Abstract
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality globally. In recent years, studies have shown that the origins of CVD may be traced to vascular and metabolic processes in early life. Retinal vascular imaging is a new technology that allows detailed non‐invasive in vivo assessment and monitoring of the microvasculature. In this systematic review, we described the application of retinal vascular imaging in children and adolescents, and we examined the use of retinal vascular imaging in understanding CVD risk in early life. We reviewed all publications with quantitative retinal vascular assessment in two databases: PubMed and Scopus. Early life CVD risk factors were classified into four groups: birth risk factors, environmental risk factors, systemic risk factors and conditions linked to future CVD development. Retinal vascular changes were associated with lower birth weight, shorter gestational age, low‐fibre and high‐sugar diet, lesser physical activity, parental hypertension history, childhood hypertension, childhood overweight/obesity, childhood depression/anxiety and childhood type 1 diabetes mellitus. In summary, there is increasing evidence supporting the view that structural changes in the retinal microvasculature are associated with CVD risk factors in early life. Thus, the retina is a useful site for pre‐clinical assessment of microvascular processes that may underlie the future development of CVD in adulthood.
Abbreviations
- AVR
arteriole‐to‐venule ratio
- CRP
C‐reactive protein
- CVD
cardiovascular disease
- DVA
dynamic vessel analyzer
- IUGR
intra‐uterine growth retardation
- NO
nitric oxide
- OCT
optical coherence tomography
- sFLT‐1
fms‐like tyrosine kinase‐1
- T1DM
type 1 diabetes mellitus
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality globally. There is increasing evidence that the origins of CVD may be traced to vascular, metabolic and other processes that start in early life. This viewpoint is sometimes referred to as the salt hypothesis (Backes et al. 2013), the Dorner hypothesis (Kaess et al. 1975), or the Barker hypothesis (Barker et al. 1989). The effects of early life conditions and diseases that may influence the development of CVD in later life have been studied in several longitudinal studies (Barker et al. 1990, 2009; Napoli et al. 1999; Harding, 2001; Eriksson et al. 2003). In Barker hypothesis, also known as the ‘thrifty phenotype hypothesis’ (Ellison, 2005), intrauterine growth restriction due to fetal adaptation to metabolic and vascular processes is linked to the development of major CVD in late‐life (Barker, 2004 b).
Population‐based studies have also suggested that early life factors may be important determinants of trends and geographical differences in CVD mortality across populations (Forsdahl, 1979; Barker & Osmond, 1986; Ben‐Shlomo & Smith, 1991; Elford et al. 1992; Dorling et al. 2000; Leon & Davey Smith, 2000). Postulated mechanisms include persistent vascular and metabolic damage due to the exposure to CVD risk factors (e.g. high‐fat diet, obesity, elevated blood pressure) in early life. This in turn may trigger other epigenetic modifications leading to morphological, pathological and metabolic alterations in major tissues (e.g. fatty tissue), and organs (e.g. liver, pancreas, brain and kidney) (Fig. 1). Consistent with epidemiological studies are autopsy studies from early childhood showing atherosclerosis with fatty streaks in the aorta, and coronary and carotid arteries (Berenson et al. 1998; McGill et al. 2000 a,b).
Figure 1. Early life risk factors and adult cardiovascular disease .
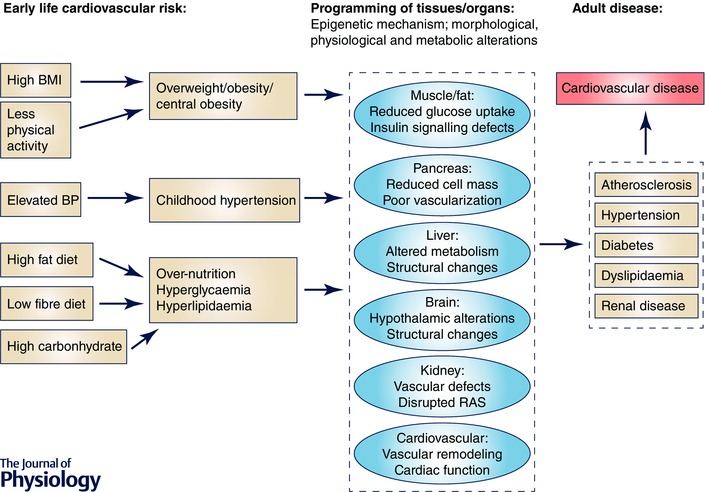
Early‐life risk factors shown in the first panel have been suggested to be associated with tissue or organ programming later in life. Such programming, due to the adaptation to existing environmental conditions, results in different phenotypes shown in the second panel. All together, they are known risk factors for future development of systemic disease and cardiovascular disease.
While these studies have provided some evidence for vascular damage in early life, most data are cross‐sectional in nature, making causal inferences from these studies difficult. Thus, the key question of which pathophysiological mechanisms explain the development and progression of CVD from early to later life remains unanswered. One pathway in the process of vascular damage involves endothelial dysfunction. Endothelial dysfunction generally refers to the reduction of nitric oxide (NO) bioavailability through decreased endothelial nitric oxide synthase expression (Griendling & FitzGerald, 2003). Animal studies have shown that endothelial damage leads to inhibition and promotion of the proliferation of smooth muscle cells. This further activates the aggregation of platelet and inflammatory cells disrupting the integrity of the microvasculature (Villar & Belizan, 1982; Nuyt, 2008). However, this area of research requires systematic and continuous long‐term monitoring of CVD risk factors and assessment of vascular changes over time.
In the past few decades, novel modalities including retinal vascular imaging have been developed to examine the systemic microvasculature in clinical studies (Liew et al. 2008 c; Strain et al. 2012; Struijker‐Boudier et al. 2012). Due to the non‐invasive nature of retinal imaging, it has been applied in a wide range of population‐based and clinical studies in persons of different ages (Strain et al. 2012). The morphology of retinal microvasculature is represented by a series of vascular parameters such as calibre of retinal arterioles and venules, and their tortuosity, branching angle and fractal dimension (Cheung et al. 2012). These parameters have been associated with a range of systemic risk factors (e.g. elevated blood pressure, hyperglycaemia, obesity) (Nguyen et al. 2008 b; Cheung et al. 2009 b, 2012; Jensen et al. 2010; Li et al. 2012, 2013; Gopinath et al. 2013 b; Xiao et al. 2015), and appear to predict the incidence of CVD, including stroke and heart disease, and are related to vascular and metabolic conditions (e.g. hypertension, diabetes, metabolic syndrome) (Wong et al. 2002 a,b; Ikram et al. 2006 a,b; McGeechan et al. 2008; Kawasaki et al. 2009; Nguyen et al. 2008 a). Furthermore, these morphological changes in the retinal vessels have been linked to several basic mechanisms involved in the development of CVD, such as inflammation, dyslipidaemia and endothelial dysfunction (Klein et al. 2006; Wong et al. 2006; Van Hecke et al. 2008; Gopinath et al. 2009; Yim‐Lui Cheung et al. 2010; Hanssen et al. 2012). In view of these developments, retinal vascular imaging can also be used as a potential tool to study early life factors related to CVD.
The use of retinal vascular imaging as a tool to study early life CVD risk factors was initiated by Hellstrom et al. and others, who proposed the concept of studying the retinal microvasculature in children in the 1990s (Hellstrom et al. 1997,1998). All these studies found a series of retinal vasculature abnormalities in children with low birth weight and even intra‐uterine growth retardation (IUGR), including lower branching points, narrower bifurcation angles, narrower retinal arteriolar calibre and wider retinal venular calibre (Chapman et al. 1997; Hellstrom et al. 1998, 2004; Minicucci et al. 1999; Kandasamy et al. 2012 a,b). These initial studies suggested that such vascular alteration might be associated with increased circulatory energy costs and suboptimal vascular architecture leading to an impairment of fetal development, which subsequently provides a mechanistic link to an increased risk of CVD in late‐life. Since then an increasing number of studies have used retinal vascular imaging to elucidate the role of the microvasculature in early life. Therefore, the aim of this systematic review is to summarize the results of retinal vascular imaging applied in studies of children and adolescents, and to determine the relationship of retinal vascular changes to CVD risk factors in early life.
Methods
Data source and study selection
We conducted a systematic review of publications with quantitative retinal vascular assessment in early life performed through two major online searching engines – PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) and Scopus (http://www.scopus.com). The following key words were used in the search criteria: retinal arterioles, retinal venules, retinal vascular calibre, retinal vessel diameter, retinal vessels, retinal microcirculation, retinal microvasculature, retinal vasculature, retinal imaging, childhood, early life, children and adolescents. Relevant papers published until 27 February 2015 were screened by their titles and abstracts. There were nearly 400,000 papers shown on both search engines with keyword searching. After combining the searching schemes, nearly 9000 papers were eligible (Fig. 2). Inclusion criteria of our systemic review were: epidemiological and/or clinical study, studies on children and/or adolescents, written in English, full text available through National University of Singapore library portal, quantitative assessment of retinal vascular parameters, and early life CVD risk factors. Early life CVD risk factors were classified into four groups: birth risk factors (e.g. low birth weight, shorter gestational weeks), environmental risk factors (e.g. parental hypertension, low physical activity, high‐fat diet), systemic risk factors (e.g. elevated blood pressure, overweight, obesity), and diseases linked to future CVD development (e.g. diabetes). Finally, 55 papers fitted the criteria and were included for data extraction.
Figure 2.
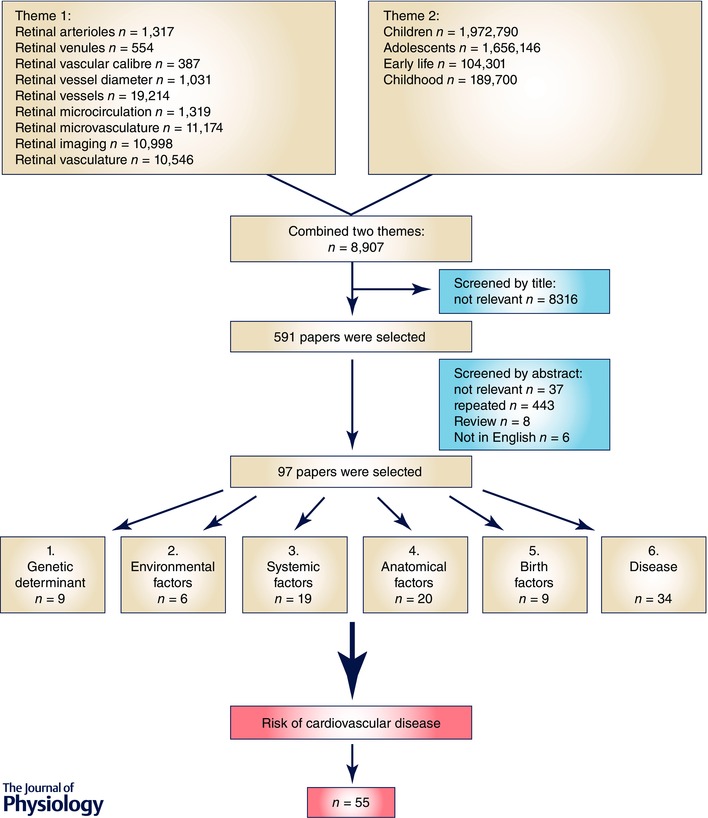
Flow chart illustrating the selection of research papers
Data extraction and table summary
A standard extraction form was used to summarize all the key findings from 55 papers: information included in the extraction form was first author's name, year of publication, country where data were collected, study design, sample size, response rate (if applicable), age, and changes in exposure and outcomes (either quantitative or qualitative assessment on retinal imaging or CVD risk).
Fundus photography and retinal vessel assessment
Retinal fundus examination allows for non‐invasive evaluation of retinal microvasculature. Recent population‐based studies have used computer‐assisted programmes to measure individual arterioles and venules and to combine them according to formulas developed firstly by Parr & Spears (1974 a,b), subsequently modified by Hubbard et al. (1999), and further improved by Knudtson et al. (2003). The use of computer‐assisted programmes differs in all population‐based epidemiological studies. For example, Computer Assisted Image Analysis of the Retina program (CAIAR) and Retinal Image MultiScale Analysis was used in UK adult studies (Mahal et al. 2009; Owen et al. 2011), retinal Imaging Software Fractal (IRIS‐Fractal) was used in an Australian children study (Gopinath et al. 2012 a, 2013 a), Non‐mydriatic Vessel Analyser (SVA‐T) was used in a German children study (Hanssen et al. 2012), Interactive Vessel Analysis (IVAN) was widely used in US studies (Wong et al. 2004; Liew et al. 2008 a) and Asian studies (Li et al. 2011 b), while Singapore I Vessel Assessment (SIVA) was newly developed and applied in recent Singaporean studies (Wong et al. 2002 b; Cheung et al. 2011 a).
Figure 3. Retinal microvasculature assessment on the grading platform .
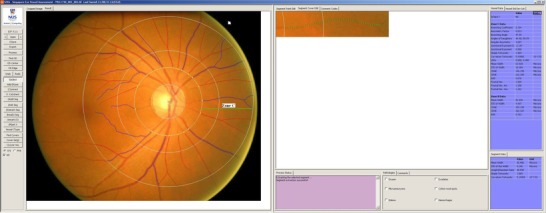
A screenshot of a computer‐assisted programme for measurement of new geometrical retinal vascular parameters from retinal fundus photograph. Zone C is marked in SIVA software by 0.5 to 2.0 optic disc diameters away from the margin of the optic disc. All retinal arterioles and venules larger than 25 μm are marked and assessed within zone C.
Retinal imaging analysis has enabled reproducible assessment of retinal microvascular parameters to quantify structural vascular morphological changes precisely (Wong et al. 2004). With the advancement of grading software such as SIVA (Singapore I Vessel Analysis, version 3.0, Singapore) (Fig. 4), a range of retinal static vascular parameters have been explored and widely used, such as retinal vascular calibre, retinal vascular tortuosity, retinal vascular branching angle and retinal vascular fractal dimension. A brief description of these parameters is provided below:
Retinal vascular calibre is represented as central retinal arteriolar equivalent (CRAE) and central retinal venular equivalent (CRVE). Pathological changes in such parameters have been identified as retinal arteriolar narrowing and retinal venular widening (Fig. 4) (Ikram et al. 2013).
