Abstract
Diabetes and the ageing process independently increase the risk for cardiovascular disease (CVD). Since incidence of diabetes increases as people get older, the diabetic older adults represent the largest population of diabetic subjects. This group of patients would potentially be threatened by the development of CVD related to both ageing and diabetes. The relationship between CVD, ageing and diabetes is explained by the negative impact of these conditions on vascular function. Functional and clinical evidence supports the role of vascular inflammation induced by the ageing process and by diabetes in vascular impairment and CVD. Inflammatory mechanisms in both aged and diabetic vasculature include pro‐inflammatory cytokines, vascular hyperactivation of nuclear factor‐кB, increased expression of cyclooxygenase and inducible nitric oxide synthase, imbalanced expression of pro/anti‐inflammatory microRNAs, and dysfunctional stress‐response systems (sirtuins, Nrf2). In contrast, there are scarce data regarding the interaction of these mechanisms when ageing and diabetes co‐exist and its impact on vascular function. Older diabetic animals and humans display higher vascular impairment and CVD risk than those either aged or diabetic, suggesting that chronic low‐grade inflammation in ageing creates a vascular environment favouring the mechanisms of vascular damage driven by diabetes. Further research is needed to determine the specific inflammatory mechanisms responsible for exacerbated vascular impairment in older diabetic subjects in order to design effective therapeutic interventions to minimize the impact of vascular inflammation. This would help to prevent or delay CVD and the specific clinical manifestations (cognitive decline, frailty and disability) promoted by diabetes‐induced vascular impairment in the elderly.
Abbreviations
- AA
arachidonic acid
- BMI
body mass index
- COX
cyclooxygenase
- CRP
C‐reactive protein
- CVD
cardiovascular disease
- FMD
flow‐mediated dilatation
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LETO
Long–Evans Tokushima Otsuka
- LPS
lipopolysaccharide
- miR
microRNA
- NF‐κB
nuclear factor‐κB
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nrf2
nuclear related factor‐2
- OLETF
Otsuka Long–Evans Tokushima fatty
- PBMNC
peripheral blood mononuclear cell
- PGI2
prostaglandin I2
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- ICAM
intercellular adhesion molecule
- SIRT
sirtuin
- TNF‐α
tumour necrosis factor‐α
- TXA2
thromboxane A2
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
Introduction
Ageing and diabetes are two well‐established and outstanding risk factors for the development of cardiovascular disease (CVD) (Lakatta & Levy, 2003; Sarwar et al. 2010). This is due, in part, to the presence of altered endothelial function, manifested by reduced endothelium‐dependent vasodilatation. In fact, endothelial dysfunction is considered the primary antecedent for atherosclerotic diseases. Therefore, exploring the underlying mechanisms implicated in the genesis of this alteration in physiological or pathological conditions is of the utmost importance in developing adequate strategies to prevent or retard the clinical manifestations of CVD. In this sense, a large body of experimental and clinical evidence indicates a prominent role of vascular inflammation in the development of endothelial dysfunction. Within arterial wall the interplay between the pro‐inflammatory and pro‐oxidant milieus, which both act synergistically, accelerates the formation of atherosclerotic plaque and, therefore, increases the risk of arterial disease (Hajjar & Gotto, 2013). In fact, inflammation is now considered a key event in vascular dysfunction and the development of CVDs (Okazaki et al. 2014; Libby & Hansson, 2015; Rein et al. 2015). Such a concept is supported by the fact that anti‐inflammatory cytokines protect endothelial function (Shao et al. 2014) and interventions resulting in reduced inflammation prevent vascular dysfunction and cardiovascular events (Santos‐Parker et al. 2014; Moreira et al. 2015; van Bussel et al. 2015). Thus, the focus of this review is to address the role of inflammation and its mechanisms in vascular dysfunction associated with ageing and diabetes, by describing the impact of these conditions separately and, then, analysing the vascular impairment and inflammatory mechanisms generated when ageing and diabetes co‐exist. First, we will briefly describe the impact of these two cardiovascular risk factors on vascular function.
Vascular dysfunction in ageing
Ageing is considered the major risk factor and driver of CVD. In fact, the incidence and severity of subclinical and clinical manifestations of CVD steeply increase with age (Lakatta & Levy, 2003; Paneni et al. 2015), even in the absence of traditional risk factors (Wu et al. 2014). Both clinical and preclinical data have shown that vascular ageing is associated with functional and structural changes that take place at different levels: the endothelium and vascular smooth muscle cells, as well as the extracellular matrix of vessel walls (Rubio‐Ruiz et al. 2014). Salient aspects of age‐associated changes in vasculature include intima and media thickening, increased arterial stiffness and dilatation of central elastic arteries resulting in a reduced ability to expand and contract in response to pressure changes (Kotsis et al. 2011). Several data have highlighted a linear relationship between arterial stiffness and age (Wen et al. 2015). Others have also found accelerated stiffening between 50 and 60 years of age (McEniery et al. 2005).
In fact, arterial stiffness is always preceded by an impaired endothelial vasodilatation suggesting that this arterial alteration is also linked to endothelial dysfunction (Scuteri et al. 2008). Furthermore, the presence of an altered endothelial function may, in turn, aggravate media thickness and fibrosis (Paneni et al. 2015). Thus, endothelial dysfunction represents a key step in the initiation and maintenance of atherosclerosis and is an independent predictor of cardiovascular events (Steyers & Miller, 2014). Indeed, a vast number of published data show that the ageing process is associated with endothelial dysfunction, manifested by a reduction of the endothelium‐dependent vasodilatation, in both the micro‐ and the macrovasculature derived from animal models (Lakatta & Levy, 2003; Matz & Andriantsitohaina, 2003; Brandes et al. 2005; Dal‐Ros et al. 2012; Gano et al. 2014) and humans (Matz & Andriantsitohaina, 2003; Brandes et al. 2005; Rodríguez‐Mañas et al. 2009; Toda, 2012; Walker et al. 2014). Therefore, the maintenance of a correct function of the vascular bed seems to be an essential determinant of healthy ageing (Virdis et al. 2010; Toda, 2012).
Vascular dysfunction in diabetes
Diabetes represents an important risk factor for CVD (Sarwar et al. 2010; Lind et al. 2014; Peters et al. 2014). Like ageing, diabetes impacts vascular function. In fact, the impairment of endothelium‐dependent vasodilatation is a frequent finding in arteries from diabetic animals and patients in both in vivo and ex vivo settings (Rodríguez‐Mañas et al. 1998; Angulo et al. 1998; Kim et al. 2002; Rodríguez‐Mañas et al. 2003; Angulo et al. 2003; Molnar et al. 2005; Schjørring et al. 2012). Furthermore, endothelial dysfunction is a predictor of CVD in diabetic patients (van Slotten et al. 2014). Even in young diabetic patients, a decrease in endothelium‐dependent, flow‐mediated, dilatation contributes to early atherosclerotic changes (Jin et al. 2008; Naylor et al. 2011). In this sense, endothelial dysfunction seems to represent an early stage in the development of vascular complications in patients with either type 1 or type 2 diabetes (Xu & Zou, 2009; Ladeia et al. 2014). Remarkably, some comparative studies have shown greater impairment of endothelial function in subjects with type 2 diabetes (Ohsugi et al. 2014). This finding could be related to the deleterious effect that insulin resistance exerts on endothelial function (Avogaro et al. 2013), which is supported by recent data showing that only mesenteric microvessels obtained from obese subjects with insulin resistance display impaired endothelial vasodilatation, even after exclusion of diabetic cases (El Assar et al. 2013). Thus, hyperglycaemia and insulin resistance could simultaneously compromise endothelial function in type 2 diabetic patients. In fact, insulin resistance estimated by Homeostasis Model Assessment (HOMA) is independently associated with subsequent symptomatic CVD in the general population (Bonora et al. 2007).
Although reduced endothelial vasodilatation is a hallmark of vascular dysfunction related to diabetes, arterial stiffness is frequently detected in diabetic patients, especially in those with advanced age (Prenner & Chirinos, 2015). Moreover, pulse wave velocity has been found to increase in type 2 diabetic patients (Cruickshank et al. 2002; Lukich et al. 2010; Zhang et al. 2011), suggesting that arterial stiffness is associated with diabetes as another manifestation of vascular dysfunction. In fact, pulse wave velocity in diabetic patients increases as the age increases (Naka et al. 2012).
Inflammation related to either ageing or to diabetes
The mechanisms underlying vascular ageing and diabetes are complex and involve multiple pathways and factors (Fig. 1 and Table 1). It is well established that nitric oxide (NO) is a crucial factor for the proper functioning of endothelial cells. Emerging evidence derived from experimental animal and human models has emphasized a central role of two main mechanisms responsible for reduced NO bioavailability and endothelial dysfunction with ageing and with diabetes: oxidative stress and inflammation (El Assar et al. 2013; Hamilton & Watts, 2013), which are bi‐directionally associated (Seals et al., 2014). Since inflammation is a determinant factor in vascular dysfunction in ageing as well as in diabetes, we are going to delineate the inflammatory mechanisms contributing to vascular dysfunction in either ageing or diabetes and to evaluate the functionality of modulatory systems that play a significant role in the control of vascular inflammation.
Figure 1. Ageing‐ or diabetes‐induced inflammatory mechanisms cause vascular dysfunction increasing cardiovascular risk .
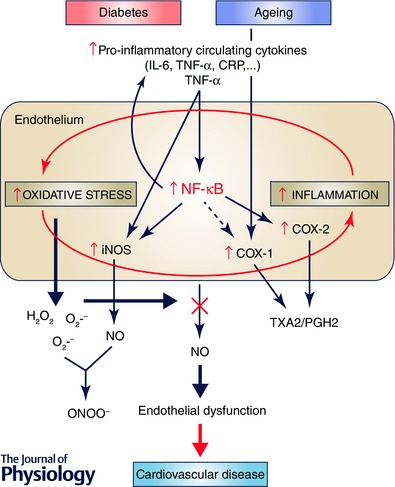
Ageing or diabetes is associated with a disruption in the endothelial environment due to the presence of high levels of both reactive oxygen species (for example, superoxide (O2.−) and hydrogen peroxide (H2O2)), and pro‐inflammatory mediators produced locally or systemically. A pro‐oxidative stress scenario impacts negatively on endothelial function by quenching nitric oxide (NO) and therefore reducing its availability. Circulating pro‐inflammatory cytokines (for examples TNF‐α) also contribute to endothelial malfunction through direct induction of iNOS or through the activation of a redox‐sensitive pro‐inflammatory nuclear factor κB (NF‐κB), which in turn activates different enzymes (iNOS, COX‐2) leading to peroxynitirite (ONOO−) formation and increased production of contractile factors. A regulation of COX‐1 is also observed in ageing. A close interaction between oxidative stress and chronic low‐grade inflammation develops in the microenvironment of aged or diabetic arteries, exacerbating one another and creating a vicious cycle. This translates into an endothelial phenotype characterized by the presence of endothelial dysfunction that consequently makes aged or diabetic individuals prone to develop cardiovascular disease. COX, cyclooxygenase; CRP, C reactive protein; IL‐6, interleukin‐6; IL‐1β, interleukin‐1β; iNOS, inducible nitric oxide synthase; PGH2, prostaglandin H2; TNF‐α, tumor necrosis factor‐α; TXA2, thromboxane A2.
Table 1.
Mediators of inflammation involved in vascular dysfunction associated with ageing, with diabetes and with the presence of both conditions
| Ageing + | |||
|---|---|---|---|
| Ageing | Diabetes | diabetes | |
| Systemic inflammation | |||
| Pro‐inflammatory cytokines | |||
| IL‐6 | + | + | ++ |
| IL‐1β | + | + | ++ |
| vTNF‐ α | + | + | ++ |
| Anti‐inflammatory cytokines | |||
| Adiponectin | ± | – | ± |
| Local inflammation | |||
| iNOS | + | + | ++ |
| COX | + | + | ++ |
| NF‐kB | + | + | ++ |
| microRNAs | |||
| miR‐27a | – | ± | ? |
| miR‐34a | + | + | ? |
| miR‐155 | – | – | ? |
| miR‐146a | + | – | ? |
| Modulatory systems | |||
| Sirtuins | |||
| SIRT1 | – | – | ? |
| SIRT 6 | – | – | ? |
| Nrf‐2 | – | – | ? |
COX, cyclooxygenase; IL‐6, interleukin‐6; IL‐1β, interleukin‐1β; iNOS, inducible nitric oxide synthase; NF‐κB, nuclear factor‐κB; TNF‐α, tumour necrosis factor‐α; +: increased/up‐regulated; –, decreased/down‐regulated; ±, not clear; ++, further increased in ageing plus diabetes with respect to separate conditions; ?, unknown.
