Significance
Programmed replication fork arrest (PFA) at specific terminator sites and the proteins that bind to these sites functionally interconnect replication, transcription, and recombination. PFA prevents collision between replication and transcription that can cause genome instability, promotes intrachromatid recombination at ribosomal (r)DNA that controls replicative life span, and maintains rDNA homeostasis. This work reveals the mechanism of PFA by showing that the Tof1 protein of budding yeast remains associated with the replication fork only when it is phosphorylated, and uses the CMG helicase that drives the replication fork as a landing pad. Tof1–Csm3 promotes PFA by preventing the terminator protein Fob1 from getting displaced by other factors such as the Rrm3 helicase by counteracting the latter. This important mechanism appears to be evolutionarily conserved.
Keywords: DDK, Ter, Fob1, Tof1, programmed fork arrest
Abstract
Several important physiological transactions, including control of replicative life span (RLS), prevention of collision between replication and transcription, and cellular differentiation, require programmed replication fork arrest (PFA). However, a general mechanism of PFA has remained elusive. We previously showed that the Tof1–Csm3 fork protection complex is essential for PFA by antagonizing the Rrm3 helicase that displaces nonhistone protein barriers that impede fork progression. Here we show that mutations of Dbf4-dependent kinase (DDK) of Saccharomyces cerevisiae, but not other DNA replication factors, greatly reduced PFA at replication fork barriers in the spacer regions of the ribosomal DNA array. A key target of DDK is the mini chromosome maintenance (Mcm) 2–7 complex, which is known to require phosphorylation by DDK to form an active CMG [Cdc45 (cell division cycle gene 45), Mcm2–7, GINS (Go, Ichi, Ni, and San)] helicase. In vivo experiments showed that mutational inactivation of DDK caused release of Tof1 from the chromatin fractions. In vitro binding experiments confirmed that CMG and/or Mcm2–7 had to be phosphorylated for binding to phospho-Tof1–Csm3 but not to its dephosphorylated form. Suppressor mutations that bypass the requirement for Mcm2–7 phosphorylation by DDK restored PFA in the absence of the kinase. Retention of Tof1 in the chromatin fraction and PFA in vivo was promoted by the suppressor mcm5-bob1, which bypassed DDK requirement, indicating that under this condition a kinase other than DDK catalyzed the phosphorylation of Tof1. We propose that phosphorylation regulates the recruitment and retention of Tof1–Csm3 by the replisome and that this complex antagonizes the Rrm3 helicase, thereby promoting PFA, by preserving the integrity of the Fob1–Ter complex.
Replication fork arrest can either occur at random sites (1) or, in some cases, is physiologically programmed to occur at specific sequences such as the Ter sites of Escherichia coli and Saccharomyces cerevisiae (also called replication fork barriers; RFBs), which bind to specific terminator proteins. The proteins Tus and Fob1 bind to the Ter sites of E. coli and S. cerevisiae, respectively, and cause polar replication fork arrest (2, 3) (reviewed in ref. 4). The arrested fork, with the help of DNA polymerase, helicase, and topoisomerase (5), merges with the fork approaching Ter from the opposite direction, and the resulting catenated daughter molecules are resolved by topoisomerase IV in bacteria (6) and topoisomerase II in eukaryotes (7, 8). In budding yeast, the unloading of the replisomal components from the template after replication termination is facilitated by Cdc48 and a ubiquitin ligase (9).
Eukaryotic cells of diverse types arrest and prevent replication fork entry into the highly transcribed ribosomal (r)DNA locus (reviewed in ref. 4). In budding yeast, a single replication terminator protein called Fob1 binds to the Ter sites (RFBs) located in nontranscribed spacer 1 (NTS1) of rDNA to cause polar fork arrest (10, 11), which prevents transcription–replication collision, fork stalling, and genome instability. Fission yeast has three known terminator proteins: Rtf1, which functions at the mating-type switch locus (12, 13), and Reb1 (Sp. Reb1) and Sap1, which act at the Ter sites of rDNA spacers (14–18). Sp. Reb1 also functions at Ter sites located in the other two chromosomes and promotes “chromosome kissing” (19).
Several studies have shown that a complex of three proteins (Tof1, Csm3, and Mrc1) called the fork protection complex (FPC) acts at replication forks to ensure normal fork progression and stabilization of forks stalled at nonhistone protein barriers and at sites of DNA damage (20). We and others have shown that two members of the heterotrimeric FPC are needed for stable fork arrest at Ter sites and at other nonhistone protein barriers. All eukaryotic cells contain homologs of these two proteins, called Tof1 and Csm3 of budding yeast (21–23), Swi1 and Swi3 of fission yeast (17, 24), and TIM and TIPIN of mammalian cells (25, 26). The third member of the complex, called Mrc1 and claspin in budding and fission yeast and mammalian cells, respectively, is dispensable for maintenance of programmed replication fork arrest (PFA) (22, 23). The FPC binds to mini chromosome maintenance (Mcm)2-7 and inhibits its DNA-dependent ATPase activity and also the helicase activity of the CMG complex (27). We have previously reported that Tof1 and Csm3 promote stable fork arrest by antagonizing the Rrm3 helicase (23, 28), which removes nonhistone protein barriers from in front of moving forks throughout the budding yeast genome (29).
PFA promotes several important physiological functions. In E. coli, two sets of Ter sites of opposite polarity are located at the antipode (with respect to the origin) of the circular chromosome, forming a replication trap. Fork convergence occurs within this trap, at or near the dimer resolution and segregation sites (called dif) that are necessary for reduction of the daughter chromosomes to monomers and their proper separation preceding cell division. Ter sites also prevent a topological shift from a Cairns-type to a rolling-circle replication, thereby contributing to genome stability (reviewed in ref. 4). In eukaryotes, PFA is required for (i) prevention of collision between a replication fork and an actively transcribing RNA polymerase approaching from the opposite direction, thereby preventing genome instability (30–33), (ii) promoting genetic imprinting and cellular differentiation in fission yeast (12), and (iii) controlling replicative life span in budding yeast (34, 35).
The two cell-cycle kinases called cyclin-dependent kinase (CDK) and Dbf4-dependent kinase (DDK) are required for replication initiation (36–39). CDK (40) but not DDK (41, 42) is involved in regulating the S-phase checkpoint. Replication initiation is a two-step process that involves the assembly of a pre-replication complex (RC) through recruitment of Mcm2–7 by an origin-bound Orc1–6 complex with the help of the Cdt1 and Cdc6 proteins. The inactive Mcm2–7 is then phosphorylated by DDK, which initiates a multistep assembly process involving the heterotetrameric GINS (Go, Ichi, Ni, and San) complex (43) and Cdc45 (cell division cycle gene 45) (44, 45) to generate the CMG complex, which has vigorous helicase activity (46–48). CDK and DDK also restrict DNA replication to only once per cell cycle (49).
In budding yeast, the requirement for DDK can be bypassed by a suppressing point mutation called mcm5-bob1 (50). The N-terminal region of Mcm4 contains an inhibitory domain that is neutralized by DDK-catalyzed phosphorylation. Consequently, deletion of this domain bypasses the need for DDK for CMG assembly (51–53). Recently, it was shown that RIF1, which controls the timing of origin firing in S. cerevisiae and recruitment of the PP1 phosphatase, causes dephosphorylation of the residues of Mcm2–7 that are phosphorylated by DDK. Deletion of RIF1 suppresses the cdc7-1 mutation and allows growth to occur at the semipermissive temperature of 30 °C (54).
The present work shows that in vitro phosphorylation of not only Tof1 but also Mcm2–7 by DDK is essential for recruitment/retention of Tof1 at the replisome, which is obligatory for PFA. Consistent with this observation, mutational inactivation of DDK in vivo caused either the release of Tof1 or its failure to be retained in the chromatin fractions. The apparent need for DDK to cause PFA was bypassed by suppressor mutations that bypass Mcm2–7/CMG function. Together, these results indicate that in addition to the known role of DDK in CMG assembly and helicase activation, it also contributes to the assembly of the CMG platform that recruits phosphorylated but not dephosphorylated Tof1 to the replisome. Together with our earlier results that Tof1–Csm3 antagonizes the Rrm3 helicase, which displaces Fob1 from Ter sites, we propose that phosphorylation of Tof1 and Mcm2–7 promotes stable association of the FPC with the replisome and is needed for functional PFA.
Results
Inactivation of DDK Caused Significant Reduction of PFA.
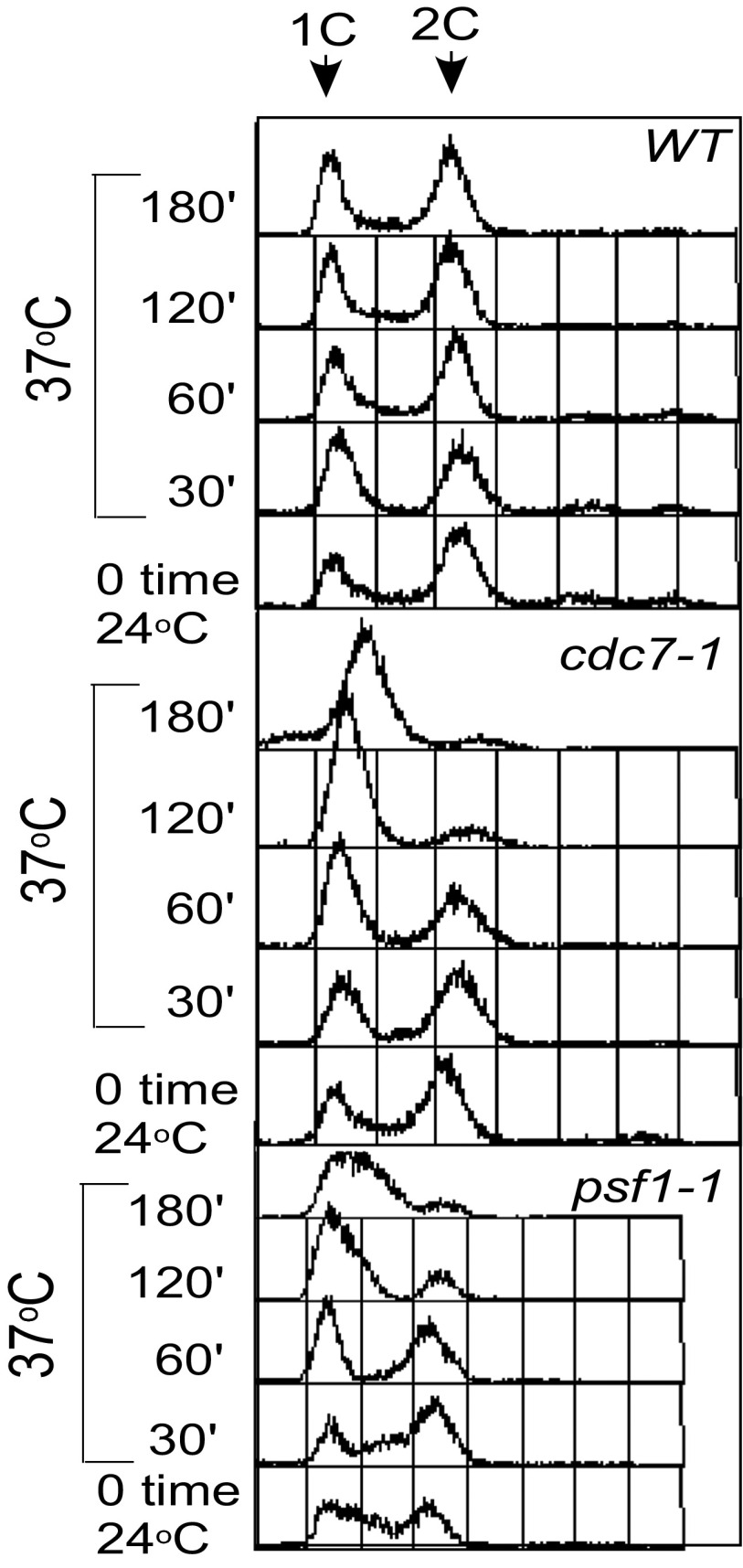
Does DDK, besides its roles in replication initiation, limiting replication to once per cell cycle, and checkpoint control, also regulate PFA? We addressed this question by investigating replication fork arrest at the Ter site of the rDNA of budding yeast in temperature-sensitive mutants of DDK called cdc7-1 and dbf4-1 of its two subunits at the permissive (24 °C) and nonpermissive temperatures (37 °C). We used Brewer–Fangman 2D gel electrophoresis (55), as modified (23), to follow fork progression in the spacer regions of rDNA. Our stratagem was to let initiation occur at 24 °C and then allow fork progression at 37 °C, which should prevent any new origins from firing. Asynchronous cultures were grown at 24 °C to midlog phase and then shifted to 37 °C for various time periods (1–2 h) and the cells were harvested. This approach, in our hands, resulted in higher yields of scarce replication intermediates, particularly from the mutant cells. Control experiments were similarly performed at 24 °C but without a shift to 37 °C. FACS analyses of the asynchronous cultures used for the experiments are shown (Fig. S1). It should be noted that although all of the 2D gels showed Y-shaped arc (Y-arc), the pattern corresponding to bubble arc was not observed because the experiments were not designed to capture and display the early replication intermediates. Fig. 1A shows the topography of NTS1 containing a single ars and tandem Ter sites present in each rDNA repeat. Replication fork arrest at the twin Ter sites, as revealed by five independent sets of 2D gels, as expected showed normal fork arrest at Ter in WT cells at both temperatures (Fig. 1B), but there was a significant reduction in the relative intensity of the Ter spots at the nonpermissive temperature (Fig. 1 C and D) in cdc7-1 and dbf4-1 cells, as determined by quantification of the images using the NIH ImageJ program. We concluded from these experiments that temperature-dependent inactivation of either the cdc7-1 or dbf4-1 component of DDK significantly reduced the magnitude of PFA at the Ter sites. Is unhindered fork progression through a protein barrier a general property of temperature-sensitive (ts) mutations at the nonpermissive temperature in several replication proteins, or is it specific to DDK? Is this caused by direct action of DDK on replication termination, or is it an indirect and relatively trivial consequence of changes in the rate of fork progression as a result of loss of DDK activity that might have caused fewer forks to reach the Ter sites? Was this possibly caused by an increase in the rate of fork progression that somehow overpowered the Fob1–Ter barriers? Alternatively, could this have been caused by the displacement of Fob1 from Ter sites precipitated by the loss of DDK activity? These questions have been systematically addressed below.