Retinal vascular tortuosity is defined as the integral of the curvature square along the path of the vessel normalized by the total path length; it takes into account the bowing and points of inflection (Fig. 5) (Cheung et al. 2011 b). Increment of retinal vessel curvature tortuosity reflects a curvier vessel and has been identified as part of the pathological changes (Ikram et al. 2013).
Retinal vascular fractal dimension, which quantifies the complexity of the branching pattern of the retinal vascular tree, is defined as the gradient of logarithms of the number of boxes and the size of the boxes (Fig. 5) (Liew et al. 2008 b). A lower value for the fractal dimension reflects a sparser vascular network and has been found in diseases such as stroke and Alzheimer's disease (Cheung et al. 2014 a,b, 2015; Hilal et al. 2014; Ikram et al. 2013; Ong et al. 2014, 2015).
Retinal vascular branching angle is defined as the first angle subtended between two daughter vessels at each bifurcation (Fig. 5) (Cheung et al. 2011 a). Larger vessel branching angle might be indicative for pathological changes in retinal vascular geometry (Ikram et al. 2013).
Figure 4. Retinal arteriolar narrowing and retinal venular widening .
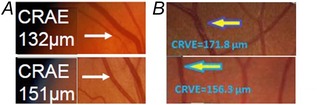
Retinal arteriolar narrowing is shown in (a). Retinal arteriolar caliber listed in the image on top has narrower (132 μm) than the image in the bottom (151 μm). Retinal venular widening is shown in (b). Retinal venular caliber listed in the image on top has wider (171.8 μm) than the image in the bottom (156.3 μm).
Figure 5. Retinal vascular geometry parameters .
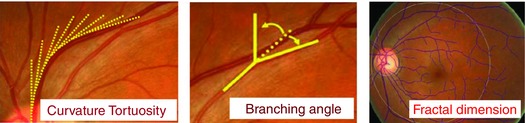
Retinal vascular tortuosity, retinal vascular branching angle and retinal vascular fractal dimension are shown. Tortuosity is derived from the integral of the curvature square along the path of the vessel, normalized by the total path length, which takes into account the bowing and points of inflection. Branching angle is defined as the first angle subtended between two daughter vessels at each bifurcation. Fractal dimension quantifies the complexity of the whole branching pattern of the retinal vascular tree and is defined as the gradient of logarithms of the number of boxes and the size the boxes.
Results
Birth risk factors and retinal microvasculature
A total of six papers (Cheung et al. 2007 a; Tapp et al. 2007; Mitchell et al. 2008; Sun et al. 2009 a; Gopinath et al. 2010 b; Kandasamy et al. 2011; Gishti et al. 2015 a) and one letter‐to‐the‐editor (Cheung et al. 2008) were published on the relationship between birth factors and retinal microvasculature (Table 1). Subjects ranging from newborn babies to adolescents around 16 years were included; these subjects were mainly of Asian and European origin. All studies were designed in a longitudinal and school‐based way. Consistent and significant findings were reported on the association between smaller birth size indexes and retinal arteriolar narrowing and/or retinal venular widening in these children and adolescents (Cheung et al. 2007 a, 2008; Tapp et al. 2007; Mitchell et al. 2008; Sun et al. 2009 a; Gopinath et al. 2010 b). Furthermore, subjects in the UK with lower birth weight and subjects in Australia with smaller head circumference had higher tortuosity and optimality deviance in retinal arterioles (Tapp et al. 2007) and lower retinal vascular fractal dimension (Gopinath et al. 2010 b). However, a recently published clinical study reported that infants born with lower birth weight tend to have both retinal arterioles and venules dilatation (Kandasamy et al. 2011). The difference might be due to the small sample size (n = 24) of this study.
Table 1.
Birth risk factors and retinal vasculature in early life
| Study population | Sample size | Arteriolar | Venular | ||||
|---|---|---|---|---|---|---|---|
| and study | and response | Age, | Cardiovascular | parameters β or | parameters β or | ||
| Study | design | rate | male % | risk factors | mean, 95 % CI | mean, 95 % CI | |
| 1 | Olta G | Population‐based prospective cohort study | 4122 | 6.2 years | Birth weight: | 0.26 SDS ↓ | n.s. |
| (2015) | Generation R | 61% | 50% | Low vs. Normal | (P trend ≤0.001) | ||
| 2 | Kandasamy Y | Clinical observational study | 24 newborn infants | Term newborn | Birth weight: | Calibre: | Calibre: |
| (2011) | Low vs. Normal | 113.1 vs. 86.4 μm | 151.7 vs. 128.4 μm | ||||
| (P = 0.0009) | (P = 0.0040) | ||||||
| 3 | Gopinath B | School‐based, longitudinal study | 2353 out of 3144 | 12.7 years | Birth weight: | Calibre: | Calibre: |
| (2010) | SCES | 75.3% | 50.4% | 1st vs. 4th quartile | 149.4 vs. 151.9 μm | n.s. | |
| (P trend = 0.001) | |||||||
| Birth length: | Calibre: | Calibre: | |||||
| 1st vs. 4th quartile | 148.9 vs. 151.2 μm | n.s. | |||||
| (P trend = 0.005) | |||||||
| Head circum: | Calibre: | Calibre: | |||||
| 1st vs. 4th quartile | 150.0 vs. 152.3 μm | n.s. | |||||
| (P trend = 0.03) | |||||||
| Birth weight: | Fractal dimension: | ||||||
| 1st vs. 4th quartile | n.s. | ||||||
| Birth length: | n.s. | ||||||
| 1st vs. 4th quartile | |||||||
| Head circum: | 1.466 vs. 1.469 (P trend = 0.03) | ||||||
| 1st vs. 4th quartile | |||||||
| 4 (2009a) | Sun C TEST | Population‐based, longitudinal | 266 twins (49 monozygotic and 84 dizygotic pairs) | 5–16 years MZ: 43% DZ: 53% | Birth length: each 5 cm ↓ | Calibre: −7.27 μm (−11.54, −3.01) (P < 0.001) | Calibre: n.s. |
| Head circum: | Calibre: | Calibre: | |||||
| each 2 cm ↓ | −2.55 μm | n.s. | |||||
| (−4.92, −0.18) | |||||||
| (P = 0.04) | |||||||
| Birth weight: | Calibre: | Calibre: | |||||
| each 1 kg ↓ | n.s. | n.s. | |||||
| 5 | Mitchell P | Population‐based, longitudinal study | 1369 out of 2238 | 12.7 years | Birth weight: | Calibre: | Calibre: |
| (2008) | SCES | 78.7% | 50.4% | each SD ↓ | −1.28 μm (−2.23, −0.32) | n.s. | |
| (P = 0.01) | |||||||
| SD = 569 g | |||||||
| Birth weight: | Calibre: | Calibre: | |||||
| Low vs. Normal | −3.73 μm (−7.09, −0.38) | n.s. | |||||
| (P = 0.03) | |||||||
| Birth length: | Calibre: | Calibre: | |||||
| each SD ↓ | −1.00 μm (−1.89, −0.12) | n.s. | |||||
| SD = 3.1 cm | (P = 0.03) | ||||||
| Head circum: | Calibre: | Calibre: | |||||
| each SD ↓ | −1.24 μm (−2.09, −0.38) | n.s. | |||||
| SD = 1.8 cm | (P = 0.006) | ||||||
| Gestational age: | Calibre: | Calibre: | |||||
| Premature vs. | −3.43 μm (−5.95, −0.91) | n.s. | |||||
| Full‐term | (P = 0.009) | ||||||
| 6 | Cheung N | School‐based, longitudinal study | 561 out of 768 children | 7–9 years | Birth weight: | Calibre: | Calibre: |
| (2008) | SCORM | 73.0% | 52.5% | Each SD ↓ | n.s. | 1.49 μm (0.18, 2.80) | |
| (SD = 453 g) | (P = 0.03) | ||||||
| Birth Weight: | 171.8 μm | ||||||
| Very low, < 2500 g | n.s | 167.6 μm | |||||
| Low, 2500–2999 g | 165.0 μm (P trend = 0.006) | ||||||
| Normal, ≥ 3000 g | |||||||
| 7 | Tapp RJ | Population‐based, longitudinal study | 166 children | 9 years | Birth weight: | Optimality deviance: | Optimality deviance: |
| (2007) | ALSPAC | 40% | each 1 kg ↓ | 0.190 | n.s. | ||
| (P = 0.021) | |||||||
| Simple tortuosity: | Simple tortuosity: | ||||||
| 0.179 | n.s. | ||||||
| (P = 0.026) | |||||||
| Bifurcation angle: | Bifurcation angle: | ||||||
| n.s. | n.s. | ||||||
| Bifurcation angle: | Length–diameter ratio: | ||||||
| n.s. | n.s. |
Abbreviation: 95% CI, 95% confidence interval; SD, standard deviation; circum, circumference; TEST, the Twins Eye Study in Tasmania; SCES: Sydney Children Eye Study; SCORM: the Singapore Cohort Study of the Risk Factors of Myopia; ALSPAC, the Avon Longitudinal Study of Parents and Children.
Environmental risk factors and retinal microvasculature
Seven papers (Gopinath et al. 2011 a,c, 2012 b, 2014; Hanssen et al. 2011; Poon et al. 2013; Islam et al. 2014) and one letter‐to‐the‐editor (Lim et al. 2009) published on associations between environmental risks and retinal microvasculature were part of the analysis (Table 2). The environmental risks included diet, parental hypertension and physical activity. All associations between environmental risks and retinal microvasculature were designed in a population/family‐based and cross‐sectional way. Four papers explored the relationship between unhealthy diet and retinal vasculature; however, the findings were not consistent. The Sydney Children Eye Study (SCES) found that children and adolescents with unhealthy diet including higher intake of sugar and carbohydrate and lower intake of yoghurt and fibre tend to have retinal arteriolar narrowing and suboptimal retinal arteriolar fractal dimension (Gopinath et al. 2012 b, 2014). However, some of findings could not be repeated in Singaporean children (Lim et al. 2009). Furthermore, a recent study done on 481 children and adolescents with type 1 diabetes reported no association between vitamin D intake and a series of retinal vascular parameters including calibre, tortuosity, length‐to‐diameter ratio, branching angle and fractal dimension; the results were the same for association with vitamin D deficiency as well (Poon et al. 2013). Two papers found a consistent relationship between parental blood pressure/hypertension history and changes of retinal microvasculature such as higher optimality deviation and larger arteriole‐to‐venule ratio (AVR) in children (Gopinath et al. 2011 a; Islam et al. 2014). Interestingly, physical activity and sedative activity such as TV viewing were also reflected by retinal imaging differently (Gopinath et al. 2011 c; Hanssen et al. 2011). Children with less outdoor physical activity and more TV viewing time had narrower retinal arterioles than had their counterparts.
Table 2.
