Significance
Tridymite, a SiO2 mineral that crystallizes at low pressures and high temperatures (>870 °C) from high-SiO2 materials, was detected at high concentrations in a sedimentary mudstone in Gale crater, Mars. Mineralogy and abundance were determined by X-ray diffraction using the Chemistry and Mineralogy instrument on the Mars Science Laboratory rover Curiosity. Terrestrial tridymite is commonly associated with silicic volcanism where high temperatures and high-silica magmas prevail, so this occurrence is the first in situ mineralogical evidence for martian silicic volcanism. Multistep processes, including high-temperature alteration of silica-rich residues of acid sulfate leaching, are alternate formation pathways for martian tridymite but are less likely. The unexpected discovery of tridymite is further evidence of the complexity of igneous petrogenesis on Mars, with igneous evolution to high-SiO2 compositions.
Keywords: Mars, tridymite, Gale crater, lake, volcanism
Abstract
Tridymite, a low-pressure, high-temperature (>870 °C) SiO2 polymorph, was detected in a drill sample of laminated mudstone (Buckskin) at Marias Pass in Gale crater, Mars, by the Chemistry and Mineralogy X-ray diffraction instrument onboard the Mars Science Laboratory rover Curiosity. The tridymitic mudstone has ∼40 wt.% crystalline and ∼60 wt.% X-ray amorphous material and a bulk composition with ∼74 wt.% SiO2 (Alpha Particle X-Ray Spectrometer analysis). Plagioclase (∼17 wt.% of bulk sample), tridymite (∼14 wt.%), sanidine (∼3 wt.%), cation-deficient magnetite (∼3 wt.%), cristobalite (∼2 wt.%), and anhydrite (∼1 wt.%) are the mudstone crystalline minerals. Amorphous material is silica-rich (∼39 wt.% opal-A and/or high-SiO2 glass and opal-CT), volatile-bearing (16 wt.% mixed cation sulfates, phosphates, and chlorides−perchlorates−chlorates), and has minor TiO2 and Fe2O3T oxides (∼5 wt.%). Rietveld refinement yielded a monoclinic structural model for a well-crystalline tridymite, consistent with high formation temperatures. Terrestrial tridymite is commonly associated with silicic volcanism, and detritus from such volcanism in a “Lake Gale” catchment environment can account for Buckskin’s tridymite, cristobalite, feldspar, and any residual high-SiO2 glass. These cogenetic detrital phases are possibly sourced from the Gale crater wall/rim/central peak. Opaline silica could form during diagenesis from high-SiO2 glass, as amorphous precipitated silica, or as a residue of acidic leaching in the sediment source region or at Marias Pass. The amorphous mixed-cation salts and oxides and possibly the crystalline magnetite (otherwise detrital) are primary precipitates and/or their diagenesis products derived from multiple infiltrations of aqueous solutions having variable compositions, temperatures, and acidities. Anhydrite is post lithification fracture/vein fill.
The Mars Science Laboratory (MSL) rover, Curiosity, has been exploring sedimentary rocks within Gale crater since landing in August 2012, and progressing upward through a sequence of strata exposed along the lower slopes of Aeolis Mons (informally known as Mount Sharp). This traverse has taken Curiosity through sequences of fluvial, deltaic, and lacustrine sediments (e.g., ref. 1). On sol 1057 (the number of martian days since landing), Curiosity began preparations for collecting drill powder from high-silica sedimentary rock at a location named Buckskin. We describe the geologic setting of the Buckskin sample location, the detection of significant amounts of the silica polymorph tridymite by the MSL Chemistry and Mineralogy (CheMin) X-ray diffraction instrument, and its implications for volcanism on Mars.
Geologic Setting
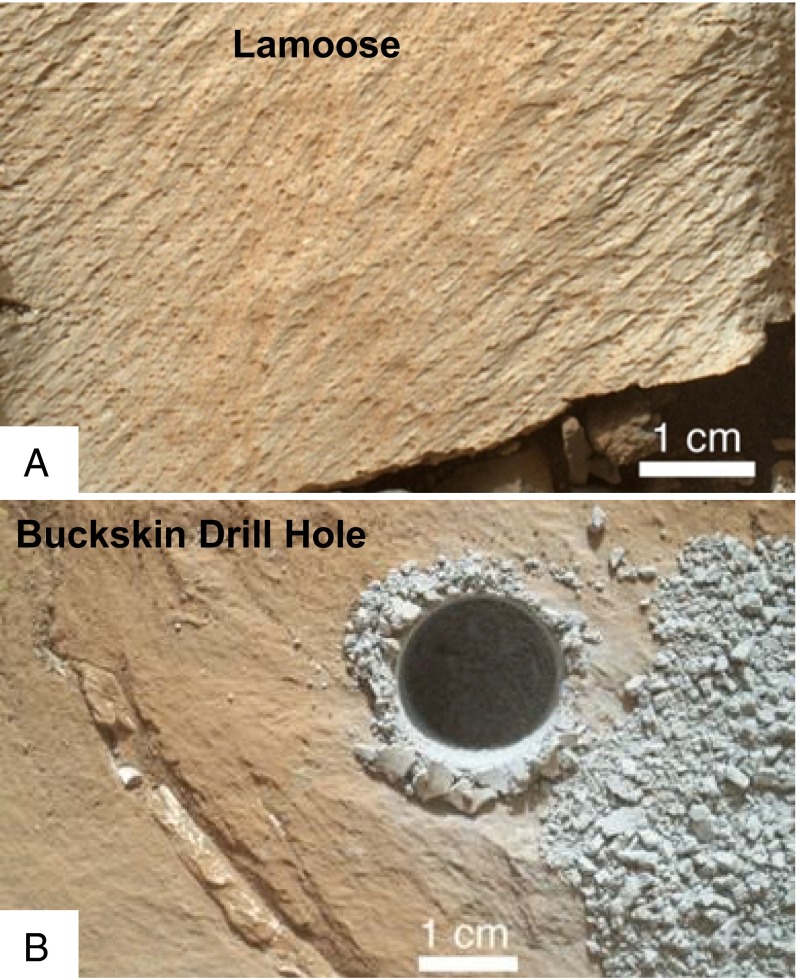
The Buckskin outcrop is part of the Murray formation of lacustrine mudstones exposed in the Marias Pass area (SI Appendix, Figs. S1 and S2). The formation was previously studied in the Pahrump Hills (1), where three drill samples were analyzed by CheMin [Confidence Hills, Mojave2, and Telegraph Peak (2)]. With the assumption that bedding is approximately horizontal, the Marias Pass outcrop is ∼2–3 m thick and ∼6 m higher in stratigraphic position than the highest outcrop sampled in the Pahrump Hills (Telegraph Peak) (1). Mudstone, generally characterized by fine lamination, is the dominant depositional facies in the Pahrump Hills (1) and also at Marias Pass, as shown by the Mars Hand Lens Imager images of Marias Pass bedrock and the dust-free float rock Lamoose (Fig. 1A). Here the laminae have a mean thickness of ∼0.5 mm and are characteristically parallel; individual laminae are laterally continuous for at least several tens of centimeters. The mudstone facies lacks evidence for cross-stratification at any scale, sediment transport (or erosion) by traction currents, sediment-filled mudcracks, intraclasts that indicate reworking of mud by currents, or clasts. The observed features are characteristic of lacustrine sedimentation and contrast strongly with subaerial settings associated with eolian, fluvial, volcanic, or impact processes (1).
