Abstract
The ability to sustain a given absolute submaximal workload declines with advancing age, likely to be due to a lower level of blood flow and O2 delivery to the exercising muscles. Given that physical inactivity mimics many of the physiological changes associated with ageing, separating the physiological consequences of ageing and physical inactivity can be challenging; yet, observations from cross‐sectional and longitudinal studies on the effects of physical activity have provided some insight. Physical activity has the potential to offset the age‐related decline in blood flow to contracting skeletal muscle during exercise where systemic blood flow is not limited by cardiac output, thereby improving O2 delivery and allowing for an enhanced energy production from oxidative metabolism. The mechanisms underlying the increase in blood flow with regular physical activity include improved endothelial function and the ability for functional sympatholysis – an attenuation of the vasoconstrictor effect of sympathetic nervous activity. These vascular adaptations with physical activity are likely to be an effect of improved nitric oxide and ATP signalling. Collectively, precise matching of blood flow and O2 delivery to meet the O2 demand of the active skeletal muscle of aged individuals during conditions where systemic blood flow is not limited by cardiac output seems to a large extent to be related to the level of physical activity.
Introduction
Blood flow through skeletal muscle at rest and during contraction, and the mechanisms underlying this regulation, have been subjects of great interest since the early experiments by Sadler in 1869 and Gaskell in 1877 demonstrating an increase in blood flow in response to skeletal muscle contraction (Sadler, 1869; Gaskell, 1877). Oxidative metabolism is the dominant source of energy for skeletal muscle, and blood flow and O2 delivery to skeletal muscle are closely related to workload and the O2 demand of the contracting muscles (Andersen & Saltin, 1985; Gonzalez‐Alonso et al. 2002, 2008; Mortensen et al. 2008). Furthermore, during knee‐extensor exercise, perfusion of skeletal muscle can increase from resting values of ∼4 to ∼250 ml min−1 (100 g)−1 in sedentary humans and ∼400 ml min−1 (100 g)−1 in endurance‐trained individuals (Andersen & Saltin, 1985; Richardson et al. 1993; Saltin, 2007). This precise matching of blood flow and metabolism and enormous vasodilator capacity is essential for physical performance as it ensures that any increase in muscle work is precisely matched by increases in O2 delivery.
An association between ageing and reduced blood flow to the exercising lower limb was first documented in 1974 (Wahren et al. 1974), and later studies have confirmed a reduction in blood flow and O2 delivery to the exercising upper and lower extremity in aged subjects (Proctor et al. 1998 b; Lawrenson et al. 2003; Poole et al. 2003; Kirby et al. 2012; Nyberg et al. 2012 a). The mechanisms underlying the reduction in exercise hyperaemia in ageing have not been resolved but may include structural alterations in the vasculature, reductions in skeletal muscle mass and/or quality, increased skeletal muscle sympathetic neural outflow, and alterations in the balance of locally formed vasodilators and vasoconstrictors (Proctor & Parker, 2006). In addition, maximal cardiac output is decreased with advancing age (Ogawa et al. 1992; Proctor et al. 1998 a; Beere et al. 1999) and this decrement in central capacity is likely to affect peripheral blood flow. Importantly, many biological changes associated with advancing age are due to complex and integrated alterations in physiological systems that are influenced by genetic and life‐style factors. One important lifestyle factor is the level of physical activity, as physical inactivity mimics many of the cardiovascular changes associated with ageing (Saltin et al. 1968; McGuire et al. 2001). Moreover, it has been proposed that being physically active is the default requirement for maintaining health and physiological function throughout the life span (Lazarus & Harridge, 2010). Hence, it may be that the reduced blood flow to the exercising limb with advancing age is not merely an effect of sand running through the hourglass; could the magnitude of exercise hyperaemia be more dependent on the level of physical activity than age?
The close relationship between cardiac output and systemic O2 uptake has been shown to be unaffected by ageing and training status (Ogawa et al. 1992; Proctor et al. 1998 a). Thus, as an effect of the decrease in maximal cardiac output with advancing age (Ogawa et al. 1992; Proctor et al. 1998 a; Beere et al. 1999), peripheral blood flow during intense exercise engaging a large muscle mass is expected to be compromised in even well‐trained older individuals. This central limitation to skeletal muscle blood flow in ageing is important to bear in mind for whole body performance. The focus of this review is, however, the control of blood flow and O2 delivery to contracting skeletal muscle in ageing during exercise where systemic blood flow is not limited by cardiac output.
