Abstract
Key points
The dilatory role for sensory innervation of mesenteric arteries (MAs) is impaired in Old (∼24 months) versus Young (∼4 months) mice. We investigated the nature of this impairment in isolated pressurized MAs.
With perivascular sensory nerve stimulation, dilatation and inhibition of sympathetic vasoconstriction observed in Young MAs were lost in Old MAs along with impaired dilatation to calcitonin gene‐related peptide (CGRP).
Inhibiting NO and prostaglandin synthesis increased CGRP EC50 in Young and Old MAs. Endothelial denudation attenuated dilatation to CGRP in Old MAs yet enhanced dilatation to CGRP in Young MAs while abolishing all dilatations to ACh.
In Old MAs, sensory nerve density was reduced and RAMP1 (CGRP receptor component) associated with nuclear regions of endothelial cells in a manner not seen in Young MAs or in smooth muscle cells of either age.
With advanced age, loss of dilatory signalling mediated through perivascular sensory nerves may compromise perfusion of visceral organs.
Abstract
Vascular dysfunction and sympathetic nerve activity increase with advancing age. In the gut, blood flow is governed by perivascular sensory and sympathetic nerves but little is known of how their functional role is affected by advanced age. We tested the hypothesis that functional sensory innervation of mesenteric arteries (MAs) is impaired for Old (24 months) versus Young (4 months) C57BL/6 male mice. In cannulated pressurized MAs preconstricted 50% with noradrenaline and treated with guanethidine (to inhibit sympathetic neurotransmission), perivascular nerve stimulation (PNS) evoked dilatation in Young but not Old MAs while dilatations to ACh were not different between age groups. In Young MAs, capsaicin (to inhibit sensory neurotransmission) blocked dilatation and increased constriction during PNS. With no difference in efficacy, the EC50 of CGRP as a vasodilator was ∼6‐fold greater in Old versus Young MAs. Inhibiting nitric oxide (l‐NAME) and prostaglandin (indomethacin) synthesis increased CGRP EC50 in both age groups. Endothelial denudation reduced the efficacy of dilatation to CGRP by ∼30% in Old MAs yet increased this efficacy ∼15% in Young MAs while all dilatations to ACh were abolished. Immunolabelling revealed reduced density of sensory (CGRP) but not sympathetic (tyrosine hydroxylase) innervation for Old versus Young MAs. Whereas the distribution of CGRP receptor proteins was similar in SMCs, RAMP1 associated with nuclear regions of endothelial cells of Old but not Young MAs. With advanced age, the loss of sensory nerve function and diminished effectiveness of CGRP as a vasodilator is multifaceted and may adversely affect splanchnic perfusion.
Key points
The dilatory role for sensory innervation of mesenteric arteries (MAs) is impaired in Old (∼24 months) versus Young (∼4 months) mice. We investigated the nature of this impairment in isolated pressurized MAs.
With perivascular sensory nerve stimulation, dilatation and inhibition of sympathetic vasoconstriction observed in Young MAs were lost in Old MAs along with impaired dilatation to calcitonin gene‐related peptide (CGRP).
Inhibiting NO and prostaglandin synthesis increased CGRP EC50 in Young and Old MAs. Endothelial denudation attenuated dilatation to CGRP in Old MAs yet enhanced dilatation to CGRP in Young MAs while abolishing all dilatations to ACh.
In Old MAs, sensory nerve density was reduced and RAMP1 (CGRP receptor component) associated with nuclear regions of endothelial cells in a manner not seen in Young MAs or in smooth muscle cells of either age.
With advanced age, loss of dilatory signalling mediated through perivascular sensory nerves may compromise perfusion of visceral organs.
Abbreviations
- ACh
acetylcholine
- CGRP
calcitonin gene‐related peptide
- CRLR
calcitonin receptor‐like receptor
- EC
endothelial cell
- EDH
endothelial dependent hyperpolarization
- ID
internal diameter
- MA
mesenteric artery
- NA
noradrenaline
- NO
nitric oxide
- OD
outer diameter
- PKA
protein kinase A
- PNS
perivascular nerve stimulation
- PSS
physiological salt solution
- PVN
perivascular nerve
- RAMP1
receptor activity modifying protein 1
- ROI
region of interest
- SMC
smooth muscle cell
- SNP
sodium nitroprusside
- TH
tyrosine hydroxylase
- TRP
transient receptor potential
- TTX
tetrodotoxin
Introduction
Ageing leads to changes in physiological regulation throughout the body. Cardiovascular ageing is of para‐mount concern as > 70% of people older than ∼60 years have cardiovascular disease with a corresponding number of deaths in people older than 75 years (Go et al. 2013). Within the vasculature, advancing age is associated with arterial stiffening (Lakatta & Levy, 2003; Izzo & Mitchell, 2007) and endothelial dysfunction (Gates et al. 2009; Seals et al. 2011; Behringer et al. 2013), which both contribute to impaired vasomotor function.
Perivascular nerves (PVNs) are integral to blood flow control in healthy individuals as well as during age‐related changes in vascular function. Arteries of all sizes across vascular beds and species are enmeshed by PVNs (reviewed in Westcott & Segal, 2013 b). Activation of sympathetic PVNs typically produces vasoconstriction with a reduction in tissue blood flow while activation of sensory PVNs evokes vasodilatation with an increase in tissue perfusion (Westcott & Segal, 2013 b). Sensory PVNs are capable of both orthodromic and antidromic signalling, thereby mediating vasodilatation in response to a range of physiological stimuli including changes in temperature, acid–base status, chemical irritation, mechanical stimulation and vasoactive agents (reviewed in Burnstock & Ralevic, 1994). In addition to their effects on vessel diameter and tissue blood flow, PVNs may exert reciprocal negative feedback, with stimulation of either sympathetic or sensory nerves inhibiting neurotransmitter release from the other (Kawasaki et al. 1990; Coffa & Kotecha, 1999; Kawasaki, 2002). Thus age‐related decline in PVN function may be manifested through alterations in the activation of vascular smooth muscle cells (SMCs) and endothelial cells (ECs) and through impaired neuromodulation. Increased sympathetic nerve activity with advancing age is well‐documented (Dinenno et al. 1999; Esler et al. 2002; Seals & Dinenno, 2004). The consequences include greater noradrenaline (NA) release, restricted tissue blood flow and desensitized adrenoreceptors (Buchholz et al. 1998; Dinenno & Joyner, 2006; Proctor & Parker, 2006; Jackson et al. 2010), which may in turn affect the activation of sensory PVNs. However, the effects of ageing on sensory PVNs are poorly understood and are the focus of the present study.
Calcitonin gene‐related peptide (CGRP) is the primary neurotransmitter released from sensory nerves and is a potent vasodilator when activating its receptors on either SMCs or ECs (Brain et al. 1985). The functional CGRP receptor requires three subunits: (1) calcitonin receptor‐like receptor (CRLR) protein, (2) a chaperone known as receptor activity modifying protein (RAMP1) and (3) receptor component protein (McLatchie et al. 1998). Age‐related changes in any of these components may impair sensory nerve function mediated through the actions of CGRP. In SMCs from mesenteric (Nelson et al. 1990), renal (Reslerova & Loutzenhiser, 1998) and other arteries (Brain & Cox, 2006), CGRP promotes relaxation via increasing cAMP, leading to protein kinase A (PKA)‐mediated activation of k + channels. In turn, endothelium‐dependent dilatation to CGRP has been demonstrated in aorta (Gray & Marshall, 1992), coronary and mammary arteries (Raddino et al. 1997), and pulmonary arteries (Wisskirchen et al. 1998), with SMC relaxation typically resulting from the actions of nitric oxide (NO) via cAMP and PKA. Endothelial NO production contributes further to SMC relaxation via cGMP leading to decreased SMC [Ca2+]i (Brain & Grant, 2004). In a complementary role, prostaglandins released through EC cyclooxygenase activity also contribute to vasodilatation through increased production of NO, cAMP and cGMP (Armstead, 1995). With ageing, a decline in dilatation to CGRP occurs in the aorta, where CGRP acts through endothelial production of NO (Gray & Marshall, 1992; Lu et al. 2002). However, the signalling events underlying CGRP‐mediated vasodilatation may vary with the vascular bed, as the vasomotor response can reflect simultaneous activation of multiple signalling pathways in SMCs and ECs.