Fig. 1.
(A) Lamoose is a float block of Murray formation, rotated and eroded by wind to reveal very fine lamination in addition to fine grain size. Wind-induced surface striations trend obliquely to primary depositional lamination. (B) The Buckskin drill hole penetrates tridymitic mudstone of the Murray formation at Marias Pass. Image courtesy of NASA/JPL-Caltech/MSSS.
Buckskin Mineralogical Composition
Targets for chemical analyses by the Alpha Particle X-Ray Spectrometer (APXS) at the Buckskin site included tailings from the test drill hole (sol 1059), the sample acquisition hole (sol 1064; Fig. 1B), and dump piles from emptying the Collection and Handling for In-Situ Martian Rock Analysis tool before (sol 1065) and after (sol 1091) the collected drill powder was sieved to <150 μm [see MSL mission overview for background (3)]. The chemical similarity of APXS analyses of the presieve and postsieve dump piles (Table 1) indicates that mineralogical fractionation between bulk sample and CheMin-analyzed sample (<150-μm postsieve dump pile) is minimal.
Table 1.
Composition of presieve and postsieve dump piles (from APXS) and calculated compositions of crystalline and amorphous materials
| Presieve, wt.% | Postsieve, wt.% | Crystalline,* wt.% | Amorphous,* wt.% | |
| SiO2 | 74.43 | 73.65 | 68.20 | 77.01 |
| TiO2 | 1.66 | 1.57 | 0.12 | 2.54 |
| Al2O3 | 5.45 | 5.66 | 13.91 | 0.04 |
| Cr2O3 | 0.08 | 0.10 | 0.00 | 0.17 |
| FeOT | 4.94 | 5.49 | 7.27 | 4.71 |
| MnO | 0.08 | 0.09 | 0.00 | 0.15 |
| MgO | 0.72 | 0.82 | 0.16 | 1.26 |
| CaO | 2.99 | 3.05 | 5.25 | 1.54 |
| Na2O | 2.01 | 2.08 | 2.80 | 1.58 |
| K2O | 0.99 | 0.96 | 1.35 | 0.69 |
| P2O5 | 1.29 | 1.25 | 0.00 | 2.09 |
| SO3 | 4.85 | 4.80 | 1.06 | 7.31 |
| Cl | 0.28 | 0.29 | 0.00 | 0.49 |
| Total | 99.77 | 99.81 | 100.12 | 99.60 |
Amorphous material composition calculated using APXS postsieve dump pile analysis as bulk composition, 40 wt.% crystalline material, and 60 wt.% amorphous material.
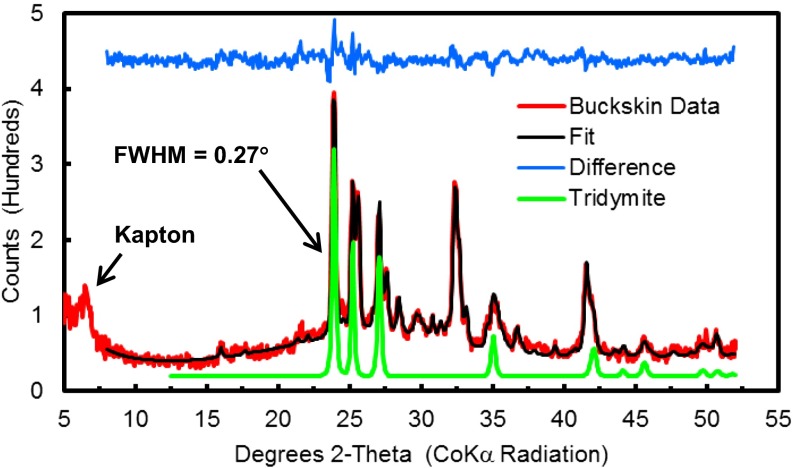
CheMin is a transmission X-ray diffraction (XRD) and X-ray fluorescence instrument that uses cobalt radiation to produce two-dimensional Co Kα diffraction images (Debye ring pattern) (4). Samples are normally measured over the course of three nights, producing 45 total images that are summed and converted to a one-dimensional diffraction pattern over an angular range of 2–52° 2θ. The diffraction pattern used for Buckskin quantitative XRD analysis was the sum of the first 4 of 45 diffraction images, and tridymite peaks are clearly evident (Fig. 2). The remaining 41 images are affected by on-ring and off-ring diffraction spots. Coincident with the appearance of abundant spots are a decrease in the intensities of tridymite diffraction peaks (by ∼50%) and a concomitant increase in the intensities of anhydrite diffraction peaks. This observation is based on a comparison of diffraction patterns for the sum of images 1 through 4 and 16 through 45, with each normalized to 45 images (i.e., the same integration time) (SI Appendix, Fig. S3). The explanation for the change in the nature of the diffraction patterns after image 4, possibly resulting from poor grain motion and particle clumping, is under investigation.
Fig. 2.
Buckskin diffraction pattern (red) from sum of first four diffraction images, calculated pattern from Rietveld analysis (black), difference pattern (blue), and refined tridymite pattern (offset green); see SI Appendix, Fig. S4 for refined patterns of all minerals. Full width at half maximum intensity (FWHM) of the tridymite peak at 23.9° 2θ is 0.27° 2θ, which corresponds to CheMin 2θ resolution measured on Earth before launch (ref. 4, figure 18). Peak at ∼6.5° 2θ is from the Kapton cell window.
