Abstract
Key points
This study aimed to provide molecular insight into the differential effects of age and physical inactivity on the regulation of substrate metabolism during moderate‐intensity exercise.
Using the arteriovenous balance technique, we studied the effect of immobilization of one leg for 2 weeks on leg substrate utilization in young and older men during two‐legged dynamic knee‐extensor moderate‐intensity exercise, as well as changes in key proteins in muscle metabolism before and after exercise.
Age and immobilization did not affect relative carbohydrate and fat utilization during exercise, but the older men had higher uptake of exogenous fatty acids, whereas the young men relied more on endogenous fatty acids during exercise.
Using a combined whole‐leg and molecular approach, we provide evidence that both age and physical inactivity result in intramuscular lipid accumulation, but this occurs only in part through the same mechanisms.
Abstract
Age and inactivity have been associated with intramuscular triglyceride (IMTG) accumulation. Here, we attempt to disentangle these factors by studying the effect of 2 weeks of unilateral leg immobilization on substrate utilization across the legs during moderate‐intensity exercise in young (n = 17; 23 ± 1 years old) and older men (n = 15; 68 ± 1 years old), while the contralateral leg served as the control. After immobilization, the participants performed two‐legged isolated knee‐extensor exercise at 20 ± 1 W (∼50% maximal work capacity) for 45 min with catheters inserted in the brachial artery and both femoral veins. Biopsy samples obtained from vastus lateralis muscles of both legs before and after exercise were used for analysis of substrates, protein content and enzyme activities. During exercise, leg substrate utilization (respiratory quotient) did not differ between groups or legs. Leg fatty acid uptake was greater in older than in young men, and although young men demonstrated net leg glycerol release during exercise, older men showed net glycerol uptake. At baseline, IMTG, muscle pyruvate dehydrogenase complex activity and the protein content of adipose triglyceride lipase, acetyl‐CoA carboxylase 2 and AMP‐activated protein kinase (AMPK)γ3 were higher in young than in older men. Furthermore, adipose triglyceride lipase, plasma membrane‐associated fatty acid binding protein and AMPKγ3 subunit protein contents were lower and IMTG was higher in the immobilized than the contralateral leg in young and older men. Thus, immobilization and age did not affect substrate choice (respiratory quotient) during moderate exercise, but the whole‐leg and molecular differences in fatty acid mobilization could explain the age‐ and immobilization‐induced IMTG accumulation.
Key points
This study aimed to provide molecular insight into the differential effects of age and physical inactivity on the regulation of substrate metabolism during moderate‐intensity exercise.
Using the arteriovenous balance technique, we studied the effect of immobilization of one leg for 2 weeks on leg substrate utilization in young and older men during two‐legged dynamic knee‐extensor moderate‐intensity exercise, as well as changes in key proteins in muscle metabolism before and after exercise.
Age and immobilization did not affect relative carbohydrate and fat utilization during exercise, but the older men had higher uptake of exogenous fatty acids, whereas the young men relied more on endogenous fatty acids during exercise.
Using a combined whole‐leg and molecular approach, we provide evidence that both age and physical inactivity result in intramuscular lipid accumulation, but this occurs only in part through the same mechanisms.
Abbreviations
- ACC2
acetyl‐CoA carboxylase 2
- AMPK
AMP‐activated protein kinase
- ATGL
adipose triglyceride lipase
- AUC
area under the curve
- CON
control leg
- CS
citrate synthase
- FA
fatty acid
- FABPpm
plasma membrane‐associated fatty acid binding protein
- FATP
fatty acid transport protein
- HAD
β‐hydroxyacyl‐CoA dehydrogenase
- HSL
hormone‐sensitive lipase
- IM
immobilized leg
- IMTG
intramuscular triglyceride
- LLM
leg lean mass
- IP
immunoprecipitate
- LPL
lipoprotein lipase
- mTORC1
mammalian target of rapamycin complex 1
- PDC
pyruvate dehydrogenase complex
- RER
respiratory exchange ratio
- RQ
respiratory quotient
carbon dioxide output
oxygen uptake
maximal oxygen uptake
- Wattmax
maximal work capacity
Introduction
Age and inactivity have been associated with ectopic intramuscular triglyceride (IMTG) accumulation (Cree et al. 2004, 2010; Bergouignan et al. 2009), although it is currently not known whether one factor is secondary to the other or if the effects are additive. This has major health implications because ectopic IMTG accumulation may lead to impaired muscle insulin sensitivity and, thus, to the development of type 2 diabetes (Unger, 2002; Moro et al. 2008).
A low maximal oxygen uptake (; Henriksson, 1977; Martin et al. 1993; Helge et al. 2007) and age (Meredith et al. 1989; Sial et al. 1996; Levadoux et al. 2001) are associated with greater glucose oxidation. Hence, the age‐ and inactivity‐related substrate choice during exercise potentially promotes IMTG accumulation.
Several intramuscular steps in metabolism are also likely to play a role in the age‐ and inactivity‐mediated lipid accumulation, e.g. through impaired fatty acid (FA) uptake, storage and regulation of mitochondrial substrate uptake. Fatty acid uptake in muscle occurs through (at least) three proteins, namely plasma membrane‐associated fatty acid binding protein (FABPpm), CD36 and fatty acid transport protein (FATP). Of these, only the first is influenced (increased) by physical training (Kiens et al. 1997, 2004). However, the impact of age and physical inactivity on the expression of FABPpm is not known.
The regulation of lipolysis is probably another pivotal factor in lipid accumulation. At rest, ∼50% of the FA taken up by the skeletal muscle is stored in intramuscular lipid droplets, and there is a complete turnover of the lipid pool in ∼29 h (Sacchetti et al. 2004). Therefore, the influence of acute and chronic activity and inactivity on the regulation of storage and release of FA from the lipid droplets for oxidation have received considerable attention. In young men, endurance training increases protein levels of adipose triglyceride lipase (ATGL) and the activity of hormone‐sensitive lipase (HSL) through increased Ser660 and decreased Ser565 phosphorylation of HSL, but not HSL protein level (Alsted et al. 2009), whereas inactivity imposed by bed rest decreases HSL activity by an increased Ser565 phosphorylation (Alibegovic et al. 2010). However, the effect of age on ATGL and HSL protein levels not known.
