Abstract
Brain radiotherapy is frequently used successfully to treat brain tumors. However, radiotherapy is often associated with declines in short-term and long-term memory, learning ability, and verbal fluency. We previously identified a downregulation of the brain-derived neurotrophic factor (BDNF) following cranial irradiation in experimental animals. In the present study, we investigated whether targeting the BDNF high affinity receptor, tropomysin receptor kinase B (TrkB), could mitigate radiation-induced cognitive deficits. After irradiation, chronic treatment with a small molecule TrkB agonist, 7,8-dihydroxyflavone (DHF) in mice led to enhanced activation of TrkB and its downstream targets ERK and AKT, both important factors in neuronal development. DHF treatment significantly restored spatial, contextual, and working memory, and the positive effects persisted for at least 3 months after completion of the treatment. Consistent with preservation of cognitive functions, chronic DHF treatment mitigated radiation-induced suppression of hippocampal neurogenesis. Spine density and major components of the excitatory synapses, including glutamate receptors and postsynaptic density protein 95 (PSD-95), were also maintained at normal levels by DHF treatment after irradiation. Taken together, our results show that chronic treatment with DHF after irradiation significantly mitigates radiation-induced cognitive defects. This is achieved most likely by preservation of hippocampal neurogenesis and synaptic plasticity.
Keywords: cognitive function, TrkB, irradiation, neurogenesis, synaptic plasticity, hippocampus
Introduction
Cranial irradiation is a powerful therapeutic tool for patients with primary or metastatic brain tumors (Bovi and White 2012, Gondi et al. 2010). Prophylactic cranial irradiation has also been used successfully to reduce metastatic brain tumors and increase survival in patients with small-cell lung cancer, metastatic breast cancer, and leukemia (Bovi and White 2012, Jabbour et al. 2010, Rodriguez and Lilenbaum 2010). Despite its effectiveness in cancer therapy, cranial irradiation has been shown to cause immediate and chronic defects in cognitive functions (McTyre et al. 2013, Padovani et al. 2012). Neurocognitive late effects, which manifest months to years after completion of cancer treatment, are common sequelae in patients undergoing cranial irradiation, and they include decreases in spatial memory, verbal memory, and short-term memory. Decline in learning, memory, and spatial processing in patients following cranial irradiation points to hippocampal damage; however the pathogenic mechanism for the various cognitive defects are not completely understood (Gondi et al. 2010, Lee et al. 2012). Studies with animal models have suggested that radiation-induced hippocampal defects include decreases in neurogenesis (Monje et al. 2002, Raber et al. 2004), increases in neuronal apoptosis (Kim et al. 2011, Monje et al. 2003), and altered dendritic structures and spine densities (Chakraborti et al. 2012, Corniola et al. 2012).
There are currently no effective clinical interventions for radiation-induced cognitive defects (D’Antonio et al. 2014). Radiologists have tried alternatives to conventional cranial radiotherapy, such as hyper-fractionated radiotherapy and stereotactic radiosurgery. Both procedures have resulted in sparing of normal brain tissues and reduced radiation-induced cognitive impairments. However, there are mixed reports to the effectiveness of hyper-fractionated radiotherapy, depending on the tumor location (Murphy et al. 2015, Pan et al. 2012, Waber et al. 2004). There is also the bystander effect, which describes the radiating defect on normal tissues due to signals from nearby irradiated tissues (Marin et al. 2015). Experimentally, several approaches to protecting the brain from radiation damage have been explored: enhancing antioxidant capacity (Zou et al. 2012), reducing neuroinflammation (Belarbi et al. 2013, Jenrow et al. 2013), systemic hypoxic treatment (Warrington et al. 2012), inhibition of polyamine synthesis (Allen et al. 2014), or stem cell implant (Acharya et al. 2015) has exhibited some success in rescuing hippocampal neurogenesis or cognitive functions in rodent models. However, the complications in clinical implementation of these therapies, such as surgery or bypassing the blood-brain-barrier, makes further investigation of these experimental approaches difficult.
Neurotrophic factors, including brain-derived neurotrophic factor (BDNF), neurotrophin 3 and 4 (NT-3 and NT-4), and nerve growth factor (NGF), are crucial for proper brain development and function. BDNF binds at high affinity to its receptor, tropomyosin-related kinase receptor B (TrkB), and activates its downstream signaling pathways such as the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways (30). Recently, a small molecule, 7,8-dihydroxyflavone (DHF), was identified as a TrkB agonist (Jang et al. 2010). When administered systemically, DHF was shown to cross the blood-brain-barrier and specifically activated the TrkB receptor in the brain (Jang et al. 2010), leading to long-lasting activation of BDNF signaling pathways (Liu et al. 2014). Its therapeutic potential was demonstrated in rodent models in several areas of neurological defects, including promoting motor neuron survival in an amyotrophic lateral sclerosis model (Korkmaz et al. 2014), preventing synaptic loss and cognitive defects in an Alzheimer disease model (Zhang et al. 2014), inhibiting return of fear memory following extinction (Baker-Andresen et al. 2013), and enhancing cognitive functions in aged rats (Zeng et al. 2012). However, the therapeutic potential of DHF on the cognitive defects from cranial irradiation has yet to be investigated.
We previously observed a loss in BDNF expression in the hippocampus following radiation in a mouse model of cranial irradiation (Zou et al. 2012), and the defects correlated with reduced hippocampal neurogenesis and cognitive impairments (Zou et al. 2012). In this study, the efficacy of DHF in mitigating hippocampal dysfunctions following cranial irradiation was examined. Mice received a single dose of 5 Gy gamma irradiation to the head and were treated with DHF for 3 weeks starting one week after irradiation. Cognitive functions, hippocampal neurogenesis, and changes in synaptic proteins were investigated.
Materials and Methods
Animal Studies
All animal protocols were reviewed and approved by the IACUC committee at the VA Palo Alto Health Care System (Palo Alto, CA, USA) and were consistent with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
Experimental Groups and DHF treatment
Two month old male C57BL/6J mice were sham-irradiated or received 5 Gy of cranial irradiation using a Mark I Cesium Irradiator (JL Shepherd, San Fernando, CA, USA) as described previously (Corniola et al. 2012, Zou et al. 2012). The dose rate was approximately 58.8 cGy/min. One week after irradiation, 5 mg/kg DHF was administered daily by subcutaneous (SQ) injection for three weeks. For TrkB pathway immunoblotting, DHF was administered for 10 days. DHF was initially dissolved in DMSO and then diluted in PBS to achieve a concentration of 0.5 mg/ml. The final DMSO concentration was approximately 17%. Accordingly, vehicle control mice received daily SQ injection of 17% DMSO prepared in PBS. DHF was either synthesized by Dr. Keqiang Ye (Emory University, Atlanta, GA, USA) or purchased from Santa Cruz biotechnology (Santa Cruz, CA, USA).
