Highlights
-
•
The prognosis of anaplastic thyroid cancer (ATC) is poor with a mean survival time of six months following diagnosis.
-
•
A generally accepted and effective treatment strategy for ATC has not been established yet.
-
•
Pre-therapeutic in vitro investigation of novel drugs could succeed for a personalized antitumor therapy in patients affected by ATC.
-
•
Individualized tumor therapy gives explanation concerning the mechanisms regulating the biology of ATC.
-
•
In vitro drug evaluation of individual tumor cells might be a promising tool to ameliorate the prognosis of ATC patients.
Keywords: Anaplastic thyroid carcinoma, Case report, Individualized therapy, Tyrosine kinase inhibitors, Multimodal treatment strategy
Abstract
Introduction
The prognosis of anaplastic thyroid cancer (ATC) is poor with a mean survival time of six months following diagnosis. Despite various attempts to modify common treatment modalities including surgery, external beam radiation and chemotherapy, an effective treatment is not available yet. We report, here, a patient who achieved long-term survival based on multimodal treatment, including in vitro evaluation of drug response of his tumor cells.
Presentation of case
A 42 years old male patient underwent total thyroidectomy with central and lateral neck dissection for ATC (pT4b, pN0 (0/36), L0, V0, Pn1, R0 cM0 – UICC-Stage: IV b). From the tumor tissue a primary cell culture was established. While the patient received a combined radio-chemotherapy cell viability assays were performed using Sorafenib, Vandetanib und MLN8054 (Aurora kinase inhibitor) as inhibitors. Cell viability was determined by MTT-assay after 72 and 144 h of treatment.
Discussion
All the three compounds affected cell viability in a time- and dose dependent manner. These effects were most pronounced by Sorafenib. Based on in vitro findings, the patient was treated daily with 400 mg Sorafenib for 75 days. 43 months after initial diagnosis, the patient had no evidence of disease as shown by MRI, CT and FDG-PET-CT imaging.
Conclusion
In the setting of multimodal treatment, in vitro drug evaluation of individual tumor cells of patients might be a promising tool to ameliorate the fatal prognosis of selected ATC patients.
1. Introduction
Although anaplastic thyroid carcinoma (ATC) accounts only for 2% of thyroid carcinomas, it is one of the most aggressive diseases with a median survival time of 6 months after the diagnosis and a mortality rate higher than 90% [1]. Effective treatment strategies to overcome this fatal prognosis are still lacking. Multimodality treatment consisting of surgical resection, if possible, in combination with radio- and/or chemotherapy is generally recommended [2]. Nevertheless new therapeutic strategies are urgently required to overcome the poor prognosis of ATC [3].
New insights into the biological behavior, the genetic and molecular pathogenesis of ATC might offer the possibility of novel targeted therapies [4].
But as more new systemic agents become available, it is important to get information on the drug response of different compounds on individual ATC tumor cells.
Based on the current literature and on the results of our own investigations, three compounds (Aurora kinase inhibitor MLN8054, multikinase inhibitors Vandetanib and Sorafenib) were selected to be evaluated in this setting.
The BRAF- and multikinase inhibitor Sorafenib (Nexavar®, BAY49-3006) has proven to inhibit multiple intracellular signaling pathways leading to cell cycle arrest and initiation of apoptosis in thyroid carcinoma cell lines regardless of their tumor subtype origin or the BRAF-status [5]. Several studies evaluated the effect of Sorafenib in thyroid cancer and reported positive effects [6], [7]. Based on these results, Sorafenib got granted marketing authorization since 2014 for the treatment of patients with progressive, locally advanced or metastatic, differentiated thyroid carcinoma in Europe.
Vandetanib (AstraZeneca, Macclesfield, UK) is an oral multikinase inhibitor that selectively targets RET, VEGFR and EGFR tyrosine kinases [8], [9]. Its efficacy was determined in a phase II-trial enrolling patients with poorly differentiated thyroid carcinoma (PDTC). It was shown that patients receiving Vandetanib had longer progression free survival (PFS 11 months) compared to the placebo group (5 months) [10].