Mechanisms of vascular inflammation in ageing
Circulating cytokines in ageing
Low‐grade chronic inflammation is a well‐known contributing factor for the pathogenesis of arterial ageing, this concept having been referred to as ‘Inflammageing’ (Cevenini et al. 2013). A huge amount of data demonstrate that there is a profound modification of the cytokine network as age increases that occurs in the absence of any microorganism, characterized by a general increase in plasma levels and cell capability of producing pro‐inflammatory cytokines and a reduction of anti‐inflammatory cytokines. In fact, increased levels of tumour necrosis factor‐α (TNF‐α), interleukin (IL)‐1β and members of the superfamily of interleukin‐6 (IL‐6), as well as higher levels of C‐reactive protein (CRP) have been detected in plasma of older subjects when compared to young adults (Ferrucci et al. 2005). It has been shown that higher levels of these cytokines are correlated with an increasing risk of morbidity and mortality, not only in a frail population but also in non‐frail elderly (Michaud et al. 2013). This increase is positively correlated with age, independently of the presence of other cardiovascular risk factors and comorbid conditions (Miles et al. 2008).
Evidence also shows a relationship between endothelial dysfunction associated with ageing and elevated systemic pro‐inflammatory cytokines such as TNF‐α, IL‐6 or hs‐CRP both in animals and in humans (Donato et al. 2008; Lesniewski et al. 2011; LaRocca et al. 2012). In contrast, high concentrations of the anti‐inflammatory cytokine, IL‐10, are associated with less presence of carotid atherosclerosis and coronary calcification in very old individuals (> 80 years) (Freitas et al. 2011). IL‐10 seems, in fact, to protect against ageing‐induced endothelial dysfunction since this manifestation was only present in carotid arteries from old mice lacking the IL‐10 gene (Kinzenbaw et al. 2013). Furthermore, knockout mice for the IL‐10 gene develop vascular inflammation, and cardiac and vascular dysfunction with increasing age (Sikka et al. 2013). On the other hand, an inverse strong association between the anti‐inflammatory adipokine, adiponectin, and incidence of coronary heart disease was found in healthy middle‐aged males (Pischon et al. 2004), while others described a moderate association in other populations studied (Sattar et al. 2006). Furthermore, high adiponectin concentrations were significantly associated with increased all‐cause and cardiovascular mortality in an elderly cohort (Choi et al. 2015), although others observed no association (Kizer et al. 2012).
One possible mechanism explaining pro‐inflammatory activation and cytokine overproduction in arteries with ageing is increased immune cell infiltration, including macrophages and T lymphocytes. These immune cells produce inflammatory cytokines that can in turn initiate and sustain vascular inflammation (Weber et al. 2008). In this sense, sporadic clustering of macrophages in the aortic wall is more common in older compared with young human donors (Wang et al. 2007). There is also evidence of a marked increase in macrophages and T lymphocytes in the adventitia and perivascular fat tissue of old mice (Lesniewski et al. 2011), and of polymorphonuclear leukocytes accumulation in aorta of old F344 rats (Zou et al. 2006).
Vascular hyperactivation of NF‐κB in ageing
NF‐кB is a key factor in vascular inflammation. It activates a series of target genes critically involved in inflammation of vascular wall (Collins & Cybulsky, 2001). Moreover, recently, Walker et al. have suggested that there might be an association between endothelial nuclear factor‐κB (NF‐κB) signalling and oxidative stress‐related impairment of endothelium‐dependent vasodilatation in healthy sedentary subjects (Walker et al. 2014).
The proinflammatory status associated with vascular ageing (Csiszar et al. 2003; Ungvari et al. 2004; Csiszar et al. 2008; Song et al. 2012) leads to the activation of NF‐κB, which in turn has the potential to establish a complex self‐amplifying feedback loop. NF‐κB signalling seems to be the culprit of inflammageing, as this signalling system integrates the intracellular regulation of immune responses in both ageing and age‐related diseases (Salminen et al. 2008). Furthermore, several recent studies have shown that antagonizing NF‐κB signalling can delay the ageing phenotype, demonstrating the key role that this signalling pathway plays in the ageing process of various tissues (Tilstra et al. 2012). Furthermore, the activation of NF‐κB is mediated in part by the close interplay with the age‐related oxidative stress driven by elevated levels of reactive oxygen species (ROS), which can activate NF‐κB signalling in the endothelium and promote chronic vascular inflammation (Csiszar et al. 2008). Activated NF‐κB regulates multiple inflammatory molecules including TNF‐α, interleukins (IL‐1β, and IL‐6), chemokines (IL‐8 and RANTES), adhesion molecules (intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM)), and enzymes (inducible nitric oxide synthase (iNOS) and cyclooxygenase‐2 (COX‐2)) (El Assar et al. 2013).
iNOS and COX in ageing
Studies in rodent vasculature have shown that advancing age is associated with an increase in iNOS protein expression (Kim et al. 2009; Gong et al. 2014). Furthermore, the expression of this inducible isoform is enhanced in different human cell types derived from the elderly, ranging from mesothelial cells (Rodriguez‐Mañas et al. 2006) to microvessels (Rodriguez‐Mañas et al. 2009). In this sense, data obtained from aged human microvessels and from aged animal arteries have shown improved endothelium‐dependent dilatation when iNOS was selectively inhibited (Rodriguez‐Mañas et al. 2009; Tian et al. 2010). Furthermore, the pleiotropic inflammatory cytokine TNF‐α, involved in a variety of biological processes including vascular dysfunction, regulates NOS expression and/or activity and thereby exerts direct effects on NO production (Zhang et al. 2009). Previous data have demonstrated an up‐regulation of iNOS mRNA expression when human aortic endothelial cells were treated with TNF‐α (MacNaul et al. 1993). The iNOS‐derived reactive nitrogen species leads to nitrosative stress and impaired endothelial function (Zhang et al. 2009). Another enzyme involved in the altered endothelium responses associated with vascular ageing is COX and its derivatives. In fact, many studies have demonstrated the positive effect exerted by COX inhibitors on the blunted endothelium vasodilatation caused by ageing, indicating the implication of COX‐derived vasoconstrictor factors in this process. In this sense, indomethacin, a non‐selective COX inhibitor, augmented the relaxation in isolated arteries in older patients (Vanhoutte & Tang, 2008) and aged animals (Shi et al. 2008). The contribution of the contractile factor thromboxane A2 (TXA2) in the malfunction of the endothelial layer has been confirmed in human mesenteric arteries and in rat aorta by acting on its specific receptor (Matz et al. 2000; Rodriguez‐Mañas et al. 2009). Nevertheless, the lack of prostaglandin I2 (PGI2)‐mediated vasodilatation has been documented both in vivo (Schrage et al. 2007) and in mesenteric arteries in vitro (Rodriguez‐Mañas et al. 2009). Regarding which COX isoform is implicated in the endothelial dysfunction associated with the ageing process, no consensus has been established. While some studies reported the capacity of a COX‐2 inhibitor to enhance acetylcholine‐induced vasodilatation in mesenteric arteries and aorta of aged rats (Alvarez de Sotomayor et al. 2005, 2007), others found no effect of this same compound in mesenteric arteries from aged rats (Matz et al. 2000).
microRNAs in ageing
microRNAs (miRNAs) are a family of highly conserved, small (∼21–3 nucleotides) non‐coding RNAs that regulate gene expression at the post‐transcriptional level. In general, miRNAs bind to complementary sites located on 3′ untranslated regions of target mRNAs leading to a negative regulation of mRNA stability and translation (Ameres & Zamore, 2013). The ability of miRNA to regulate many targets at the same time makes them good candidates to control many physiological processes, especially multifactorial ones like ageing and diabetes (Chen et al. 2010). Furthermore, several lines of evidence have demonstrated that some miRNAs are involved in regulating inflammation (Oliveri et al. 2013 b). In fact, some miRNAs contribute to the regulation of NF‐κB signalling and/or are regulated by this factor (Jiang et al. 2012; Gantier et al. 2012; Song et al. 2013).
Growing evidence supports the idea of a crucial role of miRNAs as mediators of vascular ageing both in animal models and in humans (Boon et al, 2011; Menghini et al. 2014). In this sense, the expression of various miRNAs, such as miR‐155, which displays anti‐inflammatory effects in endothelial cells (Cheng et al. 2014), was down‐regulated in peripheral blood mononuclear cells (PBMNCs) of elderly people when compared with young individuals suggesting that miR‐155 and its predicted target have the potential to be diagnostic indicators of age and age‐related diseases (Noren Hooten et al. 2010).
Furthermore, reduced expression of miR‐27a was observed in older mice and it was also found to be decreased in older individuals, suggesting a role of this miRNA in regulating longevity (Noren Hooten et al. 2010; Jansen et al. 2015). Meanwhile, an ageing‐associated increase of miR‐34a expression levels was detected in proliferative human aortic smooth muscle cells, which promotes vascular smooth muscle senescence and inflammation through down‐regulation of the longevity‐associated protein, Sirtuin‐1 (SIRT 1). The latter, in conjunction with other factors, may lead to arterial dysfunction associated with ageing (Badi et al. 2014).
Additionally, a marked overexpression of miR‐146a has been detected in senescent endothelial cells from human umbilical vein, aorta and coronary artery, being associated with an inflammatory phenotype and showing significant negative correlation with telomere length and telomerase activity (Olivieri et al. 2013 a). However, studies in senescent human fibroblasts and macrophages from aged mice suggest that miR‐146a is expressed in response to rising inflammatory cytokine levels and NF‐κB activation as a negative feedback loop (Bhaumik et al. 2009J; iang et al. 2012).
Modulatory systems counteracting vascular inflammation in ageing
Vascular dysfunction associated with ageing has been related to alterations in several critical cellular homeostatic and stress resistance pathways that suppress oxidative stress and inflammation (Seals et al. 2014). Sirtuins have been shown to counterbalance the NF‐κB transcription system. SIRT1 has emerged as a key factor in the interplay between inflammation and ageing by inhibiting NF‐κB (Salminen et al. 2008). SIRT1 inhibits the transcriptional activity of NF‐κB by deacetylating RelA/p65 (Yeung et al. 2004). Consistently, the decline in SIRT1 activity and expression in skeletal muscle with ageing is accompanied by increased inflammation and oxidative stress (Pardo & Boriek, 2011). The role of SIRT1 during ageing seems to involve the orchestration of different stress response pathways (Kourtis & Tavernarakis, 2011). In this sense, SIRT1 plays an important role in the anti‐oxidant and anti‐inflammatory effects driven by caloric restriction in the vasculature of aged rats (Csiszar et al. 2009). Like SIRT1, SIRT6 is able to repress NF‐κB activity and has also been proposed to be involved in the process of ageing (Lombard et al. 2008). Deletion of SIRT6 in mice results in an accelerated ageing phenotype (Mostoslavsky et al. 2006). SIRT6 protects human endothelial cells from senescence (Cardus et al. 2013), whereas down‐regulation of SIRT 6 mediates oxidative stress‐induced senescence in these cells (Liu et al. 2014). In fact, SIRT6 seems to counteract inflammatory responses in human endothelial cells since its knockdown promotes transcriptional activity of NF‐κB, pro‐inflammatory cytokine production and COX expression. In contrast, overexpression of SIRT6 inhibits NF‐κB activity (Lappas, 2012). Interestingly, inflammatory stimuli decrease SIRT6 expression, a fact that would allow for speculation on a possible down‐regulation of SIRT6 under pro‐inflammatory conditions such as ageing. The same could be applied to SIRT1 since inflammatory stimuli in cells and inflammatory conditions in rodents decrease SIRT1 activity through its S‐nitrosylation resulting in increased NF‐κB activity (Shinozaki et al. 2014). Pharmacological activation of SIRT1 reverses vascular inflammation and endothelial dysfunction in aged mice (Gano et al. 2014).
On the other hand, an important role for nuclear related factor‐2 (Nrf2) in regulating the ageing process by orchestrating the cellular response to oxidative stress has been proposed (Lewis et al. 2010). Diverse Nrf2 activators, such as phenethyl isothiocyanate, attenuate lipopolysaccharide (LPS)‐induced NF‐κB activation, suggesting that Nrf2 and NF‐κB behave as antagonistic pathways (Jeong et al. 2004). In fact, Nrf2 promotes suppression of NF‐κB signalling by inhibiting IκB kinase (IKK)/inhibitor of κB (IκB) phosphorylation and p65 NF‐κB subunit nuclear translocation (Xu et al. 2005). Conversely, NF‐κB represses Nrf2 signalling at the transcription level by competing for transcription co‐activator CREB binding protein (Wakabayashi et al. 2010). Despite an increase in superoxide production, aged rat aortae display a decrease in Nrf2 activity that inversely correlates with the expression of NF‐кB target genes (Marmol et al. 2007). Similar results were observed in carotid arteries from aged non‐human primates (Ungvari et al. 2011). In this sense, the prevention by caloric restriction of Nrf2 dysfunction associated with ageing in cerebromicrovascular endothelial cells in rats is accompanied by reversion of ageing‐related NF‐κB activity up‐regulation and pro‐inflammatory phenotype in these cells (Csiszar et al. 2014). Thus, the ageing‐related defective functionality of modulatory systems responsible for counteracting vascular inflammation is reasonably proven.
