy (FA), which reflects the degree of water diffusion’s directional dependency, shows increased FA values as various skills are learned (5–7, 9–12). Another DTI index, radial diffusivity (RD), reflects water diffusion in a direction perpendicular to the fiber tracts. Increased FA and decreased RD values were associated with improved reading skills in children (11, 13). A recent study showed that a polymorphism in the catechol-O-methyltransferase (COMT) gene is related to FA values in children and adolescents (14). Taken together, these observations led us to explore the relationship between FA values and college students’ participation in an English immersion class. We further explored whether COMT polymorphisms can change the observed relationships.
COMT encodes an enzyme that degrades catecholamines (for a review, see ref. 15) and the protein of COMT is present in neuron and glial cells, including oligodendrocyte cells that produce myelin (16). COMT activity is also found in the cerebromicrovascular endothelial and smooth muscle walls, as well as in the capillary walls in brains (17, 18). A common polymorphism of COMT affects enzyme activity (19–21): the Methionine (Met) variant at position 158 in COMT leads to lower thermostability than the Valine (Val) variant. COMT-deficient mice showed increased dopamine or dopamine metabolite (3,4-Dihydroxyphenylacetic acid, DOPAC), but not norepinephrine, in the cortex compared with the heterozygotes and wild-type littermates (22, 23). Using family data, COMT activity was first shown to be recessive (24) and later confirmed to be the two codominant alleles at the same locus (25), suggesting three potential phenotypes corresponding to three genotypes. Consistent with this idea, COMT activity measured in whole-blood samples were shown to be highest in individuals with the Val/Val genotype, the intermediate level for Val/Met genotype, and the lowest for the Met/Met genotype (26). Studies using functional imaging techniques have suggested that the COMT genotype may have effects on brain activation responses (for a review, see ref. 27). For example, subjects with the Met/Met genotype showed lower prefrontal activation responses than the subjects with the Met/Val and Val/Val genotypes (28) or Val/Val genotype alone (29). One additional study also showed a different relationship between the midbrain dopamine synthesis and prefrontal activity compared with subjects with the Met/Val and Val/Val genotypes (30).
In this study, we: (i) characterize whether diffusion properties of white matter fiber tracts are related to subjects’ participation in a short-term English immersion program; (ii) examine whether diffusion properties of white matter fiber tracts vary between subjects with different COMT genotypes; and finally, (iii) predict subjects’ class grades in the immersion program based on individuals’ brain diffusion properties of white matter and their COMT genotype.
We recruited Chinese freshmen students who enrolled in an intensive English immersion program at the University of Washington to prepare for college-level instruction (n = 44). These were the experimental subjects. The program consisted of 16 training lessons, each of 3.5-h duration. Thus, students experienced a total of 56 h of immersion by the time they completed the program. The program focused on advanced skills in English comprehension and writing. In addition, we recruited Chinese students at the University of Washington who arrived in the United States at the same time but did not enroll in the immersion program (n = 35). These were the control subjects. The experimental (E) and control (C) student participants were matched for their prior exposure to English, including their age of first exposure to English, their parental/sibling English proficiency levels, and the amount of time they lived outside of China (see Materials and Methods for details).
We performed brain scans during a 14-d period, beginning 7 d after students entered the United States (11 d after the short-term immersion program began for students enrolled in the immersion program). All subjects had one brain scan. All brain scans were completed 8 d after the immersion program ended.
Our preliminary DTI analyses on E subjects revealed that FA/RD measures varied depending on whether the brain scan occurred while the immersion experience was still ongoing, versus after the immersion experience ended. Taking this into account, we separated subjects’ FA and RD data based on when the brain scans occurred. For E subjects who had been in the immersion program at the time of the scan, we examined FA and RD values as a function of the number of days (ND1) they had been in class. For subjects who had already finished the immersion class at the time of the scan, we examined the FA and RD values as a function of the number of days after the immersion program ended (ND2). We expected different trends to be observed in ND1 and ND2 data.
Results
Brain White Matter Fiber Tracts.
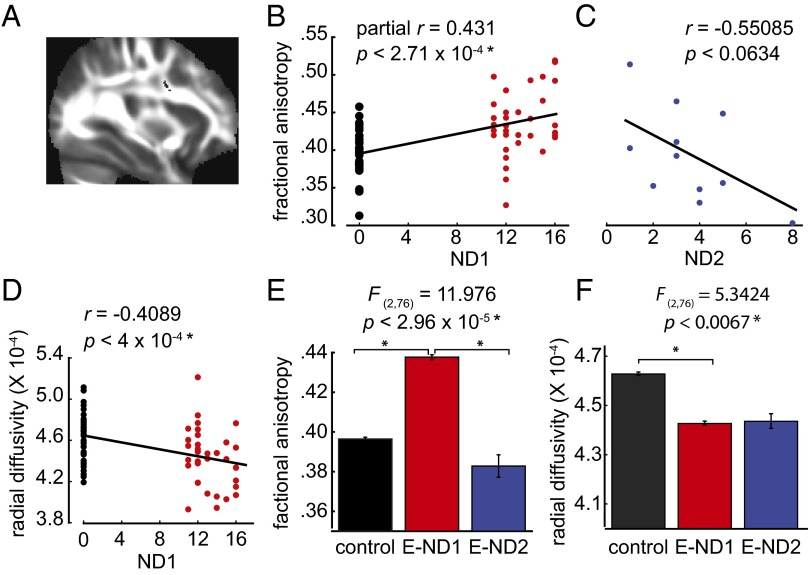
We examined E subjects who had a brain scan before the end of immersion program as a function of the number of days they had participated in the immersion program at the time of brain scan (E-ND1, n = 32). Results from whole-brain tract-based spatial statistics showed a cluster of brain voxels located in the right superior longitudinal fasciculus (SLF) (Fig. 1A) in which the FA values were significantly positively correlated with ND1, after we controlled for age (partial correlation coefficient = 0.431, P < 2.71 × 10−4) (Fig. 1B). For E subjects who underwent brain scans after finishing the class, we examined FA values in the SLF as a function of the number of days after completion of the immersion program (E-ND2, n = 12). The results showed a marginally negative correlation (Fig. 1C) (Pearson’s r = −0.55085, P < 0.063). We also examined the FA values in a homologous area in the left hemisphere, and found a marginally significant relationship between the FA values and ND1 (Fig. S1) (Pearson’s r = 0.2114, P < 0.081), suggesting that SLF in the left hemisphere may also be involved during immersion.