Behavioral studies
Behavioral studies were performed four weeks after the completion of DHF treatment. Mice (n=12 per group) were tested sequentially in open field, Y maze, elevated zero maze, and fear conditioning. Approximately five weeks after the completion of contextual fear conditioning, the same groups of mice were tested again in open field, Y maze, and fear conditioning. Barnes maze was then used to investigate spatial learning. One week after the completion of Barnes maze, half of each cohort (n=6) was exposed to a novel environment for 5 minutes and sacrificed 90 minutes later to examine activation of the immediate early gene c-Fos. The remaining population (n=6 each) of each cohort served as cage controls and were sacrificed on the same day. Unless stated differently, all arenas were cleaned with 70% ethanol between each trial and test subject. All behavioral studies were recorded and analyzed by TopScan or FreezeScan (CleverSys Inc., Reston, VA, USA). Behavioral test procedures are explained in greater detail in Supplementary Material.
Hippocampal neurogenesis
A separate set of mice were used for hippocampal neurogenesis studies. At the completion of DHF treatment, half of each cohort (n=6 each) was subjected to daily bromodeoxyuridine (BrdU, 50 mg/kg, IP) injection for five consecutive days and sacrificed four weeks after the first BrdU injection. The other half (n=6 each) was injected with two doses of BrdU, eight hours apart, and sacrificed for tissue collection the next day. BrdU was incorporated by replicating cells, and the long washout period in the long-term study allowed for identification of newborn cells with the capacity for long-term survival, while the short turnaround time in the short-term study allowed for examination of progenitor cell proliferation during the period of BrdU administration.
Immunohistochemical staining, imaging, and analysis
Brain tissues used for immunohistochemical analyses were processed and analyzed as described (Corniola et al. 2012, Zou et al. 2012). Antibodies used are listed in Table 1. The systemic random sampling principal was applied as described (Corniola et al. 2012, Zou et al. 2012) to estimate total number of newborn neurons in hippocampal formation. One in every 6th sections was used for BrdU staining for the estimation of total newborn cells; one in every 12th sections was used for doublecortin (Dcx) or triple (BrdU/NeuN/GFAP) staining for the estimation of total numbers of immature neurons or assessment of differentiation of newborn cells, respectively.
Table 1. Antibodies used in the study.
| Primary antibodies | |||||
|---|---|---|---|---|---|
| Target | Antigen | Antibody | Dilution | Source | Cat number |
| Astroglial cells | Glial fibrillary acidic protein (GFAP) |
Rabbit anti-GFAP | 1:1000 | DAKO | Z0334 |
| Brain derived neurotrophic factor receptor |
Tropomyosin receptor kinase B (TrkB) |
Rabbit anti-TrkB | 1:1000 | Cell Signaling |
#4603 Clone 80E3 |
| Gamma- aminobutyric acid (GABA) receptor |
GABA-A receptor alpha1 (GABAARA-1) |
Mouse anti-GABA- AR α-1 IgG1 |
1:10 | NeuroMab | 73-136 Clone N95/35 |
| Glutamate/NMDA receptor |
Glutamate/NMDA receptor epsilon2 (GluN2B/NR2B) |
Mouse anti- GluN2B/NR2B |
1:1000 | NeuroMab | 73-097 Clone N59/20 |
| Housekeeping protein |
β-Actin | Mouse anti-β- actin IgG1 |
1:10000 | Abcam | Ab6276 Clone AC-15 |
| Housekeeping protein |
α-Tubulin | Mouse anti-α- tubulin IgG1 |
1:10000 | Abcam | Ab7291 Clone DM1A |
| Immature neurons | Double cotrin (Dcx) | Goat anti-Dcx | 1:250 | Santa Cruz | SC-8066 |
| Immediate early gene |
c-Fos | Rabbit anti-c-Fos | 1:1000 | EMD | PC38 |
| Mature neurons | Neuronal nuclear protein (NeuN) |
Mouse anti-NeuN | 1:1000 | Millipore | MAB377 |
| Phosphorylated TrkB (Y816) |
pTrkB (Y816) | Rabbit anti-pTrkB (Y816) |
1:1000 | Abcam | Ab75173 |
| Phosphorylated TrkB downstream protein |
Phospho-extracellular- signal-regulated kinase (pErk) |
Rabbit Anti-pErk (Thr202/Tyr204) |
1:1000 | Cell Signaling |
#9101 |
| Phosphorylated TrkB downstream protein |
Phospho-protien kinase B (pAkt) |
Rabbit Anti-pAkt (Ser473) |
1:1000 | Cell Signaling |
#9271 |
| Postsynaptic protein |
Postsynaptic density protein 95 (PSD-95) |
Mouse anti-PSD- 95 IgG2a |
1:100 | NeuroMab | 73-028 Clone K28/43 |
| Presynaptic protein | Synaptophysin | Mouse anti- synaptophysin |
1:2000 | Alomone labs |
ANR-013 |
| Replicating cells | Bromodeoxyuridine (BrdU) |
Rat anti-BrdU | 1:1000 | Abcam | Ab6326 |
| TrkB downstream protein |
Extracellular-signal- regulated kinase (Erk) |
Rabbit Anti-Erk | 1:1000 | Assay designs |
KAP-MA001 |
| TrkB downstream protein |
Protien kinase B (Akt) | Mouse Anti-Akt | 1:1000 | Cell Signaling |
#2920S |
| Secondary antibodies | |||||
|---|---|---|---|---|---|
| Label | Host | Target | Dilution | Source | Cat Number |
| Alexa 488 | Goat | Mouse IgG | 1:1000 | Invitrogen | A11001 |
| Alexa 647 | Goat | Rabbit IgG | 1:1000 | Invitrogen | A21244 |
| Alexa 647 | Donkey | Goat IgG | 1:1000 | Invitrogen | A10522 |
| Biotin | Rabbit | Goat IgG | 1:1000 | Vector | BA5000 |
| Biotin | Goat | Rabbit IgG | 1:1000 | Vector | BA1000 |
| Cy 3 | Goat | Rat IgG | 1:1000 | Invitrogen | A21447 |
| IR Dye 680 | Goat | Mouse IgG | 1:20000 | Li-Cor | 926-32210 |
| IR Dye 800 | Goat | Mouse IgG | 1:20000 | Li-Cor | 926-32220 |
| IR Dye 680 | Goat | Rabbit IgG | 1:20000 | Li-Cor | 926-32221 |
| IR Dye 800 | Goat | Rabbit IgG | 1:20000 | Li-Cor | 926-32211 |
Immunoblotting
For quantification of TrkB, ERK, AKT, and synaptic proteins in the hippocampus following irradiation and DHF treatment, western blot analyses were performed as previously described (Zou et al. 2012), or following manufacturers’ recommendations. Detailed procedures are described in Supplementary Material and antibody information is provided in Table 1.