Environmental risk factors and retinal microvasculature in early life
| Sample size and | Environmental | Arteriolar parameters β | Venular parameters β | ||||
|---|---|---|---|---|---|---|---|
| Study | Study design | response rate | Age, male % | factors | or mean, 95% CI | or mean, 95% CI | |
| 1 | Gopinath B | School‐based, cross‐sectional study | 888 | 17 years | Yoghurt, serves/day: | 160.9 vs. 162.2 μm | 237.9 vs. 235.9 μm |
| (2014) | SCES | 49.4% | 1st vs. 3rd tertile | (P trend = 0.05) | (P trend = 0.04) | ||
| 2 | Poon M | Clinical study, cross‐sectional | 481 | mean age: | Vitamin D deficiency: | Calibre/tortuosity/length–diameter ratio/ | |
| (2013) | 14.9 years | branching angle/fractal dimension: | |||||
| 52% | n.s. | ||||||
| 3 | Gopinath B | School‐based, cross‐sectional study | 2353 | 12 years | Carbohydrate intake: | Calibre: | Calibre: |
| (2012) | SCES | 49.4% | 1st vs. 3rd tertile | Girls: | Boys: | ||
| 153.1 vs. 151.7 μm | 217.6 vs. 219.9 μm | ||||||
| (P trend = 0.03) | (P trend = 0.02) | ||||||
| Fractal dimension: | Fractal dimension: | ||||||
| Girls: | Boys and girls: | ||||||
| 1.461 vs. 1.465 | n.s. | ||||||
| (P trend = 0.003) | |||||||
| Total sugar intake: | Calibre: | Calibre: | |||||
| 1st vs. 3rd tertile | Boys: | Boys and girls: | |||||
| 150.5 vs. 148.7 μm | n.s. | ||||||
| (P trend = 0.04) | Fractal dimension: | ||||||
| Fractal dimension: | Boys and girls: | ||||||
| Girls: | n.s. | ||||||
| 1.462 vs. 1.465 | |||||||
| (P trend = 0.002) | |||||||
| Total fibre intake: | Calibre: | Calibre: | |||||
| 1st vs. 3rd tertile | Boys and girls: | Boys and girls: | |||||
| n.s. | n.s. | ||||||
| Mean dietary GI: | Calibre: | Calibre: | |||||
| 1st vs. 3rd tertile | Girls: | Boys and girls: | |||||
| 153.5 vs. 151.7 μm | n.s. | ||||||
| (P trend = 0.03) | |||||||
| Mean dietary GL: | Calibre: | Calibre: | |||||
| 1st vs. 3rd tertile | Girls: | Boys and girls: | |||||
| 153.5 vs. 151.9 μm | n.s. | ||||||
| (P trend = 0.05) | Fractal dimension: | ||||||
| Fractal dimension: | Boys and girls: | ||||||
| Girls: | n.s. | ||||||
| 1.461 vs. 1.464 | |||||||
| (P trend = 0.01) | |||||||
| 4 | Cheung N | School‐based, cross‐sectional study | 823 | 12.8 years | Fibre/sugar/total fat/protein/energy intake: | Calibre: | Calibre: |
| (2009) | SCORM | 52.5% | n.s. | n.s. | |||
| 5 | Islam M | Family‐based, cross‐sectional study | 751 | 9–14 years | Paternal SBP: | Optimality deviation: | |
| (2014) | 49.6% | 1st vs. 5th quintile | 115.1 vs. 145.7 μm (P trend = 0.032) | ||||
| each 10 mmHg ↑ | 0.0053 (0.0001, 0.0106) (P = 0.047) | ||||||
| Paternal DBP: | 115.5 vs. 145.4 (P trend = 0.010) | ||||||
| 1st vs. 5th quintile | 0.0109 (0.0025, 0.0193) (P = 0.011) | ||||||
| each 10 mmHg ↑ | AVR: | ||||||
| Maternal SBP: | 0.83 vs. 0.80 (P trend = 0.013) | ||||||
| 1st vs. 5th quintile | 0.82 vs. 0.80 (P trend = 0.008) | ||||||
| Maternal DBP: | −0.0102 (−0.0198, −0.00007) (P = 0.035) | ||||||
| 1st vs. 5th quintile | |||||||
| each 10 mmHg ↑ | |||||||
| 6 | Gopinath B | School‐based, cross‐sectional study | 1739 out of 2238 | 6 years | Parental HTN: | Calibre: | Calibre: |
| (2011) | SCES | 77.7% | 50.4% | Yes vs. No | Girls: | Boys and girls: | |
| 161.6 vs. 165.9 μm | n.s. | ||||||
| (P = 0.0004) | |||||||
| Parental HTN: | Calibre: | Calibre: | |||||
| Yes vs. No | Girls: | Boys and girls: | |||||
| 161.6 vs. 165.9 μm | n.s. | ||||||
| (P = 0.0004) | |||||||
| Maternal HTN: | Calibre: | Calibre: | |||||
| Yes vs. No | Girls: | Boys and girls: | |||||
| 161.2 vs. 165.7 μm | n.s. | ||||||
| (P = 0.01) | |||||||
| 7 | Hanssen H | School‐based, cross‐sectional study | 578 out of 792 | 11.1 years | Physical inactivity: | AVR: | |
| (2011) | JuvenTUM 3 | 73.0% | 41.5% | each 1 h/week ↑ | <0.001 (< 0.001, < 0.001) (P = 0.032) | ||
| (2008) | |||||||
| 8 | Gopinath B | School‐based, cross‐sectional study | 1765 out of 2238 | 6 years | Outdoor sporting activities: | Calibre: | Calibre: |
| (2011) | SCES | 50.4% | Low vs. High | 162.5 vs. 164.7 μm | n.s. | ||
| Television viewing: | (P trend = 0.004) | ||||||
| 1st vs. 4th quartile | Calibre: | Calibre: | |||||
| 164.2 vs. 161.9 μm | n.s. | ||||||
| (P trend = 0.003) | |||||||
Abbreviation: 95% CI, 95% confidence interval; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; AVR, arteriovenous ratio; SCES: Sydney Children Eye Study; SCORM: the Singapore Cohort Study of the Risk Factors of Myopia.
Systemic risk factors and retinal microvasculature
Elevated blood pressure
A body of evidence has confirmed the relationship between elevated blood pressure, childhood hypertension and retinal microvasculature. Thirteen papers have published consistent findings from pre‐schoolers to adolescents (Table 3) (Mitchell et al. 2007; Gopinath et al. 2010 a, 2013 b; Tapp et al. 2007, 2013; Li et al. 2011 b; Owen et al. 2011; Hanssen et al. 2012; Kurniawan et al. 2012; Murgan et al. 2013; Sasongko et al. 2010; Zheng et al. 2013; Gishti et al. 2015 b). A wide range of retinal vascular parameters were studied among all these original articles, such as retinal vessel width, fractal dimension, tortuosity and length‐to‐diameter ratio. Elevated peripheral and central blood pressure and subsequent childhood hypertension were associated with narrower retinal arteriolar calibre, wider retinal venules, more tortuous retinal arterioles and lower retinal arteriolar fractal dimension and length‐to‐diameter ratio (Mitchell et al. 2007; Gopinath et al. 2010 a, 2013 b; Sasongko et al. 2010; Li et al. 2011 b; Owen et al. 2011; Hanssen et al. 2012; Kurniawan et al. 2012; Murgan et al. 2013; Tapp et al. 2013; Zheng et al. 2013). In a group of 166 UK children aged 9 years, increased heart rate was also found to be associated with lower simple tortuosity (Tapp et al. 2007).
Table 3.
Elevated blood pressure and retinal vasculature in early life
| Study population | Sample size and | Arteriolar parameters β | Venular parameters β | ||||
|---|---|---|---|---|---|---|---|
| Study | and study design | response rate | Age, male % | Blood pressure | or mean, 95 % CI | or mean, 95 % CI | |
| 1 | Olta G | Population‐based, cross‐sectional study | 4007, 61.4% | 6.0 | per SDS ↓ | per SDS ↑ | |
| (2015) | Generation R | 50.2% | SBP: | 0.19 SDS ↑ (P < 0.05) | 0.05 SDS ↑ (P < 0.05) | ||
| DBP: | 0.13 SDS ↑ (P < 0.05) | n.s. | |||||
| Pulse pressure: | 0.10 SDS ↑ (P < 0.05) | 0.05 SDS ↑ (P < 0.05) | |||||
| Carotid femoral | n.s. | 0.04 SDS ↑ (P < 0.05) | |||||
| pulse wave | |||||||
| velocity: | |||||||
| 2 | Gopinath B | Population‐based, cross‐sectional study | 1077 | 3–6 years | SBP: | Calibre: | Calibre: |
| (2013) | SCES | 50.8% | each 10 mm Hg ↑ | −1.70 μm (P = 0.02) | n.s. | ||
| 3 | Zheng YF | Population‐based, cross‐sectional study | 1035 twin pairs | 7–19 years | MAP: | Calibre: | Calibre: |
| (2013) | The Guangzhou Twin Eye Study | (657 MZ, 378 DZ) | 51.8% | each 10 mm Hg ↑ | −1.48 μm (P < 0.005) | n.s. | |
| 4 | Tapp RJ | Population‐based, cross‐sectional study | 1067 | 12 years | SBP at 9 years: | Calibre: | Calibre: |
| (2013) | ALSPAC | 49% | each SD ↑ | −0.21 μm (−0.33, −0.09) | n.s. | ||
| DBP at 9 years: | (P < 0.001) | n.s. | |||||
| each SD ↑ | −0.16 μm (−0.27, −0.05) | Calibre: | |||||
| SBP at 11 years: | (P = 0.004) | n.s. | |||||
| each SD ↑ | Calibre: | n.s. | |||||
| DBP at 11 years: | −0.20 μm (−0.13, −0.08) | ||||||
| each SD ↑ | (P = 0.001) | ||||||
| −0.13 μm (−0.24, −0.02) | |||||||
| (P = 0.019) | |||||||
| 5 | Murgan I | Clinical research, cross‐sectional study | 121 | 16 years | Central SBP: | Calibre: | |
| (2013) | 56.2% | each 1 mm Hg ↑ | −0.096 (P = 0.023) | ||||
| 6 | Hanssen H | School‐based, cross‐sectional study | 578 out of 792 | 11.1 | SBP: | Calibre: | Calibre: |
| (2012) | JuvenTUM 3 | 73.0% | 41.5% | each 10 mm Hg ↑ | −1.34 μm (−2.61, −0.06) | n.s. | |
| DBP: | (P = 0.040) | n.s. | |||||
| each 10 mm Hg ↑ | −2.53 μm (−4.15, −0.92) | ||||||
| (P = 0.002) | |||||||
| 7 | Li LJ | Population‐based, cross‐sectional study | 385 | 4–5 years | SBP: | Calibre: | Calibre: |
| (2011) | STARS | 50.7% | each 10 mm Hg ↑ | −2.00 μm (−3.61, −0.39) | 2.51 (0.35, 4.68) | ||
| DBP: | (P = 0.02) | (P = 0.02) | |||||
| each 10 mm Hg ↑ | n.s. | n.s. | |||||
| MABP: | −1.39 μm (−2.95, 0.17) | 2.15 (0.06, 4.24) | |||||
| each 10 mm Hg ↑ | (P = 0.08) | (P = 0.04) | |||||
| Hypertensive | 160.15 vs. 156.06 μm | n.s. | |||||
| stage: | (P = 0.02) | ||||||
| No vs. Yes | |||||||
| 8 | Owen CG | School‐based, cross‐sectional study | 986 | 10–11 | DBP: | Tortuosity: | Tortuosity: |
| (2011) | CHASE | Years | each SD ↑ | 2.3% (0.1%, 4.6%) | n.s. | ||
| 46.9% | SD = 9.1 mm Hg | (P = 0.01) | |||||
| 9 | Kurniawan ED | School‐based, cross‐sectional study | 1174 | 10–14 years | SBP: | Fractal dimension: | Fractal dimension: |
| (2011) | SCORM | 49.1% | each 10 mm Hg ↑ | −0.74 (−1.37, −0.11) | n.s | ||
| DBP: | (P = 0.021) | ||||||
| each 10 mm Hg ↑ | n.s. | ||||||
| MABP: | −0.71 (−1.31, −0.10) | ||||||
| each 10 mm Hg ↑ | (P = 0.022) | ||||||
| 10 | Gopinath B | Australian adolescents | 2353 out of 3144 | 12.7 years | SBP: | Calibre: | Calibre: |
| (2010) | SCES | Population‐based, cross‐sectional study | 75.3% | 50.4% | 1st vs. 4th quartile | 153.9 vs. 149.7 μm | n.s. |
| DBP: | (P trend < 0.0001) | n.s. | |||||
| 1st vs. 4th quartile | 153.0 vs. 149.4 μm | n.s. | |||||
| MABP: | (P trend < 0.0001) | n.s. | |||||
| 1st vs. 4th quartile | 153.4 vs. 149.6 μm | ||||||
| Hypertensive | (P trend < 0.0001) | ||||||
| stage: | 151.9 vs. 149.9 μm | ||||||
| No vs. Yes | (P = 0.002) | ||||||
| 11 | Sasongko MB | Clinic‐based, cross‐sectional study | 944 out of 1159 | 12–20 | SBP: | Length–diameter ratio: | Length–diameter ratio: |
| (2010) | SPDS | 81.4% | years | each SD ↑ | −10.0 (−16.3, −3.69) | n.s. | |
| with type 1 diabetes | 43.7% | (SD = 12 mm Hg) | (P = 0.003) | ||||
| 12 | Tapp RJ | Population‐based, cross‐sectional study | 166 children | 9 years | Heart rate: | Simple tortuosity: | Optimality deviance/ simple tortuosity/ bifurcation angle/ length–diameter ratio: |
| (2007) | ALSPAC | 40% | each 1 BPM ↑ | −0.190 | n.s. | ||
| (P = 0.019) | |||||||
| 13 | Mitchell P | School‐based, cross‐sectional study | 1572 Australian children | SCES: | SBP: | Calibre: | Calibre: |
| (2007) | SCES, SCORM | 380 Singapore | 6–8 years | each 10 mm Hg ↑ | SCES: −2.08 μm | SCES: −1.12 μm | |
| children | 50.8% | DBP: | (−2.79, −1.38) | (−2.00, −0.24) | |||
| SCORM: | each 10 mm Hg ↑ | (P < 0.0001) | (P = 0.013) | ||||
| 7–9 years | MABP: | SCORM: −1.43 μm | SCROM: n.s. | ||||
| 56.8% | each 10 mm Hg ↑ | (−2.59, −0.27) | SCES: n.s. | ||||
| (P = 0.016) | SCORM: n.s. | ||||||
| SCES: −1.46 | SCES: −0.99 | ||||||
| (−2.16, −0.78) | (−1.95, −0.04) | ||||||
| (P < 0.001) | (P = 0.041) | ||||||
| SCORM: −2.30 | SCORM: n.s. | ||||||
| (−4.00, −0.59) | |||||||
| (P = 0.008) | |||||||
| SCES: −2.00 | |||||||
| (−2.76, −1.24) | |||||||
| (P < 0.001) | |||||||
| SCORM: −2.45 | |||||||
| (−4.11, −0.79) | |||||||
| (P = 0.004) |
Abbreviation: 95% CI: 95% confidence interval; SD, standard deviation; SBP, systolic blood pressure; DBP, diastolic blood pressure; MABP: mean arterial blood pressure; BPM, beats per minute; SCES, Sydney Children Eye Study; ALSPAC, the Avon Longitudinal Study of Parents and Children; STARS, the Strabismus, Amblyopia and Refractive Error Study in Singapore Chinese Preschoolers; SCORM, the Singapore Cohort Study of the Risk Factors for Myopia; CHASE, the Child Heart and Health Study in England; SPDS, Sydney Pediatric Diabetes Study.
Anthropometric indexes
As a phenotypic indication for overweight and obesity, anthropometric indexes and retinal microvasculature was widely investigated across all races and ages (Table 4) (Cheung et al. 2007 b; Tapp et al. 2007, 2013; Taylor et al. 2007; Sasongko et al. 2010; Gopinath et al. 2011 b, 2013 b; Li et al. 2011 a; Owen et al. 2011; Hanssen et al. 2012; Zheng et al. 2013; Siegrist et al. 2014; Xiao et al. 2015; Gishti et al. 2015 d). Among children and adolescents, BMI, ponderal index, waist circumference, skinfold thickness indexes, fat mass index, body water percentage and trunk fat percentage parameters were all used to evaluate the body composition. It was observed that if a child or adolescent had higher index for body composition or fat deposition, he/she had retinal venular widening consistently with or without retinal arteriolar narrowing. Interestingly, retinal venular calibre seemed to be the most sensitive index to reflect body composition among all retinal vascular parameters such as retinal arteriolar calibre, fractal dimension, tortuosity, length‐to‐diameter ratio and optimality deviation. Aside from structural retinal vasculature, functional changes were also investigated in 77 children and adolescents with either type 1 diabetes mellitus (T1DM) or overweight or obesity. Retinal venular dilatory response under flicker light examination was found to be associated with increased BMI (Schiel et al. 2009).