AMP‐activated protein kinase (AMPK) is considered a master switch in muscle metabolism and key in the regulation of transport of fuel into the mitochondria for oxidation. During exercise, increased AMPK activity stimulates FA utilization (Fentz et al. 2015) and inhibits other energy‐consuming processes (Jørgensen et al. 2004; Jensen et al. 2009; Richter & Ruderman, 2009; Hardie, 2011). Furthermore, AMPK may partly favour FA oxidation through inhibition of acetyl‐CoA carboxylase 2 (ACC2; Stephens et al. 2002), although this may not be a limiting factor (Dzamko et al. 2008). Additionally, older individuals seem to have augmented AMPK activation in response to acute exercise (Drummond et al. 2008; Mortensen et al. 2009). Given that AMPK also inhibits pyruvate dehydrogenase complex (PDC) activity (Klein et al. 2007), which controls the rate of pyruvate transport into the mitochondria (Constantin‐Teodosiu et al. 1992), this may partly account for the lower rate of glucose oxidation in older compared with young individuals during exercise. Immobilization for 2 weeks does not change AMPKα1, α2, β2 or α‐subunit content in skeletal muscle (Eijnde et al. 2005). Overall, the effect of inactivity and age on AMPK, ACC2 and PDC protein levels and activity remains to be elucidated fully.
To date, most studies have investigated the effect of endurance training on FA storage and oxidation in young and older men, but information on the effect of inactivity in these groups is lacking. Furthermore, there is a gap in our knowledge concerning whether age and inactivity affect the same or different mechanisms that control fuel mobilization and oxidation. Collectively, this information would add information on the development of ectopic IMTG accumulation. To address this question, we immobilized one leg in both young and older men for 2 weeks, while the other leg served as the control. After immobilization, the participants performed 45 min isolated knee‐extensor exercise at moderate intensity with both legs. The arteriovenous balance technique was used to estimate relative FA and glucose utilization (by indirect calorimetry) and the net balance of substrates mobilized for oxidation in the legs. Our hypothesis was that both age difference and inactivity would be associated with changes that could be related to ectopic IMTG accumulation. We hypothesized that this would be associated with an increased relative glucose utilization (i.e. the opposite adaptation of endurance training) and a derangement of several key proteins regulating substrate metabolism in skeletal muscle. This would lead to more FA being mobilized from the circulation without being oxidized, which would lead to IMTG accumulation.
Methods
Subjects
Seventeen young and 15 older men were included. Age inclusion criteria were 20–27 and 60–75 years, respectively. The participants were selected to have average (42–49 and 25–35 ml O2 min−1 kg−1), body mass index (22–27 and 20–29 kg m−2) and whole‐body fat percentage (15–25 and 20–30% for young and older men, respectively) for their age group according to the Danish Health Examination Survey (Eriksen et al. 2011; Table 1). The study was performed according to the Declaration of Helsinki and was approved by the Ethics Committee of Copenhagen (H‐4‐2010‐85). All subjects were carefully informed (verbal and written material) about the possible risks and discomfort involved before written consent to participate was obtained. The subjects received remuneration for participation, and all transportation costs during the immobilization and to and from meetings at the department were reimbursed.
Table 1.
Characteristics of the young and older men at inclusion and after immobilization for 2 weeks
| Inclusion | After 2 weeks of immobilization | |||
|---|---|---|---|---|
| Characteristic | Young men (n = 16) | Older men (n = 15) | Young men | Older men |
| Age (years) | 23 ± 1* | 68 ± 1 | ||
| Weight (kg) | 80 ± 2 | 83 ± 2 | 80 ± 2 | 83 ± 2 |
| Body mass index (kg m−2) | 24 ± 1* | 27 ± 1 | 24 ± 1* | 27 ± 1 |
| Whole‐body fat (%) | 21 ± 1* | 28 ± 1 | 21 ± 1* | 29 ± 1 |
| Lean body mass (kg) | 60 ± 1(*) | 57 ± 2 | 60 ± 1(*) | 56 ± 2 |
| Glycated haemoglobin (mmol mol−1) | 5.2 ± 0.1* | 5.6 ± 0.1 | 5.2 ± 0.1* | 5.5 ± 0.1 |
| Maximal O2 uptake (ml O2 min−1 kg−1) | 48 ± 1* | 33 ± 2 | 44 ± 1* | 32 ± 2† |
Data are mean values ± SEM. *P < 0.05 and (*)0.05 < P > 0.1 young vs. older men same time point; † P < 0.05 vs. inclusion in the young men.
Experimental protocol
The experimental protocol has been described previously in detail (Nørregaard et al. 2015). Both young and older men underwent a clinical examination prior to recruitment to exclude individuals with diabetes (assessed as glycated haemoglobin >6.5 mmol mol−1), musculoskeletal disease, cardiovascular disease (resting ECG in the older men) or known predisposition to deep venous thrombosis. None of the young men took medication, but some of the older men were in medical treatment for hypertension (n = 2; thiazide diuretic plus angiotensin II inhibitor and angiotensin II receptor antagonist, respectively), prostate enlargement (n = 2; α‐blocker), mild asthma (n = 1; anticholinergic pro re nata), mild depression (n = 1; selective serotonin reuptake inhibitor) and attention deficit hyperactive disorder (n = 1; modafinil). None of the participants was a smoker.
The participants were instructed to eat a weight‐maintaining diet throughout the study, following the national guidelines for macronutrient composition (Fogelholm, 2013). In the 3 days before each biopsy sampling, the subjects were instructed to abstain from alcohol intake. In addition, the participants were instructed to avoid strenuous exercise for 3 days before the test days.
The present study is a part of a larger study on the effect of immobilization and aerobic retraining in young and older men. The previous studies have investigated interluekin‐6 and tumour necrosis factor‐α release during exercise in young men (Reihmane et al. 2013) and changes in mitochondrial respiration and H2O2 production (Gram et al. 2014, 2015), leg function (e.g. leg lean mass, strength and muscle fibre type composition; Vigelsø et al. 2015 b) and plasma lipid profile (Nørregaard et al. 2015) with immobilization and retraining. It follows that most of the descriptive data on these subjects have been reported previously (Reihmane et al. 2013; Gram et al. 2014; Nørregaard et al. 2015; Vigelsø et al. 2015 b,c), and this is clearly cited in the present paper.
Anthropometric measurements
Body composition was determined by dual‐energy X‐ray absorptiometry scanning (Lunar iDXA; GE Medical Systems, Madison, WI, USA) at inclusion and after immobilization. EnCORE software (enCORE software version 14.10.022; GE Medical Systems) automatically determined the regions of interest (e.g. the legs).
At inclusion and after immobilization, a graded test and a test of the maximal work capacity (Wattmax) of each leg were performed. The was achieved when the following two criteria were met: plateau in oxygen consumption in spite of increasing workload; and respiratory exchange ratio (RER) >1.15 for the young and >1.05 for the elderly on average over 20 s. Polar RS400 heart rate monitors (Polar Electro Oy, Kempele, Finland) were used to measure heart rate. At the Wattmax test prior to the experiment, the participants were accustomed to exercise in the knee‐extension ergometer. The Wattmax of each leg was then determined. In brief, a graded test (starting at 10 W, with 2 min at 20 W, followed by 5 W, 2 min increments) was performed, and pulmonary oxygen uptake (), carbon dioxide ouput () and heart rate were measured. The workload at which exercise could not be sustained without involving additional muscle was defined as one‐leg Wattmax.