Role of physical activity on the hyperaemic response to exercise in aged individuals
In a recent study from our lab, the role of lifelong physical activity on peripheral haemodynamic and metabolic responses to exercise was addressed (Nyberg et al. 2012 a; Mortensen et al. 2012 b) (Fig. 1). Here it was shown that a lifelong sedentary lifestyle was associated with a reduced leg blood flow and O2 uptake during submaximal knee‐extensor exercise compared to young sedentary subjects. In parallel with the reduced O2 uptake, release of lactate from the leg was increased in the older sedentary subjects, indicating that anaerobic metabolism was increased to compensate for the lower oxidative metabolism. In the lifelong physically active older subjects, blood flow was not different from the older sedentary group; however, due to a higher O2 extraction, O2 uptake of the leg was maintained in the active older subjects so that it was not different from that of young sedentary subjects. Exercise training has been shown to lower blood flow to the exercising leg in both young and middle‐aged subjects (Kiens et al. 1993; Proctor et al. 2001; Nyberg et al. 2012 b), which is thought to be due to training adaptations within skeletal muscle such as increased capillarization that results in an optimized blood flow distribution and conditions for O2 diffusion (Saltin et al. 1976; Kalliokoski et al. 2001; Proctor et al. 2001). These training‐induced adaptations are in line with the higher O2 extraction in the physically active subjects. Notably, impaired mitochondrial and contractile efficiency has been documented in the human quadriceps muscle of aged subjects (Conley et al. 2013; Layec et al. 2015) and a higher oxidative cost of cycling has also been reported (Conley et al. 2013). The similar O2 uptake in the young sedentary and lifelong physically active subjects could, therefore, indicate that O2 delivery was inadequate; however, the contribution from anaerobic metabolism appeared to be similar, as evidenced by uptake rather than release of lactate by the exercising leg of the older active subjects. This suggests that well‐trained aged individuals have a similar cost of contraction as young, which could be explained by a training‐induced improvement in mechanical efficiency (Hopker et al. 2013) that counteracts the age‐related decline in mitochondrial and contractile efficiency (Conley et al. 2013; Layec et al. 2015). This effect of lifelong physical activity may be related to fibre type distribution, as the vastus lateralis muscle of these subject consisted of ∼75% type I fibres (M. Nyberg, S. P. Mortensen and Y. Hellsten, unpublished observation), which have been suggested to be more efficient than type II fibres (Coyle et al. 1992; Krustrup et al. 2004; Krustrup et al. 2008).
Figure 1. Femoral arterial blood flow (A), leg a‐vO2 difference (B), leg O2 uptake (C) and leg lactate release (D) in young sedentary, older lifelong sedentary and older lifelong physically active subjects during submaximal knee‐extensor exercise performed at the same absolute workload .
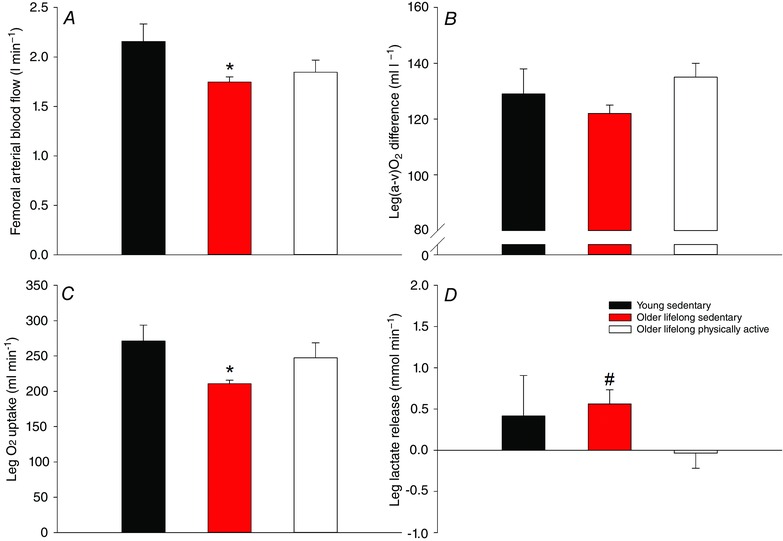
Adapted from Nyberg et al. (2012 a). Significant difference from young sedentary: *P < 0.05; significant difference from resting conditions: # P < 0.05.