In mesenteric arteries (MAs) of the anaesthetized mouse, advanced age (e.g. ∼24 versus 4 months; corresponding to mid‐60 s and mid‐20 s, respectively, in humans; Flurkey et al. 2007) was found to enhance sympathetic vasoconstriction in association with a diminished role for sensory nerves (Westcott & Segal, 2013 a). However, the mechanism underlying such changes is unknown. The goal of the present study was to resolve the basis of ageing‐related dysfunction in perivascular sensory nerves. We tested the hypothesis that advanced age leads to changes in both the functional anatomy of sensory nerves (i.e. innervation density) and their downstream actions on receptor activation and vasomotor function. Our new findings illustrate that advanced age is associated with both structural and functional changes in perivascular sensory nerves of MAs. In contrast to Young mice, MAs from Old mice failed to dilate in response to electrical stimulation of sensory PVNs and were desensitized to exogenous CGRP. Optimal dilatation to CGRP in MAs from Old (but not Young) mice relied on the endothelium irrespective of NO and prostaglandin production. Immunolabelling revealed that relative to Young mice, MAs from Old mice have diminished sensory (but not sympathetic) innervation along with altered RAMP1 localisation within ECs but not SMCs. We suggest that multiple mechanisms contribute to dysfunctional sensory innervation of MAs with advanced age.
Methods
Ethical approval and animal use
All procedures were approved by the Animal Care and Use Committee of the University of Missouri and performed in accord with the Guide for the Care and Use of Laboratory Animals (National Research Council; 8th edn, 2011). Experiments were performed on Young (3–6 months) and Old (24–28 months) C57BL/6 mice bred at colonies maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA, USA). Each experimental protocol included MAs from three to seven mice of respective age groups, with most experiments utilizing at least two vessel segments (studied independently on the same day) per mouse. Experiments on Young and Old mice were randomized across days to avoid any order effect. Mice were anaesthetized using pentobarbital sodium (60 mg kg−1, intraperitoneal injection) and abdominal fur was removed by shaving. Following surgical procedures, the anaesthetized mouse was killed with an overdose of pentobarbital via intracardiac injection followed by cervical dislocation or by exsanguination.
Tissue preparation and vessel dissection
The intact mesentery and small intestine were carefully excised and placed in a dissection chamber maintained at 4°C filled with Ca2+‐free physiological salt solution (PSS) comprised of (in mm): 140 NaCl, 5 KCl, 1 MgCl2, 10 Hepes, 10 glucose (pH 7.4). A loop of intestine was gently spread and pinned onto a block of transparent silicone rubber (Sylgard 184, Dow Corning; Midland, MI, USA) to isolate a mesenteric arterial network. An unbranched second‐order MA was dissected free from surrounding tissue and transferred to a tissue chamber (RC‐27 N; Warner Instruments; Hamden, CT, USA) using a Wiretrol pipette (Drummond Scientific; Broomall, PA, USA). The tissue chamber was at room temperature (∼23°C) and secured within a stage platform containing a heated chamber (PH‐6) and in‐line heater (SH‐27B) controlled by a dual temperature controller (TC‐344B, Warner Instruments). A three‐axis micromanipulator (DT3‐100; Siskiyou Design; Grants Pass, OR, USA) positioned at each end of the platform secured micropipettes for vessel cannulation.
Vessel cannulation
The intact MA was cannulated onto micropipettes pulled (P‐97; Sutter Instruments; Novato, CA, USA) from borosilicate glass capillaries (Warner Instruments; internal diameter (ID) = 0.94 mm; outer diameter (OD) = 1.2 mm) with heat‐polished tips (OD, ∼100 μm) and secured at each end by tying with a single strand isolated from braided 10‐0 silk suture. The stage platform with the cannulated MA was mounted on a microscope (Olympus BX51W1; Olympus America Inc.; Center Valley, PA, USA). During a 30 min equilibration period, the vessel was warmed to 37°C and pressurized to 100 cmH2O (∼75 mmHg). For all functional studies, vessels were superfused continuously at ∼5 ml min−1 with PSS (above) that contained 2 mm CaCl2.
Diameter measurements
For the initial nerve stimulation experiments and CGRP concentration–response curves, vessel images were acquired using a 20× water immersion objective (NA = 0.5) and a charge‐coupled device camera (PTI IC‐100; Photon Technology International; Edison, NJ, USA) coupled to a digital monitor. Internal diameter of each vessel was measured using a video calliper calibrated against a stage micrometer (100 × 0.01 = 1 mm; Graticules Ltd.; Tonbridge, UK). Final magnification on the monitor face was 1300× with spatial resolution of ∼1 μm. For all further experiments, vessel images were acquired using a 10× water immersion objective (NA = 0.3) and a Firewire camera (DMK 21AF04; TheImagingSource; Charlotte, NC, USA) interfaced with a personal computer to measure IDs using custom edge‐tracking software (LabView 2009; National Instruments, Austin, TX, USA) graciously provided by Dr Michael J. Davis at the University of Missouri.
Perivascular nerve stimulation (PNS)
Perivascular nerves were stimulated in an electrical field between two wire electrodes (90% platinum–10% iridium; diameter, 250 μm) connected to a stimulation isolation unit (SIU5, Grass; Quincy, MA, USA) driven by a square wave stimulator (S48, Grass). An electrode was positioned on either side of the cannulated MA and ID was measured > 100 μm away from the site of stimulation. In accord with experiments performed in anaesthetized mice (Westcott & Segal, 2013 a), electrical pulses (30 V, 2 ms) were delivered at 8 Hz to activate PVNs until a stable response diameter was achieved (10–15 s). PNS was repeated in the presence of guanethidine (10 μm) to block sympathetic neurotransmission by depleting NA from synaptic vesicles (Kadzielawa, 1962) and/or capsaicin (10 μm) to block sensory neurotransmission via transmitter depletion (Eid & Cortright, 2009). To confirm that the evoked vasomotor responses were secondary to evoking action potentials along nerve fibres, PNS was also performed in the presence of tetrodotoxin (TTX, 1 μm). Guanethidine, capsaicin and/or TTX were included in the superfusion solution for 20 min prior to evaluating their effects on vasomotor responses to PNS.
Concentration–response curves
All concentration–response experiments were cumulative, with appropriate volumes of concentrated drug solutions added to the 25 ml reservoir of superfusion solution. Final drug concentrations are given as those to which vessels were exposed and were 10 nm to 10 μm for NA, 1 nm to 10 μm for ACh and 0.1 nm to 1 μm for CGRP. A vessel was exposed to each concentration for at least 5 min before recording stable response IDs. For evaluating responses to ACh and CGRP, MAs were first preconstricted using the appropriate EC50 concentration of NA that we determined for Young and Old MAs. To determine the role of endothelium‐derived autacoids in dilatations to CGRP, after the initial concentration–response curve was determined, superfusion with control PSS was resumed until the initial level of tone (in the presence of EC50 NA) was restored, then the CGRP concentration–response relationship was re‐evaluated in the presence of l‐NAME (100 μm) + indomethacin (1 μm) to inhibit nitric oxide (NO) and prostaglandin synthesis, respectively. Vasomotor responses to NA, ACh and CGRP were each studied in separate MAs. Maximum ID for each MA was obtained at the end of every experiment during superfusion with Ca2+‐free PSS containing sodium nitroprusside (SNP, 10 μm).
Endothelial denudation
Second‐order MAs were isolated and positioned onto a tungsten wire (OD, 50 μm; Goodfellow; Huntington, UK). To denude the endothelium, an MA was pulled slowly over the wire three times. The denuded vessel was cannulated and used for evaluating responses to CGRP only if it constricted ∼50% to an EC50 concentration of NA (confirming SMC integrity) and failed to dilate to 1 μm ACh (confirming EC ablation). Constrictions to NA were verified following CGRP exposure to confirm SMC viability throughout the experimental protocol.
Immunofluorescence
Second‐order MAs were isolated as above then transferred to a 12‐well plate coated with Sylgard. For immunolabelling of PVNs and of RAMP1/CRLR in SMCs, MAs were pinned intact. For immunolabelling of RAMP1/CRLR in ECs, MAs were opened longitudinally and secured with the endothelium exposed (i.e. en face) using fine tungsten wire pins (OD = 25 μm; Goodfellow). Respective preparations were fixed in 4% paraformaldehyde for 20 min and washed in phosphate‐buffered saline (PBS, Sigma, cat. no. P5368) Subsequent incubations were performed at 4°C with primary and secondary antibodies diluted in PBS + 0.2% Triton X‐100.
Preparations were incubated for 60 min in PBS with 10% normal goat serum and 0.2% Triton X‐100, then placed overnight in solutions containing the primary antibody against tyrosine hydroxylase (TH; 1:250; cat. no. T2928, Sigma), CGRP (1:250, cat. no. PC205L, EMD Millipore), CRLR (V‐20, 1:250 for ECs and 1:100: for SMCs; cat. no. sc‐18007, Santa Cruz Biotechnology), or RAMP1 (1:250 for ECs and 1:100: for SMCs; cat. no. sc‐11379, Santa Cruz Biotechnology; generated using the full‐length amino acid sequence of the protein to exclude RAMP2/3 isoforms). Preparations were then placed into PBS with 0.2% Triton X‐100 + 10% normal goat serum for 60 min and incubated for 90 min in the respective secondary antibody (Alexa Fluor‐488 or ‐546; Molecular Probes/Life Technologies; Carlsbad, CA, USA) each at 1:500 dilution. After a final wash in PBS, preparations were mounted on slides using ProLong Gold (Invitrogen) and a coverslip. Each experiment included controls that omitted the primary antibody and these consistently demonstrated a lack of non‐specific binding or fluorescence.