Statistical Analysis
Statistical analysis was performed with Prism® 6 (GraphPad Software, La Jolla, CA, USA) using two-way ANOVA with Bonferroni post-test or Students’ T-test where appropriate. Outliers were identified by the Grubbs’ test and excluded from final analyses. All data were presented as Mean ± SEM. Results from two-way ANOVA Bonferroni post-tests of all experimental end points are summarized in Supplementary Material Table S1.
Results
DHF treatment activated TrkB signaling pathway
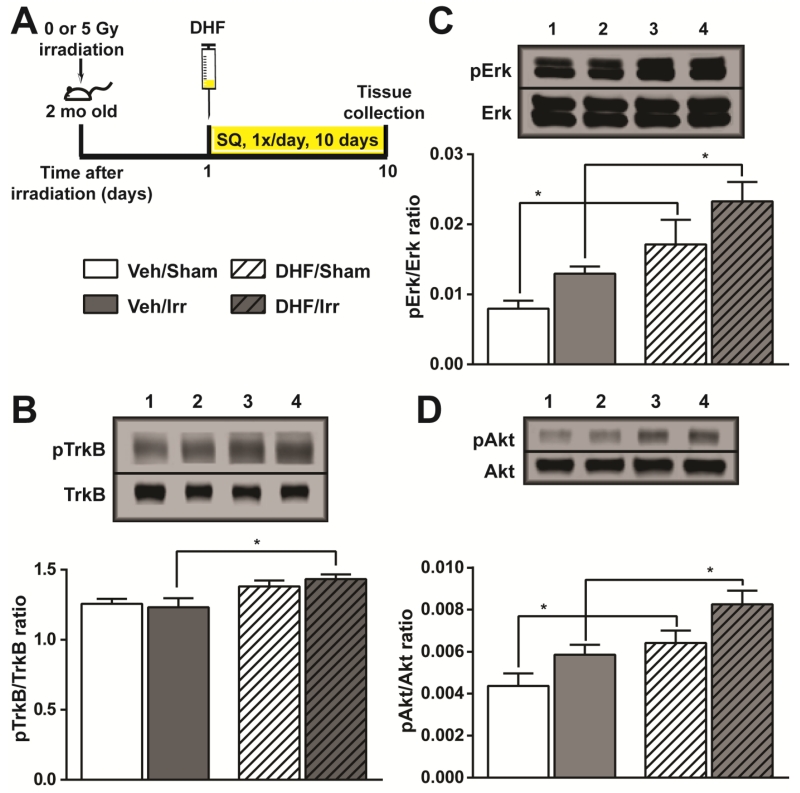
DHF binding to and activation of TrkB has been well established in vitro (Jang et al. 2010). In vivo activation of TrkB by DHF administration has also been demonstrated in a number of experimental systems (Tzeng et al. 2013, Zhang et al. 2014). With 5 mg/kg/day DHF administration for 10 days (Fig. 1A), we observed increased pTrkB/TrkB ratio and a concomitant increase in its downstream targets pErk and pAkt in sham and irradiated mice (Fig. 1B-1D). Whereas the ratio of pTrkB/TrkB was increased by 10% and 16% in DHF-treated sham and irradiated mice, respectively, the ratio of pErk/Erk increased by 115% and 80%, and pAkt/Akt increased by 47% and 41% in DHF treated cohorts. The data suggested in vivo activation of TrkB signaling pathway by daily DHF treatment, and the activation was not negatively affected by cranial irradiation.
Fig. 1.
In vivo activation of TrkB and its downstream targets. Hippocampal formation collected from the four experimental cohorts after irradiation and DHF treatment were examined for the extent of TrkB activation. A, experimental timeline for irradiation, DHF treatment, and tissue collection. B, the ratio of phosphoTrkB (pTrkB) and total TrkB. C and D, the ratio of phosphoErk (pErk) and phosphoAkt (pAkt) to total Erk and Akt, respectively. Results from two-way ANOVA with Bonferroni’s post-hoc analysis are shown. *, p<0.05. N=5 each. Western blot lanes 1-4: Veh/Sham; Veh/Irr; DHF/Sham; DHF/Irr.
DHF treatment restored cognitive functions following cranial irradiation
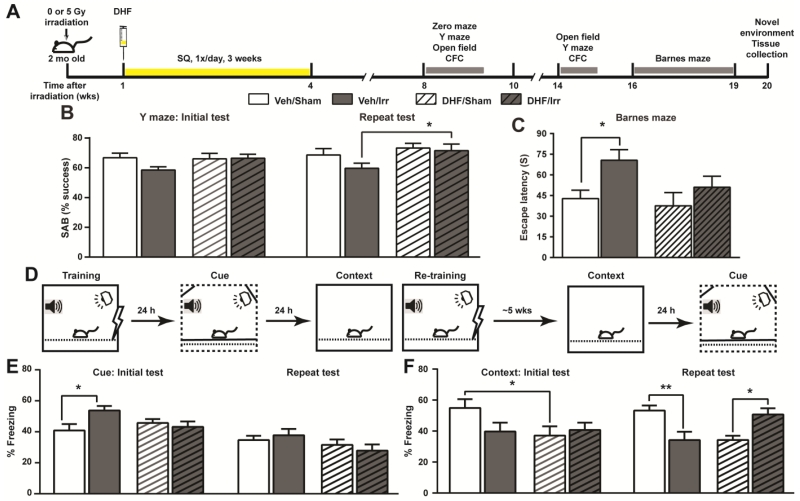
To investigate whether chronic DHF treatment can ameliorate the negative impact of irradiation on cognitive functions, mice were exposed to a 5 Gy gamma irradiation in the head and received daily DHF treatment (5 mg/kg/day) one week later for a total of 3 weeks. A series of behavioral studies were carried out 4 weeks after the completion of DHF treatment (Fig. 2A). The experimental groups included vehicle-treated sham (Veh/Sham) or irradiated (Veh/Irr), and DHF-treated sham (DHF/Sham) or irradiated (DHF/Irr) mice.
Fig. 2.