In a study by Proctor and co‐workers, it was shown that blood flow to the legs during cycling exercise at the same absolute workload was similar between young and older subjects who were recreationally active (Proctor et al. 2003). In a more recent study, measurements of skeletal muscle blood flow in the knee‐extensor muscles with the use of positron emission tomography revealed that blood flow per unit of muscle mass was higher in moderately active older subjects compared to young subjects matched for physical activity (Rudroff et al. 2014). When interpreting these findings two factors need to be taken into account. Firstly, ageing is associated with a decline in haemoglobin levels and arterial O2 content (Ershler et al. 2005), as also reported in the study by Proctor and co‐workers (Proctor et al. 2003). Secondly, as mentioned above, a greater metabolic cost of contraction with ageing has also been shown. These haemodynamic and metabolic changes associated with aging suggest that blood flow would need to be higher to compensate for the lower arterial O2 content and also in order to meet the increased metabolic cost of contraction. To what extent the higher blood flow in the study by Rudroff and co‐workers (Rudroff et al. 2014) was sufficient to deliver an amount of O2 that matched the O2 demand is uncertain as skeletal muscle O2 uptake was not reported. Furthermore, although the precise O2 uptake of the leg was not reported in the subjects performing cycling exercise (Proctor et al. 2003), blood flow and a‐vO2 values seem to indicate a higher O2 uptake of the leg in the aged subjects at the same absolute workload. Whether this was sufficient to meet the O2 demand is unclear, but quantification of both oxidative and anaerobic metabolism could have provided more insight into this.
In agreement with the cross‐sectional studies showing an association between physical activity level and the magnitude of blood flow, a 3 month period of exercise training increased leg blood flow and O2 uptake during submaximal cycling exercise in older previously sedentary subjects (Beere et al. 1999). This increase in the haemodynamic and metabolic response to exercise was evident despite no differences in these variables being detected between the young and older subjects before training. These findings are in agreement with an age‐related decline in mitochondrial and contractile efficiency with advancing age (Conley et al. 2013; Layec et al. 2015) as it suggest that the increase in O2 uptake of the leg with exercise training in the older subjects was a consequence of a compromised oxidative metabolism before training due to insufficient O2 delivery and potentially reduced mitochondrial function (Joseph et al. 2012).
Effect of physical activity on endothelial function in ageing: implications for exercise hyperaemia
One hallmark of ageing is the development of decreased endothelial function as evidenced by a lowered vasodilator response to arterial infusion of the endothelium‐dependent vasodilator ACh along with an unaltered responsiveness to the endothelium‐independent vasodilator sodium nitroprusside (Taddei et al. 1997; Mortensen et al. 2012 b). Physical activity has the potential to improve endothelial function, and in older animals and humans exercise training has been shown to increase the vasodilator response to ACh (Taddei et al. 2000; Trott et al. 2009; Mortensen et al. 2012 b) (Fig. 2). This effect of physical activity was in part suggested to be a result of an increased nitric oxide (NO) bioavailability in the trained state (Taddei et al. 2000; Trott et al. 2009). In line with this finding, lifelong physical activity was shown to prevent a reduction in arterial and skeletal muscle NO bioavailability (Nyberg et al. 2012 a). NO is important for vascular tone at rest and during recovery from exercise in both the leg (Radegran & Saltin, 1999; Heinonen et al. 2011) and the forearm (Vallance et al. 1989; Panza et al. 1993; Gilligan et al. 1994), and inhibition of NO synthesis during forearm exercise reduces blood flow (Schrage et al. 2004, 2007). However, NO does not appear to be obligatory for exercise hyperaemia in the exercising leg (Frandsen et al. 1996; Radegran & Saltin, 1999; Bradley et al. 1999; Kingwell et al. 2002; Schrage et al. 2010; Heinonen et al. 2011). Although NO may not be essential for exercise hyperaemia in the leg, the finding that simultaneous inhibition of NO and prostanoid (Mortensen et al. 2007) formation reduces blood flow to the exercising leg suggests a redundancy between these two systems where a compensatory formation of one vasodilator ensures that adequate blood flow is achieved when the function of the other is reduced. Accordingly, the function of the prostanoid system appears to decline with advancing age (Schrage et al. 2007; Barnes et al. 2012), indicating that the reduced blood flow to the exercising leg could be a result of impairments in both the NO and the prostanoid systems. In this scenario, improved NO signalling would be likely to increase blood flow and O2 delivery to the contracting skeletal muscles and this mechanism of action could, at least in part, explain an improved perfusion of active skeletal muscle with physical exercise training.