Slides were imaged using a Leica SP5 confocal laser‐scanning microscope (Leica Microsystems; Buffalo Grove, IL, USA). Staining for TH and CGRP was visualized using an HCX PL APO 40× oil immersion objective (NA = 1.25; Leica) with a 2× optical zoom and 1 μm Z‐slices. Staining for CRLR and RAMP1 was visualized using an HCX PL APO 60× glycerol immersion objective (NA = 1.3; Leica), a 4× optical zoom in ECs, a 6× optical zoom in SMCs, and 0.5 μm Z‐slices. For all slide sets, Young and Old MAs were imaged using similar laser power and gain settings.
The density of PVNs was determined by quantifying the fluorescence area of TH and CGRP staining on intact MA segments using ImageJ (Abramoff, 2004). To analyse the fluorescence of each antibody, a maximum Z‐stack projection was created in ImageJ through the uppermost half of the vessel wall. This was done by taking the maximum intensity value of each pixel through the series of confocal slices and combining these pixel values into a single image. A region of interest (ROI) was then placed around the entire projection, background fluorescence (from a region located between nerve fibres) was subtracted and the remaining fluorescent pixels were quantified (irrespective of intensity) relative to the total number of pixels in the ROI. This analysis yielded the percent fluorescence area within the ROI for each antibody. Controls confirmed lack of imaging bleed‐through between respective secondary antibodies.
Chemicals and reagents
CGRP was purchased from AnaSpec (Fremont, CA, USA). Guanethidine was purchased from BOC Sciences (New York). All other reagents were purchased from Sigma‐Aldrich (St Louis, MO, USA) unless otherwise stated. Solutions were prepared fresh for each day's experiments. Indomethacin was dissolved in DMSO and diluted to its final concentration in PSS with ≤0.1% DMSO. All solutions were used at pH 7.4.
Data presentation and statistical analysis
Data for PNS experiments and agonist concentration–response curves are presented as percent constriction or dilatation from baseline ID, calculated as follows: % Constriction = [(IDbase – IDresp) / IDbase] × 100 (thus 100% indicates closure of the vessel lumen); %Max (maximal) Dilatation = [(IDresp – IDbase) / (IDmax – IDbase] × 100, where IDbase = baseline ID under control conditions, IDresp = response ID in the presence of a given agonist concentration, and IDmax = maximal ID in Ca2+‐free PSS + 10 μm SNP. Vasomotor tone (in PSS containing 2 mm Ca2+) was calculated as the percentage difference between IDbase and IDmax, and thus % tone = [(IDmax – IDbase) / IDmax] × 100, and was evaluated before (i.e. ‘spontaneous’) and during preconstruction with NA. Data were analysed using Student's t‐test or analysis of variance, with repeated measures when appropriate (Prism 5, GraphPad Software; La Jolla, CA, USA). When significant F‐ratios were obtained with analysis of variance, post hoc comparisons were made using Bonferroni tests. Summary data are mean values ± SE. Differences were considered statistically significant with P ≤ 0.05.
Results
Loss of vasodilatation to perivascular nerve stimulation with advanced age
Across all experiments, the IDs of Young MAs (n = 23) and Old MAs (n = 24) were not significantly different at rest under control conditions (198 ± 5 versus 189 ± 5 μm, respectively). Respective maximal IDs (214 ± 6 versus 201 ± 6 μm) were also not different between age groups, nor was the level of spontaneous vasomotor tone (8 ± 1 versus 6 ± 1%).
Perivascular nerve stimulation produced similar levels of MA constriction (∼50%) for both age groups (Fig. 1 A). To resolve the vasodilator effect of sensory nerve activation, PNS was repeated for MAs preconstricted ∼50% with phenylephrine (1 μm) and exposed to guanethidine (10 μm). From similar preconstricted baseline IDs (Fig. 1, legend), PNS dilated MAs from Young but not Old mice (Fig. 1 B, P < 0.05). During preconstriction with phenylephrine, repeating PNS in the presence of capsaicin (10 μm) in addition to guanethidine inhibited vasomotor responses of MAs from both Young and Old mice (Fig. 1 C). Neither guanethidine nor capsaicin treatment altered preconstricted baseline vessel diameters. With capsaicin treatment alone, vasoconstriction to PNS was enhanced in MAs from Young mice but not in MAs from Old mice (Fig. 2 A). Treatment with TTX (1 μm) blocked PNS‐induced constrictions across age groups (Fig. 2 B). Collectively, these data indicate a loss in the ability of perivascular sensory nerves to dilate MAs or to attenuate sympathetic vasoconstriction with advanced age.
Figure 1. Impaired dilatation of MAs to sensory nerve stimulation with advanced age .
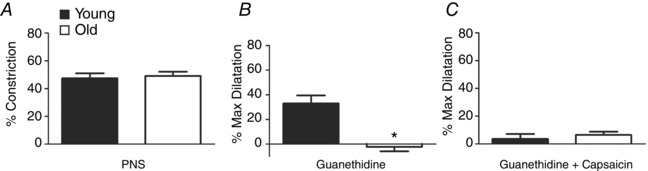
A, from resting control IDs (Young, 208 ± 12 μm; Old, 172 ± 20 μm), constriction during PNS (8 Hz @ 30 V, 2 ms) was not different between MAs from Young versus Old mice (IDs decreased to 110 ± 11 μm and 89 ± 13 μm, respectively). B, for MAs preconstricted with phenylephrine (1 μm), IDs were 96 ± 4 μm (Young) and 87 ± 10 μm (Old). Following treatment with guanethidine (10 μm), PNS dilated MAs of Young (to 156 ± 20 μm) but not Old mice (86 ± 11 μm). C, in MAs preconstricted as in B, treatment with capsaicin (10 μm) in addition to guanethidine prevented dilatation to PNS (Young, 103 ± 11 μm; Old, 78 ± 10 μm). n = 4–5 MAs per age group. % Constriction or % Max Dilatation calculated as described in Methods. *P < 0.05 compared to Young.
Figure 2. Sensory nerves limit constriction to perivascular nerve stimulation for Young but not Old MAs .
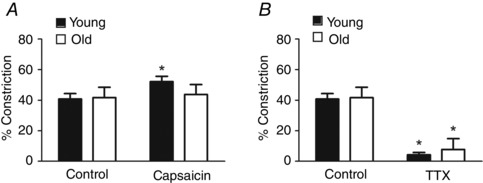
A, under control conditions, PNS caused similar constrictions in Young MAs (ID rest, 199 ± 6 μm; ID response, 120 ± 10 μm) and Old MAs (ID rest, 183 ± 14 μm; ID response, 113 ± 13 μm). Repeating PNS during capsaicin treatment (10 μm) increased constriction in Young (ID rest, 200 ± 7 μm; ID response, 97 ± 10 μm) but not Old MAs (ID rest, 182 ± 17 μm; ID response, 110 ± 12 μm). B, tetrodotoxin (TTX, 1 μm) blocked constriction to PNS in Young (ID rest, 201 ± 6; ID response, 193 ± 8 μm) and Old MAs (ID rest, 188 ± 17; ID response, 180 ± 15 μm). % Constriction calculated as described in Methods. n = 4 MAs per age group in A and n = 3 MAs per age group in B. *P < 0.05 compared to Control in same age group.
Increased EC50 for NA with no change for ACh
To determine whether MAs of Young and Old mice have the same ability to constrict and relax during activation of smooth muscle and endothelium, concentration–response curves were evaluated for NA and ACh, respectively. For NA, EC50 increased with advanced age with no change in efficacy (Fig. 3 A and Table 1). For ACh, dilatations were not different between MAs from Young versus Old mice at any concentration (Fig. 3 B), nor were respective EC50 values (Table 1). Thus, despite reduced EC50 of NA as a vasoconstrictor during advanced age, diminished dilatations of MAs to PNS (Fig. 1 B) cannot be attributed to differences in vasomotor tone or impaired endothelium‐dependent vasodilatation.
Figure 3. Increased EC50 of constriction to NA but not dilatation to ACh .
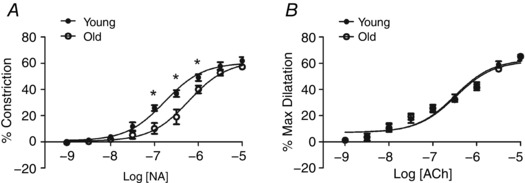
A, concentration‐dependent constriction to NA is shifted to the right in Old MAs compared to Young MAs. Initial resting IDs were 199 ± 12 for Young MAs and 187 ± 10 μm for Old MAs; respective IDs were 76 ± 7 and 80 ± 6 μm during peak constriction to NA and were 217 ± 12 and 206 ± 11 μm during maximal dilatation. B, concentration‐dependent dilatation to ACh is similar in Old and Young MAs. Initial baseline IDs during preconstriction with NA (1 μm) were not different between age groups (Young, 107 ± 7 μm; Old, 101 ± 9 μm). Peak IDs during vasodilatation to ACh were 174 ± 2 and 166 ± 12 μm, respectively; corresponding maximal IDs were 211 ± 3 μm and 201 ± 12 μm. % Constriction and % Max dilatation calculated as described in Methods. n = 5 MAs per age group. *P < 0.05, Young versus Old MAs at same NA concentration.