Mechanisms of vascular inflammation in diabetes
Inflammation is tightly linked to diabetic conditions. This association exists in both directions since chronic inflammation seems to promote the development of diabetes. This is supported by the fact that elevated baseline levels of markers of inflammation predict the incidence of diabetes. High leukocyte count and, more specifically, high neutrophil count are correlated with a worsening of insulin sensitivity and incident diabetes (Vozarova et al. 2002). On the other hand, overt diabetes promotes inflammatory responses that mediate vascular dysfunction, CVD and end organ damage. In this sense, it has been suggested that targeting inflammation in diabetes improves glycaemic control, and decreases vascular complications (Agrawal & Kant, 2014). As shown in Fig. 1 and Table 1, inflammatory mechanisms leading to vascular dysfunction and CVD in diabetes are similar to those occurring in vascular ageing.
Circulating cytokines in diabetes
Elevation of inflammatory cytokines IL‐1β and IL‐6 is associated with an increased risk of type 2 diabetes mellitus (Spranger et al. 2003). IL‐6 levels were also found to be high in type 1 diabetes mellitus patients, regardless of adiposity and glycaemic control (Spranger et al. 2003). Higher levels of the inflammatory adipokine, leptin, increase the risk of developing type 2 diabetes (Julia et al. 2014) while an elevated concentration of the anti‐inflammatory adipokine, adiponectin, reduce such risk (Lindberg et al. 2015). On the other hand, anti‐inflammatory interventions targeted to inhibit IL‐1β improved glycaemic control in type 2 diabetic patients (Larsen et al. 2007; Ridker et al. 2012; Hensen et al. 2013). Similar results were observed when type 2 diabetic patients were treated with salsalate, a precursor of salicylate that inhibits the inflammatory transcription factor NF‐κB (Goldfine et al. 2010). Thus, it seems reasonable to suggest that inflammation developed with the ageing process could contribute to the higher incidence of diabetes in older people.
Conversely, elevation of inflammatory markers in diabetic patients is related to the incidence of CVD in this population. Higher plasma levels of CRP were associated with an increased risk of incident cardiovascular events among patients with type 2 diabetes (Schulze et al. 2004; Friedman et al. 2005; Krzyzanowska et al. 2007). Analogously, higher levels of soluble TNF‐α receptor II increased the probability of developing coronary heart disease in 929 women with type 2 diabetes (Shai et al. 2005). Furthermore, higher levels of adiponectin not only reduced the incidence of type 2 diabetes, but also protected from subsequent cardiovascular events in diabetic patients (Lindberg et al. 2015). The influence of inflammation on the development of diabetic vascular complications was supported by data obtained from the Diabetes Control and Complications Trial (DCCT) (Lin et al. 2008).
The presence of systemic inflammation relates to the development of endothelial dysfunction in diabetes. In this sense, vascular dysfunction is associated with increasing circulating concentrations of TNF‐α and IL‐6 in type 2 diabetic patients (Natali et al. 2006). Moreover, forearm skin blood flow is reduced in pregnant women with gestational diabetes and negatively correlates with TNF‐α and IL‐6, while it is positively correlated with circulating levels of adiponectin (Mrizak et al. 2013). Regarding the influence of inflammation on clinical outcomes, studies performed in patients with diabetic neuropathy and nephropathy reveal that the further reduction of endothelial vasodilatation observed in diabetic patients with vascular complications is associated with increased concentrations of inflammatory markers (Doupis et al. 2009; Taslipinar et al. 2011). Consistent with the involvement of inflammation on diabetic vascular dysfunction, some therapeutic interventions, such as rosiglitazone and atorvastatin, which lower systemic inflammatory factors, also improve vascular function in diabetic patients (Esposito et al. 2006; Konduracka et al. 2008). However, vasodilatory responses in obese type 2 diabetic patients did not improve after etanercept (anti‐TNF‐α) administration despite causing significant reduction of plasma CRP and IL‐6 (Domínguez et al. 2005).
An important amount of experimental evidence in animal models supports the causative role of inflammation on diabetic vascular dysfunction. Vascular tissues from type 2 diabetic rats display inflammatory activation confirmed by increased NF‐κB activity and up‐regulation of TNF‐α and ICAM and augmented myeloperoxidase activity (Bitar et al. 2010). The improvement in endothelial vasodilatation of coronary arteries accomplished by exercise training is related to down‐regulation of TNF‐α expression in cardiac tissue and reduction of circulating IL‐6 in diabetic mice (Lee et al. 2011 b), while beneficial effects in aortic vasodilatation are partly dependent on adiponectin up‐regulation in these animals (Lee et al. 2011 a).
The anti‐inflammatory cytokine IL‐10 protects from endothelial dysfunction in diabetes since the deletion of its gene in mice results in exacerbated impairment of endothelium‐dependent vasodilatation caused by the induction of diabetes (Gunnett et al. 2002). Supporting this idea, increased serum levels of IL‐10 were associated with lower risk of erectile dysfunction in type 2 diabetic patients (Araña‐Rosaínz et al. 2011). Inhibition of inflammatory cytokine production with semapimod treatment restored endothelial vasodilatation in obese Zucker rats in correlation with the reduction of serum concentrations of TNF‐α, IL‐6 and CRP while acute administration of TNF‐α suppresses endothelium‐dependent relaxations in lean control rats (Nishimatsu et al. 2008), confirming the previously reported involvement of TNF‐α in endothelial dysfunction in this model of insulin resistance (Picchi et al. 2006) that was also observed in type 2 diabetic mice (Yang et al. 2009 a).
Vascular hyperactivation of NF‐κB in diabetes
NF‐кB represents an important link between vascular oxidative stress and inflammation in the vascular damage caused by hyperglycaemia (Nishikawa et al. 2000). NF‐кB is one major intracellular target of hyperglycaemia (Barnes & Karin, 1997; Mohamed et al. 1999). Hyperglycaemia induces ROS formation in endothelial cells, which triggers NF‐кB transcriptional activity. This activation of NF‐кB seems to be an initial signalling event in the vascular inflammatory response, while many products of the genes targeted by NF‐кB (vascular endothelial growth factor (VEGF), TNF‐α, IL‐1β), in turn, activate this factor, representing a positive feedback loop (Evans et al. 2002). In different animal models of diabetes, vascular NF‐кB hyperactivation has been detected whereas different pharmacological approaches resulting in NF‐кB inhibition led to reduced vascular inflammation and improved vascular function (Pieper et al. 2002; Yang et al. 2009 b; Murthy et al. 2010; Bruder‐Nascimento et al. 2015). In diabetic patients, mononuclear cell expression of NF‐кB in peripheral blood was related to glycaemic control and diabetic complications (Hofmann et al. 1998; 1999). In this sense, in patients with chronic kidney disease, the presence of diabetes is associated with an increasing activation of NF‐кB in epigastric arteries that is associated with more severe vascular injury and greater levels of IL‐6, monocyte chemoattractant protein‐1 and VCAM‐1 (Triñanes et al. 2012). Thus, pathological activation of the NF‐кB system is likely to contribute to vascular inflammation in diabetes.
iNOS/COX in diabetes
Induction of diabetes in rats results in increased expression of iNOS in vascular tissue (Ahmad et al. 2005). Furthermore, deletion of the iNOS gene prevents the impairment of endothelial vasodilatation caused by diabetes in carotid arteries of mice (Gunnett et al. 2003) and preserves cerebral arteriolar vasomotor function in diabetic mice (Kitayama et al. 2006). Diabetes also interferes with the activity of vascular COX leading to altered synthesis of prostanoids. In this sense, the conversion of arachidonic acid (AA)‐induced relaxations mediated by PGI2 in mesenteric arteries from non‐diabetic dogs into contractions driven by AA and mediated by TXA2 in diabetic animals (Sterin‐Borda et al. 1984). More recent evidence has shown the up‐regulation of COX‐2 and increased TXA2 production that results in enhanced vascular smooth muscle tone in aorta and skeletal muscle arterioles from diabetic (db/db) mice (Guo et al. 2005; Bagi et al. 2005). Increased vascular expression of COX‐2 was also detected in coronary arterioles from diabetic patients but, in this case, associated with elevated production of PGE2 and PGI2 (Szerafin et al. 2006). In plasma from type 1 diabetic patients, levels of PGE2 were also found to be elevated (Chen et al. 2009). In addition, human diabetes is associated to enhanced expression of COX‐2 in atherosclerotic plaques (Baldan et al. 2014). High glucose concentrations increase COX‐2 expression in human endothelial and vascular smooth muscle cells (Cosentino et al. 2003; Gordillo‐Moscoso et al. 2013). These evidences point to a relevant role of iNOS and COX in the inflammatory mechanisms leading to vascular dysfunction in diabetes.
microRNAs in diabetes
Similarly to that observed in ageing, down‐regulation of miR‐155 was also reported in PBMNCs from patients with type 2 diabetes with respect to healthy control subjects. In fact, lower levels of miR‐155 and its significant correlation with glycaemic control suggest a role for this miRNA in the pathogenesis of type 2 diabetes (Corral‐Fernández et al. 2013). A similar pattern of expression in PBMNCs from type 2 diabetic patients was observed for miR‐146a (Corral‐Fernández et al. 2013). In other study comparing type 2 diabetic patients with non‐diabetic control subjects with similar overweight and dyslipidaemia, levels of miR‐146a but not those of miR‐155 were significantly reduced in diabetes without association to glycaemia, BMI or dyslipidaemia, but correlating with levels of inflammatory cytokine, IL‐8 (Baldeón et al. 2014). In fact, the reduced expression of miR‐146a in PBMNCs of diabetic patients was previously reported to be negatively correlated with both glycaemic control and insulin resistance, and NF‐κB expression and plasmatic levels of TNF‐α and IL‐6, linking subclinical inflammation in type 2 diabetes with impaired expression of miR‐146a (Balasubramanyam et al. 2011). The functional role of miR‐146a was supported by the fact that hyperglycaemia reduced levels of miR‐146a in human aortic endothelial cells while overexpression of miR‐146 inhibited inflammatory phenotype induced by high glucose in these cells (Wang et al. 2014 a). Likewise, human retinal endothelial cells from diabetic donors display reduced miR‐146a expression and increased inflammatory cytokines. Inhibition of miR‐146a augments the expression of ICAM‐1, which is reduced by treating with a miR‐146a mimic (Wang et al. 2014 b). Moreover, streptozotocin‐induced diabetes results in downregulation of miR‐146a in rat aorta associated with increased mRNA levels of NF‐κB (Emadi et al. 2014). Although further research is required, it could be speculated that the induction of miR‐146a to restrain inflammatory status is dysfunctional in diabetes.
Dysregulation of miR‐27a has also been detected in diabetic patients in correlation with fasting glucose (Karolina et al. 2012) and in tissues from type 2 diabetic rats (Herrera et al. 2010). Increased levels of miR‐34a have been detected in serum of type 2 diabetic subjects (Kong et al. 2011). Reduced SIRT‐1 activity by miR‐34a upregulation is also observed in obesity (Choi et al. 2013). Furthermore, high glucose in vitro and diabetes in animals result in increased expression of miR‐34a, an event that could be related to the development of diabetic nephropathy (Zhang et al. 2014).
miRNAs have the potential to be clinically relevant biomarkers for inflammatory responses and inflammation‐related conditions such as ageing and diabetes (Olivieri et al. 2013 b), but could also be promising targets for intervention, especially miR‐146a, since they are involved in many steps of vascular inflammation.
Modulatory systems counteracting vascular inflammation in diabetes
The potential anti‐inflammatory effects of SIRT1 in the diabetic vasculature have been proposed (Winnik et al. 2012). In this sense, SIRT1 expression is reduced in vascular smooth muscle cells after induction of diabetes in rats (Toniolo et al. 2013). As well, SIRT6 has also been suggested to be involved in metabolic disorders (Lombard et al. 2008). In contrast, overexpression of SIRT6 inhibits NF‐κB activity (Lappas, 2012).
On the other hand, expression of Nrf2 also decreases in diabetic vasculature while pharmacological induction of Nrf2 prevents structural alterations and vascular inflammation in the aorta of diabetic rats (Miao et al. 2013; Wang et al. 2014 c). In addition, gestational diabetes causes dysregulation of the Nrf2 system in fetal endothelial cells by preventing the Nrf2‐mediated transcriptional activity in response to exposure to lipid peroxides (Cheng et al. 2013). Accordingly, it has been proposed that hyperglycaemia‐induced inactivation of the Nrf2 defence pathway in endothelial cells would result in endothelial dysfunction and the development of diabetic complications (Cheng et al. 2011). Although less strongly supported by the existing literature than in ageing, the systems in charge of counteracting vascular inflammation, such as sirtuins and Nrf2, are also compromised in diabetes.
Inflammation and vascular dysfunction when both ageing and diabetes co‐exist
The presence of diabetes in adults 65 years or older confers on this group special characteristics and needs. This situation is rather usual as diabetes is present in 25% of the older adults (at 65 years old or older) and 50% of the people with diabetes are older than 65 (Sinclair et al. 2015). We have already reviewed the role of inflammation as an agent involved in the pathophysiology of the vascular damage produced when ageing or diabetes occurs separately. But, what happens when these two conditions co‐exist in the same individual? Is there further damage to the vascular system? Are the same mechanisms involved in vascular damage? We will now try to address these questions.