Figure 2. Association between age and ACh‐induced change in leg vascular conductance in sedentary and active healthy subjects .
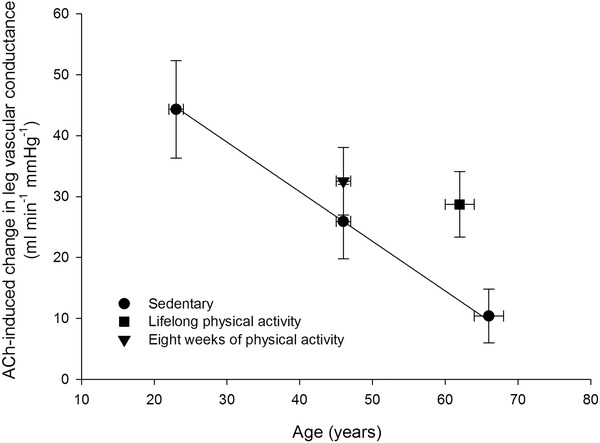
ACh was infused at a rate of 100 μg min−1 (kg leg mass)−1. Adapted from Nyberg et al. (2012 b) and Mortensen et al. (2012 b).
Functional sympatholysis, physical activity and ageing
During exercise, sympathetic nervous activity increases (Alam & Smirk, 1937; Seals & Victor, 1991) in both resting and contracting skeletal muscle (Hansen et al. 1994; Ray & Mark, 1995; Strange, 1999). In inactive tissues, the increase in sympathetic drive during exercise causes vasoconstriction (Bevegard & Shepherd, 1966; Rowell, 1993). However, in young healthy individuals the vasoconstrictor effect of an increase in sympathetic nervous activity or pharmacologically induced noradrenaline release can be attenuated or even abolished in active skeletal muscle (Hansen et al. 1996; Tschakovsky et al. 2002; Rosenmeier et al. 2004; Mortensen et al. 2012 a), termed functional sympatholysis (Remensnyder et al. 1962). Data obtained from both longitudinal (Mortensen et al. 2012 a, 2014; Jendzjowsky & DeLorey, 2013) and cross‐sectional (Mortensen et al. 2012 b) studies have provided strong evidence that the ability for functional sympatholysis is related to the training status of the skeletal muscle. This effect of physical activity appears to be independent of age as the vasoconstriction caused by pharmacologically induced noradrenaline release from sympathetic nerves during exercise has been shown to be abolished in lifelong physically active subjects (Mortensen et al. 2012 b) (Fig. 3). Since functional sympatholysis is thought to allow for adequate perfusion and O2 delivery to the contracting fibres (Saltin & Mortensen, 2012), improved ability for functional sympatholysis may be an important mechanism underlying the effect of physical activity on the precise matching of blood flow and O2 delivery to oxidative metabolism in aged individuals.
Figure 3. Tyramine‐induced change in femoral arterial blood flow during submaximal knee‐extensor exercise in sedentary and active healthy young (∼20 years) and older subjects (∼65 years) .
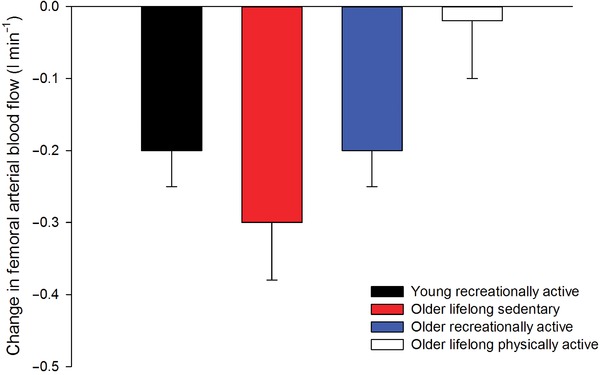
Adapted from Mortensen et al. (2012 b) and unpublished observations by M. Nyberg, S. P. Mortensen and Y. Hellsten.