Aurora kinases (A–C) are serine/threonine kinases that play a crucial role in cell division. They are overexpressed in many human tumors including ATC, where they account for aberrant cell proliferation. MLN8054 has shown profound antitumor activity in ATC cells in vitro and in vivo [11], [12].
Here we report a patient suffering from ATC, who was free of disease 43 months after multimodal treatment with surgery, radio-chemotherapy and individualized targeted therapy with Sorafenib based on in vitro testing of drug efficacy in his tumor cells. This approach might represent an effective strategy for an optimized, tailored treatment of ATC.
2. Presentation of case
A 42-year-old man was referred to our institution in September 2012, two weeks after he underwent a subtotal thyroidectomy in an external hospital for a rapidly growing scintigraphically cold nodule in the right thyroid lobe. Histopathological examination stated an ATC in the right lobe showing a negative staining for thyroid transcription factor 1 (TTF-1) and thyroglobulin (Tg). Postoperative laryngoscopy demonstrated paresis of the laryngeal nerve on the right side.
At this time, CT scan of the thorax, MRI scan of the neck, thyroid scintigraphy and ultrasonography demonstrated a persisting lesion of 21 × 18 mm on the right side of the neck without evidence of distant metastatic disease, but with some enlarged suspicious lymph nodes (max. 26 × 20 mm). Fine needle aspiration biopsy confirmed remnants of an ATC. Bronchoscopy and gastroscopy showed no evidence of infiltration of the esophagus or the trachea.
After multidisciplinary tumor board decision, the patient underwent a multimodal therapeutic strategy, including an individualized targeted therapy.
The patient first underwent completion thyroidectomy with a bilateral cervicocentral and cervicolateral lymphadenectomy in September 2012. The tumor was classified as ATC pT4b, pN0 (0/36), L0, V0, Pn1, R0 cM0 – UICC-Stage: IV b. A second expert confirmed the diagnosis of an ATC, immunonegative for TTF1 and TG and without a BRAFV600E mutation. Ki-67 index was 60–70%. Four weeks after surgical resection, a combined radio-chemotherapy was started for four weeks with four cycles of Cisplatin 25 mg/m2 and Docetaxel 20 mg/m2 combined with an external radiation beam therapy for a total dose of 64.8 Gy. A MRI-scan, performed after receiving the combined therapy, was negative for metastases or tumor recurrence.
From fresh tumor tissue a primary cell culture was established and in vitro analysis of three different drugs demonstrated Sorafenib as the most effective one (Fig. 1A–C). Thus, Sorafenib was administered as an individual treatment strategy in off-label use to the patient, with 400 mg twice a day starting in January 2013. Because of adverse events (polyneuropathy, pain in muscles and bones) the initial dose was reduced to 400 mg once a day. Sorafenib was given in three cycles over a period of 75 days.
Fig. 1.
