Abstract
Environmental factors can induce epigenetic alterations in the germ cells that can potentially be transmitted transgenerationally. This non-genetic form of inheritance is termed epigenetic transgenerational inheritance and has been shown in a variety of species including plants, flies, worms, fish, rodents, pigs, and humans. This phenomenon operates during specific critical windows of exposure, linked to the developmental biology of the germ cells (sperm and eggs). Therefore, concepts of the developmental origins of transgenerational inheritance of phenotypic variation and subsequent disease risk need to include epigenetic processes affecting the developmental biology of the germ cell. These developmental impacts on epigenetic transgenerational inheritance, in contrast to multigenerational exposures, are the focus of this Perspective.
Keywords: transgenerational, epigenetic, disease, generational, critical windows
Introduction
The majority of environmental factors and toxicants do not have the ability to alter DNA sequence or promote genetic mutations directly [ 1 ]. However, many environmental factors can promote abnormal phenotypes or increase the risk of disease. Early life exposures during critical windows of development are one of the most important aspects of this process [ 2 ]. These environmental impacts on phenotype and disease risk are often not directly mediated through classical genetic mechanisms, even over the course of many generations; instead epigenetic mechanisms which can affect phenotype from one generation to the next are important [ 3 ]. The epigenetic mechanisms involved include DNA methylation, histone modifications, non-coding RNAs (ncRNAs), and chromatin structure [ 3 ]. The environmental exposures that directly influence these epigenetic processes can range from nutrition, temperature, and stress to large numbers of environmental toxicants [ 1 , 3 ], Table 1 . The majority of environmental exposures result in direct actions on the somatic cells of specific tissues during critical windows of development, for example to affect the numbers of cardiomyocytes or nephrons which affect the later risk of the exposed individuals to adult onset disease [ 2 ], Table 2 . While these effects on somatic cells can have dramatic consequences for the individual, in classical thinking they do not have the ability to pass this phenotype transgenerationally: this is known as Weissman’s barrier, which suggests that only the germline can transmit genetic information between generations [ 4 ], Table 2 . Therefore, germ cell alterations are required to transmit molecular information to the next generation.
Table 1.
exposure induced epigenetic transgenerational inheritance
| Toxicants | Species | Generation | References |
|---|---|---|---|
| Vinclozolin (agricultural fungicide) | Rat and mouse | F4 | [ 5 , 9 , 10 ] |
| Methoxychlor (agricultural pesticide) | Rat | F4 | [ 5 , 11 ] |
| TCDD/dioxin (industrial contaminant) | Rat, mouse, fish | F3 | [ 12 , 13 , 40 ] |
| Plastics (bisphenol-A, phthalate-DEHP and DBP) | Rat | F3 | [ 6 , 14 , 15 ] |
| Jet fuel [JP8] (hydrocarbon mixture) | Rat | F3 | [ 47 ] |
| Permethrin and DEET pesticide and insect repellent | Rat | F3 | [ 48 ] |
| DDT (pesticide) | Rat | F4 | [ 16 ] |
| Bisphenol A (BPA) (plastic toxicant) | Rat, mouse, fish | F3 | [ 49 , 50 , 98 ] |
| Phthalates (plastic toxicant) | Rat | F3 | [ 17 ] |
| Tributyltin (industrial toxicant) | Rat | F3 | [ 18 ] |
| Nutrition | |||
| Folate (nutrition) | Mouse | [ 25 ] | |
| High fat diet (nutrition) | Mouse and rat | F2, F3 | [ 23 , 24 ] |
| Caloric restriction (nutrition) | Human, rat, mouse, pig, worm, flies | F2, F3 | [ 19–22 , 36 , 37 , 39 , 42 ] |
| Other types exposures | |||
| Temperature and drought (plant flowering and health) | Plant | F2, F3 | [ 26–29 ] |
| Stress (behavioral) | Mouse, rat, human | F2, F3 | [ 30 , 31 , 44–46 ] |
| Smoking (health) | Human | F2, F3 | [ 32 , 33 ] |
| Nicotine (health) | Rat | F3 | [ 34 ] |
| Alcohol (health) | Rat | F3 | [ 35 ] |
Table 2.
sites of action and phenotypes of environmental factors
| Site of action | Biological response and toxicology |
|---|---|
| Somatic cells | Allows tissue-specific toxicology and critical for adult onset disease in the individual exposed but not capable of transmitting a transgenerational phenotype. |
| Germ cells | Allows transmission between generations and in the absence of direct exposure to promote a transgenerational phenotype. |
More recently, studies showing the ability of environmental exposures to alter the epigenetics of the germline have revealed the potential to promote a transgenerational phenotype [ 5 ]. The heritable transmission of environmentally induced phenotypes is referred to as epigenetic transgenerational inheritance [ 3 , 5–7 ] ( Table 3 ) and is of particular interest as it may transmit risk of disease across generations in the absence of continued environmental exposures. This non-genetic or non-Mendelian form of inheritance requires epigenetic alterations of a germ cell (sperm or egg) to transmit the environmentally induced phenotypes between generations [ 1 , 3 , 8 ]. The focus of this Perspective is on the developmental origins of these germline changes and their role in epigenetic transgenerational inheritance, not the direct effects involved in multigenerational exposures. The ability to directly expose a germ cell to induce effects in the offspring (i.e. multigenerational exposure) are important, but the ability to produce a permanent epigenetic alteration in the germ cells which is maintained in the absence of the continued environmental exposure suggests a novel form of inheritance which could have a much greater impact on biology, disease etiology, and evolution. Therefore, the literature reviewed will focus on epigenetic transgenerational inheritance, in contrast to multigenerational exposures.