Fig. 1.
Relationship between immersion experience and FA values in the SLF. (A) Cluster of voxels in the SLF, with coordinates of peak voxels: x = 32, y = 3, z = 26. Size of cluster = 57 voxels. Voxels are overlaid on the mean FA image from all subjects. (B) Significant positive correlation between the FA values and number of days each subject had been in the class at the time of brain scan (ND1) (P < 2.71 × 10−4). C subjects not enrolled in immersion are represented as 0 d. C subjects are shown in black and E subjects scanned before the end of the immersion are shown in red. (C) Negative correlation between the FA values and number of days after the class ended (ND2) (P < 0.063). (D) Significant negative correlation between the RD values and ND1 (P < 4 × 10−4). (E) Significant difference in mean FA values between C subjects and E-ND1, and between E-ND1 and E-ND2 subjects (P < 2.96 × 10−5). (F) Significant difference in the mean RD values between C and E-ND1 subjects (P < 0.0067).
Fig. S1.
The relationship between the FA values in the left SLF and ND1.
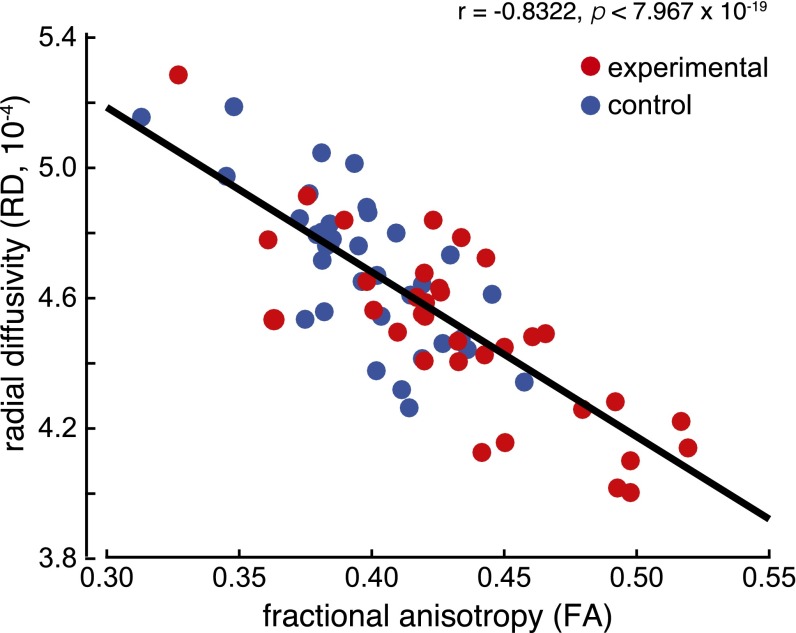
Using the same SLF voxels, and as expected, we found that RD values significantly negatively correlated with ND1 (Fig. 1D) (Pearson’s r = −0.4089, P < 4 × 10−4), and that RD and FA values were significantly negatively correlated (Fig. S2).
Fig. S2.
In the SLF, RD values were significantly negatively correlated with FA values. Black dots represent control subjects and red dots represent E-ND1 subjects.
We compared the mean FA and RD values in the following three groups of subjects: E-ND1 and E-ND2 groups and the C subjects who did not enroll in the immersion program. We found a significant main effect of group on the FA values [F(2, 76) = 11.976, P < 2.96 × 10−5]. Post hoc analysis revealed significant differences in the FA values between the C and E-ND1, and between the E-ND1 and E-ND2 groups, but no significant difference between the C and E-ND2 groups (Fig. 1E). Similarly, we found a significant main effect of group on the RD values [F(2, 76) = 5.3424, P < 0.0067]. Post hoc analysis revealed a significant difference in the RD values between the C and E-ND1 groups (Fig. 1F).
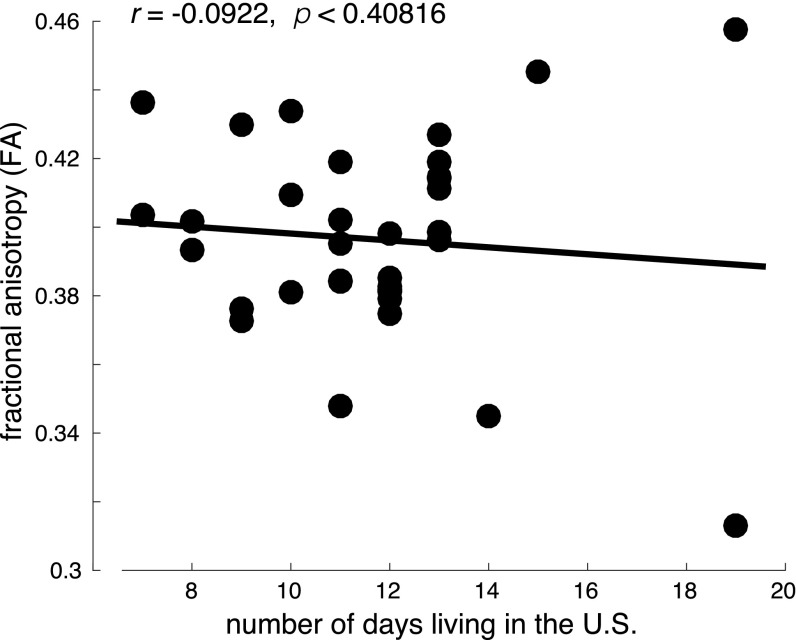
To test whether the observed FA differences in E subjects were attributable to the immersion experience and not simply to living in the United States, we examined the relationship between the number of days that the C subjects (n = 35) had resided in the United States at the time of their brain scans and their FA values. We did not observe a significant relationship (Pearson’s r = −0.0922, P < 0.4082) (Fig. S3).
Fig. S3.
The FA values in the SLF for control subjects.
Influence of COMT on Brain–Behavior Relationships.