For quantitative analysis of CheMin XRD data, we used the full pattern-fitting capability (Rietveld) in JADE (Materials Data Inc.), with refinement of literature cell parameters (5) for well-crystalline minerals present at >5 wt.% abundance, and FULLPAT analysis for poorly crystalline and XRD-amorphous phases (6–9). The combined results from both analyses are listed in Table 2. Unit cell parameters were refined for tridymite, plagioclase, sanidine, and magnetite (Table 3). The two most abundant crystalline minerals are plagioclase (43 wt.% of crystalline material; ∼An41) and tridymite (34 wt.%). All tridymite principal diffraction intensities (10) are present and well defined at instrument resolution (Table 4 and Fig. 2). They are indicative of fully ordered monoclinic tridymite, originally formed at high temperatures, as opposed to a disordered material like opal-CT, which has broad diffraction peaks that represent portions of a disordered tridymite structure in the 4.05–4.10 Å and 2.50 Å regions and with a weak diffraction peak at 4.25–4.35 Å (11). There are suggestions in the literature that opal-T can exist as a substance more ordered toward tridymite than opal-CT, which would be recognized by a broad diffraction peak near 4.107 Å and weaker broad diffraction peaks near 4.328 Å and 2.50 Å (12). However, the Buckskin tridymite shows none of these characteristics. Refined diffraction patterns for plagioclase, tridymite, sanidine, and magnetite are illustrated in SI Appendix, Fig. S4, as well as patterns of cristobalite and anhydrite whose unit cell parameters were fixed during refinement.
Table 2.
Mineralogy of Buckskin drill sample relative to crystalline material and relative to bulk sample using XRD- and APXS-derived concentrations for the amorphous material
| Crystalline material* | Bulk sample | ||
| XRD* | APXS* | ||
| Plagioclase | 42.8 ± 3.0 | 21.4 ± 1.5 | 17.1 ± 1.2 |
| Sanidine | 8.4 ± 1.8 | 4.2 ± 0.9 | 3.4 ± 0.7 |
| Magnetite | 6.9 ± 0.8 | 3.5 ± 0.4 | 2.8 ± 0.3 |
| Anhydrite | 1.8 ± 0.6 | 0.9 ± 0.3 | 0.7 ± 0.2 |
| Tridymite | 34.1 ± 2.0 | 17.1 ± 1.0 | 13.6 ± 0.8 |
| Cristobalite | 6.0 ± 0.8 | 3.0 ± 0.4 | 2.4 ± 0.3 |
| Amorphous | — | 50 ± 15† | 60†ǂ |
| Total | 100 | 100 | 100 |
Units = wt.%; uncertainties are 2σ.
Includes contributions from the broad hump centered near 26° 2θ [modeled as opal-A plus obsidian (33 wt.% of bulk sample) and opal-CT (6 wt.%)] and the broad hump centered near 40° 2θ modeled as ferrihydrite plus low-angle scattering (21 wt.%) (SI Appendix, Fig. S5).
Lower limit; constrained by APXS chemistry.
Table 3.
Space groups, unit cell parameters, and compositions from Rietveld analysis
| Tridymite | Plagioclase | Sanidine | Magnetite | |
| Space group | Cc | C | C2/m | Fd m |
| a, Å | 4.996 ± 0.002 | 8.155 ± 0.006 | 8.54 ± 0.02 | 8.359 ± 0.002 |
| b, Å | 8.671 ± 0.005 | 12.862 ± 0.006 | 13.01 ± 0.02 | 8.359 ± 0.002 |
| c, Å | 8.194 ± 0.003 | 7.106 ± 0.004 | 7.15 ± 0.02 | 8.359 ± 0.002 |
| α, deg | 90 | 93.32 ± 0.03 | 90 | 90 |
| β, deg | 90.43 ± 0.02 | 116.28 ± 0.03 | 115.8 ± 0.1 | 90 |
| γ, deg | 90 | 90.10 ± 0.04 | 90 | 90 |
| V, Å3 | 352.8 ± 0.3 | 666.9 ± 0.5 | 716 ± 3 | 584.04 ± 0.4 |
Tridymite and sanidine are monoclinic; plagioclase is triclinic; magnetite is cubic. Chemical compositions calculated from unit cell parameters are An41 ± 4Ab59 ± 4 (plagioclase), Ab32 ± 16Or68 ± 16 (sanidine), and Fe2.78 ± 0.05O4 (cation-deficient magnetite). Uncertainties are 1σ.
Table 4.
Peak intensities and lattice d-spacings of refined tridymite in Buckskin sample compared with monoclinic Cc tridymite (10)
| hkl* | Intensity | d, Å | ||
| Ref. 10 | Buckskin | Ref. 10 | Buckskin | |
| 110 | 100 | 100 | 4.3260 | 4.3222 |
| 020 | 45.9 | 46.2 | 4.3002 | 4.3060 |
| 002 | 75.9 | 75.6 | 4.1070 | 4.0972 |
| −111 | 29.7 | 29.0 | 3.8641 | 3.8276 |
| 021 | 28.6 | 28.7 | 3.8097 | 3.8118 |
| 111 | 23.5 | 23.9 | 3.7921 | 3.8184 |
| −112 | 10.9 | 10.6 | 3.0130 | 2.9779 |
| 022 | 13.5 | 13.4 | 2.9701 | 2.9682 |
| 112 | 7.3 | 7.6 | 2.9452 | 2.9692 |
| 200 | 10.8 | 11.2 | 2.5026 | 2.4986 |
| 130 | 17.7 | 17.2 | 2.4877 | 2.4892 |
| −131 | 2.9 | 3.1 | 2.3896 | 2.3828 |
| −113 | 2.8 | 2.9 | 2.3378 | 2.3121 |
| 023 | 3.9 | 3.8 | 2.3096 | 2.3066 |
| 113 | 6.7 | 6.8 | 2.2901 | 2.3060 |
hkl are the Miller indices.
The poorly crystalline or XRD amorphous material was modeled by contributions from opal-A, obsidian, and opal-CT to account for the diffuse diffraction feature centered near 26° 2θ and a low-angle scattering component that also includes a diffuse ferrihydrite-like diffraction feature centered near 40° 2θ (SI Appendix, Fig. S5). Instrumental artifacts, including a peak near 6.6° 2θ (∼15 Å) from the Kapton cell window, were modeled using CheMin patterns from analyses of empty cells with Kapton windows. Although opal-A was used to model the amorphous material, any high-SiO2 phase with a similar XRD pattern can be used, including SiO2 glass, gem opal, and silica gel. (See SI Appendix, Fig. S6 for XRD patterns of amorphous high-SiO2 materials produced by synthetic and natural processes.) The amount of amorphous material in the bulk sample based on FULLPAT is 50 ± 15 wt.% (Table 2).