Immobilization
The immobilized leg was chosen by randomization. The chosen leg was immobilized (IM) with a DonJoy® knee brace (DJO Nordic, Malmö, Sweden) locked at 60 deg for 2 weeks. The other leg served as the control (CON). The DonJoy® knee brace was secured with plastic strips that, if broken, would reveal that the brace had been removed. The subjects were given a pair of crutches and were repeatedly instructed not to engage in any weightbearing activity with the immobilized leg. However, they ambulated freely during the entire 2 weeks. The subjects reported to the laboratory at least once during the 2 week immobilization to control and adjust the DonJoy® brace. All subjects received 75 mg acetylsalicylic acid per day in the first 10 days to reduce the risk of deep venous thrombosis. This treatment was withdrawn for the last 4 days before the experimental days to remove potential interference with the measurements.
Acute exercise
After 2 weeks of immobilization of one leg, the subjects reported to the laboratory in the morning after an overnight fast (12 h). A dual‐energy X‐ray absorptiometry scan was performed to determine the impact of immobilization on leg muscle mass.
A muscle biopsy was obtained from both legs. The procedure was carried out, after local anaesthesia (lidocaine; 5 mg ml−1; Amgros I/S, Copenhagen, Denmark) of the skin and the superficial muscle fascia, using the Bergström needle modified with suction. The biopsy was immediately frozen in liquid nitrogen and stored at −80°C for further analysis.
Thereafter, catheters were placed in the brachial artery (20 gauge arterial cannula; Becton Dickinson A/S, Albertslund, Denmark) and both femoral veins (14 gauge catheter; Arrow International, ViCare Medical, Birkeroed, Denmark) under local anaesthesia. The catheters were inserted into the femoral vein distal to the inguinal ligament and in the anterograde direction. The catheters were kept patent by a slow drip of isotonic sodium chloride infusate. On one occasion, a subject was unable to perform acute exercise because of a vasovagal syncope, and on five occasions, we were unable to insert a catheter in one of the legs. Hence, the data presented here are for 14 and 13 control legs and for 16 and 14 immobilized legs in the young and older men, respectively.
After 1 h of rest, the subject was positioned in a custom‐made isolated one‐leg knee extension ergometer in a semi‐supine position. Blood was sampled simultaneously from the brachial artery and femoral veins 15 min before (−15 min) and immediately before the start of exercise at time 0 min. The subjects performed 45 min of isolated dynamic knee‐extensor exercise with both legs, with each leg in a separate one‐legged ergometer. The absolute leg workload was set to 50% of Wattmax, which was determined before the immobilization, i.e. both legs performed the same absolute amount of work. During exercise, blood was sampled at 15, 30 and 45 min. Femoral arterial blood flow was measured at all time points in both legs by Doppler ultrasound (ACUSON S2000; Siemens Healthcare, Ballerup, Denmark). Heart rate was recorded continuously before and during the exercise. Furthermore, subjects were requested to report perceived workload on a scale from 1 to 10 (1 was ‘can go on forever’ and 10 was ‘I have to stop within seconds’) after 5, 25 and 40 min of exercise. Whole‐body oxygen consumption was measured from 20 to 27 min of exercise using an Oxycon Pro (Jaeger, CareFusion GmbH, Hoechberg, Germany). Throughout the experiment, participants had free access to water, and exercise was performed at an ambient temperature of 20°C. Another muscle biopsy was obtained from both legs immediately (5–10 min) after the acute exercise.
The participants reported to the laboratory the next day in order to determine changes in Wattmax after immobilization. The test was performed the day after the acute exercise so that it could not interfere with the effects of immobilization.
Analytical procedures
Blood was sampled anaerobically and distributed into tubes containing heparin or Trasylol/EDTA. The heparinized samples were immediately analysed for haematocrit (ABL800 Flex; Radiometer, Copenhagen, Denmark). Plasma for determination of glucose, glycerol and FA was cooled down and separated by centrifugation at 2000g at 4°C for 10 min, frozen on dry ice, and stored at −80°C until further analysis. Plasma glucose, free FA and glycerol were analysed on a Cobas 6000 c 501 (Roche, Glostrup, Denmark).
Calculations
The plasma concentrations of O2 and CO2 were calculated as previously described (Siggaard‐Andersen et al. 1988; Peronnet & Massicotte, 1991). The Fick principle was used to calculate the leg uptake or release of O2, CO2, glucose, lactate, FA and glycerol across the legs at rest and during exercise [i.e. the brachial arterial and femoral venous plasma concentration differences multiplied by plasma flow (blood flow × (1 − haematocrit)]. Indirect calorimetry was used to calculate the total energy contribution of glucose and FA oxidation (Peronnet & Massicotte, 1991). The energy contribution from FA uptake and glycerol release was calculated by converting the rate of oxidation (in micromoles per minute) to its molar mass equivalent (272 and 860 g mol−1, respectively; Jeukendrup & Wallis, 2005) and by assuming that oxidation of 1 g of triglycerides yields 9.75 calories (Jeukendrup & Wallis, 2005). The area under the curve (AUC) was calculated by the trapezoid method, with the x‐axis as a baseline. However, AUC for leg glycerol release is presented with the resting value as a baseline because the young men had a net release, whereas the older men had a net uptake (see Fig. 2 C).
Figure 2. Utilization of exogenous substrates, measured as net balance over the leg .