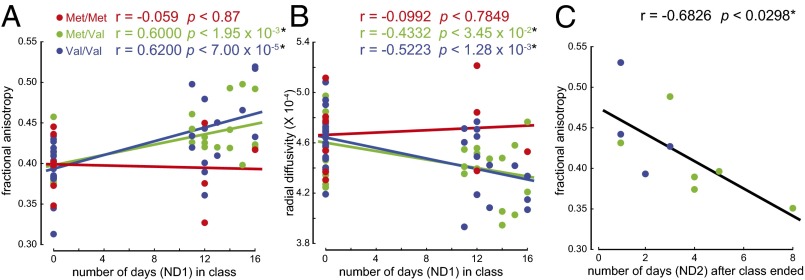
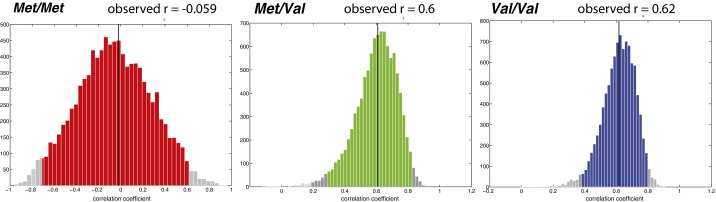
We next investigated whether the Val158Met polymorphisms in COMT were related to white matter fiber-tract properties for students participating in the immersion program. Using linear regression (figure 3 in ref. 31), we entered the E-ND1 and COMT genotype (categorical variable) to investigate whether the relationships between the FA values and E-ND1 differ among subjects with different COMT genotypes. We found significant effects of COMT on the positive correlation between FA and E-ND1 (R2 = 0.35, P < 3.32 × 10−6). Subjects with the Met/Val and Val/Val genotypes showed a significantly positive correlation between the FA values and E-ND1, whereas the subjects with the Met/Met genotype did not (Fig. 2A) (Pearson’s r = −0.059, P < 0.872 for Met/Met genotype; r = 0.6, P < 1.95 × 10−3 for Met/Val genotype; and r = 0.62, P < 7 × 19−5 for Val/Val genotype). The same method was used to examine the effect of COMT on the negative correlation between RD and ND1. We found a significant effect of COMT genotype on the negative correlation between the RD values and ND1 (Fig. 2B) (R2 = 0.253, P < 2.13 × 10−3). Subjects with the Met/Val and Val/Val genotypes showed a significant negative relationship between the RD values and ND1, whereas the subjects with the Met/Met genotype did not (Pearson’s r = −0.0992, P < 0.7849 for Met/Met genotype; r = −0.4332, P < 3.45 × 10−2 for Met/Val genotype; and r = −0.5223, P < 1.28 × 10−3 for Val/Val genotype).
Fig. 2.
Relationship between immersion and white matter brain structure differed with COMT genotype. (A) Subjects with Met/Val and Val/Val genotypes showed a significant positive correlation between the FA values in the SLF and number of days each subject had been in the class at the time of brain scan (ND1), whereas Met/Met subjects did not (P < 0.872 for Met/Met genotype; P < 1.95 × 10−3 for Met/Val genotype; and P < 7 × 10−5 for Val/Val genotype). (B) Subjects with Met/Val and Val/Val genotypes showed a significant negative relationship between the RD values in the SLF and ND1 (P < 0.7849 for Met/Met genotype; P < 3.45 × 10−2 for Met/Val genotype; and P < 1.28 × 10−3 for Val/Val genotype). (C) Subjects with the Met/Val and Val/Val genotypes showed a significant negative relationship in FA values in the SLF with number of days after the class ended (ND2) (P < 0.0298).
We also tested whether the relationships between the E-ND2 and FA or RD values in the SLF varied between subjects with different COMT genotypes. Subjects with the Met/Val and Val/Val genotypes showed a significant negative correlation between FA values in the SLF and E-ND2 (Pearson’s r = −0.6826, P < 0.0298) (Fig. 2C). We did not see a significant effect of COMT genotype on the relationship between the RD values in the SLF and ND2 (Pearson’s r = 0.2836, P < 0.3718).
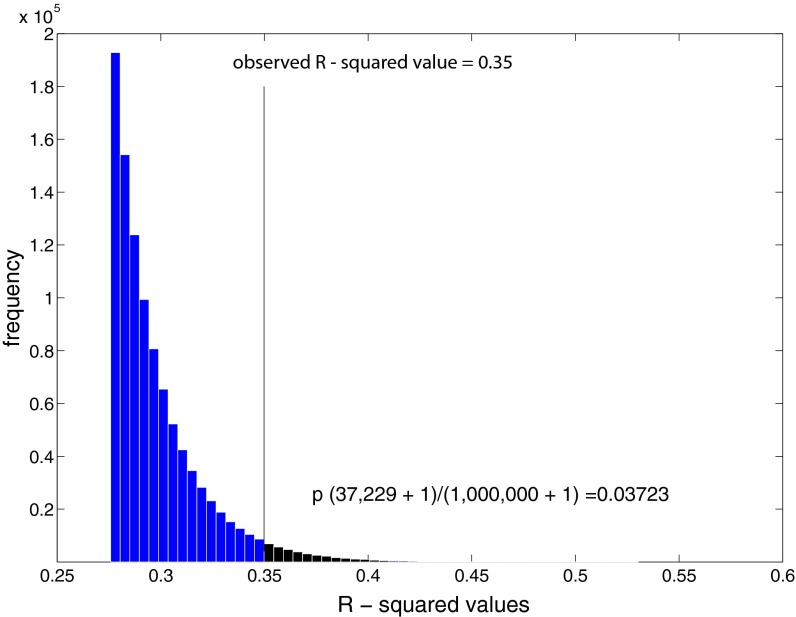
We performed two additional statistical tests to confirm the observed difference in the relationship between the FA values and E-ND1 in subjects with different COMT genotypes. First, we used a permutation test with 1 × 106 iterations to test whether the observed R2 value of 0.35 representing the significant interaction between the COMT genotype and ND1 (Fig. 2A) is statistically significant. The probability of obtaining the observed R2 value would be random if caused by a statistical artifact. The permutation test suggests that obtaining an R2 value of 0.35 or greater is unlikely to be due to chance (P = 0.03723) (Fig. S4).
Fig. S4.
Frequency distribution of R2 values from the permutation test. P value was calculated using the total number of occurrence (n = 37,229) when the R2 value was equal to or greater than the observed R2 value (0.35) of 1 × 106 iterations.