Fig. S1.
FACS analyses of the strains used for 2D gel analyses of replication fork progression in nonsynchronous cultures. The cells were held at 37 °C for 3 h before release at the permissive (24 °C) temperature.
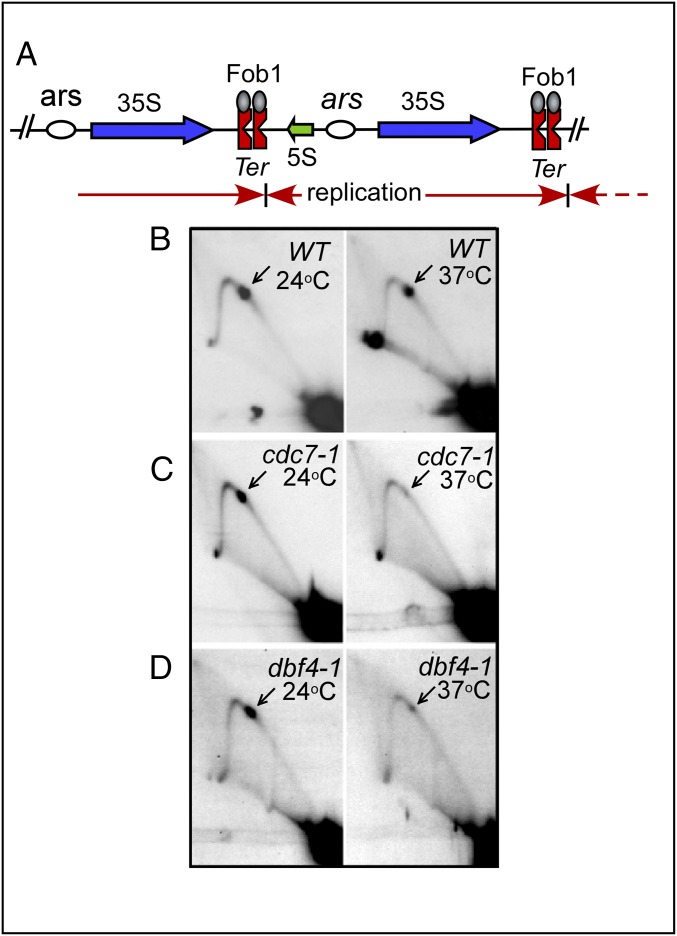
Fig. 1.
Replication termination in the WT and cells with temperature-sensitive mutants of DDK. (A) Schematic diagram of the rDNA repeats showing the locations of the Ter (RFBs) and ars sites. Blue arrows show the direction of rRNA transcripts; red arrows show the direction of replication fork progression. (B–D) Images of Brewer–Fangman 2D gels showing replication fork progression (Y-arc) and the fork arrest at the twin Ter sites (arrows). The cells were grown for 4 h at 24 °C to reach early log phase, transferred to 37 °C for 2–3 h, and then rapidly chilled and treated with Na azide as described in the text and replication intermediates were prepared. Note that whereas fork arrest occurred in the WT cells at both temperatures, it was greatly reduced in the cdc7-1 and dbf4-1 cells at 37 °C.
Abolition of PFA Is Not a General Property of Several Mutant Forms of Proteins Known to Be Defective in Replication Initiation.
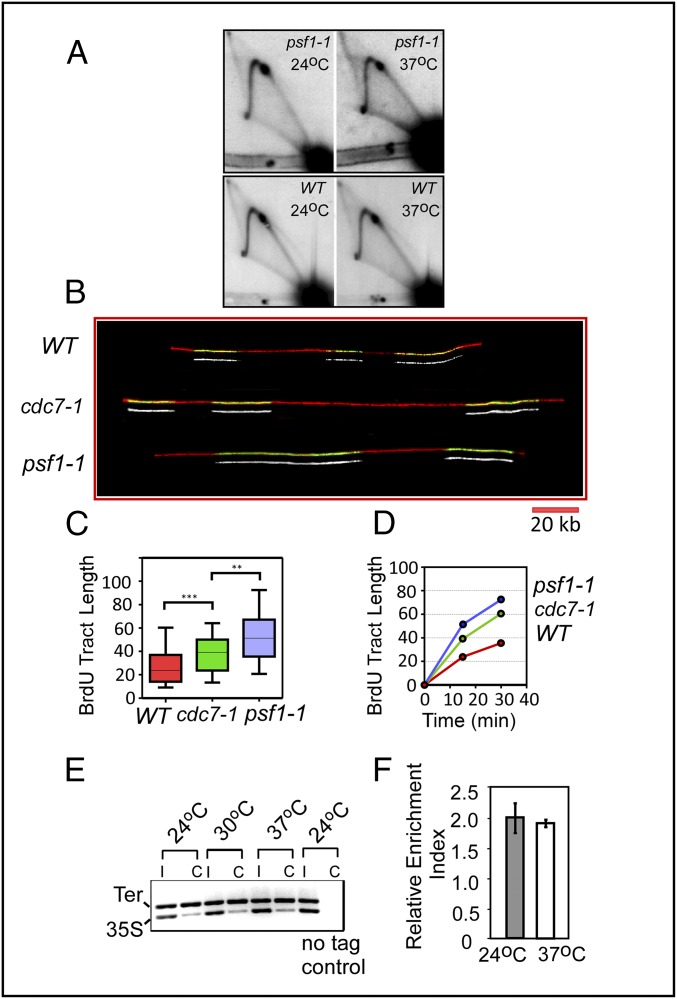
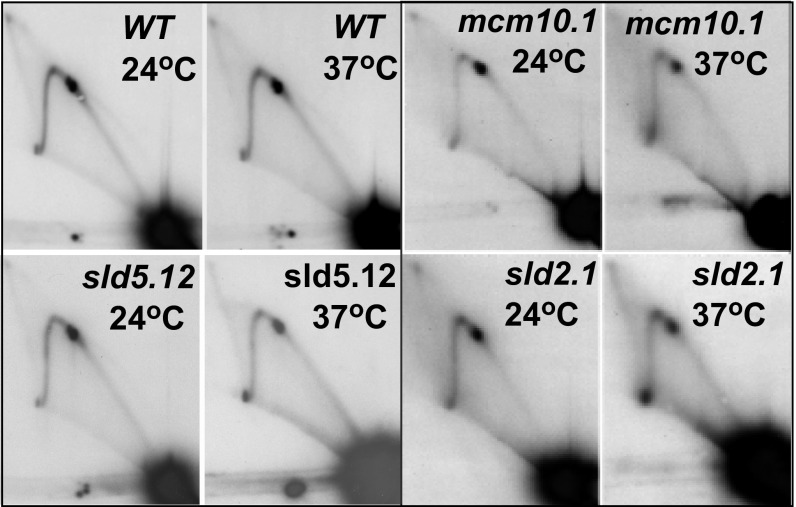
We investigated whether the loss of fork arrest upon inactivation of DDK was a specific or general defect caused by reduction in the rates of initiation and ongoing replication by ts mutations in other replication proteins. Two-dimensional gel analyses of fork movement using several ts mutants in proteins known to be involved in initiation (e.g., psf1-1, psf2-1, sld2-2, sld5-12, and mcm10-1) showed that PFA at Ter sites in these mutants was not significantly diminished at 37 °C in comparison with that of the WT cells (Fig. 2A and Fig. S2). As an example, the psf-1 mutant is known to be nonleaky (43). Published 2D gel analyses of its replication intermediates clearly show significant reduction of the bubble arc in 2D gels in comparison with that of the WT, clearly indicative of loss of replication initiation at 37 °C (43). Therefore, loss of PFA is not an inevitable consequence of a loss of initiation function.
Fig. 2.
DNA combing to measure the rate of replication fork progression in the WT and cdc7-1 and psf1-1 mutants at 37 °C and ChIP analysis of Fob1 occupancy at Ter. (A) Two-dimensional gel analysis of psf1-1 DNA. (B) Representative images of the DNA pulse-labeled with BrdU and combed, showing the extent of replication during the pulse period in WT, cdc7-1, and psf1-1 cells. (C and D) Data analysis of BrdU tract length in WT, cdc7-1, and psf1-1 cells. The cells were grown overnight at 24 °C and shifted to 37 °C for 15 or 30 min in the presence of 200 µg/mL BrdU. DNA fibers were stretched on silanized coverslips by DNA combing and BrdU incorporation was visualized by immunofluorescence. DNA fibers from WT, cdc7-1, and psf1-1 cells after 15 min of BrdU pulse at 37 °C. Green, anti-BrdU; red, anti-ssDNA. (Scale bar, 20 kb.) The bottom track of each of the 6 pairs of tracks in Fig. 2B show BrdU green channel. Distribution of BrdU tract length in WT, cdc7-1, and psf1-1 cells after a 15-min BrdU pulse at 37 °C. Boxes represent 25th to 75th percentiles; horizontal lines in the boxes represent median lengths; whiskers span 10th to 90th percentiles. **P < 0.01; ***P < 0.001. Median BrdU tract length in WT, cdc7-1, and psf1-1 cells after 15 and 30 min at 37 °C (Table 1). (E and F) ChIP analyses showing that Fob1 occupies the Ter sites in the cdc7-1 cells at both the permissive (24 °C) and nonpermissive (37 °C) temperatures. The DNA segment from the transcribed region of 35S rRNA was used as an internal (loading) control. Error bars represent standard error.
Fig. S2.
Replication fork progression. PFA in WT and temperature-sensitive mutant strains at the twin Ter sites located in intergenic spacer 1 of S. cerevisiae at 24 °C and 37 °C.
DNA Combing Showed No Correspondence Between Replication Fork Arrest and the Relative Differences in Fork Movement.
To investigate whether a relative difference in the rate of fork progression in the two mutants compared with that of WT cells could cause PFA or its abolition, we performed DNA combing experiments with the WT, cdc7-1, and psf1-1 cells at the nonpermissive temperature (Fig. 2B). The cells of the three genotypes were first grown at 24 °C, shifted to 37 °C, and pulse-labeled with BrdU. The DNA was gently extracted to minimize hydrodynamic shear and spread on silanized coverslips and visualized by antibodies against BrdU (green) and single-stranded DNA (red). Several hundred molecules were recorded under a fluorescence microscope, tract lengths were measured, statistical analyses were performed (Table 1), and the data were plotted as described in Materials and Methods and the legend to Fig. 2. The relative differences in the rates of fork movement were small but consistent, with the magnitudes as follows: psf1-1 > cdc7-1 > WT (Fig. 2 B–D). Keeping in mind that fork arrest at Ter occurred in psf1-1 and WT cells but not in cdc7-1 at 37 °C, the data suggested that there was no detectable correlation between the relative rate of fork progression and fork arrest at Ter. It should be noted that the psf1 and WT tracts are longer in the DNA combing profile because initiation was blocked in cdc7-1 and psf1-1 cells, and those that initiated, presumably because of less competition for existing initiation factors, cause somewhat faster fork movement (56).
Table 1.
Mean BrdU tract length (kilobases)
| Time, min | WT | cdc7-1 | psf1-1 |
| 0 | 0 | 0 | 0 |
| 15 | 28.45 | 40.21 | 53.52 |
| 30 | 46.19 | 62.16 | 79.92 |
Failure to Arrest Forks in cdc7-1 Cells at 37 °C Was Not Due to Displacement and Loss of Fob1 from the Ter Sites.
We wished to investigate whether the absence of DDK activity caused Fob1 to dissociate from the Ter sites by measuring the magnitudes of Fob1 occupancy at Ter in the cdc7-1 strains at 24 °C, 30 °C, and 37 °C for 3 h. ChIP analyses of Fob1 binding to Ter in vivo were performed using a segment of DNA from the 35S transcribing region as the control. The data showed that Fob1 remained bound to Ter sites in cdc7-1 cells at all temperatures tested (Fig. 2 E and F).
Key Role for DDK-Catalyzed Phosphorylation in PFA.
