Abstract
Spinal cord injury (SCI) impairs sensory systems causing allodynia. Measuring the development of allodynia in rodent models of SCI is challenging due to spinal shock and marked motor impairments. Assessment of SCI-induced allodynia is not standardized across labs, making interpretation of results difficult. Therefore, we validated sensory threshold assessment after SCI and developed a novel assessment of allodynia prior to motor recovery in a rat SCI model. One hundred fifty-six Sprague–Dawley rats received T8 laminectomy or mild to moderate SCI using the OSU SCI device (0.3 mm to 1.3mm cord displacement). To determine tactile thresholds, von Frey hairs (VFH) were applied in Up–Down or ascending order to the dorsal or plantar hindpaw. The most efficient and valid procedures that maintain high sensitivity and specificity were identified. Ten Up–Down VFH applications yielded stable thresholds; reducing the risk of threshold decay and unnecessary exposure to painful stimuli. Importantly, distraction of SCI-rats with food revealed differential decay of thresholds than when distraction is not provided. The new test uses dorsal VFH stimulation and is independent of trunk or hindlimb control. Acute dorsal VFH thresholds collected before recovery of hindlimb weight support accurately predicted plantar VFH thresholds measured at late timepoints (χ2=8.479; p<0.05). Thus, standardized testing early after SCI using the dorsal VFH test or later using 10 stimuli in the Up–Down test produces valid measures of tactile sensation across many SCI severities. Early detection of allodynia in experimental SCI will allow identification of mechanisms responsible for pain development and determine targets for therapeutic interventions.
Keywords: von Frey Hair, Allodynia, Rats, Withdrawal threshold, Distraction
Introduction
Over two-thirds of individuals with spinal cord injury (SCI) experience devastating effects of neuropathic pain on their daily lives (Crozier et al., 1991; Finnerup et al., 2001; Siddall et al., 2003, 1999a; Widerstrom-Noga et al., 2001). Allodynia, a common form of SCI-induced pain, occurs when normally innocuous stimuli produce painful responses.
While several models of SCI exist, the requisite cognitive and attentive aspects of pain in each model remain unclear. Aversive paw withdrawal to tactile stimulation serves as a proxy for pain but factors which influence movement will confound the measurement of sensation (i.e. naturally-occurring diurnal fluctuations in activity (Gobel and Cordes, 1990; Lemmer, 1991) and experimentally-induced paralysis or paresis). Thus, standardized procedures which control seemingly unrelated factors are especially important in SCI testing. However, many sensory testing approaches exist for SCI often with little description of key parameters like acclimation methods, testing environment, initial VFH force and number of VFH applications (Table 1). To date only one procedure appears to be standardized—all testing paradigms require hind limb motor control before testing begins. By necessity then, the earliest readout of sensation occurs weeks after SCI. Whether acute assessments can be developed remains unclear but represents an important, uncharacterized time point in below-level neuropathic pain development.
Table 1.
Assessments of below-level allodynia in rat models of spinal cord injury*.
| SCI model | Test methodology
|
Threshold
|
References | |||||
|---|---|---|---|---|---|---|---|---|
| Approach | Force | # of Stimuli | Behavioral response | Threshold determination | Non-Allodynic | Allodynic | ||
| Contusion | ||||||||
| Infinite Horizons 100, 150, 200 kdyne | Repetitive | 0.50, 1.01, 20.8 | 10/VFH | Withdrawal | – | – | – | 1 |
| Up–Down | ? | ? | Paw withdrawal | Dixon 1980 | 35–75 | 8–11 | 1,2 | |
| New York University/MASCIS Device 6.25, 12.5, 25, 50 mm wt. drop | Repetitive | 0.4, 1.0, 25.9 | 10/VFH | Withdrawal | – | – | – | 3 |
| Repetitive | 0.50, 1.01, 20.8 | 10/VFH | Withdrawal + SS | – | – | – | 4–6 | |
| Ascending | ? | ? | Withdrawal | 3/5 + responses | 26 | ~10 | 7,8 | |
| Ascending | ? | ? | Withdrawal + Vocalization | 5/10 + responses | 60 | 8–15.1 | 9–11 | |
| Up–Down | 4.97–108.3 | 10 | Withdrawal | Lowest VFH with >50% + responses | 20.82–50.45 | 9.80–14.32 | 12 | |
| Up–Down | 0.4–26 | 10 | Withdrawal | Dixon, 1980; Chaplan, 1994 | 14–22 | 3–8 | 13–19 | |
| Ohio State University electromagnetic device (0.3, 0.5, 0.7, 0.9, 1.1, 1.25, 1.3 mm cord displacement) | Up Down 20 with food distraction | 5.18–? | 20 | Withdrawal | Lowest VFH with >50% + responses | 75.858 | <28.84 | 20–22 |
| Excitotoxic | ||||||||
| Quisqualic acid microinjection | Electronic VFH | – | 3 | Withdrawal | Mean force of trials | 25–38 | 8–15 | 23–25 |
| Ischemic | ||||||||
| Photochemical (Xu 1992) | Ascending | 0.21–410 | 5–10/VFH | Vocalization | Lowest VFH with >75% vocalization | 73–90 | 4.6–10 | 26–38 |
| Clip compression (20–50 g) | Repetitive | 1.5 | 10 | Withdrawal + SS | – | – | – | 39–42 |
| Ascending | 0.41–5.51 | 10/VFH | Withdrawal + SS | VFH w/at least 5/10 + responses | 2.04–5.51 | 1.22–3.67 | 39 | |
| Up–Down | ?–15 | ? | Withdrawal | Chaplan 1994 | N15 | 2–9 | 43–47 | |
| Knife lesions | ||||||||
| T13 Lateral hemisection | Repetitive | 0.50, 1.01, 12.44 | 10/VFH | Withdrawal | – | – | – | 48 |
| Repetitive | 0.50, 1.01, 5.09 | 10/VFH | Withdrawal | – | – | – | 49 | |
| Repetitive | 0.50, 1.01, 20.8 | 10/VFH | Withdrawal | – | – | – | 50–52 | |
| Repetitive | 0.45, 1.01, 20.8 | 10/VFH | Withdrawal + SS | – | – | – | 53 | |
| Repetitive | 2.59 | 10 | Withdrawal | – | – | – | 54 | |
| Repetitive | 1.01 | 10 | Withdrawal | – | – | – | 55 | |
| Repetitive | 0.43, 1.01 | 10/VFH | Withdrawal | – | – | – | 56 | |
| Repetitive | 2 | 10 | Withdrawal | – | – | – | 57,58 | |
| Up–Down | 0.407–15 | ? | Withdrawal | Chaplan, 1994 | 17 g | 3–5 g | 59–63 | |
| Electronic VFH | – | 1 | Withdrawal | electronic readout | ~35 | ~15 | 64,65 | |
| Dorsal column lesion | Electronic VFH | – | 3 | Withdrawal | Mean force of trials | ~35 | – | 66,67 |
–=Not Applicable;
?=Not Provided;
SS=Supraspinal
12% of mechanistic papers fail to report sensory behavior.
(Hains et al., 2003, Hains and Waxman, 2006, Voda et al., 2007, Zhao et al., 2007b,a, Tan et al., 2008, Tan et al., 2009),
(Hao et al., 1991, Xu et al., 1992, Hao et al., 1996a,b, Yu et al., 1997, Hao et al., 1998, von Heijne et al., 1998, von Heijne et al., 1999, Hao et al., 2000, Xu et al., 2001, Kouya et al., 2002, Colpaert et al., 2004),
(Gwak et al., 2003, Gwak et al., 2007, Gwak et al., 2008, Liu et al., 2008, Gwak and Hulsebosch, 2009),
Currently, three methods detect below-level allodynia after experimental SCI by manipulating the frequency, quality or intensity of von Frey hair monofilaments (VFH) applied to the hindpaw (Table 1). The most common method relies on frequency of responses to sub- or supra-threshold stimulus. To be considered allodynia, more responses to sub-threshold stimulation must occur relative to normal. The two remaining paradigms define allodynia as a marked reduction in tactile thresholds. The ascending method applies VFHs in consecutive, ascending order until paw withdrawal occurs at least 50% of VFH applications (Chaplan et al., 1994). The Up–Down method determines the threshold by alternating VFHs according to the presence or absence (pattern) of paw withdrawal. Up–Down thresholds are calculated in two ways—using mathematical formulas established by Dixon (Chaplan et al., 1994; Dixon, 1965, 1980) or the 50% response threshold (Lindsey et al., 2000). While good validity exists for ascending and Dixon-derived thresholds in peripheral nerve injury models of allodynia (Chaplan et al., 1994), no validation for SCI exists.