Table 4.
Anthropometric indexes and retinal vasculature in early life
| Study population and | Sample size and | Age, male | Anthropometric | Arteriolar parameters β | Venular parameters β | ||
|---|---|---|---|---|---|---|---|
| Study | study design | response rate | % | measurements | or mean, 95 % CI | or mean, 95 % CI | |
| 1 | Gishti O | Population‐based, cross‐sectional study | 4145 | 6.0 years | Per SDS ↓: | n.s.. | |
| (2015) | Generation R | 61% | 50% | BMI: | 0.06 SDS ↑ (P < 0.05) | ||
| Total body fat mass: | 0.05 SDS ↑ (P < 0.05) | ||||||
| 2 | Xiao Wei | Population‐based, cross‐sectional study | 444 twins | 12–19 years | Fat mass index | Calibre: | Calibre: |
| (2015) | The Guangzhou Twin Eye Study | 46.4% | (kg m−2) | 147.2 vs. 148.4 μm | 211.7 vs. 218.2 μm | ||
| 1st vs. 4th quartile | (P trend = 0.005) | (P trend = 0.005) | |||||
| Body water %, | Calibre: | Calibre: | |||||
| 1st vs. 4th quartile | 148.3 vs. 147.9 μm | 218.9 vs. 213.2 μm | |||||
| Trunk fat %, | (P trend = 0.009) | (P trend = 0.001) | |||||
| 1st vs. 4th quartile | Calibre: | Calibre: | |||||
| Triceps skinfold, mm | 147.0 vs. 148.7 μm | 211.7 vs. 218.8 μm | |||||
| 1st vs. 4th quartile | (P trend = 0.007) | (P trend = 0.001) | |||||
| BMI (kg m−2) | Calibre: | Calibre: | |||||
| 1st vs. 4th quartile | 147.7 vs. 148.2 μm | 211.5 vs. 218.0 μm | |||||
| (P trend = 0.007) | (P trend = 0.002) | ||||||
| Calibre: | Calibre: | ||||||
| 149.4 vs. 147.1 μm | 213.1 vs. 216.3 μm | ||||||
| (P trend = 0.026) | (P trend = 0.020) | ||||||
| 3 | Siegrist M | Randomized controlled school‐and family‐based lifestyle interventional trial | 792 | 10–11 years | BMI: | AVR: | |
| (2014) | 58.5% | each 1 kg m−2 | −0.003 (−0.004, −0.001) | ||||
| (P = 0.003) | |||||||
| 4 | Zheng YF | Population‐based, | 1035 twin pairs | 7–19 years | BMI: | Calibre: | Calibre: |
| (2013) | The Guangzhou Twin Eye Study | cross‐sectional study | (657 MZ, 378 DZ) | 51.8% | each 1 kg m−2 ↑ | −0.30 μm (P = 0.01) | 0.43 μm (P < 0.05) |
| 5 | Tapp RJ | Population‐based, cross‐sectional study | 1067 | 12 years | Fat mass at 9 years: | Calibre: | Calibre: |
| (2013) | ALSPAC | 49% | each SD ↑ | n.s. | 0.19 (0.03, 0.35) | ||
| Fat mass at 11 | Calibre: | (P = 0.022) | |||||
| years: | n.s. | Calibre: | |||||
| each SD ↑ | 0.25 (0.10, 0.40) | ||||||
| (P = 0.001) | |||||||
| 6 | Gopinath B | Australian pre‐schoolers, population‐based, cross‐sectional study | 1077 | 3–6 years | Healthy vs. Overweight vs. Obese | Calibre: | Calibre: |
| (2013) | SCES | 159.5 μm vs. 156.3 μm | 214.1 μm vs. 218.7 μm | ||||
| 50.5% | vs. 153.4 μm | vs.220.5 μm | |||||
| (P trend = 0.01) | (P trend = 0.01) | ||||||
| 7 | Hanssen H | German school children at 5th grade School‐based cross‐sectional study | 578 out of 792 | 11.1 years | BMI : | Calibre: | Calibre: |
| (2012) | JuvenTUM 3 | 73.0% | 41.5% | each 1 kg m−2 ↑ | −0.374 μm (−0.724, −0.025) | 0.369 μm (0.007, 0.731) | |
| (P = 0.036) | (P = 0.046) | ||||||
| Obese vs. Overweight vs. | AVR: | ||||||
| Normal weight | 0.85 vs. 0.87 vs. 0.89 (P ≤ 0.05) | ||||||
| PBF: | AVR: | ||||||
| each 1% ↑ | −0.001 (−0.002, <0.001) (P = 0.002) | ||||||
| Waist circum: | AVR: | ||||||
| each 1 mm ↑ | −0.001 (−0.002, <0.001) (P < 0.001) | ||||||
| 8 | Li LJ | Population‐based, cross‐sectional study | 136 | 6–16 years | BMI: | Calibre: | Calibre: |
| (2012) | STARS | 45.6% | each SD ↑ | n.s. | 3.40 μm | ||
| SD = 3.53 kg m−2 | n.s. | (P = 0.005) | |||||
| Above vs. Below | n.s. | 227.38 vs. 218.05 | |||||
| threshold | n.s. | (P = 0.021) | |||||
| TSF: | 2.94 μm | ||||||
| each SD ↑ | (P = 0.012) | ||||||
| SD = 4.49 mm | 227.96 vs. 217.75 | ||||||
| Above vs. Below threshold | (P = 0.001) | ||||||
| 9 | Gopinath B | Australian adolescents | 2353 out of 3144 | 12.7 years | BMI: | Calibre: | Calibre: |
| (2011) | SCES | Population‐based, cross‐sectional study | 75.3% | 50.4% | 4th vs. 1st quartile | 150.0 vs. 152.8 μm | 221.1 vs. 216.9 μm |
| (P < 0.0001) | (P = 0.0009) | ||||||
| Fractal dimension: | |||||||
| n.s. | |||||||
| Obese vs. Overweight vs. | Calibre: | Calibre: | |||||
| Normal weight | 149.2 vs. 150.6 vs. 152.0 μm | 222.6 vs. 220.4 vs. 218.1 μm | |||||
| (P = 0.01) | (P = 0.01) | ||||||
| 10 | Schiel R | Clinical study, cross‐sectional | 77 | 6–16 Years | BMI (kg m−2) | Dilatation: | Dilatation: |
| (2009) | n.s. | r = 0.336 (P = 0.026) | |||||
| 11 | Taylor | Population‐based, cross‐sectional study | 1608 out of 1740 | 6 years | BMI: | Calibre: | Calibre: |
| SCES | 92.4% | 50.8% | each SD ↑ | −0.76 μm↓ | 1.13 μm | ||
| (2007) | SD = 2.14 kg m−2 | (−1.43, −0.08) | (0.11, 2.15) | ||||
| Above vs. Below BMI threshold | Calibre: | Calibre: | |||||
| 162.0 vs. 163.7 μm | 231.7 vs. 229.0 | ||||||
| (P = 0.0029) | (P = 0.0007) | ||||||
| Waist circum: | Calibre: | Calibre: | |||||
| each SD ↑ | n.s. | 0.99 μm (0.15, 1.84) | |||||
| SD = 5.14 cm | |||||||
| BSA: | Calibre: | Calibre: | |||||
| each SD ↑ | n.s. | 1.97 μm (0.86, 3.09) | |||||
| SD = 0.099 m2 | |||||||
| 12 | Cheung N | Singapore Chinese | 768 | 7–9 Years | BMI: | Calibre: | Calibre: |
| (2006) | SCORM | School‐based, cross‐sectional study | each SD ↑ | n.s. | 2.19 (0.23, 4.15) | ||
| 52.5% | SD = 3.1 kg m−2 | (P = 0.03) | |||||
| 13 | Owen CG | School‐based, cross‐sectional study | 986 | 10–11 years | Ponderal index: | Tortuosity: | Tortuosity: |
| (2011) | CHASE | 46.9% | each 1 kg m−3 ↑ | n.s. | n.s. | ||
| Waist circum: | n.s. | n.s. | |||||
| each 1 cm ↑ | n.s. | n.s. | |||||
| Sum of skinfolds: | n.s. | n.s. | |||||
| each 1 mm ↑ | |||||||
| Fat mass index: | |||||||
| each 1 kg m−5 ↑ | |||||||
| 14 | Sasongko MB | Clinic‐based, cross‐sectional study | 944 out of 1159 | 12–20 years | BMI: | Tortuosity/branchingnangle/optimality deviation/length–diameter ratio: | |
| (2010) | SPDS | 81.4% | 43.7% | each SD ↑ | n.s. | ||
| with type 1 diabetes | SD = 3.5 kg m−2 | ||||||
| 15 | Tapp RJ | Population‐based, cross‐sectional study | 166 children | 9 years | BMI: | All vascular parameters: | All vascular parameters: |
| (2007) | ALSPAC | 40% | each 1 kg m−2 ↑ | n.s. | n.s. | ||
Abbreviation: 95% CI: 95% confidence interval; SD, standard deviation; PBF, percentage body fat; BMI, body mass index; circum, circumference; BSA, body surface area; SCES, the Sydney Children Eye Study; ALSPAC, the Avon Longitudinal Study of Parents and Children; STARS, the Strabismus, Amblyopia and Refractive Error Study in Singapore Chinese Preschoolers; SCORM, the Singapore Cohort Study of the Risk Factors for Myopia; CHASE, the Child Heart and Health Study in England; SPDS, Sydney Pediatric Diabetes Study.
Inflammation, hyperglycaemia, dyslipidaemia and angiogenesis
Systemic conditions such as inflammation, hyperglycaemia, dyslipidaemia and angiogenesis were well recognized to be on the path of developing atherosclerosis and future CVD. In the last 5 years, researchers have started to look into the early indication of such a process in children and adolescents. Five papers published cross‐sectional data and one paper published longitudinal data on relevant topics (Table 5) (Owen et al. 2011; Sasongko et al. 2010; Hanssen et al. 2012; Siegrist et al. 2014; Gishti et al. 2015 c,d). Inflammation biomarkers such as C‐reactive protein (CRP), hyperglycaemia (indicated by high glucose, HbA1C and insulin level), dyslipidaemia (indicated by cholesterol and low density lipoprotein, high density lipoprotein, leptin and triglyceride) were all associated with retinal venular widening and more tortuous retinal arterioles (Sasongko et al. 2010; Owen et al. 2011; Hanssen et al. 2012; Siegrist et al. 2014). Interestingly, a group of Dutch researchers also found significant associations between indexes for maternal angiogenesis (e.g. placental growth factor, soluble fms‐like tyrosine kinase‐1 (sFLT‐1)) and their ongoing impact on children turning 6 years (Gishti et al. 2015 c).
Table 5.
Inflammation, dyslipdemia and angiogenesis and retinal microvasculature in early life
| Study population and | Sample size and | Age, male | Arteriolar parameters β | Venular parameters β | |||
|---|---|---|---|---|---|---|---|
| Study | study design | response rate | % | Serum biomarkers | or mean, 95 % CI | or mean, 95 % CI | |
| 1 | Gishti O | Population‐based, longitudinal study | 3505 | 6 years | PIGF at 2nd trimester: | Calibre: | Calibre: |
| (2015) | Generation R | 61% | 50% | < 145.80 pg ml−1 vs. > 145.80 pg ml−1 (reference) | −0.11 (−0.19, −0.03) | n.s. | |
| sFLT‐1 at 2nd trimester: | (P = 0.008) | Calibre: | |||||
| > 7.35 ng ml−1 vs. < 7.35 ng ml−1 (reference) | Calibre: | 0.09 (0.01, 0.17) | |||||
| Per SDS↑ in CRP: | 0.10 (0.02, 0.17) | (P = 0.03) | |||||
| (P = 0.02) | 0.09 SDS ↑ | ||||||
| n.s. | (P < 0.05) | ||||||
| 2 | Siegrist M | Randomized controlled school‐and family‐based lifestyle interventional trial | 10–11 years | Adiponectin/IL‐6: | Calibre: | Calibre: | |
| (2014) | 792 | 58.5% | each unit ↑ | n.s. | n.s. | ||
| Insulin: | Calibre: | Calibre: | |||||
| each 1 μU ml−1 ↑ | 0.152 (0.001, 0.305) | n.s. | |||||
| Leptin: | (P = 0.046) | Calibre: | |||||
| 1st vs. 4th quartile | Calibre: | 232.8 vs. 239.9 μm | |||||
| n.s. | (P trend = 0.009) | ||||||
| 3 | Hanssen H | School‐based, cross‐sectional study | 578 out of 792 | 11.1 years | CRP: | Calibre: | Calibre: |
| (2012) | JuvenTUM 3 | 73.0% | 41.5% | each 1 mg l−1 ↑ | n.s. | 11.01 μm | |
| (4.164, 17.859) | |||||||
| (P = 0.002) | |||||||
| HDL‐C: | Calibre: | Calibre: | |||||
| each 1 unit ↑ | n.s. | n.s. | |||||
| Triglyceride: | Calibre: | Calibre: | |||||
| each 1 unit ↑ | n.s. | n.s. | |||||
| Glucose: | Calibre: | Calibre: | |||||
| each 1 unit ↑ | n.s. | n.s. | |||||
| 4 | Owen C | Cross‐sectional study | 968 UK children | 10–11 years | Triglyceride: | Tortuosity: | Tortuosity: |
| (2011) | CHASE | 46.9% | each 1 mmol l−1 ↑ | 3.8% | n.s. | ||
| (1.0%, 6.6%) | |||||||
| (P = 0.01) | |||||||
| Total cholesterol: | Tortuosity: | Tortuosity: | |||||
| each 1 mmol l−1 ↑ | 3.2% ↑ | n.s. | |||||
| (0.8%, 5.7%) | |||||||
| (P = 0.01) | |||||||
| LDL‐C: | Tortuosity: | Tortuosity: | |||||
| each 1 mmol l−1 ↑ | 2.9% ↑ | n.s. | |||||
| (0.4%, 5.5%) | |||||||
| (P = 0.02) | |||||||
| Glucose/HbA1C/ | Tortuosity: | Tortuosity: | |||||
| insulin/CRP/HDL‐C: | n.s. | n.s. | |||||
| each unit ↑ | |||||||
| 5 | Sasongko MB | Clinic‐based, cross‐sectional study | 944 out of 1159 | 12–20 years | HbA1C: | Tortuosity: | Tortuosity: |
| (2010) | SPDS | 81.4% | 43.7% | ≤ 8.5% vs. > 8.5% | 1.75 (0.45, 3.05) | n.s. | |
| (P = 0.014) | |||||||
| Cholesterol: | Tortuosity: | Tortuosity: | |||||
| each SD ↑ | n.s. | n.s. | |||||
| SD = 0.90 mmol l−1 | Branching angle: | Branching angle: | |||||
| n.s. | n.s. | ||||||
| optimality deviation (×102): | optimality deviation (×102): | ||||||
| n.s. | −1.90 (−3.75, −0.06) | ||||||
| LDR: | (P = 0.015) | ||||||
| 6.92 (0.19, 13.6) | LDR: | ||||||
| (P = 0.041) | n.s. |
Abbreviation: 95% CI: 95% confidence interval; SD, standard deviation; CRP, C‐reactive protein; HDL‐C, high density lipoprotein cholesterol; LDL‐C, low density lipoprotein cholesterol; HbA1C, glycosylated haemoglobin; LDR, length‐to‐diameter ratio; CHASE, the Child Heart and Health Study in England; SPDS, Sydney Pediatric Diabetes Study.