Examination of cognitive functions following irradiation and DHF treatment. A, experimental timeline showing the chronological order of different treatments and behavioral tests. B, success rate of SAB in the Y maze. Mice were tested at 8 and 14 weeks after irradiation. C, Barnes maze test for spatial learning and memory approximately 16-19 weeks after irradiation. Escape latencies from the final trial were plotted. D, experimental design for contextual fear conditioning. E and F, cued (E) and contextual (F) freezing measured approximately 9 and 15 weeks after irradiation. Results from two-way ANOVA with Bonferroni’s post-hoc analysis are shown. *, p<0.05; **, p<0.01. N=8 per group for Barnes maze and 12 per group for all other tests.
The spontaneous alteration behavior (SAB) (Hughes 2004) in Y maze exploration was used to measure the hippocampal-dependent working spatial memory (Fig. 2B). Whereas irradiation led to a 12.4% reduction in SAB in Veh/Irr mice, the level of SAB in DHF/Irr mice remained at a similar level as that in Veh/Sham and DHF/Sham mice. Defects in SAB in Veh/Irr mice increased with time, and the mice performed significantly worse than DHF/Irr mice when re-examined 5 weeks later (Fig. 2B).
To further investigate changes in spatial memory and spatial navigation, mice were subjected to a modified Barnes maze test (Faizi et al. 2012) roughly 12 weeks after completion of DHF treatment. The training protocol was challenging to all cohorts on the first day with gradual improvements on the subsequent days, especially as the mice moved into the 4th trial of each day (Supplementary Material Fig. S1A). Significant improvements (escape latency from trial 1 vs. trial 4) were observed in DHF/Irr mice on day 3 and in DHF/Sham and Veh/Sham mice on day 4 (Supplementary Material Fig. S1A). Additionally, a significant difference in escape latencies was observed in the 4th trial on the last day of the test, where Veh/Irr mice spent significantly more time (65.2%) to find the escape tunnel compared to Veh/Sham mice (Fig. 2C). Irradiated mice treated with DHF, on the other hand, continued to exhibit well-preserved cognitive functions with only an increased trend (26.5%) in the escape latency compared to that of DHF/Sham mice. Collectively, the results showed the persistent benefits of DHF in mitigating the negative impacts of cranial irradiation on spatial learning and spatial memory, which were behavioral readouts that relied heavily on hippocampal functions.
The delayed fear conditioning paradigm was used to examine hippocampal-dependent contextual memory and limbic system-dependent cue memory, and the study was designed to test recent and distant memory recalls (Fig. 2D). Baseline freezing during the training period was not significantly different among all cohorts (Supplementary Material Fig. S1B). Whereas, Veh/Irr mice showed a heightened level of cued freezing compared to the Veh/Sham controls (Fig. 2E), no significant difference in cued freezing was observed between DHF/Sham and DHF/Irr mice. The enhanced fear memory in Veh/Irr mice was transient and had diminished five weeks later upon re-testing. Consistent with increased cued freezing in the fear conditioning test, elevated zero maze and open field tests also showed transiently increased anxiety in Veh/Irr mice that returned to levels similar to the other three cohorts five weeks later (Supplementary Material Fig. S1D & E).
In contrast to the transient nature of the enhanced fear memory, defects in contextual memory was more persistent. Veh/Irr mice exhibited a trend in reduced contextual memory (Fig. 2F) during the recent memory recall phase, and a significant reduction five weeks later upon re-examination. DHF treatment lowered the level of contextual freezing in DHF/Sham mice when compared to that of Veh/Sham controls. However, irradiation did not lead to a further reduction in contextual memory in DHF/Irr mice. Recall of the distant contextual memory remained at a similar level for DHF/Sham mice. However, contextual freezing was increased by 24.7% in DHF/Irr mice 5 weeks later (Fig. 2F) although the change did not reach the statistically significant level when analyzed with two-way ANOVA Bonferroni post-hoc analysis.
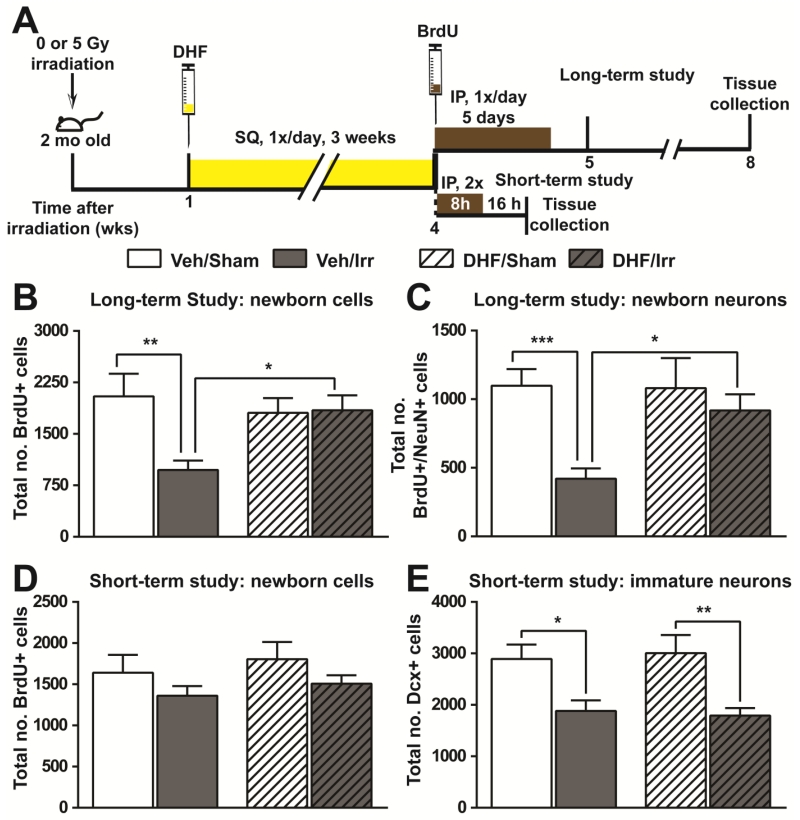
DHF treatment enhanced long-term survival of newborn neurons following irradiation
To understand the cellular basis for the observed changes in learning and memory, we investigated the effects of DHF on the production of newborn neurons in the subgranular zone (SGZ) of hippocampus. Following the same irradiation and DHF treatment schedule as that used for behavioral studies, BrdU administration was used to label newborn cells (Fig. 3A). BrdU positive (BrdU+) cells in the hippocampal SGZ 3-4 weeks after BrdU administration represented long-term survival of newborn cells in this location. Whereas irradiation resulted in a 46% reduction in the number of BrdU+ cells in Veh/Irr mice, DHF treatment prevented this deficit and DHF/Irr mice had a comparable number of BrdU+ cells as that observed in DHF/Sham and Veh/Sham mice (Fig. 3B). Irradiation also caused a significant change in the lineage selection during differentiation of newborn cells in Veh/Irr mice with a lower percentage of BrdU+ cells possessing the mature neuronal marker, neuronal-specific nuclear protein (NeuN) (Supplementary Material Fig. S2A). Consequently, a significant reduction in the number of BrdU+/NeuN+ cells was observed in Veh/Irr mice (Fig. 3C). DHF treatment was able to prevent this change, and the number of newborn mature neurons in DHF/Irr mice was comparable to that in DHF/Sham and Veh/Sham controls (Fig. 3C).