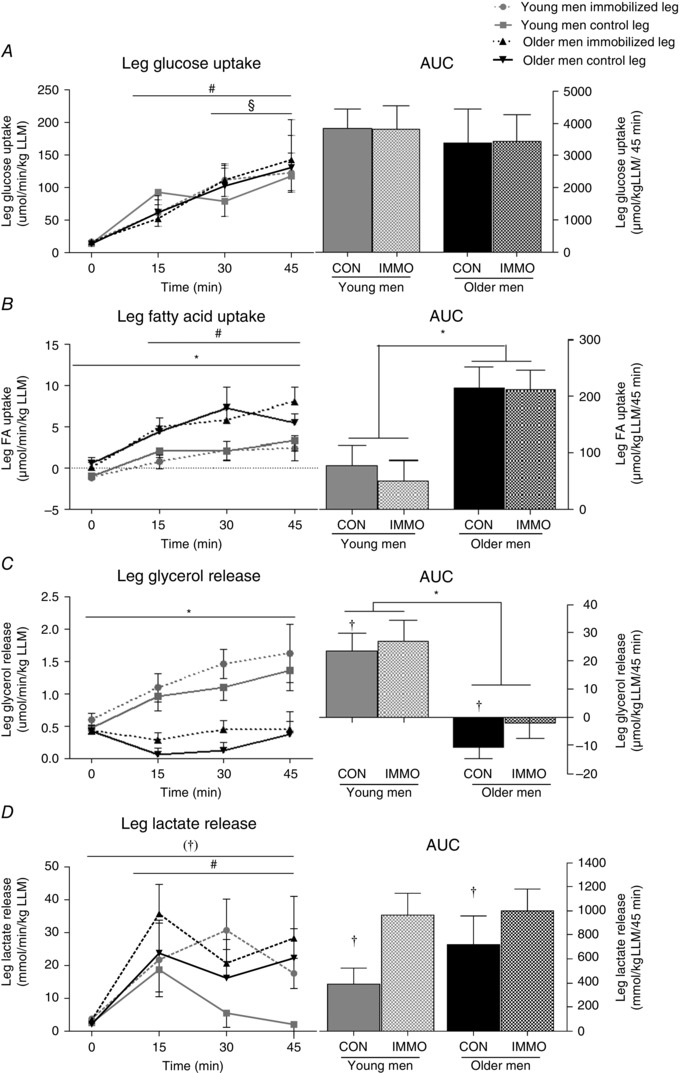
Leg glucose uptake (A), fatty acid (FA) uptake (B), glycerol release (C) and lactate release (D) in young and older men after 2 weeks of unilateral immobilization (IMMO) with the other leg serving as the control (CON) during 45 min moderate‐intensity exercise with both legs, with the corresponding area under curve (AUC). *P < 0.05 young vs. older men (main effect); † P < 0.05 and (†)0.05 < P > 0.1 immobilized leg vs. control leg (main effect); # P < 0.05 0 min vs. 15, 30 and 45 min; § P < 0.05 15 min vs. 30 and 45 min. Data are mean values ± SEM.
Western blotting
Two laboratories performed the Western blotting analysis of protein content; therefore, two Western blotting procedures are described. The protein content was analysed in the biopsies obtained before the acute exercise, and phosphorylation of proteins was analysed in the biopsies obtained both before and after the exercise (i.e. ACC2 and pACC Ser221).
Lipid metabolism‐related proteins
The analysis was performed as previously described in detail (Larsen et al. 2015; Vigelsø et al. 2015 a). In brief, 4.0–4.5 mg (dry weight) of skeletal muscle was homogenized in cold RIPA buffer enriched with protease and phosphatase inhibitors [50 mm Tris pH 8.0, 150 mm NaCl, 1% NP‐40, 0.5% sodium deoxycholate, 0.1% SDS, 2.5 mm phenylmethylsulfonyl fluoride (PMSF), 20 mm β‐glycerophosphate, 10 mm pyrophosphate and 2 mm sodium orthovanadate, including a mini EDTA‐free protease inhibitor tablet according to the instructions of the manufacturer (Roche Diagnostics, Mannheim, Germany)]. Protein concentration was measured by bicinchoninic acid assay (Pierce, Rockford, IL, USA) in triplicate, and a maximal coefficient of variation of 5% between replicates was accepted.
Twenty micrograms of protein lysate in sample buffer was heated to 95°C for 10 min and separated on 12% Criterion TGX Stain‐Free polyacrylamide SDS precast gels (Criterion, BioRad, Copenhagen, Denmark). After SDS electrophoresis, the gels were activated with ultraviolet light for 5 min, followed by a 1 s image in an LAS 4000 image analyser (GE Healthcare, Little Chalfont, UK). The activated gel was transferred to a polyvinylidene fluoride membrane (0.2 μm pores; BioRad, Copenhagen, Denmark) using the Trans‐Blot Turbo Transfer System (BioRad, Copenhagen, Denmark) with Trans‐Blot Turbo Midi Transfer Packs. After the transfer, another 1 s image of the membrane and gel with ultraviolet light to visualize protein transfer was taken.
The membranes were blocked for 1.5 h at room temperature with either skimmed milk or bovine serum albumin (BSA) diluted in Tris‐buffered saline (10 mm Tris base and 150 mm NaCl, pH 7.4) plus 0.05% Tween 20. The membranes were then probed with the following primary antibodies: anti‐ATGL (Ab109251; Abcam, Cambridge, UK, 1:1000), anti‐FABPpm (GOT2, Ab93928; Abcam, Cambridge, UK, 1:1000), anti‐HSL (G7, sc‐74489; Santa Cruz biotechnology, Inc., Heidelberg, Germany, 1:200) and anti‐lipoprotein lipase (H53, sc‐32885; Santa Cruz Biotechnology, Inc., Heidelberg, Germany, 1:200) overnight at 4°C. Thereafter, a horseradish peroxidase‐conjugated secondary goat antibody against rabbit was added (Dako, Glostrup, Denmark, all 1:2000). After primary and secondary antibody incubations, the membranes were washed three times, each for 10 min, in Tris‐buffered saline with or without 0.05% Tween 20.
Blots were developed in ECL detection reagents (GE Healthcare), and the chemiluminescence emitted from immune complexes was visualized with an LAS 4000 image analyser (GE Healthcare). The images of the membranes and stain‐free gels were quantified using ImageQuant TL software version 7.0 (GE Healthcare), see representative blots in Figure 7. Given that we have observed previously that glyceraldehyde‐3‐dehydrogenase, β‐actin and α‐tubulin are not suitable as loading controls in this data set (Vigelsø et al. 2015 a), the intensities of the bands of interest were normalized to the total Stain‐Free fluorescence (total protein).
Figure 7. Representative Western blots for Table 3 and Figs 2 and 3 .
Abbreviations: ACC2, acetyl‐CoA carboxylase 2 [and phosphorylation (p) at serine 221 (Ser221)]; AMPK, AMP‐activated protein kinase; ATGL, adipose triglyceride lipase; CON, control leg; FABPpm, plasma membrane‐associated fatty acid binding protein; HSL, hormone‐sensitive lipase; IM, immobilized leg; and LPL, lipoprotein lipase.
Muscle lysate preparation for AMPK analysis
Lysates were prepared from 20 mg freeze‐dried muscle, dissected free of visible connective tissue, blood and fat and homogenized in 50 mm Hepes (pH 7.5), 10% glycerol, 20 mm sodium pyrophosphate, 150 mm NaCl, 1% NP‐40, 20 mm β‐glycerophosphate, 10 mm NaF, 2 mm PMSF, 1 mm EDTA, 1 mm EGTA, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 2 mm Na3VO4 and 3 mm benzamidine. Homogenates were rotated end over end at 4°C for 1 h. Lysates were prepared by centrifugation of the homogenates for 20 min at 16,000g. Total lysate protein content was analysed by the bicinchoninic acid method (Pierce Biotechnology).
SDS‐PAGE and Western blotting
AMPK subunit isoform protein levels, AMPK (Thr172) and pACC2 Ser221 phosphorylation were measured on lysate mixed in sample buffer (350 mm Tris–HCl, pH 6.8, 30% glycerol, 350 mm SDS, 600 mm DTT, 0.2 mm Bromophenol Blue) and heated for 5 min at 96°C. Fifteen micrograms of protein was separated using 7.5 and 10% Criterion TGX Stain‐Free Precast Gels (BioRad, Copenhagen, Denmark) and transferred (semi‐dry) at 20 V for 30 min (Trans‐Blot Turbo Transfer System, BioRad, Copenhagen, Denmark) to polyvinylidene fluoride membranes (Immobilon Transfer Membrane; Millipore, Denmark).