Our primary aim is to determine sensory testing methodologies which deliver sensitive, valid and accurate estimates of sensation across a range of motor impairments, SCI severities and acute through chronic time points. For acute assessment, we developed and report validity results for VFH testing on the dorsum of the paw as early as 1 week post SCI. For subacute and chronic assessments, we employed our database of over 150 rats tested with Up–Down procedures to attain necessary design parameters. Analyzing this large population allowed for standardized testing conditions, thereby minimalizing the influence of small random effects, and the applicability of testing methods across the broad distribution of injury severity seen after SCI. We addressed four experimental objectives (Table 2) using tests of criterion-related validity, convergent validity, responsivity, and threshold stability. Importantly, the Up–Down measures were compared to two gold standard assessments ascending VFH and Dixon’s estimate of sensory thresholds (Chaplan et al., 1994; Dixon, 1965, 1980). This work was reported in abstract form (Clark et al., 2009; Detloff et al., 2008a).
Table 2.
Study objectives, types of sensory tests and distribution of rats by sex and SCI severity.
| Experimental objective | Sensory testing method | SCI severity/sex/rats |
|---|---|---|
| Determine the degree of equivalence for sensory thresholds derived from up down method and ascending method | Up down method with 20 stimuli vs. ascending with up to 90 stimuli n=11 |
SCI, Female n=5 Naïve, Female n=6 |
| Validate acute vs chronic sensory thresholds | Dorsal VFH testing at 7 dpi vs. plantar VFH testing at 28 dpi n=35 | 0.5 SCI, Male n=3 0.7 SCI, Female n=3 0.9 SCI, Male n=4 Female n=8 1.1SCI, Male n=3 Female n=8 Naïve, Female n=6 |
| Determine the fewest number of VFH stimuli required to produce valid sensory thresholds | Plantar VFH thresholds from 6, 10, 15 or 20 touches vs. Dixon’s mathematical estimates n=156 |
0.3 SCI, Female n=11 0.5 SCI, Female n=20 Male n=3 0.7 SCI, Female n=20 0.9 SCI, Female n=28 Male n=4 1.1 SCI, Female n=38 Male n=3 1.25 SCI, Female n=6 1.3 SCI, Female n=5 LAM, Female n=8 Naïve, Female n=10 |
| Determine whether sensory thresholds decay during plantar VFH testing | Plantar VFH testing with 20 stimuli with food distraction vs. without food distraction on a consecutive day n=167 |
0.3 SCI, Female n=11 0.5 SCI, Female n=19 Male n=3 0.7 SCI, Female n=20 0.9 SCI, Female n=28 Male n=4 1.1 SCI, Female n=43 Male n=3 1.25 SCI, Female n=6 1.3 SCI, Female n=5 |
Methods
Subjects and surgeries
One hundred fifty-six adult Sprague–Dawley rats (146 female, 10 male; 209–310 g; Harlan, Indianapolis, IN) were randomly assigned to one of nine groups: Naïve, laminectomy control (LAM), or SCI groups with injuries caused by 0.3 mm, 0.5 mm, 0.7 mm, 0.9 mm, 1.1 mm, 1.25 mm, 1.3 mm spinal cord displacements. By using a wide range of displacements, we assessed whether techniques to quantify tactile sensation are valid and perform consistently across injury severities with axonal loss from 0 (naïve, LAM) to >90% (1.25 or 1.3 mm). The distribution of rats for our specific research aims appears in Table 2. Rats were housed 2–3 per cage in a 12-h light-dark cycle with food and water at all times. All experimental procedures were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
Laminectomy and spinal cord contusion surgeries were performed as described previously (Kloos et al., 2005). Rats were anesthetized with ketamine (80 mg/kg)–xylazine (20 mg/kg) and given prophylactic antibiotics (gentocin, 1 mg/kg). After removing the T8 lamina, the spinal cord was rapidly displaced 0.3–1.3 mm using The Ohio State University Electromagnetic Spinal Cord Injury Device creating a broad range of injury severities from Mild to Severe (Jakeman et al., 2000). The incision was closed in layers and 5 cc of sterile saline was administered subcutaneously to prevent dehydration. All rats received antibiotics daily for 1 week following SCI and bladders were manually expressed twice daily until self-voiding occurred. Oral Vitamin C was given to all rats daily until sacrifice to prevent urinary tract infections (Behrmann et al., 1992).
Histology
Rats underwent transcardiac perfusion with 0.1 M phosphate buffered saline then 4% paraformaldehyde followed by sucrose cryoprotection. The lesion site was transversely sectioned (20um) and stained for myelin using luxol fast blue or eriochrome cyanine. The section with the largest lesion and least amount of stained white matter represented the lesion epicenter. The area of stained white matter at the epicenter divided by the total cross sectional area of the cord served as a measure of injury severity (Kloos et al., 2005).
Behavioral measures
All behavioral tests were conducted by raters who were masked to SCI severity. To determine valid and responsive measures of below-level sensation across a broad range of SCI, we compared 3 different techniques—two approaches that are well-established for peripheral nerve injury (ascending, Up–Down) and a new modified ascending technique for the dorsum of the paw (dVFH). All sensory testing techniques were conducted using von Frey hair monofilaments (VFH, Stoelting Co., Wood Dale, Illinois) with bending forces calibrated from 6 g to 125.9 g. To eliminate diurnal variation on sensation, all sensory testing for each technique occurred at the same time of day (Gobel and Cordes, 1990; Lemmer, 1991). Additionally, all rats were isolated in a separate room during testing to prevent unfavorable visual, olfactory and ultrasonic auditory cues between rats that produce anticipatory hypervigilant behavior and aberrant sensory results (Basso, 2004; Brudzynski and Chiu, 1995; Brudzynski and Ociepa, 1992; Calvino et al., 1996; Cuomo et al., 1992).
Ascending von Frey Testing Procedures
Testing environment
Rats were placed in an inverted Plexiglas cage (20 cm×9 cm×10 cm) with a wire mesh bottom (0.635 cm grid size) allowing access to the plantar surface of the hind paws. To prevent visualization of VFH application to the paw and reduce apprehension and freezing behavior, sugared cereal was provided throughout acclimation and testing. Rats were tested only when eating sugared cereal. Rats actively eat before, during and after the stimulus thereby preventing the conditions for Pavlovian learning and providing us with a method to standardize attention across animals with different injury severities.
Application of the tactile stimuli
The purpose of the ascending method is to begin sensory testing in a range well below the sensory threshold and gradually increase the stimulus strength until the sensory threshold is crossed. The lowest VFH (6.0 g) was applied perpendicularly to the mid-plantar hindpaw approximately 1 cm posterior to the footpad of the middle phalange at a slow, consistent speed until it bent, then it was removed (~1 s). Immediate, brisk withdrawal of the paw to VFH application constituted a positive response (Detloff et al., 2008b; Hutchinson et al., 2004; Kloos et al., 2005; Lindsey et al., 2000). The VFH was applied to the paw 10 times with each touch separated by 30–60 s to avoid wind-up. The number of positive responses to 10 touches was recorded. Any stimulus that lifted the paw, thereby producing proprioceptive rather than tactile input, was discarded and retested after an interstimulus delay of at least 30–60 s. The bending force of the VFH stimulus was logarithmically increased every 10 applications until a≥50% paw withdrawal response occurred or the highest VFH was reached (125.9 g). A total of 90 stimulus applications are possible with the ascending method (9 VFHs applied 10 times each). The tactile sensory threshold equaled the weakest force to elicit paw withdrawal on at least 50% of the stimulus applications (Chaplan et al., 1994).
Testing schedule
Rats were habituated to the testing environment for twenty minutes once a day for 5 days prior to testing. After SCI, plantar VFH testing began when evidence of hind limb motor control (i.e. weight support) recovered as evidenced by a score of 9 during Basso Beattie Bresnahan Locomotor Rating (BBB; (Basso et al., 1995)). Recovery of hind limb weight support is dependent upon lesion severity, with the majority of SCI-rats recovering stepping between 7 and 21 days after injury. This criterion ensured that all rats had sufficient trunk control to enable hindpaw withdrawal from an unpleasant stimulus. The dependence of plantar testing on moderate motor recovery necessarily precludes early sensory testing (Detloff et al., 2008b; Hutchinson et al., 2004; Kloos et al., 2005; Lindsey et al., 2000).
Up–Down testing procedures
Testing environment
The testing environment described for ascending methods was used for Up–Down testing.
Application of tactile stimuli
The purpose of the Up–Down method is to cross the sensory threshold several times and successively titrate the stimulus strength in both sub- and supra-threshold ranges to reach the true sensory threshold. Testing began with the 15.14 g VFH applied perpendicularly at a slow, consistent rate (~1 s) to the L5 plantar dermatome of the paw (1 cm posterior to the foot pads, as described above in the Ascending Testing Procedures). When a brisk, immediate paw withdrawal occurred, the next lower VFH was applied. When no hindpaw withdrawal occurred, the next higher VFH was applied. Twenty stimulus applications were used and approximately 30–60 s separated each touch. In the rare occurrence when a VFH lifted the paw, the trial was discarded and retested following the standard interstimulus interval. The tactile sensory threshold was defined as the lowest gram force to produce hind paw withdrawal on at least 50% of its applications.