Chemistry of Crystalline and Amorphous Materials
The chemical compositions of Buckskin plagioclase, tridymite, sanidine, and magnetite were estimated from unit cell parameters calculated by Rietveld refinement (Table 3). However, mineral unit cell parameters are insensitive to abundances of trace and minor elements (e.g., Fe in plagioclase). To limit artificial assignments of these elements to the amorphous material, we adopted compositions of naturally occurring materials for the chemical compositions of plagioclase, tridymite, and sanidine (13–15), subject to the constraint that their compositions are consistent with those derived from XRD unit cell parameters (Table 3 and SI Appendix, Table S1). This approach was more difficult for magnetite, because its refined cell (a = 8.359 ± 0.001 Å; Table 3) is too small for stoichiometric magnetite [Fe3O4; a = 8.394 Å (5)]. The cell parameters and bulk rock composition are most consistent with cation-deficient magnetite (Fe2.78O4) to accommodate the observed magnetite unit cell parameters. Alternatives to reduce the magnetite unit cell parameters that are not reasonable based on the bulk composition of the rock (Table 1) include a chromite [Fe2+(Cr,Fe3+)2O4] component (too little Cr) and hercynite (FeAl2O4) or spinel (MgAl2O4) components (all Al is associated with feldspar). An ulvöspinel (Fe2TiO4) component gives a larger cell parameter rather than the smaller one observed. Stoichiometric compositions were used for cristobalite and anhydrite (SI Appendix, Table S1), and their unit cell parameters were fixed to literature values (5) during Rietveld analysis because these minerals were not present in sufficiently high concentrations (Table 3) to permit meaningful refinement of unit cell parameters.
The chemical composition of crystalline material in Buckskin (Table 1) was calculated from mineral chemical compositions (SI Appendix, Table S1) and their abundances (Table 2). The high SiO2 concentration (∼68 wt.%) in the crystalline material reflects the high relative proportion of tridymite and feldspars. The bulk composition of the Buckskin sample (Table 1), the composition of the crystalline component (Table 1), and the weight proportion of the amorphous material from FULLPAT (a relatively uncertain 50 ± 15 wt.%) enable calculation (by mass balance) of the chemical composition of the noncrystalline (i.e., XRD amorphous) material (Table 1). However, the value of 50 wt.% results in an amorphous material with a calculated negative Al2O3 concentration (∼-2.6 wt.%), which is not physically possible. Increasing the abundance of the amorphous material in the calculations results in a positive Al2O3 concentration (0.04 wt.%; Table 1) at 60 wt.%. We take this value, which is within the uncertainty limits of the FULLPAT value, to represent the proportion of amorphous material, recognizing that the value could be higher. The amorphous material has a calculated value of ∼77 wt.% SiO2, which is consistent with the observed broad diffraction hump centered near 26° 2θ that is expected for high-SiO2 amorphous materials (SI Appendix, Fig. S6).
The calculated composition of the amorphous material also has a high anion concentration (SO3 + P2O5 + Cl ∼10 wt.%) (Table 1). Charge balance in the amorphous material is achieved if these anions occur predominantly as mixed-cation sulfate, phosphate, and chloride−perchlorate−chlorate XRD-amorphous salts. This calculation excludes SiO2 and TiO2, which do not readily form salts, and results in a small oxide excess (∼2 wt.%) taken as amorphous Fe2O3.
Tridymite Formation Pathways
Tridymite is the stable polymorph of SiO2 at low pressures (up to ∼0.4 GPa) between ∼870 °C and ∼1,470 °C (e.g., refs. 16 and 17) and is thus metastable under ambient terrestrial (and martian) surface conditions. It is readily produced under laboratory conditions within its stability field, especially with alkali fluxes (18–27). We are not aware of any geologically reasonable, low-temperature formation pathway for preparation of tridymite outside of its thermochemical stability field. In the following paragraphs, we focus on natural occurrences of tridymite and evaluate their applicability to the origin of abundant tridymite in the Buckskin sample. Unless otherwise noted, the tridymite crystal structure is unspecified in the cited references.
Many terrestrial tridymite occurrences are associated with silicic volcanism, which, by its nature, has the requisites of high temperatures and high-SiO2 lava compositions (dacite and rhyolite) for tridymite formation within its stability field. Vapor-phase formation of tridymite (along with sanidine and iron oxides) occurs in vugs and lithophysae in rhyolitic ash-flow tuff (28–35). For example, the mineral stratigraphy within compound cooling units of a rhyolitic ash-flow tuff showed tridymite is most abundant (∼25 wt.%) in the central zone where temperatures were highest and occurs along with sanidine as crystals growing into voids; cristobalite and sanidine occur in cooler zones where replacement of glass predominates (30). Tridymite derived from oxidation of fayalite has also been reported in a rhyolite flow (Oregon) (36). At the Iwodake rhyolitic cone of Satsuma-Iwojima volcano (Japan), a tridymite formation pathway is alteration of rhyolite by volcanic vapors and acidic hydrothermal fluids at temperatures up to 900 °C (37–40). Acid sulfate leaching of rhyolite also occurred there, increasing the SiO2 concentration from ∼71 wt.% to ∼97 wt.%.
The temperature regime associated with basaltic and intermediate volcanism can also provide a formation pathway for tridymite when the requisite high-SiO2 material is present. Pyroclastic flows and associated ash plumes generated by partial lava dome collapse at andesitic Soufriere Hills volcano (Montserrat) have 10–27 wt.% tridymite plus cristobalite with minor quartz in their <10-μm size fraction (41). The tridymite and cristobalite are inferred to have formed by vapor-phase crystallization and devitrification of rhyolitic glass (∼78 wt.% SiO2) within the lava dome. In altered basalts from Stromboli volcano (Aeolian Islands, Italy) and andesites from Augustine volcano (Alaska), the high-SiO2 host for tridymite is the residue after acid sulfate leaching of primary mafic rock (42, 43). Pyrometamorphic ejecta (buchite) blocks (tridymite−clinopyroxene facies) have up to ∼90 vol.% tridymite at Stromboli volcano. Occurrences of quartz paramorphs after tridymite have been reported in contact metamorphic assemblages between high-SiO2 rocks and basaltic lava (e.g., refs. 44–46); however, quartz and not tridymite would be detected by CheMin for an equivalent assemblage at Gale crater. The high concentrations of tridymite (∼14 wt.% of bulk sample) and SiO2 (∼74 wt.%) for the Buckskin sample are not reasonably sourced from low-silica volcanics for which tridymite concentrations would be very low when averaged over the volumetric equivalent of a “Lake Gale” catchment involving entirely basaltic to intermediate volcanics.
Tridymite has been reported at terrestrial impact structures where target lithologies are SiO2-rich and heated to elevated temperatures by the impact event (47, 48). Monoclinic tridymite was reported (abundance not quantified) in a core from the Chesapeake Bay impact structure, but only in a silica-rich 5-cm interval (∼70 wt.% SiO2 relative to whole rock) (48). In cores from the Popigai impact structure (gneiss target lithology), we calculate from ref. 47 that the mean tridymite concentration of melt rocks is ∼1 vol.%, although up to ∼5 vol.% tridymite is reported for several individual melt rocks. The impact that created Gale crater (∼150 km diameter) generated a melt pool possibly of 0.5–1 km thickness (49) and presumably injected impact melt into fractured crater walls and central uplift. Even if the preimpact target lithology were SiO2-rich (an assumption implying silicic volcanism predating the Gale impact), the high tridymite concentration in the Buckskin laminated mudstones is unlikely sourced from a Lake Gale catchment of impact melt rocks given the low tridymite abundance of known impactites.