The mechanisms underlying the effect of physical activity on functional sympatholysis are still largely unresolved, but several compounds have been suggested to play a role. It has been suggested that NO mediates functional sympatholysis in rat skeletal muscle (Thomas & Victor, 1998), and data from rodents also indicate that functional sympatholysis is augmented through a NO‐dependent mechanism (Jendzjowsky & DeLorey, 2013). In humans, NO has also been suggested to be important for functional sympatholysis in the forearm (Sander et al. 2000; Chavoshan et al. 2002). Ageing has been proposed to be associated with increased levels of reactive oxygen species (ROS) that scavenge NO, thereby decreasing its bioavailability (Taddei et al. 2001). Interestingly, oxidative stress impairs functional sympatholysis in skeletal muscle of rat hindlimb and human forearm (Fadel et al. 2012), and as exercise training effectively up‐regulates antioxidant systems in both blood and skeletal muscle (Gomez‐Cabrera et al. 2008; Gliemann et al. 2013 a,2013 b; Nyberg et al. 2012 a, 2014), which allows for a greater removal of ROS, this adaptation to physical activity may contribute to the improved functional sympatholysis in skeletal muscle. Despite these findings in support of a role of NO, infusion of an NO donor does not blunt sympathetic vasoconstriction in the forearm of young men (Rosenmeier et al. 2003) and increasing NO availability in older men with impaired functional sympatholysis does not increase leg exercise hyperaemia (Nyberg et al. 2012 a; Mortensen et al. 2012 b). Hence, although improved ROS handling and NO availability in aged individuals are attractive candidates in the search for mechanisms involved in the improved ability for functional sympatholysis in aged individuals with physical activity, more evidence is needed to confirm these mechanisms.
ATP increases in the plasma of the arterial inflow and venous drainage of active skeletal muscle (Gonzalez‐Alonso et al. 2002; Mortensen et al. 2011), and when infused, ATP can significantly blunt sympathetic α‐adrenergic vasoconstriction in both young (Rosenmeier et al. 2004; Kirby et al. 2008) and older subjects (Mortensen et al. 2012 b). One important source of intravascular ATP is thought to be the erythrocyte (Sprague & Ellsworth, 2012), and an attenuated release of ATP from erythrocytes and local vasodilatation have been demonstrated in older sedentary subjects (Kirby et al. 2012). Hence, an improved release of ATP from erythrocytes may be one mechanism by which functional sympatholysis is improved in aged individuals with physical activity; however, as with NO, direct evidence is needed to support this role of ATP in humans.
Future directions
Ageing has consistently been shown to be associated with a reduced blood flow to the exercising limb. However, when accounting for physical activity level, it appears that physical activity has the potential to offset the age‐related decline in blood flow during conditions where systemic blood flow is not limited by cardiac output. More importantly, precise matching of blood flow and O2 delivery to meet the O2 demand of the active skeletal muscle of aged individuals seems to a large extent to be related to the level of physical activity (see Fig. 4). An important physiological aspect to consider is the age‐related decline in arterial O2 content and mechanical efficiency. Hence, future studies on the effects of physical activity should focus less on blood flow and more on whether the O2 delivery is sufficient to meet the O2 demand of the exercising muscles. Accordingly, methods that accurately quantify both oxidative and anaerobic metabolism will provide valuable insight into whether regular physical activity in aged individuals will allow for adequate O2 delivery to meet the O2 demand of the contracting skeletal muscle fibres. Furthermore, interventions that increase or decrease O2 delivery to contracting skeletal muscle in older individuals will also be very useful for establishing the extent to which O2 delivery is limiting oxidative metabolism in skeletal muscle.
Figure 4.