Testing schedule
All rats were habituated for one twenty minute session per day across 5 days before baseline testing occurred. Postoperative Up–Down testing began once rats demonstrated weight support and some hind limb motor control during open field BBB testing (BBB score of 9).
Dorsal von Frey Testing Procedures
Testing environment
The purpose of dVFH testing is to identify tactile sensory thresholds during the acute period of SCI, before weight support or trunk control recovered. The head and forequarters of each rat were wrapped loosely in a clean hand towel which prevented visualization of the VFH stimulus, provided stability and trunk control and produced a calming effect (possibly due to the dark environment). Using the towel wrap, the examiner supported the rat so that the hindpaw lightly rested on a table surface.
Application of tactile stimulus
Assessment began with the 6.0 g VFH and continued in an ascending progression. Each VFH was applied perpendicular to the dorsal surface in the same dermatome as testing on the plantar surface of the hind paw between the first and second metatarsal approximately 1 cm proximal to the joint. Aversive hind paw withdrawal consisted of paw movement posteriorly or externally while flexing the hind limb. Each VFH was presented 3 times and the number of hind paw withdrawals was recorded. The next larger VFH was applied unless paw withdrawal occurred in at least two of the three applications or until the largest VFH was reached (125.9 g). The initial stimulus was applied only after the rat was calm and quiet in the hand towel for at least 10 s. The standard interstimulus interval of 30–60 s was applied for every subsequent stimulus application. Tactile sensory threshold was determined as the lowest force which elicited paw withdrawal at least 66% of the time. We used 3 trials per VFH in the dVFH test because it reduced testing time, prevented the risk of wind up and minimized exposure to potentially nociceptive stimuli.
Testing schedule
Rats were acclimated to the testing environment for one ten minute session per day across 5 days before testing occurred. Because of the shorter testing time, acclimation times to the testing environment was shortened due to the fact that assessment using dVFH methods requires less time than conventional plantar VFH techniques. Importantly, by the end of the 5 sessions, rats were calm and comfortable with the testing environment. Testing began at 7 dpo to avoid the period of spinal shock.
Mathematically derived threshold estimate
Methods used in peripheral nerve injury models suggest that the pain threshold can be estimated with as few as 6 Up–Down VFH applications (Chaplan et al., 1994; Dixon, 1965, 1980). Since we do not know whether tactile sensory thresholds can be estimated with so few trials after SCI, we utilized one of two formulas established by Dixon to estimate thresholds when at least 6 VFH stimuli are applied (Dixon, 1965, 1980). Both formulas are designed to estimate sensory thresholds based on the pattern and frequency of withdrawal responses during Up–Down VFH testing. A negative (−) response requires the next higher force be applied, while a positive (+) response leads to a lower applied force on the successive VFH application. N′ is the absolute number of responses recorded in the hind paw test (For Fig. 1, N′=20). Although all N′ responses were recorded in the hindpaw test, the Dixon threshold estimation procedures only include stimuli that are close to the actual threshold (Dixon, 1980). That is, a string of like responses (i.e. all negatives (−) or all positives (+)) produced at the beginning of the trial (i.e. as the threshold is being approached) are excluded from the calculation of the mathematical estimate. N is the number of stimulus applications included in the threshold estimate analysis. It is the total number of stimuli applied reduced by one less than the string of like responses at the beginning of the series. Importantly, the last stimuli before the sensory threshold is first crossed (the first hindpaw withdrawal) is included in the sample N. In other words, the two stimuli that first straddle the pain threshold are included in the sample N and are integral in accurately assessing Dixon’s threshold estimates (for Fig. 1, N=19). Conceptually, the first hindpaw withdrawal indicates the sensory threshold has been crossed and subsequent delivery of sub and suprathreshold VFHs should produce alternating + and − responses. The true sensory threshold is bracketed by the positive and negative responses to graded stimulus strengths (Fig. 1; 58.82 g force; solid line). When N=6 stimuli are included, the following formula was used: 50% g Threshold = xf + kd; where xf is the force of the last VFH applied, k is a tabular value based on the pattern of + and − responses to stimuli presented after the sensory threshold is crossed from a table presented by Dixon (Dixon, 1980), and d is the mean difference (in log units) between applied stimuli (i.e. highest minus lowest applied force (Chaplan et al., 1994; Dixon, 1980)). For example, xf=28.84 g, k=0.737, and d=0.42 and the 50% g Threshold estimate is 58.82 (Fig. 1).
Fig. 1.
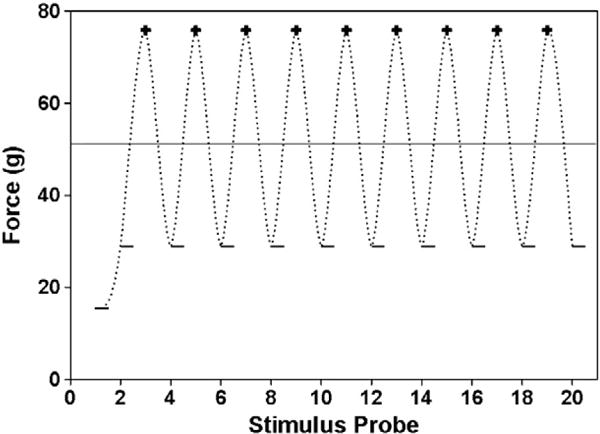
Presence (+) or absence (−) of paw withdrawal to 20 successive von Frey stimuli. In the Up–Down 20 and Dixon derived thresholds, the behavior of the hind paw to the stimulus influences the next force which is to be applied. When paw withdrawal is absent, stronger stimuli are applied until withdrawal returns. Then, lighter stimuli are delivered until the paw fails to withdraw. The perceptual threshold lies between the positive and negative responses. Once the threshold is crossed, this method should produce alternating positive and negative responses. The reported pain threshold for the Up–Down 20 method in this case would be 75.86 g (the lowest gram force that produced hind paw withdrawal at least 50% of its applications), while Dixon’s mathematical threshold estimate is bracketed by the positive and negative responses (53.17, solid line). Importantly, the precision of the derived threshold depends on the range of applied forces. Under normal conditions, there is a large range in applied forces due to the logarithmic design of the von Frey stimuli, producing less precise thresholds.
To determine whether higher accuracy of the threshold estimate could be obtained, we examined estimates when N′=10, 15, or 20 applied stimuli. Using similar criteria, a string of like responses at the beginning of the test were excluded, and the remaining stimuli were incorporated into the following formula that accommodates larger data sets: 50% g Threshold=(ΣXi/N)+(d/N)×(A+C), where N is the number of trials after the threshold is first crossed, Xi is the force applied during each trial, d is the mean difference between applied stimuli, and values for A and C can be determined from a table published by Dixon (Dixon, 1965). In the table, n0 refers to the number of O’s and nx refers to the number of X’s in the final N trials. For example, when N=19, ΣXi=107.52, d=0.42, A=1.53 and C=0.16 leading to a threshold estimate of 49.69 g force (Fig. 1). These threshold estimates were determined for N=6 in replication of methods established by Chaplan (Chaplan et al., 1994) and for N′=10, 15 or 20 responses and compared to the thresholds obtained by the Up–Down method mentioned above. The term Up–Down 20 means that 20 stimuli were applied and so forth.
Analysis of threshold decay
Tactile sensory thresholds can decay during a testing session if too many stimuli are applied, if stimuli are applied in rapid succession (wind-up) or if the rat sees the stimulus as it is applied. For Up–Down testing, we defined threshold decay as 3 or more consecutive positive responses to successively lower VFH forces after crossing the threshold (first +; see Fig. 4B). In 292 hind paw Up–Down 20 tests (20 trials) with food, we determined the prevalence of threshold decay, at what point in the stimulus train threshold decay occurred, and whether threshold decay changed the tactile sensory classification (i.e. Allodynic or Non-Allodynic). In a subset of rats (n=11), we compared the incidence of threshold decay with and without food measured 5–7 days apart at 28–35 dpo.
Fig. 4.