Table 3.
transgenerational versus multigenerational phenotypes
| Phenotype | Exposure | Definition |
|---|---|---|
| Multigenerational | Direct | Coincident direct exposure of multiple generations to an environmental factor promoting alterations in the multiple generations exposed. |
| Transgenerational | None, except the initial generation | After the initial exposure, the transgenerational phenotype is transmitted through the germ line in the absence of direct exposure. |
Although direct exposure multigenerational observations have been made, the initial observation of an environmental factor promoting epigenetic transgenerational inheritance of disease involved an agricultural fungicide, vinclozolin [ 5 , 9 ]. A wide variety of environmental factors from nutrition to toxicants have now been shown to promote the epigenetic transgenerational inheritance of disease or phenotypic variation [ 3 ] ( Table 1 ). The largest group of environmental exposures are toxicants including vinclozolin [ 5 , 9 , 10 ], methoxychlor [ 5 , 11 ], dioxin [ 12 , 13 ], the plasticizer compound bisphenol A (BPA) [ 6 , 14 , 15 ], the pesticide dichlorodiphenyltrichloroethane (DDT) [ 16 ], phthalates [ 14 , 17 ], and tributyltin [ 18 ]. Unbalanced nutrition, ranging from calorie or protein restriction [ 19–22 ] to high fat diets [ 23 , 24 ], as well as manipulation of micronutrients, such as folate [ 25 ], have also been shown to promote the epigenetic transgenerational inheritance of disease. Other environmental factors such as temperature [ 26–29 ], stress [ 30 , 31 ], smoking [ 32 , 33 ], nicotine [ 34 ], and alcohol [ 35 ] have also been studied ( Table 1 ). Therefore, a wide variety of different environmental exposures involving different signal transduction mechanisms can promote the epigenetic transgenerational inheritance of disease and phenotypic variation.
Environmental factors have also been shown to promote the epigenetic transgenerational inheritance of disease or phenotypic variation in a wide variety of different species ( Table 1 ). Extremes of temperature, salinity, and drought promote abnormal transgenerational phenotypes in plants [ 26–29 ]. Nutritional challenges in worms ( C. elegans ) [ 36 , 37 ], flies (drosophila) [ 38 , 39 ], fish [ 40 ], birds [ 41 ], pigs [ 42 ], rodents [ 5 , 43 ], and humans [ 19 , 20 ] have all been shown to promote transgenerational phenotypes. Smoking [ 32 , 33 ] or nicotine [ 34 ] and alcohol- [ 35 ] induced transgenerational phenotypes in rodents or humans have been observed. Environmental stress has also been shown to influence transgenerational phenotypes in rodents [ 30 , 31 ] and humans [ 44–46 ]. Environmental toxicants have been shown to promote transgenerational phenotypes in rodents [ 5 , 6 , 9 , 11–14 , 16–18 , 47–50 ], fish [ 40 ], plants [ 26–29 ], and humans [ 32 , 33 ], Table 1 . In all the species investigated environmental factors have been shown to promote the epigenetic transgenerational inheritance of disease or phenotypic variation. Therefore, the phenomenon is highly conserved among species, supporting an important role for this form of epigenetic transgenerational inheritance.
The ability of environmental factors to promote the epigenetic transgenerational inheritance of diseases and phenotypic variation has significant impact on concepts of the etiology of disease, especially the non-communicable diseases (NCDs) – diabetes, cardiovascular and chronic lung disease and some forms of cancer, and obesity. Epidemiological and molecular studies have shown that fixed genetic variations such as single-nucleotide polymorphisms do not explain a substantial fraction of the risk for these diseases at the population level ( Table 4 ). Nor can such variations explain the dramatic increase seen in the prevalence of NCDs over a few decades globally or the different patterns of disease between monozygotic twins [ 51 ], Table 4 . The environmental exposures associated with disease listed above have not been shown to promote genetic mutations causing disease [ 3 ]. Environmentally induced epigenetic effects can help explain many of these observations.
Table 4.
environmental epigenetic impacts on biology and disease
| • Worldwide differences in regional disease frequencies |
| • Low frequency of genetic component of disease as determined with genome wide association studies (GWAS) |
| • Dramatic increases in disease frequencies over past decades |
| • Identical twins with variable and discordant disease frequency |
| • Environmental exposures associated with disease |
| • Regional differences and rapid induction events in evolution |
Evolutionary biology studies have also demonstrated a number of observations that cannot easily be explained by classical genetics alone. These include rapid evolutionary events or microevolution involving disparate phenotypic variations within a species [ 52–54 ]. The fusion of classical Darwinian concepts, neo-Darwinian genetic concepts, and neo-Lamarckian environmental epigenetic concepts has suggested a more holistic theory for evolutionary molecular mechanisms [ 54 ]. For example, recently the ability of environmental exposures to promote the epigenetic transgenerational inheritance of sperm epimutations has been shown to promote the development of genetic mutations in later generations [ 55 ]. Therefore, a combination of epigenetics and genetics will likely influence the long term transgenerational phenotype [ 55 ]. Previously, it has been shown that altered epigenetics can increase genome instability to promote nearly all forms of genetic mutations [ 55 , 56 ]. This suggests many evolutionary processes such as genetic assimilation may be in part a function of earlier alterations in epigenome [ 54 , 55 , 57 ]. Therefore, environmentally induced epigenetic transgenerational inheritance is a non-genetic inheritance mechanism that has dramatic impacts on a wide range of areas of science and medicine [ 1–3 , 54 , 58 ]. Critical elements that need to be considered include the developmental impacts and experimental limitations of epigenetic transgenerational inheritance studies.