Table 1.
Mesenteric artery EC50 values and peak responses to vasoactive agents
| EC50 | EC50 | Peak response | Peak response | |
|---|---|---|---|---|
| Compound | Young (nm) | Old (nm) | Young (%) | Old (%) |
| NA | 167 ± 7 | 600 ± 87* | 62 ± 3 | 57 ± 2 |
| ACh | 370 ± 120 | 360 ± 91 | 64 ± 2 | 65 ± 2 |
| CGRP | 8 ± 2 | 52 ± 19* | 75 ± 2 | 70 ± 5 |
| CGRP + l‐NAME/Indo | 48 ± 15 | 160 ± 37* | 62 ± 3 | 62 ± 8 |
| CGRP (denuded) | 10 ± 3 | 18 ± 13 | 90 ± 3† | 48 ± 7*, † |
EC50 values are in nanomolar; peak response values to NA are % constrictions from resting baseline; all other peak response values are % maximum dilatations from preconstriction with NA, calculated as described in Methods. Peak response values for NA and ACh are from Fig. 3 and those for CGRP are from Fig. 4. Summary data are means ± SE. *P < 0.05 compared to Young. † P < 0.05 compared to intact MAs of the same age group. n = 4–11 per age group.
Increased EC50 for CGRP
To determine whether the decrease in perivascular sensory nerve function reflects diminished responses to the sensory neurotransmitter CGRP, concentration–response curves were evaluated for Young and Old MAs preconstricted with NA. Because the EC50 was greater with advanced age (Table 1, Fig. 3 A, P < 0.05), respective EC50 values for NA were used to obtain equivalent functional baselines of preconstriction from which to evaluate CGRP. Exogenous CGRP produced concentration‐dependent vasodilatation with similar efficacy in MAs from both Young and Old mice (Fig. 4 A). However, as illustrated by the shift to the right for Old MAs, the EC50 of CGRP increased significantly when compared to Young MAs (Fig. 4 A; Table 1). Vasodilatations to CGRP were also more variable for Old versus Young MAs.
Figure 4. Impaired dilatation to CGRP despite greater dependence on endothelium .
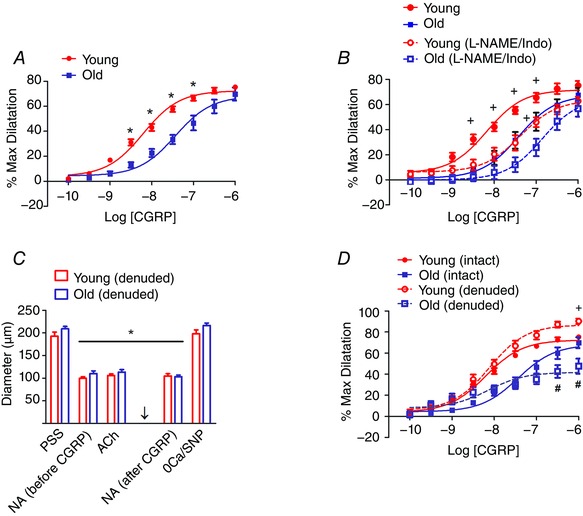
A, concentration‐dependent dilatation to CGRP is shifted to the right for Old MAs compared to Young MAs. Intact MAs were preconstricted with NA using EC50 values determined for respective age groups (Table 1). From preconstricted baseline IDs of 111 ± 8 μm (Young) and 108 ± 2 μm (Old), CGRP evoked peak IDs of 193 ± 11 μm and 184 ± 9 μm, respectively; n = 11 per age group. *P < 0.05, Young vs. Old MAs at same CGRP concentration. B, inhibiting endothelium‐derived autacoids with l‐NAME (100 μm) + indomethacin (Indo, 10 μm) shifted response curves to the right for intact MAs of both age groups (dashed lines); n = 6 per age group. Control data (continuous lines) are a subset of values shown in A with same statistical differences.+ P < 0.05, Control versus l‐NAME/Indo. C, sequence of evaluations following endothelial denudation. IDs were not different between Young versus Old MAs with control PSS, during EC50 constrictions with NA before and after evaluating responses to CGRP (arrow) during exposure to ACh (1 μm added during preconstriction with EC50 NA; note loss of endothelium‐dependent dilatation), or during maximal dilatation with 0 Ca2+ + 10 μm SNP (0Ca/SNP). n = 6 per age group; *P < 0.05 versus resting control diameter within same age group. D, the efficacy of dilatation to CGRP is decreased in Old MAs and increased in Young MAs. Control (intact) data re‐plotted from A for reference. n = 6 (denuded) or 11 (intact) per age group. + P < 0.05, Young denuded versus Young intact. # P < 0.05, Old denuded versus Old intact.
Role of endothelium in responses to CGRP
To determine the role of endothelium‐derived autacoids (NO and prostaglandins) in CGRP‐mediated vasodilatation, concentration–response curves to CGRP were performed before and during treatment with the nitric oxide synthase inhibitor l‐NAME (100 μm) in combination with the cyclooxygenase inhibitor indomethacin (10 μm). For Young MAs, autacoid inhibition shifted the CGRP response curve to the right (Table 1, Fig. 4 B, n = 5, P < 0.05) such that it was nearly superimposed on the control curve for Old MAs. Treatment with l‐NAME + indomethacin also shifted the CGRP response curve to the right for Old MAs (Table 1, Fig. 4 B). As there were no differences in efficacy between experimental conditions or age groups, these data indicate that autacoid production lowers the EC50 of dilatation to CGRP but is not essential for its efficacy as a vasodilator in either age group.
To further explore the role of the endothelium in CGRP‐mediated dilatation in Young and Old MAs, concentration–response curves to CGRP were repeated in MAs following endothelial denudation. Resting and maximum diameters were not different between age groups (Fig. 4 C). The viability of denuded MAs was confirmed by the maintenance of vasoconstriction to respective EC50 concentrations of NA while the success of denudation was verified by the absence of vasodilatation to 1 μm ACh (Fig. 4 C). When compared to intact vessels, endothelial denudation increased the efficacy of dilatation to CGRP by ∼15% in Young MAs (P < 0.05; Fig. 4 D and Table 1). In striking contrast, the efficacy of CGRP to dilate Old MAs was reduced by ∼30% (Fig. 4 D and Table 1). Thus, following endothelial denudation, the efficacy of dilatation to CGRP in Old MAs was ∼50% of that in Young MAs. Confirming the integrity of SMCs in denuded MAs, constrictions to NA were maintained after evaluating the responses to CGRP (Fig. 4 C).
Density of sympathetic and sensory nerves
Immunofluorescence labelling was performed to determine whether advanced age is associated with changes in the density of either sympathetic or sensory PVNs. The density of sensory nerves was significantly lower in Old MAs compared to Young MAs (Figs. 5 and 6 A). In contrast, the density of sympathetic nerves was not different between MAs of respective age groups (Figs 5 and 6 B). Thus functional impairments in vasomotor responses to sensory nerve stimulation with advanced age (Fig. 1 B) are associated with diminished sensory innervation.
Figure 5. Immunofluorescence of perivascular nerves .
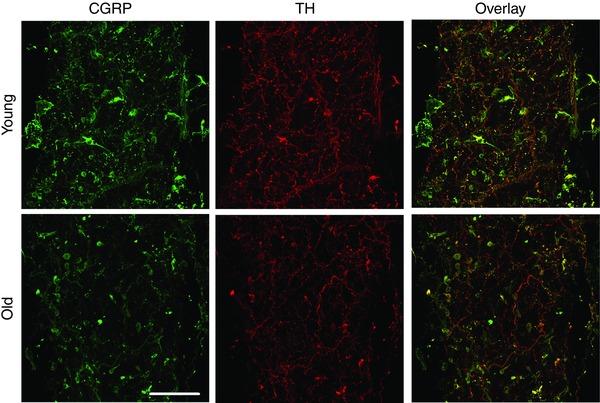
Images are representative maximum Z‐projections of MAs double‐stained for both sensory nerves (left panels, labelled for CGRP) and sympathetic nerves (centre panels, labelled for tyrosine hydroxylase, TH) from Young (top row) and Old MAs (bottom row). Right panels are overlays of the fluorescence from respective images. Scale bar = 100 μm and applies to all panels. Images are representative of n = 9–16 MAs from 4–5 mice per age group.