Disease linked to future CVD and retinal microvasculature
There are a series of diseases in adults that have been identified as being associated with vascular damage with the possibility of leading to future cardiovascular disease, such as hypertension, diabetes, depression and metabolic syndrome. A total of 14 papers were published on a wide range of early life diseases including childhood hypertension, T1DM, metabolic syndrome, carotid plaque, and microvascular complications (e.g. retinopathy and nephropathy) in T1DM paediatric patients (Table 6) (Alibrahim et al. 2006; Kifley et al. 2007; Cheung et al. 2009 a; Gopinath et al. 2010 a; Sasongko et al. 2010, 2011, 2012; Benitez‐Aguirre et al. 2011, 2012; Li et al. 2011 b, 2014; Bronson‐Castain et al. 2012; Hosking et al. 2013; Meier et al. 2014; Yau et al. 2014; Gishti et al. 2015 b). Except for four studies that were longitudinal (on T1DM young patients) (Kifley et al. 2007; Benitez‐Aguirre et al. 2011, 2012; Cheung et al. 2009 a), the rest of the studies were published on cross‐sectional data (four on T1DM, two on hypertension, one on depression and anxiety, one on metabolic syndrome, and one on carotid plaque) (Bronson‐Castain et al. 2012; Gopinath et al. 2010 a; Hosking et al. 2013; Li et al. 2011 b, 2014; Meier et al. 2014; Sasongko et al. 2010, 2011, 2012; Yau et al. 2014). The majority of the papers (9 out of 14) had investigated microvascular changes and complications in T1DM (Kifley et al. 2007; Cheung et al. 2009 a; Sasongko et al. 2010, 2011, 2012; Benitez‐Aguirre et al. 2011, 2012; Bronson‐Castain et al. 2012; Hosking et al. 2013). Consistent cross‐sectional findings in five papers suggested that retinal venular calibre widening was commonly seen in T1DM. Moreover, longer duration of T1DM was also associated with more tortuosity of retinal arterioles and higher retinal arteriolar and venular optimality deviation (Bronson‐Castain et al. 2012; Hosking et al. 2013; Sasongko et al. 2010, 2011, 2012). Abnormal retinal vascular morphology such as wider retinal venular calibre, higher retinal arteriolar tortuosity and larger length‐to‐diameter ratio was related to concurrent and incident microvascular complications in both nephropathy and retinopathy among T1DM young patients (Kifley et al. 2007; Cheung et al. 2009 a; Benitez‐Aguirre et al. 2011, 2012). As for childhood‐specific hypertension, two studies on Singaporean pre‐schoolers and Sydney adolescent children reported similar findings on significant narrowing of retinal arterioles (Gopinath et al. 2010 a; Li et al. 2011 b). There were three papers published on mental health, carotid plaque and metabolic syndrome in children and adolescents. Narrowing in retinal arteriolar calibre was suggested to be associated with higher risks in carotid plaque (Li et al. 2014), smaller white matter volume (Yau et al. 2014) and presence of metabolic syndrome (Yau et al. 2014), yet with lower risks in depression and anxiety (Meier et al. 2014).
Table 6.
Disease linked to future CVD and retinal microvasculature in early life
| Study population | Sample size and | Diseases that will | Arteriolar parameters β | Venular parameters β | |||
|---|---|---|---|---|---|---|---|
| Study | and study design | response rate | Age, male % | linked to CVD | or mean, 95 % CI | or mean, 95 % CI | |
| 1 | Olta G | population‐based, cross‐sectional study | 4007, 61.4% | 6.0 | per SDS ↓ | per SDS ↓ | |
| (2015) | Generation R | 50.2% | Childhood HTN: | OR: 1,35 (1.21, 1.45) | OR: 1.19 (1.00, 1.32) | ||
| 2 | Meier MH | Population‐based, longitudinal study | 865 | mean age: 16.5 years | Depression/axiety: | Calibre: | Calibre: |
| (2014) | Brisbane Twin Study | 42.8% | each 1 score↑ | 0.08 μm (SE, 0.036) | n.s. | ||
| Overall mental health: | (P = 0.025) | Calibre: | |||||
| each 1 score ↑ | Calibre: | n.s. | |||||
| 0.08 μm (SE, 0.036) | |||||||
| (P = 0.028) | |||||||
| 3 | Li LX | Clinical study, cross‐sectional | 2970 | 15–90 years | Carotid intima‐media thickness (CIMT): | Retinal microvascular abnormalities (retinal arteriolar narrowing, retinopathy) | |
| (2014) | 55.8% | 0.078 mm (0.080, 0.262) (P < 0.001) | Presence | ||||
| Carotid plaque: | Presence | ||||||
| OR = 1.72 (1.32, 2.24) (P < 0.001) | |||||||
| 4 | Yau PL | Clinical research, cross‐sectional study | 90 obese adolescents | 14–21 years | Mets: | Calibre: | |
| (2014) | 39 with Mets | 42.2% | |||||
| 51 without Mets | Presence vs. Non‐presence | 182.35 vs. 198.62 μm | |||||
| Mets criteria present: | (P < 0.01) | ||||||
| each 1 score ↑ | Calibre: | ||||||
| White matter: | −8.61, P < 0.001 | ||||||
| smaller than 3150 voxel | Calibre: | ||||||
| Reduction (β not mentioned) | |||||||
| (P < 0.001) | |||||||
| 5 | Hosking SPM | Clinical study | 26 T1DM | 8–18 years | Microvascular complications: | AVR: | |
| (2013) | cross‐sectional design | 50% | Absent vs. Present | n.s. | |||
| 6 | Benitez‐Aguirre P | Hospital based, | 511 baseline with T1DM | 12–20 years | Renal dysfunction: | Length–diameter ratio: | Length–diameter ratio: |
| (2012) | Clinical research | prospective cohort study | 174 developed renal dysfunction | 48.6% | OR = 1.69 | n.s. | 4th vs. 1–3rd quartile |
| Median 3.7 years’ follow‐up | (1.17, 2.44) | ||||||
| (P = 0.02) | |||||||
| OR = 1.55 | Simple tortuosity: | Simple tortuosity | |||||
| (1.08, 2.22) | n.s. | ||||||
| (P = 0.02) | 1st vs. 2–4th quartile | ||||||
| 7 | Bronson‐Castain KW | Clinical observational, cross‐sectional study | 26 control | Ctrl: 17.6 | Type 2 DM: | Calibre: | Calibre: |
| (2012) | Clinical study | 32 T1 DM | 38.5% | Yes vs. No | n.s. | 283.4 vs. 270.9 μm | |
| 15 T2 DM | T1 15.6 | (P < 0.05) | |||||
| 56.2% | |||||||
| T2: 16.0 | |||||||
| 40% | |||||||
| 8 | Sasongko MB | Clinic‐based, cross‐sectional study | 944 out of 1159 | 12–20 years | Early retinopathy: | Tortuosity: | Tortuosity: |
| (2011–2012) | SPDS | 81.4% | 43.7% | OR = 1.42 | each SD ↑ | n.s. | |
| (1.11, 1.83) | SD = 10.2 (×103) | n.s. | |||||
| (P = 0.005) | each SD ↑ | n.s. | |||||
| Early kidney dysfunction: | SD = 10.2 (×103) | ||||||
| OR = 1.56 | each SD ↑ | ||||||
| (1.06, 2.28) | SD = 10.2 (×103) | ||||||
| (P = 0.023) | |||||||
| Both: | |||||||
| OR = 1.46 | |||||||
| (1.13, 1.89) | |||||||
| (P = 0.004) | |||||||
| 9 | Benitez‐Aguirre P | Hospital based, | 736 baseline with T1DM | 12–20 years | DR: | Simple tortuosity: | Simple tortuosity: |
| (2011) | Clinical research | prospective cohort study | 287 developed retinopathy | 48.6% | OR = 1.5 | 4th vs. 1st quartile | n.s. |
| Median 3.8 years’ follow‐up | (1.0, 2.2) | Length–diameter ratio: | Length–diameter ratio: | ||||
| (P = 0.03) | 4th vs. 1st quartile | 4th vs. 1st quartile | |||||
| n.s. | |||||||
| Study | Study population and study design | Sample size and response rate | Age, | Diseases that will linked to CVD | Arteriolar parameters | Venular parameters | |
| male % | β or mean, 95 % CI | β or mean, 95 % CI | |||||
| 10 | Li LJ | Population‐based, cross‐sectional study | 385 | 4–5 years | Hypertensive stage: | Calibre: | Calibre: |
| (2011) | STARS | 50.7% | No vs. Yes | 160.15 vs. 156.06 μm | n.s. | ||
| (P = 0.02) | |||||||
| 11 | Sasongko MB | Clinic‐based, cross‐sectional study | 944 out of 1159 | 12–20 years | T1DM duration: | Tortuosity: | Tortuosity: |
| (2010) | SPDS | 81.4% | 43.7% | each 3.3 years ↑ | n.s. | n.s. | |
| Branching angle: | Branching angle: | ||||||
| 1.15 (0.52, 1.78) | n.s. | ||||||
| (P = 0.045) | Optimality deviation (×102): | ||||||
| Optimality deviation (×102): | 1.29 (0.22, 2.35) | ||||||
| 1.29 (0.22, 2.35) | (P = 0.007) | ||||||
| (P = 0.007) | Length–diameter ratio: | ||||||
| Length–diameter ratio: | n.s. | ||||||
| n.s. | |||||||
| 12 | Gopinath B | Australian adolescents | 2353 out of 3144 | 12.7 years | Hypertensive stage: | Calibre: | Calibre: |
| (2010) | SCES | Population‐based, cross‐sectional study | 75.3% | 50.4% | No vs. Yes | 151.9 vs. 149.9 μm | n.s. |
| (P = 0.002) | |||||||
| 13 | Cheung N | Hospital based, | 645 baseline with T1DM | 12–20 years | DR: | Calibre: | Calibre: |
| (2009) | prospective cohort study | 274 developed retinopathy | 44.2% | HR = 1.46 | each SD ↑ | n.s. | |
| Median 2.5 years’ follow‐up | (1.22, 1.74) | SD = 18.90 μm | each SD ↑ | ||||
| (P < 0.001) | SD = 22.63 μm | ||||||
| HR = 0.82 | |||||||
| (0.69, 0.98) | |||||||
| (P = 0.028) | |||||||
| 14 | Alibrahim E | Hospital based, | 668 baseline with T1DM | 12–20 | DR: | Calibre: | Calibre: |
| (2006) | prospective cohort study | 172 developed retinopathy | Years | OR = 1.44 | each SD ↑ | n.s. | |
| Median 3.1 years’ follow‐up | 180 age, sex‐matched control | 48.2% | (1.11. 1.86) | ||||
| (P = 0.006) |
Abbreviation: 95% CI: 95% confidence interval; SD, standard deviation; DR, diabetic retinopathy; HR, hazard ratio; STARS, the Strabismus, Amblyopia and Refractive Error Study in Singapore Chinese Preschoolers; SPDS, Sydney Pediatric Diabetes Study.
Discussion
CVD is the leading cause of mortality, morbidity and hospitalization worldwide (Visentin et al. 2014). Although the clinical manifestation is acute, CVD is a chronic disease that evolves gradually and may interfere with quality of life, physical disability, and lifelong dependence on health services and medications (Visentin et al. 2014). Establishing the mechanisms that link these factors with vascular and metabolic changes could provide essential insights for the development of preventative and therapeutic strategies.
Early life CVD risk factors might exert their influence through a series of complicated mechanisms, including adverse in utero programming (Barker, 2004 a, 2005), IUGR (Barker, 2004 a, 2005), lack of physical activity (Malina, 1996), unbalanced nutrition (Barclay et al. 2008), childhood hypertension and obesity (Berenson et al. 1998; Brion et al. 2007), depression (Glassman & Shapiro, 1998; Nemeroff & Goldschmidt‐Clermont, 2012) and type 1 diabetes (de Ferranti et al. 2014 a,b; Nathan et al. 2005). All CVD risk factors will impose an adverse impact on endothelium and subsequently lead to vascular remodelling. In this systematic review, we summarized 55 papers published on the topic of retinal imaging and early life CVD risk factors. Consistent and strong trends were found in children and adolescents between the presence of early life CVD risk factors and suboptimal structural changes in the retinal microvasculature.