Developmental Biology
Understanding of developmental biology has now moved on substantially from the concept of a genetic “programme” for development, which protected the embryo and fetus from the influences of environmental factors [ 59 ]. In retrospect, it is hard to understand why this idea took so long to be revised to include epigenetic processes. The concept of Waddington (who coined the word “epigenetic” in about 1942) of canalization emphasized that, while it protected development from extraneous influences, it was nonetheless a mutable process which provided a degree of developmental plasticity [ 60 ]. Today, the fields of evolutionary developmental biology and ecological developmental biology are well established [ 61 , 62 ]. The transmission of phenotypic variation to offspring via developmental plasticity induced through parental cues forms the basis for parental effects reported in many species [ 63 ]. An area in which these concepts have been particularly influential in medicine is the Developmental Origins of Health and Disease. Developmental Origins of Health and Disease research has shown how a range of aspects of the developmental environment, especially those mediated via the parents such as maternal (and to some extent paternal) diet, body composition, and health-related behaviors, can affect the development of the offspring. These processes operate within the normal range, not only of diets and lifestyle in contemporary societies but also in terms of prenatal development. They are not therefore necessarily accompanied by overt differences in phenotype visible at birth, even though epigenetic changes to organs, systems, and control mechanisms may have occurred. These physiological phenotype changes are then associated with differences in responses to later environmental challenges such as living in an obesogenic environment which affects the individual’s risk of NCDs (for review see [ 2 ]). Environmental and parental stimuli inducing developmental plasticity operate over critical periods, and these have been shown to commence even before conception on the germline [ 64 ] and to involve the early embryo [ 65 ], the fetus [ 66 ], and the newborn [ 67 ]. During each of these periods, epigenetic processes have been invoked [ 68 ].
Epigenetic transgenerational inheritance requires the germ-line transmission of altered epigenetic information between generations [ 1 , 3 , 5 ]. Therefore, the cell types and critical windows of exposure to consider involve sperm and egg development and differentiation. The onset of gonadal sex determination corresponds to cell fate determination when a primordial germ cell (PGC) differentiates into an egg or sperm cell lineage [ 1 , 3 , 69 ]. This occurs during embryonic or fetal days E8–E14 in the rodent or weeks 6–18 of gestation in the human [ 1 , 3 ]. This fetal development of sex determination period in mammals is the initial critical window of exposure, and for other species, there is a comparable time of embryonic development when germ cell differentiation is initiated. The other critical window for germ cell development is gametogenesis when differentiated sperm or egg develop. The egg develops later in development when it is arrested in meiosis and differentiates during follicle development [ 70 ]. The oogonia that are arrested in the adult female are not actively developing but do provide a potential target for environmental factors. The susceptibility of the egg to epigenetic alterations at this adult stage of development needs to be further investigated. In contrast, the sperm actively undergo cell differentiation during spermatogenesis in the adult, and so they are potential targets for epigenetic change [ 1 , 3 , 70 ]. The majority of studies have demonstrated that the fetal period of gonadal sex determination is a critical window for environmentally induced epigenetic transgenerational inheritance [ 5 , 6 , 9 , 11–14 , 16–18 , 47–50 ]. However, recent studies have also demonstrated that effects on the adult male’s spermatogenesis can promote epigenetic transgenerational inheritance of altered phenotypes [ 46 ]. They include stress-induced behavioral effects and nutritionally induced metabolic conditions in rodents [ 30 , 31 ]. Therefore, these critical developmental periods for germ cell differentiation are the windows of sensitivity for environmental factors to promote epigenetic transgenerational inheritance.
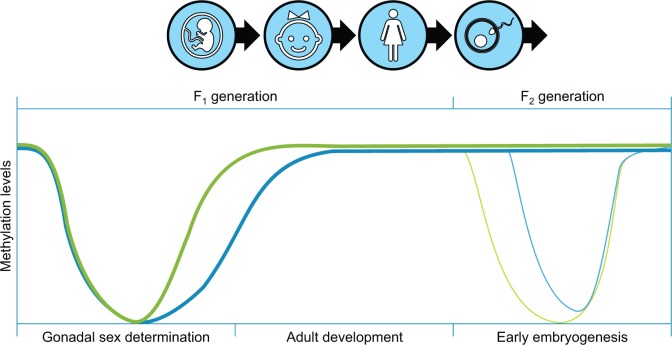
The sperm and egg periods of development directly correspond to the germ cell epigenetic programming windows [ 1 , 3 , 70 ]. The stem cells for the germ cells are PGCs that early in development migrate to colonize the fetal gonad prior to gonadal sex determination [ 69 , 70 ]. During this migration and colonization, the DNA methylation in the PGC is erased for the most part to negligible levels, then, at the onset of gonadal sex determination, the germ-line DNA initiates a re-methylation of the DNA in a sex-specific manner [ 1 , 3 , 69 ] ( Fig. 1 ). The completion of the male germ cell DNA re-methylation is later in fetal gonadal development and after birth in the female germ cell, but the initiation of re-methylation is at gonadal sex determination for both sexes [ 1 , 3 , 69 ], Fig. 1 . Therefore, environmental exposures at this period of gonadal sex determination have the capacity to alter the epigenetic programming of the germ cells [ 3 ]. Although the female germ cell (oocyte) does not have a dramatic regulation of epigenetic processes in the adult, the male germ cell does have epigenetic programming during spermatogenesis as spermatogonia develop into spermatozoa in the adult testis [ 3 ]. Environmental exposures have been shown to alter this epigenetic programming [ 6 , 7 , 55 ] and to promote the epigenetic transgenerational inheritance of abnormal physiology [ 5 , 6 , 9 , 11–14 , 16–18 , 47–50 ]. Therefore, alterations in epigenetic programming of the germ cells at these critical developmental windows promote transgenerational phenotypes and alteration in the epigenetics of the germ cells.