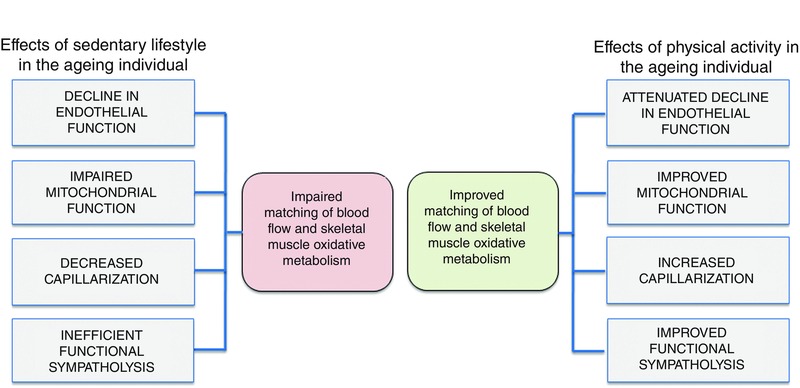
Observed effects of a sedentary and physically active lifestyle on the matching of blood flow and oxidative metabolism in active skeletal muscle of ageing individuals
Additional information
Competing interests
None declared.
Funding
This study was supported by a grant from The Danish Council for Independent Research and the Lundbeck Foundation.
Biographies
Michael Nyberg is a postdoctoral research fellow working within the cardiovascular group at the department of Nutrition, Exercise and Sports, University of Copenhagen where he also earned his PhD.

Professor Ylva Hellsten is the head of the cardiovascular group at the department of Nutrition, Exercise and Sports, University of Copenhagen. Their research investigates the regulation of blood flow to exercising skeletal muscle and how this regulation is altered in ageing and in disease states.
References
- Alam M & Smirk FH (1937). Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P & Saltin B (1985). Maximal perfusion of skeletal muscle in man. J Physiol 366, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JN, Schmidt JE, Nicholson WT & Joyner MJ (2012). Cyclooxygenase inhibition abolishes age‐related differences in cerebral vasodilator responses to hypercapnia. J Appl Physiol 112, 1884–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW & Higginbotham MB (1999). Aerobic exercise training can reverse age‐related peripheral circulatory changes in healthy older men. Circulation 100, 1085–1094. [DOI] [PubMed] [Google Scholar]
- Bevegard BS & Shepherd JT (1966). Reaction in man of resistance and capacity vessels in forearm and hand to leg exercise. J Appl Physiol 21, 123–132. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Kingwell BA & McConell GK (1999). Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48, 1815–1821. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG & Thomas GD (2002). Nitric oxide‐dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Cress ME & Esselman P (2013). Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp Physiol 98, 768–777. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF & Beltz JD (1992). Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc 24, 782–788. [PubMed] [Google Scholar]
- Ershler WB, Sheng S, McKelvey J, Artz AS, Denduluri N, Tecson J, Taub DD, Brant LJ, Ferrucci L & Longo DL (2005). Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc 53, 1360–1365. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Farias IM, Gallagher KM, Wang Z & Thomas GD (2012). Oxidative stress and enhanced sympathetic vasoconstriction in contracting muscles of nitrate‐tolerant rats and humans. J Physiol 590, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen U, Lopez‐Figueroa M & Hellsten Y (1996). Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun 227, 88–93. [DOI] [PubMed] [Google Scholar]
- Gaskell WH (1877). Changes of the blood‐stream in muscles through stimulation of their nerves. J Anat Physiol 11, 360–402. [PMC free article] [PubMed] [Google Scholar]
- Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR & Quyyumi AA (1994). Contribution of endothelium‐derived nitric oxide to exercise‐induced vasodilation. Circulation 90, 2853–2858. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Nyberg M & Hellsten Y (2013. a). Nitric oxide and reactive oxygen species in limb vascular function: what is the effect of physical activity? Free Radic Res 48, 71–83. [DOI] [PubMed] [Google Scholar]
- Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard H & Hellsten Y (2013. b). Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol 591, 5047–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Cabrera MC, Domenech E & Vina J (2008). Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med 44, 126–131. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA & Dufour SP (2008). Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586, 2405–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J, Olsen DB & Saltin B (2002). Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ & Victor RG (1996). Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest 98, 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Jacobsen TN & Victor RG (1994). Muscle metaboreflex triggers parallel sympathetic activation in exercising and resting human skeletal muscle. Am J Physiol Heart Circ Physiol 266, H2508–H2514. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K & Hellsten Y (2011). Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol 300, H1510–H1517. [DOI] [PubMed] [Google Scholar]