Fig. 3.
Hippocampal neurogenesis following irradiation and DHF treatment. A, experimental timeline. Long-term and short-term study indicates the long and short interval, respectively, between BrdU injection and tissue collection. The long interval allowed examination of the long-term survival of newborn cells and neurons in the SGZ, while the short interval allowed assessment of progenitor cell proliferation at the time of BrdU administration. B and C, total number of newborn cells (BrdU+, B) and newborn mature neurons (BrdU+/NeuN+, C) in the SGZ of hippocampal dentate gyrus from the long-term study. D and E, total number of newborn cells (BrdU+, D) and immature neurons (Dcx+, E) in the SGZ of hippocampal dentate gyrus. Results from two-way ANOVA with Bonferroni’s post-hoc analysis are shown. *, p<0.05; **, p<0.01; ***, p<0.001. N=6 each.
To know if the normalized hippocampal neurogenesis was a result of normal progenitor cell proliferation and production of immature neurons in DHF/Irr mice, the number of BrdU+ cells and immature neurons (doublecortin positive cells, Dcx+) in the hippocampal SGZ was examined at the completion of DHF treatment. BrdU+ cells in the hippocampal SGZ collected 16 hours after the last BrdU administration reflected proliferation of neural progenitor cells at this location. Irradiation resulted in a modest reduction (17%) in the number of BrdU+ cells in both cohorts of irradiated mice (Veh/Irr and HDF/Irr) (Fig. 3D). Additionally, the number of Dcx+ cells was reduced by irradiation by 35-40% (F(1,20)=18.42, p=0.0004) in Veh/Irr and DHF/Irr mice (Fig. 3E). The data suggested that DHF treatment starting one week after irradiation had no effect on progenitor cell proliferation or the production of immature neurons, and that normalized hippocampal neurogenesis in DHF/Irr mice was a result of enhanced survival of newborn neurons.
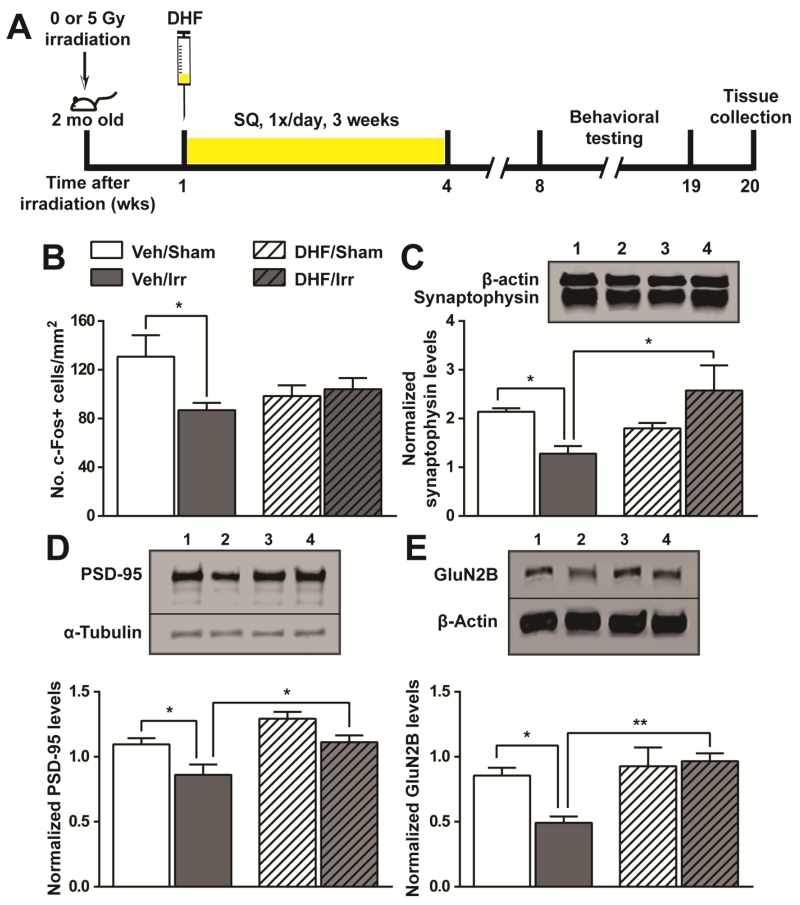
DHF treatment prevented loss of neuronal activities in the post-irradiation environment
To explore the molecular basis for the preserved cognitive functions in DHF/Irr mice, hippocampal neuronal activation upon exposure to a novel environment was examined (Fig. 2A & Fig. 4A). Expression of the transcription factor c-Fos is quickly activated in behaving animals and is often used as a measurement for neuronal activities (Cruz et al. 2013). Compared to Veh/Sham mice, irradiation led to a 33% reduction in c-Fos positive (c-Fos+) cells in the hippocampal dentate gyrus of Veh/Irr mice (Fig. 4B). The data showed a strong concordance between cognitive functions and neuronal activities in vehicle-treated cohorts. Interestingly, DHF treatment resulted in a lower number of c-Fos+ cells in DHF/Sham mice. However, irradiation did not cause a further reduction in DHF/Irr mice. Given the divergent results in cognitive functions and the number of c-Fos+ cells in DHF-treated mice, there might be unidentified interactions between DHF and synaptic inputs. The number of c-Fos+ cells in all groups exposed to the novel environment was 4-5 times higher than that in cage controls (data not shown).
Fig. 4.
Changes in neuronal activities and synaptic proteins following irradiation and DHF treatment. A, experimental timeline highlighting time points of irradiation, DHF treatment, and tissue collection. B, expression of the immediate early gene, c-Fos, upon exposure to a novel environment. The number of c-Fos positive cells in the dentate gyrus was normalized to the area examined. C-E, the levels of synaptophysin (C), PSD-95 (D), and glutamate receptor (GluN2B) (E) in the synaptosomes isolated from the hippocampal formation. Results from two-way ANOVA with Bonferroni’s post-hoc analysis are shown. *, p<0.05; **, p<0.01. N=6 each. Western blot lanes 1-4: Veh/Sham; Veh/Irr; DHF/Sham; DHF/Irr.