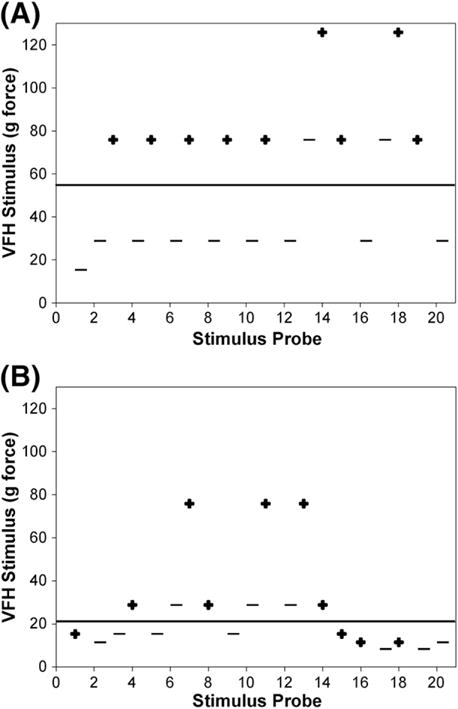
Positive and negative responses to von Frey stimuli throughout a single testing session. (A) Representative example of non-allodynic response pattern during Up–Down 20 testing after spinal cord injury. The hind paw responds with alternating positive (+) and negative (−) responses successively, thereby bracketing the Dixon mathematical threshold estimate (solid line; 57.51). (B) Response patterns demonstrating 3 successive positive responses or threshold decay (denoted by the bracket) after SCI. Threshold decay drastically reduces the pain threshold and heightens sensitivity to tactile stimuli (Dixon estimate=20.99; solid line). It most often occurs in the latter half of the test session, during stimulation of the second hind paw. Normal oscillatory and decay patterns are seen irrespective of non-allodynic or allodynic classification.
Statistical analyses
Tests of criterion-related and convergent validity included Cohen’s Kappa, Chi-Square analysis, regression and other descriptive statistics (sensitivity, specificity, negative predictive value, positive predictive value, percent agreement) were calculated to determine the fidelity of the Up–Down 20 testing methodology to gold standard techniques as well as compare the predictability of early dorsal VFH for later plantar testing. Threshold stability was compared using a one-way ANOVA with Tukey’s post hoc was performed to determine if food during VFH testing altered the paw withdrawal threshold. Correlations were determined using Spearman’s Rank test. Data are represented as mean±SEM. To determine responsiveness, we compared actual threshold values to Dixon estimates for 6, 10 and 15 VFH applications.
Results
The Up–Down method accurately identifies normal and allodynic-like sensation after SCI
To identify tactile withdrawal thresholds across SCI severities, we observed sensory recovery in 156 rats after mild, moderate, or severe SCI. We selected 28.84 g as the cut off for the allodynic-like threshold. The mean threshold for responses to below-normal stimuli in SCI rats ranged from 12.4 to 20.4 g. For 5 of 7 SCI severities examined in this meta-analysis, the threshold lay between 15 and 28 g; therefore, we selected 28.84 g as the cut off to indicate allodynic-like thresholds. This threshold represents a reduction of stimulus strength greater than 50 g which still elicits paw withdrawal.
Analysis of spared white matter revealed that the incidence of tactile allodynia increased with injury severity (Fig. 2). After SCI, paw tactile sensitivity dropped by 63% to at least 28.8 g force compared to 75 g force of naïve rats (χ2=7.103, p<0.01). Using 28.8 g force as the allodynic threshold, 50–83% of rats with moderate to severe SCIs developed below-level hypersensitivity compared to only 7% of rats with mild injuries and 0% of Naïve or LAM groups (Fig. 2). If white matter sparing was greater than 10–15%, then rats have a 1 in 5 chance of developing hind paw allodynia; however, if less than 10% white matter is spared after SCI, then 4 out of 5 rats will develop below-level hypersensitivity. The Up–Down technique yielded almost no false positive withdrawals due to lifting the paw with the VFH (2.5±0.6%). The rate was ~1 lift in 60 VFH applications. To establish criterion validity, we compared Up–Down 20 derived sensory thresholds to the gold standard Ascending method. Rats were classified into allodynic and non-allodynic groups based on a 28.8 g (+5 g) force cut-off. The Up–Down 20 method demonstrated perfect agreement (100%) with the gold standard classifications (κ=1.0). The thresholds from Up–Down 20 strongly correlated to ascending thresholds (Fig. 3; r=0.90; p<0.0001). High sensitivity, specificity, negative and positive predictive values (100%, for each variable) confirmed that Up–Down 20 performs synonymously to the Ascending method.
Fig. 2.
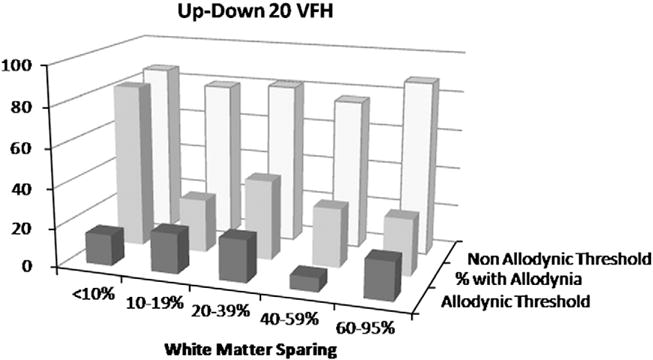
Mean allodynic and non-allodynic tactile sensory thresholds grouped by percent white matter sparing (WMS) for von Frey Up–Down testing with 20 stimulus applications. The highest percentage of rats displaying allodynic thresholds (middle row) had <10% WMS. Note that the allodynic threshold remains constant regardless of the severity of SCI. Non-allodynic thresholds remained stable across all SCI severities despite a wide percentage of those responding (17–72%).
Fig. 3.
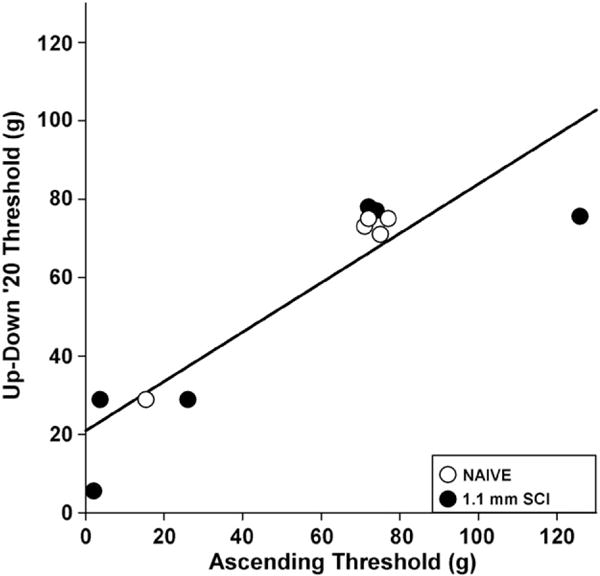
Validation of Up–Down ‘20 von Frey hair testing protocol. Similar tactile sensory thresholds were derived when applying the Up–Down ‘20 VFH protocol or the gold standard Ascending method (n=12; R=0.90, p<0.001). Categorization of hind limb responses using Up–Down ‘20 and Ascending methods into allodynic or non-allodynic groups resulted in perfect agreement between the two testing paradigms (κ=1).
Accurate tactile sensory thresholds are derived with only ten VFH applications after SCI
To establish convergent validity of the Up–Down 20 methods, we compared actual sensory thresholds to mathematically-derived threshold estimates using the Dixon equation for increased number of stimuli (Dixon, 1980). Dixon estimates have been validated for allodynic sensory thresholds after peripheral nerve injury (Chaplan et al., 1994). Hind paws were classified into allodynic and non-allodynic clusters based on Up–Down 20 actual thresholds. Excellent fidelity occurred between actual Up–Down 20 thresholds after SCI and mathematic estimates for both allodynic (97.56% agreement) and non-allodynic clusters (78.44% agreement; Table 3). Thus, the Up–Down 20 thresholds strongly agreed with validated threshold estimates for each cluster (κ=0.71).
Table 3.
Sensory Thresholds derived from Dixon Formulas or 50% withdrawal responses for 6–20 Up–Down stimulus applications and their agreement to standardized Up– Down ‘20 tests.
| Allodynic threshold
|
Non-allodynic threshold
|
|||||
|---|---|---|---|---|---|---|
| Stimuli | Mean±SEM | n | Agreement with Up–Down 20 | Mean±SEM | n | Agreement with Up–Down 20 |
| Dixon method | ||||||
| N=6 | 24.86±2.47 | 123 | 80.49% | 61.36±3.30 | 169 | 75.74% |
| N′=10 | 18.86±0.92 | 123 | 93.50% | 47.23±1.38 | 169 | 76.92% |
| N′=15 | 18.22±0.80 | 123 | 95.93% | 46.06±1.29 | 169 | 72.18% |
| N′=20 | 18.34±0.78 | 123 | 97.56% | 47.48±1.19 | 167* | 78.44% |
| Up–Down 20 method | ||||||
| N=6 | 25.60±1.93 | 123 | 86.17% | 67.16±1.93 | 169 | 79.29% |
| N′=10 | 25.03±1.80 | 123 | 88.62% | 74.52±2.02 | 169 | 84.62% |
| N′=15 | 21.15±1.56 | 123 | 92.68% | 73.18±2.08 | 169 | 82.84% |
| N′=20 | 18.75±0.76 | 123 | 81.48±1.22 | 167* | ||
n=2 removed due to non-biologic threshold estimate.