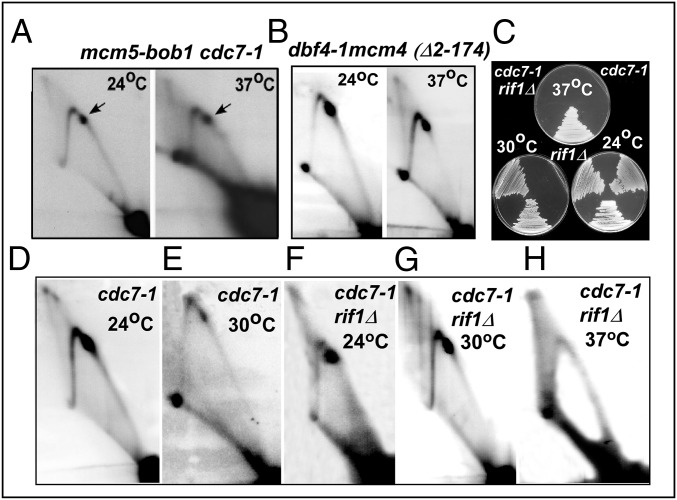
It is known that the requirement for DDK-catalyzed phosphorylation of Mcm2–7 for cell survival can be bypassed by several different suppressor mutations. For example, cdc7-1 or a cdc7Δ deletion can be suppressed by the mcm5-bob1 suppressor, which maintains cell viability in the absence of DDK (50). We compared and contrasted PFA at the Ter site of rDNA in strains containing cdc7-1 mcm5-bob1 and cdc7Δ mcm5-bob1 and the WT control. The experiments were repeated twice. The images of the 2D gels showed that PFA was restored in cdc7-1 mcm5 bob1 and cdc7Δ mcm5-bob1 cells at 37 °C (Figs. 3A and 4 A, iii) as contrasted with the cdc7-1 single mutant, which failed to arrest forks at Ter at 37 °C (Fig. 1C). The results were consistent with the conclusion that (i) DDK was dispensable for fork arrest in the presence of mcm5-bob1 and (ii) alteration in the Mcm2–7 structure caused by the suppressors controlled PFA, probably by assembling CMG independent of DDK. Additional pathways of suppression of DDK and their effect on fork arrest are described below. It should be noted that although in some of the 2D gels the sizes of the Y-arcs seem to be different, these are caused by image processing and there are no real differences.
Fig. 3.
PFA in various suppressors of cdc7-1 or dbf4-1 at permissive (24 °C), semipermissive (30 °C), and nonpermissive temperatures (37 °C). (A and B) Two-dimensional gel analysis of (A) mcm5-bob1 cdc7-1 cells at 24 °C and 37 °C and (B) dbf4-1 mcm4 (Δ2–174) cells at 24 °C and 37 °C. (C) Growth patterns of cdc7-1 rif1Δ cells at three different temperatures. (D–H) Two-dimensional gel analysis of fork movement in cdc7-1 cells at (D) 24 °C and (E) 30 °C and fork movement and PFA of cdc7-1rif1Δ at (F) 24 °C, (G) 30 °C, and (H) 37 °C.
Fig. 4.
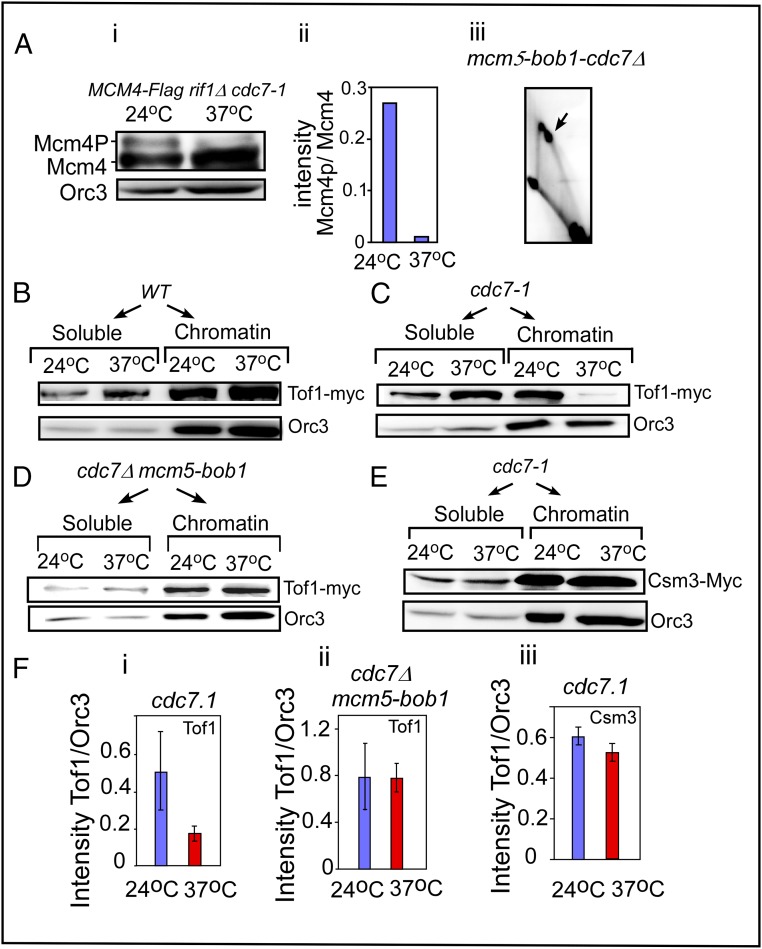
Western blots showing Tof1 retention in the chromatin fraction in cells with the designated genotypes at 24 °C and 37 °C. (A, i) Phosphorylation of Mcm4 in cdc7-1 at 24 °C and 37 °C. (A, ii) Quantification of Mcm4 phosphorylation. (A, iii) Two-dimensional gel showing that PFA occurs in mcm5-bob1 cdc7Δ cells at 37 °C. (B) WBs showing the relative proportions of Tof1-myc present in the soluble vs. chromatin fractions of WT cells at 24 °C and 37 °C; Orc3 was used as a loading control. (C) The same as B except that cdc7-1 cells were used. (D) The same as C except that cdc7Δ mcm5-bob1 cells were used. (E) Relative distribution of Csm3-myc in the soluble vs. chromatin fractions of cdc7-1 cells. (F) Quantification of the WBs (replicated four to seven times) and the data plotted with SE bars. (F, i) Tof1 in chromatin fractions of cdc7-1 at 24 °C and 37 °C. (F, ii) Same as i except that cdc7-1 mcm5-bob1 cells were used. (F, iii) Retention of Csm3-myc in the chromatin fractions at the two temperatures in cdc7-1 cells.
Deletion of the Inhibitory Domain in the N-Terminal Region of Mcm4 Restored Fork Arrest in DDK ts Mutants at 37 °C.
The N-terminal region of Mcm4 contains an anchoring domain for Dbf4 recruitment, and residues 2–174 of Mcm4 also contain an inhibitory domain (52). Because the main function of Mcm4 phosphorylation by DDK is believed to be neutralization of the inhibitory domain of Mcm4, a ts dbf4-1 mutation, as expected, is suppressed by the N-terminal deletion (Δ2–174) of Mcm4 (51–53). If PFA is controlled by DDK through phosphorylation of Mcm4, required to form the CMG complex, it should be manifested in stable fork arrest in a dbf4-1 mcm4 (Δ2–274) double mutant at both 24 °C and 37 °C for 3 h. Two-dimensional gel analysis confirmed this prediction by showing that, whereas the single mutant dbf4-1 showed significantly reduced fork arrest at 37 °C (Fig. 1D), a dbf4-1 mcm4 (Δ2–174) double mutant showed no defect in replication fork arrest at 37 °C (Fig. 3B). Thus, the results provided additional support for the conclusion that although Mcm2–7 phosphorylation by DDK was required for stable fork arrest, the N-terminal deletion of Mcm4 facilitated PFA, most likely by permitting formation of active CMG complex in the absence of DDK activity.
Deletion of Rif1 Suppressed the cdc7-1 Mutation at 30 °C and Restored PFA at the Semipermissive Temperature.
It was recently reported that Rif1 can erase Mcm2–7 phosphorylation by recruiting the protein phosphatase PP1 to the replisome. Therefore, rif1Δ suppressed a cdc7-1 mutation at 30 °C and restored cell growth at that temperature (54, 57, 58). To investigate whether there was a correspondence between suppression of DDK mutants by rif1Δ and restoration of PFA, we first performed growth analysis and confirmed that rif1Δ could suppress a cdc7-1 mutation at 30 °C but not at 37 °C (Fig. 3C). We took this result to mean that whereas a moderate defect in cdc7-1 observed at 30 °C was suppressible by rif1Δ, a more severe phenotype manifested at 37 °C was not suppressed. We then performed 2D gel analyses of replication intermediates of a rif1Δ cdc7-1 double mutant and the cdc7-1 single mutant at all three temperatures and observed fork arrest in the double mutant at 24 °C and 30 °C but not at 37 °C for 3 h (Fig. 3 F–H). In contrast, similar analysis using a cdc7-1 single mutant revealed, as expected, a clear reduction in fork arrest at both 30 °C (Fig. 3 D and E) and 37 °C (Fig. 1C). Together, the data from three separate suppressors of DDK mutants support the conclusion that phosphorylated Mcm2–7, but not its dephosphorylated form, plays a critical role in promoting PFA, and that the requirement for Mcm2–7 phosphorylation is dispensable in mutants that can initiate replication in the absence of DDK.
DDK-Catalyzed Phosphorylation of the Mcm2–7 Complex Was Necessary for the Retention of Tof1 at the Replisome.
How does DDK-dependent CMG (Mcm2–7) phosphorylation control fork arrest at Ter? We previously reported that Tof1 and Csm3, but not Mrc1, of the FPC are necessary for stable fork arrest at Ter sites and protein barriers elsewhere in the chromosome and that Tof1–Csm3 protects the arrested fork by counteracting the Rrm3 helicase, which evicts Fob1 protein from the Ter sites in the absence of either Tof1 or Csm3 (23, 28). We hypothesized that a phosphorylated Mcm2–7 complex was necessary for the recruitment and/or retention of Tof1 at the replisome and that inactivation of DDK by a ts mutation at the nonpermissive temperature was predicted to evict Tof1 from, or fail to recruit it to, the replisome. We expected this to cause unhindered fork passage not only through the Fob1–Ter complexes but also past many other DNA–protein barriers. Furthermore, we expected a suppressor such as mcm5-bob1 to restore PFA, presumably by blocking removal of Tof1 from the replisome at 37 °C. We tested these predictions as described below.
First, we wished to determine whether Mcm4 phosphorylation was reduced by inactivation of DDK in vivo. We performed Western blots (WBs) using FLAG-tagged Mcm4 in cdc7-1 rif1Δ to monitor dephosphorylation of Mcm4 by gel electrophoresis at 24 °C and 37 °C. We used a rif1Δ strain to enhance the yield of phosphorylated Mcm4 for easier detection, and the data showed that inactivation of cdc7-1 at 37 °C caused a significant reduction in the ratio of the dephosphorylated to the phosphorylated form of Mcm4 (Fig. 4 A, i and ii).
To follow the degrees of retention of Tof1 protein in the nuclear vs. cytoplasmic fractions in the presence and absence of active DDK, we synchronized the cells of the different genotypes by growing them at 24 °C until reaching log phase and then arrested the cells in G1 phase with α-factor, released the block by proteolyzing the α-factor, and allowed the cells to grow for an additional 30 min at either 24 °C or 37 °C. Spheroplasts were prepared and fractionated into soluble and chromatin fractions, and the presence or absence of Tof1-myc in each fraction was measured by WBs using anti-myc antibodies. Measurement of Orc3 from the same fractions, using anti-Orc3 antibodies, was used as an internal control. In WT cells, Tof1 was associated with the chromatin fraction at both 24 °C and 37 °C (Fig. 4B). By contrast, in cdc7-1 cells, we consistently observed, in six replicates of the experiment, that Tof1 remained chromatin-bound at 24 °C but was greatly reduced in the chromatin fractions at 37 °C (Fig. 4 C and F, i).
Similar experiments were performed in a cdc7Δ mcm5-bob1 strain. As predicted, Tof1-myc was retained in the chromatin fraction even after incubation at 37 °C (Fig. 4 D and F, ii). Surprisingly, in the congenic cdc7-1 strain a myc-tagged Csm3 was retained in the chromatin fraction (Fig. 4 E and F, iii). We propose that trapping of Csm3 by secondary interactions with other proteins, for example with the replication protein A [single-strand DNA-binding protein (59)], might have prevented its release from the chromatin fraction. In the presence of the mcm5-bob1 suppressor, these cells grew without DDK and also displayed replication fork arrest at Ter at both 24 °C and 37 °C (Figs. 3A and 4 A, iii). The data suggested that direct interaction between DDK and Tof1 was not obligatory for PFA when Mcm2–7 was activated in the suppressor mutant. We therefore investigated why Tof1 was not recruited or retained in the chromatin fraction upon inactivation of DDK in the biochemical experiments described below.
Protein–Protein Interaction Between Tof1 and the Replisome.
We wished to test the hypothesis that, in addition to the known role of phosphorylation of Mcm2–7 by DDK for CMG assembly, Mcm2–7 phosphorylation also contributes to the function of CMG as a landing pad for Tof1 recruitment. We performed in vitro protein–protein interaction experiments using phosphorylated and dephosphorylated CMG and Tof1–Csm3, and also examined the Mcm2–7 complex (both the phosphorylated and dephosphorylated forms) and separately Cdc45 and GINS for binding to Tof1–Csm3. The purity of the various proteins used was determined by Coomassie blue-stained SDS/PAGE profiles (Fig. S3). The experiments provided two significant insights into the regulation of PFA: (i) Tof1 bound to the phosphorylated CMG and Mcm2–7 but not to their dephosphorylated forms or to Cdc45 or GINS, and (ii) only phospho-Tof1 was competent to interact with CMG and Mcm2–7. The evidence for these conclusions is presented below.