As with terrestrial occurrences, tridymite in extraterrestrial samples has also been inferred to result from high-temperature igneous and/or impact processes on parent bodies or to form by direct condensation at high temperatures in the solar nebulae (e.g., refs. 24 and 50–58). Tridymite, cristobalite, quartz, and/or silica-rich glass are reported as minor constituents of lunar samples (e.g., ref. 57) and martian meteorites (e.g., refs. 51, 53, and 59). A granitic microclast in polymict ureilite EET 87720 contains a phase tentatively identified as tridymite by micro-XRD, with ∼98 wt.% SiO2 (58). The bulk composition of the microclast (excluding interstitial glass) has ∼77 wt.% SiO2, and the interstitial glass is silica-rich (SiO2 ∼73 wt.%) and volatile-rich (∼11 wt.% SO3 and ∼1 wt.% Cl). According to ref. 58, this microclast is evidence for an evolved melt on an unknown parent body with isotopic and volatile element data possibly pointing to Mars.
Tridymite has been synthesized in the laboratory by heating amorphous SiO2 at 196 °C in glycol solutions (60). Aside from the fact that the chemical composition of the solutions used in these experiments is not geologically realistic, the temperature of the experiments is higher than those inferred so far for diagenesis at Gale crater [<∼80 °C (8)], and the relative intensities of the diffraction peaks for the tridymite synthesized by ref. 60 differ markedly from those for the well-crystalline Buckskin tridymite (Table 4). These experiments indicate that low-temperature synthesis of metastable tridymite is possible and should be further explored. The apparent absence in nature of tridymite formation at low temperatures, based on studies of terrestrial and extraterrestrial rocks over many years (61−72) (see SI Appendix), suggests that this is an unlikely explanation for the large quantities of tridymite observed in the Buckskin sample.
Significance of Tridymite in the Murray Formation
In the absence of a geologically credible, low-temperature formation pathway for tridymite, and based on the consistency of its occurrences in natural samples as discussed in Tridymite Formation Pathways, we infer that tridymite in the Murray formation (Buckskin drill sample) is a marker for low-pressure and high-temperature (>870 °C) processes, consistent with its stability field, and high-SiO2 precursor lithologies. Silicic volcanism fits the geologic, thermal, and mineralogical context best, with tridymite being incorporated into the Murray formation laminated mudstone (i.e., tridymitic mudstone) at Buckskin as detrital sediment from erosion of silicic volcanic rocks. It follows that, if cogenetic, the cristobalite, sanidine, plagioclase, and any amorphous high-SiO2 glass are also detrital. The high-SiO2 glass would constitute some unknown fraction of the amorphous silica material determined by CheMin (33 wt.% of bulk as opal-A and/or high-SiO2 glass). However, we infer, from the presence of opal-CT in the Buckskin amorphous material, that any glass has undergone diagenesis at least in part if not wholly to opal-A and opal-CT. This diagenesis could have occurred in the volcanic source area or subsequent to lithification of tridymitic mudstone at Marias Pass. If the diagenesis took place in the source region, the opaline material is detrital.
Lacustrine sediments in cores from Lake Tecocomulco (Mexico), with a catchment basin that includes basaltic−andesitic, andesitic, and rhyolitic volcanic rocks (73), provide an appropriate terrestrial analog to an ancient Lake Gale and the Murray formation. Sedimentary units in core T-A95 from that lake include both fine-grained clastic sediments and coarser-grained dacite−rhyolite air-fall tephra. The highest combined abundance of tridymite plus cristobalite (up to >30% with no quartz) are in unit IIIa, a silty sand clastic sediment with ∼60 wt.% SiO2. Tridymite and cristobalite are present at generally lower abundances (5–30%) in two air-fall tephra layers in unit IIIb (∼70 wt.% SiO2) from Plinian eruptions 8–60 km away (73). The Lake Tecocomulco analog shows that sediments with high abundances of tridymite and cristobalite, like those in the Buckskin sample, are obtainable in lake sediments with a catchment that includes silicic volcanism.
By partial analogy with tridymite-bearing sediments in Lake Tecocomulco (73), silicic volcanic rocks exposed within the catchment of ancient Lake Gale could have contributed the detrital tridymite and cogenetic material to the Murray formation. Fine-grained sediments are most readily formed by downstream fining in alluvial settings, and evidence for this is preserved in the Gale crater moat (74). The even and fine lamination observed in the Murray formation at Marias Pass is inconsistent with direct fallout of distal volcanic ash, even through a water column (1). For the lower Murray formation at Pahrump Hills, these regular laminae were interpreted to result from the settling of fines derived from distal deltas, likely as plumes (1). Therefore, we suggest that the tridymite-bearing material was derived by erosion and transport from a silicic source area in a manner similar to Lake Tecocomulco. Unlike the Lake Tecocomulco analog, which has units of interstratified silicic volcanic ash (73), there are no known silicic volcanic rocks interstratified with Gale sedimentary rocks, so we appeal to crater rim and central peak regions as sources of tridymite-bearing material that may predate the Gale crater impact.
Recent studies have suggested that the Gale crater rim/wall may host igneous lithologies more evolved than other known martian igneous rocks, ranging up to ∼67 wt.% SiO2 (75). The Lake Gale basin would have captured sediments from these terrains. Mineralogical observations across Mars made by orbital remote sensing have also been interpreted as evidence for magma evolution to compositions more silicic than basaltic (76–78). The microclast in the ureilite meteorite EET 87720, which has a Buckskin-like mineralogy and composition, is also consistent with an evolved magma on a parent body that is possibly Mars (58).
The mixed-cation salts, minor oxides, and any precipitated silica in Buckskin amorphous material can be interpreted as cements precipitated during deposition and/or from later infiltrations by aqueous solutions having variable chemistries, temperatures, and acidities. The crystalline cation-deficient magnetite could also be present as a primary precipitate and cement, but there are insufficient constraints available to exclude igneous detritus or diagenesis of Fe-bearing materials. Anhydrite is interpreted as late fracture and vein fill as reported for other drill samples (8), implying its precipitation postdates lithification of the tridymite-bearing sedimentary rock.