Possible mechanisms for retinal vascular changes
In the general adult population, changes in the retinal microvasculature may reflect different changes in the systemic microvasculature. A range of morphological changes has been studied and they may reflect different underlying physiological and pathological states.
For example, generalized retinal arteriolar narrowing has been suggested to be related to hypertension. The pathophysiological changes in retinal arteriolar narrowing are related to initial vasospasm, followed by chronic arteriosclerotic changes in relation to elevated blood pressure (Wong & Mitchell, 2007; Sun et al. 2009 b). As systemic blood pressure remains chronically elevated, generalized retinal arteriolar narrowing will develop as a consequence of an auto‐regulatory process and result in intimal thickening, media‐wall hyperplasia and hyaline degeneration (Sun et al. 2009 b).
As for retinal venular dilatation, inflammatory‐induced NO‐dependent endothelial dysfunction has been mostly postulated (Sun et al. 2009 b). One animal study found that administration of lipid hydroperoxide into the vitreous humour of rats increased the number of leucocytes in the retinal microvasculature, which led to retinal venular dilatation (Tamai et al. 2002). In human subjects, low dosage of an injected Escherichia coli endotoxin will cause an increase in peripheral white blood cell count and dilatation in retinal venules (Kolodjaschna et al. 2004).
As described earlier, besides retinal vascular calibre, retinal vascular geometry represents different parameters of the retinal blood vessel network. These parameters include tortuosity, branching angle, fractal dimension and a series of others. Although the exact pathophysiological substrates of all these vascular geometric parameters are not fully understood, the main idea is that they reflect increased circulatory energy costs and a decreased efficancy in the distribution of blood to the tissue (e.g. retina).
There are also other ways to assess structural and functional changes of retinal microcirculation through different newly developed and advanced retinal imaging tools, such as ultra‐wide field retinal imaging, retinal oximetry and scanning laser Doppler flowmetry. All these new techniques can measure and analyse peripheral retinal vasculature, foveal capillary network, retinal oxygen saturation, retinal blood flow and choroidal vasculature (Cheung et al. 2012).
Other methods to measure retinal microcirculation
Optical coherence tomography. In the past few years, optical coherence tomography (OCT) has made accessible in‐depth high‐resolution information on the retina with its vessels, including quantitative analysis of the vessel diameter. The retinal vasculature in OCT scans can be derived from direct recognition of the smooth musculature of the vessel wall and the vessel lumen. With advances in software algorithms, there are possibilities to perform OCT angiography, which provides a better approach to invasively visualize blood flow in the retina and the choroid capillary network and to detect the growth of neovascularization (Spaide et al. 2015). Therefore, future application of the OCT technique may be quite promising to image the capillary network around the optic nerve head either by vascular static parameters (e.g. OCT scan (Muraoka et al. 2013; Schuster et al. 2015)) or by vascular dynamic parameters (e.g. fourier‐domain OCT (Wang et al. 2008), Doppler OCT (Konduru et al. 2012; Tan et al. 2012) and en face OCT angiography (Dansingani et al. 2015)).
Dynamic vessel analyzer
Dynamic vessel analyzer (DVA) is a new technology to determine dynamic retinal vessel responses through a series of stimulation techniques including flickering light (Nagel & Vilser, 2004), carbogen and oxygen inhalation (Wimpissinger et al. 2004; Heitmar et al. 2010), and intravenous vasoactive substance infusions (Jeppesen et al. 2007). After stimulation with flickering light, DVA analysis software calculates maximum retinal vessel response to 20 s of flickering light over three stimulation cycles. The average response, within a 17–23 s window after the start of the stimulation, is taken to be the maximum diameter response. This analysis generates a maximum artery dilatory response index, as well as similar outputs for minimum response, peak response and maximum venous dilatory response to flicker (Heitmar et al. 2010).
Adaptive optics imaging
Adaptive optics imaging is an opto‐electronic technology that improves the resolution of fundus images (Koch et al. 2014). Current adaptive optics‐based fundus cameras enable visualization of microstructures such as photoreceptors (Liang et al. 1997), capillaries (Martin & Roorda, 2005) and vascular wall (Chui et al. 2012) noninvasively in humans.
However, these imaging techniques may be difficult to implement in children, since most of them require full understanding by the subject of the instructions given by the examiner. In view of these limitations, thus far retinal fundus imaging is the most feasible and widely used technique in children and young adults. Future child‐friendly technologies with quantitative measurements targeting structural (e.g. En Face OCT (Dansingani et al. 2015; Savastano et al. 2015) and speckle variance OCT (Chan et al. 2015)) and functional (e.g. DVA (Lim et al. 2013) and oximetry) aspects of the microvasculature may yield promising results.
The current gap in research and future perspectives
There is increasing interest in epidemiological studies of early origins of CVD. In the past three decades, Barker's hypothesis has been widely debated and modified. Early life CVD risks such as IUGR, malnutrition, T1DM, elevated blood pressure and obesity may lead to damage in several target organs, subsequently increasing the risk of a variety of diseases later in life. Microvascular changes have been implicated as one of the pathways through which these early life factors may be related to the risk of CVD in late‐life. However, thus far the exploration of various pathways related to the microvasculature has been limited due to the inability to employ any invasive examinations in these young subjects. To some extent the implementation of retinal imaging has made it now possible to interrogate the role of the microvasculature non‐invasively during early life. In this systematic review, we summarized 55 papers published on the topic of retinal imaging and early life CVD risks. We found a consistent and strong trend that children and adolescents exposed to early life CVD risk factors had suboptimal structural changes in the retinal microvasculature. Furthermore, all these studies have shown the feasibility, safety and reliability of retinal imaging in young children. However, there are also a few major gaps in the current research employing retinal imaging: first, most studies thus far are cross‐sectional, hence limiting our ability to draw causal inferences and examine the predictive value of retinal imaging; second, functional retinal imaging has so far been difficult to implement due to limited cooperation of study subjects; and third, retinal imaging has not been implemented in the very young (< 3 years old).
Despite these limitations, the current literature shows strong ‘proof of concept’ that retinal imaging can provide additional information on the status of the microvasculature in children. Besides, the need for longitudinal studies to assess the additional value of retinal imaging, there is a need to implement some of the advanced retinal imaging techniques described earlier, which may allow us to examine functional aspects of the microvasculature.
Conclusion
In summary, there is now substantial evidence that CVD risk factors are associated with structural changes in the retinal microvasculature in early life. The retinal microvasculature may therefore be an indicator of future CVD risk. These findings emphasize early life predisposition to microvascular damage due to the presence of CVD risk factors during that period. Further longitudinal studies are required to investigate specific pathophysiological mechanisms, and to examine the additional value of retinal imaging in early life in the prediction of CVD during adulthood.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
L.‐J.L. received funding from the Singapore Ministry of Health's National Medical Research Council (NMRC/TA/0027/2014). M.K.I. received funding from the Singapore Ministry of Health's National Medical Research Council (NMRC/CSA/038/2013) and the Netherlands Organisation for Health Research and Development (ZonMW; VENI project number: 91612163).
Acknowledgements
The authors appreciate the support of Duke‐NUS/ SingHealth Academic Medicine Research Institute and Ms Taara Madhavan (Associate, Clinical Sciences, Duke‐NUS Graduate Medical School) in editing the manuscript.
Biographies
Ling‐Jun Li is a clinical research fellow currently working on clinical and epidemiological projects at Singapore Eye Research Institute (SERI) and also collaborating on relevant research at the National University of Singapore, KK Women's and Children's Hospital and Tan Tock Seng Hospital. She has been trained and involved in basic research, clinical practice and epidemiological studies as a whole. The eye is a ‘window’ to the human circulation and inflammation. Her current research links retinal imaging data, using advanced and state‐of‐the‐art computer‐based image analysis, to monitor maternal health (e.g. maternal gestational diabetes and gestational hypertension) and children's health (e.g. fetal growth restriction, Kawasaki disease), and even to predict drug effects (e.g. HIV/AIDS patients undergoing anti‐viral therapy).

Mohammad Kamran Ikram completed dual training in Epidemiology (PhD 2005) and Neurology (Dutch Specialist Board Certified 2010) at the Erasmus Medical Center Rotterdam, the Netherlands. He is currently Associate Professor at the Duke‐NUS Graduate Medical School, National University of Singapore and working at the Singapore Eye Research Institute. He is Principal Investigator of the Epidemiology of Dementia in Singapore (EDIS) Study, which is part of the Memory Aging and Cognition Centre (MACC) at the National University Health System in Singapore. His current scientific activities cover a broad spectrum of epidemiological research both in Neurology and Ophthalmology. These include cerebrovascular diseases, dementia, stroke, novel retinal imaging and diabetic retinopathy. He has published more than 100 international peer‐reviewed papers. As Medical Director of SNEC,
Tien Y. Wong helms one of the largest tertiary eye hospital in Asia, with a faculty of >70 ophthalmologists managing >300,000 outpatient visits and >27,000 major surgeries annually. SNEC's research division, the Singapore Eye Research Institute (SERI), is one of the world's leading eye research institutes. Prof Wong balances clinical practice in ophthalmology, focusing on retinal diseases such as diabetic retinopathy and age‐related macular degeneration, with a broad‐based research program comprising epidemiological, clinical and translational studies of Asian retinal diseases, and on the use of retinal imaging to predict disease risk. He has published >1,000 peer‐reviewed papers, including papers in the New England Journal of Medicine and the Lancet. He has given >200 invited plenary, symposium and named lectures globally, and received >US$50 million in grant funding as Principal Investigator.
This review was presented at the symposium “Microvascular plasticity and developmental priming: Impact on human health”, which took place at the 10th World Congress for Microcirculation in Kyoto, Japan, 25–27 September 2015.
References
- Alibrahim E, Donaghue KC, Rogers S, Hing S, Jenkins AJ, Chan A & Wong TY (2006). Retinal vascular caliber and risk of retinopathy in young patients with type 1 diabetes. Ophthalmology 113, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Backes CH, Nelin T, Gorr MW & Wold LE (2013). Early life exposure to air pollution: how bad is it? Toxicol Lett 216, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay AW, Petocz P, McMillan‐Price J, Flood VM, Prvan T, Mitchell P & Brand‐Miller JC (2008). Glycemic index, glycemic load, and chronic disease risk–a meta‐analysis of observational studies. Am J Clin Nutr 87, 627–637. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2004. a). The developmental origins of chronic adult disease. Acta Paediatr Suppl 93, 26–33. [DOI] [PubMed] [Google Scholar]
- Barker DJ (2004. b). The developmental origins of well‐being. Philos Trans R Soc Lond B Biol Sci 359, 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C & Simmonds SJ (1990). Fetal and placental size and risk of hypertension in adult life. BMJ 301, 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ & Osmond C (1986). Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E & Eriksson JG (2005). Trajectories of growth among children who have coronary events as adults. N Engl J Med 353, 1802–1809. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Kajantie E & Eriksson JG (2009). Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann Hum Biol 36, 445–458. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B & Simmonds SJ (1989). Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580. [DOI] [PubMed] [Google Scholar]
- Ben‐Shlomo Y & Smith GD (1991). Deprivation in infancy or in adult life: which is more important for mortality risk? Lancet 337, 530–534. [DOI] [PubMed] [Google Scholar]
- Benitez‐Aguirre P, Craig ME, Sasongko MB, Jenkins AJ, Wong TY, Wang JJ, Cheung N & Donaghue KC (2011). Retinal vascular geometry predicts incident retinopathy in young people with type 1 diabetes: a prospective cohort study from adolescence. Diabetes Care 34, 1622–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez‐Aguirre PZ, Sasongko MB, Craig ME, Jenkins AJ, Cusumano J, Cheung N, Wong TY & Donaghue KC (2012). Retinal vascular geometry predicts incident renal dysfunction in young people with type 1 diabetes. Diabetes Care 35, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE & Wattigney WA (1998). Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338, 1650–1656. [DOI] [PubMed] [Google Scholar]
- Brion MA, Ness AR, Davey Smith G & Leary SD (2007). Association between body composition and blood pressure in a contemporary cohort of 9‐year‐old children. J Hum Hypertens 21, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson‐Castain KW, Bearse MA Jr, Neuville J, Jonasdottir S, King‐Hooper B, Barez S, Schneck ME & Adams AJ (2012). Early neural and vascular changes in the adolescent type 1 and type 2 diabetic retina. Retina 32, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan G, Balaratnasingam C, Xu J, Mammo Z, Han S, Mackenzie P, Merkur A, Kirker A, Albiani D, Sarunic MV & Yu DY (2015). In vivo optical imaging of human retinal capillary networks using speckle variance optical coherence tomography with quantitative clinico‐histological correlation. Microvasc Res 100, 32–39. [DOI] [PubMed] [Google Scholar]