Fig. S3.
Coomassie blue-stained SDS/PAGE patterns of the purified proteins used in this work. A–E show Tof1-Csm3, CMG complex, Mcm2–7, Cdc45, and the GINS complex, respectively, purified as described in the text.
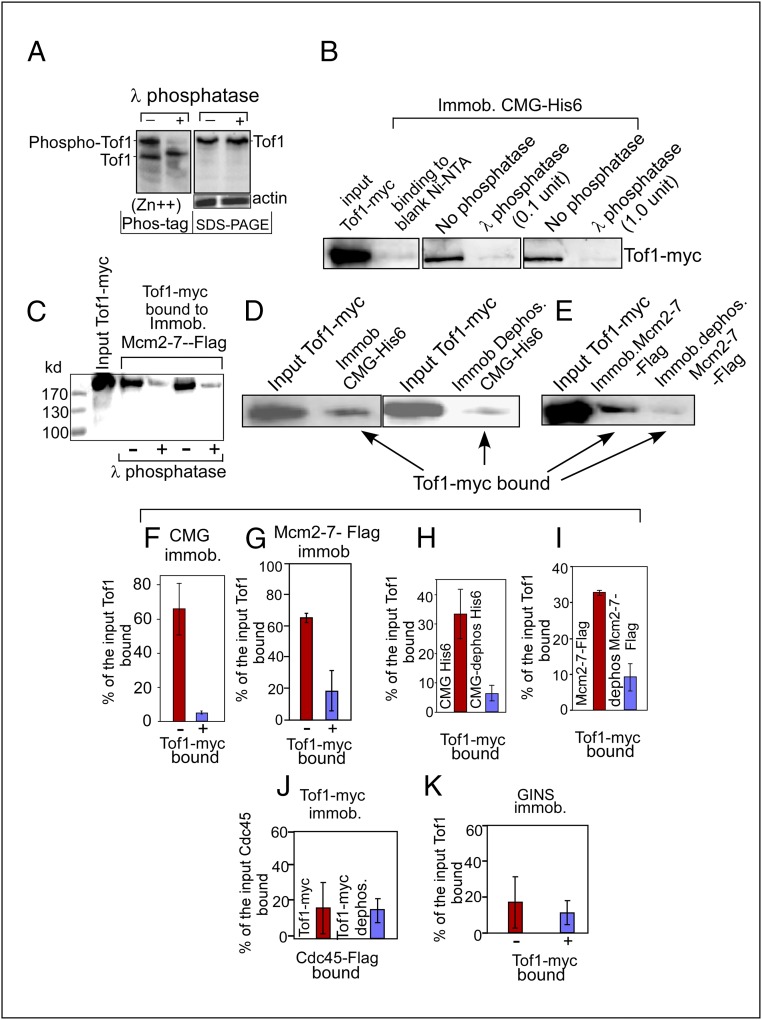
We purified the Tof1–13myc–Csm3 complex, taking care to protect the proteins from possible dephosphorylation during purification by using multiple phosphatase inhibitors (SI Materials and Methods). We tested the purified complex to determine its phosphorylated state by Zn2+-Phos-tag PAGE analysis (60), which revealed that ∼50% of the purified Tof1 was phosphorylated, as indicated by the upward mobility shift of the protein bands and loss of these shifted bands after dephosphorylation (Fig. 5A, Left). A control SDS/PAGE of the same protein without Phos-tag is shown in Fig. 5A, Right, with actin as the loading control. The Tof1 protein, before and after dephosphorylation, had almost the same mobility, commensurate with its molecular mass, by SDS/PAGE. The gels were replicated three times with nearly identical results. The data suggested that in vitro dephosphorylation did not cause incidental proteolytic degradation of Tof1. To measure the possible effect of dephosphorylation on binding to phospho-CMG, an aliquot of Tof1-myc was pretreated with λ-phosphatase and the enzyme was inactivated by a mixture of phosphatase inhibitors (PhosSTOP) before loading onto a CMG affinity column. We made sure that pretreatment of Tof1-myc with the phosphatase followed by addition of inhibitors was effective in inactivating the enzyme by carrying out standard phosphatase assays, with little or no residual phosphatase activity detectable by standard colorimetric phosphatase assays (SI Materials and Methods and Fig. S4). It should be noted that Csm3 is also a phosphoprotein (PhosphoGRID, www.phosphogrid.org/), and could also be contributing to Tof1–Csm3 interaction(s) with CMG. Approximately equal picomoles of phospho-Tof1 and the dephosphorylated form were applied to the CMG affinity columns, each containing the same amount of the affinity matrix. Control binding to the affinity matrix showed only low background levels of retention of Tof1 (Fig. 5B). The Tof1-myc that bound to the immobilized CMG columns (as measured in seven replicates of WBs using three different protein preparations) was quantified as a percentage of the input protein bound to immobilized CMG. The data consistently revealed an approximately eightfold increase in binding of phosphorylated (untreated) Tof1 to the CMG matrix compared with the dephosphorylated Tof1 protein (Fig. 5B; see quantification in Fig. 5F). Does Tof1–Csm3 in the phosphorylated form bind to the separate components of the CMG complex? We separately purified Mcm2–7, Cdc45, and GINS as described in SI Materials and Methods. Mcm2–7 included a FLAG-tagged Mcm5 subunit. The complex was immobilized on anti-FLAG affinity beads and challenged with phosphorylated (untreated with λ-phosphatase) and dephosphorylated (treated with 1 unit λ-phosphatase at 30 °C for 1 h) Tof1 protein. WBs of the bound Tof1 protein visualized with anti-Myc antibodies revealed that whereas phosphorylated Tof1–Csm3 readily bound to the immobilized phospho-Mcm2–7, the dephosphorylated form of Tof1 showed significantly reduced binding to the affinity matrix (Fig. 5 C and G; - and + indicate no treatment or dephosphorylation with λ phosphatase, respectively). Similar binding experiments using Cdc45 and GINS complex showed 15–20% binding by either phospho-Tof1–Csm3 or its dephosphorylated form (Fig. 5 J and K). We conclude from the data that phospho-Tof1 specifically binds to Mcm2–7 but not to Cdc45 and GINS.
Fig. 5.
Interactions of phosphorylated and dephosphorylated forms of CMG and Tof1 with each other as revealed by Western blotting. (A, Left) Zn2+ Phos-tag gel of phosphorylated and dephosphorylated Tof1. (A, Right) SDS/PAGE profiles of both untreated Tof1-myc and that treated with λ-phosphatase. Actin loading controls are also shown. (B) Interaction of Tof1-myc–Csm3 complex untreated and treated with λ-phosphatase with immobilized CMG-His6. (C) WBs showing binding of Tof1-myc untreated (-) and treated with λ-phosphatase (+) to immobilized FLAG-tagged Mcm2–7. (D) Interaction of Tof1-myc with immobilized untreated CMG-His6 (Left) and dephosphorylated immobilized CMG-His6 (Right). (E) Interaction of Tof1-myc with untreated immobilized Mcm2–7-FLAG and with dephosphorylated and immobilized Mcm2–7-FLAG. (F) Quantification of binding of Tof1-myc untreated (-) and dephosphorylated (+) to immobilized CMG. (G) Binding of untreated (-) and dephosphorylated Tof1-myc to immobilized Mcm2–7-FLAG (not dephosphorylated with λ-phosphatase). (H) Binding of untreated Tof1-myc to immobilized untreated CMG-His6 and to the same after dephosphorylation with λ-phosphatase. (I) Quantification of binding of Tof1-myc to untreated immobilized Mcm2–7-FLAG and to the same after dephosphorylation with λ-phosphatase. (J) Quantification of binding of Cdc45 in solution to untreated immobilized Tof1-myc or to the same after treatment with λ-phosphatase. (K) Quantification of the binding of immobilized GINS complex to Tof1-myc in solution before (-) and after (+) treatment with λ-phosphatase. Error bars represent standard error.
Fig. S4.
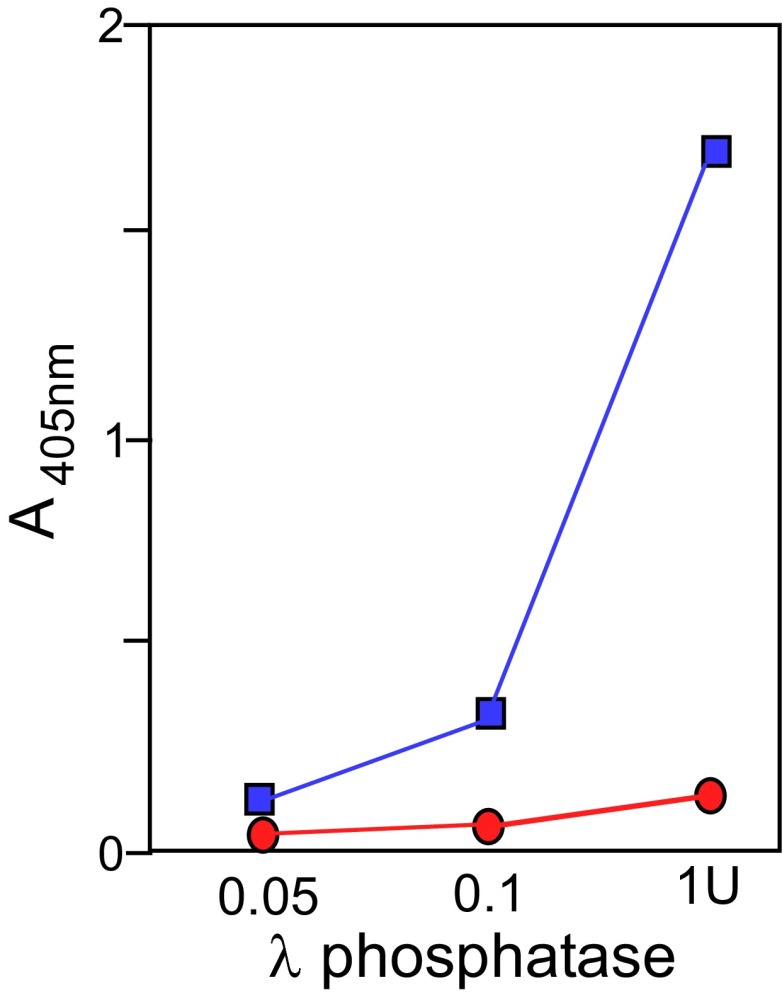
Phosphatase assay showing any residual phosphatase activity left after treatment of Tof1-myc with λ-phosphatase and subsequent treatment with the phosphatase inhibitor mixture PhosSTOP. Blue squares indicate phosphatase activity as measured colorimetrically with the designated amounts of the enzyme. The designated amounts of the enzyme without PhosSTOP for 30 min at 30 °C. A second aliquot of the reaction mixture was treated with PhosSTOP, mixed, and reassayed for residual phosphatase activity with the same substrate for 30 min at 30 °C. The residual phosphatase activity is shown as red circles.
Do CMG and Mcm2–7 require phosphorylation to bind to phospho-Tof1? To investigate this question, purified CMG was extensively treated with λ-phosphatase followed by removal of the phosphatase by a gel-filtration step. Aliquots of the dephosphorylated CMG and separately the same amounts of the phosphorylated form were immobilized on Ni-NTA columns. Equal amounts of Tof1-myc–Csm3 complex were applied to each affinity column, and the proteins were eluted, resolved by SDS/PAGE, and visualized by WBs using anti-Myc Ab. The results showed that CMG, after dephosphorylation, showed a significant reduction in binding to Tof1-GST (Fig. 5 D and H). Therefore, a continued phosphorylated state of CMG, even after complex formation, is required for optimal binding to and retention of Tof1–Csm3 at the replisome. Similarly, we determined that immobilized phospho-Mcm2–7 but not its dephosphorylated form was able to bind to Tof1-myc–Csm3 complex (Fig. 5 E and I).
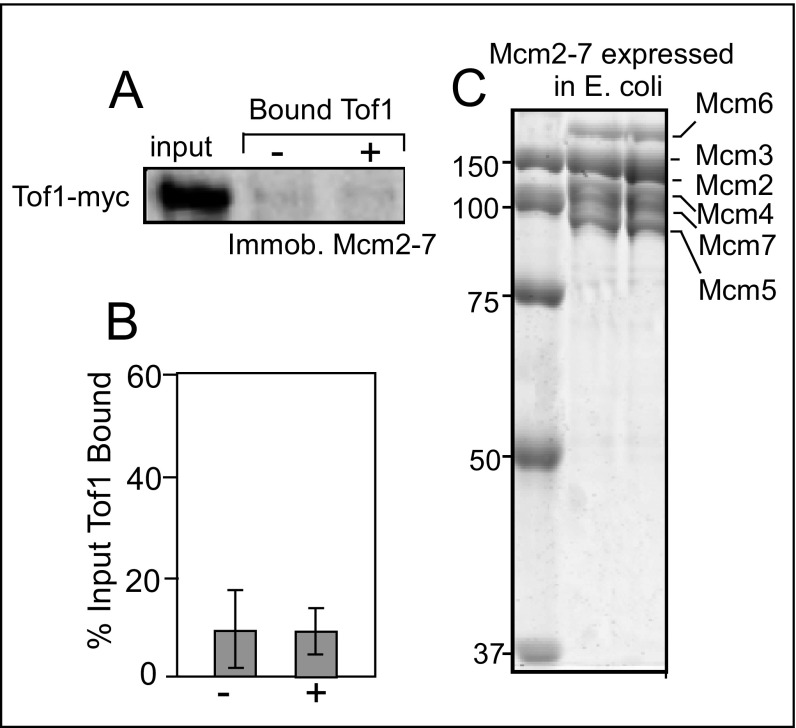
Independent support for the aforementioned conclusion is provided by the expression and purification of Mcm2–7 subunits in E. coli reconstitution into an Mcm2–7 complex [that had limited helicase activity (61)]. The rationale was that Mcm2–7 expressed in E. coli is not expected to be phosphorylated because of a lack of a DDK or DDK-like kinase. Therefore, Mcm2–7E. coli should not bind to Tof1. We confirmed this expectation by attempting to bind phosphorylated Tof1 to immobilized Mcm2–7E. coli but observed only low background levels of binding with both phosphorylated and dephosphorylated Tof1 (Fig. S5).