The origin of the opal-A is not constrained. It could have formed as an alteration product of high-SiO2 volcanic glass, as a residue from acid sulfate leaching of silicates in the sediment source region, or as similar residue from leaching in the sediment itself. Another alternative is primary precipitation of silica gel in the Gale lake environment followed by diagenesis to opal-A and opal-CT after burial. CheMin would not be able to distinguish between silica gel and opal-A, however (SI Appendix, Fig. S6). At present, there are insufficient constraints to partition the high-SiO2 amorphous material among the aforementioned possible formation pathways, although silicic volcanism and diagenesis can account for all opal-A and opal-CT.
Implications for Martian Petrogenesis
The Buckskin tridymitic mudstone sample contains abundant SiO2 phases (∼55 wt.% of bulk sample) consisting of 14 wt.% tridymite, 2 wt.% cristobalite, 6 wt.% opal-CT, 33 wt.% opal-A and/or high-SiO2 glass, and no quartz. The monoclinic tridymite is well crystallized and not a mineraloid, such as opal with partial tridymite stacking, which might be attributed to a low-temperature origin. Because the martian crust is largely basaltic, we explored other options for the origin of Buckskin tridymite. Of all of the options, derivation from silicic volcanic rocks is the most parsimonious within available constraints, the most important being its high formation temperature (∼870 °C to ∼1,470 °C).
Although the general view of Mars is that of a basaltic planet, tridymite at Buckskin adds support to previous studies that describe regions of Mars where occurrences of high-SiO2 igneous rocks have been inferred (76–78). Furthermore, the Buckskin tridymitic mudstone is yet another indication of evolved volcanic compositions in sedimentary source regions of Gale crater (75). The unexpected discovery of tridymite at Gale crater is further evidence of the complexity of igneous petrogenesis on Mars, with igneous evolution to high-silica compositions.
Supplementary Material
Acknowledgments
We acknowledge the unwavering support of the JPL engineering and MSL operations staff. This research was supported by the NASA Mars Science Laboratory Mission. The MSL APXS was financed and managed by the Canadian Space Agency, with MacDonald Dettwiler & Assoc., Brampton, as prime subcontractor for the construction of the instrument. Operation of the MSL APXS is supported by CSA Contract 9F052-110786 and by NASA. Some of this research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. J.D.F. thanks the Danish Villum Foundation for support. S.P.S. acknowledges UK Space Agency funding.
Footnotes
The authors declare no conflict of interest.
Data deposition: CheMin and APXS experiment data records and CheMin diffraction patterns have been deposited with the NASA Planetary Data System at pds-geosciences.wustl.edu/msl/msl-m-chemin-4-rdr-v1/mslcmn_1xxx/ for the CheMin data and pds-geosciences.wustl.edu/msl/msl-m-apxs-4_5-rdr-v1/mslapx_1xxx/ for the APXS data.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607098113/-/DCSupplemental.
References
- 1.Grotzinger JP, et al. Deposition, exhumation, and paleoclimate of an ancient lake deposit, Gale crater, Mars. Science. 2015;350(6257):aac7575. doi: 10.1126/science.aac7575. [DOI] [PubMed] [Google Scholar]
- 2.Rampe EB, et al. Diagenesis in the Murray Formation, Gale Crater, Mars. Lunar Planet Sci. 2016;47:Abstr 2543. [Google Scholar]
- 3.Grotzinger JP, et al. Mars Science Laboratory mission and science investigation. Space Sci Rev. 2012;170(1-4):5–56. [Google Scholar]
- 4.Blake D, et al. Characterization and calibration of the CheMin mineralogical instrument on Mars Science Laboratory. Space Sci Rev. 2012;170(1):341–399. [Google Scholar]
- 5.Lafuente B, Downs RT, Yang H, Stone N. The power of databases: The RRUFF project. In: Armbruster T, Danisi RM, editors. Highlights in Mineralogical Crystallography. De Gruyter; Berlin: 2015. pp. 1–30. [Google Scholar]
- 6.Chipera SJ, Bish DL. FULLPAT: A full-pattern quantitative analysis program for x-ray powder diffraction using measured and calculated patterns. J Appl Cryst. 2002;35:744–749. [Google Scholar]
- 7.Bish DL, et al. MSL Science Team X-ray diffraction results from Mars Science Laboratory: Mineralogy of Rocknest at Gale crater. Science. 2013;341(6153):1238932. doi: 10.1126/science.1238932. [DOI] [PubMed] [Google Scholar]
- 8.Vaniman DT, et al. MSL Science Team Mineralogy of a mudstone at Yellowknife Bay, Gale crater, Mars. Science. 2014;343(6169):1243480. doi: 10.1126/science.1243480. [DOI] [PubMed] [Google Scholar]
- 9.Treiman AH, et al. Mineralogy, provenance, and diagenesis of a potassic basaltic sandstone on Mars: CheMin X-ray diffraction of the Windjana sample (Kimberley area, Gale Crater) J Geophys Res Planets. 2016;121(1):75–106. doi: 10.1002/2015JE004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graetsch H, Topalovic-Dierdorf I. 29Si MAS NMR spectrum and superstructure of modulated tridymite L3-To(MX-1) Eur J Mineral. 1996;8(1):103–113. [Google Scholar]
- 11.Elzea JM, Odom IE, Miles WJ. Distinguishing well ordered opal-CT and opal-C from high temperature cristobalite by X-ray diffraction. Anal Chim Acta. 1994;286(1):107–116. [Google Scholar]
- 12.Eversull LG, Ferrell RE. Disordered silica with tridymite-like structure in the Twiggs clay. Am Mineral. 2008;93(4):565–572. [Google Scholar]
- 13.Mason B. Tridymite and christensenite. Am Mineral. 1953;38(9-10):866–867. [Google Scholar]
- 14.Grant RW. New data on tridymite. Am Mineral. 1967;52(3-4):536. [Google Scholar]
- 15.Papike JJ, Karner JM, Shearer CK, Burger PV. Silicate mineralogy of martian meteorites. Geochim Cosmochim Acta. 2009;73(24):7443–7485. [Google Scholar]
- 16.Swamy V, Saxena SK, Sundman B, Zhang J. A thermodynamic assessment of silica phase diagram. J Geophys Res Solid Earth. 1994;99(B6):11787–11794. [Google Scholar]
- 17.Koike CR, et al. Infrared spectra of silica polymorphs and the conditions of their formation. Astrophys J. 2013;778(1):60. [Google Scholar]
- 18.Holmquist S. Conversion of quartz to tridymite. J Am Ceram Soc. 1961;44(2):82–86. [Google Scholar]
- 19.Sato M. X-ray study of tridymite (1) Mineral J. 1963;4(2):115–130. [Google Scholar]
- 20.Sato M. X-ray study of low tridymite (2) Mineral J. 1963;4(2):131–146. [Google Scholar]
- 21.Nukui A, Nakazawa H, Akao M. Thermal changes in monoclinic tridymite. Am Mineral. 1978;63(11-12):1252–1259. [Google Scholar]
- 22.Carpenter MA, Wennemer M. Characterization of synthetic tridymites by transmission electron microscopy. Am Mineral. 1985;70(5-6):517–528. [Google Scholar]
- 23.Garofalini SH, Miller AD. Kinetics of tridymite formation. J Cryst Growth. 1986;78(1):85–96. [Google Scholar]
- 24.Fabian D, Jäger C, Henning T, Dorschner J, Mutschke H. Steps toward interstellar silicate mineralogy. V. Thermal evolution of amorphous magnesium silicates and silica. Astron Astrophys. 2000;364:282–292. [Google Scholar]
- 25.Chen S, Jak E, Hayes PC. Phase equilibria in the cristobalite, tridymite and pyroxene primary phase fields in the MgO-”FeO”-SiO2 system in equilibrium with metallic iron. ISIJ Int. 2005;45(6):791–797. [Google Scholar]
- 26.Hirose T, Kihara K, Okuno M, Fujinami S, Shinoda K. X-ray, DTA and Raman studies of monoclinic tridymite and its higher temperature orthorhombic modification with varying temperature. J Mineral Petrol Sci. 2005;100(2):55–69. [Google Scholar]
- 27.Dapiaggi M, et al. The formation of silica high temperature polymorphs from quartz: Influence of grain size and mineralising agents. J Eur Ceram Soc. 2015;35(16):4547–4555. [Google Scholar]
- 28.Ross CS, Smith RL. 1961. Ash Flow Tuffs: Their Origin, Geologic Relations and Identification, USGS Professional Paper (US Geol Surv, Boulder, CO), Vol 366.