- Chapman N, Mohamudally A, Cerutti A, Stanton A, Sayer AA, Cooper C, Barker D, Rauf A, Evans J, Wormald R, Sever P, Hughes A & Thom S (1997). Retinal vascular network architecture in low‐birth‐weight men. J Hypertens 15, 1449–1453. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ikram MK, Sabanayagam C & Wong TY (2012). Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 60, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Hilal S, Ikram MK, Low S, Ong YL, Venketasubramanian N, Yap P, Seow D, Chen CL & Wong TY (2015). Retinal ganglion cell analysis using high‐definition optical coherence tomography in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 45, 45–56. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Ikram MK, Chen C & Wong TY (2014. a). Retinal microvasculature in Alzheimer's disease. J Alzheimers Dis 42 Suppl 4, S339–S352. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP & Wong TY (2014. b). Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement 10, 135–142. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Tay WT, Mitchell P, Wang JJ, Hsu W, Lee ML, Lau QP, Zhu AL, Klein R, Saw SM & Wong TY (2011. a). Quantitative and qualitative retinal microvascular characteristics and blood pressure. J Hypertens 29, 1380–1391. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Zheng Y, Hsu W, Lee ML, Lau QP, Mitchell P, Wang JJ, Klein R & Wong TY (2011. b). Retinal vascular tortuosity, blood pressure, and cardiovascular risk factors. Ophthalmology 118, 812–818. [DOI] [PubMed] [Google Scholar]
- Cheung N, Donaghue KC, Liew G, Rogers SL, Wang JJ, Lim SW, Jenkins AJ, Hsu W, Li Lee M & Wong TY (2009. a). Quantitative assessment of early diabetic retinopathy using fractal analysis. Diabetes Care 32, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Islam FM, Saw SM, Shankar A, de Haseth K, Mitchell P & Wong TY (2007. a). Distribution and associations of retinal vascular caliber with ethnicity, gender, and birth parameters in young children. Invest Ophthalmol Vis Sci 48, 1018–1024. [DOI] [PubMed] [Google Scholar]
- Cheung N, Rogers S, Mosley TH, Klein R, Couper D & Wong TY (2009. b). Vital exhaustion and retinal microvascular changes in cardiovascular disease: atherosclerosis risk in communities study. Psychosom Med 71, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Saw SM, Islam FM, Rogers SL, Shankar A, de Haseth K, Mitchell P & Wong TY (2007. b). BMI and retinal vascular caliber in children. Obesity (Silver Spring) 15, 209–215. [DOI] [PubMed] [Google Scholar]
- Cheung N, Wong TY, Liew G & Saw SM (2008). Low birth weight and retinal vascular caliber in young children. Pediatrics 121, 862–863; author reply 863. [DOI] [PubMed] [Google Scholar]
- Chui TY, Vannasdale DA & Burns SA (2012). The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express 3, 2537–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansingani KK, Naysan J & Freund KB (2015). En face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation). Eye (Lond) 29, 703–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B & Eckel RH (2014. a). Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130, 1110–1130. [DOI] [PubMed] [Google Scholar]
- de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B & Eckel RH (2014. b). Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 37, 2843–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling D, Mitchell R, Shaw M, Orford S & Smith GD (2000). The ghost of Christmas past: health effects of poverty in London in 1896 and 1991. BMJ 321, 1547–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford J, Shaper AG & Whincup P (1992). Early life experience and cardiovascular disease–ecological studies. J Epidemiol Community Health 46, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT (2005). Evolutionary perspectives on the fetal origins hypothesis. Am J Hum Biol 17, 113–118. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Osmond C & Barker DJ (2003). Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care 26, 3006–3010. [DOI] [PubMed] [Google Scholar]
- Forsdahl A (1979). Are poor living conditions in childhood and adolescence and important risk factor for arteriosclerotic heart disease? Int J Rehabil Res 2, 238–239. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Duijts L, Steegers E, Reiss I, Hofman A, Wong TY, Ikram MK & Gaillard R (2015. a). Impact of birth parameters and early life growth patterns on retinal microvascular structure in children: The Generation R Study. J Hypertens 33, 1429–1437. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Felix JF, Klaver CC, Hofman A, Wong TY, Ikram MK & Gaillard R (2015. b). Retinal microvasculature and cardiovascular health in childhood. Pediatrics 135, 678–685. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Felix JF, Reiss I, Hofman A, Ikram MK, Steegers EA & Gaillard R (2015. c). Influence of maternal angiogenic factors during pregnancy on microvascular structure in school‐age children. Hypertension 65, 722–728. [DOI] [PubMed] [Google Scholar]
- Gishti O, Jaddoe VW, Hofman A, Wong TY, Ikram MK & Gaillard R (2015. d). Body fat distribution, metabolic and inflammatory markers and retinal microvasculature in school‐age children. The Generation R Study. Int J Obes (Lond) 39, 1482–1487. [DOI] [PubMed] [Google Scholar]
- Glassman AH & Shapiro PA (1998). Depression and the course of coronary artery disease. Am J Psychiatry 155, 4–11. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Hardy LL, Wang JJ, Teber E, Wong TY & Mitchell P (2011. a). Parental history of hypertension is associated with narrower retinal arteriolar caliber in young girls. Hypertension 58, 425–430. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Pfund N, Burlutsky G & Mitchell P (2013. a). Differences in association between birth parameters and blood pressure in children from preschool to high school. J Hum Hypertens 27, 79–84. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Teber E, Liew G, Wong TY & Mitchell P (2011. b). Effect of obesity on retinal vascular structure in pre‐adolescent children. Int J Pediatr Obes 6, e353–359. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Wang JJ, Hardy LL, Teber E, Kifley A, Wong TY & Mitchell P (2011. c). Influence of physical activity and screen time on the retinal microvasculature in young children. Arterioscler Thromb Vasc Biol 31, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Wang JJ, Teber E, Liew G, Cheung N, Wong TY & Mitchell P (2010. a). Blood pressure is associated with retinal vessel signs in preadolescent children. J Hypertens 28, 1406–1412. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Baur LA, Wang JJ, Teber E, Liew G, Cheung N, Wong TY & Mitchell P (2010. b). Smaller birth size is associated with narrower retinal arterioles in early adolescence. Microcirculation 17, 660–668. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Flood VM, Burlutsky G, Louie JC, Baur LA & Mitchell P (2014). Dairy food consumption, blood pressure and retinal microcirculation in adolescents. Nutr Metab Cardiovasc Dis 24, 1221–1227. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Flood VM, Rochtchina E, Baur LA, Smith W & Mitchell P (2012. a). Influence of high glycemic index and glycemic load diets on blood pressure during adolescence. Hypertension 59, 1272–1277. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Flood VM, Wang JJ, Smith W, Rochtchina E, Louie JC, Wong TY, Brand‐Miller J & Mitchell P (2012. b). Carbohydrate nutrition is associated with changes in the retinal vascular structure and branching pattern in children. Am J Clin Nutr 95, 1215–1222. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Wang JJ, Flood VM, Burlutsky G, Wong TY & Mitchell P (2009). The associations between blood levels of homocysteine, folate, vitamin B12, and retinal vascular caliber. Am J Ophthalmol 148, 902–909. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Wang JJ, Kifley A, Tan AG, Wong TY & Mitchell P (2013. b). Influence of blood pressure and body mass index on retinal vascular caliber in preschool‐aged children. J Hum Hypertens 27, 523–528. [DOI] [PubMed] [Google Scholar]
- Griendling KK & FitzGerald GA (2003). Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 108, 1912–1916. [DOI] [PubMed] [Google Scholar]
- Hanssen H, Nickel T, Drexel V, Hertel G, Emslander I, Sisic Z, Lorang D, Schuster T, Kotliar KE, Pressler A, Schmidt‐Trucksass A, Weis M & Halle M (2011). Exercise‐induced alterations of retinal vessel diameters and cardiovascular risk reduction in obesity. Atherosclerosis 216, 433–439. [DOI] [PubMed] [Google Scholar]
- Hanssen H, Siegrist M, Neidig M, Renner A, Birzele P, Siclovan A, Blume K, Lammel C, Haller B, Schmidt‐Trucksass A & Halle M (2012). Retinal vessel diameter, obesity and metabolic risk factors in school children (JuvenTUM 3). Atherosclerosis 221, 242–248. [DOI] [PubMed] [Google Scholar]
- Harding JE (2001). The nutritional basis of the fetal origins of adult disease. Int J Epidemiol 30, 15–23. [DOI] [PubMed] [Google Scholar]
- Heitmar R, Blann AD, Cubbidge RP, Lip GY & Gherghel D (2010). Continuous retinal vessel diameter measurements: the future in retinal vessel assessment? Invest Ophthalmol Vis Sci 51, 5833–5839. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Dahlgren J, Marsal K & Ley D (2004). Abnormal retinal vascular morphology in young adults following intrauterine growth restriction. Pediatrics 113, e77–80. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Hard AL, Chen Y, Niklasson A & Albertsson‐Wikland K (1997). Ocular fundus morphology in preterm children. Influence of gestational age, birth size, perinatal morbidity, and postnatal growth. Invest Ophthalmol Vis Sci 38, 1184–1192. [PubMed] [Google Scholar]
- Hellstrom A, Hard AL, Niklasson A, Svensson E & Jacobsson B (1998). Abnormal retinal vascularisation in preterm children as a general vascular phenomenon. Lancet 352, 1827. [DOI] [PubMed] [Google Scholar]
- Hilal S, Ong YT, Cheung CY, Tan CS, Venketasubramanian N, Niessen WJ, Vrooman H, Anuar AR, Chew M, Chen C, Wong TY & Ikram MK (2014). Microvascular network alterations in retina of subjects with cerebral small vessel disease. Neurosci Lett 577, 95–100. [DOI] [PubMed] [Google Scholar]
- Hosking SP, Bhatia R, Crock PA, Wright I, Squance ML & Reeves G (2013). Non‐invasive detection of microvascular changes in a paediatric and adolescent population with type 1 diabetes: a pilot cross‐sectional study. BMC Endocr Disord 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD & Cai J (1999). Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 106, 2269–2280. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Janssen JA, Roos AM, Rietveld I, Witteman JC, Breteler MM, Hofman A, van Duijn CM & de Jong PT (2006. a). Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes 55, 506–510. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Ong YT, Cheung CY & Wong TY (2013). Retinal vascular caliber measurements: clinical significance, current knowledge and future perspectives. Ophthalmologica 229, 125–136. [DOI] [PubMed] [Google Scholar]
- Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A & de Jong PT (2006. b). Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension 47, 189–194. [DOI] [PubMed] [Google Scholar]
- Islam M, Jafar TH, Bux R, Hashmi S, Chaturvedi N & Hughes AD (2014). Association of parental blood pressure with retinal microcirculatory abnormalities indicative of endothelial dysfunction in children. J Hypertens 32, 598–605. [DOI] [PubMed] [Google Scholar]
- Jensen RA, Shea S, Ranjit N, Diez‐Roux A, Wong TY, Klein R, Klein BE, Cotch MF & Siscovick DS (2010). Psychosocial risk factors and retinal microvascular signs: the multi‐ethnic study of atherosclerosis. Am J Epidemiol 171, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P, Knudsen ST, Poulsen PL, Mogensen CE, Schmitz O & Bek T (2007). Response of retinal arteriole diameter to increased blood pressure during acute hyperglycaemia. Acta Ophthalmol Scand 85, 280–286. [DOI] [PubMed] [Google Scholar]
- Kaess H, Kuntzen O, Teckentrupp U & Dorner M (1975). The influences of propranolol on serum gastrin concentration and hydrochloric acid secretion in response to hypoglycemia in normal subjects. Digestion 13, 193–200. [DOI] [PubMed] [Google Scholar]
- Kandasamy Y, Smith R & Wright IM (2011). Retinal microvasculature measurements in full‐term newborn infants. Microvasc Res 82, 381–384. [DOI] [PubMed] [Google Scholar]
- Kandasamy Y, Smith R & Wright IM (2012. a). Retinal microvascular changes in low‐birth‐weight babies have a link to future health. J Perinat Med 40, 209–214. [DOI] [PubMed] [Google Scholar]
- Kandasamy Y, Smith R, Wright IM & Hartley L (2012. b). Relationship between birth weight and retinal microvasculature in newborn infants. J Perinatol 32, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki R, Cheung N, Wang JJ, Klein R, Klein BE, Cotch MF, Sharrett AR, Shea S, Islam FA & Wong TY (2009). Retinal vessel diameters and risk of hypertension: the Multiethnic Study of Atherosclerosis. J Hypertens 27, 2386–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifley A, Wang JJ, Cugati S, Wong TY & Mitchell P (2007). Retinal vascular caliber, diabetes, and retinopathy. Am J Ophthalmol 143, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Knudtson MD, Wong TY & Tsai MY (2006). Are inflammatory factors related to retinal vessel caliber? The Beaver Dam Eye Study. Arch Ophthalmol 124, 87–94. [DOI] [PubMed] [Google Scholar]
- Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R & Klein BE (2003). Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 27, 143–149. [DOI] [PubMed] [Google Scholar]
- Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet‐Riffaud P, Girerd X, Rossant F & Paques M (2014). Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens 32, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodjaschna J, Berisha F, Lung S, Schaller G, Polska E, Jilma B, Wolzt M & Schmetterer L (2004). LPS‐induced microvascular leukocytosis can be assessed by blue‐field entoptic phenomenon. Am J Physiol Heart Circ Physiol 287, H691–694. [DOI] [PubMed] [Google Scholar]
- Konduru RK, Tan O, Nittala MG, Huang D & Sadda SR (2012). Reproducibility of retinal blood flow measurements derived from semi‐automated Doppler OCT analysis. Ophthalmic Surg Lasers Imaging 43, 25–31. [DOI] [PubMed] [Google Scholar]
- Kurniawan ED, Cheung N, Cheung CY, Tay WT, Saw SM & Wong TY (2012). Elevated blood pressure is associated with rarefaction of the retinal vasculature in children. Invest Ophthalmol Vis Sci 53, 470–474. [DOI] [PubMed] [Google Scholar]