Fig. S5.
Yeast Mcm2–7 complex expressed in E. coli, purified, and reconstituted does not interact with Tof1–Csm3 in vitro. (A) Binding in vitro of immobilized Mcm2–7 with phospho-Tof1–Csm3 and the dephosphorylated form of the protein; Tof1 was myc-tagged; - and + indicate the phosphorylated and dephosphorylated forms of Tof1–Csm3, respectively. (B) Quantification of binding of phosphorylated and dephosphorylated Tof1–Csm3 with immobilized Mcm2–7. (C) Coomassie blue-stained SDS/PAGE profile of purified MCM2–7 expressed in E. coli.
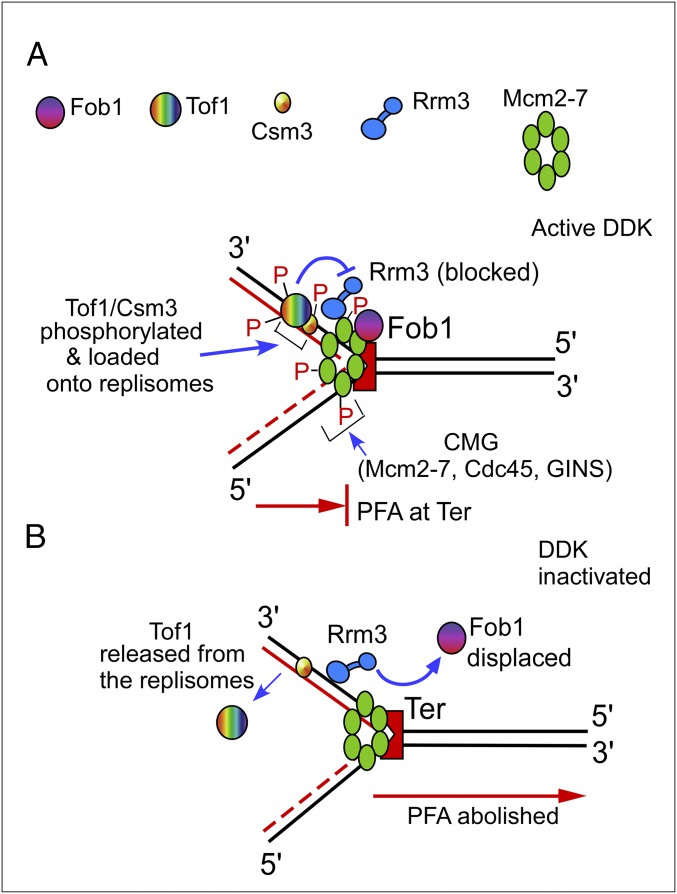
The proposed mechanism of PFA, supported by these data, is schematically shown in Fig. 6. The scheme takes into consideration our previous discovery that the Tof1–Csm3 complex counteracts the Rrm3 “sweepase” from displacing Fob1 from Ter, thereby maintaining stable fork arrest (Fig. 6A) (23, 28). In summary, the model posits that DDK phosphorylates Mcm2–7, which, along with Cdc45 and GINS, forms the CMG helicase. The CMG complex then recruits the phosphorylated but not the dephosphorylated form of Tof1–Csm3 to the replisome. Although Tof1 also requires phosphorylation for its recruitment by CMG, the kinase responsible for Tof1 phosphorylation remains unclear at this time. We propose that inactivation of DDK prevents the CMG complex from recruiting or retaining Tof1, thereby allowing Rrm3 to displace Fob1, resulting in unhindered fork progression past Ter (Fig. 6B). Why does PFA still work in cdc7-1 mcm5-bob1 cells at the nonpermissive temperature? We speculate that in the absence of DDK, CMG assembles in an alternative conformation that, as has been suggested, may be dependent on Cdc28, Clb2, and Clb5 (62).
Fig. 6.
Schematic representations of a model illustrating the mechanism of programmed fork arrest. Cdc7, GINS, and Cdc45 are not shown, to simplify the figure. (A) DDK phosphorylates Mcm2–7 and, along with Cdc45 and GINS, assembles CMG, which recruits phosphorylated Tof1–Csm3. Tof1–Csm3 antagonizes Rrm3 to prevent displacement of Fob1 from Ter. (B) In the absence of DDK, Tof1 is no longer maintained at Ter, causing unhindered fork passage. See the text for further details.
SI Materials and Methods
Strain Construction.
Strain construction was carried out by standard genetic techniques. Epitope tagging was done by using standard PCR-based insertion procedures (69). Transformation of temperature-sensitive (ts) strains was done with a modification at the heat shock step (37 °C for 9 min), followed by a snap chill on ice for 5 min. For phleomycin selection, transformed cells (ts) were incubated at 24 °C overnight in YPD (pH 7.0) and then plated on phleomycin plates. Retention of the ts phenotype was checked periodically. For Myc tagging, a cassette containing 13 copies of Myc epitope was amplified from strain YFL921 and cotransformed with a 1.3-kb phleomycin resistance gene PCR product. Primers used are 6F, 6R, 7F, 7R, 8F, and 8R, and are listed in Table S2.
Table S2.
Oligonucleotides
| Primer | Sequence, 5′-3′ |
| 1F | AGGCGTCCTTGTGGCGTCGCTGAACCATAG |
| 1R | CTGAACATGTCTGGACCCTGCCCTCATATC |
| 2F | CAGGGTAATGGAGATCAAACAAGAGACTTTGGCACATCAATGGAATTGTCCATGGAA AAGAGAAG |
| 2R | GCGTACGAAGCTTCAGCTGTCAGGTTGACTTCCCCGCGGAATTCG |
| 3F | CGAATTCCGCGGGGAAGTCAACCTGACAGCTGAAGCTTCGTA |
| 3R | TTCACCTATGGTGACTCCTCCTTTCATTCTATCCTACATATTAGCATAGGCCAC TAGTGGATCTG |
| 4F | CTCTGGAACTTGCCATCATCATTC |
| 4R | GCAAAGATGGGTTGAAAGAGAAGG |
| 5F | TTCACCTACGGAAACCTTGTT |
| 5R | TGGCCGAGAGGTCTTGGTAAT |
| 6F | GCAAAAAGTCTAGAGTTGTTTTGAGCCAAGGTGATAGTGATGATCATGCCACTCTCGTCT TCGATGTGGAG |
| 6R | GCGTACGAAGCTTCAGCTGATTACCCTGTTATCCCTAGCGGATCTGC |
| 7F | TGACCACACCTCTACCGGCAGATCCGCTAGGGATAACAGGGTAATCAGCTGAAGCTT CGTACGC |
| 7R | AAAATTACACGTATTAAAGGGATTAATTACTACATATTCATTCGCATAGGCCACTAG TGGATCTG |
| 8F | GGCGTAGATCAAGATGAGTTGGATGCTATGAAGGAAATGGGCTTTCATGCCACT CTCGTCTTCGATGTGGAG |
| 8R | TAGATGCCCACACGCACGTGTGGATTATTACCTTCAATGACATTGGCATAGGCC ACTAGTGGATCTG |
| 9F | TCCTCCAAAATCGGATCTGGAAGTTCTGTTCCAGGGTCCCGGGGGAGATCCACATATGTCTGCTGATTTGCAACAAGGCACTAC |
| 9R | TGATCTATCGATTTCAATTCAATTCAATTTATTTCCCGGAAGATCATTACCCTGTTATCCCTAGCGGATCTGC |
F, forward; R, reverse.
Integrations of BrdU cassettes into the strains were done as described elsewhere using the plasmids p404–BrdU–Inc and p405–BrdU–Inc (70). To disrupt the Bar1 locus by URA3 incorporation, the pbar1::Ura3 plasmid was digested with BamHI and BglII and transformed into the appropriate strain and selected on Ura dropout plates. The Tof1 ORF was cloned as a cassette Tof1-13x Myc in the overexpression vector pBJ842 (a gift from Satya Prakash, University of Texas Medical Branch at Galveston, Galveston, TX). The primer pair 9F and 9R was used to make the above construct.
Chromatin Fractionation and Western Blots.
Chromatin fractionation was done with some modifications as described (51). The serial expansion of culture was done in YPD medium at 24 °C and the culture was diluted (1:20) in 600 mL of YPD (OD 0.2) and incubated at 24 °C for 2 h. At OD 0.4, α-factor (360 nM; Sigma) was added and the culture was incubated for 2 more hours at 24 °C. After 2 h of growth, the culture was divided into two flasks (300 mL each) and one flask was shifted to a 37-°C water bath for heat shock (swirling movements) for 5 min. The control culture was grown at 24 °C without a temperature shift. Both cultures were incubated further for 1 h at 24 °C/37 °C. The cells were pelleted and washed with YPD that was maintained at 24 °C/37 °C. The cell pellet was resuspended and mixed thoroughly by vortexing and added to YPD media (300 mL, 24 °C/37 °C) containing Pronase E (200 µg/mL; Sigma). The incubation was continued at the respective temperature and harvested after 30 min, washed twice with chilled water containing 1 mM PMSF, frozen in liquid nitrogen, and stored at −80 °C.
The cells were processed for chromatin fractionation as described previously (51) with some modifications. Cells were sonicated on ice at 30% amplitude for 25 s (5-s intervals) and centrifuged for 10 min at 12,000 rpm (Eppendorf, fixed-angle rotor# F-45-24-11), and the supernatant containing chromatin-bound proteins was recovered. Aliquots of the soluble and chromatin fractions were mixed with SDS gel loading dye, resolved by SDS/PAGE, and blotted against c-Myc antibody clone 9E10 (Sigma) and ORC3 (SB3) mouse monoclonal antibody (a gift from Bruce Stillman, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). A 146-kDa band of Tof1-13Myc and an ∼68-kDa band of Orc3 were visualized by Western blots.
Protein Purification.
CMG, Mcm2–7, and Cdc45 were purified by expression in yeast strains containing integrated genes as described (47, 48). For the Mcm2–7 complex, an N-terminal 3×FLAG tag was cloned in-frame with the Mcm5 gene and integrated into the chromosome along with the other five Mcm genes as described. Cells were grown at 30 °C in 12 flasks of 1 L YP-glycerol and induced for 6 h at OD600 ∼0.7 with 20 g galactose per 1-L culture. Frozen cell pellets were ground into powder in a SPEX cryogenic grinding mill (6970 EFM). Mcm2–7 was purified by the same procedure as described for CMG through the FLAG-column step. To obtain Cdc45-FLAG, yeast expressing CMG (His6 and 3×FLAG tags on the N terminus of Mcm5 and C terminus of Cdc45, respectively) was used, which, due to poor assembly of components into CMG, contained a large excess of recombinant Cdc45-FLAG. This enabled purification of Cdc45-FLAG in three steps. First the FLAG column, then the flow-through of the Ni column of the CMG preparation, and finally Cdc45-FLAG were further purified on a MonoQ column using a 100–500 mM KCl gradient in 20 mM Hepes (pH 7.5), 10% (vol/vol) glycerol, 1 mM EDTA, 2 mM DTT.
Four-subunit GINS with an N-terminal hexahistidine kinase tag on Psf1 was overexpressed in E. coli (Lucigen) from the plasmids pCDF-Duet Psf2, pET Duet Psf3+Sld5, and pLANT/RIL hk/p Psf1 by the addition of 1 mM isopropyl β-d-1-thiogalactopyranoside, incubated at 15 °C overnight, and purified as follows. A 6-L equivalent of frozen cell pellet was resuspended in buffer L (20 mM Tris⋅HCl, pH 7.9, 10% glycerol) plus 80 mM NaCl and lysed in a cooled French press. Spermidine was added to the lysed cell extract to a final concentration of 30 mM and the extract was spun 40 min at 12,000 rpm in a Sorvall RC6 centrifuge with an SLA-1500 rotor to pellet insoluble material. Soluble cell extract (∼150 mL) containing overexpressed Psf1, Pfs2, Psf3, and Sld5 was applied to 25 mL Fast Flow Q Sepharose resin (GE Healthcare) on an XK 26 column (Pharmacia Biotech) at 1 mL/min. The column was washed with buffer L, 80 mM NaCl and eluted with a 250-mL gradient of 80 mM NaCl to 1 M NaCl in buffer L, 1 M NaCl. Peak fractions containing four-subunit GINS, which eluted at a conductivity equivalent centered around 300 mM NaCl, were pooled (60 mL) and adjusted to 500 mM NaCl, 5 mM imidazole by addition of concentrated stocks. The pool was loaded onto a 5-mL HiTrap Chelating HP column (GE Healthcare) that had been previously charged with 50 mM nickel sulfate and equilibrated in buffer L with 500 mM NaCl and 5 mM imidazole. The column was washed with 30 mL buffer L containing 500 mM NaCl and 60 mM imidazole to remove nonspecifically bound proteins and eluted with a 50-mL gradient from 60 mM to 500 mM imidazole in buffer L, 500 mM NaCl. Peak fractions of pure GINS were combined into two separate pools and dialyzed overnight against 1 L 20 mM Tris⋅HCl (pH 7.9), 500 mM NaCl to remove the imidazole. The pools were then dialyzed for 6 h against 1 L buffer A, 100 mM NaCl for storage at −80 °C (buffer A consisted of 20 mM Tris⋅HCl, pH 7.5, 10% glycerol, 2 mM DTT, and 0.5 mM EDTA). Total yield of pure GINS was 25.8 mg (pool 1, 4.2 mg/mL in 3.0 mL; pool 2, 2.5 mg/mL in 5.3 mL).