Figure 6. Decreased perivascular sensory but not sympathetic nerve density .
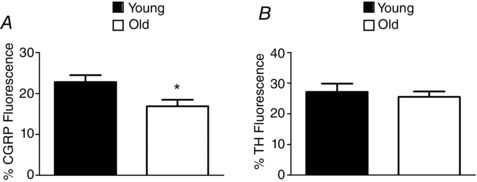
A, quantifying CGRP fluorescence following immunolabelling indicates a significant reduction in the density of sensory nerves surrounding Old versus Young MAs. *P < 0.05 versus Young. B, quantifying TH fluorescence following immunolabelling indicates no difference in the density of sympathetic nerves surrounding Old versus Young MAs. n = 9–16 MAs from 4–5 mice per age group.
Localisation of CGRP receptor proteins
Immunofluorescence labelling was performed to determine whether age‐related differences in CGRP‐mediated vasodilatation are associated with changes in functional expression of CGRP receptors. In MAs from both age groups, ECs exhibited punctate fluorescence for RAMP1 and CRLR with considerable overlap in apparent localisation. However, only ECs of MAs from Old mice exhibited accumulation of RAMP1 in the nuclear region (Fig. 7). In contrast, both age groups exhibited similar punctuate staining for RAMP1 and CRLR throughout SMCs, with a high degree of overlap between respective proteins (Fig. 8).
Figure 7. Nuclear association of RAMP1 with endothelial cell nuclei of Old but not Young MAs .
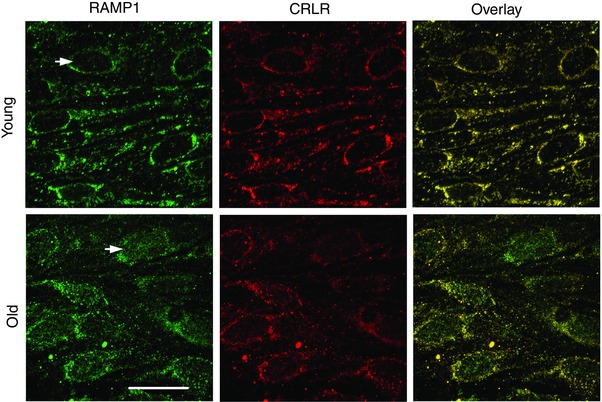
Images are maximum Z‐projections double‐stained for RAMP1 and CRLR. Panels at right are overlays; yellow indicates correspondence in localisation of respective CGRP receptor proteins and is most consistent in endothelium of Young MAs. Staining for RAMP1 is associated with endothelial cell nuclei of Old MAs that is not present in Young MAs (arrows). Scale bar = 20 μm and applies to all panels. Representative of n = 9–12 MAs from 3 mice per age group.
Figure 8. Similar expression of RAMP1 and CRLR proteins in smooth muscle cells of Young and Old MAs .
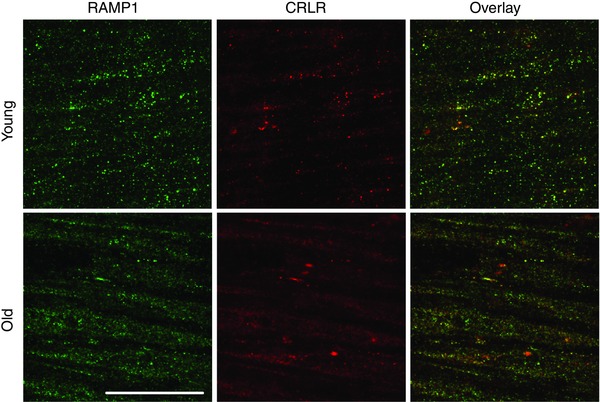
Images are maximum Z‐projections double‐stained for RAMP1 and CRLR. Panels at right are overlays; yellow indicates correspondence in localisation of respective CGRP receptor proteins. Scale bar = 20 μm and applies to all panels. Representative of n = 8–10 MAs from 4 mice per age group.
Discussion
Advanced age decreases perivascular sensory nerve function of MAs controlling blood flow to the small intestine of mice (Westcott & Segal, 2013 a). The present study used isolated MAs to gain insight into the mechanisms underlying this functional deficit. Our new findings highlight the loss of vasodilatation in response to sensory nerve stimulation with advanced age and have identified multiple adaptations that contribute to this decline in function. In cannulated pressurized MAs, the EC50 of CGRP as a dilator increased with advanced age, requiring a higher concentration of CGRP to evoke a given level of dilatation when compared to MAs of Young mice. Nevertheless, endothelium‐derived autacoids lower the EC50 of CGRP as a dilator of MAs in both age groups. Remarkably, endothelial denudation had opposite effects on the efficacy of CGRP as a vasodilator in MAs of respective age groups, reducing peak dilatation in Old MAs while enhancing peak dilatation in Young MAs. Fluorescence immunolabelling revealed that, compared to Young MAs, Old MAs have diminished perivascular density of sensory nerves containing CGRP but not of sympathetic nerves containing TH. Further, an integral CGRP receptor protein (RAMP1) was found to be associated with the nuclear region of ECs in Old MAs but not Young MAs. As the expression of these proteins was similar in SMCs of MAs from both age groups, the assembly of functional CGRP receptors may thus be compromised in ECs with advanced age. Collectively, the present findings illustrate that multiple components mediating the ability of sensory nerves to promote blood flow through the splanchnic circulation are altered during advanced age.
Loss of vasodilatation to sensory nerve activation
Under control conditions, Young and Old MAs had similar diameters, spontaneous vasomotor tone and constrictions to PNS (Fig. 1 A). To study vasodilatation as a response to PNS, we elevated the level of vasomotor tone from < 10% (spontaneous) to ∼50% by applying the α1‐adrenoreceptor agonist phenylephrine, with guanethidine added to inhibit sympathetic neurotransmission. Under these conditions, Young MAs dilated by ∼30% during PNS, while Old MAs did not (Fig. 1 B). In turn, the dilatation observed in Young MAs was blocked by capsaicin (Fig. 1 C), confirming that SMC relaxation induced by PNS resulted from the activation of sensory nerves. With sympathetic nerves intact, capsaicin treatment alone increased vasoconstriction to PNS in Young but not Old MAs (Fig. 2 A). Our finding of impaired sensory nerve function in MAs of Old mice is consistent with diminished dilatation of MAs from Old (25 months) rats during electrical field stimulation (Sullivan & Davison, 2001) and loss of the ability of sensory nerves to inhibit sympathetic vasoconstriction of MAs controlling blood flow to the intestine in Old (24 months) mice (Westcott & Segal, 2013 a). We confirmed that the effects of PNS do not reflect direct activation of SMCs, as treatment with TTX prevented vasomotor responses to PNS (Fig. 2 B). Nor did the loss of PNS‐induced vasodilatation of MAs from Old mice reflect the inherent loss of endothelium‐dependent dilatation because ACh dilated MAs from both age groups with similar EC50 and efficacy (Table 1 and Fig. 3 B) and dilatation to ACh was lost following endothelial denudation.
The effect of ageing on ACh‐mediated vasodilatation in resistance arteries is variable between studies and vascular beds. While our laboratory (Bearden et al. 2004; Sinkler & Segal, 2014) and others (Barton et al. 1997; Sullivan et al. 2004; Kinzenbaw et al. 2013) have found that ACh‐mediated vasodilatation is preserved in advanced age, impaired vasodilatations to ACh during ageing have been reported in rodents (Yang et al. 2009; Zhou et al. 2010) as well as humans (Taddei et al. 1995). Nevertheless, our finding that dilatations to ACh were not different between MAs from Young and Old mice (Fig. 3 B) and that these responses were abolished following endothelial denudation (Fig. 4 C) confirms that endothelium‐dependent vasodilatation persists during advanced age. We therefore conclude that the vasodilator role of sensory nerves in MAs is compromised selectively in Old versus Young mice.
Increased EC50 of sympathetic and sensory neurotransmitters
The maintenance of vasoconstriction and loss of vasodilatation in response to PNS led us to evaluate respective receptor‐mediated events. We first tested whether the response to activation of α‐adrenoreceptors was affected by ageing. Concentration–response curves to the sympathetic neurotransmitter NA (which acts on both α1‐ and α2‐adrenoreceptors to produce vasoconstriction) revealed an increase in EC50, implying a loss of sensitivity with advanced age (Fig. 3 A) in accord with earlier findings in vivo (Westcott & Segal, 2013 a). Based upon these results, additional experiments were performed in which MAs from Young and Old mice were preconstricted to the same functional baseline using respective EC50 concentrations of NA (Table 1). Under these conditions, the EC50 for CGRP as a dilator was significantly greater with advanced age (Table 1) implying a loss of sensitivity (Fig. 4 A).