The standard Up–Down 20 method relies on 20 applications to quantify sensory thresholds after SCI, but in peripheral nerve injury models, far fewer stimuli are needed. We determined the fewest Up–Down applications needed to accurately identify sensory thresholds after SCI. For each cluster, we calculated Dixon’s mathematical threshold estimate for 6, 10, or 15 stimuli and compared them to the standard Up–Down 20 threshold (Table 3). Thresholds derived from 10 or 15 stimulus applications using Dixon’s mathematical estimates showed nearly perfect agreement to actual thresholds for the allodynic cluster (93.5–95.93%, Table 3). Non-allodynic thresholds also demonstrated 72.18–76.92% agreement between estimated and actual values for 10 or 15 applications. Taken together, as few as 10 Up–Down stimuli are needed to accurately detect allodynic and non-allodynic sensory thresholds after SCI. Sensory thresholds derived from only 6 stimuli after SCI had the lowest agreement with Up–Down 20 (κ=0.48) compared to estimates using 10, 15, or 20 stimulus applications (κ=0.63, 0.65, 0.7,1 respectively).
Testing without food distraction increases tactile threshold decay
In the Up–Down method, failure to remove the paw from the stimulus indicates a lack of painful sensation and necessitates stimulation with a larger, more forceful VFH until withdrawal occurs. It assumes that the first positive response after a negative stimulus is near the pain threshold and all subsequent Up–Down trials will alternate around this threshold. Thus, a string of positive responses indicates a decay in the sensory threshold possibly due to wind-up, heightened attention and/or hypersensitization. We define threshold decay as three or more consecutive positive responses (+) to successively lower VFH forces after a single negative (−) response (Fig. 4). Under standard Up–Down 20 methods, 22% of hind paws exhibit threshold decay. Of those with decay, 71% occurred in allodynic ranges (<28.8 g) and 29% occurred in non-allodynic ranges. This discrepancy likely reflects the smaller range of forces tested within the allodynic thresholds compared to the broad logarithmic range in non-allodynic thresholds. A typical Up–Down 20 test classified as allodynic may span only 8 g force over 4 VFHs; while a non-allodynic test would cover 110 g force over 4 VFHs, making decay less likely to occur. Interestingly, in instances of decay, the classification of the sensory threshold as allodynic or non-allodynic rarely changed (27%).
To ensure that limb withdrawal is due to tactile stimulation, visualization of stimulus application by the subject must be avoided. Current lab protocols maximize distraction and preclude visualization of the VFH by ensuring that the rat accepts and eats sugared cereal throughout testing. However, pairing food with a withdrawal response may impose Pavlovian learning. To determine whether the presence or absence of food during testing affected Up–Down 20 thresholds after SCI, we compared the same rats 5–7 days apart. Seventy-three percent of rats were classified identically whether thresholds were obtained with or without food distraction. Surprisingly, withholding food increased sensory decay to 55% of hind limbs compared to 27% with food provided (Fig. 5). Since the incidence of threshold decay was different when the rat was tested in the presence or absence of food suggests that supraspinal modulation of the paw withdrawal reflex occurs after incomplete SCI.
Fig. 5.
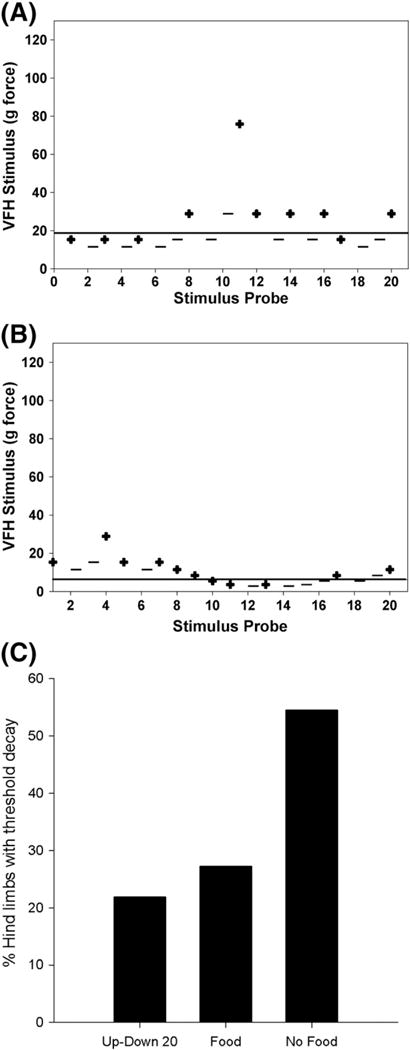
Maximizing distraction reduces the incidence of threshold decay. (C) Twenty-two percent of hind paws (n=292) tested with the Up–Down 20 method showed evidence of tactile threshold decay. In a subset of rats (n=11), tactile thresholds were measured with and without food distraction in the same SCI and naive rats. As expected, providing food distraction did not alter sensory thresholds. However, absence of food during sensory testing increased threshold decay by 28% (p<0.01). (A, B) Representative histograms depict a hind paw tested using Up–Down 20 methods 5 days apart with food in A and without food distraction in B. Note the robust threshold decay that occurs when food is not provided (string of positive (+) responses denoted by the bracket). The differential response that is elicited when distraction is or is not provided is indicative of some level of supraspinal processing.
Novel dorsal VFH test accurately and reliably predicts Up–Down thresholds at chronic timepoints
Dorsal VFH thresholds obtained acutely at 7 days after SCI had high agreement (79–80%) with those derived using Up–Down methods (Table 4; κ=0.32). Dorsal thresholds maintained high agreement across a broad range of contusion severities (Fig. 6A), in both males and females (data not shown). These data confirm previous findings from our laboratory that allodynic thresholds are an all-or-none response which occur in more severe contusions (Kloos et al., 2005). Importantly, regression analysis revealed that dorsal thresholds at 7 dpo strongly predicted plantar thresholds at chronic time points (Fig. 6B; r=0.67; p<0.01). Chi square, sensitivity and specificity analyses showed that the new Dorsal VFH method identifies tactile sensory thresholds acutely as well as predict chronic hypersensitivity after SCI with 100% accuracy (Fig. 6B; χ2 = ; sensitivity = 1; specificity =1; positive predictive value=1; negative predictive value=1). Normal sensory thresholds were predicted with 100% accuracy when compared to the Up–Down 20.
Table 4.
Novel dorsal VFH method at 7 days after SCI predicts chronic tactile sensory thresholds detected via Up–Down *20 paradigm.
| Allodynic threshold
|
Non-allodynic threshold
|
|||||
|---|---|---|---|---|---|---|
| Stimuli | Mean±SEM | n | Agreement with Up–Down 20a | Mean±SEM | n | Agreement with Up–Down 20a |
| Dorsal VFH | 15.59±2.48 | 10 | 80.00% | 43.03±4.48 | 33 | 78.79% |
| Up–Down 20 | 15.53±1.25 | 10 | 63.07±4.27 | 33 | ||
Percent hind limbs with the same threshold classification using both methods.
Fig. 6.
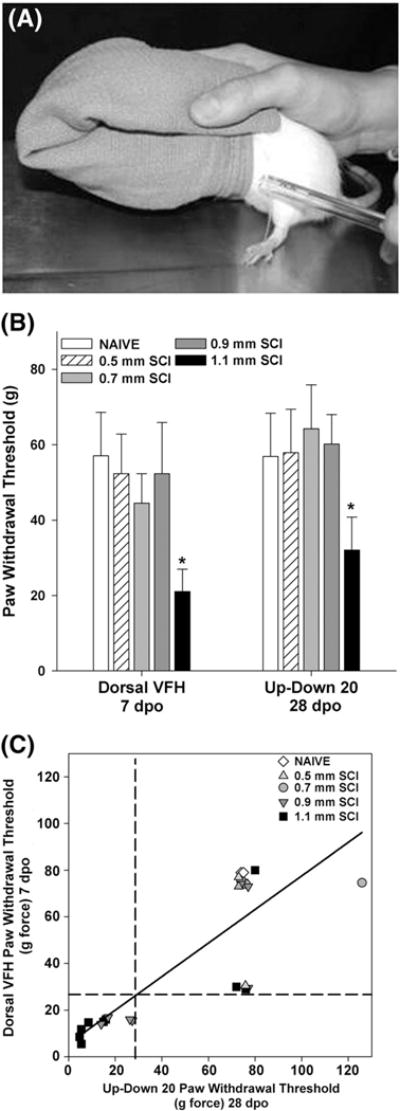
Early dorsal von Frey Hair (DVFH) sensory thresholds predict allodynic and non-allodynic tactile sensitivity across injury severities at later time points. (A) A side view of the technique to hold the rat while applying von Frey hairs to the dorsal surface of the hind paw. (B) Comparable sensory thresholds occurred between 7 dpo Dorsal VFH and 28 dpo Up–Down 20 measures after SCI. Remarkable similarity occurred for a wide range of SCI, with significant allodynia noted in more severe SCI (p<0.05 1.1 mm displacement vs. all other groups). (C) Strong agreement between the novel Dorsal VFH test and standard Up–Down methods is evident in a significant positive correlation (n=44; Pearson Coefficient=0.503, p<0.01, r2=0.67). Dashed lines show the cutoff threshold for allodynic sensation using either the new Dorsal VFH or Up–Down 20 techniques and indicate that allodynic thresholds determined at 7 dpo using the DVFH test predicts chronic below-level allodynia determined by Up–Down 20 methods.