Tof1–Csm3 was purified as follows. Tof1 ORF with 13Myc was cloned into the vector pBJ842 (74). Tof1 was expressed in S. cerevisiae as a GST fusion protein in this vector and purified on a Glutathione Sepharose 4B (GE Healthcare) column as described below. The plasmid pBJ842-Tof1 was transformed into the protease-deficient yeast strain BJ5464, and cells were grown in SD/Leu− plates as described for the pBJ842-derived clones (74). Colonies from SD/Leu− plates were inoculated in SD/Leu− medium containing 2% glucose, 2% glycerol, and 1.8% lactate. Overnight cultures were washed and then inoculated into fresh SD/Leu− medium containing 2% glycerol and 1.8% lactate with a 1:20 dilution. An 8-L culture was incubated for 16 h and galactose was added to a final concentration of 2% for the induction. At the time of induction, 1% peptone, 0.5% yeast extract, and 1 mM Na pyrophosphate were also added. The culture was induced for 6 h and harvested in a Sorvall RC-5C centrifuge. The cell pellets were washed in breaking buffer [50 mM Tris⋅HCl, pH 7.5, 500 mM KCl, 0.5 mM benzamidine, 0.5 mM PMSF, EDTA-free protease inhibitor mixture, PhosSTOP (Roche Applied Science)], centrifuged, frozen in liquid nitrogen, and stored at −80 °C. The cell pellets were then suspended in breaking buffer with 10% sucrose. The cell suspension was frozen in liquid nitrogen, thawed, and lysed by a bead beater. The lysate was centrifuged at 40,000 rpm for 30 min at 4 °C in a Beckman Ti70 rotor. The clear straw-color supernatant was collected and a 70% ammonium sulfate precipitation was done, and a protein pellet was recovered by centrifugation. The protein pellet was resuspended in column buffer (50 mM Tris⋅HCl, pH 7.5, 10% glycerol, 1 mM DTT, 0.5 mM benzamidine, 0.5 mM PMSF, protease inhibitor mixture, and PhosSTOP). The conductivity of this solution was measured and adjusted to the conductivity of the column buffer by dilution. Soluble protein solution was slowly loaded onto a Glutathione Sepharose 4B column preequilibrated with column buffer at 4 °C. The column was washed with three column volumes (CVs) of wash buffer (50 mM Tris⋅HCl, pH 7.5, 10% glycerol, 500 mM KCl, 1 mM DTT, 0.01% Tween 20, 0.5 mM benzamidine, 0.5 mM PMSF, EDTA-free protease inhibitor mixture, PhosSTOP) and with three CVs of column buffer containing 50 mM Tris⋅HCl (pH 7.5), 10% glycerol, 100 mM KCl, 1 mM DTT, and 0.01% Tween 20. The GST–Tof1-myc protein bound to the beads was eluted with freshly prepared elution buffer (50 mM Tris⋅HCl, pH 7.5, 10% glycerol, 100 mM KCl, 1 mM DTT, 0.01% Tween 20, 40 mM reduced glutathione) at 4 °C for 30 min. Eluates were collected and snap-frozen in liquid nitrogen and stored at −80 °C. Aliquots were checked by SDS/PAGE and protein was quantified. SDS/PAGE analysis revealed the presence of equimolar amounts of Csm3 that copurified with the Tof1 protein.
The degrees of purification of the proteins and protein complexes used in this work are shown in Fig. S3.
Tof1-Myc Dephosphorylation Reaction.
For a 100-µL reaction mixture, 2 pmol purified protein GSTT of 1 Myc in 2× binding buffer (40 mM Tris⋅HCl, pH 7.5, 20% glycerol, 100 mM KCl, 2 mM DTT, 2 mM MgCl2), 1× reaction buffer [protein metallo phosphatase (PMP)], 1 mM MnCl2, and 2 units λ-phosphatase was added and incubated in a 30-°C water bath for 1 h. At the completion of the reaction, 2 µL PhosSTOP stock solution was added to inhibit phosphatase at room temperature for 10 min.
Protein Binding Assay.
(i) Two picomoles of each protein (CMG complex, Mcm2–7, and GINS) was immobilized on Ni-NTA affinity beads (40 µL as a slurry) preequilibrated with binding buffer (20 mM Tris⋅HCl, pH 7.5, 10% glycerol, 50 mM KCl, 1 mM DTT, 1 mM MgCl2) and bound at 4 °C for 1 h in Eppendorf tubes. (ii) For Mcm2–7, binding buffer was supplemented with 3 mM ATP, 10 µM ZnCl2; MgCl2 was substituted with 10 mM Mg acetate. Beads were washed and fresh binding buffer was added. Two picomoles of Tof1-myc with and without dephosphorylation was added to the respective beads in Eppendorf tubes (200 µL) and allowed to interact at 4 °C for 1 h. (iii) For the Cdc45-Tof1 binding assay, 2 pmol GST–Tof1-myc with λ-phosphatase–treated and –untreated reaction mixtures (150 µL) was immobilized on Glutathione Sepharose 4B beads and then 2 pmol of Cdc45 was loaded onto the GST–Tof1-bound beads (200 µL total reaction volume) at 4 °C for 1 h. After the binding reaction, protein-bound beads were loaded onto microcolumns, which were washed three times with wash buffer (binding buffer + 0.01% Tween 20) and the beads were extracted into Eppendorf tubes. Sample buffer was added, boiled at 95 °C, and resolved by 8% SDS/PAGE.
Western Blot Analyses.
The resolved protein samples were transferred onto membrane (Bio-Rad), and the blots were blocked with 1% BSA and incubated in the presence of anti–c-Myc antibody (1:5,000 dilution) and anti-FLAG antibody (1:1,000 dilution) (Sigma) overnight at 4 °C. Secondary antibody treatment, washing, and detection by chemiluminescence were performed (EMD Millipore). All of the experiments were done in triplicate. The image acquisition and quantification was done by ImageJ software (NIH).
All of the controls for the purified protein were done; GST and GST–Tof1-myc was immobilized to Glutathione Sepharose 4B, and equimolar CMG complex was allowed to interact. We ensured that the CMG complex specifically bound to GST–Tof1-myc but not to the GST-only column. In another set, GST–Tof1-myc protein was loaded onto a Ni-NTA column and there was, as expected, no binding or low levels of nonspecific binding to the beads. Purified E. coli-expressed Mcm2–7 and GINS, and CMG proteins expressed in yeast were diluted and applied to the Ni-NTA beads, and bound protein Mcm2–7 was checked by SDS/PAGE and Coomassie blue staining. CMG and Cdc45 were checked by Western blots probed with anti-FLAG antibody (Sigma). To check the effectiveness and efficiency of λ-phosphatase and PhosSTOP (1 tablet per mL; 1 µL inhibits 4 units λ-phosphatase in a 50-μL reaction mixture), the enzyme reaction was carried out in vitro on an ELISA plate to experimentally determine and standardize the reaction conditions for the subsequent experiments.
Zn2+ Phos-Tag SDS/PAGE.
Cells expressing 13Myc-tagged Tof1 were lysed in RIPA buffer (50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, 0.5 mM Na3VO4, 1 mM NaF, 1 mM PMSF, complete protease inhibitor mix) using glass beads. The lysates were quantified. and equal amounts were separated by 5% neutral-pH SDS/PAGE containing 50 μM Phos-tag (Wako) and 100 μM Zn(NO3)2. The gel consisted of a separating gel [5% (wt/vol) polyacrylamide and 357 mM Bis-Tris⋅HCl, pH 6.8] and staking gel [4.5% (wt/vol) polyacrylamide and 357 mM Bis-Tris⋅HCl, pH 6.8] and run in 1× NuPAGE 3-(N-Morpholino) propanesulfonic (Mops) running buffer [50 mM Mops, 50 mM Tris base, 0.1% SDS, pH 7.8, 5 mM sodium bisulfite (added fresh from a 1-M stock solution)]. After electrophoresis, gels were soaked in transfer buffer (25 mM Bicine, 25 mM Tris base, 1 mM EDTA, pH 7.2, 10% methanol, 5 mM sodium bisulfite) with gentle agitation for 30 min and transferred overnight onto PVDF membrane (Bio-Rad). The blots were incubated overnight at 4 °C in the presence of monoclonal anti–c-Myc antibody (1:5,000; Sigma). Secondary antibody treatment, washing, and detection by chemiluminescence were performed as directed by the supplier’s manual (EMD Millipore). Dephosphorylation of Tof1 was done by using λ-protein phosphatase (NEB) at 37 °C for 1 h.
Discussion
The biochemical mechanism of control of PFA in eukaryotes and its mode of regulation are poorly understood (4). This mechanism is of considerable general interest because of the many important physiological transactions mediated by the process. The cell-cycle kinases CDK and DDK are required for replication initiation, and CDK is involved in limiting replication to only once per cell cycle and in S-phase checkpoint control (37, 39, 40, 49, 63). The work reported here demonstrates that DDK is also required for PFA at a nonhistone protein barrier. This mechanism has been illuminated by our observation that inactivation of cdc7-1 not only caused abolition of PFA at Ter sites of rDNA but that the phosphorylated form of Mcm2–7, besides its well-known function in the assembly of the CMG complex, was also necessary for binding to Tof1 in vitro and its recruitment in vivo. Furthermore, phospho-Tof1 but not its dephosphorylated form bound to phospho-CMG and phospho-Mcm2–7 but not to the dephosphorylated protein complexes. Consistent with the in vitro binding data, Tof1 in vivo was retained in the chromatin fraction only in the presence of active DDK in the cell milieu.
Which kinase phosphorylates Tof1–Csm3? The data in Fig. 4 show that Tof1 is still recruited to chromatin in a cdc7-1 mutant in the presence of the mcm5-bob1 suppressor at the nonpermissive temperature, which suggests that DDK-catalyzed Tof1 phosphorylation is not necessary in this genetic background and probably another kinase phosphorylates Tof1. It is also possible that a phosphorylated Tof1 is not needed in this genetic background. Nevertheless, several lines of evidence support the possibility that DDK is important for Tof1 function. First, the Schizosaccharomyces pombe homolog of Tof1, called Swi1, interacts with the homologous DDK complex (Hsk1–Dfp1) in the two-hybrid system and the proteins are also shown to form a complex by coimmunoprecipitation (64). Second, Tof1–Csm3 and DDK from S. cerevisiae also form a complex, as shown by coimmunoprecipitation, and Tof1–Csm3 is believed to recruit DDK to the replication fork during meiotic DNA replication (65). The requirement of Tof1–Csm3 for subsequent meiotic double-strand-break formation could be bypassed by physically connecting DDK to Cdc45/CMG within the replication fork, indicating that Tof1–Csm3 serves as a platform for recruitment of DDK. Whether this interaction also leads to DDK phosphorylation of Tof1 remains to be determined. It is possible that in WT yeast, DDK is recruited by Mcm2–7 and not only phosphorylates Mcm2–7 but also Tof1, but that in the absence of DDK the mcm5-bob1 mutant form of Mcm2–7 is able to recruit another kinase, perhaps Cdc28-Clb5 (62), that helps carry out PFA and replication. Further work is needed to resolve this issue.
Tof1 along with Csm3 plays a critical role in stabilizing stalled replication forks at protein barriers even in the absence of S-phase checkpoint activation, and this is central to its function in PFA, although DDK is not necessary for S-phase checkpoint activations (41, 42). However, unlike most instances, in which replication forks stall transiently while awaiting resolution of an impediment to fork progression, fork stalling in PFA is physiologically programmed and longer-lasting. This raises the question of how PFA differs mechanistically from transient fork stalling. We showed previously that Tof1 plays an additional role in PFA by counteracting the effects of the Rrm3 helicase. Rrm3 is known to act as a sweepase for its function in removing nonhistone DNA-binding proteins, thereby allowing unrestrained replication fork movement through protein-bound DNA. In the absence of Rrm3 in yeast, replication fork stalling is observed at over 1,000 sites that are not observed in wild-type cells (66). Many of these sites are known to be densely populated with DNA-binding proteins, including centromeres and tRNA-coding genes, among many others. However, in contrast with PFA, the data from the rrm3Δ strain suggest that these DNA-bound proteins are readily removed by the action of the Rrm3 sweepase, so how does Tof1 antagonize the action of Rrm3 in the rDNA array? In this context, it should be kept in mind that a deletion of rrm3Δ only partially rescues PFA in cells harboring either tof1Δ or csm3Δ (23). This suggests that besides Rrm3, other factors are also involved in the abolition of PFA in tof1Δ and csm3Δ cells.