After blocking [in Tris‐buffered saline plus 0.05% Tween‐20 (TBST) plus 2% skimmed milk] for 45 min at room temperature, the membranes were incubated with primary antibodies (in TBST plus 2% skimmed milk) overnight. The antibodies used were was follows: α1AMPK (ab32047; Abcam, Cambridge, UK, 1:1000), β1AMPK (Arexis, AB, Uppsala, Sweden; Mahlapuu et al. 2004, 1:1000), β2AMPK (donated by Dr. Grahame Hardie, College of Life Sciences, The University of Dundee, UK), AMPKγ1 (ab32508; Abcam, Cambridge, UK, 1:1000), AMPKα2 (SC19131, Santa Cruz biotechnology, Inc., Heidelberg, Germany, 1 μg/ml), AMPKγ3 (sc‐20166; Santa Cruz Biotechnology, Inc., Heidelberg, Germany, 1:200), pACCSer221 (07‐303; Millipore, Hellerup, Denmark, 1:500) and pAMPKThr172 (#2531; Cell Signalling, Danvers, MA, US, 1:1000). Membranes were incubated with the appropriate horseradish peroxidase‐conjugated secondary antibody for 45 min at room temperature (TBST plus 2% skimmed milk; DAKO, Glostrup, Denmark or Jackson ImmunoResearch, West Grove, PA, USA). Detection of ACC was performed by incubating the blocked (3% BSA) membrane with horseradish peroxidase‐conjugated streptavidin (P0397; Dako, Glostrup, Denmark; in 3% BSA) overnight. Blots were developed in ECL detection reagent (ECL, Millipore ECL Forte) and visualized by a charge‐coupled device camera (ChemiDocTM MP System; BioRad, Copenhagen, Denmark). Band densitometry was performed using BioRad ImageLab (version 4.0). The protein content was expressed in arbitrary units, background subtracted and related to the mean of a human skeletal muscle standard sample loaded twice on the corresponding gel in order to minimize assay variation. By loading a control sample in different amounts, it was ensured for each particular protein probed for that quantification was within the linear response range, see representative blots in Figure 7.
One membrane (25–50 kDa) was sequentially probed for γ1 (38 kDa) and then β2 (30 kDa) because they have distinguishable molecular weights and bands. Two membranes were reprobed with an alternative antibody (pACC → ACC and α1 → α2) after being incubated for 60 min at 50°C in stripping buffer (62.3 mm Tris–HCl, 69.4 mm SDS, double distilled H2O and 0.8% β‐mercaptoethanol) and tested for signal from the first antibody.
AMPK activity assay
AMPK complex specific activities were measured on heterotrimeric complexes isolated by a sequential immunoprecipitation. A mixture of 300 μg of muscle lysate protein, a γ3 isoform‐specific antibody (Dr. Grahame Hardie, College of Life Sciences, The University of Dundee, UK) and protein G–agarose beads (16‐266; Milipore) in immunoprecipitation buffer (50 mm NaCl, 1% Triton‐X 100, 50 mm NaF, 5 mm sodium pyrophosphate, 20 mm Trizma base pH 7.5, 500 μm PMSF, 2 mm DTT, 4 μg ml−1 leupeptin, 50 μg ml−1 soybean trypsin inhibitor T9128, 6 mm benzamidine and 250 mm sucrose) rotated end over end overnight at 4°C. The samples were centrifuged twice, each for 60 s at 520g at 4°C. The immunoprecipitate with bound α2β2γ3 was washed once in immunoprecipitation buffer, once in 480 mm Hepes (pH 7.0) and 240 mm NaCl, and twice in 240 mm Hepes (pH 7.0) and 120 mm NaCl, leaving only the agarose after last wash. The kinase reaction ran for 30 min at 30°C in a total volume of 30 μl containing 833 μm DTT, 200 μm AMP, 100 μm AMARA‐peptide, 5 mm MgCl2, 200 μm ATP and 2 μCi of ATP[γ‐33P] (Perkin Elmer, DK). The reaction was stopped by adding 10 μl of 1% phosphoric acid to the reaction, after which 20 μl was spotted onto P81 filter paper (Whatman, GE Healthcare, Brøndby, Denmark), which was then washed 4 × 15 min in 1% phosphoric acid. The dried filter paper was analysed for activity using a Storm 840 PhosphoImager (Molecular Dynamics). The α2β2γ1 activity was analysed on an α2 (Dr Hardie, Dundee) IP on supernatant immunodepleted for α2β2γ3. The α1β2γ1 activity was measured on an α1 (Dr. Grahame Hardie, College of Life Sciences, The University of Dundee, UK) IP on supernatant immunodepleted for both α2β2γ3 and α2β2γ1 as performed previously (Birk & Wojtaszewski, 2006).
β‐Hydroxyacyl‐CoA dehydrogenase activity
β‐Hydroxyacyl‐CoA dehydrogenase (HAD) was measured using spectrophotometry. Approximately 2 mg of the dissected tissue was homogenized in 600 μl of 0.3 m K2HPO4, 0.05% BSA, pH 7.7 for 2 min on a Tissuelyzer (Qiagen, Venlo, Limburg, The Netherlands). Six microlitres of 10% Triton was added, and the samples were left on ice for 15 min before they were stored at −80°C for later analysis. The homogenate was diluted 70 times in a solution containing 0.33 mm acetoacetyl‐CoA, 180 μm NADH, 41.7 μm EDTA and 27.1 mm imidazole (pH 7.0). The changes in NADH at 37°C were measured spectrophotometrically at 340 nm (Bergmeyer, 1974) on an automatic analyser, Cobas 6000, C 501 (Roche Diagnostics). Enzyme activities are expressed as micromoles of substrate per minute per gram dry weight of muscle tissue.
Muscle PDC enzyme activity assay
A small portion of frozen ‘wet’ muscle was used to determine PDC activity as previously described (Constantin‐Teodosiu et al. 1991). Briefly, the activity of PDC in its dephosphorylated active form (PDCa) was assayed in a buffer containing NaF and dichloroacetic acid and was expressed as a rate of acetyl‐CoA formation (in millimoles per minute per kilogram of wet muscle) at 37°C.