Normal cognitive functions were associated with well-preserved excitatory synapses in DHF/Irr mice
Synaptic proteins and neurotransmitter receptors play an important role in neuronal activation and consequently, in learning and memory (Kim and Diamond 2002). To further identify the mechanisms underlying DHF-mediated preservation of hippocampal functions following cranial irradiation, changes in synaptic proteins and dendritic spines were examined.
Irradiation significantly reduced two important synaptic proteins in Veh/Irr mice: the major synaptic vesicle protein synaptophysin (68% reduction) and the post-synaptic protein 95 (PSD-95) (27% reduction) in the pre- and post-synapses, respectively (Fig. 4C & 4D). DHF treatment prevented this negative effect from irradiation, and the level of both proteins in DHF/Irr mice were comparable to that of Veh/Sham controls. PSD-95 is an anchoring protein for glutamate receptors and is associated with long-term potentiation in excitatory synapses. To further investigate the relationship between changes in PSD-95 and cognitive outcomes in this experimental system, we investigated changes in the glutamate receptor GluN2B/NR2B, which is critical for spatial memory (Brim et al. 2013). Consistent with reduced PSD-95 levels, Veh/Irr mice had a significantly reduced level of GluN2B/NR2B receptor compared to Veh/Sham controls. DHF/Irr mice, on the other hand, showed no significant changes from that of DHF/Sham and Veh/Sham controls (Fig. 4E). PSD-95 is the most abundant protein at the post-synaptic density, which is a cytoskeletal structure at the synaptic junction of dendritic spines. Consistent with the abundance of PSD-95, a 5-week continuous DHF administration with osmotic pumps prevented dendritic spine loss in hippocampal granule cells in DHF/Irr mice (Supplementary Material Fig. S3A). Taken together, elements important for synaptic plasticity, including synaptic proteins, glutamate receptor, and dendritic spines, were well maintained in the hippocampus by DHF treatment after cranial irradiation, and this most likely supported normal cognitive functions seen in DHF/Irr mice.
Discussion
In this study, we showed that DHF, when given one week after a single dose of 5 Gy cranial irradiation, activated TrkB and its downstream signaling pathways in the hippocampus. The treatment modulated the adverse effects of cranial irradiation on cognitive functions, especially in the hippocampal-dependent functions of spatial learning and spatial memory. We identified enhanced long-term survival of newborn neurons and well-maintained synaptic structures in the hippocampal dentate gyrus as the potential mechanisms supporting DHF-mediated preservation of hippocampal functions after cranial irradiation.
We observed abnormalities in both hippocampal-dependent and hippocampal-independent behaviors following irradiation in this experimental system. However, while hippocampal-independent defects (enhanced anxiety and fear memories) appeared to be transient, hippocampal-dependent defects (spatial and contextual memories) lasted for at least 4 months after irradiation. The persistence in hippocampal defects was consistent with previous findings (Belarbi et al. 2013, Raber et al. 2013) and reinforced the need for interventions to mitigate hippocampal defects in patients receiving cranial irradiation therapy. DHF treatment normalized several irradiation-induced behavioral defects, including anxiety and fear memory (Fig. 2E, Supplementary Material Fig. S1), working spatial memory, contextual memory, and spatial learning (Fig. 2B, 2C, 2F). It was interesting to note that DHF/Irr mice showed improvement in their ability to recall a distant contextual memory following re-conditioning (Fig. 2F), whereas the ability for fear memory recall declined with time (Fig. 2E). Since memories depend on different brain compartments (Curzon et al. 2009), it seems that memory consolidation and retrieval was affected by irradiation and DHF treatment to various degrees, where DHF/Irr mice showed noticeable improvements in re-consolidation and retrieval of distant memories.
Hippocampal neurogenesis is important for certain aspects of hippocampal-dependent learning and memory, and recent evidence suggests its involvement in the fine discrimination of similar contexts (Kheirbek et al. 2012, Sahay et al. 2011). In this experimental system, we observed a positive correlation between hippocampal neurogenesis and hippocampal-dependent functions. Interestingly, the normalized hippocampal neurogenesis in DHF/Irr mice was due to enhanced survival of newborn neurons, which compensated for the upstream loss of immature neurons from irradiation. One mechanisms contributing to the improved long-term survival of newborn neurons in DHF-treated mice could be due to the preservation of synaptic proteins (Fig. 4) and increase in dendritic spine density (Supplementary Material Fig. S3A), which allowed newborn neurons to establish synaptic connections and ensured their survival. The effect of DHF on hippocampal neurogenesis following cranial irradiation was similar to that observed in the mouse model expressing high levels of extracellular superoxide dismutase (EC-SOD) (Zou et al. 2012) in that both experimental systems enhanced the long-term survival of newborn neurons without affecting progenitor cell proliferation or the generation of immature neurons in the SGZ of hippocampal dentate gyrus. Anti-inflammatory approaches have also been shown to be effective in mitigating irradiation-induced cognitive defects and reduced hippocampal neurogenesis (Belarbi et al. 2013, Lee et al. 2012, Monje et al. 2003). In addition to TrkB activation, DHF has been shown to exhibit antioxidant and anti-inflammatory effects in vitro when used before or at the time of oxidative insults or LPS treatment (Kang et al. 2015, Park et al. 2014, Ryu et al. 2014). However, its antioxidant and anti-inflammatory effects in the in vivo system have not been evaluated. Additional studies will need to be performed to establish if antioxidant or anti-inflammatory mechanisms also contributed to the positive effects of DHF observed in this experimental system.
Synaptic connections in the hippocampus are dynamic, where communication via synapses typically strengthens synaptic transmissions and promotes further synaptic plasticity (Tronson and Taylor 2007). Processes such as long-term potentiation (LTP) is critical for memory consolidation and recollection (Lynch 2004), and chronic DHF treatment has been shown to enable LTP induction by enhancing glutamate-NMDA receptors in learning impaired aged rats and a concomitant improvement in cognitive functions (Zeng et al. 2012). Our findings showed the preservation of the same glutamate receptors in DHF/Irr mice, suggesting the likelihood of restoring LTP in this cohort as well. Collectively, the maintenance of normal levels of synaptic proteins in DHF/Irr mice likely contributes to the observed normal hippocampal-dependent functions of learning and memory.