As with most regulatory functions in biology, the answer appears to lie in a balancing act between positive and negative regulators and also in the evolutionary tradeoff between genome stability and genome adaptability. The DNA at stalled replication forks is subject to breakage followed by recombination that can lead to genome rearrangements, particularly in the case of repeated DNA sequences. At the same time, collisions between DNA replication and DNA transcription can also lead to replication fork collapse. The Fob1-mediated DNA replication barrier in the rDNA of S. cerevisiae strikes a balance between these imperatives by preventing collisions between RNA and DNA synthesis and by using the components of the replication fork protection complex to prevent collapse of the replication fork stalled at the barrier. Although other organisms, as well as human cells, appear to use various mechanisms to prevent collisions between replication and transcription in the highly transcribed ribosomal RNA genes, they all use Tof1–Csm3 or the homologous human Tim/Tipin components of the fork protection complex to prevent collapse of the paused replication forks in the rDNA locus (reviewed in ref. 67).
Finally, although it is well-established that DDK-catalyzed phosphorylation of Mcm2–7 is needed for CMG assembly in WT cells, it is not known whether continued phosphorylation of the CMG complex is necessary for fork progression. Our data showing that even after a CMG complex has been formed its dephosphorylation causes failure to recruit the fork protection complex in vitro indicate that continued maintenance of the phosphorylated state of CMG seems to be necessary for error-free fork progression. It is known that under genotoxic stress, in the absence of Tof1 or Mrc1, the helicase and polymerase of yeast dissociate from each other in the replisome, causing fork collapse (68). Therefore, one would infer that continued maintenance of the phosphorylated state of CMG in the postinitiation step would be critical for genome stability.
Materials and Methods
Strain Construction.
Yeast strains used in this study are listed in Table S1. All strains are from either the A364a or W303 background unless otherwise noted. Strains were constructed by standard genetic and molecular genetic techniques. Epitope tagging was performed using standard PCR-based insertion procedures (69). Incorporation of BrdU cassettes into strains was done as described elsewhere using the plasmids p404–BrdU–Inc and p405–BrdU–Inc (70). To disrupt the Bar1 locus by Ura3 incorporation, the pbar1::Ura3 plasmid was digested with BamHI and BglII and then transformed into the appropriate strain and selected on Ura dropout plates.
Table S1.
Yeast strains
| Strain | Relevant genotype (background) | Source |
| W303-1A | MATa ade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 (phi+) ura3-1(W303) | Bruce Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) |
| BTY100 | MATa mcm10-1 (P269L) W303-1a | Y. Kawasaki (Osaka University, Osaka, Japan) |
| HYLS108 | MATa cdc7-1 MCM4-6His-3FLAG::nat1 CDC45-6HA::hphNT1 (A364a) | A. D. Donaldson (University of Aberdeen, Scotland, UK) |
| SHY360 | MATa cdc7-1 rif1Δ::LEU2 MCM4-6His-3FLAG::nat1(A364a) | A. D. Donaldson |
| BW303-1A | 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15 ybp1-1, leu2-3:: BrdU-Inc (LEU2) (W303-1A) | This study |
| BYB556 | cdc7-1, 4xw303, leu2-3:: BrdU-Inc (LEU2) (W303) | This study |
| BYYT1 | Mata 112 trp1-1, can1-100, ade2-1, his3-11, ura3-1, 15leu2-3 BrdU-Inc (LEU2), bar1 psf1-1 | This study |
| RSy1298 | ade2-1 ura3-1 his3-11, 15 trp1-1 leu2-3, 112 can1-100 RAD5 bar1::hisG ars608::HIS3 ars609::TRP1 ars305::BrdU-Inc (TRP1) cdc7-1 | O. Aparicio (University of Southern California, Los Angeles) |
| RSY311T | MATa bar1 trp1 leu2 ura3 can1 his6 cyh2 TOF1-13XMYC::ble (A364a background). | This study |
| HYLS108T | bar1 MATa cdc7-1 MCM4-6His-3FLAG::nat1 CDC45-6HA::hphNT1 TOF1-13XMYC::ble (A364a background) | This study |
| YB349 | dbf4-1 backcrossed into W303-1 (ade2-1 trp1-1 can1-100 leu2-3,112 his3-11,15 ura3-1), ura3 trp1 leu2 ade2-1 | Bruce Stillman |
| YB556 | CDC7-1, 4xw303 | Bruce Stillman |
| YFL921 | MATa ade2-1 trp1-1 can1-100 leu2-3,112 his3-11 ura3 rad5-535 rad9ΔBRCT::2xFKBP-13MYC:kanMX6 (W303). | M. Falconi (University of Milan, Milan, Italy) |
| YB529B | W303 (MATa ade2-1 his3-11,15 TRP1 can1-100 leu2-3,112 ura3-1) bob1cdc7Δ::HIS3 bar1::URA3 TOF1-13XMYC::ble | This study |
| YB1186 | MATa mcm4(Δ2–174) dbf4-1 ade2-1 his3-11,15 trp1-1 can1-100 leu2-3,112 ura3-1 | Bruce Stillman |
| YYT1 | MATa ade2-1 can1-100 his3-11, 15 leu2-3,112 trp1-1 ura3-1 bar1 psf1-1 | H. Araki (National Institute of Genetics, Research Organization of Information and Systems, Shizuoka, Japan) |
| YYK38 | MATa ade2-1 can1-100 his3-11, 15 leu2-3,112 trp1-1 ura3-1 bar1 sld5-12 | H. Araki |
| HYLS108C | bar1 MATa cdc7-1 MCM4-6His-3FLAG::nat1 CDC45-6HA::hphNT1 CSM3-13XMYC::ble (A364a background) | This study |
| YB556T | cdc7-1, Fob1-TAP::ble, 4xW303 | This study |
| Y799 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100, sld2.1 (drc1-1) | S. Elledge (Harvard Medical School, Boston) |
Oligonucleotides.
Oligonucleotides are listed in Table S2.
Analysis of Mcm4 Phosphorylation.
Chromatin fractions were used to analyze in vivo Mcm4 phosphorylation (54, 58). Phenylmethylsulfonyl fluoride (PMSF), sodium fluoride (NaF), and sodium orthovanadate (Na3VO4) were added directly to cultures of MCM4-6His-3FLAG cells to a final concentration of 1, 1, and 0.5 mM, respectively. We collected cells by a brief centrifugation (Sorvall 5C Plus; rotor# HS-4) at 5,000 rpm for 5 min and washed them with an ice-cold solution of 1 mM PMSF, 2 mM NaF, and 1 mM Na3VO4. Cells were collected by centrifugation and protein extracts were prepared as described above for the chromatin fractionation assay [51]. Proteins were analyzed by electrophoresis on 6% SDS/PAGE, followed by Western blotting using monoclonal anti-FLAG antibody (M2; Sigma-Aldrich) as the primary antibody.
ChIP Assays.
ChIP assays were performed in the cdc7-1 strain (YB556) in which Fob1 was tagged with TAP at the C terminus (primers 2F, 2R, 3F and 3R), using cells grown at 24 °C and 37 °C (23, 71). Each step has been described before (23, 71), except that cells grown at 37 °C were quickly chilled for 5 min on ice before adding 1% formaldehyde. PCR was performed using the primers 4F, 4R, 5F, and 5R (Table S2).
Two-Dimensional Agarose Gel Electrophoresis.
Brewer–Fangman 2D agarose gel electrophoresis was performed to visualize replication intermediates within cells obtained from cultures grown at 24 °C and 37 °C as previously described (55). Primers 1F and 1R were used to amplify the 1.4-kb region encompassing the rDNA NTS region, which was used as a probe to visualize replication intermediates.
Overnight starter cultures were inoculated into yeast, peptone, dextrose (YPD) medium at a starting OD600 of 0.2 and then grown for 2 h at 24 °C. Half of this culture was poured into another flask and swirled for 5 min in a water bath at 37 °C and then grown a further 2–3 h at 37 °C before being harvested. The control culture was grown 2 h more at 24 °C after reaching an OD600 of 0.2 and then harvested. Genomic DNA was prepared and digested with EcoRV and BglII restriction enzymes to yield a 4.5-kb region of rDNA that included the replication origin.
DNA Content Analysis by FACS.
FACS analysis was performed on either a synchronous or asynchronous culture grown at 24 °C or 37 °C as described (56).
Chromatin Fractionation.
Chromatin fractionation was performed to determine the level of chromatin-bound Tof1 or Csm3 in cells at 24 °C or 37 °C. The detailed method is described in SI Materials and Methods. Briefly, cells were harvested after Pronase treatment. Proteins from the chromatin and soluble fractions were prepared and resolved by SDS/PAGE and blotted to Nytran membranes, and the membranes were developed with anti-myc and anti-ORC3 antibodies.
DNA Combing.
DNA combing was performed as described (72). BrdU was detected with a rat monoclonal antibody (AbCys; clone BU1/75) and goat anti-rat coupled to Alexa 488 (Invitrogen). DNA fibers were counterstained using anti-mouse MAB 3034 (Millipore) and goat anti-mouse coupled to Alexa 546. Images were recorded on a Leica DM6000 microscope equipped with a 40× objective and a CoolSNAP HQ CCD camera (Roper Scientific). Images were processed as described (73). BrdU tracts were measured with MetaMorph (Molecular Devices). Statistical analyses of differences between samples were performed with GraphPad Prism 6.0 using the Mann–Whitney rank-sum test. More details of data collection and statistical analysis are provided in the legend to Fig. 2.
Protein Purification.
CMG, Mcm2–7, and Cdc45 were purified by expression in yeast strains containing integrated genes as described in greater detail in SI Materials and Methods. Tof1–Csm3 was purified as follows. Tof1 ORF with 13c-Myc was cloned into the vector pBJ842 (74). Tof1 was expressed in S. cerevisiae as a GST fusion protein in this vector and purified on a glutathione-agarose column as described in detail in SI Materials and Methods.
Protein–Protein Interaction in Vitro.
Details are described in SI Materials and Methods.
Phos-Tag Gels.
Phosphorylated Tof1 protein was detected by Zn2+ Phos-tag gels as described in detail in SI Materials and Methods following a published procedure (60, 75).
Phosphatase Assay.
The phosphatase assay was performed as described to not only detect phosphatase activity of λ-phosphatase at different dilutions but to check the effectiveness of the phosphatase inhibitor (PhosSTOP; Roche). A stock solution (1 tablet per mL) was prepared and 1 μL/50 μL reaction mixture was added to stop dephosphorylation. For the colorimetric assay, 4-nitrophenyl phosphate was used as substrate and color was detected with an ELISA plate reader (76).
Acknowledgments
We thank Robert Sclafani, Bruce Stillman, Bonita Brewer, M. K. Raghuraman, and Anne Donaldson for plasmids and strains and Bruce Stillman for Orc3 antibodies. We thank Ryan Mayle for λ-phosphatase–treated CMG. We also thank Oscar Aparicio for FACS analyses. This work was supported by National Institute of General Medical Sciences Grant 5 R01-GM-098013 (to D.B.), grants from Agence Nationale pour la Recherche and Ligue contre le Cancer (Équipe Labellisée) (to P.P.), NIH Grant GM-115809 (to M.E.O.), and the Howard Hughes Medical Institute (M.E.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607552113/-/DCSupplemental.