- 29.Keith TE, Muffler L. Minerals produced during cooling and hydrothermal alteration of ash flow tuff from Yellowstone drill hole Y-5. J Volcanol Geotherm Res. 1978;3(3):373–402. [Google Scholar]
- 30.Broxton DE, Heiken GH, Chipera S, Byers FM., Jr 1995. Stratigraphy, petrography, and mineralogy of Bandelier Tuff and Cerro Toledo deposits. Earth Science Investigations for Environmental Restoration—Los Alamos National Laboratory, Technical Area 21, eds Broxton DE, Eller GG (Los Alamos Natl Lab, Los Alamos, NM), Rep LA-12934-MS, pp 33−63.
- 31.Streck M, Grunder A. Crystallization and welding variations in a widespread ignimbrite sheet; the Rattlesnake Tuff, eastern Oregon, USA. Bull Volcanol. 1995;57(3):151–169. [Google Scholar]
- 32.Bargar KE, Keith TE. 1999. Hydrothermal Mineralogy of Core from Geothermal Drill Holes at Newberry Volcano, Oregon, USGS Professional Paper (US Geol Surv, Boulder, CO), Vol 1578.
- 33.Chigira M, Nakamoto M, Nakata E. Weathering mechanisms and their effects on the landsliding of ignimbrite subject to vapor-phase crystallization in the Shirakawa pyroclastic flow, northern Japan. Eng Geol. 2002;66(1):111–125. [Google Scholar]
- 34.Breitkreuz C. Spherulites and lithophysae—200 years of investigation on high-temperature crystallization domains in silica-rich volcanic rocks. Bull Volcanol. 2013;75(4):1–16. [Google Scholar]
- 35.Galan LDP, Doval M, La Iglesia A, Soriano J, Chavez L. Occurrence of silica polymorphs nanocrystals in tuffaceous rocks, Province of the Mesa Central, Mexico, and their formation from subcritical Si-rich fluids. Am Mineral. 2013;98(5-6):977–985. [Google Scholar]
- 36.Martin AM, et al. Fayalite oxidation processes in Obsidian Cliffs rhyolite flow, Oregon. Am Mineral. 2015;100(5-6):1153–1164. [Google Scholar]
- 37.Hamasaki S. Volcanic-related alteration and geochemistry of Iwodake volcano, Satsuma-Iwojima, Kyushu, SW Japan. Earth Planets Space. 2002;54(3):217–229. [Google Scholar]
- 38.Shinohara H, Kazahaya K, Saito G, Matsushima N, Kawanabe Y. Degassing activity from Iwodake rhyolitic cone, Satsuma-Iwojima volcano, Japan: Formation of a new degassing vent, 1990–1999. Earth Planets Space. 2002;54(3):175–185. [Google Scholar]
- 39.Kazahaya K, Shinohara H, Saito G. Degassing process of Satsuma-Iwojima volcano, Japan: Supply of volatile components from a deep magma chamber. Earth Planets Space. 2002;54(3):327–335. [Google Scholar]
- 40.Matsushima N, Kazahaya K, Saito G, Shinohara H. Mass and heat flux of volcanic gas discharging from the summit crater of Iwodake volcano, Satsuma-Iwojima, Japan, during 1996–1999. J Volcanol Geotherm Res. 2003;126(3):285–301. [Google Scholar]
- 41.Baxter PJ, et al. Cristobalite in volcanic ash of the Soufriere Hills volcano, Montserrat, British West Indies. Science. 1999;283(5405):1142–1145. doi: 10.1126/science.283.5405.1142. [DOI] [PubMed] [Google Scholar]
- 42.Del Moro S, Renzulli A, Tribaudino M. Pyrometamorphic processes at the magma–hydrothermal system interface of active volcanoes: Evidence from buchite ejecta of Stromboli (Aeolian Islands, Italy) J Petrol. 2011;52(3):541–564. [Google Scholar]
- 43.Getahun A, Reed MH, Symonds R. Mount St. Augustine volcano fumarole wall rock alteration: Mineralogy, zoning, composition and numerical models of its formation process. J Volcanol Geotherm Res. 1996;71(2):73–107. [Google Scholar]
- 44.Wager L, Weedon D, Vincent E. A granophyre from Coire Uaigneich, Isle of Skye, containing quartz paramorphs after tridymite. Mineral Mag. 1953;30(223):263–275. [Google Scholar]
- 45.Black GP. The significance of tridymite in igneous and metamorphic petrogenesis. Mineral Mag. 1954;30(227):518–524. [Google Scholar]
- 46.Holness MB. Spherulitic textures formed during crystallization of partially melted arkose, Rum, Scotland. Geol Mag. 2002;139(06):651–663. [Google Scholar]
- 47.Whitehead J, Grieve RAF, Spray JG. Mineralogy and petrology of melt rocks from the Popigai impact structure, Siberia. Meteorit Planet Sci. 2002;37(5):623–647. [Google Scholar]
- 48.Jackson JC, Horton JW, Chou I-M, Belkin HE. Monoclinic tridymite in clast-rich impact melt rock from the Chesapeake Bay impact structure. Am Mineral. 2011;96(1):81–88. [Google Scholar]
- 49.Schwenzer SP, et al. Gale Crater: Formation and post-impact hydrous environments. Planet Space Sci. 2012;70(1):84–95. [Google Scholar]
- 50.Bridges J, et al. Cristobalite- and tridymite-bearing clasts in Parnallee (LL3) and Farmington (L5) Meteoritics. 1995;30(6):715–727. [Google Scholar]
- 51.Xirouchakis D, Draper DS, Schwandt CS, Lanzirotti A. Crystallization conditions of Los Angeles, a basaltic Martian meteorite. Geochim Cosmochim Acta. 2002;66(10):1867–1880. [Google Scholar]
- 52.Treiman AH, Lanzirotti A, Xirouchakis D. Ancient water on asteroid 4 Vesta: Evidence from a quartz veinlet in the Serra de Magé eucrite meteorite. Earth Planet Sci Lett. 2004;219(3-4):189–199. [Google Scholar]