A recent in vitro study of DHF demonstrated its high affinity binding and sustained activation of TrkB (Liu et al. 2014). Although the positive effects of DHF can be attributed to the activation of BDNF signaling pathways (Jang et al. 2010, Zeng et al. 2012), prolonged or excessive activation could be problematic in causing decreased dendritic and axonal branching (Danzer et al. 2002). Consequently, BDNF was thought to play an important role in epileptogenesis for its function in strengthening excitatory synapses and at the same time weakening inhibitory synapses (Binder and Scharfman 2004). In this experimental system, irradiation decreased both glutamate and GABA receptor levels in the hippocampus (Fig. 4, Supplementary Material Fig. S3B); however, DHF treatment appeared to be only efficacious in normalizing the level of glutamate receptors. Additionally, excitatory-associated synaptic protein PSD-95 levels were increased with DHF treatment in DHF/Sham mice (Fig. 4). This imbalance between excitatory and inhibitory neurotransmission has the potential to cause side effects, especially in DHF-treated, sham-irradiated controls. Finally, TrkB is expressed in various neuronal and glial populations and its activation has the potential to affect excitatory and inhibitory circuits, as well as neuroinflammation, in different brain regions (Huang and Reichardt 2003, Zhang et al. 2003, Zhang et al. 2014). A clear understanding of the effects of DHF on different cell populations will be crucial to designing effective treatments for various neurological disorders.
Conclusion
This study showed that chronic treatment with DHF after cranial irradiation activated TrkB and its downstream signaling pathways in the hippocampal formation. Concomitant to this observation was the rescued behavioral, cellular, and synaptic defects induced by radiation, and the effects persisted for at least 3 months after the completion of DHF treatment. Our experimental plan was designed to facilitate recovery following radiation rather than protecting tissues at the time of radiation to broaden the scope of use. Given its efficacy in post-exposure administration, DHF could potentially be used to treat accidental radiation exposure or space radiation damages. The pharmacokinetics of DHF via oral administration have been characterized in mice (Liu et al. 2014), and it will simplify the dosing method at the point of care in the future as the drug is tested for clinical use (Liu et al. 2016). Given the post-exposure efficacy of DHF in minimizing the late effects of cognitive impairments from radiation, it will be important to see if DHF can be combined with other agents to enhance protection and recovery of normal tissues without necessarily increasing protection to tumor tissues in the design of radiation therapy.
Supplementary Material
Highlights.
7,8-dihydroxyflavone (DHF) activates BDNF receptor, TrkB, in hippocampus
Chronic DHF treatment after cranial irradiation mitigates neurocognitive defects
DHF improves long-term survival of newborn neurons in hippocampal dentate gyrus
DHF treatment after cranial irradiation also enhances excitatory synaptic proteins
Treatment with DHF can be efficacious in preserving cognition after radiotherapy
Acknowledgements
We thank Hsun Yang and Melody Khosravi for technical assistance in tissue processing, and Huy Nugyen for critical reading of the manuscript and assistance in graphical design. This work was supported by funding from the Department of Veterans Affairs Merit review BX-0024-71 (to TTH), National Institutes of Health Grant NS046051 (to JRF), and the resources and facilities at the Veteran’s Affairs Palo Alto Health Care System.
Abbreviations
- DHF
7,8-dihydroxyflavone
- BDNF
brain-derived neurotrophic factor
- TrkB
tropomycin receptor kinase B
- ERK
extracellular signal-regulated kinase
- AKT
protein kinase B
- PSD-95
post-synaptic density 95
- SGZ
subgranular zone
- BrdU
bromodeoxyuridine
- Dcx
double cortin
- NeuN
neuronal nuclear antigen
- GFAP
glial fibrillary acidic protein
- Irr
irradiation
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya MM, Martirosian V, Christie LA, Riparip L, Strnadel J, Parihar VK, Limoli CL. Defining the optimal window for cranial transplantation of human induced pluripotent stem cell-derived cells to ameliorate radiation-induced cognitive impairment. Stem Cells Transl Med. 2015;4(1):74–83. doi: 10.5966/sctm.2014-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AR, Eilertson K, Sharma S, Baure J, Allen B, Leu D, Rosi S, Raber J, Huang TT, Fike JR. Delayed administration of alpha-difluoromethylornithine prevents hippocampus-dependent cognitive impairment after single and combined injury in mice. Radiat Res. 2014;182(5):489–498. doi: 10.1667/RR13753.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Andresen D, Flavell CR, Li X, Bredy TW. Activation of BDNF signaling prevents the return of fear in female mice. Learn Mem. 2013;20(5):237–240. doi: 10.1101/lm.029520.112. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Arellano C, Fike JR, Rosi S. CCR2 Deficiency Prevents Neuronal Dysfunction and Cognitive Impairments Induced by Cranial Irradiation. Cancer Res. 2013;73(3):1201–1210. doi: 10.1158/0008-5472.CAN-12-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovi JA, White J. Radiation therapy in the prevention of brain metastases. Curr Oncol Rep. 2012;14(1):55–62. doi: 10.1007/s11912-011-0208-6. [DOI] [PubMed] [Google Scholar]
- Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, Foster TC, Magnusson KR. Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238:211–226. doi: 10.1016/j.bbr.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 2012;7(7):e40844. doi: 10.1371/journal.pone.0040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corniola R, Zou Y, Leu D, Fike JR, Huang TT. Paradoxical Relationship between Mn Superoxide Dismutase Deficiency and Radiation-Induced Cognitive Defects. PLoS One. 2012;7(11):e49367. doi: 10.1371/journal.pone.0049367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, Hope BT. New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci. 2013;14(11):743–754. doi: 10.1038/nrn3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Rustay NR, Browman KE. In: Cued and fear conditioning for rodents. Methods of Behavioral analysis in neuroscience. Buccafusco JJ, editor. CRC Press; Boca Raton, FL: 2009. [PubMed] [Google Scholar]