References
- 1.Hawkins M, et al. High-resolution replication profiles define the stochastic nature of genome replication initiation and termination. Cell Reports. 2013;5(4):1132–1141. doi: 10.1016/j.celrep.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatri GS, MacAllister T, Sista PR, Bastia D. The replication terminator protein of E. coli is a DNA sequence-specific contra-helicase. Cell. 1989;59(4):667–674. doi: 10.1016/0092-8674(89)90012-3. [DOI] [PubMed] [Google Scholar]
- 3.Lee EH, Kornberg A, Hidaka M, Kobayashi T, Horiuchi T. Escherichia coli replication termination protein impedes the action of helicases. Proc Natl Acad Sci USA. 1989;86(23):9104–9108. doi: 10.1073/pnas.86.23.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastia D, Zaman S. Mechanism and physiological significance of programmed replication termination. Semin Cell Dev Biol. 2014;30:165–173. doi: 10.1016/j.semcdb.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zechiedrich EL, Cozzarelli NR. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev. 1995;9(22):2859–2869. doi: 10.1101/gad.9.22.2859. [DOI] [PubMed] [Google Scholar]
- 6.Madabhushi R, Marians KJ. Actin homolog MreB affects chromosome segregation by regulating topoisomerase IV in Escherichia coli. Mol Cell. 2009;33(2):171–180. doi: 10.1016/j.molcel.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter J, Diffley JF. Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast. Mol Cell. 2008;30(6):790–802. doi: 10.1016/j.molcel.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Fachinetti D, et al. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol Cell. 2010;39(4):595–605. doi: 10.1016/j.molcel.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maric M, Maculins T, De Piccoli G, Labib K. Cdc48 and a ubiquitin ligase drive disassembly of the CMG helicase at the end of DNA replication. Science. 2014;346(6208):1253596. doi: 10.1126/science.1253596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohanty BK, Bastia D. Binding of the replication terminator protein Fob1p to the Ter sites of yeast causes polar fork arrest. J Biol Chem. 2004;279(3):1932–1941. doi: 10.1074/jbc.M309078200. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T. The replication fork barrier site forms a unique structure with Fob1p and inhibits the replication fork. Mol Cell Biol. 2003;23(24):9178–9188. doi: 10.1128/MCB.23.24.9178-9188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalgaard JZ, Klar AJ. A DNA replication-arrest site RTS1 regulates imprinting by determining the direction of replication at mat1 in S. pombe. Genes Dev. 2001;15(16):2060–2068. doi: 10.1101/gad.200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eydmann T, et al. Rtf1-mediated eukaryotic site-specific replication termination. Genetics. 2008;180(1):27–39. doi: 10.1534/genetics.108.089243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krings G, Bastia D. Sap1p binds to Ter1 at the ribosomal DNA of Schizosaccharomyces pombe and causes polar replication fork arrest. J Biol Chem. 2005;280(47):39135–39142. doi: 10.1074/jbc.M508996200. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Gorostiaga A, López-Estraño C, Krimer DB, Schvartzman JB, Hernández P. Transcription termination factor reb1p causes two replication fork barriers at its cognate sites in fission yeast ribosomal DNA in vivo. Mol Cell Biol. 2004;24(1):398–406. doi: 10.1128/MCB.24.1.398-406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mejía-Ramírez E, Sánchez-Gorostiaga A, Krimer DB, Schvartzman JB, Hernández P. The mating type switch-activating protein Sap1 is required for replication fork arrest at the rRNA genes of fission yeast. Mol Cell Biol. 2005;25(19):8755–8761. doi: 10.1128/MCB.25.19.8755-8761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krings G, Bastia D. swi1- and swi3-dependent and independent replication fork arrest at the ribosomal DNA of Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 2004;101(39):14085–14090. doi: 10.1073/pnas.0406037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krings G, Bastia D. Molecular architecture of a eukaryotic DNA replication terminus-terminator protein complex. Mol Cell Biol. 2006;26(21):8061–8074. doi: 10.1128/MCB.01102-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SK, Sabatinos S, Forsburg S, Bastia D. Regulation of replication termination by Reb1 protein-mediated action at a distance. Cell. 2010;142(6):868–878. doi: 10.1016/j.cell.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bando M, et al. Csm3, Tof1, and Mrc1 form a heterotrimeric mediator complex that associates with DNA replication forks. J Biol Chem. 2009;284(49):34355–34365. doi: 10.1074/jbc.M109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calzada A, Hodgson B, Kanemaki M, Bueno A, Labib K. Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 2005;19(16):1905–1919. doi: 10.1101/gad.337205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tourrière H, Versini G, Cordón-Preciado V, Alabert C, Pasero P. Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol Cell. 2005;19(5):699–706. doi: 10.1016/j.molcel.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty BK, Bairwa NK, Bastia D. The Tof1p-Csm3p protein complex counteracts the Rrm3p helicase to control replication termination of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2006;103(4):897–902. doi: 10.1073/pnas.0506540103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalgaard JZ, Klar AJ. swi1 and swi3 perform imprinting, pausing, and termination of DNA replication in S. pombe. Cell. 2000;102(6):745–751. doi: 10.1016/s0092-8674(00)00063-5. [DOI] [PubMed] [Google Scholar]
- 25.Gotter AL, et al. A time-less function for mouse Timeless. Nat Neurosci. 2000;3(8):755–756. doi: 10.1038/77653. [DOI] [PubMed] [Google Scholar]
- 26.Gotter AL. Tipin, a novel Timeless-interacting protein, is developmentally co-expressed with Timeless and disrupts its self-association. J Mol Biol. 2003;331(1):167–176. doi: 10.1016/s0022-2836(03)00633-8. [DOI] [PubMed] [Google Scholar]
- 27.Cho WH, et al. Human Tim-Tipin complex affects the biochemical properties of the replicative DNA helicase and DNA polymerases. Proc Natl Acad Sci USA. 2013;110(7):2523–2527. doi: 10.1073/pnas.1222494110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohanty BK, Bairwa NK, Bastia D. Contrasting roles of checkpoint proteins as recombination modulators at Fob1-Ter complexes with or without fork arrest. Eukaryot Cell. 2009;8(4):487–495. doi: 10.1128/EC.00382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivessa AS, et al. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol Cell. 2003;12(6):1525–1536. doi: 10.1016/s1097-2765(03)00456-8. [DOI] [PubMed] [Google Scholar]
- 30.Bermejo R, Lai MS, Foiani M. Preventing replication stress to maintain genome stability: Resolving conflicts between replication and transcription. Mol Cell. 2012;45(6):710–718. doi: 10.1016/j.molcel.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Brewer BJ. When polymerases collide: Replication and the transcriptional organization of the E. coli chromosome. Cell. 1988;53(5):679–686. doi: 10.1016/0092-8674(88)90086-4. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande AM, Newlon CS. DNA replication fork pause sites dependent on transcription. Science. 1996;272(5264):1030–1033. doi: 10.1126/science.272.5264.1030. [DOI] [PubMed] [Google Scholar]
- 33.Lin YL, Pasero P. Interference between DNA replication and transcription as a cause of genomic instability. Curr Genomics. 2012;13(1):65–73. doi: 10.2174/138920212799034767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Defossez PA, et al. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol Cell. 1999;3(4):447–455. doi: 10.1016/s1097-2765(00)80472-4. [DOI] [PubMed] [Google Scholar]
- 35.Choudhury M, Zaman S, Jiang JC, Jazwinski SM, Bastia D. Mechanism of regulation of ‘chromosome kissing’ induced by Fob1 and its physiological significance. Genes Dev. 2015;29(11):1188–1201. doi: 10.1101/gad.260844.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annu Rev Genet. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui K, On KF, Diffley JF. Regulating DNA replication in Eukarya. Cold Spring Harb Perspect Biol. 2013;5(9):a012930. doi: 10.1101/cshperspect.a012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka S, Araki H. Helicase activation and establishment of replication forks at chromosomal origins of replication. Cold Spring Harb Perspect Biol. 2013;5(12):a010371. doi: 10.1101/cshperspect.a010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji T, Lau E, Chiang GG, Jiang W. The role of Dbf4/Drf1-dependent kinase Cdc7 in DNA-damage checkpoint control. Mol Cell. 2008;32(6):862–869. doi: 10.1016/j.molcel.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pessoa-Brandão L, Sclafani RA. CDC7/DBF4 functions in the translesion synthesis branch of the RAD6 epistasis group in Saccharomyces cerevisiae. Genetics. 2004;167(4):1597–1610. doi: 10.1534/genetics.103.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandão LN, Ferguson R, Santoro I, Jinks-Robertson S, Sclafani RA. The role of Dbf4-dependent protein kinase in DNA polymerase ζ-dependent mutagenesis in Saccharomyces cerevisiae. Genetics. 2014;197(4):1111–1122. doi: 10.1534/genetics.114.165308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takayama Y, et al. GINS, a novel multiprotein complex required for chromosomal DNA replication in budding yeast. Genes Dev. 2003;17(9):1153–1165. doi: 10.1101/gad.1065903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou L, Mitchell J, Stillman B. CDC45, a novel yeast gene that functions with the origin recognition complex and Mcm proteins in initiation of DNA replication. Mol Cell Biol. 1997;17(2):553–563. doi: 10.1128/mcb.17.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou L, Stillman B. Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin. Science. 1998;280(5363):593–596. doi: 10.1126/science.280.5363.593. [DOI] [PubMed] [Google Scholar]
- 46.Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci USA. 2006;103(27):10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgescu RE, et al. Mechanism of asymmetric polymerase assembly at the eukaryotic replication fork. Nat Struct Mol Biol. 2014;21(8):664–670. doi: 10.1038/nsmb.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langston LD, et al. CMG helicase and DNA polymerase ε form a functional 15-subunit holoenzyme for eukaryotic leading-strand DNA replication. Proc Natl Acad Sci USA. 2014;111(43):15390–15395. doi: 10.1073/pnas.1418334111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen VQ, Co C, Li JJ. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature. 2001;411(6841):1068–1073. doi: 10.1038/35082600. [DOI] [PubMed] [Google Scholar]
- 50.Hardy CF, Dryga O, Seematter S, Pahl PM, Sclafani RA. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proc Natl Acad Sci USA. 1997;94(7):3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheu YJ, Stillman B. Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell. 2006;24(1):101–113. doi: 10.1016/j.molcel.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheu YJ, Stillman B. The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature. 2010;463(7277):113–117. doi: 10.1038/nature08647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheu YJ, Kinney JB, Lengronne A, Pasero P, Stillman B. Domain within the helicase subunit Mcm4 integrates multiple kinase signals to control DNA replication initiation and fork progression. Proc Natl Acad Sci USA. 2014;111(18):E1899–E1908. doi: 10.1073/pnas.1404063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiraga S, et al. Rif1 controls DNA replication by directing protein phosphatase 1 to reverse Cdc7-mediated phosphorylation of the MCM complex. Genes Dev. 2014;28(4):372–383. doi: 10.1101/gad.231258.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewer BJ, Fangman WL. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhong Y, et al. The level of origin firing inversely affects the rate of replication fork progression. J Cell Biol. 2013;201(3):373–383. doi: 10.1083/jcb.201208060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattarocci S, et al. Rif1 controls DNA replication timing in yeast through the PP1 phosphatase Glc7. Cell Reports. 2014;7(1):62–69. doi: 10.1016/j.celrep.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Davé A, Cooley C, Garg M, Bianchi A. Protein phosphatase 1 recruitment by Rif1 regulates DNA replication origin firing by counteracting DDK activity. Cell Reports. 2014;7(1):53–61. doi: 10.1016/j.celrep.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kemp MG, et al. Tipin-replication protein A interaction mediates Chk1 phosphorylation by ATR in response to genotoxic stress. J Biol Chem. 2010;285(22):16562–16571. doi: 10.1074/jbc.M110.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinoshita E, Kinoshita-Kikuta E. Improved Phos-tag SDS-PAGE under neutral pH conditions for advanced protein phosphorylation profiling. Proteomics. 2011;11(2):319–323. doi: 10.1002/pmic.201000472. [DOI] [PubMed] [Google Scholar]
- 61.Davey MJ, Indiani C, O’Donnell M. Reconstitution of the Mcm2-7p heterohexamer, subunit arrangement, and ATP site architecture. J Biol Chem. 2003;278(7):4491–4499. doi: 10.1074/jbc.M210511200. [DOI] [PubMed] [Google Scholar]
- 62.Sclafani RA, Tecklenburg M, Pierce A. The mcm5-bob1 bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdk1-dependent steps in Saccharomyces cerevisiae. Genetics. 2002;161(1):47–57. doi: 10.1093/genetics/161.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bousset K, Diffley JF. The Cdc7 protein kinase is required for origin firing during S phase. Genes Dev. 1998;12(4):480–490. doi: 10.1101/gad.12.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto S, Ogino K, Noguchi E, Russell P, Masai H. Hsk1-Dfp1/Him1, the Cdc7-Dbf4 kinase in Schizosaccharomyces pombe, associates with Swi1, a component of the replication fork protection complex. J Biol Chem. 2005;280(52):42536–42542. doi: 10.1074/jbc.M510575200. [DOI] [PubMed] [Google Scholar]
- 65.Murakami H, Keeney S. Temporospatial coordination of meiotic DNA replication and recombination via DDK recruitment to replisomes. Cell. 2014;158(4):861–873. doi: 10.1016/j.cell.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ivessa AS, Zhou JQ, Schulz VP, Monson EK, Zakian VA. Saccharomyces Rrm3p, a 5′ to 3′ DNA helicase that promotes replication fork progression through telomeric and subtelomeric DNA. Genes Dev. 2002;16(11):1383–1396. doi: 10.1101/gad.982902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dalgaard JZ, Godfrey EL, MacFarlane RJ. Eukaryotic replication barriers: How, why and where forks stall. In: Seligmann H, editor. DNA Replication—Current Advances. InTech; Rijeka, Crotia: 2011. pp. 14–24. [Google Scholar]
- 68.Katou Y, et al. S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature. 2003;424(6952):1078–1083. doi: 10.1038/nature01900. [DOI] [PubMed] [Google Scholar]
- 69.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12(3):259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 70.Viggiani CJ, Aparicio OM. New vectors for simplified construction of BrdU-incorporating strains of Saccharomyces cerevisiae. Yeast. 2006;23(14–15):1045–1051. doi: 10.1002/yea.1406. [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Moazed D. Association of the RENT complex with nontranscribed and coding regions of rDNA and a regional requirement for the replication fork block protein Fob1 in rDNA silencing. Genes Dev. 2003;17(17):2162–2176. doi: 10.1101/gad.1108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bianco JN, et al. Analysis of DNA replication profiles in budding yeast and mammalian cells using DNA combing. Methods. 2012;57(2):149–157. doi: 10.1016/j.ymeth.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Pasero P, Bensimon A, Schwob E. Single-molecule analysis reveals clustering and epigenetic regulation of replication origins at the yeast rDNA locus. Genes Dev. 2002;16(19):2479–2484. doi: 10.1101/gad.232902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson RE, Prakash L, Prakash S. Yeast and human translesion DNA synthesis polymerases: Expression, purification, and biochemical characterization. Methods Enzymol. 2006;408:390–407. doi: 10.1016/S0076-6879(06)08024-4. [DOI] [PubMed] [Google Scholar]
- 75.Kinoshita E, Kinoshita-Kikuta E, Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat Protoc. 2009;4(10):1513–1521. doi: 10.1038/nprot.2009.154. [DOI] [PubMed] [Google Scholar]
- 76.Zhang ZY, et al. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J Biol Chem. 1992;267(33):23759–23766. [PubMed] [Google Scholar]