Discussion
This study strived to establish behavioral approaches necessary to elucidate relevant mechanisms of neuropathic pain in animal models of SCI. Without clinically-relevant SCI models and valid sensory testing paradigms, the risk of investigating mechanisms which are not applicable to people with SCI-induced pain remains high. The present study demonstrates excellent convergent and criterion-related validity of the Up–Down 20 method for below-level tactile thresholds after SCI having complete agreement with the Ascending method and strong agreement with Dixon’s mathematical threshold estimates. Furthermore, good validity and accuracy of sensory thresholds occurs with only 10 Up–Down VFH applications, thereby limiting exposure to noxious stimuli and increasing efficiency. Providing food distraction reduced threshold decay and improved the accuracy of Up–Down testing. An inherent limitation of plantar Up–Down testing is the dependence on motor recovery, trunk stability and weight support. We developed a novel dorsal VFH test which overcomes these limitations and derives accurate and valid tactile thresholds of the paw as early as 7 days after SCI. It detects tactile thresholds within allodynic and non-allodynic ranges across lesion severities and predicts chronic Up–Down thresholds with good fidelity.
Evidence for using a rat contusion model to study SCI-induced allodynia
The OSU spinal cord contusion model is a valid model of below-level allodynia with strong similarities to human SCI. Lesion shape, size and type in experimental SCI correspond to clinical presentation (Bresnahan et al., 1991; Bunge, 1994; Bunge et al., 1997). The resulting sensory impairments in experimental models mimic those in people with SCI and may foreshadow similar cellular responses (Basso et al., 1996; Detloff et al., 2008b; Kloos et al., 2005; Siddall et al., 2003, 1999a). Remote inflammation after SCI may be one cellular mechanism of neuropathic pain given its common incidence in people and animals (Detloff et al., 2008b; Hains and Waxman, 2006; Peng et al., 2006; Zhao et al., 2007a,b) with SCI-induced allodynia. That light, innocuous touch evokes nocifensive responses in rats and pain responses in people with SCI further strengthens the translational relevance of our experimental model (Detloff et al., 2008b; Hutchinson et al., 2004; Kloos et al., 2005; Siddall and Loeser, 2001). Evidence that food distraction stabilizes sensory thresholds after experimental SCI provides support for the translational relevance of contusion models and points to retention of supraspinal processing in allodynic conditions.
The relative alignment of behavioral and cellular evidence of pain between experimental SCI and clinical presentation does not fully establish that aversive withdrawal to innocuous stimuli indicates allodynia. A critical distinction unresolved by the current study is whether withdrawal is due to hyperreflexia or allodynia. For this paper, our interest was to identify valid, behavioral testing techniques which provide a stable measure of threshold detection in rats with SCI. Whether those thresholds reflect hyperreflexia or pain remains to be determined. Regardless, valid behavioral measures which are responsive to change enable scientists to identify mechanisms and treatments for either of these two devastating clinical conditions.
Current methods to assess below-level allodynia
It is critical to accurately identify and classify aberrant sensory behavior after SCI; yet no assessments of below-level allodynia to date have been validated. Widely-used approaches quantify the shift in sensory threshold from normal to hypersensitivity using Ascending or Up–Down techniques. An alternative approach uses withdrawal frequency to normally innocuous and noxious stimuli and does not determine sensory threshold (Table 1). Since SCI-induced allodynia is an all-or-none response (Kloos et al., 2005), small improvements in tactile thresholds may represent the difference between chronic severe pain and near normal quality of life for people with SCI. Thus, methods which derive presumptive pain thresholds may facilitate better translation of mechanistic studies to the clinic. Stable thresholds can be attained in experimental SCI models under well-controlled testing conditions. By minimizing confounders like time of day, stress, anticipation, and inter-rat communication, we generated remarkably similar sensory thresholds using three threshold-testing paradigms—Up–Down, Ascending and dorsal VFH. Thresholds ranged from 2.04 to 28.8 g for allodynic rats and 38.68 to 125.8 g for non-allodynic rats across the three paradigms. Moreover, we classified rats into allodynic and non-allodynic classes with near-perfect agreement across the three methods (κ=0.71–1.0; Figs. 2 and 3 and Table 3). That is, using Up– Down and Ascending methods yield the same numerical threshold. Importantly, our standard Up–Down 20 method demonstrated high criterion-related validity with the ascending approach and strong convergent validity with Dixon threshold estimates. That the Up–Down paradigm could generate the same paw withdrawal threshold as the Ascending method with at times 30 fewer stimulations, limits confounders like anxiety and stress on the animal’s well-being as well as the threshold value.
Differences in normal sensory thresholds exist between SCI studies and peripheral nerve injury (Table 1, (Chaplan et al., 1994)). Normal thresholds generated in our SCI studies are markedly higher indicating that stronger VFH forces produced patterned withdrawals. It does not appear that our thresholds are specific to SCI given that similar allodynic and non-allodynic thresholds occurred for SCI and spinal nerve ligation (Detloff et al., 2008a).
Effect of cognitive distraction on stabilization of below-level sensory thresholds
To generate authentic sensory thresholds after SCI, our data suggest that reducing the frequency of stimulation and providing food distraction improve validity and reduce threshold decay. Threshold decay represents an artificially low sensory threshold typified by a string of withdrawal responses to successively lighter tactile stimuli (Chaplan et al., 1994). Normally, the Up–Down method produces an alternating response pattern as stimulus strength increases or decreases. During decay, the alternating pattern is replaced by a train of positive responses to ever lighter stimuli. Threshold decay in naïve rats occurred with 81 stimuli and approximated neuropathic pain levels (Chaplan et al., 1994). In SCI rats with threshold decay, 15 stimuli initiated this decay when no distraction was provided. Therefore, fewer than 15 VFH applications should be used to derive thresholds when no food distraction is given. Up to 30 stimuli can be used with food distraction before decay occurs. While Dixon and others suggest that 6 stimuli are sufficient to generate valid threshold estimates (Chaplan et al., 1994; Dixon, 1965, 1980), we found 6 to be the least effective as it produced the highest variability, lowest convergent validity and least agreement with other testing methods. We show good agreement and convergent validity for below-level sensory thresholds derived when 10 stimuli are applied. Whether Up–Down testing with only 6 stimuli produces valid tactile thresholds for above- or at-level allodynia after SCI remains to be determined. Taken together, our data suggest that any paradigm (response frequency or threshold derivation) which applies more than 10 stimuli to an undistracted SCI-rat or 30 stimuli to a distracted rat will likely result in invalid sensory assessments.
The cellular mechanisms of threshold decay have not been elucidated, however we show that supraspinal processing protects against decay. By providing food distraction during testing, sensory thresholds were more stable and decay occurred in relatively low numbers (<25% for an individual study and across our population to date). Threshold decay was twice as likely when distraction was not provided. The robust increase in the incidence of decay cannot be easily explained by stimulus visualization, stress, or anxiety as there was no freezing behavior, pyloerection of the fur, or porphorin release in the non-distracted condition. Rather, food distraction may be minimizing hypervigilance, an anticipatory state in which the threat of pain activates fear and emotional centers in the brain (Brown and Jones, 2008; Burgmer et al., 2009; Ushida et al., 2005). Perhaps the most dramatic example of hypervigilance in rodents occurs when naive animals observe painful responses in cagemates and then execute hyperalgesic responses themselves (Langford et al., 2006). A variety of interventions at the supraspinal, cognitive processing level, including food distraction, stabilizes sensory perception in humans and animals by tempering hypervigilant behavior (Aminabadi et al., 2008; Casey and Morrow, 1983; Ford et al., 2008; Hoffman et al., 2008) and potentially modulating mu opioid receptors (Fields, 2004; Mason, 2005). While food distraction does not completely eliminate threshold decay in our hands, it provides important evidence that our below-level testing methods are likely capturing supraspinal involvement associated with the withdrawal response. Without distraction, these testing paradigms may be more heavily dependent on spinal cord mediated mechanisms which requires further study.