Next, we investigated whether the relationship between the FA values and E-ND1 within each COMT genotype was influenced by potential outliers. We performed three independent bootstrap tests with 1 × 104 iterations for each genotype. These tests showed that the observed Pearson’s correlation coefficients for Met/Met, Met/Val, and Val/Val groups were within the confidence intervals for each respective genotype distribution (Fig. S5). These results suggest that the observed correlation coefficients of three COMT genotypes shown in Fig. 2A were not driven by outliers.
Fig. S5.
Results from bootstrap tests showed that the observed Pearson’s correlation coefficients (r) representing each COMT genotypes in Fig. 2 were within the confidence interval of each respective genotype distribution (two-tailed, P < 0.05). Shaded areas represent areas outsides the confidence interval at P level 0.05 (two-tail).
To assess whether the COMT genotype had an effect on the difference in the mean FA values shown in Fig. 1E, we added COMT genotype as an additional factor in the ANOVA analysis. We found a significant Group × COMT interaction (Table S1) [F(4, 70) = 4.2166, P < 4.1 ×10−3], indicating that the mean FA values shown in control, E-ND1, and E-ND2 groups depend on the COMT genotype.
Table S1.
ANOVA results
| Factors | DOF | Mean sq | F stats | P value |
| Group | 2 | 0.0057 | 3.9454 | 0.0238* |
| COMT genotype | 2 | 0.0022 | 1.5373 | 0.2221 |
| Group × COMT genotype | 4 | 0.0061 | 4.2166 | 0.0041* |
COMT genotype and group assignment were entered as factors in a two-way ANOVA analysis. It yielded a significant effect of Group, and a significant interaction between Group and COMT genotype on the FA values in the SLF. DOF, degree of freedom; Mean sq, mean square error; P < .05 corrected for multiple comparisons.
We confirmed that there was no significant difference in either FA or RD values among COMT genotype groups in the C subjects (Fig. S6), indicating that the higher FA and lower RD values observed in the experimental subjects were likely associated with the immersion experience.
Fig. S6.
The Val158Met polymorphisms in COMT did not have effects on (A) the FA values or (B) the RD values in the SLF in control subjects. Central mark in red within each box represents the median of the respective group, and the edges of the box are the 25th and 75th percentiles.
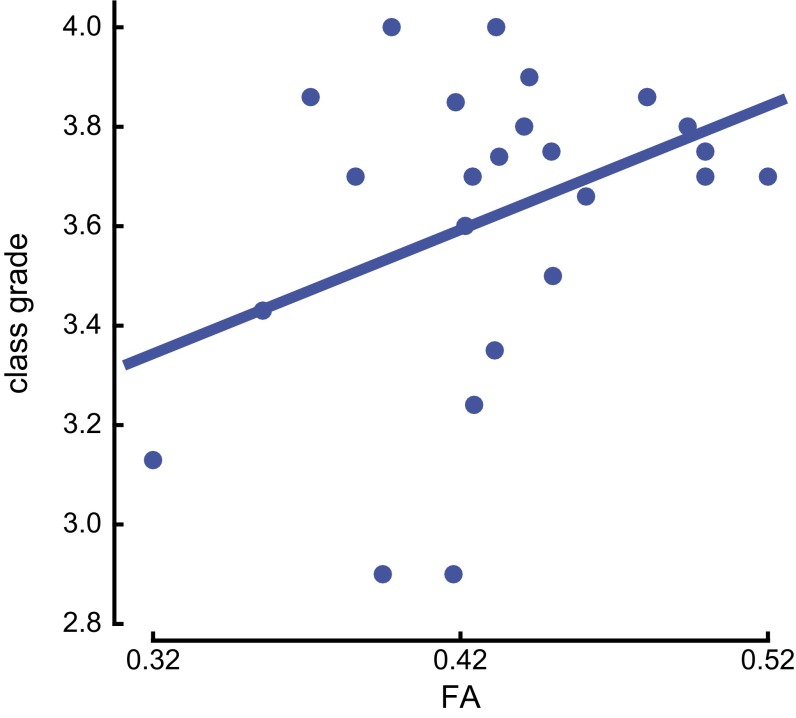
Finally, we created a statistical model that incorporates students’ FA values in the SLF and their COMT genotype. We obtained the class grades from 25 student participants who enrolled in the immersion program, and we tested the FA values in the SLF, adjusting for ND1, as a predictor of class grades, and confirmed a significant relationship (Fig. S7) (R2 = 0.186, P < 0.0315). Adding the COMT genotype as an additional predictor increased the total variance explained by more than 100%, from 18.6% to 46.2% (R2 = 0.462, P < 0.027). We did not observe a significant interaction between the COMT genotype and FA (P < 0.1741).
Fig. S7.
Relationship between student class grades and the FA values in the SLF. The FA values in the SLF and COMT genotype significantly predicted students’ class grades (R2 = 0.462, P < 0.017).
Discussion
The present study sought to determine the relationship between genetic variation, brain white matter fiber-tract properties, and second-language learning. We focused on adult Chinese students, who enrolled in an intensive short-term English immersion program as preparation for entering the university as freshmen, and matched controls. Our results provided two new findings. First, the data illustrate the relationship between the diffusion properties of brain white matter and immersion: higher FA values and lower RD values were observed across students as a function of the amount of time each one had spent in the immersion program. Moreover, in students scanned after the immersion experience ended, we observed lower FA values across individual students as a function of the number of days since immersion ended. These findings occurred in a fiber tract known to be important to language processing, the SLF (for a review, see ref. 32), which forms a major connection between the prefrontal cortex and language areas (33, 34). Second, we showed, to our knowledge for the first time, that polymorphisms of the COMT gene are significantly related to the FA and RD values in the SLF during immersion.
What possible cellular events could explain the variations we observed in white matter fiber tracks as a function of an immersion experience with a new language, and their relationship with the COMT gene? We propose three possible explanations, explained below.
Higher FA May Be Related to Increased Brain Myelination.
First, increased myelination may result in higher FA and lower RD values in the cortex, as previously shown in rodents (35, 36). It is possible that the subjects in our study increased myelination in the SLF, as it was previously suggested that increases in myelination are associated with learning (11, 13, 37). This hypothesis is also consistent with the difference in the FA values we observed between the E-ND1 and E-ND2 groups. Previous studies have suggested that the myelination process is dynamic and dependent on neural activity (38–46). It is thought that signals released from strongly active axons can trigger myelin production (41), and that the increased myelination facilitates signal transduction along the axonal fibers, allowing better information transfer across brain regions to accomplish the required tasks (for a review, see ref. 47). Animals showed an increased number of myelin sheaths (44) or increased rates of oligodendrogenesis when measured 6 h after stimulation (37). Similar mechanisms may explain higher FA values in subjects in the E-ND1 group. Consistent with this notion, diminished neural activity after the immersion program ended may contribute to the lower FA values in the E-ND2 group.