Many of the potential inflammatory mechanisms implicated in vascular alteration associated with ageing overlap with those present in diabetes. However, the inflammatory mechanisms of vascular damage prevailing when both conditions are present are relatively unknown (Table 1). Since vascular damage exists secondary to either ageing or diabetes, two hypotheses arise from the interaction between these conditions when they are jointly present: (1) as mentioned above, both entities share most of mechanisms of inflammatory injury and, thus, their co‐existence could cause no further impact on vascular function; and (2) the presence of both advanced age and diabetes results in greater vascular damage than in the presence of just one condition. Epidemiological and clinical data strongly suggest that ageing maximizes the effects of diabetes on vascular tissue, but is this impact simply the final outcome of the additive effects of both ageing and diabetes or is it the effect of different new mechanisms that are only present when both conditions are present? Evidence supporting that the vascular system is more damaged when ageing and diabetes are simultaneously present is discussed below.
Greater impact on vascular function
Ample evidence has shown that older people with diabetes are at higher risk for both acute and chronic microvascular and cardiovascular complications that will negatively impact the independence, self‐care capacity and, therefore, quality of life of older adults (Kirkman et al. 2012). In line with this, some studies have reported higher risk of death in diabetic people at all ages younger than 80 years when compared with those without diabetes, this risk being higher in woman than men (Sinclair et al. 2015). Moreover, higher prevalence of vascular diseases (ischaemic heart disease, cerebrovascular disease and peripheral vascular disease) in the elderly with diabetes versus those without diabetes (Nakano & Ito, 2007) has been reported. On the other hand, although some studies described lower mortality associated with diabetes at an older than younger age (Barnett et al. 2006; Sinclair et al. 2015), an excess mortality associated with diabetes has been detected in American elderly, even in those aged 85 years or more (Bertoni et al. 2002). This would be consistent with the higher prevalence of diabetic vascular complications described in the elderly with diabetes with respect to middle‐aged people with diabetes (Nakano & Ito, 2007). Furthermore, for a given duration of diabetes, cardiovascular complications and mortality have been shown to steeply increase with advanced age (Huang et al. 2014). This consistent epidemiological evidence allows the discarding of the hypothesis that the vascular damage caused by co‐existing ageing and diabetes is not greater than that already generated by either of the two conditions on its own.
Although limited in number, there are some studies that aimed to analyse the influence of both diabetes and ageing on human vascular function. Defective vasodilatation in middle‐aged (46–60 years old) type 1 diabetic patients is still patent with respect to age matched controls (Grzelak et al. 2011). Similarly, a greater impairment of flow‐mediated dilatation (FMD) was induced by type 2 diabetes at all ages despite the existence of an ageing‐induced impairment (Petrofsky & Lee, 2005). This would suggest that, despite sharing pathophysiological mechanisms, ageing and diabetes additively impact vascular function. In this sense, the skin blood flow increase in response to heat stimulus was blunted in older subjects with respect to younger, but diabetic older adults displayed a further reduction of blood flow response with respect to non‐diabetic older adults, despite having similar age and BMI (Petrofsky et al. 2013). Furthermore, brachial artery FMD was impaired in middle‐aged/older adults (62 ± 1 years) with normal fasting glucose with respect to young (24 ± 1 years), but the presence of impaired fasting glucose was related to a further reduction of FMD in middle‐aged/older subjects (64 ± 1 years). In fact, taking together these middle‐aged/older subjects, FMD was inversely correlated with fasting plasma glucose concentrations. Interestingly, impairments caused by ageing and high glucose level were both prevented by regular aerobic exercise (Devan et al. 2013).
Evidence from animal models also suggests that the severity of vascular dysfunction increases in aged diabetic animals with respect to those only diabetic or only aged. Old type 2 diabetic rats show an exacerbated reduction of endothelium‐dependent relaxations in aorta, mesenteric artery and corpus cavernosum (Miyata et al. 1992; Witte et al. 2002; Gür et al. 2005). While endothelial vasodilatation is preserved in aorta of lean non‐diabetic (Long–Evans Tokushima Otsuka; LETO) rats up to 40 weeks of age (middle‐age), this response is significantly reduced in obese diabetic (Otsuka Long–Evans Tokushima fatty; OLETF) rats at 20 weeks and further reduced at 40 weeks of age, an impairment that was prevented by performing physical activity (Bunker et al. 2010). Thus, epidemiological and functional evidence clearly shows that the presence of ageing and diabetes promotes a greater vascular impairment than that driven by any of the separate conditions. Although the mechanism(s) leading to this event have not been completely elucidated, we propose inflammation as a key factor in the exaggerated vascular damage produced when ageing and diabetes co‐exist.
Inflammation as a contributor to the exaggerated vascular dysfunction
The role of inflammation in the profound impairment of vascular function driven by concomitant diabetes and ageing is also supported by evidence derived from animal models. In aorta of aged OLETF rats (60–65 weeks old), the reduction of endothelium‐dependent relaxation and the enhancement of endothelium‐dependent contraction in comparison with aged LETO rats were associated with an increase in COX‐1/2 expression and prostanoid production (Matsumoto et al. 2007). Using the same model, it was demonstrated that increased transcriptional activity of NF‐кB was related to increased production of COX‐derived prostanoids, TXA2 and PGE2, and to enhanced endothelium‐dependent contractions that are reversed by NF‐кB inhibition (Matsumoto et al. 2009). In this sense, blunted endothelium‐dependent relaxation of mesenteric arteries in Zucker diabetic fatty (ZDF) rats was improved after COX‐2 inhibition but only in aged animals (12 months old). Although diabetes was associated with increased ROS generation and COX‐2 expression in mesenteric arteries from young rats, these effects of diabetes were exacerbated in aged animals (Vessières et al. 2013). It is interesting to remark that, even in elderly populations, the presence of diabetes is associated with elevated systemic levels of COX‐derived prostanoids (Helmersson et al. 2004). Exaggerated inflammation in aortic tissue from 22‐ to 24‐month‐old diabetic Goto Kakizaki rats with respect to age‐matched Wistar rats has also been reported. Increased production of TNF‐α and ICAM, myeloperoxidase activity (leukocyte infiltration) and NF‐кB activity were detected (Bitar et al. 2010). Moreover, the mild impairment of endothelium‐dependent relaxation observed in aortae from young mice fed with a high fat diet that renders the animals obese and hyperglycaemic turns into a strong reduction of vasodilatation when the high fat diet was administered to aged (24 months) mice. This is associated with exaggerated aortic inflammation and ROS production. Furthermore, exacerbated inflammation is also detected in periaortic adipose tissue from aged obese/hyperglycaemic mice that is able to induce a strong inflammatory phenotype in control aortae (Bailey‐Downs et al. 2013). All this evidence would suggest that the vascular inflammation induced by diabetes is exacerbated when an inflammatory background is already present in the aged vasculature, resulting in a further impairment of vascular function. This idea is graphically represented in Fig. 2.
Figure 2. Exaggerated vascular dysfunction when ageing and diabetes co‐exist: role of basal low‐grade inflammation .
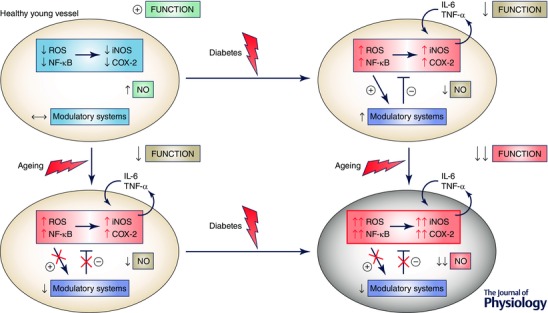
In young arteries (upper left), the development of diabetes induces oxidative stress and inflammation that is counteracted by an anti‐inflammatory/stressor response by modulatory systems (Nrf2, sirtuins, etc.) that mitigates in some degree the impact of diabetes on NO availability and vascular function (upper right). In aged arteries, there exists a low‐grade inflammation that moderately impacts vascular function and NO availability (lower left). In this situation, the development of diabetes induces further oxidative stress and inflammation, but the basal oxidant and inflammatory conditions prevent a balanced response by defective modulatory systems resulting in exacerbated NO deficit and vascular dysfunction (lower right). A similar situation could result when a diabetic condition persists and the ageing process further impairs vascular function. COX, cyclooxygenase; IL‐6, interleukin‐6; iNOS, inducible nitric oxide synthase; NF‐κB, nuclear factor‐κB; ROS, reactive oxygen species; TNF‐α, tumour necrosis factor‐α.
Chronic low‐grade inflammation associated with vascular ageing, in addition to directly interfering with the adequate function of the vasculature, could prevent the required actions in response to a stressor condition like oxidative stress or acute inflammation. In this sense, constitutive expression of redox‐sensitive transcription factors such as NF‐κB in old skeletal muscles impedes the additional transcription of cytoprotective genes following contraction, making these muscles vulnerable to oxidative stress (Jackson & McArdle, 2011). Similarly, elevated basal expression of NF‐κB‐related pro‐inflammatory molecules prevents the response triggered by high concentrations of early glycosylated proteins (Amadori adducts) in human mesothelial cells from old subjects (Rodríguez‐Mañas et al. 2006). This evidence allows for the proposal that basal low‐grade inflammation and constitutive elevation of related transcription factors present in aged vasculature would prevent an adequate response when stressor situations occur, as could be the case for diabetes or other metabolic disturbances. The inadequate response to pro‐inflammatory and oxidant stress could be aggravated by a down‐regulation of anti‐inflammatory/anti‐oxidant pathways by ageing and/or diabetes. As depicted in Fig. 2, a metabolic stressor such as diabetes would increase inflammatory pathways but also would trigger pathways counteracting inflammatory insult such as Nrf2 or sirtuins in a young vessel. In contrast, these modulatory systems are defective in aged vasculature that lacks adequate control of inflammatory pathways stimulated by diabetes which results in exacerbated vascular impairment.
Figure 3 illustrates how the inflammatory mechanisms induced by diabetes might interact with low‐grade inflammation in aged vasculature to give different outcomes from in young vessels. Potentially, either a lack of further effect over the ageing‐induced vascular damage (Fig. 3 A) or an exaggerated inflammatory insult could be produced (Fig. 3 B). The first hypothesis is based on the loss of further oxidative stress or inflammatory response with age in human mesothelial cells exposed to glycated haemoglobin (Rodríguez‐Mañas et al. 2006). As mentioned above, the absence of additional inflammatory injury in this case would be accompanied by the lack of an adequate anti‐inflammatory response that would explain the greater vascular impairment induced by diabetes in aged vessels. On the other hand, the presence of basal inflammatory stimuli in aged vascular cells would trigger the deleterious effects of diabetes on vasculature as indicated by the injury exerted by hyperglycaemia in endothelial and smooth muscle cells only when inflammatory stimuli are present. In this sense, vascular smooth muscle cells are relatively resistant to hyperglycaemic conditions, but when an inflammatory stimulus is added to a high glucose environment vascular cells suffer from marked alterations. Elevated concentrations of glucose (22 mm) do not induce NF‐κB or iNOS expression but exacerbate the pro‐inflammatory effects of IL‐1β on these cells (Lafuente et al. 2008). Similarly, ICAM‐1 and VCAM‐1 expression and leukocyte adhesion are not modified by high glucose in endothelial cells (human umbilical vascular endothelial cells; Azcutia et al. 2010), but the induction of NF‐κB, ICAM‐1 and VCAM‐1 expression and the increase in leukocyte adhesion driven by IL‐1β in these cells is potentiated by hyperglycaemic conditions. Furthermore, in vivo intraperitoneal injection of glucose causes leukocyte rolling, adhesion and migration in rat mesenteric arteries only when IL‐1β was co‐administered (Azcutia et al. 2010). Such evidence would be consistent with the epidemiological data that show an increase in the probability of developing coronary heart disease in women with type 2 diabetes with both elevated soluble tumour necrosis factor receptor II and glycosylated haemoglobin (HbA1C) with respect to those who presented elevation of only one parameter (Shai et al. 2005). Thus, a low‐grade inflammation associated with vascular ageing would not only represent additive deleterious effects by mechanisms shared with diabetes, but also trigger diabetes‐induced vascular damage that would not be manifested in young (non‐inflamed) vessels, suggesting that the simultaneous presence of diabetes and ageing compromises vascular function by additional mechanisms which are not present when these conditions appear separately. Exemplification of two possible modifications of the effects induced by the same condition (diabetes) on aged with respect to young vessels (i.e. loss of effect/response because of the alterations already induced by aging vs. the generation of vascular damage only in aged vessels favoured by the presence of ageing‐induced inflammation) is schematized in Fig. 3. This assumption should condition new research directions, but could also influence the clinical perspectives concerning the elderly population with diabetes.
Figure 3. Ageing‐induced modification of the vascular inflammatory insult driven by diabetes .