- Hopker JG, Coleman DA, Gregson HC, Jobson SA, Von der Haar T, Wiles J & Passfield L (2013). The influence of training status, age, and muscle fiber type on cycling efficiency and endurance performance. J Appl Physiol 115, 723–729. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG & DeLorey DS (2013). Short‐term exercise training enhances functional sympatholysis through a nitric oxide‐dependent mechanism. J Physiol 591, 1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E & Leeuwenburgh C (2012). The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high‐ and low‐functioning elderly individuals. Aging Cell 11, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski KK, Oikonen V, Takala TO, Sipila H, Knuuti J & Nuutila P (2001). Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance‐trained men. Am J Physiol Endocrinol Metab 280, E1015–E1021. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen‐Gustavsson B, Christensen NJ & Saltin B (1993). Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol 469, 459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell BA, Formosa M, Muhlmann M, Bradley SJ & McConell GK (2002). Nitric oxide synthase inhibition reduces glucose uptake during exercise in individuals with type 2 diabetes more than in control subjects. Diabetes 51, 2572–2580. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF & Dinenno FA (2012). Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE & Dinenno FA (2008). Graded sympatholytic effect of exogenous ATP on postjunctional α‐adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586, 4305–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Secher NH, Relu MU, Hellsten Y, Soderlund K & Bangsbo J (2008). Neuromuscular blockade of slow twitch muscle fibres elevates muscle oxygen uptake and energy turnover during submaximal exercise in humans. J Physiol 586, 6037–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M & Bangsbo J (2004). Slow‐twitch fiber glycogen depletion elevates moderate‐exercise fast‐twitch fiber activity and O2 uptake. Med Sci Sports Exerc 36, 973–982. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P & Richardson RS (2003). Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285, H1023–H1031. [DOI] [PubMed] [Google Scholar]
- Layec G, Hart CR, Trinity JD, Le FY, Jeong EK & Richardson RS (2015). Skeletal muscle work efficiency with age: the role of non‐contractile processes. Clin Sci (Lond) 128, 213–223. [DOI] [PubMed] [Google Scholar]
- Lazarus NR & Harridge SD (2010). Exercise, physiological function, and the selection of participants for aging research. J Gerontol A Biol Sci Med Sci 65, 854–857. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B & Mitchell JH (2001). A 30‐year follow‐up of the Dallas Bedrest and Training Study: I. Effect of age on the cardiovascular response to exercise. Circulation 104, 1350–1357. [PubMed] [Google Scholar]
- Mortensen SP, Damsgaard R, Dawson EA, Secher NH & Gonzalez‐Alonso J (2008). Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high‐intensity whole‐body exercise in humans. J Physiol 586, 2621–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Gonzalez‐Alonso J, Damsgaard R, Saltin B & Hellsten Y (2007). Inhibition of nitric oxide and prostaglandins, but not endothelial‐derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Morkeberg J, Thaning P, Hellsten Y & Saltin B (2012. a). Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol 302, H2074–H2082. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Gliemann L, Thaning P, Saltin B & Hellsten Y (2014). Exercise training modulates functional sympatholysis and α‐adrenergic vasoconstrictor responsiveness in hypertensive and normotensive individuals. J Physiol 592, 3063–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K & Saltin B (2012. b). Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590, 6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Thaning P, Nyberg M, Saltin B & Hellsten Y (2011). Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y & Mortensen SP (2012. a). Lifelong physical activity prevents an age‐related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590, 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y & Mortensen SP (2012. b). Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high‐intensity aerobic training. J Physiol 590, 1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Cabo H, Gomez‐Cabrera MC, Vina J & Hellsten Y (2014). Roles of sedentary aging and lifelong physical activity in exchange of glutathione across exercising human skeletal muscle. Free Radic Biol Med 73C, 166–173. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH III, Kohrt WM, Schechtman KB, Holloszy JO & Ehsani AA (1992). Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86, 494–503. [DOI] [PubMed] [Google Scholar]
- Panza JA, Casino PR, Badar DM & Quyyumi AA (1993). Effect of increased availability of endothelium‐derived nitric oxide precursor on endothelium‐dependent vascular relaxation in normal subjects and in patients with essential hypertension. Circulation 87, 1475–1481. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C & Richardson RS (2003). Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284, H1251–H1259. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR & Joyner MJ (1998. a). Influence of age and gender on cardiac output‐VO2 relationships during submaximal cycle ergometry. J Appl Physiol 84, 599–605. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Miller JD, Dietz NM, Minson CT & Joyner MJ (2001). Reduced submaximal leg blood flow after high‐intensity aerobic training. J Appl Physiol 91, 2619–2627. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA & Leuenberger UA (2003). Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol 94, 1859–1869. [DOI] [PubMed] [Google Scholar]