Few studies have examined how ageing may affect the vasoactive properties of CGRP in MAs, and the effect of ageing on CGRP responses has been controversial. In rats, for example, dilatation of MAs to exogenous CGRP was reduced at 18 versus 3 months (Amerini et al. 1994). In contrast, using transmural nerve stimulation, CGRP‐mediated dilatation of MAs was not different between rats at 6 and 27 months (Li & Duckles, 1993). However, in the perfused rat mesentery, a decline in capsaicin‐induced CGRP release was found at 18 versus 2 months (Sun et al. 1998). Such variability across earlier studies may reflect differences in the age groups being compared as well as the experimental approach. The age groups evaluated in the present study are based upon previous studies of advanced age from our laboratory (Behringer et al. 2013; Sinkler & Segal, 2014; Socha et al. 2015) and the functional experiments performed here encompassed both nerve stimulation and the application of exogenous CGRP. With both approaches, the present findings confirm dysfunctional sensory neuroeffector signalling for MAs of Old mice when compared to MAs of Young mice. It is reassuring to note that the present results are consistent with studies of rat thoracic aorta preconstricted with PE, where CGRP‐mediated dilatation was lower in vessels from 24‐month‐old animals compared to those 6 months of age (Lu et al. 2002). Ageing‐related decreases in CGRP‐mediated dilatation have also been demonstrated in rat caudal arteries (Chan & Fiscus, 2002), rabbit coronary arteries (Corr et al. 1991) and rabbit basilar arteries (Brizzolara et al. 1994). In light of reduced CGRP immunolabelling (Figs. 5 and 6) and altered RAMP1 distribution in ECs of MAs from Old versus Young mice (Fig. 7), the impaired functional role for perivascular sensory nerves in the intact system (Westcott & Segal, 2013 a) further reflects a decrease in neuropeptide availability and altered receptor function. Thus, ageing exerts a multifaceted effect on suppressing the role of sensory nerves in mediating vasodilatation along with their ability to attenuate sympathetic vasoconstriction.
Altered dependence on endothelium for vasodilatation to CGRP
A decline in CGRP‐mediated vasodilatation with ageing has been suggested to be specific to vessels in which CGRP effects are endothelium (and particularly eNOS) dependent (Lu et al. 2002). Therefore, we performed concentration–response curves to exogenous CGRP in MAs before and during treatment with l‐NAME + indomethacin to inhibit production of endothelium‐derived NO and prostaglandins. Inhibition of these vasodilator autacoids resulted in a significant shift to the right in responses to CGRP of MAs from both Young and Old mice (see Table 1 of EC50 values). Thus the higher EC50 of CGRP in Old versus Young MAs persisted during autacoid inhibition (Table 1 and Fig. 4 B). Our finding that the dilatory response to CGRP at least partially reflects the production of endothelium‐derived autacoids contrasts with previous studies in MAs (Li & Duckles, 1992; Sullivan & Davison, 2001; Ralevic, 2002); however these earlier experiments were all performed with rat models under experimental conditions different from those used here in mice. Nevertheless, the present data indicate that irrespective of age, the sensitivity to CGRP as a vasodilator is enhanced by the production of endothelium‐derived autacoids (Fig. 4 B).
The role of the endothelium in CGRP‐mediated vasodilatation varies between vascular beds and animal species. Thus, coronary arteries of pigs and humans (Gupta et al. 2006), rat thoracic aorta (Wisskirchen et al. 1999) and equine colonic arteries (Moore et al. 2005) exhibited endothelium‐dependent dilatation to CGRP. In contrast, CGRP dilatation was independent of the endothelium in human subcutaneous arteries (Edvinsson et al. 2014), porcine coronary arteries (Wisskirchen et al. 1999) and guinea pig basilar arties (Jansen‐Olesen et al. 2001). Vasodilatation during the inhibition of NO and prostaglandin synthesis is taken to reflect endothelium‐derived hyperpolarization (EDH), with activation of K+ channels in ECs leading to relaxation of SMCs (Garland et al. 2011). The specific K+ channels involved may vary with vascular bed and/or vessel size. Large‐ and intermediate‐conductance Ca2+‐activated K+ channels (BKCa and IKCa, respectively) were involved in CGRP‐mediated dilatation of rat coronary arteries (Sheykhzade & Berg Nyborg, 2001) but not in human subcutaneous arteries (Edvinsson et al. 2014). In rabbit MAs, KATP channels were integral to dilatation in response to CGRP (Standen et al. 1989). While further studies are needed to characterize autacoid‐independent dilatations to CGRP in MAs of both Young and Old mice, several possibilities are suggested by the present findings as considered below.
A key goal of the present study was to elucidate the respective roles for SMCs and ECs in CGRP‐mediated vasodilatation. A striking difference between age groups emerged following endothelial denudation. For MAs from Young mice, dilatation to CGRP was enhanced by ∼15% at the highest concentrations whereas dilatation of MAs from Old mice was attenuated by ∼30% compared to respective intact vessels (Fig. 4 D). Thus, removing the endothelium resulted in a 2‐fold greater efficacy of CGRP as a vasodilator in Young versus Old MAs. Our finding that dilatation to CGRP prevails following endothelial denudation in both age groups indicates a direct effect of CGRP in promoting SMC relaxation via receptor activation and downstream signalling (Brain & Grant, 2004). This differential effect of ablating the endothelium on respective age groups may in turn reflect modification of common signalling pathways and our studies point to the endothelium as the primary source of age‐related changes.
The efficacy of dilatation to CGRP persisted during autacoid inhibition yet CGRP remained less potent in MAs from Old versus Young mice (Fig. 4 B). In turn, we suggest that adaptations in CGRP signalling through EDH may underlie the age‐related differences observed in the role of endothelium in CGRP dilatation. Thus, activation of PKA by CGRP may lead to phosphorylation of ion channels in ECs, altering their activity and thereby affecting vasomotor control. For example, in MAs stimulated with ACh, increased adenylyl cyclase activity was found to reduce EDH via PKA‐mediated inhibition of IKCa (Dora et al. 2008). This finding is consistent with studies of neurons showing decreased IKCa activity following phosphorylation by PKA (Vogalis et al. 2003). In such manner, PKA activity may thereby limit EDH‐mediated dilatation to CGRP in Young MAs, as suggested by enhanced vasodilatation following endothelial denudation (Fig. 4 D). In the absence of the endothelium, enhanced vasodilatation in Young MAs may in turn be explained by the activation of K+ channels on SMCs (Nelson et al. 1990), which may be down‐regulated (Marijic et al. 2001; Kang et al. 2009) with advanced age. Additional studies will be required to resolve these functional interactions alluded to from our present findings.
In contrast to diminished IKCa activity resulting from PKA stimulation, endothelial transient receptor potential (TRP) channel activity may increase with ageing due to phosphorylation by PKA in response to CGRP. For example, TRPV4 channel activity was increased by phosphorylation in HEK293 cells (Fan et al. 2009) and recent studies have demonstrated that activation of only a few TRPV4 channels in myoendothelial projections can produce near‐maximal vasodilatation of MAs (Sonkusare et al. 2012). TRPA1 channels in myoendothelial projections can produce similarly potent vasodilatations (Earley et al. 2009) and demonstrate increased activity following phosphorylation (Lapointe & Altier, 2011). In light of these studies, changes in endothelial ion channel function (and therefore EDH of SMCs) may underlie both the functional deficits in CGRP responses and the greater dependence upon the endothelium for dilatation in response to CGRP with advanced age. Thus, defining the role(s) and regulation of respective ion channels in ECs and SMCs represents another key area for future studies aimed at determining how advanced age affects vasodilatation in response to CGRP and the activation of perivascular sensory nerves.
Differential effects of advanced age on perivascular sympathetic and sensory nerve density
Immunofluorescence studies of MAs from Young and Old mice showed that advanced age was associated with a ∼25% decrease in the density of perivascular sensory nerves. This loss with ageing appears specific for sensory innervation, as sympathetic nerve density of MAs was not different between age groups (Figs 5 and 6). These data suggest that the decrease in sensory nerve function (Fig. 1 B) (Westcott & Segal, 2013 a) is due, at least in part, to the loss of sensory innervation and imply that the remaining sensory nerves do not release a compensatory amount of CGRP at a given level of PNS. The maintenance of sympathetic nerve density during advanced age found here is consistent with data from mouse mesenteric, femoral, gracilis and carotid arteries (Long & Segal, 2009) as well as guinea pig mesenteric and carotid arteries (Dhall et al. 1986). In turn, the decrease in sensory (CGRP‐ergic) nerve density with advanced age is consistent with studies of guinea pig renal and femoral arteries (Dhall et al. 1986) and MAs from spontaneously hypertensive rats (Hobara et al. 2005). A reduction in sensory nerve density with advanced age may have important consequences on integrated vasomotor control. Because perivascular sympathetic and sensory nerves exert reciprocal inhibition (Kawasaki et al. 1990; Coffa & Kotecha, 1999; Kawasaki, 2002), the specific decline in sensory nerve density (Figs 5 and 6) may contribute to ageing‐related increases in systemic sympathetic nerve activity (Dinenno et al. 1999; Seals & Dinenno, 2004) including elevated blood pressure as shown in mice (Gros et al. 2002) as well as humans (Lakatta & Levy, 2003). This interpretation is consistent with finding that deletion of the CGRP gene leads to hypertension in mice (Gangula et al. 2000).