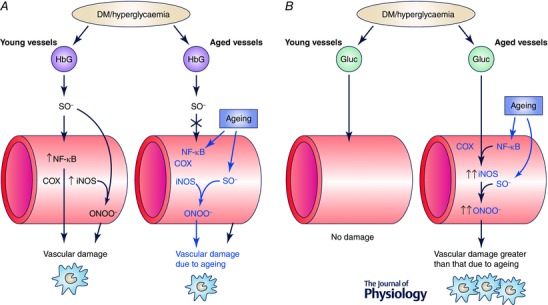
Exemplification of two possible interactions between vascular ageing and diabetes. A, the vascular damage resulting from glycated haemoglobin (HbG) increase is associated with diabetic conditions (diabetes mellitus (DM)/hyperglycaemia) in young and aged vessels. In young vessels (left), superoxide anion (SO−) generated by HbG induces NF‐κB expression and its downstream inflammatory mediators such as cyclooxygenase (COX) and inducible nitric oxide synthase (iNOS). This inflammatory response is translated into vascular damage through mechanisms involving, for example, peroxinitrite (ONOO−) formation. In contrast, aged vessels (right) manifest chronic low‐grade inflammation with up‐regulation of NF‐кB and inflammatory mediators as well as increased SO− generation that results in ageing‐induced vascular damage. Under these conditions, diabetic insult leading to SO− generation by HbG does not further induce vascular inflammatory response and no appreciable additional vascular damage. B, by contrast, there is a lack of vascular damage due to diabetes‐induced high glucose concentrations in the absence of inflammatory conditions in young vessels (left), while the chronic low‐grade inflammation caused by ageing in the vasculature (right) amplifies the deleterious effects driven by hyperglycaemia, which results in an exacerbated vascular damage, greater than that caused by normoglycaemic ageing. Alterations driven by ageing are highlighted in blue. Epidemiological and functional evidence in older diabetic animals and humans suggest that the interaction exemplified in B predominates over that in A.
Future research directions and clinical perspectives
Although there is substantial knowledge on the inflammatory mechanisms that contribute to vascular dysfunction in either ageing or diabetes, there is a critical lack of information regarding the interaction of these mechanisms when both ageing and diabetes co‐exist. As mentioned above, the fact that co‐existence of ageing and diabetes results in greater inflammation and vascular damage is reasonably established, but the chain of events giving such a result is far from being elucidated. Dissection of specific inflammatory pathways determinant of vascular dysfunction in older diabetic subjects with respect to either old or diabetic ones is required for designing targeted interventions to prevent or reverse vascular damage. Exploring the functional and molecular impact as well as the mechanisms of inflammation in the vasculature of aged animals when exposed to diabetic/metabolic stress in comparison to young animals exposed to the same stressor is also needed. This would help to determine if new mechanisms of inflammation and vascular damage arise when aged vasculature is exposed to diabetic stress.
Advances in the identification of inflammatory pathways responsible for vascular damage in older diabetic patients would certainly have therapeutic implications. Therapeutic approaches (anti‐inflammatory, hypoglycaemic and cardiovascular drugs, among others) should consider the specific characteristics of this population. For instance, antidiabetic drugs with known anti‐inflammatory activities such as metformin (Kim & Choi 2012) could be beneficial in older people with diabetes (Ng et al. 2014). On the other hand, specific characteristics of inflammatory pathways altered by ageing and diabetes could influence the therapeutic outcome of anti‐inflammatory drugs such as NSAIDs. For instance, diabetes preferentially (if not exclusively) up‐regulates COX‐2 (Bagi et al. 2005; Mokhtar et al. 2013), while both COX‐1 and COX‐2 isoforms are up‐regulated in aged vascular tissue (Heymes et al. 2000; Matz et al. 2000). This could result in enhanced cardiovascular adverse effects by COX‐2 inhibitors in the elderly since these compounds would promote COX‐1 up‐regulation, which is already up‐regulated in aged vessels. Furthermore, identification of novel therapeutic targets would specifically address the requirements of the older diabetic subjects. In addition, non‐pharmacological interventions should be considered for this population, including healthy lifestyle changes such as exercise, adjusted diet and nutritional supplements.
Finally, it should be considered that the vascular damage in diabetic persons of advanced age probably results in different clinical manifestations when compared to young adults. In this sense, cardiovascular disease is associated with cognitive impairment in older people (Hayes et al. 2014; Jefferson et al. 2015) and the presence of diabetes largely favours the presence and prognosis of cognitive impairment and dementia in these individuals (Talley et al. 2015). Moreover, endothelial dysfunction may also be involved in the development of physical disability and dependency in activities of daily living that has been shown in older adults with diabetes (Wong et al. 2013). In this regard, the levels of asymmetric dimethylarginine, a marker of endothelial dysfunction, are associated with frailty in elderly populations (Alonso‐Bouzón et al. 2014). Concerning the last statement, development of new animal models calibrating phenotypic manifestations of vascular dysfunction in ageing and diabetes is required in order to determine the mechanisms responsible for increased disability in older people with diabetes.
Conclusion
There is a huge amount of evidence regarding pathological changes in the vascular wall, including inflammation, which impair vascular function and prompt the manifestation of CVD in diabetes and during the ageing process. However, there is a noteworthy lack of data (obtained mainly from animal models), if any, about the role of these mechanisms in the major group of patients with diabetes: the older adults. This raises the necessity of filling the information gap in order to provide relevant targets for the treatment of this population group.
Additional information
Conflict of interest
The authors declare that no conflict of interest exists.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Acknowledgements
The present work was funded by grants from the Ministry of Economy and Competitiveness and cofunded by Fondos FEDER (Instituto de Salud Carlos III, PI11/01068, RETICEF RD12/0043), Spanish Government; and by the MID‐FRAIL (278803‐2) Study funded by the European Union (FP7‐HEALTH‐2011‐two‐stage).
Biographies
Mariam El Assar is a biologist researcher at the Instituto de Investigación Sanitaria del Hospital de Getafe (Madrid). Her PhD was from the Universidad Autónoma of Madrid. Her research interests are in vascular ageing and diabetes with special emphasis on the mechanisms involved in endothelial dysfunction associated to these processes.
Javier Angulo is a staff researcher at the Instituto Ramón y Cajal de Investigación Sanitaria (Madrid), Honorary Professor at Department of Pharmacology and Therapeutics of Universidad Autónoma de Madrid, and Scientific Collaborator at Instituto de Investigación Sanitaria del Hospital de Getafe. He has focused a substantial part of his research on identifying pharmacological targets for the treatment of sexual dysfunction, and his contributions to this field allowed him to obtain the Award of Excellence of the European Society for Sexual Medicine in 2005. Along his career he has combined the research in this field with that in vascular pharmacology and pathophysiology, with special interest in vascular alterations due to diabetes and ageing process.
Leocadio Rodríguez‐Mañas is the Head of the Department of Geriatrics at the Hospital de Getafe (Madrid), and Professor of Geriatric Medicine at the European University of Madrid. He is Coordinator of the Spanish Collaborative Research Network on Aging and Frailty‐RETICEF (Ministry of Science) and Leader of the subgroup on Frailty of the A3 Group (Frailty and Functional Decline) of the European Innovation Partnership on Active Healthy Aging. His research interest focuses on both the clinical investigation and the mechanisms of vascular damage in ageing and diabetes. He is currently leading several EU‐funded projects on different aspects of ageing, frailty and diabetes.

References
- Agrawal NK & Kant S (2014). Targeting inflammation in diabetes: newer therapeutic options. World J Diabetes 5, 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS & Abraham NG (2005). Heme oxygenase‐1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy‐le‐grand) 51, 371–376. [PubMed] [Google Scholar]
- Alonso‐Bouzón C, Carcaillon L, García‐García FJ, Amor‐Andrés MS, El Assar M & Rodríguez‐Mañas L (2014). Association between endothelial dysfunction and frailty: the Toledo Study for Healthy Aging. Age (Dordr) 36, 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Sotomayor M, Mingorance C & Andriantsitohaina R (2007). Fenofibrate improves age‐related endothelial dysfunction in rat resistance arteries. Atherosclerosis 193, 112–120. [DOI] [PubMed] [Google Scholar]
- Alvarez de Sotomayor M, Perez‐Guerrero C, Herrrera MD, Jimenez L, Marin R, Marhuenda E & Andriantsitohaina R (2005). Improvement of age‐related endothelial dysfunction by simvastatin: effect on NO and COX pathways. Br J Pharmacol 146, 1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres SL & Zamore PD (2013). Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol 14, 475–488. [DOI] [PubMed] [Google Scholar]
- Angulo J, Cuevas P, Fernández A, Gabancho S, Allona A, Martín‐Morales A, Moncada I, Videla S, Sáenz de Tejada I (2003). Diabetes impairs endothelium‐dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun 312, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Angulo J, Rodríguez‐Mañas L, Peiró C, Neira M, Marín J & Sánchez‐Ferrer CF (1998). Impairment of nitric oxide‐mediated relaxations in anaesthetized autoperfused streptozotocin‐induced diabetic rats. Naunyn Schmiedebergs Arch Pharmacol 358, 529–537. [DOI] [PubMed] [Google Scholar]
- Araña‐Rosaínz MJ, Ojeda MO, Acosta JR, Elías‐Calles LC, González NO, Herrera OT, García‐Alvarez CT, Rodríguez EM, Báez ME, Seijas EÁ & Valdés RF (2011). Imbalanced low‐grade inflammation and endothelial activation in patients with type 2 diabetes mellitus and erectile dysfunction. J Sex Med 8, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Avogaro A, de Kreutzenberg SV, Federici M & Fadini GP (2013). The endothelium abridges insulin resistance to premature aging. J Am Heart Assoc 2, e000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcutia V, Abu‐Taha M, Romacho T, Vázquez‐Bella M, Matesanz N, Luscinkas FW, Rodríguez‐Mañas L, Sanz MJ, Sánchez‐Ferrer CF & Peiró C (2010). Inflammation determines the pro‐adhesive properties of high extracelular D‐glucose in human endotelial cells in vitro and rat microvessels in vivo. PLoS One 5, e10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badi I, Burba I, Ruggeri C, Zeni F, Bertolotti M, Scopece A, Pompilio G & Raucci A (2014). MicroRNA‐34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age‐associated pro‐inflammatory secretory factors. J Gerontol A Biol Sci Med Sci 70, 1304–1311. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Erdei N, Toth A, Li W, Hintze TH, Koller A & Kaley G (2005). Type 2 diabetic mice have increased arteriolar tone and blood pressure. Enhanced release of COX‐2‐derived constrictor prostaglandins. Arterioscler Thromb Vasc Biol 25, 1610–1616. [DOI] [PubMed] [Google Scholar]
- Bailey‐Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A & Ungvari Z (2013). Aging exacerbates obesity‐induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci 68, 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathiskumar C, Ranjani H & Mohan V (2011). Impaired miR‐146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Mol Cell Biochem 351, 197–205. [DOI] [PubMed] [Google Scholar]
- Baldan A, Ferronato S, Olivato S, Malerba G, Scuro A, Veraldi GF, Gelati M, Ferrari S, Mariotto S, Pignatti PF, Mazzucco S & Gómez‐Lira M (2014). Cyclooxygenase 2, toll‐like receptor 4 and interleukin 1β mRNA expression in atherosclerotic plaques of type 2 diabetic patients. Inflamm Res 63, 851–858. [DOI] [PubMed] [Google Scholar]
- Baldeón RL, Weigelt K, de Wit H, Ozcan B, van Oudenaren A, Sempértegui F, Sijbrands E, Grosse L, Freire W, Drexhage HA & Leenen PJ (2014). Decreased serum level of miR‐146a as sign of chronic inflammation in type 2 diabetic patients. PLoS One 9, e115209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ & Karin M (1997). Nuclear factor‐кB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336, 1066–1071. [DOI] [PubMed] [Google Scholar]
- Barnett KN, McMurdo ME, Ogston SA, Morris AD & Evans JM (2006). Mortality in people diagnosed with type 2 diabetes at an older age: a systematic review. Age Ageing 35, 463–468. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Anderson GF, Krop JS & Brancati FL (2002). Diabetes‐related morbidity and mortality in a national sample of U.S. elders. Diabetes Care 25, 471–475. [DOI] [PubMed] [Google Scholar]
- Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ & Campisi J (2009). MicroRNAs miR‐146a/b negatively modulate the senescence‐associated inflammatory mediators IL‐6 and IL‐8. Aging (Albany NY) 1, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar MS, Ayed AK, Abdel‐Halim SM, Isenovic ER & Al‐Mulla F (2010). Inflammation and apoptosis in aortic tissues of aged type II diabetes: amelioration with α‐lipoic acid through phosphatidylinositol 3‐kinase/Akt‐dependent mechanism. Life Sci 86, 844–853. [DOI] [PubMed] [Google Scholar]
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, Bonadonna RC & Muggeo M (2007). Insulin resistance as estimated by Homeostasis Model Assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care 30, 318–324. [DOI] [PubMed] [Google Scholar]
- Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM & Dimmeler S (2011). MicroRNA‐29 in aortic dilation: implications for aneurysm formation. Circ Res 109, 1115–1119. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Fleming I & Busse R (2005). Endothelial aging. Cardiovasc Res 66, 286–294. [DOI] [PubMed] [Google Scholar]