- Proctor DN & Parker BA (2006). Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13, 315–327. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL & Joyner MJ (1998. b). Reduced leg blood flow during dynamic exercise in older endurance‐trained men. J Appl Physiol 85, 68–75. [DOI] [PubMed] [Google Scholar]
- Radegran G & Saltin B (1999). Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276, H1951–H1960. [DOI] [PubMed] [Google Scholar]
- Ray CA & Mark AL (1995). Sympathetic nerve activity to nonactive muscle of the exercising and nonexercising limb. Med Sci Sports Exerc 27, 183–187. [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH & Arnoff SJ (1962). Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11, 370–380. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Fritzlar SJ, Dinenno FA & Joyner MJ (2003). Exogenous NO administration and α‐adrenergic vasoconstriction in human limbs. J Appl Physiol 95, 2370–2374. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J & Gonzalez‐Alonso J (2004). Circulating ATP‐induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB (1993). Human Cardiovascular Control Oxford University Press, New York. [Google Scholar]
- Rudroff T, Weissman JA, Bucci M, Seppanen M, Kaskinoro K, Heinonen I & Kalliokoski KK (2014). Positron emission tomography detects greater blood flow and less blood flow heterogeneity in the exercising skeletal muscles of old compared with young men during fatiguing contractions. J Physiol 592, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler W (1869). Arbeiten aus der phys, 12th edn. Anstalt Zu, Leipzig. [Google Scholar]
- Saltin B (2007). Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol 583, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K & Chapman CB (1968). Response to exercise after bed rest and after training. Circulation 38, VII1–VII78. [PubMed] [Google Scholar]
- Saltin B & Mortensen SP (2012). Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol 590, 6269–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B & Gollnick D (1976). The nature of the training response; peripheral and central adaptations of one‐legged exercise. Acta Physiol Scand 96, 289–305. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD & Victor RG (2000). Functional muscle ischemia in neuronal nitric oxide synthase‐deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 97, 13818–13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH & Joyner MJ (2007). Ageing reduces nitric‐oxide‐ and prostaglandin‐mediated vasodilatation in exercising humans. J Physiol 579, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ & Dinenno FA (2004). Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Wilkins BW, Johnson CP, Eisenach JH, Limberg JK, Dietz NM, Curry TB & Joyner MJ (2010). Roles of nitric oxide synthase and cyclooxygenase in leg vasodilation and oxygen consumption during prolonged low‐intensity exercise in untrained humans. J Appl Physiol 109, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR & Victor RG (1991). Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev 19, 313–349. [PubMed] [Google Scholar]
- Sprague RS & Ellsworth ML (2012). Erythrocyte‐derived ATP and perfusion distribution: role of intracellular and intercellular communication. Microcirculation 19, 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S (1999). Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol 514, 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C & Salvetti A (2000). Physical activity prevents age‐related impairment in nitric oxide availability in elderly athletes. Circulation 101, 2896–2901. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A & Salvetti A (2001). Age‐related reduction of NO availability and oxidative stress in humans. Hypertension 38, 274–279. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I & Salvetti A (1997). Hypertension causes premature aging of endothelial function in humans. Hypertension 29, 736–743. [DOI] [PubMed] [Google Scholar]
- Thomas GD & Victor RG (1998). Nitric oxide mediates contraction‐induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Gunduz F, Laughlin MH & Woodman CR (2009). Exercise training reverses age‐related decrements in endothelium‐dependent dilation in skeletal muscle feed arteries. J Appl Physiol 106, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z & Joyner MJ (2002). Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Collier J & Moncada S (1989). Effects of endothelium‐derived nitric oxide on peripheral arteriolar tone in man. Lancet 2, 997–1000. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L & Pernow B (1974). Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33, 79–86. [DOI] [PubMed] [Google Scholar]