We observed a positive relationship between FA values and the amount of immersion experience in subjects with the Met/Val and Val/Val genotypes, but not in subjects with the Met/Met genotype. A possible explanation may reside in dopamine-related brain myelination. Dopamine has been shown to regulate myelination. Through the activation of dopamine D3 receptors, dopamine inhibits oligodendrocyte precursor cells as well as immature oligodendrocytes (48) from maturing into oligodendrocytes that can produce myelin sheaths around axonal fibers in the central nervous system (for a review, see ref. 49). In the present study, we used a common variant in the COMT gene that allows us to infer brain dopamine levels in student subjects. It was previously shown that COMT-deficient mice show elevated levels of dopamine at steady state in the frontal cortex (23), or increased dopamine metabolite (DOPAC), when administered with l-dopa and carbidopa compared with wild-type controls and the heterozygotes (22, 23). Individuals with the Met/Met genotype, compared with those with the Met/Val and Val/Val genotypes, would be expected to have elevated brain dopamine during immersion as a result of reduced COMT activity (27). We argue that the elevated brain dopamine during immersion may have prevented subjects with the Met/Met genotype from undergoing axonal myelination in the SLF. Interestingly, we did not observe statistically significant differences in FA values in the control subjects with different COMT genotypes, suggesting that COMT only affects the FA values of the brain voxels in the SLF during acute experience and is not a result of basal state differences.
Although we did not directly measure dopamine release while subjects were participating in the short-term immersion, we speculate that the subjects with the Met/Met genotype would have higher dopamine levels in the cortex. Previous studies have shown that transcranial and direct electrical stimulation in the prefrontal cortex can trigger dopamine release in other cortical regions in human and rat brains (50, 51). Thus, we argue that an increased dopamine efflux could be attributable to language experience during the intense immersion program, which in turn contributed to brain myelination in the SLF.
Our findings are consistent with a previous study in which subjects showed increased FA values in their brain white matter structure following 15-h training on juggling over a 6-wk period (52). Our student participants had 56 h of immersion experiences at the completion of the program. Their immersion experience may contribute to higher FA values in the SLF. However, another study using a 2-h training program showed changes in the mean diffusivity in the brains of healthy volunteers, which may not be attributable to myelination. Taking these data together, it remains to be determined whether brain myelination is contributing to the variation of FA values in subjects’ brains after a 1-wk foreign language immersion.
Higher FA May Be Related to Axonal Branching/Growth.
Second, it is also possible that higher FA values reflect axonal branching/growth. In human and nonhuman primate brains, axonal growth during development is correlated with increased FA values in the association fibers (53, 54). Neural activity has been shown to result in the growth and branching of axonal fibers (55–60). This type of activity-dependent axonal growth may have occurred in subjects during immersion, which may contribute to higher FA values.
According to this explanation, the effect of the COMT genotype could be a result of different brain activity levels in the prefrontal cortex in subjects with different COMT genotypes. It is generally agreed that stronger brain activity suggests higher neural activity measured by functional MRI (for a review, see ref. 61). Previous studies have shown that subjects with the Met/Met genotype had the least prefrontal activity at rest compared with subjects with the Val/Val genotype (62). Subjects with the Met/Met genotype also showed reduced prefrontal brain responses, or a different relationship between midbrain dopamine synthesis and prefrontal activity compared with subjects with the Met/Val and Val/Val genotype during cognitive tasks (28, 30). In other words, it is possible that subjects’ prefrontal activity during immersion depends on their COMT genotype. Increased prefrontal activity during immersion would have resulted in increased axonal growth, as suggested by these previous studies. If so, higher FA values shown in the subjects with the Met/Val and Val/Val genotype during immersion relative to the subjects with the same genotypes but without immersion experience may be related to the increased prefrontal activity. Similarly, higher FA values shown in the subjects with the Met/Val and Val/Val genotypes may be related to the increased prefrontal activity during immersion compared with the subjects with the Met/Met genotype.
COMT-dependent frontal activity has also been shown to interact with another polymorphism in the dopamine transporter gene (DAT). Individuals with the Val/Val genotype in COMT and 9-repeat alleles in the DAT show the highest prefrontal activity compared with individuals with the Met/Met genotype in the COMT and the 10-repeat alleles in the DAT (63). Future studies examining multiple genes involved in regulating dopamine levels in the cortex will be necessary before we can fully understand how brain white matter fiber-tract properties are influenced by cortical dopamine during learning.
Higher FA May Be Related to Reduced Volume of the Extracellular Space.
The third possible explanation is that the higher FA values we observed may be related to reduced volume in the extracellular space. Reduced volume in the extracellular space can result from swelling in nerve fibers and blood vessel dilation (for a review, see ref. 64). It has been shown that electrical stimulation can result in swelling in nerve fibers and tissue in vitro (for a review, see ref. 65). Such an event may reduce the volume in the extracellular space and restrict water movement in vivo. It is possible that our subjects may have experienced swelling in the SLF during the intensive immersion experience. However, whether restricted water movement will increase FA values in the SLF as observed remains to be determined.
Increased neural activity can also result in an increase in blood flow in a highly restricted region (for a review, see ref. 66). Signals released from strongly active neurons can cause the dilation of microvessels and it can be observed within seconds following stimulation (67). This type of dilation may have happened in the microvessels adjacent to the SLF during the intensive immersion experience. Interestingly, COMT activity is present in the cerebromicrovascular endothelial and smooth muscle walls, as well as in the capillary walls (17, 18). Low COMT activity in the cerebral cortex was found in rats with hypertension compared with normal controls (68), suggesting that COMT activity may regulate blood flow in the cortex. In line with this idea, a recent population study shows that subjects with the Met/Met genotype have significantly higher incidences of cardiovascular disease than subjects with the Val/Val genotype (69). In the present study, subjects with the Met/Met genotype who have lower COMT activity may have experienced less blood vessel dilation during immersion compared with the subjects with the Val/Val genotype. Reduced blood vessel dilation may have resulted in less shrinkage of extracellular volume, which reflects lower FA values.