- Bruder‐Nascimento T, Callera GE, Montezano AC, He Y, Antunes TT, Cat AN, Tostes RC & Touyz RM (2015). Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: role of Rac1/2‐sensitive Nox‐dependent pathways. Clin Sci (Lond) 128, 411–423. [DOI] [PubMed] [Google Scholar]
- Bunker AK, Arce‐Esquivel AA, Rector RS, Booth FW, Ibdah JA & Laughlin MH (2010). Physical activity maintains aortic endothelium‐dependent relaxation in the obese type 2 OLETF rat. Am J Physiol Heart Circ Physiol 298, H1889–H1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardus A, Uryga AK, Walters G & Erusalimsky JD (2013). SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc Res 97, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevenini E, Monti D & Franceschi C (2013). Inflamm‐ageing. Curr Opin Clin Nutr Metab Care 16, 14–20. [DOI] [PubMed] [Google Scholar]
- Chen LH, Chiou GY, Chen YW, Li HY & Chiou SH (2010). MicroRNA and aging: a novel modulator in regulating the aging network. Ageing Res Rev 9 (Suppl 1), S59–S66. [DOI] [PubMed] [Google Scholar]
- Chen SS, Jenkins AJ & Majewski H (2009). Elevated plasma prostaglandins and acetylated histone in monocytes in type 1 diabetic patients. Diabet Med 26, 182–186. [DOI] [PubMed] [Google Scholar]
- Cheng HS, Njock MS, Khyzha N, Dang LT & Fish JE (2014). Noncoding RNAs regulate NF‐κB signaling to modulate blood vessel inflammation. Front Genet 5, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, Mayr M, Siow RC & Mann GE (2013). Gestational diabetes mellitus impairs Nrf2‐mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes 62, 4088–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Siow RC & Mann GE (2011). Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor‐E2‐related factor 2‐Kelch‐like ECH‐associated protein 1 defense pathway. Antioxid Redox Signal 14, 469–487. [DOI] [PubMed] [Google Scholar]
- Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B & Kemper JK (2013). Elevated microRNA‐34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 12, 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Ku EJ, Hong ES, Lim S, Kim KW, Moon JH, Kim KM, Park YJ, Park KS & Jang HC (2015). High serum adiponectin concentration and low body mass index are significantly associated with increased all‐cause and cardiovascular mortality in an elderly cohort, “adiponectin paradox”: The Korean Longitudinal Study on Health and Aging (KLoSHA). Int J Cardiol 183, 91–97. [DOI] [PubMed] [Google Scholar]
- Collins T & Cybulsky MI (2001). NF‐кB: pivotal mediator or innocent bystander in atherogenesis? J Clin Invest 107, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral‐Fernández NE, Salgado‐Bustamante M, Martínez‐Leija ME, Cortez‐Espinosa N, García‐Hernández MH, Reynaga‐Hernández E, Quezada‐Calvillo R & Portales‐Pérez DP (2013). Dysregulated miR‐155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 121, 347–353. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der Loo B, Bachsmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M & Lüscher TF (2003). High glucose causes upregulation of cyclooxygenase 2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation 107, 1017–1023. [DOI] [PubMed] [Google Scholar]
- Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G & Gosling RC (2002). Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 106, 2085–2090. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE & Ungvari Z (2014). Caloric restriction confers persistent anti‐oxidative, pro‐angiogenic, and anti‐inflammatory effects and promotes anti‐aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307, H292–H306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R & Ungvari Z (2009). Anti‐oxidative and anti‐inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130, 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG & Kaley G (2003). Aging‐induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J 17, 1183–1185. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta EG & Ungvari Z (2008). Inflammation and endothelial dysfunction during aging: role of NF‐κB. J Appl Physiol 105, 1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal‐Ros S, Bronner C, Auger C & Schini‐Kerth VB (2012). Red wine polyphenols improve an established aging‐related endothelial dysfunction in the mesenteric artery of middle‐aged rats: role of oxidative stress. Biochem Biophys Res Commun 419, 381–387. [DOI] [PubMed] [Google Scholar]
- Devan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE & Seals DR (2013). Regular aerobic exercise protects against impaired fasting plasma glucose‐associated vascular endothelial dysfunction with aging. Clin Sci (Lond) 124, 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez H, Storgaard H, Rask‐Madsen C, Steffen‐Hermann T, Ihlemann N, Baunbierg Nielsen D, Spohr C, Kober L, Vaag A & Torp‐Pedersen C (2005). Metabolic and vascular effects of tumor necrosis factor‐α blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res 42, 517–525. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB & Seals DR (2008). Aging is associated with greater nuclear NFκB, reduced IκBα, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 7, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T & Veves A (2009). Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 94, 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Assar M, Angulo J & Rodriguez‐Mañas L (2013). Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 65, 380–401. [DOI] [PubMed] [Google Scholar]
- El Assar M, Ruiz de Adana JC, Angulo J, Pindado Martínez ML, Hernández Matías A & Rodríguez‐Mañas L (2013). Preserved endothelial function in human obesity in the absence of insulin resistance. J Transl Med 11, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi SS, Soufi FG, Khamaneh AM & Alipour MR (2014). MicroRNA‐146a expression and its intervention in NF‐кB signaling in diabetic rat aorta. Endocr Regul 48, 103–108. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA & Grodsky GM (2002). Oxidative stress and stress‐activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23, 599–622. [DOI] [PubMed] [Google Scholar]
- Esposito K, Ciotola M, Carleo D, Schisano B, Saccomano F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A & Giugliano D (2006). Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care 29, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM & Longo DL (2005). The origins of age‐related proinflammatory state. Blood 105, 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas WM, Quaglia LA, Santos SN, Soares AA, Japiassú AV, Boaventura V, dos Santos Barros E, Córdova C, Nóbrega OT & Sposito AC (2011). Association of systemic inflamatory activity with coronary and carotid atherosclerosis in the very elderly. Atherosclerosis 216, 212–216. [DOI] [PubMed] [Google Scholar]
- Friedman AN, Hunsicker LG, Selhub J & Bostom AG; Collaborative Study Group (2005). C‐reactive protein as a predictor of total arteriosclerotic outcomes in type 2 diabetic nephropathy. Kidney Int 68, 773–778. [DOI] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL & Seals DR (2014). The SIRT1 activator SRT1720 reverses vascular endotelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307, H1754–H1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantier MP, Stunden HJ, McCoy CE, Behlke MA, Wang D, Kaparakis‐Liaskos M, Sarvestani ST, Yang YH, Xu D, Corr SC, Morand EF & Williams BR (2012). A miR‐19 regulon that controls NF‐κB signaling. Nucleic Acids Res 40, 8048–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA & Shoelson SE; TINSAL‐T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team (2010). The effects of salsalate on glycaemic control in patients with type 2 diabetes. Ann Intern Med 152, 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Ma Y, Ruan Y, Fu G & Wu S (2014). Long‐term atorvastatin improves age‐related endothelial dysfunction by ameliorating oxidative stress and normalizing eNOS/iNOS imbalance in rat aorta. Exp Gerontol 52, 9–17. [DOI] [PubMed] [Google Scholar]
- Gordillo‐Moscoso A, Ruiz E, Carnero M, Reguillo F, Rodríguez E, Tejerina T & Redondo S (2013). Relationship between serum levels of triglycerides and vascular inflammation, measured as COX‐2, in arteries from diabetic patients: a translational study. Lipids Health Dis 12, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelak P, Czupryniak L, Olszycki M, Majos A & Stefanczyk L (2011). Age effect on vascular reactivity in Type 1 diabetes. Diabet Med 28, 833–837. [DOI] [PubMed] [Google Scholar]
- Gunnett CA, Heistad DD & Faraci FM (2002). Interleukin‐10 protects nitric oxide‐dependent relaxation during diabetes: role of superoxide. Diabetes 51, 1931–1937. [DOI] [PubMed] [Google Scholar]
- Gunnett CA, Heistad DD & Faraci FM (2003). Gene‐targeted mice reveal a critical role for inducible nitric oxide synthase in vascular dysfunction during diabetes. Stroke 34, 2970–2974. [DOI] [PubMed] [Google Scholar]
- Guo Z, Su W, Allen S, Pang H, Dougherty A, Smart E & Gong MC (2005). Up‐regulation and smooth muscle contractile hyperreactivity in spontaneous diabetic db/db mice. Cardiovasc Res 67, 723–735. [DOI] [PubMed] [Google Scholar]
- Gür S, Karahan ST, Oztürk B, Badilli M (2005). Effect of ascorbic acid treatment on endothelium‐dependent and neurogenic relaxation of corpus cavernosum from middle‐aged non‐insulin dependent diabetic rats. Int J Urol 12, 821–828. [DOI] [PubMed] [Google Scholar]
- Hajjar DP & Gotto AM Jr (2013). Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am J Pathol 182, 1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SJ & Watts GF (2013). Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud 10, 133–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Alosco ML & Forman DE (2014). The effects of aerobic exercise on cognitive and neural decline in aging and cardiovascular disease. Curr Geriatr Rep 3, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmersson J, Vessby B, Larsson A & Basu S (2004). Association of type 2 diabetes with cyclooxygenase‐mediated inflammation and oxidative stress in an elderly population. Circulation 109, 1729–1734. [DOI] [PubMed] [Google Scholar]
- Hensen J, Howard CP, Walter V & Thuren T (2013). Impact of interleukin‐1β antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo controlled trial. Diabetes Metab 39, 524–531. [DOI] [PubMed] [Google Scholar]
- Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernández C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI & Lindgren CM (2010). Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J & Boulanger CM (2000). Cyclo‐oxygenase‐1 and ‐2 contribution to endothelial dysfunction in ageing. Br J Pharmacol 131, 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Schiekofer S, Isermann B, Kanitz M, Henkels M, Joswig M, Treusch A, Morcos M, Weiss T, Borcea V, Abdel Khalek AK, Amiral J, Tritschler H, Ritz E, Wahl P, Ziegler R, Bierhaus A & Nawroth PP (1999). Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative stress sensitive transcription factor NF‐кB. Diabetologia 42, 222–232. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Schiekofer S, Kanitz M, Klevesath MS, Joswig M, Lee V, Morcos M, Tritschler H, Ziegler R, Wahl P, Bierhaus A & Nawroth PP (1998). Insufficient glycaemic control increases nuclear factor‐кB binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care 21, 1310–1316. [DOI] [PubMed] [Google Scholar]
- Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH & Karter AJ (2014). Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 174, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MJ & McArdle A (2011). Age‐related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589, 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F, Yang X, Nickenig G, Werner N & Vasa‐Nicotera M (2015). Role, function and therapeutic potential of microRNAs in vascular aging. Curr Vasc Pharmacol 13, 324–330. [DOI] [PubMed] [Google Scholar]
- Jefferson AL, Hohman TJ, Liu D, Haj‐Hassan S, Gifford KA, Benson EM, Skinner JS, Lu Z, Sparling J, Sumner EC, Bell S & Ruberg FL (2015). Adverse vascular risk is related to cognitive decline in older adults. J Alzheimer Dis 44, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong WS, Kim IW, Hu R & Kong AN (2004). Modulatory properties of various natural chemopreventive agents on the activation of NF‐kappaB signaling pathway. Pharm Res 21, 661–670. [DOI] [PubMed] [Google Scholar]
- Jiang M, Xiang Y, Wang D, Gao J, Liu D, Liu Y, Liu S & Zheng D (2012). Dysregulated expression of miR‐146a contributes to age‐related dysfunction of macrophages. Aging Cell 11, 29–40. [DOI] [PubMed] [Google Scholar]
- Jin SM, Noh CI, Yang SW, Bae EJ, Shin CH, Chung HR, Kim YY & Yun YS (2008). Endothelial dysfunction and microvascular complications in type 1 diabetes mellitus. J Korean Med Sci 23, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julia C, Czernichow S, Charnaux N, Ahluwalia N, Adreeva V, Touvier M, Galan P & Fezeu L (2014). Relationships between adipokines, biomarkers of endothelial function and inflammation and risk of type 2 diabetes. Diabetes Res Clin Pract 105, 231–238. [DOI] [PubMed] [Google Scholar]
- Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF & Jeyaseelan K (2012). Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 97, E2271–E2276. [DOI] [PubMed] [Google Scholar]
- Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, Santhanam L, Webb A, Camara A, Sikka G, Nyhan D, Shoukas AA, Ilies M, Christianson DW, Champion HC & Berkowitz DE (2009). Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol 107, 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA & Choi HC (2012). Metformin inhibits inflammatory response via AMPK‐PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun 425, 866–872. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW & Kim CD (2002). Vascular NADH oxidase is involved in impaired endothelium‐dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 51, 522–527. [DOI] [PubMed] [Google Scholar]