Early detection of sensory dysfunction after SCI
The Dorsal VFH test was created to determine pain thresholds of the hindpaw acutely after SCI when below-level function is limited. The Dorsal VFH test employs a modified ascending method which minimizes the number of painful stimulations (at most 3) and reduces the possibility of wind-up, hypervigilance and threshold decay. Using predictive validity tests, we established that Dorsal VFH testing as early as 1 week after SCI accurately estimates chronic Up–Down pain thresholds across a range of injury severities (Table 4; Fig. 6) making it a powerful tool in determining allodynic mechanisms after SCI. Indeed, we recently showed that proinflammatory cytokines in L5 dorsal horn were positively correlated with allodynia measured dorsally, further validating the technique (Detloff et al., 2008b). To our knowledge, this is the only below-level tactile sensory test capable of producing valid thresholds during acute SCI.
Conclusions
Below-level allodynia is a complex perception that results from a plethora of SCI-induced factors which drive its development and persistence. Ameliorative treatment of below-level allodynia will likely require combinatorial strategies to both prevent and combat mechanisms responsible for this disease. Early detection of allodynia in experimental SCI will for the first time allow researchers to identify the cellular substrates which underlie below-level neuropathic pain, thereby providing potential targets for therapeutic or rehabilitative interventions in the clinic.
Acknowledgments
Our sincerest thanks to Patricia Walters, A. Todd Lash, Qin Yin, Zhen Guan, David Hassenzahl, and Emily Hoschouer of The Ohio State University’s Center for Brain and Spinal Cord Repair (CBSCR) for their assistance with animal care, surgery and behavioral procedures. Support for this work was contributed by NINDS # NS43798 (DMB), F31 # NS058138 (MRD), P30-NS045758 (CBSCR) and the Paralyzed Veterans of America #2451 (DMB).
References
- Aminabadi NA, Farahani RM, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33–40. [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma. 1992;9:197–217. doi: 10.1089/neu.1992.9.197. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- Bresnahan JC, Beattie MS, Stokes BT, Conway KM. Three-dimensional computer-assisted analysis of graded contusion lesions in the spinal cord of the rat. J Neurotrauma. 1991;8:91–101. doi: 10.1089/neu.1991.8.91. [DOI] [PubMed] [Google Scholar]
- Brown CA, Jones AK. A role for midcingulate cortex in the interruptive effects of pain anticipation on attention. Clin Neurophysiol. 2008;119:2370–2379. doi: 10.1016/j.clinph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Bruce JC, Oatway MA, Weaver LC. Chronic pain after clip-compression injury of the rat spinal cord. Exp Neurol. 2002;178:33–48. doi: 10.1006/exnr.2002.8026. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Chiu EM. Behavioural responses of laboratory rats to playback of 22 kHz ultrasonic calls. Physiol Behav. 1995;57:1039–1044. doi: 10.1016/0031-9384(95)00003-2. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiol Behav. 1992;52:655–660. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Clinical implications of recent advances in neurotrauma research. In: Salzman SK, Faden AI, editors. The enurobiology of central nervous system trauma. Oxford University Press; New York: 1994. pp. 329–339. [Google Scholar]
- Bunge RP, Puckett WR, Hiester ED. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv Neurol. 1997;72:305–315. [PubMed] [Google Scholar]
- Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. Neuroimage. 2009;44:502–508. doi: 10.1016/j.neuroimage.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Calvino B, Besson JM, Boehrer A, Depaulis A. Ultrasonic vocalization (22–28 kHz) in a model of chronic pain, the arthritic rat: effects of analgesic drugs. Neuroreport. 1996;7:581–584. doi: 10.1097/00001756-199601310-00049. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ. Nocifensive responses to cutaneous thermal stimuli in the cat: stimulus-response profiles, latencies, and afferent activity. J Neurophysiol. 1983;50:1497–1515. doi: 10.1152/jn.1983.50.6.1497. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Clark LM, Detloff MR, Fisher LC, Diebert RJ, Kloos AD, Hutchinson KJ, et al. Novel and standard tests of tactile sensation are valid and reliable after spinal cord injury in rats. Combined Sections Meeting of American Physical Therapy Association; Las Vegas, NV. 2009. [Google Scholar]
- Colpaert FC, Wu WP, Hao JX, Royer I, Sautel F, Wiesenfeld-Hallin Z, et al. High-efficacy 5-HT1A receptor activation causes a curative-like action on allodynia in rats with spinal cord injury. Eur J Pharmacol. 2004;497:29–33. doi: 10.1016/j.ejphar.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Crozier KS, Graziani V, Ditunno JF, Jr, Herbison GJ. Spinal cord injury: prognosis for ambulation based on sensory examination in patients who are initially motor complete. Arch Phys Med Rehabil. 1991;72:119–121. [PubMed] [Google Scholar]
- Cuomo V, Cagiano R, De Salvia MA, Mazzoccoli M, Persichella M, Renna G. Ultrasonic vocalization as an indicator of emotional state during active avoidance learning in rats. Life Sci. 1992;50:1049–1055. doi: 10.1016/0024-3205(92)90100-4. [DOI] [PubMed] [Google Scholar]
- Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008a;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Clark LM, Fisher LC, Deibert RJ, Enquist LW, Basso DM. Validation of optimal assessments for tactile allodynia after spinal cord injury in rats. Society for Neuroscience; Washington, DC: 2008b. [Google Scholar]
- Dixon WJ. The up-and-down method for small samples. Am J Stat Assoc. 1965;60 [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Wolfe SQ. Clinical feasibility for cell therapy using human neuronal cell line to treat neuropathic behavioral hypersensitivity following spinal cord injury in rats. J Rehabil Res Dev. 2009;46:145–165. [PubMed] [Google Scholar]
- Eaton MJ, Wolfe SQ, Martinez M, Hernandez M, Furst C, Huang J, et al. Subarachnoid transplant of a human neuronal cell line attenuates chronic allodynia and hyperalgesia after excitotoxic spinal cord injury in the rat. J Pain. 2007;8:33–50. doi: 10.1016/j.jpain.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Erschbamer M, Pernold K, Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci. 2007;27:6428–6435. doi: 10.1523/JNEUROSCI.1037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Johannesen IL, Sindrup SH, Bach FW, Jensen TS. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord. 2001;39:256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- Ford GK, Moriarty O, McGuire BE, Finn DP. Investigating the effects of distracting stimuli on nociceptive behaviour and associated alterations in brain monoamines in rats. Eur J Pain. 2008;12:970–979. doi: 10.1016/j.ejpain.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Gobel H, Cordes P. Circadian variation of pain sensitivity in pericranial musculature. Headache. 1990;30:418–422. doi: 10.1111/j.1526-4610.1990.hed3007418.x. [DOI] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Attenuation of mechanical hyperalgesia following spinal cord injury by administration of antibodies to nerve growth factor in the rat. Neurosci Lett. 2003;336:117–120. doi: 10.1016/s0304-3940(02)01251-x. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hains BC, Johnson KM, Hulsebosch CE. Effect of age at time of spinal cord injury on behavioral outcomes in rat. J Neurotrauma. 2004;21:983–993. doi: 10.1089/0897715041650999. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;23:1111–1124. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Kang J, Leem JW, Hulsebosch CE. Spinal AMPA receptor inhibition attenuates mechanical allodynia and neuronal hyperexcitability following spinal cord injury in rats. J Neurosci Res. 2007;85:2352–2359. doi: 10.1002/jnr.21379. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Chastain KM, Everhart AW, McAdoo DJ, Hulsebosch CE. Transplants of adrenal medullary chromaffin cells reduce forelimb and hindlimb allodynia in a rodent model of chronic central pain after spinal cord hemisection injury. Exp Neurol. 2000;164:426–437. doi: 10.1006/exnr.2000.7439. [DOI] [PubMed] [Google Scholar]