Figure 1.
epigenetic (DNA methylation) programming in the germline during various developmental periods. The green line is the male germline developmental pattern and blue line the female germline developmental pattern (modified from [ 1 ])
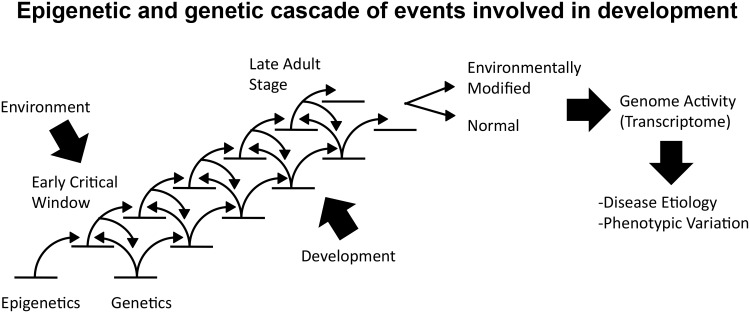
Environmental factors can readily alter epigenetic processes but not readily alter DNA sequence [ 1 ]. However, the genetic background will directly impact the influence of altered epigenetics. Therefore, any cellular, physiological, or biological phenomena will involve a cascade of genetic and epigenetic events to produce a differentiated cell or tissue [ 71 ] ( Fig. 2 ). A cascade of genetic events leading to a progression of gene expression profiles will interact with the corresponding cascade of epigenetic alterations that occur during cellular differentiation. Although the genetic cascade is less sensitive to environmental factors, the epigenetic processes in an early critical window can be modified such that the subsequent interactions between the epigenetic and genetic events lead to altered differentiation ( Fig. 2 ). This in turn leads to an altered gene expression profile and changed susceptibility for disease [ 71 , 72 ]. The integration of epigenetics and genetics during the early stages of development provides a molecular mechanism for the disease susceptibility and phenotypic variation observed [ 72 ]. In addition, these molecular events provide a mechanism for the developmental origins of disease and phenotypic variation [ 2 , 73 ]. Although most exposures will directly act on somatic cell development and alter the exposed individual’s later life physiology and disease [ 2 ], in the event the exposure affects the germ-line and does not get erased at fertilization, the development of an epigenetic transgenerational inheritance phenotype occurs.
Figure 2.
epigenetic and genetic cascade of events (arrows) from a stem cell state to a differentiated adult state involved in development. A normal or environmentally modified state promotes genome alterations associated with an increase susceptibility for disease and phenotypic variation. The critical window of early development is when environmental factors have the ability to alter the epigenome (modified from [ 71 ])
To test this hypothesis, several transgenerational disease models have been used to determine if the somatic cells critical to the disease have an altered epigenome and transcriptome. The first is the transgenerational male fertility effects involving spermatogenic cell apoptosis in the adult testis [ 5 ]. The adult testis somatic cell that supports the developing spermatogenic cells is the Sertoli cell forming the seminiferous tubule. Sertoli cells from control versus the vinclozolin F3 generation lineage (great grand-offspring) males were found to have a dramatic alteration in both gene expression and epigenetic DNA methylation profiles [ 74 ]. The gene expression profile identified could lead to altered pyruvate production which could explain the spermatogenic cell apoptosis observed [ 74 ]. A second example involved a female polycystic ovarian follicles (PCO) transgenerational model [ 75 ]. Many environmental toxicants promote this PCO transgenerational disease [ 75 ]. The granulosa cells within the ovarian follicles prior to the development of the PCO were found to have altered epigenetic DNA methylation profiles and transcriptomes in exposed lineage animals [ 75 ]. Many of the genes with altered expression had been previously shown to be associated with PCO [ 75 ]. These experiments demonstrate the importance of direct measurements in the germ cells and relevant somatic cell types themselves. Observations support the hypothesis that transgenerational germ-line epigenetic alterations acting through the embryonic stem cells can promote disease susceptibility in a wide variety of cells and tissues [ 76 ].
Critical Experimental Limitations
A number of experimental considerations need to be made in the design of epigenetic transgenerational inheritance studies. The first is to consider the critical windows of exposure discussed above in regards to germ cell development. Studies have demonstrated that the critical window of gestation and fetal development identified in many studies [ 5 , 6 , 9 , 11–14 , 16–18 , 47–50 ] needs to be considered in the experimental design of transgenerational studies. Exposure to a stimulus during a window that preceded or followed the critical window of gonadal sex determination may produce effects other than those on germ cell epigenetic programming. For example, a recent study of vinclozolin actions used an exposure window that did not include the entire gonadal sex determination window (embryonic day E7–13 in mouse and E8–14 in rat) and found no transgenerational effect [ 77 ]. In contrast to statements in a recent review [ 78 ], this does not constitute a negative result, but simply an experimental design that was not suitable.
Another critical experimental consideration is the impact of inbreeding within the experimental model used. A number of studies that did not induce epigenetic transgenerational inheritance were performed using inbred strains of rodents [ 77 , 79 , 80 ]. Previously, literature has demonstrated inbreeding depression of environmentally induced phenotypes, particularly toxicant actions [ 81 ] and suppression of epigenetic processes has been shown to be involved [ 82–84 ]. A study that compared inbred and outbred lines of mice found transgenerational phenotypes in the outbred but not the inbred strains [ 43 ]. Therefore, recent studies that have used inbred strains and obtained negative observations may in part be confounded by such inbreeding [ 77 , 78 ]. The molecular nature of this inbreeding depression on epigenetics remains to be established.