These three explanations are not mutually exclusive. Further advancements in MR technology will be necessary before we can characterize in more detail the dynamic interactions among neurons, glial cells, and blood vessels during learning.
Anatomically, we observed higher FA coupled with lower RD in the SLF during immersion. The SLF constitutes a major portion of the dorsal pathway connecting the frontal cortex to language areas (for a review, see ref. 70). The SLF is known for its role in processing complex syntactic structure (for a review, see ref. 32). This type of processing is critical for our subjects because the English immersion program focused on advanced English comprehension and writing skills. Other studies have also reported higher FA values in the SLF or higher gray matter density in brain regions associated with the SLF in adults who regularly use their second language as opposed to those who use it less (12, 71, 72). These results suggest that intensive second-language learning and use in adults is associated with functional connectivity between the prefrontal cortex and language area through the SLF.
What is the functional significance of brain white matter fiber-tract properties and genetic factors for success in second-language learning? A statistical model incorporating individuals’ FA values and their COMT genotype highlighted the role of genes on brain white matter fiber-tract properties related to experience and reflecting learning. Using the class grades we obtained from 25 participants (11 Val/Val, 11 Met/Val, and 3 Met/Met) in the immersion program, we found that our model showed that although students’ class grades were significantly correlated with their FA values in language areas of the brain, the association accounted for only 18% of the total variance. However, COMT genotype and the FA values jointly accounted for 46.2% of the total variance. We did not observe a significant interaction between COMT genotype and FA values in our model. It is possible that a higher number of subjects is needed to observe differences in the relationships.
The results of the present study demonstrate an interaction between genes, brain, and behavior, interactions that are likely to underlie much of human learning. Future studies integrating molecular, genetic, and behavioral assays may uncover other factors that affect brain white matter fiber-tract properties during learning. Our findings suggest that second-language learning in adults is accompanied by diffusion properties in white matter in an area of the brain known to be important for language learning, and that these properties during immersion are significantly influenced by the COMT gene.
Materials and Methods
Selection Criteria.
All participants were new full-time students enrolled at the University of Washington. Experimental procedures were approved by the Institute Review Board of the University of Washington, and written informed consent was obtained from each participant. We matched experimental and control participants on sex, age of exposure to English, and environmental factors that influence English learning. Using screening questionnaires, subjects reported their family members’ proficiency in English speaking and listening. All subjects except for four (two E and two C) reported English exposure after the age of 6 y; four were first exposed at the age of 4 or 5 y old. Statistical comparisons for age of exposure to English for E and C subjects were nonsignificant (P > 0.05). In addition, there was no statistical difference in the fathers’, mothers’, or siblings’ English proficiency between E and C subjects (P > 0.05). We eliminated participants based on any previous residence outside of China before moving to the United States; previous participation in a student-exchange programs outside of China; both mother and father not of Chinese origin; past use of serotonin- or dopamine-related agents; a medical history of Axis I disorders, epilepsy, or brain injury; problems with normal vision or hearing; or left-handedness as assessed by the Edinburgh handedness test. For MRI safety, individuals with metallic or cardiac implants or tattoos were additionally excluded from the study.
Study Design.
Participants (n = 193) were Chinese students enrolled at the University of Washington. Genotyping was performed on all recruited subjects to evaluate the COMT genotype. This was done to ensure a sufficient representation of the Met/Met genotype in the analysis, which is present in less than 13% of Chinese Han individuals (International HapMap Project). Based on these genotype measures, we invited 44 students enrolled in the 16-d English language immersion class and 35 students not enrolled in an English language learning class (mean age = 20.02, SD = 3.35, 17 females) to participate in the brain-imaging component of this study (see Table S2 for the genotype distribution of subjects who were assessed with brain imaging). Brain-imaging data collection began 2 wk after recruitment. We began the brain scanning on the 11th day of the class. All brain-imaging data acquisition was completed within 8 d after the last day of the immersion class.
Table S2.
Distribution of COMT genotype
| Variable | Met/Met | Met/Val | Val/Val |
| n | 13 (female = 8) | 29 (female = 11) | 37 (female = 17) |
| Age (y) (mean ± SEM) | 21.5 ± 0.7 | 20.4 ± 0.9 | 20.4 ± 0.6 |
English Language Immersion Program.
The English language immersion class (course title: English 108, offered by the Department of English at the University of Washington) was comprised of a total of 16 d, 3.5-h sessions, which were held 4 d a week (Monday through Thursday). Student participants had a total of 56-h immersion by the completion of the program. The class began 1 d after the orientation event and ended 3.5 wk later.
Data Analysis.
Detailed information about the DNA extraction, brain-imaging processing, and genetic, brain, and behavioral analysis is provided in the SI Materials and Methods.
SI Materials and Methods
Genotyping.
DNA extraction.
Study participants provided saliva samples at an initial screening appointment. DNA was extracted from these samples using the Oragene DNA kit, following instructions provided by the manufacturer (DNA Genotek). All participants refrained from eating, chewing gum, drinking, or smoking 30 min before saliva collection.
TaqMan genotyping assay.
The TaqMan Drug Metabolism Genotyping Assay (Life Technologies) was used to genotype single nucleotide polymorphisms in the rs4680 region. The probes used to discriminate the alleles consisted of an oligonucleotide with a 5′-reporter dye and a 3′-quencher dye. The probe covalently linked to the VIC dye had the sequence CCA-GCG-GAT-GGT-GGA-TTT-CGC-TGG-C, and those covalently linked to the FAM dye had the sequence TGA-AGG-ACA-AGG-TGT-GCA-TGC-CTG-A. A LightCycler 480 InstrumentLightCycler 480 (Roche Diagnostics) was used for amplification and melting analysis. PCR was performed using a 25-μL solution that included 1 μg of genomic DNA, and a premix that contained primers and probes (18 μM of each primer and 4 μM of the probes). Thermal cycling was set for 1 min at 95 °C, followed by 45 cycles of 15 s at 92 °C and 1 min at 60 °C. We ran PCR on a 96-well plate that also contained two negative and two positive control wells. An algorithm provided by the manufacturer was used to discriminate the alleles.
DTI Acquisition.