- Kinzenbaw DA, Chu Y, Peña‐Silva RA, Didion SP & Faraci FM (2013). Interleukin‐10 protects against aging‐induced endothelial dysfunction. Physiol Rep 1, e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE & Swift CS (2012). Diabetes in older adults. Diabetes Care 35, 2650–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama J, Faraci FM, Gunnett CA & Heistad DD (2006). Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke 37, 2129–2133. [DOI] [PubMed] [Google Scholar]
- Kizer JR, Benkeser D, Arnold AM, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Defilippi CR, Newman AB & Djousse L (2012). Associations of total and high‐molecular‐weight adiponectin with all‐cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation 126, 2951–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konduracka E, Galicka‐Latala D, Cieslik G, Rostoff P, Fedak D, Sieradzki J, Naskalski J & Piwowarska W (2008). Effect of atorvastatin on endothelial function and inflammation in long‐duration type 1 diabetic patients without coronary heart disease and essential hypertension. Diabetes Obes Metab 10, 719–725. [DOI] [PubMed] [Google Scholar]
- Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J & Zhao L (2011). Significance of serum microRNAs in pre‐diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48, 61–69. [DOI] [PubMed] [Google Scholar]
- Kotsis V, Stabouli S, Karafillis I & Nilsson P (2011). Early vascular aging and the role of central blood pressure. J Hypertens 29, 1847–1853. [DOI] [PubMed] [Google Scholar]
- Kourtis N & Tavernarakis N (2011). Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J 30, 2520–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska K, Mittermayer F, Wolzt M & Schernthaner G (2007). Asymmetric dimethylarginine predicts cardiovascular events in patients with type 2 diabetes. Diabetes Care 30, 1834–1839. [DOI] [PubMed] [Google Scholar]
- Ladeia AM, Sampaio RR, Hita MC & Adan LF (2014). Prognostic value of endothelial dysfunction in type 1 diabetes mellitus. World J Diabetes 5, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente N, Matesanz N, Azcutia V, Romacho T, Nevado J, Rodríguez‐Mañas L, Moncada S, Peiró C & Sánchez‐Ferrer CF (2008). The deleterious effect of high concentrations of D‐glucose requires pro‐inflammatory preconditioning. J Hypertens 26, 478–485. [DOI] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Lappas M (2012). Anti‐inflammatory properties of sirtuin 6 in human umbilical vein endothelial cells. Mediat Inflamm 2012, 597514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Henson GD, Thorbum A, Sindler AL, Pierce GL & Seals DR (2012). Translational evidence that impaired autophagy contributes to arerial ageing. J Physiol 590, 3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup‐Poulsen T & Donath MY (2007). Interleukin‐1‐receptor antagonist in type 2 diabetes mellitus. N Engl J Med 356, 1517–1526. [DOI] [PubMed] [Google Scholar]
- Lee S, Park Y, Dellsperger KC & Zhang C (2011. a). Exercise training improves endothelial function via adiponectin‐dependent and independent pathways in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 301, H306–H314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park Y & Zhang C (2011. b). Exercise training prevents coronary endothelial dysfunction in type 2 diabetic mice. Am J Biomed Sci 3, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Henson GD, Black AD, Donato AJ & Seals DR (2011). Aerobic exercise reverses arterial inflammation with aging in mice. Am J Physiol Heart Circ Physiol 301, H1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Mele J, Hayes JD & Buffenstein R (2010). Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol 50, 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P & Hansson GK (2015). Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 116, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Glynn RJ, Rifai N, Manson JE, Ridker PM, Nathan DM & Schaumberg DA (2008). Inflammation and progressive nephropathy in type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care 31, 2338–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind M, Svensson AM, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M & Rosengren A (2014). Glycemis control and excess mortality in type 1 diabetes. N Engl J Med 371, 1972–1982. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Jensen JS, Bjerre M, Pedersen SH, Frystyk J, Flyvbjerg A, Galatius S, Jeppesen J & Mogelvang R (2015). Adiponectin, type 2 diabetes and cardiovascular risk. Eur J Prev Cardiol 22, 276–283. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu H, Ha Y, Tilton RG & Zhang W (2014). Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. Biomed Res Int 2014, 902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Schwer B, Alt FW & Mostoslavsky R (2008). SIRT6 in DNA repair, metabolism, and ageing. J Intern Med 263, 128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukich E, Matas Z, Boaz M & Shargorodsky M (2010). Increasing derangement of glucose homeostasis is associated with increased arterial stiffness in patients with diabetes, impaired fasting glucose and normal controls. Diabetes Metab Res Rev 26, 365–370. [DOI] [PubMed] [Google Scholar]
- MacNaul KL & Hutchinson NI (1993). Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun 196, 1330–1334. [DOI] [PubMed] [Google Scholar]
- Marmol F, Sanchez J, Lopez D, Martinez N, Rosello‐Catafau J, Mitjavila MT & Puig‐Parellada P (2007). Loss of adaptation to oxidative stress as a mechanism for aortic damage in aging rats. J Physiol Biochem 63, 239–247. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Ishida K, Kobayashi T & Kamata K (2009). Pyrrolidine dithiocarbamate reduces vascular prostanoid‐induced responses in aged type 2 diabetic rat model. J Pharmacol Sci 110, 326–333. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Noguchi E, Kobayashi T & Kamata K (2007). Imbalance between endothelium‐derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol 293, H1480–H1490. [DOI] [PubMed] [Google Scholar]
- Matz RL & Andriantsitohaina R (2003). Age‐related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging 20, 527–550. [DOI] [PubMed] [Google Scholar]
- Matz RL, de Sotomayor MA, Schott C, Stoclet JC & Andriantsitohaina R (2000). Vascular bed heterogeneity in age‐related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol 131, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR; ACCT Investigators (2005). Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol 46, 1753–1760. [DOI] [PubMed] [Google Scholar]
- Menghini R, Stohr R & Federici M (2014). MicroRNAs in vascular aging and atherosclerosis. Ageing Res Rev 17, 68–78. [DOI] [PubMed] [Google Scholar]
- Miao X, Cui W, Sun W, Xin Y, Wang B, Tan Y, Cai L, Miao L, Fu Y, Su G & Wang Y (2013). Therapeutic effect of MG132 on the aortic oxidative damage and inflammatory response in OVE26 type 1 diabetic mice. Oxid Med Cell Longev 2013, 879516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M & Nourhashemi F (2013). Proinflammatory cytokines, aging, and age‐related diseases. J Am Med Dir Assoc 14, 877–882. [DOI] [PubMed] [Google Scholar]
- Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, Wahle KW & Calder PC (2008). Age‐related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis 196, 298–305. [DOI] [PubMed] [Google Scholar]
- Miyata N, Tsuchida K, Okuyama S, Otomo S, Kamata K & Kasuya Y (1992). Age‐related changes in endothelium‐dependent relaxation in aorta from genetically diabetic WBN/Kob rats. Am J Physiol Heart Circ Physiol 262, H1104–H1109. [DOI] [PubMed] [Google Scholar]
- Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R & Nawroth PP (1999). The role of oxidative stress and NF‐кB activation in late diabetic complications. Biofactors 10, 157–167. [DOI] [PubMed] [Google Scholar]
- Mokhtar SS, Vanhoutte PM, Leung SWS, Yusof MI, Sulaiman WAW, Saad AZM, Suppian R & Rasool AHG (2013). Reduced expression of prostacyclin synthase and nitric oxide synthase in subcutaneous arteries of type 2 diabetic patients. Tohoku J Exp Med 231, 217–222. [DOI] [PubMed] [Google Scholar]
- Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I & Dansky HM (2005). Diabetes induces endothelial dysfunction but does not increase neointimal formation in high‐fat diet fed C57BL/6J mice. Circ Res 96, 1178–1184. [DOI] [PubMed] [Google Scholar]
- Moreira DM, da Silva RL, Vieira JL, Fattah T, Lueneberg ME & Gottschall CA (2015). Role of vascular inflammation in coronary artery disease: potential of anti‐inflammatory drugs in the prevention of atherothrombosis. Inflammation and anti‐inflammatory drugs in coronary artery disease. Am J Cardiovasc Drugs 15, 1–11. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD & Alt FW (2006). Genomic instability and aging‐like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329. [DOI] [PubMed] [Google Scholar]
- Mrizak I, Arfa A, Fekih M, Debbabi H, Bouslema A, Boumaiza I, Zaouali M, Khan NA & Tabka Z (2013). Inflammation and impaired endothelium‐dependent vasodilation in non obese women with gestational diabetes mellitus: preliminary results. Lipids Health Dis 12, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SN, Desouza CV, Bost NW, Hilaire RC, Casey DB, Badejo AM, Dhaliwal JS, McGee J, McNamara DB, Kadowitz PJ & Fonseca VA (2010). Effects of salsalate therapy on recovery from vascular injury in female Zucker fatty rats. Diabetes 59, 3240–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KK, Papathanassiou K, Bechlioulis A, Kazakos N, Pappas K, Tigas S, Makriyiannis D, Tsatsoulis A & Michalis LK (2012). Determinants of vascular function in patients with type 2 diabetes. Cardiovasc Diabetol 11, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T & Ito H (2007). Epidemiology of diabetes mellitus in old age in Japan. Diabetes Res Clin Pract 77 ( Suppl 1 ), S76–S81. [DOI] [PubMed] [Google Scholar]
- Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L & Ferrannini E (2006). Clustering of insulin resistance with vascular dysfunction and low‐grade inflammation in type 2 diabetes. Diabetes 55, 1133–1140. [DOI] [PubMed] [Google Scholar]
- Naylor LH, Green DJ, Jones TW, Kalic RJ, Suriano KL, Shah M, Hopkins N & Davis EA (2011). Endothelial function and carotid intima‐medial thickness in adolescents with type 2 diabetes mellitus. J Pediatr 159, 971–974. [DOI] [PubMed] [Google Scholar]
- Ng TP, Feng L, Yap KB, Lee TS, Tan CH & Winblad B (2014). Long‐term metformin usage and cognitive function among older adults with diabetes. J Alzheimer Dis 41, 61–68. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I & Brownlee M (2000). Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790. [DOI] [PubMed] [Google Scholar]
- Nishimatsu H, Suzuki E, Takeda R, Takahashi M, Oba S, Kimura K, Nagano T & Hirata Y (2008). Blockade of endogenous proinflammatory cytokines ameliorates endothelial dysfunction in obese Zucker rats. Hypertens Res 31, 737–743. [DOI] [PubMed] [Google Scholar]
- Noren Hooten N, Abdelmohsen K, Gorospe M, Ejiogu N, Zonderman AB & Evans MK. (2010). microRNA expression patterns reveal differential expression of target genes with age. PloS One 5, e10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi K, Sugawara H, Ebina K, Shiga K, Kikuchi N, Mori M & Yokota S (2014). Comparison of brachial artery flow‐mediated dilation in youth with type 1 and type 2 diabetes mellitus. J Diabetes Invest 5, 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Sakaguchi M, Miwa K, Furukado S, Yamagami H, Yagita Y, Mochizuki H & Kitagawa K (2014). Association of interleukin‐6 with the progression of carotid atherosclerosis: a 9‐year follow‐up study. Stroke 45, 2924–2929. [DOI] [PubMed] [Google Scholar]
- Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbateccola AM, Antonicelli R, Franceschi C, Procopio AD (2013. a) MiR‐146a as a marker of senescence‐associated pro‐inflammatory status in cells involved in vascular remodelling. Age (Dordr) 35, 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri F, Rippo MR, Procopio AD & Fazioli F (2013. b). Circulating inflamma‐miRs in aging and age‐related diseases. Front Genet 4, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneni F, Costantino S & Cosentino F (2015). Molecular pathways of arterial aging. Clin Sci (Lond) 128, 69–79. [DOI] [PubMed] [Google Scholar]
- Pardo PS & Boriek AM (2011). The physiological roles of Sirt1 in skeletal muscle. Aging (Albany NY) 3, 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SA, Huxley RR & Woodward M (2014). Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta‐analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 383, 1973–1980. [DOI] [PubMed] [Google Scholar]
- Petrofsky J, Alshammari F, Bains GS, Khowailed IA, Lee H, Kuderu YN, Lodha RD, Rodrigues S, Nguyen D, Potnis PA, Deshpande PP, Yim JE & Berk L (2013). What is more damaging to vascular endothelial function: Diabetes, age, high BMI, or all of the above? Med Sci Monit 19, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrofsky J & Lee S (2005). The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit 11, CR247–CR254. [PubMed] [Google Scholar]
- Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM & Zhang C (2006). Tumor necrosis factor‐α induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99, 69–77. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Siebeneich W, Olds CL, Felix CC & Del Soldato P (2002). Vascular protective actions of a nitric oxide aspirin analog in both in vitro and in vivo models of diabetes mellitus. Free Radic Biol Med 32, 1143–1156. [DOI] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB & Rimm EB (2004). Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291, 1730–1737. [DOI] [PubMed] [Google Scholar]
- Prenner SB & Chirinos JA (2015). Arterial stiffness in diabetes mellitus. Atherosclerosis 238, 370–379. [DOI] [PubMed] [Google Scholar]