- D’Antonio C, Passaro A, Gori B, Del Signore E, Migliorino MR, Ricciardi S, Fulvi A, de Marinis F. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;6(3):101–114. doi: 10.1177/1758834014521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzer SC, Crooks KR, Lo DC, McNamara JO. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J Neurosci. 2002;22(22):9754–9763. doi: 10.1523/JNEUROSCI.22-22-09754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faizi M, Bader PL, Saw N, Nguyen TV, Beraki S, Wyss-Coray T, Longo FM, Shamloo M. Thy1-hAPP(Lond/Swe+) mouse model of Alzheimer’s disease displays broad behavioral deficits in sensorimotor, cognitive and social function. Brain Behav. 2012;2(2):142–154. doi: 10.1002/brb3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondi V, Tome WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97(3):370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28(5):497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Jabbour E, Thomas D, Cortes J, Kantarjian HM, O’Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010;116(10):2290–2300. doi: 10.1002/cncr.25008. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenrow KA, Brown SL, Lapanowski K, Naei H, Kolozsvary A, Kim JH. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 2013;179(5):549–556. doi: 10.1667/RR3026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Choi IW, Han MH, Kim GY, Hong SH, Park C, Hwang HJ, Kim CM, Kim BW, Choi YH. The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts. Int J Mol Med. 2015;36(2):501–510. doi: 10.3892/ijmm.2015.2256. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, Tannenholz L, Hen R. NR2B-dependent plasticity of adult-born granule cells is necessary for context discrimination. J Neurosci. 2012;32(25):8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim JS, Yang M, Kim J, Lee D, Kim JC, Shin T, Kim SH, Moon C. Comparison of the dose-response relationship of radiation-induced apoptosis in the hippocampal dentate gyrus and intestinal crypt of adult mice. Radiat Prot Dosimetry. 2011;148(4):492–497. doi: 10.1093/rpd/ncr191. [DOI] [PubMed] [Google Scholar]
- Korkmaz OT, Aytan N, Carreras I, Choi JK, Kowall NW, Jenkins BG, Dedeoglu A. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci Lett. 2014;566:286–291. doi: 10.1016/j.neulet.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Haditsch U, Cord BJ, Guzman R, Kim SJ, Boettcher C, Priller J, Ormerod BK, Palmer TD. Absence of CCL2 is sufficient to restore hippocampal neurogenesis following cranial irradiation. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther (Seoul) 2012;20(4):357–370. doi: 10.4062/biomolther.2012.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chan CB, Ye K. 7,8-dihydroxyflavone, a small molecular TrkB agonist, is useful for treating various BDNF-implicated human disorders. Transl Neurodegener. 2016;5:2. doi: 10.1186/s40035-015-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Obianyo O, Chan CB, Huang J, Xue S, Yang JJ, Zeng F, Goodman M, Ye K. Biochemical and biophysical investigation of the brain-derived neurotrophic factor mimetic 7,8-dihydroxyflavone in the binding and activation of the TrkB receptor. J Biol Chem. 2014;289(40):27571–27584. doi: 10.1074/jbc.M114.562561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84(1):87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Marin A, Martin M, Linan O, Alvarenga F, Lopez M, Fernandez L, Buchser D, Cerezo L. Bystander effects and radiotherapy. Rep Pract Oncol Radiother. 2015;20(1):12–21. doi: 10.1016/j.rpor.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4(Suppl 4):S236–244. doi: 10.4103/2152-7806.111301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Murphy ES, Chao ST, Angelov L, Vogelbaum MA, Barnett G, Jung E, Recinos VR, Mohammadi A, Suh JH. Radiosurgery for Pediatric Brain Tumors. Pediatr Blood Cancer. 2015 doi: 10.1002/pbc.25831. [DOI] [PubMed] [Google Scholar]
- Padovani L, Andre N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol. 2012;8(10):578–588. doi: 10.1038/nrneurol.2012.182. [DOI] [PubMed] [Google Scholar]
- Pan ZQ, He XY, Guo XM, Ye M, Zhang Z, He SQ, Liu TF. A phase III study of late course accelerated hyperfractionated radiotherapy versus conventionally fractionated radiotherapy in patients with nasopharyngeal carcinoma. Am J Clin Oncol. 2012;35(6):600–605. doi: 10.1097/COC.0b013e31822dfd55. [DOI] [PubMed] [Google Scholar]
- Park HY, Park C, Hwang HJ, Kim BW, Kim GY, Kim CM, Kim ND, Choi YH. 7,8-Dihydroxyflavone attenuates the release of pro-inflammatory mediators and cytokines in lipopolysaccharide-stimulated BV2 microglial cells through the suppression of the NF-kappaB and MAPK signaling pathways. Int J Mol Med. 2014;33(4):1027–1034. doi: 10.3892/ijmm.2014.1652. [DOI] [PubMed] [Google Scholar]
- Raber J, Allen AR, Rosi S, Sharma S, Dayger C, Davis MJ, Fike JR. Effects of whole body Fe radiation on contextual freezing and Arc-positive cells in the dentate gyrus. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Lilenbaum RC. Small cell lung cancer: past, present, and future. Curr Oncol Rep. 2010;12(5):327–334. doi: 10.1007/s11912-010-0120-5. [DOI] [PubMed] [Google Scholar]
- Ryu MJ, Kang KA, Piao MJ, Kim KC, Zheng J, Yao CW, Cha JW, Chung HS, Kim SC, Jung E, Park D, Chae S, Hyun JW. 7,8-Dihydroxyflavone protects human keratinocytes against oxidative stress-induced cell damage via the ERK and PI3K/Akt-mediated Nrf2/HO-1 signaling pathways. Int J Mol Med. 2014;33(4):964–970. doi: 10.3892/ijmm.2014.1643. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8(4):262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tzeng WY, Chuang JY, Lin LC, Cherng CG, Lin KY, Chen LH, Su CC, Yu L. Companions reverse stressor-induced decreases in neurogenesis and cocaine conditioning possibly by restoring BDNF and NGF levels in dentate gyrus. Psychoneuroendocrinology. 2013;38(3):425–437. doi: 10.1016/j.psyneuen.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Waber DP, Silverman LB, Catania L, Mautz W, Rue M, Gelber RD, Levy DE, Goldwasser MA, Adams H, Dufresne A, Metzger V, Romero I, Tarbell NJ, Dalton VK, Sallan SE. Outcomes of a randomized trial of hyperfractionated cranial radiation therapy for treatment of high-risk acute lymphoblastic leukemia: therapeutic efficacy and neurotoxicity. J Clin Oncol. 2004;22(13):2701–2707. doi: 10.1200/JCO.2004.10.173. [DOI] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 2012;7(1):e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q. 7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J Neurochem. 2012;122(4):800–811. doi: 10.1111/j.1471-4159.2012.07830.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Geula C, Lu C, Koziel H, Hatcher LM, Roisen FJ. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp Neurol. 2003;183(2):469–481. doi: 10.1016/s0014-4886(03)00222-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zeng L, Yu T, Xu Y, Pu S, Du D, Jiang W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34(3):715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Schroeder JP, Chan CB, Song M, Yu SP, Weinshenker D, Ye K. 7,8-dihydroxyflavone prevents synaptic loss and memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39(3):638–650. doi: 10.1038/npp.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Corniola R, Leu D, Khan A, Sahbaie P, Chakraborti A, Clark DJ, Fike JR, Huang TT. Extracellular superoxide dismutase is important for hippocampal neurogenesis and preservation of cognitive functions after irradiation. Proc Natl Acad Sci U S A. 2012;109(52):21522–21527. doi: 10.1073/pnas.1216913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.