Statistics
To investigate the effects of group (young and older men), leg (immobilized and control) and acute exercise [rest (−15 and 0 min), 15, 30 and 45 min] and possible interactions (group × leg × acute exercise), a mixed‐model ANOVA was performed with least‐squares post hoc tests followed by a Tukey–Kramer adjustment. Systematic effects in the model were group, leg and acute exercise, with random levels for leg nested within subject. When interactions were non‐significant, the statistical model was reduced accordingly. Data that were not normally distributed or had unequal variance were logarithmically transformed before statistical analysis. In the case of randomly missing values, the Satterthwaite approximation was used. Outliers were systematically removed from the data set if the data point was greater than the mean ± 2 SD. The level of significance was set at P < 0.05. Statistical analysis was conducted in SAS Enterprise Guide 4.3 (SAS Institutes, Cary, NC, USA). All data are presented as means ± SEM.
Results
Anthropometric data
The participants were included to be representative for their age group. Hence, the older men had higher body mass index and body fat percentage, and the young men had higher (Table 1; Reihmane et al. 2013; Gram et al. 2014; Nørregaard et al. 2015).
Maximal work capacity and workload
During the exercise, there was no difference in workload (in watts), heart rate relative to maximal heart rate or whole‐body RER between the groups (Table 2). At inclusion, there was no difference in Wattmax between the groups or the legs (Vigelsø et al. 2015 b). With immobilization, Wattmax decreased by −14 ± 5 (P < 0.05) and −9 ± 4% (P < 0.05) in the immobilized leg of the young and older men, respectively. The Wattmax did not change in the control leg in either group (Vigelsø et al. 2015 b; Table 2). Hence, the immobilized leg worked at a relatively higher workload compared with the control leg in both young and older volunteers (Table 2).
Table 2.
Whole‐body and single‐leg data after immobilization for 2 weeks and during 45 min acute isolated knee‐extensor exercise at moderate intensity in young and older men
| Whole body | Young men | Older men | ||
|---|---|---|---|---|
| Workload (W) | 20 ± 1 | 20 ± 1 | ||
| Heart rate during exercise (beats min−1) | 113 ± 4* | 95 ± 5 | ||
| Percentage of maximal heart rate (%) | 59 ± 2 | 61 ± 4 | ||
| ss (ml min−1) (20–30 min) | 1066 ± 38(*) | 874 ± 35 | ||
| ss (ml min−1) (20–30 min) | 931 ± 35(*) | 738 ± 35 | ||
| Respiratory exchange ratio | 0.87 ± 0.01 | 0.86 ± 0.01 |
| Isolated leg | Control leg | Immobilized leg | Control leg | Immobilized leg |
|---|---|---|---|---|
| Leg lean mass§ (kg) | 10.7 ± 0.3† | 10.1 ± 0.3* | 9.3 ± 0.3 | 9.2 ± 0.3 |
| Wattmax § (W) | 42 ± 3† | 37 ± 3 | 45 ± 3† | 40 ± 3 |
| Relative workload (% of Wattmax) | 50 ± 2† | 57 ± 3 | 47 ± 4† | 52 ± 3 |
| ss (ml min−1) (30–45 min) | 212 ± 9 | 220 ± 12* | 148 ± 10 | 154 ± 14 |
| IMTG at rest§ [μmol (g dry wt)−1] | 68 ± 9† | 92 ± 11* | 114 ± 13† | 174 ± 39 |
| ΔIMTG [μmol (g dry wt)−1 (45 min)−1] | −20 ± 17 | −20 ± 13 | −18 ± 18 | −18 ± 21 |
| Glycogen at rest§ [μmol (g dry wt)−1] | 221 ± 24 | 214 ± 23* | 257 ± 27 | 265 ± 37 |
| ΔGlycogen [μmol (g dry wt)−1 (45 min)−1] | 36 ± 11 | 12 ± 23*, ‡ | 41 ± 13 | 0 ± 16 |
| HAD activity§ [μmol min−1 (g dry wt)−1] | 102 ± 4† | 87 ± 5 | 101 ± 7† | 88 ± 5 |
| Reported perceived exertion (1–10) | 4.3 ± 0.4† | 4.9 ± 0.4 | 4.2 ± 0.4† | 4.3 ± 0.4 |
Data are mean values ± SEM. *P < 0.05 and (*)0.1 > P < 0.05 young vs. older men (main effect); † P < 0.05 control leg vs. immobilized leg of same age group (main effect). Abbreviations: IMTG, intramuscular triglyceride; ss, steady‐state oxygen uptake () or carbon dioxide output () during exercise, i.e. the average of 30–45 min; Wattmax, maximal work capacity. The values for ΔIMTG and Δglycogen are the changes over 45 min (pre to post); thus, the positive value of glycogen is utilization (‡ P < 0.05, pre vs. post, main effect) and the negative value of IMTG is accumulation (n.s.). §Previously published data (Reihmane et al. 2013; Gram et al. 2014; Nørregaard et al. 2015; Vigelsø et al. 2015 b).
Reported perceived exertion (1–10) for the individual legs increased (P < 0.05) in both groups (main effect) and legs (main effect) from the beginning (5 min) compared with after 25 and 40 min of exercise. Throughout the exercise, the participants reported greater perceived exertion in the immobilized leg compared with the control leg (P < 0.05; main effect, data not shown). Hence, the average perceived exertion was reported to be higher (main effect, P < 0.05) in the immobilized leg compared with the control leg (reportings ranged from 4, ‘I can continue for several hours’ to 5, ‘I have to stop within an hour’; Table 2).
Blood flow and respiratory quotient
Average blood flow during exercise was 41% greater (P < 0.05) in the young compared with the older men, with no difference between the legs, but oxygen extraction was 14% higher (P < 0.05) in the older compared with the young men. Nevertheless, the young men had 32% greater (P < 0.05) absolute leg during the exercise, but this difference disappeared when was normalized to leg lean mass (LLM; Fig. 1 A). However, the young men had higher leg O2 uptake per kilogram LLM over the complete exercise bout (area under the curve) compared with the older men (Fig. 1 B) and during the exercise (AUC for 15–45 min, P < 0.05, data not shown). In the older men, the respiratory quotient (RQ) was higher (P < 0.05) after 15 and 30 min of exercise compared with 0 (rest) and 45 min (Fig. 1 C). During exercise, the RQ for both legs in the older men increased (P < 0.05) to the level of the young men (Fig. 1 C).
Figure 1. Indirect calorimetry .