- Rein P, Saely CH, Silbernagel G, Vonbank A, Mathies R, Drexel H & Baumgartner I (2015). Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease. Atherosclerosis 239, 299–303. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J & Thuren T; CANTOS Pilot Investigative Group (2012). Effects of interleukin‐1β inhibition with canakinumab on haemoglobin A1c, lipids, C‐reactive protein, interleukin‐6, and fibrinogen: a phase IIb randomized, placebo controlled trial. Circulation 126, 2739–2748. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Mañas L, Angulo J, Peiró C, Llergo JL, Sánchez‐Ferrer A, López‐Dóriga P & Sánchez‐Ferrer CF (1998). Endothelial dysfunction and metabolic control in streptozotocin‐induced diabetic rats. Br J Pharmacol 123, 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Mañas L, El‐Assar M, Vallejo S, López‐Dóriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gómez‐Guerrero C, Peiró C & Sanchez‐Ferrer CF (2009). Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell 8, 226–238. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Mañas L, López‐Dóriga P, Petidier R, Neira M, Solís J, Pavón I, Peiró C & Sánchez‐Ferrer CF (2003). Effect of glycaemic control on the vascular nitric oxide system in patients with type 1 diabetes. J Hypertens 21, 1137–1143. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Mañas L, Sánchez‐Rodríguez C, Vallejo S, El‐Assar M, Peiró C, Azcutia V, Matesanz N, Sanchez‐Ferrer CF & Nevado J (2006). Pro‐inflammatory effects of early non‐enzymatic glycated proteins in human mesothelial cells vary with cell donor's age. Br J Pharmacol 149, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio‐Ruiz ME, Perez‐Torres I, Soto ME, Pastelin G & Guarner‐Lans V (2014). Aging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res Rev 18, 132–147. [DOI] [PubMed] [Google Scholar]
- Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K & Suuronen T (2008). Activation of innate immunity system during aging: NF‐κB signaling is the molecular culprit of inflamm‐aging. Ageing Res Rev 7, 83–105. [DOI] [PubMed] [Google Scholar]
- Santos‐Parker JR, LaRocca TJ & Seals DR (2014). Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ 38, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK & Danesh J; Emerging Risk Factors Collaboration (2010). Daibetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta‐analysis of 102 prospective studies. Lancet 375, 2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, Danesh J & Whincup PH (2006). Adiponectin and coronary heart disease: a prospective study and meta‐analysis. Circulation 114, 623–629. [DOI] [PubMed] [Google Scholar]
- Schjørring O, Kun A, Flyvbjerg A, Kirkeby HJ, Jensen JB & Simonsen U (2012). Flow‐evoked vasodilation is blunted in penile arteries from Zucker diabetic fatty rats. J Sex Med 9, 1789–1800. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH & Joyner MJ (2007). Ageing reduces nitric‐oxide‐ and prostaglandin‐mediated vasodilatation in exercising humans. J Physiol 579, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze MB, Rimm EB, Li T, Rifai N, Stampfer MJ & Hu FB (2004). C‐reactive protein and incident cardiovascular events among men with diabetes. Diabetes Care 27, 889–894. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Tesauro M, Rizza S, Iantorno M, Federici M, Lauro D, Campia U, Turriziani M, Fusco A, Cocciolillo G & Lauro R (2008). Endothelial function and arterial stiffness in normotensive normoglycaemic first‐degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis 18, 349–356. [DOI] [PubMed] [Google Scholar]
- Seals DR, Kaplo RA, Gioscia‐Ryan RA & LaRocca TJ (2014). You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology 29, 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, Mantzoros C & Hu FB (2005). A prospective study of soluble tumor necrosis factor‐alpha receptor II (sTNF‐RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care 28, 1376–1382. [DOI] [PubMed] [Google Scholar]
- Shao Y, Cheng Z, Li X, Chernaya V, Wang H & Yang XF (2014). Immunosuppressive/anti‐inflammatory cytokines directly and indirectly inhibit endothelial dysfunction – a novel mechanism for maintaining vascular function. J Hematol Oncol 7, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Man RY & Vanhoutte PM (2008). Two isoforms of cyclooxygenase contribute to augmented endothelium‐dependent contractions in femoral arteries of 1‐year‐old rats. Acta Pharmacol Sin 29, 185–192. [DOI] [PubMed] [Google Scholar]
- Shinozaki S, Chang K, Sakai M, Shimizu N, Yamada M, Tanaka T, Nakazawa H, Ichinose F, Yamada Y, Ishigami A, Ito H, Ouchi Y, Starr ME, Saito H, Shimokado K, Stamler JS & Kaneki M (2014). Inflammatory stimuli induce inhibitory S‐nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci Signal 7, ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD III, Ellis C, Ross D, Vandegaer K, Bedja D, Gabrielson K, Walston JD, Berkowitz DE & Barouch LA (2013). Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Exp Gerontol 48, 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A, Dunning T & Rodriguez‐Mañas L (2015). Diabetes in older people: new insights and remaining challenges. Lancet Diabetes Endocrinol 3, 275–285. [DOI] [PubMed] [Google Scholar]
- Song L, Lin C, Gong H, Wang C, Liu L, Wu J, Tao S, Hu B, Cheng SY, Li M & Li J (2013). miR‐486 sustains NF‐κB activity by disrupting multiple NF‐κB‐negative feedback loops. Cell Res 23, 274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Shen H, Schenten D, Shan P, Lee PJ & Goldstein DR (2012). Aging enhances the basal production of IL‐6 and CCL2 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 32, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H & Pfeiffer AFH (2003). Inflammatory cytokines and the risk to develop type 2 diabetes. Results of the prospective populatioon‐based European Prospective Investigation into Cancer and Nutrition (EPIC)‐Postdam Study. Diabetes 52, 812–817. [DOI] [PubMed] [Google Scholar]
- Sterin‐Borda L, Franchi AM, Borda ES, Del Castillo E, Gimeno MF, Gimeno AL & Sterin Borda I (1984). Augmented thromboxane generation by mesenteric arteries from pancreatectomized diabetic dogs is coincident with the vascular tone enhancement evoked by Na‐arachidonate and prostacyclin. Eur J Pharmacol 103, 211–221. [DOI] [PubMed] [Google Scholar]
- Steyers CM 3rd & Miller FJ Jr (2014). Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci 15, 11324–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerafin T, Erdei N, Fulop T, Pasztor ET, Edes I, Koller A & Bagi Z (2006). Increased cyclooxygenase‐2 expression and prostaglandin‐mediated dilation in coronary arterioles of patients with diabetes mellitus. Circ Res 99, e12–e17. [DOI] [PubMed] [Google Scholar]
- Talley M, Pryor E, Wadley V, Crowe M, Morrison S, Vance D (2015). The physiological mechanisms of diabetes and aging on brain health and cognition: implications for nursing practice and research. J Neurosci Nurs 47, E12–22. [DOI] [PubMed] [Google Scholar]
- Taslipinar A, Yaman H, Yilmaz NI, Demirbas S, Saglam M, Taslipinar MY, Agilli M, Kurt YG, Sonmez A, Azal O, Bolu E, Yenicesu M & Kutlu M (2011). The relationship between inflammation, endothelial dysfunction and proteinuria in patients with diabetic nephropathy. Scand J Clin Lab Invest 71, 606–612. [DOI] [PubMed] [Google Scholar]
- Tian J, Yan Z, Wu Y, Zhang SL, Wang K, Ma XR, Guo L, Wang J, Zuo L, Liu JY, Quan L & Liu HR (2010). Inhibition of iNOS protects endothelial‐dependent vasodilation in aged rats. Acta Pharmacol Sin 31, 1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N, Huard J, Clemens PR, Stolz DB, Guttridge DC, Watkins SC, Garinis GA, Wang Y, Niedernhofer LJ & Robbins PD (2012). NF‐kappaB inhibition delays DNA damage‐induced senescence and aging in mice. J Clin Invest 122, 2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N (2012). Age‐related changes in endothelial function and blood flow regulation. Pharmacol Ther 133, 159–176. [DOI] [PubMed] [Google Scholar]
- Toniolo A, Warden EA, Nassi A, Cignarella A & Bolego C (2013). Regulation of SIRT1 in vascular smooth muscle cells from streptozotocin‐diabetic rats. PLoS One 8, e65666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triñanes J, Salido E, Fernández J, Rufino M, González‐Posada JM, Torres A & Hernández D (2012). Type 1 diabetes increases the expression of proinflammatory cytokines and adhesion molecules in the artery wall of candidate patients for kidney transplantation. Diabetes Care 35, 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey‐Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG & Csiszar A (2011). Age‐associated vascular oxidative stress, Nrf2 dysfunction, and NF‐кB activation in the nonhuman primate Macaca mulatta . J Gerontol A Biol Sci Med Sci 66, 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A & Kaley G (2004). Vascular inflammation in aging. Herz 29, 733–740. [DOI] [PubMed] [Google Scholar]
- van Bussel BC, Henry RM, Ferreira I, van Greevenbroek MM, van der Kallen CJ, Twisk JW, Feskens EJ, Schalkwijk CG & Stehouwer CD (2015). A healthy diet is associated with less endothelial dysfunction and less low‐grade inflammation over a 7‐year period in adults at risk of cardiovascular disease. J Nutr 145, 532–540. [DOI] [PubMed] [Google Scholar]
- van Slotten TT, Henry RM, Dekker JM, Nijpels G, Unger T, Schram MT & Stehouwer CD (2014). Endothelial dysfunction plays a key role in increasing cardiovascular risk in type 2 diabetes: the Hoorn study. Hypertension 64, 1299–1305. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM & Tang EH (2008). Endothelium‐dependent contractions: when a good guy turns bad! J Physiol 586, 5295–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessières E, Guihot AL, Toutain B, Maquigneau M, Fassot C, Loufrani L & Henrion D (2013). COX‐2‐derived prostanoids and oxidative stress additionally reduce endothelium‐mediated relaxation in old type 2 diabetic rats. PLoS One 8, e68217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Giannarelli C & Taddei S. (2010). Endothelial dysfunction and vascular disease in later life. Maturitas 67, 20–24. [DOI] [PubMed] [Google Scholar]
- Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C & Tataranni PA (2002). High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51, 455–461. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Slocum SL, Skoko JJ, Shin S & Kensler TW (2010). When NRF2 talks, who's listening? Antioxid Redox Signal 13, 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE, Kaplon RE, Pierce GL, Nowlan MJ & Seals DR (2014). Prevention of age‐related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor κB. Clin Sci (Lond) 127, 645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Huang YL, Shih YY, Wu HY, Peng CT & Lo WY (2014. a). MicroRNA‐146a decreases high glucose/thrombin‐induced endothelial inflammation by inhibiting NADPH oxidase 4 expression. Mediators Inflamm 2014, 376537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R & Lakatta EG (2007). Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50, 219–227. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bozack SN, Yan Y, Boulton ME, Grant MB & Busik JV (2014. b). Regulation of retinal inflammation by ryhthmic expression of miR‐146a in diabetic retina. Invest Ophthalmol Vis Sci 55, 3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L & Sun J (2014. c). Sulforaphane attenuation of type 2 diabetes‐induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev 2014, 123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Zernecke A & Libby P (2008). The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol 8, 802–815. [DOI] [PubMed] [Google Scholar]
- Wen W, Luo R, Tang X, Tang L, Huang HX, Wen X, Hu S & Peng B (2015). Age‐related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis 238, 147–152. [DOI] [PubMed] [Google Scholar]
- Winnik S, Stein S & Matter CM (2012). SIRT1 – an anti‐inflammatory pathway at the crossroads between metabolic diseases and atherosclerosis. Curr Vasc Pharmacol 10, 693–696. [DOI] [PubMed] [Google Scholar]
- Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L & Lemmer B (2002). Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto‐Kakizaki rats: influence of age and diabetic state. Nitric Oxide 6, 85–95. [DOI] [PubMed] [Google Scholar]
- Wong E, Backholer K, Gearon E, Harding J, Freak‐Poli R, Stevenson C & Peeters A (2013). Diabetes of risk of physical disability in adults: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 1, 106–114. [DOI] [PubMed] [Google Scholar]
- Wu J, Xia S, Kalionis B, Wan W & Sun T (2014). The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res Int 2014, 615312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Shen G, Chen C, Gelinas C & Kong AN (2005). Suppression of NF‐κB and NF‐κB‐regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC‐3 cells. Oncogene 24, 4486–4495. [DOI] [PubMed] [Google Scholar]
- Xu J & Zou MH (2009). Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 120, 1266–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Park Y, Zhang H, Gao X, Wilson E, Zimmer W, Abbott L & Zhang C (2009. a). Role of MCP‐1 in tumor necrosis factor‐α‐induced endothelial dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 297, H1208–H1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC & Zhang C (2009. b). Feed‐forward signaling of TNF‐α and NF‐кB via IKK‐β pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296, H1850–H1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA & Mayo MW (2004). Modulation of NF‐κB‐dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23, 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC & Zhang C (2009). Role of TNF‐α in vascular dysfunction. Clin Sci (Lond) 116, 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, He S, Guo S, Xie W, Xin R, Yu H, Yang F, Qiu J, Zhang D, Zhou S & Zhang K (2014) Down‐regulation of mir‐34a alleviates mesangial proliferation in vitro and glomerular hypertrphy in early diabetic nephropathy mice by targeting GAS1. J Diabetes Complications 28, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Bai Y, Ye P, Luo I, Xiao W, Wu H & Liu D (2011). Type 2 diabetes is associated with increased pulse wave velocity measured at different sites of the arterial system but not augmentation index in a Chinese population. Clin Cardiol 34, 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP & Chung HY (2006). Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci 61, 232–244. [DOI] [PubMed] [Google Scholar]