- Hains BC, Johnson KM, McAdoo DJ, Eaton MJ, Hulsebosch CE. Engraftment of serotonergic precursors enhances locomotor function and attenuates chronic central pain behavior following spinal hemisection injury in the rat. Exp Neurol. 2001;171:361–378. doi: 10.1006/exnr.2001.7751. [DOI] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp Neurol. 2002a;175:347–362. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hains BC, Yucra JA, Eaton MJ, Hulsebosch CE. Intralesion transplantation of serotonergic precursors enhances locomotor recovery but has no effect on development of chronic central pain following hemisection injury in rats. Neurosci Lett. 2002b;324:222–226. doi: 10.1016/s0304-3940(02)00194-5. [DOI] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Antinociceptive effect of cannabinoid agonist WIN 55, 212-2 in rats with a spinal cord injury. Exp Neurol. 2007a;204:454–457. doi: 10.1016/j.expneurol.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res. 2007b;1185:117–128. doi: 10.1016/j.brainres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Hama A, Sagen J. Antinociceptive effects of the marine snail peptides conantokin-G and conotoxin MVIIA alone and in combination in rat models of pain. Neuropharmacology. 2009a;56:556–563. doi: 10.1016/j.neuropharm.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama A, Sagen J. Sustained antinociceptive effect of cannabinoid receptor agonist WIN 55, 212-2 over time in rat model of neuropathic spinal cord injury pain. J Rehabil Res Dev. 2009b;46:135–143. doi: 10.1682/JRRD.2008.04.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama AT, Sagen J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology. 2010;58:758–766. doi: 10.1016/j.neuropharm.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JX, Xu XJ, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991;45:175–185. doi: 10.1016/0304-3959(91)90186-2. [DOI] [PubMed] [Google Scholar]
- Hao JX, Yu W, Xu XJ, Wiesenfeld-Hallin Z. Capsaicin-sensitive afferents mediate chronic cold, but not mechanical, allodynia-like behavior in spinally injured rats. Brain Res. 1996a;722:177–180. doi: 10.1016/0006-8993(96)00216-8. [DOI] [PubMed] [Google Scholar]
- Hao JX, Yu W, Xu XJ, Wiesenfeld-Hallin Z. Effects of intrathecal vs. systemic clonidine in treating chronic allodynia-like response in spinally injured rats. Brain Res. 1996b;736:28–34. doi: 10.1016/0006-8993(96)00703-2. [DOI] [PubMed] [Google Scholar]
- Hao JX, Yu W, Xu XJ. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp Brain Res. 1998;118:259–268. doi: 10.1007/s002210050280. [DOI] [PubMed] [Google Scholar]
- Hao JX, Xu XJ, Urban L, Wiesenfeld-Hallin Z. Repeated administration of systemic gabapentin alleviates allodynia-like behaviors in spinally injured rats. Neurosci Lett. 2000;280:211–214. doi: 10.1016/s0304-3940(00)00787-4. [DOI] [PubMed] [Google Scholar]
- Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain. 2008;24:299–304. doi: 10.1097/AJP.0b013e318164d2cc. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, et al. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, et al. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- John SM, Bao F, Chen Y, Mathison RD, Weaver LC. Effects of a novel tripeptide on neurological outcomes after spinal cord injury. Neuroreport. 2006;17:1793–1796. doi: 10.1097/01.wnr.0000239963.83566.bb. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon YW, Hong SK, Na HS. Cold and mechanical allodynia in both hindpaws and tail following thoracic spinal cord hemisection in rats: time courses and their correlates. Neurosci Lett. 2003;343:200–204. doi: 10.1016/s0304-3940(03)00377-x. [DOI] [PubMed] [Google Scholar]
- Kim J, Back SK, Yoon YW, Hong SK, Na HS. Dorsal column lesion reduces mechanical allodynia in the induction, but not the maintenance, phase in spinal hemisected rats. Neurosci Lett. 2005;379:218–222. doi: 10.1016/j.neulet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol. 2005;191:251–265. doi: 10.1016/j.expneurol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Juraschek M, Blomer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- Kouya PF, Hao JX, Xu XJ. Buprenorphine alleviates neuropathic pain-like behaviors in rats after spinal cord and peripheral nerve injury. Eur J Pharmacol. 2002;450:49–53. doi: 10.1016/s0014-2999(02)02052-6. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lemmer B. Analysis of the circadian rhythm of pain. Z Rheumatol. 1991;50(Suppl 1):49–54. [PubMed] [Google Scholar]
- Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair. 2000;14:287–300. doi: 10.1177/154596830001400405. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, et al. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett. 2008;432:13–18. doi: 10.1016/j.neulet.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Macias MY, Syring MB, Pizzi MA, Crowe MJ, Alexanian AR, Kurpad SN. Pain with no gain: allodynia following neural stem cell transplantation in spinal cord injury. Exp Neurol. 2006;201:335–348. doi: 10.1016/j.expneurol.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, et al. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Mason P. Deconstructing endogenous pain modulations. J Neurophysiol. 2005;94:1659–1663. doi: 10.1152/jn.00249.2005. [DOI] [PubMed] [Google Scholar]
- Mills CD, Xu GY, Johnson KM, McAdoo DJ, Hulsebosch CE. AIDA reduces glutamate release and attenuates mechanical allodynia after spinal cord injury. Neuroreport. 2000;11:3067–3070. doi: 10.1097/00001756-200009280-00007. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hains BC, Johnson KM, Hulsebosch CE. Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma. 2001;18:743–756. doi: 10.1089/089771501316919111. [DOI] [PubMed] [Google Scholar]
- Mills CD, Johnson KM, Hulsebosch CE. Group I metabotropic glutamate receptors in spinal cord injury: roles in neuroprotection and the development of chronic central pain. J Neurotrauma. 2002;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, et al. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor D, Cousins MJ. Pain associated with spinal cord injury. Curr Opin Neurol. 1995;8:447–450. doi: 10.1097/00019052-199512000-00009. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999a;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Xu CL, Floyd N, Keay KA. C-fos expression in the spinal cord of rats exhibiting allodynia following contusive spinal cord injury. Brain Res. 1999b;851:281–286. doi: 10.1016/s0006-8993(99)02173-3. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Tan AM, Stamboulian S, Chang YW, Zhao P, Hains AB, Waxman SG, et al. Neuropathic pain memory is maintained by Rac1-regulated dendritic spine remodeling after spinal cord injury. J Neurosci. 2008;28:13173–13183. doi: 10.1523/JNEUROSCI.3142-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Zhao P, Waxman SG, Hains BC. Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury. J Rehabil Res Dev. 2009;46:123–133. [PubMed] [Google Scholar]
- Ushida T, Ikemoto T, Taniguchi S, Ishida K, Murata Y, Ueda W, et al. Virtual pain stimulation of allodynia patients activates cortical representation of pain and emotions: a functional MRI study. Brain Topogr. 2005;18:27–35. doi: 10.1007/s10548-005-7898-8. [DOI] [PubMed] [Google Scholar]
- Voda J, Hama A, Sagen J. FK506 reduces the severity of cutaneous hypersensitivity in rats with a spinal cord contusion. Neurosci Res. 2007;58:95–99. doi: 10.1016/j.neures.2007.02.004. [DOI] [PubMed] [Google Scholar]
- von Heijne M, Hao JX, Yu W, Sollevi A, Xu XJ, Wiesenfeld-Hallin Z. Reduced anti-allodynic effect of the adenosine A1-receptor agonist R-phenylisopropyladenosine on repeated intrathecal administration and lack of cross-tolerance with morphine in a rat model of central pain. Anesth Analg. 1998;87:1367–1371. [PubMed] [Google Scholar]
- von Heijne M, Hao JX, Sollevi A, Xu XJ. Intrathecal adenosine does not relieve allodynia-like behavior in spinally injured rats. Neuroreport. 1999;10:3247–3251. doi: 10.1097/00001756-199910190-00023. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Gris D, Saville LR, Oatway MA, Chen Y, Marsh DR, et al. Methylprednisolone causes minimal improvement after spinal cord injury in rats, contrasting with benefits of an anti-integrin treatment. J Neurotrauma. 2005;22:1375–1387. doi: 10.1089/neu.2005.22.1375. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Felipe-Cuervo E, Yezierski RP. Chronic pain after spinal injury: interference with sleep and daily activities. Arch Phys Med Rehabil. 2001;82:1571–1577. doi: 10.1053/apmr.2001.26068. [DOI] [PubMed] [Google Scholar]
- Wolfe SQ, Garg M, Cumberbatch NM, Furst C, Martinez M, Hernandez M, et al. Optimizing the transplant dose of a human neuronal cell line graft to treat SCI pain in the rat. Neurosci Lett. 2007;414:121–125. doi: 10.1016/j.neulet.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Aldskogius H, Seiger A, Wiesenfeld-Hallin Z. Chronic pain-related syndrome in rats after ischemic spinal cord lesion: a possible animal model for pain in patients with spinal cord injury. Pain. 1992;48:279–290. doi: 10.1016/0304-3959(92)90070-R. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Alster P, Wu WP, Hao JX, Wiesenfeld-Hallin Z. Increased level of cholecystokinin in cerebrospinal fluid is associated with chronic pain-like behavior in spinally injured rats. Peptides. 2001;22:1305–1308. doi: 10.1016/s0196-9781(01)00456-9. [DOI] [PubMed] [Google Scholar]
- Yu W, Hao JX, Xu XJ, Wiesenfeld-Hallin Z. The development of morphine tolerance and dependence in rats with chronic pain. Brain Res. 1997;756:141–146. doi: 10.1016/s0006-8993(97)00132-7. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007a;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007b;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