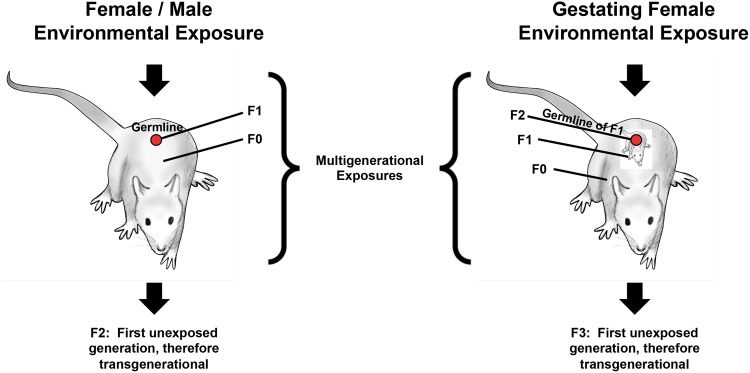
In defining epigenetic transgenerational inheritance, it is critical to consider the absence of direct environmental exposures in the transgenerational generations, as previously discussed [ 85 ] ( Table 3 ). The direct exposure of a cell or individual to an environmental exposure does not constitute a transgenerational phenotype but is due to direct exposure effects of the germ cell pre-fertilization or somatic cells early in development (embryonic, fetal, or postnatal) ( Fig. 3 ). The exposure of an F0 generation pregnant female directly exposes her, the F1 generation fetus, and the F2 generation germ-line within the F1 generation fetus [ 3 , 85 ]. Therefore, the F3 generation is the first generation that is truly a test of transgenerational inheritance, the F0, F1, and F2 generations being due rather to a multigenerational exposure [ 3 , 85 ] ( Fig. 3 ). In contrast, an adult male or non-pregnant female when exposed has direct exposure of the F0 generation adult and germ line that will generate the F1 generation, so it is the F2 generation (grandoffspring) which are the first recipients of transgenerational inheritance [ 3 , 85 ] ( Fig. 3 ), the F0 and F1 generations again being multigenerational exposures. As an example, the paternal exposure to smoking effects on the son is due to direct exposure effects on the germline and not due to a transgenerational response [ 86 ]. Many studies have been published that have claimed transgenerational inheritance of phenotype but instead have been multigenerational exposures not involving germ-line transmission of epigenetic information in the absence of direct exposure [ 1 , 3 , 87 ].
Figure 3.
environmentally induced transgenerational epigenetic inheritance: schematic of environmental exposure and affected generations for both gestating female and adult male or female. The multigenerational direct exposures are indicated in contrast to the transgenerational generation without direct exposure (modified from [ 97 ])
As listed in Table 1 , a large number of different laboratories, with a wide variety of environmental exposures in a number of different species, have demonstrated environmental induction of epigenetic transgenerational inheritance [ 5 , 6 , 9 , 11–14 , 16–18 , 47–50 ]. Several studies have reported an inability to induce transgenerational phenotypes [ 77 , 79 , 80 , 88 ] but did not consider all the experimental limitations described above. A question of the bioinformatics used [ 77 ] has also been raised [ 89 ]. The suggestion that these are negative studies [ 78 ] neglects a consideration of these critical aspects of experimental design and interpretation and therefore need to be made with caution.
Conclusions
Developmental considerations in environmentally induced epigenetic transgenerational inheritance of disease and phenotypic variation include the critical windows of exposure being linked to germ cell development. The time of gonadal sex determination when germ cells are undergoing epigenetic programming is a developmental period susceptible to induction of transgenerational phenotypes. The adult stage for males will be critical due to the epigenetic reprogramming during spermatogenesis. Other developmental stages likely exist but require further investigation. Studies not finding transgenerational phenotypes need to consider the need to affect a critical window of exposure in development.
A number of studies have more recently suggested a role for different epigenetic processes in the germline transmission of epigenetic transgenerational inheritance. Previous studies have focused on DNA methylation due to the link between epigenetic programming and DNA methylation, Table 5 . Genome wide effects on sperm DNA methylation profiles and the identification of differential DNA methylation regions have been found with a wide variety of environmental exposures [ 5–7 , 11 , 13 , 14 , 16 , 47 ], Tables 1 and 5 . More recently, ncRNAs have also been shown to be altered in sperm transgenerationally [ 31 , 90–92 ], Table 5 . Interesting studies have also used ncRNA injection into eggs to promote a transgenerational phenotype [ 31 ]. Histone retention in sperm has also been shown to be altered, and alteration in histone modifications and retention have been shown to be involved [ 93–96 ]. Although no studies have extensively studied the role of chromatin structure and epigenetic inheritance, it also has been suggested [ 95 ]. Therefore, a number of studies have suggested the combined roles of different epigenetic processes in epigenetic transgenerational inheritance [ 3 , 87 , 90 ]. Although some reports suggest one molecular process may be more important than another, a combination of them all ( Table 5 ) will likely regulate the epigenetic transgenerational inheritance phenomenon [ 3 , 87 ].
Table 5.
transgenerational germline epigenetic processes
Epigenetic transgenerational inheritance provides a non-genetic form of inheritance. The impact on biology of environmental factors which can promote transgenerational disease and phenotypic variation is significant. For evolutionary biology, the ability of environmental factors to promote phenotypic change is a neo-Lamarckian concept that can impact neo-Darwinian theory. Integration of environmental epigenetics and classical genetics provides a more robust molecular mechanism underlying evolution [ 54 ]. The combined mechanism helps explain topics such as genetic assimilation. Ancestral exposures that will have an impact on transgenerational disease susceptibility can play a critical role in disease etiology [ 3 , 6 ]. The impact of environment on biology is significantly enhanced when epigenetic transgenerational inheritance is considered.
Genetics and genetic inheritance is absolutely critical for biology. An additional consideration of epigenetic transgenerational inheritance as a non-genetic form of inheritance does not reduce the importance of genetics but rather expands the repertoire of molecular mechanisms which underlie many aspects of biology that cannot be easily explained with classical genetics alone ( Table 4 ). Therefore, complementary roles for non-genetic and genetic inheritance exist and need to be considered. This includes the regulation of any cellular, physiological, or biological system. No system will involve only genetics or only epigenetics as these molecular mechanisms are so integrated that they depend on each other [ 3 , 71 ] ( Fig. 2 ). Future elucidation of molecular and biological processes will need to consider both to understand the function of biological systems adequately.
These considerations have far reaching implications, because they indicate that environmental or lifestyle challenges not only produce effects on the individuals exposed themselves but may also be transmitted in potentially unmodified form to their offspring over several subsequent generations. The protection of future unborn generations from such risk must be a paramount consideration, raising a range of ethical as well as practical considerations. The situation is made more acute by the consideration that even if the inducing stimulus is removed, such as reducing the level of an environmental toxicant, the transgenerational phenotype and associated effects on disease risk may still be transmitted. Transgenerational epigenetic inheritance thus has a range of implications for sustainable health and economic development in many situations.