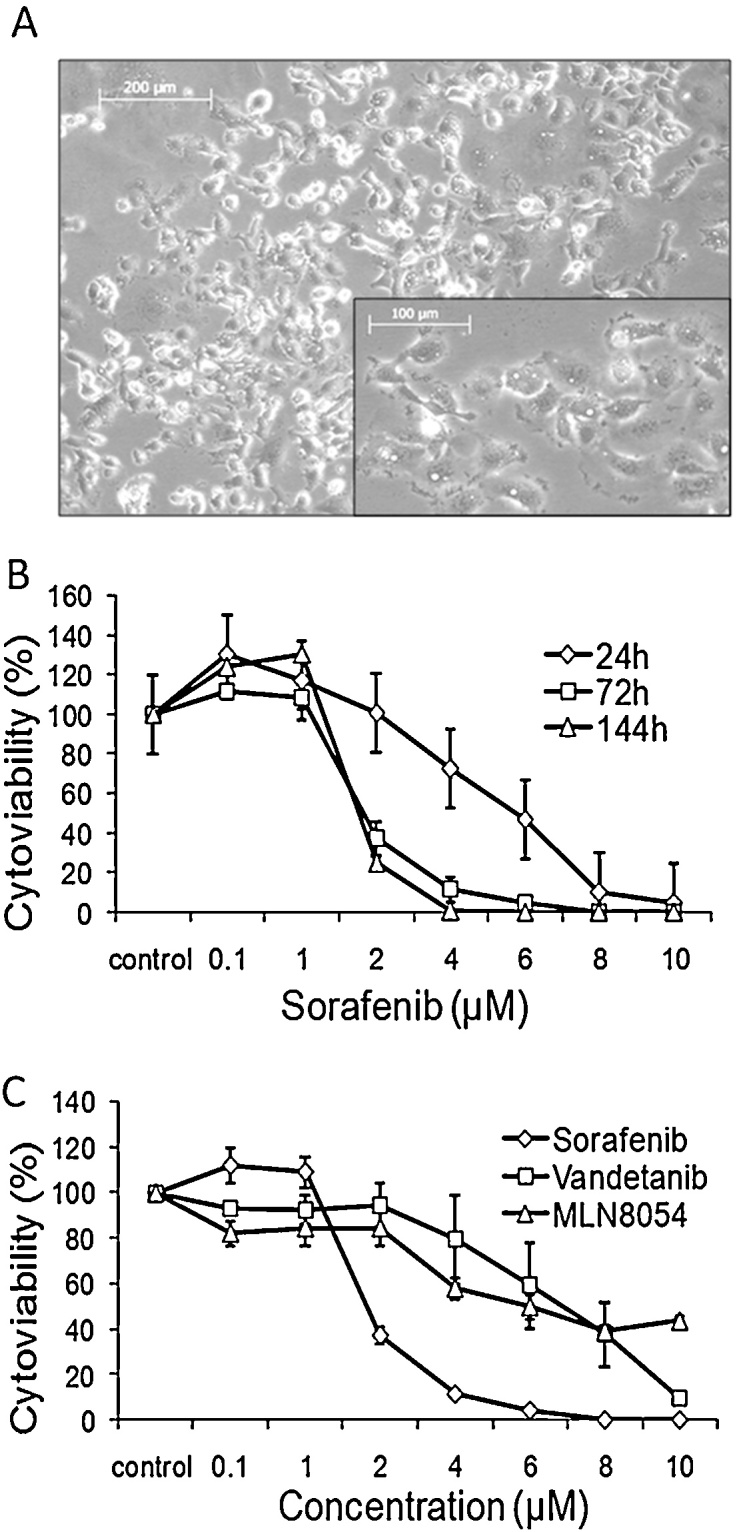
(A) Individual tumor cells established as primary culture. (B) Dose-response curve of the tumor cells established as primary culture and treated with Sorafenib for up to 144 h. Concentrations as indicated. Data reported present the mean ± SD of experiment performed in triplicates. Control: DMSO treated cells. (C) Dose-response curve of the tumor cells established as primary culture and treated with Sorafenib, Vandetanib and MLN8054 for 72 h. Concentrations as indicated. Data reported present the mean ± SD of experiment performed in triplicates. Control: DMSO treated cells.
After completion of multimodal treatment, the patient staging, evaluated by CT scan of the thorax and MRI scan of the neck, showed neither recurrent disease nor metastases.