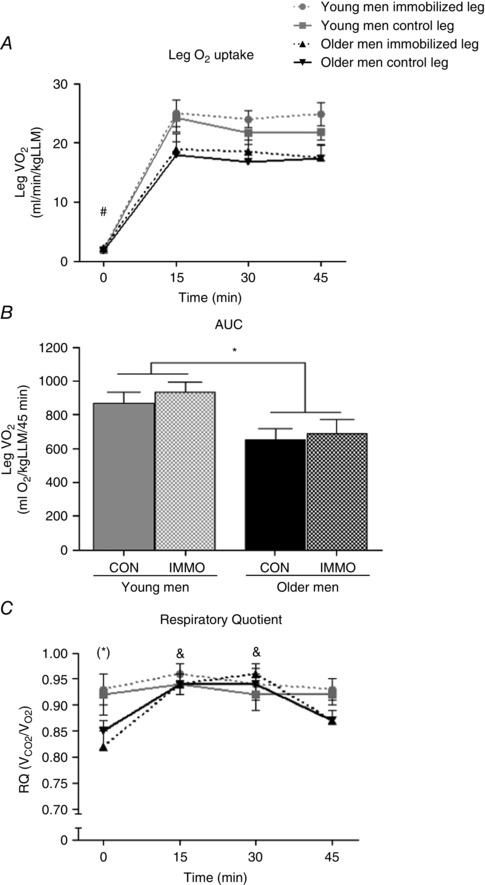
Leg O2 uptake (; A), area under the curve (AUC) for leg O2 uptake (B), C, respiratory quotient (RQ) in young and older men after 2 weeks of unilateral immobilization (IMMO) with the other leg serving as the control (CON) during 45 min moderate‐intensity exercise. *P < 0.05 and (*)0.05 > P < 0.1 young vs. older men; # P < 0.05 rest (0 min) vs. exercise (15, 30 and 45 min); & P < 0.05 15 and 30 min vs. 0 and 45 min in the older men. Data are mean values ± SEM.
Exogenous substrate utilization
Leg glucose uptake, FA uptake and glycerol release were measured as the net balance over the leg.
Leg glucose uptake increased (P < 0.05) with the onset of exercise, with no difference between groups or legs during exercise (Fig. 2 A). At rest, leg FA uptake and leg glycerol release did not differ between the legs in either young or older men (Fig. 2 B and C). From rest to exercise, leg FA uptake increased (P < 0.05) in both groups and legs. Moreover, the older men (both legs) had a greater FA uptake (P < 0.05) compared with the young men (Fig. 2 B). However, leg glycerol release only increased (P < 0.05) from rest to exercise in the young men (main effect) and not in the older men; hence, the young men had a net leg glycerol release, whereas the older men had a net glycerol uptake (Fig. 2 C).
Arterial delivery per unit leg lean mass of FA [in μmol (kg LLM)−1 45 min−1: young men, control leg, 2.0 ± 0.2 and immobilized leg, 2.2 ± 0.3; and older men, control leg, 2.1 ± 0.3 and immobilized leg, 2.0 ± 0.2] and glycerol ([in μmol (kg LLM)−1 45 min−1: young men, control leg, 281 ± 81 and immobilized leg, 319 ± 35; and older men, control leg, 252 ± 40 and immobilized leg, 299 ± 56] did not differ between the groups or the legs, respectively.
Lactate release increased (P < 0.05) in both groups and legs (main effect) from rest to exercise (Fig. 2 D). Furthermore, the lactate release was greater (main effect) in the immobilized leg compared with the control leg in both groups (Fig. 2 D).
Endogenous substrate utilization
After immobilization but before the acute exercise, the older men had 69 and 89% greater (P < 0.05) IMTG than the young men in the control and immobilized leg, respectively (Vigelsø et al. 2016; Table 2). Furthermore, IMTG was 50 ± 23 and 45 ± 20% higher (main effect, P < 0.05) in the immobilized leg compared with the control leg after immobilization in the young and older men, respectively (Table 2). We did not observe IMTG utilization during the 45 min of exercise, i.e. IMTG content did not change with acute exercise in either groups or legs (Table 2).
Muscle glycogen content was greater (main effect, P < 0.05, 17%) in the older men compared with the young men (Table 2; Vigelsø et al. 2016). Furthermore, we observed glycogen utilization during the 45 min of exercise (pre vs. post value, main effect, P < 0.05), i.e. the muscle glycogen content decreased by 10% in both legs in the young men and by 7 and 0% in the control and immobilized legs of the older men, respectively (Table 2).
Enzyme activity
Muscle HAD activity at rest did not differ between the groups and was significantly greater in the control leg than in the immobilized leg in both age groups (12 ± 6 and 10 ± 6%, respectively; P < 0.05; Table 2).
Proteins in lipid metabolism
The protein content of FABPpm tended (main effect, P = 0.08) to be 20% higher in the young compared with the older men. Additionally, there was 25% more (P < 0.05) FAPBpm protein in the control leg (main effect) compared with the immobilized leg (Fig. 3 A). The ATGL protein levels were 30% higher (P < 0.05) in the young compared with the older men. Likewise, the ATGL protein content was 20% higher (main effect, P < 0.05) in the control leg compared with the immobilized leg (Fig. 3 B). Furthermore, there was a trend (main effect, P = 0.06) of 32% higher lipoprotein lipase content in the older men (Table 3). There was no difference between the legs or groups in HSL protein content (Table 3).
Figure 3. Protein content of plasma membrane (pm)‐bound fatty acid binding protein (A) and adipose triglyceride lipase (ATGL; B) in young and older men after 2 weeks of unilateral immobilization with the other leg serving as a control .
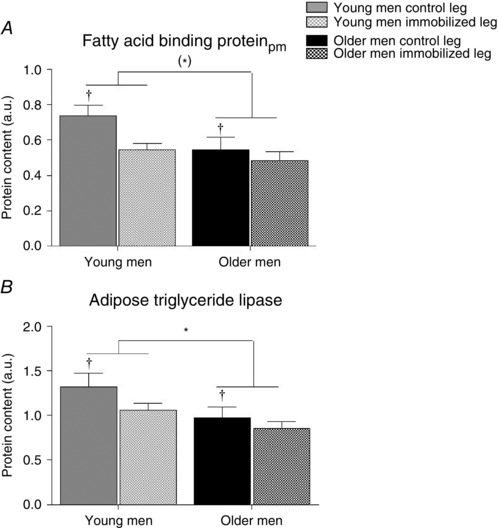
*P < 0.05 and (*)0.05 > P < 0.1 young vs. older men (main effect); † P < 0.05 immobilized leg vs. control leg of same group. Data are shown as mean values + SEM. See representative Western blots in Fig. 7.
Table 3.