References
- 1. Jirtle RL, Skinner MK . Environmental epigenomics and disease susceptibility . Nat Rev Genet 2007. ; 8 : 253 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev 2014. ; 94 : 1027 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Skinner MK . Endocrine disruptor induction of epigenetic transgenerational inheritance of disease . Mol Cell Endocrinol 2014. ; 398 : 4 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Surani MA . Human germline: a new research frontier . Stem Cell Rep 2015. ; 4 : 955 – 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anway MD, Cupp AS, Uzumcu M, et al. . Epigenetic transgenerational actions of endocrine disruptors and male fertility . Science 2005. ; 308 : 1466 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manikkam M, Guerrero-Bosagna C, Tracey R, et al. . Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures . PLoS One 2012. ; 7 : e31901 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guerrero-Bosagna C, Settles M, Lucker B, et al. . Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome . PLoS One 2010. ; 5 : e13100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skinner MK, Manikkam M, Guerrero-Bosagna C . Epigenetic transgenerational actions of environmental factors in disease etiology . Trends Endocrinol Metab 2010. ; 21 : 214 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anway MD, Leathers C, Skinner MK . Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease . Endocrinology 2006. ; 147 : 5515 – 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stouder C, Paoloni-Giacobino A . Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm . Reproduction 2010. ; 139 : 373 – 9 . [DOI] [PubMed] [Google Scholar]
- 11. Manikkam M, Haque MM, Guerrero-Bosagna C, et al. . Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult onset disease through the female germline . PLoS One 2014. ; 9 : e102091 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruner-Tran KL, Osteen KG . Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations . Reprod Toxicol 2011. ; 31 : 344 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manikkam M, Tracey R, Guerrero-Bosagna C, et al. . Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations . PLoS One 2012. ; 7 : e46249 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manikkam M, Tracey R, Guerrero-Bosagna C, et al. . Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of adult-onset disease and sperm epimutations . PLoS One 2013. ; 8 : e55387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombo M, Fernandez-Diez C, Gonzalez-Rojo S, et al. . Transgenerational inheritance of heart disorders caused by paternal bisphenol A exposure . Environ Pollut 2015. ; 206 : 667 – 78 . [DOI] [PubMed] [Google Scholar]
- 16. Skinner MK, Manikkam M, Tracey R, et al. . Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity . BMC Med 2013. ; 11 : 228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doyle TJ, Bowman JL, Windell VL, et al. . Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice . Biol Reprod 2013. ; 88 : 112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chamorro-Garcia R, Sahu M, Abbey RJ, et al. . Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice . Environ Health Perspect 2013. ; 121 : 359 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pembrey ME, Bygren LO, Kaati G, et al. . Sex-specific, male-line transgenerational responses in humans . Eur J Hum Genet 2006. ; 14 : 159 – 66 . [DOI] [PubMed] [Google Scholar]
- 20. Veenendaal MV, Painter RC, de Rooij SR, et al. . Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine . BJOG 2013. ; 120 : 548 – 53 . [DOI] [PubMed] [Google Scholar]
- 21. Burdge GC, Slater-Jefferies J, Torrens C, et al. . Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations . Br J Nutr 2007. ; 97 : 435 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Painter RC, Osmond C, Gluckman P, et al. . Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life . BJOG 2008. ; 115 : 1243 – 9 . [DOI] [PubMed] [Google Scholar]
- 23. Dunn GA, Morgan CP, Bale TL . Sex-specificity in transgenerational epigenetic programming . Horm Behav 2011. ; 59 : 290 – 5 . [DOI] [PubMed] [Google Scholar]
- 24. Burdge GC, Hoile SP, Uller T, et al. . Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition . PLoS One 2011. ; 6 : e28282 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Padmanabhan N, Watson ED . Lessons from the one-carbon metabolism: passing it along to the next generation . Reprod Biomed Online 2013. ; 27 : 637 – 43 . [DOI] [PubMed] [Google Scholar]
- 26. Song J, Irwin J, Dean C . Remembering the prolonged cold of winter . Curr Biol 2013. ; 23 : R807 – 11 . [DOI] [PubMed] [Google Scholar]
- 27. Norouzitallab P, Baruah K, Vandegehuchte M, et al. . Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model . FASEB J 2014. ; 28 : 3552 – 63 . [DOI] [PubMed] [Google Scholar]
- 28. Zheng X, Chen L, Li M, et al. . Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance . PLoS One 2013. ; 8 : e80253 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suter L, Widmer A . Environmental heat and salt stress induce transgenerational phenotypic changes in Arabidopsis thaliana . PLoS One 2013. ; 8 : e60364 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dias BG, Ressler KJ . Parental olfactory experience influences behavior and neural structure in subsequent generations . Nat Neurosci 2014. ; 17 : 89 – 96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gapp K, Jawaid A, Sarkies P, et al. . Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice . Nat Neurosci 2014. ; 17 : 667 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pembrey ME . Male-line transgenerational responses in humans . Hum Fertil 2010. ; 13 : 268 – 71 . [DOI] [PubMed] [Google Scholar]