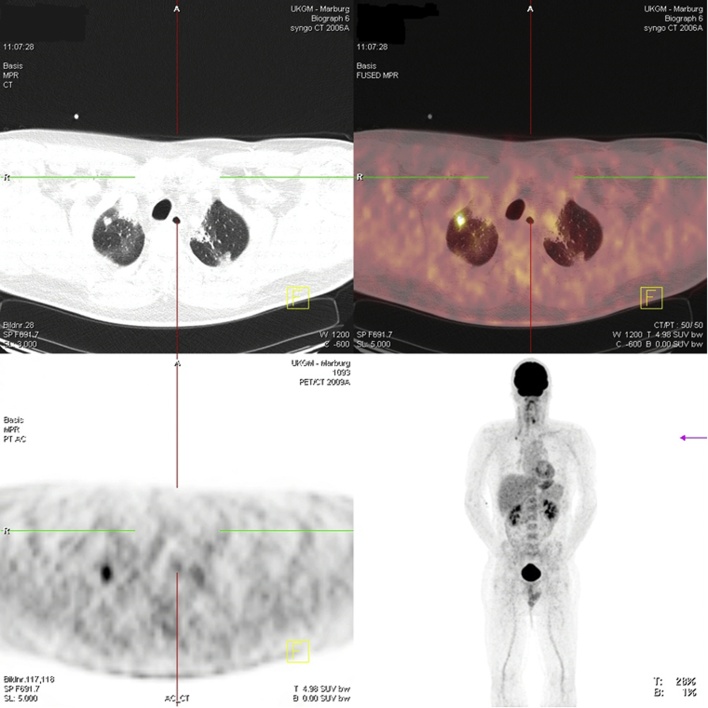
In June 2013, a complete re-staging, including MRI of the neck, CT of the thorax and FDG-PET-CT, was performed. Imaging detected a new solitary lesion of 10 × 7 mm size in the right upper lobe of the lung that was suspicious for a metastasis (Fig. 2). According to the recommendation of our tumor board, based on the absence of additional lesions, the patient underwent a video-assisted thoracoscopic wedge resection of this lesion. Histopathology of the collected fresh tissue confirmed the metastatic lesion originated from the primary ATC. Collected tissue was once again transferred to the laboratory and established as a primary cell culture. The case was discussed again in our interdisciplinary tumor board, where an additional adjuvant therapy with taxans and platin was considered, but the patient refused this therapy and preferred to undergo close surveillance with CT imaging of the thorax and MRI of the neck in 6 months intervals. At the last staging, in January 2016, 43 months after his initial operation, the patient was without evidence of recurrent or metastatic disease.
Fig. 2.
FDG-PET-CT screen of a pulmonary metastasis of ATC nine months after diagnosis.
2.1. Methods and experiments for individualized in-vitro testing of drug efficacy
2.1.1. Preparation of patient-derived human tumor tissue
Tumor cells were attained by mechanical dissociation of tumor tissue, obtained from the completion thyroidectomy and the pulmonary lesion and successfully established as primary cell culture.
2.1.2. Compounds
Sorafenib, Vandetanib and MLN8054 were used as inhibtors. Stock solutions (10 mM each) were prepared in dimethylsulfoxid (DMSO) and stored at −20 °C.
2.1.3. In vitro experiments
Primary cell culture was maintained by propagating the cells in DMEM-h21/Ham's F12 1:1 (v/v) supplemented with 10% FCS and 10U/ml penicillin and 100 μg/ml streptomycin (all: Biochrom, Berlin, Germany) under standard conditions (37 °C, 5% CO2). Before use in experiments, cell viability was assessed by Trypan blue exclusion.
To test the sensitivity of the tumor cells towards the various inhibitors, cells were seeded in 96 well plates (1 × 104 cells/well) and treated with increasing concentrations (0.1–10 μM) of Sorafenib, Vandetanib und MLN8054 for up to 144 h. Cell viability was determined by MTT-assay and dose-response curves were created.
2.2. Results
Effects on cell viability of the individual tumor cells caused by treatment with Sorafenib, Vandetanib and MLN8054
2.2.1. Cells established from the primary tumor
Cell viability of the individual tumor cells was considerably affected by all the three compounds. As documented here for Sorafenib, each compound induced a dose- and time-dependent decrease in cell viability (Fig. 1B and C). This effect was most pronounced by Sorafenib (Fig. 1B and C). Here cytoviability was reduced to about 50% at a drug concentration about 2 μM after 72 h, whereas for Vandetanib and MLN8054 IC50 values were calculated as ≈7 μM and ≈5 μM (Fig. 1B and C).
Prolonged treatment with 5 μM Sorafenib and Vandetanib resulted in 100% reduction of cell viability, MLN8054 showed a lower effect, here cell viability was decreased only to about 30% at 10 μM (Data not shown). Further, the effect of combined therapy with Sorafenib and Vandetanib was evaluated in vitro. In comparison with the single treatment, no synergistic effect or increase of efficacy could be measured (Data not shown).