- Leon DA & Davey Smith G (2000). Infant mortality, stomach cancer, stroke, and coronary heart disease: ecological analysis. BMJ 320, 1705–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Cheung CY, Chia A, Selvaraj P, Lin XY, Mitchell P, Wong TY & Saw SM (2011. a). The relationship of body fatness indices and retinal vascular caliber in children. Int J Pediatr Obes 6, 267–274. [DOI] [PubMed] [Google Scholar]
- Li LJ, Cheung CY, Ikram MK, Gluckman P, Meaney MJ, Chong YS, Kwek K, Wong TY & Saw SM (2012). Blood pressure and retinal microvascular characteristics during pregnancy: Growing Up in Singapore Towards Healthy Outcomes (GUSTO) Study. Hypertension 60, 223–230. [DOI] [PubMed] [Google Scholar]
- Li LJ, Cheung CY, Liu Y, Chia A, Selvaraj P, Lin XY, Chan YM, Varma R, Mitchell P, Wong TY & Saw SM (2011. b). Influence of blood pressure on retinal vascular caliber in young children. Ophthalmology 118, 1459–1465. [DOI] [PubMed] [Google Scholar]
- Li LJ, Ikram MK, Broekman L, Cheung CY, Chen H, Gooley JJ, Soh SE, Gluckman P, Kwek K, Chong YS, Meaney M, Wong TY & Saw SM (2013). Antenatal mental health and retinal vascular caliber in pregnant women. Transl Vis Sci Technol 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LX, Li MF, Lu JX, Jia LL, Zhang R, Zhao CC, Ren Y, Tu YF, Shen Y, Liu F, Bao YQ & Jia WP (2014). Retinal microvascular abnormalities are associated with early carotid atherosclerotic lesions in hospitalized Chinese patients with type 2 diabetes mellitus. J Diabetes Complications 28, 378–385. [DOI] [PubMed] [Google Scholar]
- Liang J, Williams DR & Miller DT (1997). Supernormal vision and high‐resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis 14, 2884–2892. [DOI] [PubMed] [Google Scholar]
- Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P & Wong TY (2008. a). Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol 126, 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, Mitchell P, Tikellis G, Taylor B & Wong TY (2008. b). The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology 115, 1951–1956. [DOI] [PubMed] [Google Scholar]
- Liew G, Wang JJ, Mitchell P & Wong TY (2008. c) . Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging 1, 156–161. [DOI] [PubMed] [Google Scholar]
- Lim LS, Cheung N, Saw SM, Yap M & Wong TY (2009). Does diet influence the retinal microvasculature in children? Stroke 40, e473–e474; author reply e475–476. [DOI] [PubMed] [Google Scholar]
- Lim M, Sasongko MB, Ikram MK, Lamoureux E, Wang JJ, Wong TY & Cheung CY (2013). Systemic associations of dynamic retinal vessel analysis: a review of current literature. Microcirculation 20, 257–268. [DOI] [PubMed] [Google Scholar]
- McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Sharrett AR, Klein BE, Wang JJ, Chambless LE & Wong TY (2008). Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 102, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill HC Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE & Strong JP (2000. a). Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 72, 1307S–1315S. [DOI] [PubMed] [Google Scholar]
- McGill HC Jr, McMahan CA, Herderick EE, Tracy RE, Malcom GT, Zieske AW & Strong JP (2000. b). Effects of coronary heart disease risk factors on atherosclerosis of selected regions of the aorta and right coronary artery. PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler Thromb Vasc Biol 20, 836–845. [DOI] [PubMed] [Google Scholar]
- Mahal S, Strain WD, Martinez‐Perez ME, Thom SA, Chaturvedi N & Hughes AD (2009). Comparison of the retinal microvasculature in European and African‐Caribbean people with diabetes. Clin Sci (Lond) 117, 229–236. [DOI] [PubMed] [Google Scholar]
- Malina RM (1996). Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport 67, S48–57. [DOI] [PubMed] [Google Scholar]
- Martin JA & Roorda A (2005). Direct and noninvasive assessment of parafoveal capillary leukocyte velocity. Ophthalmology 112, 2219–2224. [DOI] [PubMed] [Google Scholar]
- Meier MH, Gillespie NA, Hansell NK, Hewitt AW, Hickie IB, Lu Y, MacGregor S, Medland SE, Sun C, Wong TY, Wright MJ, Zhu G, Martin NG & Mackey DA (2014). Associations between depression and anxiety symptoms and retinal vessel caliber in adolescents and young adults. Psychosom Med 76, 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minicucci G, Lepore D & Molle F (1999). Abnormal retinal vascularisation in preterm children. Lancet 353, 1099–1100. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Cheung N, de Haseth K, Taylor B, Rochtchina E, Islam FM, Wang JJ, Saw SM & Wong TY (2007). Blood pressure and retinal arteriolar narrowing in children. Hypertension 49, 1156–1162. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N & Wong TY (2008). Evidence of arteriolar narrowing in low‐birth‐weight children. Circulation 118, 518–524. [DOI] [PubMed] [Google Scholar]
- Muraoka Y, Tsujikawa A, Kumagai K, Akiba M, Ogino K, Murakami T, Akagi‐Kurashige Y, Miyamoto K & Yoshimura N (2013). Age‐ and hypertension‐dependent changes in retinal vessel diameter and wall thickness: an optical coherence tomography study. Am J Ophthalmol 156, 706–714. [DOI] [PubMed] [Google Scholar]
- Murgan I, Beyer S, Kotliar KE, Weber L, Bechtold‐Dalla Pozza S, Dalla Pozza R, Wegner A, Sitnikova D, Stock K, Heemann U, Schmaderer C & Baumann M (2013). Arterial and retinal vascular changes in hypertensive and prehypertensive adolescents. Am J Hypertens 26, 400–408. [DOI] [PubMed] [Google Scholar]
- Nagel E & Vilser W (2004). Flicker observation light induces diameter response in retinal arterioles: a clinical methodological study. Br J Ophthalmol 88, 54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP & Palinski W (1999). Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet 354, 1234–1241. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P & Zinman B (2005). Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353, 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB & Goldschmidt‐Clermont PJ (2012). Heartache and heartbreak–the link between depression and cardiovascular disease. Nat Rev Cardiol 9, 526–539. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Wang JJ, Islam FM, Mitchell P, Tapp RJ, Zimmet PZ, Simpson R, Shaw J & Wong TY (2008. a). Retinal arteriolar narrowing predicts incidence of diabetes: the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes 57, 536–539. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Wong TY, Islam FM, Hubbard L, Miller J, Haroon E, Darwin C, Esser B & Kumar A (2008. b). Is depression associated with microvascular disease in patients with type 2 diabetes? Depress Anxiety 25, E158–162. [DOI] [PubMed] [Google Scholar]
- Nuyt AM (2008). Mechanisms underlying developmental programming of elevated blood pressure and vascular dysfunction: evidence from human studies and experimental animal models. Clin Sci (Lond) 114, 1–17. [DOI] [PubMed] [Google Scholar]
- Ong YT, Hilal S, Cheung CY, Venketasubramanian N, Niessen WJ, Vrooman H, Anuar AR, Chew M, Chen C, Wong TY & Ikram MK (2015). Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci Lett 584, 12–16. [DOI] [PubMed] [Google Scholar]
- Ong YT, Hilal S, Cheung CY, Xu X, Chen C, Venketasubramanian N, Wong TY & Ikram MK (2014). Retinal vascular fractals and cognitive impairment. Dement Geriatr Cogn Dis Extra 4, 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CG, Rudnicka AR, Nightingale CM, Mullen R, Barman SA, Sattar N, Cook DG & Whincup PH (2011). Retinal arteriolar tortuosity and cardiovascular risk factors in a multi‐ethnic population study of 10‐year‐old children; the Child Heart and Health Study in England (CHASE). Arterioscler Thromb Vasc Biol 31, 1933–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr JC & Spears GF (1974. a). General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol 77, 472–477. [DOI] [PubMed] [Google Scholar]
- Parr JC & Spears GF (1974. b). Mathematic relationships between the width of a retinal artery and the widths of its branches. Am J Ophthalmol 77, 478–483. [DOI] [PubMed] [Google Scholar]
- Poon M, Craig ME, Kaur H, Cusumano J, Sasongko MB, Wong TY & Donaghue KC (2013). Vitamin D deficiency is not associated with changes in retinal geometric parameters in young people with type 1 diabetes. J Diabetes Res 2013, 280691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasongko MB, Wang JJ, Donaghue KC, Cheung N, Benitez‐Aguirre P, Jenkins A, Hsu W, Lee ML & Wong TY (2010). Alterations in retinal microvascular geometry in young type 1 diabetes. Diabetes Care 33, 1331–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasongko MB, Wong TY, Donaghue KC, Cheung N, Jenkins AJ, Benitez‐Aguirre P & Wang JJ (2012). Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol 153, 176–183. [DOI] [PubMed] [Google Scholar]
- Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE & Wang JJ (2011). Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia 54, 2409–2416. [DOI] [PubMed] [Google Scholar]
- Savastano MC, Rispoli M, Savastano A & Lumbroso B (2015). En face optical coherence tomography for visualization of the choroid. Ophthalmic Surg Lasers Imaging Retina 46, 561–565. [DOI] [PubMed] [Google Scholar]
- Schiel R, Vilser W, Kovar F, Kramer G, Braun A & Stein G (2009). Retinal vessel response to flicker light in children and adolescents with type 1 diabetes mellitus and overweight or obesity. Diabetes Res Clin Pract 83, 358–364. [DOI] [PubMed] [Google Scholar]
- Schuster AK, Fischer JE, Vossmerbaeumer C & Vossmerbaeumer U (2015). Optical coherence tomography‐based retinal vessel analysis for the evaluation of hypertensive vasculopathy. Acta Ophthalmol 93, e148–153. [DOI] [PubMed] [Google Scholar]
- Siegrist M, Hanssen H, Neidig M, Fuchs M, Lechner F, Stetten M, Blume K, Lammel C, Haller B, Vogeser M, Parhofer KG & Halle M (2014). Association of leptin and insulin with childhood obesity and retinal vessel diameters. Int J Obes (Lond) 38, 1241–1247. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Klancnik JM Jr & Cooney MJ (2015). Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133, 45–50. [DOI] [PubMed] [Google Scholar]
- Strain WD, Adingupu DD & Shore AC (2012). Microcirculation on a large scale: techniques, tactics and relevance of studying the microcirculation in larger population samples. Microcirculation 19, 37–46. [DOI] [PubMed] [Google Scholar]
- Struijker‐Boudier HA, Heijnen BF, Liu YP & Staessen JA (2012). Phenotyping the microcirculation. Hypertension 60, 523–527. [DOI] [PubMed] [Google Scholar]
- Sun C, Ponsonby AL, Wong TY, Brown SA, Kearns LS, Cochrane J, MacKinnon JR, Ruddle JB, Hewitt AW, Liew G, Dwyer T, Scurrah K & Mackey DA (2009. a). Effect of birth parameters on retinal vascular caliber: the Twins Eye Study in Tasmania. Hypertension 53, 487–493. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang JJ, Mackey DA & Wong TY (2009. b). Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol 54, 74–95. [DOI] [PubMed] [Google Scholar]
- Tamai K, Matsubara A, Tomida K, Matsuda Y, Morita H, Armstrong D & Ogura Y (2002). Lipid hydroperoxide stimulates leukocyte‐endothelium interaction in the retinal microcirculation. Exp Eye Res 75, 69–75. [DOI] [PubMed] [Google Scholar]
- Tan O, Wang Y, Konduru RK, Zhang X, Sadda SR & Huang D (2012). Doppler optical coherence tomography of retinal circulation. J Vis Exp, e3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp RJ, Ness A, Williams C, Howe LD, Tilling K, Witt N, Chaturvedi N, McG Thom SA & Hughes AD (2013). Differential effects of adiposity and childhood growth trajectories on retinal microvascular architecture. Microcirculation 20, 609–616. [DOI] [PubMed] [Google Scholar]
- Tapp RJ, Williams C, Witt N, Chaturvedi N, Evans R, Thom SA, Hughes AD & Ness A (2007). Impact of size at birth on the microvasculature: the Avon Longitudinal Study of Parents and Children. Pediatrics 120, e1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B, Rochtchina E, Wang JJ, Wong TY, Heikal S, Saw SM & Mitchell P (2007). Body mass index and its effects on retinal vessel diameter in 6‐year‐old children. Int J Obes (Lond) 31, 1527–1533. [DOI] [PubMed] [Google Scholar]
- Van Hecke MV, Dekker JM, Nijpels G, Teerlink T, Jakobs C, Stolk RP, Heine RJ, Bouter LM, Polak BC & Stehouwer CD (2008). Homocysteine, S‐adenosylmethionine and S‐adenosylhomocysteine are associated with retinal microvascular abnormalities: the Hoorn Study. Clin Sci (Lond) 114, 479–487. [DOI] [PubMed] [Google Scholar]
- Villar J & Belizan JM (1982). The timing factor in the pathophysiology of the intrauterine growth retardation syndrome. Obstet Gynecol Surv 37, 499–506. [DOI] [PubMed] [Google Scholar]
- Visentin S, Grumolato F, Nardelli GB, Di Camillo B, Grisan E & Cosmi E (2014). Early origins of adult disease: low birth weight and vascular remodeling. Atherosclerosis 237, 391–399. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bower BA, Izatt JA, Tan O & Huang D (2008). Retinal blood flow measurement by circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt 13, 064003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpissinger B, Resch H, Berisha F, Weigert G, Schmetterer L & Polak K (2004). Response of choroidal blood flow to carbogen breathing in smokers and non‐smokers. Br J Ophthalmol 88, 776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR & Shahar E (2006). Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi‐ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci 47, 2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE & Hubbard LD (2002. a). Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA 287, 1153–1159. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD & Duncan BB (2002. b). Retinal arteriolar narrowing and risk of diabetes mellitus in middle‐aged persons. JAMA 287, 2528–2533. [DOI] [PubMed] [Google Scholar]
- Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM & Hubbard LD (2004). Computer‐assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 111, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Wong TY & Mitchell P (2007). The eye in hypertension. Lancet 369, 425–435. [DOI] [PubMed] [Google Scholar]
- Xiao W, Gong W, Chen Q, Ding X, Chang B & He M (2015). Association between body composition and retinal vascular caliber in children and adolescents. Invest Ophthalmol Vis Sci 56, 705–710. [DOI] [PubMed] [Google Scholar]
- Yau PL, Kim M, Tirsi A & Convit A (2014). Retinal vessel alterations and cerebral white matter microstructural damage in obese adolescents with metabolic syndrome. JAMA Pediatr 168, e142815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim‐Lui Cheung C, Wong TY, Lamoureux EL, Sabanayagam C, Li J, Lee J & Tai ES (2010). C‐reactive protein and retinal microvascular caliber in a multiethnic asian population. Am J Epidemiol 171, 206–213. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Huang W, Zhang J & He M (2013). Phenotypic and genetic correlation of blood pressure and body mass index with retinal vascular caliber in children and adolescents: the Guangzhou twin eye study. Invest Ophthalmol Vis Sci 54, 423–428. [DOI] [PubMed] [Google Scholar]