- 33. Golding J, Northstone K, Gregory S, et al. . The anthropometry of children and adolescents may be influenced by the prenatal smoking habits of their grandmothers: a longitudinal cohort study . Am J Hum Biol 2014. ; 26 : 731 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rehan VK, Liu J, Sakurai R, et al. . Perinatal nicotine-induced transgenerational asthma . Am J Physiol Lung Cell Mol Physiol 2013. ; 305 : L501 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Govorko D, Bekdash RA, Zhang C, et al. . Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations . Biol Psychiatry 2012. ; 72 : 378 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benayoun BA, Brunet A . Epigenetic memory of longevity in Caenorhabditis elegans . Worm 2012. ; 1 : 77 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kelly WG . Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans . Epigenetics Chromatin 2014. ; 7 : 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buescher JL, Musselman LP, Wilson CA, et al. . Evidence for transgenerational metabolic programming in Drosophila . Dis Model Mech 2013. ; 6 : 1123 – 32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Grentzinger T, Armenise C, Brun C, et al. . piRNA-mediated transgenerational inheritance of an acquired trait . Genome Res 2012. ; 22 : 1877 – 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker TR, Peterson RE, Heideman W . Using Zebrafish as a model system for studying the transgenerational effects of dioxin . Toxicol Sci 2014. ; 138 : 403 – 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brun JM, Bernadet MD, Cornuez A, et al. . Influence of grand-mother diet on offspring performances through the male line in Muscovy duck . BMC Genet 2015. ; 16 : 145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Braunschweig M, Jagannathan V, Gutzwiller A, et al. . Investigations on transgenerational epigenetic response down the male line in F2 pigs . PLoS One 2012. ; 7 : e30583 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guerrero-Bosagna C, Covert T, Haque MM, et al. . Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers . Reprod Toxicol 2012. ; 34 : 694 – 707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dias BG, Maddox SA, Klengel T, et al. . Epigenetic mechanisms underlying learning and the inheritance of learned behaviors . Trends Neurosci 2015. ; 38 : 96 – 107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Babenko O, Kovalchuk I, Metz GA . Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health . Neurosci Biobehav Rev 2015. ; 48 : 70 – 91 . [DOI] [PubMed] [Google Scholar]
- 46. Skinner MK . Environmental stress and epigenetic transgenerational inheritance . BMC Med 2014. ; 12 : 153 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tracey R, Manikkam M, Guerrero-Bosagna C, et al. . Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations . Reprod Toxicol 2013. ; 36 : 104 – 116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manikkam M, Tracey R, Guerrero-Bosagna C, et al. . Pesticide and insect repellent mixture (Permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations . Reprod Toxicol 2012. ; 34 : 708 – 19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salian S, Doshi T, Vanage G . Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A . Life Sci 2009. ; 85 : 11 – 8 . [DOI] [PubMed] [Google Scholar]
- 50. Wolstenholme JT, Edwards M, Shetty SR, et al. . Gestational exposure to Bisphenol A produces transgenerational changes in behaviors and gene expression . Endocrinology 2012. ; 153 : 3828 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castillo-Fernandez JE, Spector TD, Bell JT . Epigenetics of discordant monozygotic twins: implications for disease . Genome Med 2014. ; 6 : 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Day T, Bonduriansky R . A unified approach to the evolutionary consequences of genetic and nongenetic inheritance . Am Nat 2011. ; 178 : E18 – 36 . [DOI] [PubMed] [Google Scholar]
- 53. Skinner MK, Guerrero-Bosagna C, Haque MM, et al. . Epigenetics and the evolution of Darwin's finches . Genome Biol Evol 2014. ; 6 : 1972 – 89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Skinner MK . Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-lamarckian concept that facilitates neo-Darwinian evolution . Genome Biol Evol 2015. ; 7 : 1296 – 302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Skinner MK, Guerrero-Bosagna C, Haque MM . Environmentally Induced epigenetic transgenerational inheritance of sperm epimutations promote genetic mutations . Epigenetics 2015. ; 10 : 762 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wolff EM, Liang G, Jones PA . Mechanisms of disease: genetic and epigenetic alterations that drive bladder cancer . Nat Clin Pract Urol 2005. ; 2 : 502 – 10 . [DOI] [PubMed] [Google Scholar]
- 57. Soubry A . Epigenetic inheritance and evolution: a paternal perspective on dietary influences . Prog Biophys Mol Biol 2015. ; 118 : 79 – 85 . [DOI] [PubMed] [Google Scholar]
- 58. Gluckman P, Beedle A, Hanson M . Principles of Evolutionary Medicine , 1st edn . New York: : Oxford University Press; , 2009. . [Google Scholar]
- 59. Bateson P, Gluckman P, Hanson M . The biology of developmental plasticity and the predictive adaptive response hypothesis . J Physiol 2014. ; 592 : 2357 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gluckman PD, Hanson MA, Low FM . The role of developmental plasticity and epigenetics in human health . Birth Defects Res C Embryo Today 2011. ; 93 : 12 – 8 . [DOI] [PubMed] [Google Scholar]
- 61. West-Eberhard MJ . Developmental Plasticity and Evolution . New York: : Oxford University Press; , 2003. . [Google Scholar]
- 62. Gilbert SF, Epel D . Ecological Developmental Biology: The Environmental Regulation of Development, Health and Evolution , 2nd edn . Sunderland, MA: : Sinauer Associates; , 2015. . [Google Scholar]
- 63. Uller T . Developmental plasticity and the evolution of parental effects . Trends Ecol Evol 2008. ; 23 : 432 – 8 . [DOI] [PubMed] [Google Scholar]
- 64. Liu X, Chen Q, Tsai HJ, et al. . Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease . Environ Mol Mutagen 2014. ; 55 : 223 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fleming TP, Velazquez MA, Eckert JJ . Embryos, DOHaD and David Barker . J Dev Orig Health Dis 2015. ; 6 ( 5 ): 337 – 83 . [DOI] [PubMed] [Google Scholar]
- 66. Saffery R . Epigenetic change as the major mediator of fetal programming in humans: are we there yet? Ann Nutr Metab 2014. ; 64 : 203 – 7 . [DOI] [PubMed] [Google Scholar]
- 67. Garmendia ML, Corvalan C, Uauy R . Assessing the public health impact of developmental origins of health and disease (DOHaD) nutrition interventions . Ann Nutr Metab 2014. ; 64 : 226 – 30 . [DOI] [PubMed] [Google Scholar]
- 68. Hanson M, Godfrey KM, Lillycrop KA, et al. . Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms . Prog Biophys Mol Biol 2011. ; 106 : 272 – 80 . [DOI] [PubMed] [Google Scholar]