2.2.2. Cells established from the pulmonary metastasis
Tumor cells originated from the pulmonary metastasis where established as primary culture as well and treated with Sorafenib and Vandetanib similarly. As assessed by MTT-assay, comparable results were revealed. Once again after 72 h about 50% of the cells were killed at concentrations about 3 μM (Sorafenib) and 7 μM (Vandetanib), documenting a similar behaviour of the cells from the primary tumor and the metastasis (Fig. 3A and B).
Fig. 3.
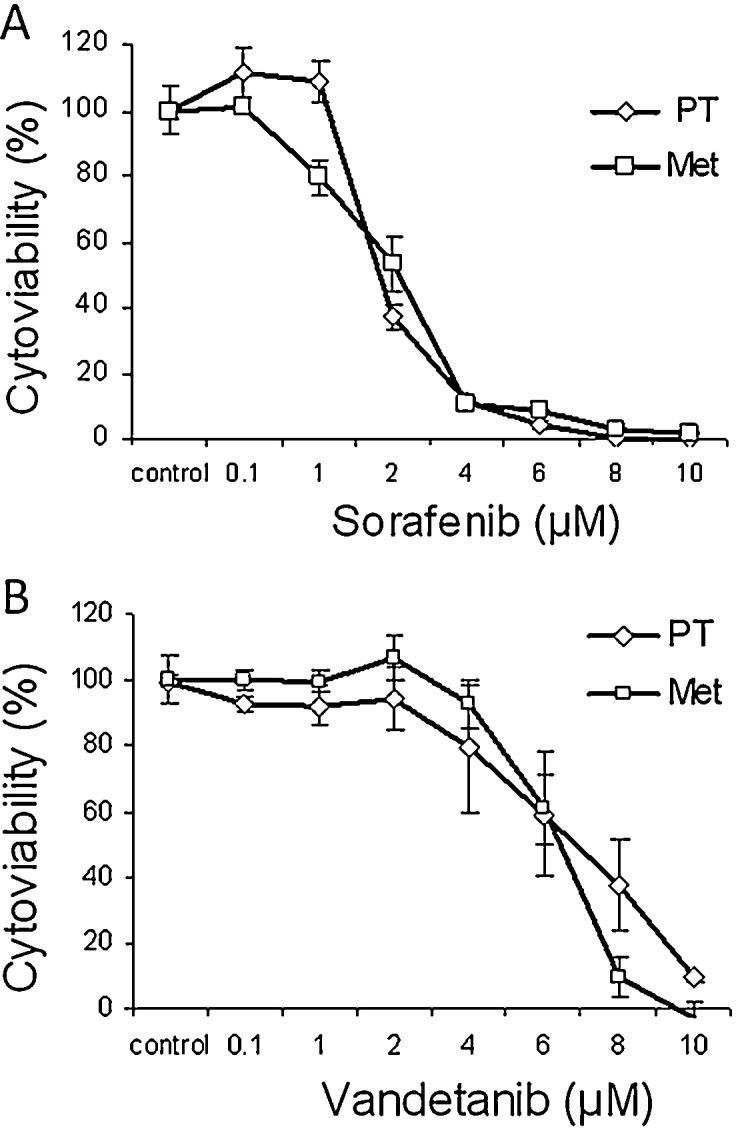
(A) Dose-response curves of tumor cells originated from the PT1 and the Met2 and treated with Sorafenib for 72 h. Concentrations as indicated. Data reported present the mean ± SD of experiment performed in triplicates. Control: DMSO treated cells. (B) Dose-response curves of tumor cells originated from the PT1 and the Met2 and treated with Vandetanib for 72 h. Concentrations as indicated. Data reported present the mean ± SD of experiment performed in triplicates. Control: DMSO treated cells.
1PT = primary tumor.
2Met = pulmonary metastasis.