Altered localisation of CGRP receptor proteins in endothelial cells
In light of a greater role for the endothelium and the diminished vasodilatation in response to exogenous CGRP in Old versus Young MAs (Fig. 4), ECs of MAs from both age groups were labelled for the CRLR and RAMP1 protein components of functional CGRP receptors. Confocal imaging revealed association of RAMP1 with the nuclear region of ECs in Old MAs that was not present in ECs of Young MAs (Fig. 7). In contrast, labelling of CRLR and RAMP1 had similar punctate appearance throughout SMCs of both age groups (Fig. 8), suggesting that age‐related changes in RAMP1 localization are specific to ECs. Nuclear association of RAMP1 in ECs with advanced age is of particular significance because this protein serves as a chaperone to route CRLR to the cell surface, which thereby creates the functional receptor for CGRP (McLatchie et al. 1998). Thus, reduced functional CGRP receptors may contribute to the greater EC50 of CGRP with advanced age observed here (Fig. 4 A). Consistent with this hypothesis, dilatation of carotid arteries to exogenous CGRP was enhanced for mice in which RAMP1 was globally overexpressed (Chrissobolis et al. 2010). Conversely, deletion of RAMP1 reduced dilatation of the aorta to CGRP (Tsujikawa et al. 2007). The present data are also consistent with a reduction in RAMP1 (but not CRLR) immunofluorescence in MAs of 12‐month‐ versus 3‐month‐old‐rats (Gangula et al. 2009) and are the first to resolve nuclear association of RAMP1 in ECs with advanced age.
Summary and perspective
We have investigated the effect of advanced age on perivascular nerve function and density in second‐order MAs of the male C57BL/6 mouse in light of endothelial and smooth muscle signalling pathways. Our findings suggest that ageing leads to a significant decline in sensory nerve function through complementary mechanisms. Thus, compared to Young mice, MAs from Old mice lose the ability to dilate in response to the activation of perivascular sensory nerves, exhibit greater EC50 values (i.e. reduced sensitivity) to exogenous CGRP and rely upon ECs to a greater extent for CGRP‐mediated vasodilatation through a mechanism that is largely independent of endothelium‐derived autacoids. Denuding the endothelium had opposite effects on MA responses to CGRP in respective age groups, attenuating dilatation in Old MAs while enhancing dilatation in Young MAs. These functional changes with advanced age occur in association with loss of perivascular sensory nerve density despite no concurrent change in sympathetic nerve density. Finding that the CGRP receptor protein RAMP1 associates with nuclear regions of ECs (but not SMCs) of MAs from Old but not Young mice suggests a deficiency in functional CGRP receptors at the cell surface in the endothelium of Old MAs. We thereby identify complementary mechanisms that help to explain how blood flow to visceral organs can be diminished with advanced age.
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
E.M.B. and S.S.S. conceived and designed the experiments. E.M.B. performed the experiments in the laboratory of S.S.S. and compiled the data. E.M.B. and S.S.S. analysed and interpreted the data. E.M.B. drafted the manuscript and figures. S.S.S. edited the manuscript and figures. Both authors reviewed and approved the final version of the article for publication.
Funding
This research was supported by National Institutes of Health grants F32‐HL118836 to E.M.B. and R01‐HL086483 to S.S.S., who is also supported by R37‐HL041026. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
The authors thank Drs Erik Behringer, Timothy Domeier and Matthew Socha for valuable discussions.
References
- Abramoff MD, Magelhaes PJ & Ram SJ (2004). Image processing with ImageJ. Biophoton Int 11, 36–42. [Google Scholar]
- Amerini S, Mantelli L, Filippi S & Ledda F (1994). Effects of aging and hypertension on vasorelaxant activity of calcitonin gene‐related peptide: a comparison with other vasodilator agents. J Cardiovasc Pharmacol 23, 432–437. [PubMed] [Google Scholar]
- Armstead WM (1995). Role of nitric oxide and cAMP in prostaglandin‐induced pial arterial vasodilation. Am J Physiol Heart Circ Physiol 268, H1436–1440. [DOI] [PubMed] [Google Scholar]
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S & Luscher TF (1997). Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 30, 817–824. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Payne GW, Chisty A & Segal SS (2004). Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561, 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer EJ, Shaw RL, Westcott EB, Socha MJ & Segal SS (2013). Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+‐activated K+ channel activation. Arterioscler Thromb Vasc Biol 33, 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD & Cox HM (2006). Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol 147 Suppl 1, S202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD & Grant AD (2004). Vascular actions of calcitonin gene‐related peptide and adrenomedullin. Physiol Rev 84, 903–934. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR & MacIntyre I (1985). Calcitonin gene‐related peptide is a potent vasodilator. Nature 313, 54–56. [DOI] [PubMed] [Google Scholar]
- Brizzolara AL, Stewart‐Lee A & Burnstock G (1994). Responses of rabbit basilar arteries to vasoconstrictor and vasodilator agents: the effects of atherosclerosis, age and sex. J Vasc Res 31, 106–113. [DOI] [PubMed] [Google Scholar]
- Buchholz J, Sexton P & Hewitt CW (1998). Impact of age on modulation of norepinephrine release from sympathetic nerves in the rat superior mesentery artery. Life Sci 62, 679–686. [DOI] [PubMed] [Google Scholar]
- Burnstock G & Ralevic V (1994). New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg 47, 527–543. [DOI] [PubMed] [Google Scholar]
- Chan GH & Fiscus RR (2002). Severe impairment of CGRP‐induced hypotension in vivo and vasorelaxation in vitro in elderly rats. Eur J Pharmacol 434, 133–139. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Zhang Z, Kinzenbaw DA, Lynch CM, Russo AF & Faraci FM (2010). Receptor activity‐modifying protein‐1 augments cerebrovascular responses to calcitonin gene‐related peptide and inhibits angiotensin II‐induced vascular dysfunction. Stroke 41, 2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffa FP & Kotecha N (1999). Modulation of sympathetic nerve activity by perivascular sensory nerves in the arterioles of the guinea‐pig small intestine. J Auton Nerv Syst 77, 125–132. [PubMed] [Google Scholar]
- Corr LA, Poole‐Wilson P & Burnstock G (1991). Responses of coronary arteries to neurotransmitters: changes with sexual maturity in the female rabbit. J Cardiovasc Pharmacol 18, 144–150. [DOI] [PubMed] [Google Scholar]
- Dhall U, Cowen T, Haven AJ & Burnstock G (1986). Perivascular noradrenergic and peptide‐containing nerves show different patterns of changes during development and ageing in the guinea‐pig. J Auton Nerv Syst 16, 109–126. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR & Tanaka H (1999). Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100, 164–170. [DOI] [PubMed] [Google Scholar]
- Dinenno FA & Joyner MJ (2006). α‐Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13, 329–341. [DOI] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A & Garland CJ (2008). Modulation of endothelial cell KCa3.1 channels during endothelium‐derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102, 1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL & Crnich R (2009). Endothelium‐dependent cerebral artery dilation mediated by TRPA1 and Ca2+‐activated K+ channels. Circ Res 104, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L, Ahnstedt H, Larsen R & Sheykhzade M (2014). Differential localization and characterization of functional calcitonin gene‐related peptide receptors in human subcutaneous arteries. Acta Physiol (Oxf) 210, 811–822. [DOI] [PubMed] [Google Scholar]
- Eid SR & Cortright DN (2009). Transient receptor potential channels on sensory nerves. Handb Exp Pharmacol, 261–281. [DOI] [PubMed] [Google Scholar]
- Esler M, Lambert G, Kaye D, Rumantir M, Hastings J & Seals DR (2002). Influence of ageing on the sympathetic nervous system and adrenal medulla at rest and during stress. Biogerontology 3, 45–49. [DOI] [PubMed] [Google Scholar]
- Fan HC, Zhang X & McNaughton PA (2009). Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem 284, 27884–27891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Currer J & Harrison DE (2007). Mouse Models in Aging Research. In: The Mouse in Biomedical Research. Edited by: Fox JG, Davisson MT, Quimbyet FW, Barthold SW, Newcomer CE & Smith AL. Burlington, American College of Laboratory Animal Medicine (Elsevier). 2nd Ed., pp. 637–672.