- 69. Seisenberger S, Peat JR, Reik W . Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells . Curr Opin Cell Biol 2013. ; 25 : 281 – 8 . [DOI] [PubMed] [Google Scholar]
- 70. Seisenberger S, Peat JR, Hore TA, et al. . Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers . Philos Trans R Soc Lond B Biol Sci 2013. ; 368 : 20110330 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skinner MK . Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability . Epigenetics 2011. ; 6 : 838 – 42 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang WW, Dietmann S, Irie N, et al. . A unique gene regulatory network resets the human germline epigenome for development . Cell 2015. ; 161 : 1453 – 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heindel JJ, Balbus J, Birnbaum L, et al. . Developmental origins of health and disease: integrating environmental influences . Endocrinology 2015. ; 156 ( 10 ): 3416 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guerrero-Bosagna C, Savenkova M, Haque MM, et al. . Environmentally induced epigenetic transgenerational inheritance of altered sertoli cell transcriptome and epigenome: molecular etiology of male infertility . PLoS One 2013. ; 8 : e59922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nilsson E, Larsen G, Manikkam M, et al. . Environmentally induced epigenetic transgenerational inheritance of ovarian disease . PLoS One 2012. ; 7 : e36129 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Skinner MK, Savenkova M, Zhang B, et al. . Gene bionetworks involved in epigenetic transgenerational inheritance of altered mate preference: environmental epigenetics and evolutionary biology . BMC Genomics 2014. ; 16 : 337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Iqbal K, Tran DA, Li AX, et al. . Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming . Genome Biol 2015. ; 16 : 59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Whitelaw E . Disputing Lamarckian epigenetic inheritance in mammals . Genome Biol 2015. ; 16 : 60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schneider S, Kaufmann W, Buesen R, et al. . Vinclozolin—the lack of a transgenerational effect after oral maternal exposure during organogenesis . Reprod Toxicol 2008. ; 25 : 352 – 60 . [DOI] [PubMed] [Google Scholar]
- 80. Inawaka K, Kawabe M, Takahashi S, et al. . Maternal exposure to anti-androgenic compounds, vinclozolin, flutamide and procymidone, has no effects on spermatogenesis and DNA methylation in male rats of subsequent generations . Toxicol Appl Pharmacol 2009. ; 237 : 178 – 87 . [DOI] [PubMed] [Google Scholar]
- 81. Brown AR, Hosken DJ, Balloux F, et al. . Genetic variation, inbreeding and chemical exposure—combined effects in wildlife and critical considerations for ecotoxicology . Philos Trans R Soc Lond B Biol Sci 2009. ; 364 : 3377 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Johannes F, Colome-Tatche M . Concerning epigenetics and inbreeding . Nat Rev Genet 2011. ; 12 : 376 . [DOI] [PubMed] [Google Scholar]
- 83. Cheptou PO, Donohue K . Epigenetics as a new avenue for the role of inbreeding depression in evolutionary ecology . Heredity 2013. ; 110 : 205 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vergeer P, Wagemaker NC, Ouborg NJ . Evidence for an epigenetic role in inbreeding depression . Biol Lett 2012. ; 8 : 798 – 801 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Skinner MK . What is an epigenetic transgenerational phenotype? F3 or F2 . Reprod Toxicol 2008. ; 25 : 2 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Northstone K, Golding J, Davey Smith G, et al. . Prepubertal start of father's smoking and increased body fat in his sons: further characterisation of paternal transgenerational responses . Eur J Hum Genet 2014. ; 22 : 1382 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Soubry A, Hoyo C, Jirtle RL, et al. . A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line . Bioessays 2014. ; 36 : 359 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schneider S, Marxfeld H, Groters S, et al. . Vinclozolin—no transgenerational inheritance of anti-androgenic effects after maternal exposure during organogenesis via the intraperitoneal route . Reprod Toxicol 2013. ; 37 : 6 – 14 . [DOI] [PubMed] [Google Scholar]
- 89. Sharma A . Variable directionality of gene expression changes across generations does not constitute negative evidence of epigenetic inheritance . Environ. Epigenetics 2015. ; 1 : 1 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yan W . Potential roles of noncoding RNAs in environmental epigenetic transgenerational inheritance . Mol Cell Endocrinol 2014. ; 398 : 24 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Grandjean V, Rassoulzadegan M . [Epigenetic inheritance of the sperm: an unexpected role of RNA] . Gynecol Obstet Fertil 2009. ; 37 : 558 – 61 . [DOI] [PubMed] [Google Scholar]
- 92. Stoeckius M, Grun D, Rajewsky N . Paternal RNA contributions in the Caenorhabditis elegans zygote . EMBO J 2014. ; 33 : 1740 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Erkek S, Hisano M, Liang CY, et al. . Molecular determinants of nucleosome retention at CpG-rich sequences in mouse spermatozoa . Nat Struct Mol Biol 2013. ; 20 : 868 – 75 . [DOI] [PubMed] [Google Scholar]
- 94. Hammoud SS, Nix DA, Hammoud AO, et al. . Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men . Hum Reprod 2011. ; 26 : 2558 – 69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van de Werken C, van der Heijden GW, Eleveld C, et al. . Paternal heterochromatin formation in human embryos is H3K9/HP1 directed and primed by sperm-derived histone modifications . Nat Commun 2014. ; 5 : 5868 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carone BR, Hung JH, Hainer SJ, et al. . High-resolution mapping of chromatin packaging in mouse embryonic stem cells and sperm . Dev Cell 2014. ; 30 : 11 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sadler-Riggleman I, Skinner MK . Environment and the epigenetic transgenerational inheritance of disease . In: Chadwick B. (ed.), Epigenetics: Current Research and Emerging Trends . Norfolk, UK: : Caister Academic Press; , 2015. , 297 – 305 . [Google Scholar]
- 98. Bhandari RK, Deem SL, Holliday DK, et al. . Effects of the environmental estrogenic contaminants bisphenol A and 17alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species . Gen Comp Endocrinol 2015. ; 214 : 195 – 219 . [DOI] [PubMed] [Google Scholar]