3. Discussion
A generally accepted treatment strategy for ATC has not been established yet [13], [14]. By analyzing 2742 cases Haymart et al. showed that the median survival of patients with stage IVb ATC is quite poor with just 6 months. Despite of a prolonged overall survival (median survival of 9.9 month) obtained by a combined multidisciplinary therapy consisting of surgery, radiation and chemotherapy, there is still a desperate need to support and accelerate clinical research [15].
In previous publications several authors pointed out the benefit of an initial complete and radical resection of the ATC [16], [17]. Passler et al. showed that patients without tumor residues (R0) had a significantly better prognosis with a median survival of 6.1 months than patients with tumor residues (R1/R2) with a median survival of 2.2 months and a 3-year survival of 50% vs. 4%. Therefore, in our case we performed a radical completion thyroidectomy with bilateral neck dissection to achieve a R0 situation.
Although several reports pointed out the inefficacy of single chemotherapy in patients with ATC [18], [19], some combined therapies improved survival [20]. That is why our patient received – corresponding to the American Thyroid Association Guidelines for Patients with Anaplastic Thyroid Carcinoma – a combined chemotherapy with docetaxel and cisplatin [4]. Both agents are known to have a great clinical activity in metastatic ATC, but it is not confirmed that they really prolonged survival or quality of life [4]. So, to complete the multimodal treatment strategy in the presented patient, surgical resection and chemotherapy were followed by radiation therapy. Retrospective analysis of the SEER data of 516 patients revealed that only the combined use of surgical resection and external beam radiotherapy were identified as independent predictors of survival in ATC patients [1].
Our patient first received a combined radio-chemotherapy. Meanwhile a primary cell culture was established and the effects of two multi-kinase inhibitors (Sorafenib, Vandetanib) and of one aurora kinase inhibitor (MLN8054) were evaluated in vitro. From these the mostly effective one was Sorafenib. Further, similar effects were shown for cells of the primary tumor and the metastasis. These results point out the feasibility of preclinical in vitro evaluation, especially, since it is well known, that the biological behavior of ATC – like other tumors – is inconsistent. Therefore a successful therapy may be more common, if the patient receives – corresponding to the individual evaluated ATC – the most effective treatment.
As younger age is associated with improved survival in patients affected by ATC [15], it must be pointed out that the positive clinical course in the presented case might have been also promoted by the limited disease at the time of diagnosis and the young age of the patient.
4. Conclusion
Our case report demonstrates – to our best knowledge – for the first time that pre-therapeutic in vitro investigation of novel drugs could succeed for a personalized antitumor therapy in a patient affected by ATC. By availability of vital tumor cells, this approach might offer the possibility for reflection of the individual tumor cell characteristics and optimize therapeutic options for patients suffering from ATC.
Conflict of interest
None.
Funding
None.
Ethical approval
This is not a research study.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors’ contributions
S. Eckhardt: analysis and interpretation of data, acquisition of data, drafting the article.
S. Hoffmann: conception and design of the study, acquisition of data.
A.I. Damanakis: final approval of the version to be submitted.
P. Di Fazio: revising the article for important intellectual content.
A. Pfestroff: analysis and interpretation of data.
M. Luster: conception and design of the study.
A. Wunderlich: analysis and interpretation of data, acquisition of data.
D.K. Bartsch: conception and design of the study, revising the article for important intellectual content.