DTI data were acquired on a Philips 3T Achieva scanner (v3.26) using an eight-channel head coil. An echo-planar diffusion spin-echo pulse sequence was used with the following parameters: 64 diffusion gradient directions, b value = 1,500 mm−2, TR = 8,986 ms, TE = 77 ms, acquisition matrix size 136 × 133 × 76, acquisition voxel size 1.76 × 1.8 × 1.8 mm3, reconstructed voxel size 1.5 × 1.5 × 1.8 mm3, EPI factor 47, receiver bandwidth 2,160 Hz, sound pressure 18.46 dB, fold-over direction AP, fat shift direction posterior (P) for TOPUP and anterior (A) for TOPDOWN, slice thickness = 1.8, SENSE factor 3 in the anterior-posterior direction, scan duration 12:12.7 min × 2 for both TOPUP and TOPDOWN.
DTI Analysis.
The FMRIB Software Library (FSL) 5.0.5 Diffusion Toolbox (FDT, fsl.fmrib.ox.ac.uk/fsl/fslwiki/fdt/) was used to process the DTI data. We used a multiple-step procedure recommended by FSL (73) that includes: (i) correcting for the motion artifact and eddy current with the “eddy” and “topup” toolbox, (ii) removing skull and nonbrain tissue from the image using the Brain Extraction toolbox, and (iii) voxel-by-voxel calculation of the diffusion tensors. FA and RD maps were generated using the DTIFit tool.
All DTI data were examined before and after preprocessing to evaluate image quality. Tract-based spatial statistics (TBSS), available in FSL (73), was used to perform voxel-wise statistical analysis. To acquire group templates accurately reflecting the effects of short-term immersion program on white matter structure, subjects who were scanned before the end of the immersion program were used in one TBSS analysis to generate its group template, and subjects who were scanned after the end of the immersion program were used in a separate TBSS analysis to generate another group template. TBSS analysis is comprised of the following steps: (i) nonlinear alignment of each subject’s FA volume to a 1 × 1 × 1 standard space; (ii) selection of a typical image to use as a group template; (iii) nonlinear transformation of image volumes previously aligned to the group template to the 1 × 1 × 1 Montreal Neurological Institute (MNI152) space; (iv) creation of a mean FA skeleton that represents the center of all tracts common to all subjects; and (v) projection of each subject’s aligned FA image onto the mean FA skeleton to generate a study specific mean FA map (mean_FA). We set the threshold at 0.2 for the mean FA map to generate a white matter-tract skeleton that represented the center of the tracts common to all subjects. We then projected each subject’s FA data onto the FA skeleton (all_FA_skeletonise) for voxel-wise statistical comparison. The “tbss_non_FA” script was then used to obtain an RD map for each study participant. We used the FEAT toolbox to identify areas where FA values were correlated with the number of days (ND1) the subjects had been in the class at the time of brain scan. In this analysis, ND1 was an exploratory variable and FA was the dependent variable. All subjects’ ND1 was demeaned to have a zero group mean. We used Randomize, which uses permutation-based nonparametric inferences, to perform statistical analysis on the FA matrix and ND1 (fsl.fmrib.ox.ac.uk/fsl/fslwiki/randomise/) (n = 1,000). We set the statistical threshold at 0.05 using the threshold-free cluster enhancement (TFCE) for the correction for multiple comparisons. The cluster of significant FA values was then used to isolate the voxels in the RD map of all subjects.
We used in-house–developed software to extract voxels from the resulting statistical images, and we obtained cluster size, peak coordinate, and FA values that exceeded the threshold (P < 0.05). We mapped the clusters to the Johns Hopkins University ICBM-DTI-81 white matter labels atlas and the Johns Hopkins University white matter tractography atlas provided by FSL to identify their anatomical location. We calculated the mean of FA of each subject by summing the FA values within the cluster and divided the number of voxels within the cluster. We used the identical method to calculate the mean of RD of each subject. The peak coordinates of the cluster are shown in Fig. 1A.
Statistical Analysis.
Multiple regression analysis using the Statistical Toolbox in MATLAB was used for assessing relationships between the mean FA and RD values, the behavioral data, and the genetic data. ANOVA analysis (unequal N) using the Statistical Toolbox in MATLAB was used to assess the mean differences in the FA and RD values between groups. Post hoc analysis identified pairs of means that differed significantly. All P values were corrected for multiple comparisons. Grades were obtained for 25 of the 44 subjects enrolled in class. To estimate the FA values as if measured at the end of the immersion program, we used the coefficient estimates from the linear regression function in MATLAB. Permutation and bootstraps tests were performed using the Statistical Toolbox in MATLAB.
Acknowledgments
We thank Jeff Stevenson for technical support; Denise Padden, Patricia Stock, Julia Mizrahi, Nicole Miles, Sahar Attar, and Spencer Sherwin for experimental assistance; John Webster and Carrie Matthew for assistance on recruitment; and Tonia Brown for manuscript editing assistance. The research was supported by National Science Foundation Science of Learning Center Grant SMA-0835854 to the University of Washington LIFE Center (to P.K.K., PI), and the University of Washington Institute for Learning and Brain Sciences’ Ready Mind Project. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606602113/-/DCSupplemental.