- 53.Imae N, Ikeda Y, Kojima H. Petrology of the Yamato nakhlites. Meteorit Planet Sci. 2005;40(11):1581–1598. [Google Scholar]
- 54.Hezel DC, Palme H, Nasdala L, Brenker FE. Origin of SiO2-rich components in ordinary chondrites. Geochim Cosmochim Acta. 2006;70(6):1548–1564. [Google Scholar]
- 55.Schrader DL, et al. Sulfide‐rich metallic impact melts from chondritic parent bodies. Meteorit Planet Sci. 2010;45(5):743–758. [Google Scholar]
- 56.Bonin B. Extra-terrestrial igneous granites and related rocks: A review of their occurrence and petrogenesis. Lithos. 2012;153:3–24. [Google Scholar]
- 57.Seddio SM, Korotev RL, Jolliff BL, Wang A. Silica polymorphs in lunar granite: Implications for granite petrogenesis on the Moon. Am Mineral. 2015;100(7):1533–1543. [Google Scholar]
- 58.Beard AD, Downes H, Chaussidon M. Petrology of a nonindigenous microgranitic clast in polymict ureilite EET 87720: Evidence for formation of evolved melt on an unknown parent body. Meteorit Planet Sci. 2015;50(9):1613–1623. [Google Scholar]
- 59.Leroux H, Cordier P. Magmatic cristobalite and quartz in the NWA 856 Martian meteorite. Meteorit Planet Sci. 2006;41(6):913–923. [Google Scholar]
- 60.Ren S, et al. The preparation of tridymite crystal by chemical processing. Phase Transit. 2008;81(5):395–402. [Google Scholar]
- 61.Herdianita NR, Browne PRL, Rodgers KA, Campbell KA. Mineralogical and textural changes accompanying ageing of silica sinter. Miner Deposita. 2000;35(1):48–62. [Google Scholar]
- 62.Campbell KA, Sannazzaro K, Rodgers K, Herdianita N, Browne P. Sedimentary facies and mineralogy of the late Pleistocene Umukuri silica sinter, Taupo Volcanic Zone, New Zealand. J Sediment Res. 2001;71(5):727–746. [Google Scholar]
- 63.Rodgers KA, et al. Silica phases in sinters and residues from geothermal fields of New Zealand. Earth Sci Rev. 2004;66(1-2):1–61. [Google Scholar]
- 64.Lynne BY, Campbell KA, Moore JN, Browne PRL. Diagenesis of 1900-year-old siliceous sinter (opal-A to quartz) at Opal Mound, Roosevelt Hot Springs, Utah, U.S.A. Sediment Geol. 2005;179(3-4):249–278. [Google Scholar]
- 65.Lynne BY, Campbell KA, Perry RS, Browne P, Moore J. Acceleration of sinter diagenesis in an active fumarole, Taupo Volcanic Zone, New Zealand. Geology. 2006;34(9):749–752. [Google Scholar]
- 66.Channing A, Butler IB. Cryogenic opal-A deposition from Yellowstone hot springs. Earth Planet Sci Lett. 2007;257(1):121–131. [Google Scholar]
- 67.Garcia-Valles M, Fernandez-Turiel J, Gimeno-Torrente D, Saavedra-Alonso J, Martinez-Manent S. Mineralogical characterization of silica sinters from the El Tatio geothermal field, Chile. Am Mineral. 2008;93(8-9):1373–1383. [Google Scholar]
- 68.Preston LJ, Benedix GK, Genge MJ, Sephton MA. A multidisciplinary study of silica sinter deposits with applications to silica identification and detection of fossil life on Mars. Icarus. 2008;198(2):331–350. [Google Scholar]
- 69.Rodgers KA, Cook KL, Browne PRL, Campbell KA. The mineralogy, texture and significance of silica derived from alteration by steam condensate in three New Zealand geothermal fields. Clay Miner. 2002;37(2):299–322. [Google Scholar]
- 70.Saishu H, Okamoto A, Tsuchiya N. Mineralogical variation of silica induced by Al and Na in hydrothermal solutions. Am Mineral. 2012;97(11-12):2060–2063. [Google Scholar]
- 71.Ramseyer K, et al. Primary silica precipitate at the Precambrian/Cambrian boundary in the south Oman salt basin, sultanate of Oman. Mar Pet Geol. 2013;39(1):187–197. [Google Scholar]
- 72.Al Rajaibi IM, Hollis C, Macquaker JH. Origin and variability of a terminal Proterozoic primary silica precipitate, Athel Silicilyte, South Oman Salt Basin, Sultanate of Oman. Sedimentology. 2015;62(3):793–825. [Google Scholar]
- 73.Roy PD, Caballero M, Lozano R, Pi T, Morton O. Late Pleistocene-Holocene geochemical history inferred from Lake Tecocomulco sediments, Basin of Mexico, Mexico. Geochem J. 2009;43(1):49–64. [Google Scholar]
- 74.Szabó T, Domokos G, Grotzinger JP, Jerolmack DJ. Reconstructing the transport history of pebbles on Mars. Nat Commun. 2015;6:8366. doi: 10.1038/ncomms9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sautter V, et al. In situ evidence for continental crust on early Mars. Nat Geosci. 2015;8:605–609. [Google Scholar]
- 76.Christensen PR, et al. Evidence for magmatic evolution and diversity on Mars from infrared observations. Nature. 2005;436(7050):504–509. doi: 10.1038/nature03639. [DOI] [PubMed] [Google Scholar]
- 77.Smith MR, Bandfield JL. Geology of quartz and hydrated silica-bearing deposits near Antoniadi Crater, Mars. J Geophys Res Planets. 2012;117(E6):E06007. [Google Scholar]
- 78.Wray JJ, et al. Prolonged magmatic activity on Mars inferred from the detection of felsic rocks. Nat Geosci. 2013;6(12):1013–1017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.