- Gangula PR, Chauhan M, Reed L & Yallampalli C (2009). Age‐related changes in dorsal root ganglia, circulating and vascular calcitonin gene‐related peptide (CGRP) concentrations in female rats: effect of female sex steroid hormones. Neurosci Lett 454, 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF & Yallampalli C (2000). Increased blood pressure in α‐calcitonin gene‐related peptide/calcitonin gene knockout mice. Hypertension 35, 470–475. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Hiley CR & Dora KA (2011). EDHF: spreading the influence of the endothelium. Br J Pharmacol 164, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PE, Strain WD & Shore AC (2009). Human endothelial function and microvascular ageing. Exp Physiol 94, 311–316. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB & Subcommittee AHASCaSS (2013). Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, 143–152. [DOI] [PubMed] [Google Scholar]
- Gray DW & Marshall I (1992). Human α‐calcitonin gene‐related peptide stimulates adenylate cyclase and guanylate cyclase and relaxes rat thoracic aorta by releasing nitric oxide. Br J Pharmacol 107, 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, Van Wert R, You X, Thorin E & Husain M (2002). Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol Heart Circ Physiol 282, H380–388. [DOI] [PubMed] [Google Scholar]
- Gupta S, Mehrotra S, Villalón CM, Garrelds IM, de Vries R, van Kats JP, Sharma HS, Saxena PR & Maassenvandenbrink A (2006). Characterisation of CGRP receptors in human and porcine isolated coronary arteries: evidence for CGRP receptor heterogeneity. Eur J Pharmacol 530, 107–116. [DOI] [PubMed] [Google Scholar]
- Hobara N, Gessei‐Tsutsumi N, Goda M, Takayama F, Akiyama S, Kurosaki Y & Kawasaki H (2005). Long‐term inhibition of angiotensin prevents reduction of periarterial innervation of calcitonin gene‐related peptide (CGRP)‐containing nerves in spontaneously hypertensive rats. Hypertens Res 28, 465–474. [DOI] [PubMed] [Google Scholar]
- Izzo JL & Mitchell GF (2007). Aging and arterial structure‐function relations. Adv Cardiol 44, 19–34. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Moore AW & Segal SS (2010). Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588, 2269–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen‐Olesen I, Kaarill L & Edvinsson L (2001). Characterization of CGRP1 receptors in the guinea pig basilar artery. Eur J Pharmacol 414, 249–258. [DOI] [PubMed] [Google Scholar]
- Kadzielawa K (1962). Mechanism of action of guanethidine. Br J Pharmacol Chemother 19, 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang LS, Kim S, Dominguez JM, Sindler AL, Dick GM & Muller‐Delp JM (2009). Aging and muscle fiber type alter K+ channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol 107, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H (2002). Regulation of vascular function by perivascular calcitonin gene‐related peptide‐containing nerves. Jpn J Pharmacol 88, 39–43. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Nuki C, Saito A & Takasaki K (1990). Adrenergic modulation of calcitonin gene‐related peptide (CGRP)‐containing nerve‐mediated vasodilation in the rat mesenteric resistance vessel. Brain Res 506, 287–290. [DOI] [PubMed] [Google Scholar]
- Kinzenbaw DA, Chu Y, Peña Silva RA, Didion SP & Faraci FM (2013). Interleukin‐10 protects against aging‐induced endothelial dysfunction. Physiol Rep 1, e00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG & Levy D (2003). Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation 107, 139–146. [DOI] [PubMed] [Google Scholar]
- Lapointe TK & Altier C (2011). The role of TRPA1 in visceral inflammation and pain. Channels (Austin) 5, 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y & Duckles SP (1993). Effect of age on vascular content of calcitonin gene‐related peptide and mesenteric vasodilator nerve activity in the rat. Eur J Pharmacol 236, 373–378. [DOI] [PubMed] [Google Scholar]
- Li YJ & Duckles SP (1992). Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur J Pharmacol 210, 23–30. [DOI] [PubMed] [Google Scholar]
- Long JB & Segal SS (2009). Quantifying perivascular sympathetic innervation: regional differences in male C57BL/6 mice at 3 and 20 months. J Neurosci Methods 184, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhu HQ, Peng J, Li NS & Li YJ (2002). Endothelium‐dependent vasorelaxation and the expression of calcitonin gene‐related peptide in aged rats. Neuropeptides 36, 407–412. [DOI] [PubMed] [Google Scholar]
- Marijic J, Li Q, Song M, Nishimaru K, Stefani E & Toro L (2001). Decreased expression of voltage‐ and Ca2+‐activated K+ channels in coronary smooth muscle during aging. Circ Res 88, 210–216. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG & Foord SM (1998). RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature 393, 333–339. [DOI] [PubMed] [Google Scholar]
- Moore RM, Sedrish SA, Holmes EP, Koch CE & Venugopal CS (2005). Role of endothelium and nitric oxide in modulating in vitro responses of colonic arterial and venous rings to vasodilatory neuropeptides in horses. Can J Vet Res 69, 116–122. [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Huang Y, Brayden JE, Hescheler J & Standen NB (1990). Arterial dilations in response to calcitonin gene‐related peptide involve activation of K+ channels. Nature 344, 770–773. [DOI] [PubMed] [Google Scholar]
- Proctor DN & Parker BA (2006). Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13, 315–327. [DOI] [PubMed] [Google Scholar]
- Raddino R, Pelà G, Manca C, Barbagallo M, D'Aloia A, Passeri M & Visioli O (1997). Mechanism of action of human calcitonin gene‐related peptide in rabbit heart and in human mammary arteries. J Cardiovasc Pharmacol 29, 463–470. [DOI] [PubMed] [Google Scholar]
- Ralevic V (2002). Endothelial nitric oxide modulates perivascular sensory neurotransmission in the rat isolated mesenteric arterial bed. Br J Pharmacol 137, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reslerova M & Loutzenhiser R (1998). Renal microvascular actions of calcitonin gene‐related peptide. Am J Physiol Renal Physiol 274, F1078–1085. [DOI] [PubMed] [Google Scholar]
- Seals DR & Dinenno FA (2004). Collateral damage: cardiovascular consequences of chronic sympathetic activation with human aging. Am J Physiol Heart Circ Physiol 287, H1895–1905. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL & Donato AJ (2011). Aging and vascular endothelial function in humans. Clin Sci (Lond) 120, 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheykhzade M & Berg Nyborg NC (2001). Mechanism of CGRP‐induced relaxation in rat intramural coronary arteries. Br J Pharmacol 132, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkler SY & Segal SS (2014). Aging alters reactivity of microvascular resistance networks in mouse gluteus maximus muscle. Am J Physiol Heart Circ Physiol 307, H830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL & Segal SS (2015). Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J Physiol 593, 2155–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill‐Eubanks DC & Nelson MT (2012). Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y & Nelson MT (1989). Hyperpolarizing vasodilators activate ATP‐sensitive K+ channels in arterial smooth muscle. Science 245, 177–180. [DOI] [PubMed] [Google Scholar]
- Sullivan JC & Davison CA (2001). Effect of age on electrical field stimulation (EFS)‐induced endothelium‐dependent vasodilation in male and female rats. Cardiovasc Res 50, 137–144. [DOI] [PubMed] [Google Scholar]
- Sullivan JC, Loomis ED, Collins M, Imig JD, Inscho EW & Pollock JS (2004). Age‐related alterations in NOS and oxidative stress in mesenteric arteries from male and female rats. J Appl Physiol (1985) 97, 1268–1274. [DOI] [PubMed] [Google Scholar]
- Sun W, Guo J, Tang Y & Wang X (1998). Alteration of capsaicin and endotoxin‐induced calcitonin gene‐related peptide release from mesenteric arterial bed and spinal cord slice in 18‐month‐old rats. J Neurosci Res 53, 385–392. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I & Salvetti A (1995). Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91, 1981–1987. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K, Yayama K, Hayashi T, Matsushita H, Yamaguchi T, Shigeno T, Ogitani Y, Hirayama M, Kato T, Fukada S, Takatori S, Kawasaki H, Okamoto H, Ikawa M, Okabe M & Yamamoto H (2007). Hypertension and dysregulated proinflammatory cytokine production in receptor activity‐modifying protein 1‐deficient mice. Proc Natl Acad Sci U S A 104, 16702–16707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR & Furness JB (2003). PKA‐mediated inhibition of a novel K+ channel underlies the slow after‐hyperpolarization in enteric AH neurons. J Physiol 548, 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EB & Segal SS (2013. a). Ageing alters perivascular nerve function of mouse mesenteric arteries in vivo . J Physiol 591, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EB & Segal SS (2013. b). Perivascular innervation: A multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 20, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisskirchen FM, Burt RP & Marshall I (1998). Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol 123, 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisskirchen FM, Gray DW & Marshall I (1999). Receptors mediating CGRP‐induced relaxation in the rat isolated thoracic aorta and porcine isolated coronary artery differentiated by hα CGRP8‐37 . Br J Pharmacol 128, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Huang A, Kaley G & Sun D (2009). eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol 297, H1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou E, Qing D & Li J (2010). Age‐associated endothelial dysfunction in rat mesenteric arteries: roles of calcium‐activated K+ channels (Kca). Physiol Res 59, 499–508. [DOI] [PubMed] [Google Scholar]