References
- 1.Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex. 2007;17(3):575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- 2.Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35(5):997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 3.Mårtensson J, et al. Growth of language-related brain areas after foreign language learning. Neuroimage. 2012;63(1):240–244. doi: 10.1016/j.neuroimage.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 4.Stein M, et al. Structural plasticity in the language system related to increased second language proficiency. Cortex. 2012;48(4):458–465. doi: 10.1016/j.cortex.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Flöel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca’s area predicts grammar learning success. Neuroimage. 2009;47(4):1974–1981. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Qi Z, Han M, Garel K, San Chen E, Gabrieli JDE. White-matter structure in the right hemisphere predicts Mandarin Chinese learning success. J Neurolinguist. 2015;33:14–28. [Google Scholar]
- 7.Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J Cogn Neurosci. 2012;24(8):1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 8.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taubert M, et al. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. J Neurosci. 2010;30(35):11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtsson SL, et al. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 11.Keller TA, Just MA. Altering cortical connectivity: Remediation-induced changes in the white matter of poor readers. Neuron. 2009;64(5):624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pliatsikas C, Moschopoulou E, Saddy JD. The effects of bilingualism on the white matter structure of the brain. Proc Natl Acad Sci USA. 2015;112(5):1334–1337. doi: 10.1073/pnas.1414183112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebauer D, et al. Differences in integrity of white matter and changes with training in spelling impaired children: A diffusion tensor imaging study. Brain Struct Funct. 2012;217(3):747–760. doi: 10.1007/s00429-011-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomason ME, et al. COMT genotype affects prefrontal white matter pathways in children and adolescents. Neuroimage. 2010;53(3):926–934. doi: 10.1016/j.neuroimage.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 16.Karhunen T, Tilgmann C, Ulmanen I, Panula P. Neuronal and non-neuronal catechol-O-methyltransferase in primary cultures of rat brain cells. Int J Deve Neurosci. 1995;13(8):825–834. doi: 10.1016/0736-5748(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 17.Helkamaa T, et al. Increased catechol-O-methyltransferase activity and protein expression in OX-42-positive cells in the substantia nigra after lipopolysaccharide microinfusion. Neurochem Int. 2007;51(6-7):412–423. doi: 10.1016/j.neuint.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Spatz M, et al. The presence of catechol-o-methyltransferase activity in separately cultured cerebromicrovascular endothelial and smooth muscle cells. Brain Res. 1986;381(2):363–367. doi: 10.1016/0006-8993(86)90090-9. [DOI] [PubMed] [Google Scholar]
- 19.Lachman HM, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lotta T, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huotari M, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002;15(2):246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 23.Gogos JA, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinshilboum RM, Raymond FA. Inheritance of low erythrocyte catechol-O-methyltransferase activity in man. Am J Hum Genet. 1977;29(2):125–135. [PMC free article] [PubMed] [Google Scholar]
- 25.Goldin LR, et al. Segregation and linkage studies of plasma dopamine-beta-hydroxylase (DBH), erythrocyte catechol-O-methyltransferase (COMT), and platelet monoamine oxidase (MAO): Possible linkage between the ABO locus and a gene controlling DBH activity. Am J Hum Genet. 1982;34(2):250–262. [PMC free article] [PubMed] [Google Scholar]
- 26.Apud JA, et al. Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology. 2007;32(5):1011–1020. doi: 10.1038/sj.npp.1301227. [DOI] [PubMed] [Google Scholar]
- 27.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Mattay VS, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100(10):6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Lindenberg A, et al. Midbrain dopamine and prefrontal function in humans: Interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 31.Green AE, et al. Using genetic data in cognitive neuroscience: From growing pains to genuine insights. Nat Rev Neurosci. 2008;9(9):710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 32.Friederici AD, Gierhan SM. The language network. Curr Opin Neurobiol. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 35.Song SK, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 36.Song SK, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Gibson EM, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344(6183):1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361(6409):258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 39.Demerens C, et al. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93(18):9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldsberry G, Mitra D, MacDonald D, Patay Z. Accelerated myelination with motor system involvement in a neonate with immediate postnatal onset of seizures and hemimegalencephaly. Epilepsy Behav. 2011;22(2):391–394. doi: 10.1016/j.yebeh.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18(5):683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishibashi T, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett. 2010;479(2):128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mensch S, et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18(5):628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18(22):9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333(6049):1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fields RD. Neuroscience. Change in the brain’s white matter. Science. 2010;330(6005):768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT. Identification of the dopamine D3 receptor in oligodendrocyte precursors: Potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18(14):5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomassy GS, Dershowitz LB, Arlotta P. Diversity matters: A revised guide to myelination. Trends Cell Biol. 2016;26(2):135–147. doi: 10.1016/j.tcb.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4(8):e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: Modulation by metabotropic glutamate receptors. J Neurosci. 1995;15(5 Pt 2):3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, et al. Rhesus monkey brain development during late infancy and the effect of phencyclidine: A longitudinal MRI and DTI study. Neuroimage. 2015;107:65–75. doi: 10.1016/j.neuroimage.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Huang H, et al. Development of human brain structural networks through infancy and childhood. Cereb Cortex. 2015;25(5):1389–1404. doi: 10.1093/cercor/bht335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Munz M, et al. Rapid Hebbian axonal remodeling mediated by visual stimulation. Science. 2014;344(6186):904–909. doi: 10.1126/science.1251593. [DOI] [PubMed] [Google Scholar]
- 56.Uesaka N, Hirai S, Maruyama T, Ruthazer ES, Yamamoto N. Activity dependence of cortical axon branch formation: A morphological and electrophysiological study using organotypic slice cultures. J Neurosci. 2005;25(1):1–9. doi: 10.1523/JNEUROSCI.3855-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama H, Fukaya M, Watanabe M, Linden DJ. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron. 2007;56(3):472–487. doi: 10.1016/j.neuron.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turney SG, Lichtman JW. Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: Evidence for synaptic competition and its mechanism. PLoS Biol. 2012;10(6):e1001352. doi: 10.1371/journal.pbio.1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Erisir A, Cline H. In vivo time-lapse imaging and serial section electron microscopy reveal developmental synaptic rearrangements. Neuron. 2011;69(2):273–286. doi: 10.1016/j.neuron.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26(13):3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 62.Tunbridge EM, Farrell SM, Harrison PJ, Mackay CE. Catechol-O-methyltransferase (COMT) influences the connectivity of the prefrontal cortex at rest. Neuroimage. 2013;68:49–54. doi: 10.1016/j.neuroimage.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caldú X, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37(4):1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 64.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88(4):1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tasaki I. Rapid structural changes in nerve fibers and cells associated with their excitation processes. Jpn J Physiol. 1999;49(2):125–138. doi: 10.2170/jjphysiol.49.125. [DOI] [PubMed] [Google Scholar]
- 66.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20(1):201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 68.Masuda M, Tsunoda M, Imai K. Low catechol-O-methyltransferase activity in the brain and blood pressure regulation. Biol Pharm Bull. 2006;29(2):202–205. doi: 10.1248/bpb.29.202. [DOI] [PubMed] [Google Scholar]
- 69.Hall KT, et al. Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34(9):2160–2167. doi: 10.1161/ATVBAHA.114.303845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friederici AD. Pathways to language: Fiber tracts in the human brain. Trends Cogn Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Kim KH, Relkin NR, Lee KM, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388(6638):171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- 72.Luk G, Bialystok E, Craik FI, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31(46):16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]