Guarantor
This is not a research study. All authors read and approved the final manuscript. Therefore, all authors are responsible for this report.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijscr.2016.06.013.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Kebebew E., Greenspan F.S., Clark O.H., Woeber K.A., McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103:1330–1335. doi: 10.1002/cncr.20936. [DOI] [PubMed] [Google Scholar]
- 2.Nagaiah G., Hossain A., Mooney C.J., Parmentier J., Remick S.C. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J. Oncol. 2011;2011:542358. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean D.S., Gharib H. Epidemiology of thyroid nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:901–911. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Smallridge R.C., Ain K.B., Asa S.L., Bible K.C., Brierley J.D. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. doi: 10.1089/thy.2012.0302. [DOI] [PubMed] [Google Scholar]
- 5.Broecker-Preuss M., Müller S., Britten M., Worm K., Kurt Werner S. Sorafenib inhibits intracellular signaling pathways and induces cell cycle arrest and cell death in thyroid carcinoma cells irrespective of histological origin or BRAF mutational status. BMC Cancer. 2015;15:184. doi: 10.1186/s12885-015-1186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas L., Lai S.Y., Dong W., Feng L., Dadu R. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014;19:251–258. doi: 10.1634/theoncologist.2013-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savvides P., Nagaiah G., Lavertu P., Fu P., Wright J.J. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlomagno F., Vitagliano D., Guida T., Ciardiello F., Tortora G. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62:7284–7290. [PubMed] [Google Scholar]
- 9.Hoffmann S., Gläser S., Wunderlich A., Lingelbach S., Dietrich C. Targeting the EGF/VEGF-R system by tyrosine-kinase inhibitors—a novel antiproliferative/antiangiogenic strategy in thyroid cancer. Langenbecks Arch. Surg. 2006;391:589–596. doi: 10.1007/s00423-006-0104-y. [DOI] [PubMed] [Google Scholar]
- 10.Leboulleux S., Bastholt L., Krause T., de la Fouchardiere C., Tennvall J. Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol. 2012;13:897–905. doi: 10.1016/S1470-2045(12)70335-2. [DOI] [PubMed] [Google Scholar]
- 11.Wunderlich A., Fischer M., Schlosshauer T., Ramaswamy A., Greene B.H. Evaluation of Aurora kinase inhibition as a new therapeutic strategy in anaplastic and poorly differentiated follicular thyroid cancer. Cancer Sci. 2011;102:762–768. doi: 10.1111/j.1349-7006.2011.01853.x. [DOI] [PubMed] [Google Scholar]
- 12.Manfredi M.G., Ecsedy J.A., Meetze K.A., Balani S.K., Burenkova O. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smallridge R.C., Copland J.A. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin. Oncol. (R. Coll. Radiol.) 2010;22:486–497. doi: 10.1016/j.clon.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granata R., Locati L., Licitra L. Therapeutic strategies in the management of patients with metastatic anaplastic thyroid cancer: review of the current literature. Curr. Opin. Oncol. 2013;25:224–228. doi: 10.1097/CCO.0b013e32835ff44b. [DOI] [PubMed] [Google Scholar]
- 15.Haymart M.R., Banerjee M., Yin H., Worden F., Griggs J.J. Marginal treatment benefit in anaplastic thyroid cancer. Cancer. 2013;119:3133–3139. doi: 10.1002/cncr.28187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlumberger M., Parmentier C., Delisle M.J., Couette J.E., Droz J.P. Combination therapy for anaplastic giant cell thyroid carcinoma. Cancer. 1991;67:564–566. doi: 10.1002/1097-0142(19910201)67:3<564::aid-cncr2820670306>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Voutilainen P.E., Multanen M., Haapiainen R.K., Leppäniemi A.K., Sivula A.H. Anaplastic thyroid carcinoma survival. World J. Surg. 1999;23:975–978. doi: 10.1007/s002689900610. discussion 978–979. [DOI] [PubMed] [Google Scholar]
- 18.Tennvall J., Tallroth E., el Hassan A., Lundell G., Akerman M. Anaplastic thyroid carcinoma. Doxorubicin, hyperfractionated radiotherapy and surgery. Acta Oncol. 1990;29:1025–1028. doi: 10.3109/02841869009091794. [DOI] [PubMed] [Google Scholar]
- 19.Levendag P.C., De Porre P.M., van Putten W.L. Anaplastic carcinoma of the thyroid gland treated by radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1993;26:125–128. doi: 10.1016/0360-3016(93)90182-u. [DOI] [PubMed] [Google Scholar]
- 20.Seto A., Sugitani I., Toda K., Kawabata K., Takahashi S. Chemotherapy for anaplastic thyroid cancer using docetaxel and cisplatin: report of eight cases. Surg. Today. 2015;45:221–226. doi: 10.1007/s00595-013-0751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.