Protein content measured by Western blotting
| Young men | Older men | ||||
|---|---|---|---|---|---|
| Protein | Control leg | Immobilized leg | Control leg | Immobilized leg | Main effect |
| Lipoprotein lipase | 0.95 ± 0.18 | 1.07 ± 0.21 | 1.30 ± 0.19 | 1.53 ± 0.22 | (*) |
| Hormone‐sensitive lipase | 0.60 ± 0.10 | 0.48 ± 0.07 | 0.53 ± 0.06 | 0.64 ± 0.14 | |
| pAMPKthr172 | 1.08 ± 0.09 | 1.15 ± 0.09 | 1.00 ± 0.12 | 1.12 ± 0.13 | |
| AMPKα1 | 0.95 ± 0.09 | 1.03 ± 0.06 | 0.91 ± 0.11 | 1.02 ± 0.10 | † |
| AMPKα2 | 0.97 ± 0.03 | 0.95 ± 0.02 | 0.93 ± 0.03 | 0.96 ± 0.04 | |
| ΑΜΡΚβ1 | 0.90 ± 0.09 | 1.14 ± 0.09 | 0.97 ± 0.12 | 1.24 ± 0.11 | † |
| ΑΜΡΚβ2 | 1.24 ± 0.08*, † | 1.11 ± 0.05 | 0.98 ± 0.06 | 0.99 ± 0.05 | |
| ΑΜΡΚγ1 | 1.35 ± 0.07 | 1.44 ± 0.09 | 1.21 ± 0.11 | 1.34 ± 0.13 | |
| ΑΜΡΚγ3 | 1.90 ± 0.17 | 1.64 ± 0.16 | 1.37 ± 0.13 | 1.21 ± 0.10 | † |
Data are mean values ± SEM. *P < 0.05 and (*)0.1 > P < 0.05 young vs. older men; † P < 0.05 immobilized leg vs. control leg. It is a main effect of either group or leg if marked in the ‘main effect’ column or an interaction between group and leg if marked in the table (AMPKβ2). See representative Western blots in Fig. 7.
Protein content of AMPK and ACC2
The AMPKα1 and α2 protein content did not differ between age groups. The protein content of AMPKβ2 was higher (P < 0.05) in the young compared with the older men (Table 3). AMPKβ1 protein content was lower (main effect, P < 0.05) in the control leg compared with the immobilized leg in both groups (Table 3). The AMPKγ1 protein content did not differ between age groups or the legs (Table 3). The AMPKγ3 protein content was higher in young compared with older men and in the control leg than in the immobilized leg in both age groups (P < 0.05; Table 3).
The ACC2 protein content in the young was higher than in the older men (Fig. 4 A). Furthermore, phosphorylation of ACCSer221 increased (main effect, P < 0.05) with acute exercise in both groups and legs (Fig. 4 B). Moreover, the acute exercise‐induced ACCSer221 phosphorylation was higher (P < 0.05) in the immobilized compared with the control leg (Fig. 4 B). Collectively, the ratio of ACCSer221 phosphorylation to ACC2 protein content increased (main effect, P < 0.05) with acute exercise and was higher in the immobilized leg (main effect, P < 0.05). Additionally, there was a trend (main effect, P = 0.07) towards higher ACCSer221 phosphorylation to ACC2 protein content ratio in the immobilized leg (Fig. 4 C).
Figure 4. Acetyl‐CoA carboxylase 2 (ACC2) activation: protein content of ACC2 (A) pACC2Ser221 (B) and relative ACC2 activation (pACC2Ser221/ACC2 ratio; C) in young and older men after 2 weeks of unilateral immobilization with the other leg serving as a control .
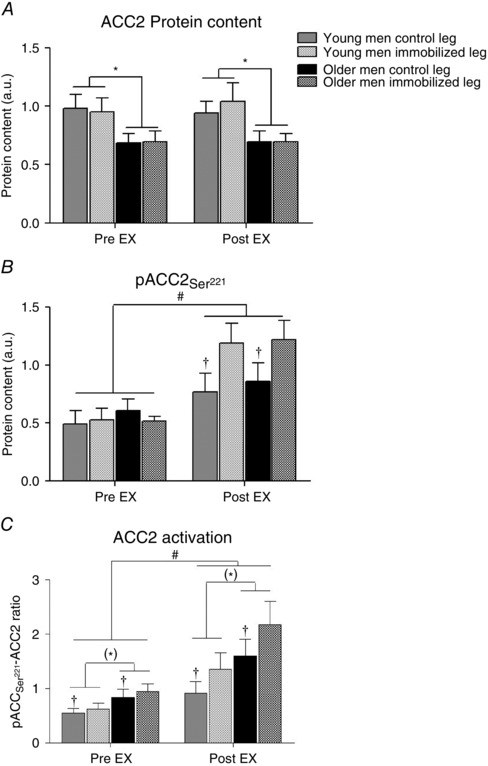
Values were measured pre and post 45 min isolated knee‐extensor exercise (EX) at moderate intensity with both legs. *P < 0.05 and (*)0.05 > P < 0.1 young vs. older men (main effect); † P < 0.05 immobilized leg vs. control leg of same group and time point; # P < 0.05 pre vs. post EX (main effect). Data are shown as mean values + SEM. See representative Western blots in Fig. 7.
AMPK complex specific activity and PDC activity
The activity of AMPKα1β2γ1 in the young men was lower (main effect, P < 0.05) than in the older men. Moreover, in response to acute exercise AMPKα1β2γ1 activity decreased (main effect, P < 0.05) in the immobilized leg (Fig. 5 A). There was no difference between the age groups, between legs or in response to acute exercise in AMPKα2β2γ1 activity (Fig. 5 B) or AMPKThr172 phosphorylation, representing total AMPK activity (Table 3). There was an increase across age and treatment groups (main effect) in AMPKα2β2γ3 activity with acute exercise (Fig. 5 C).
Figure 5. AMP‐activated protein kinase (AMPK) complex specific activity of AMPKα2β2γ3 (A) AMPKα2β2γ1 (B) and AMPKα1β2γ1 (C) in young and older men after 2 weeks of unilateral immobilization with the other leg serving as a control .
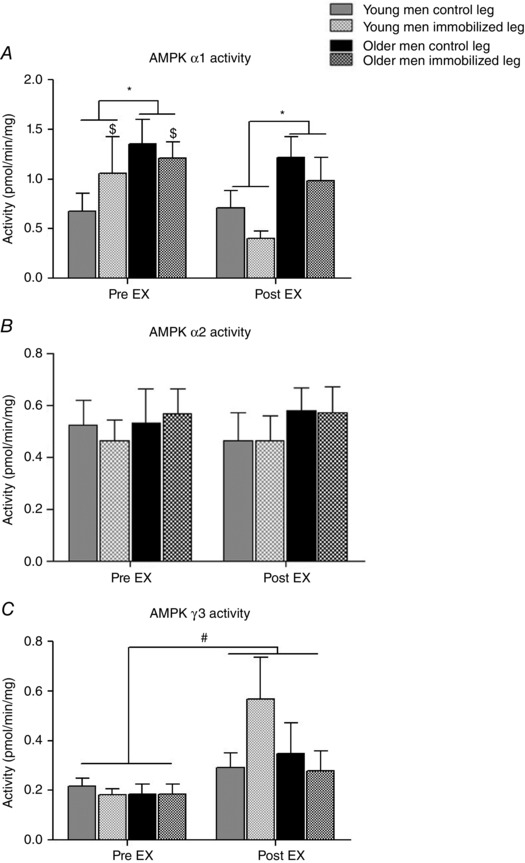
Values were measured pre and post 45 min isolated knee‐extensor exercise (EX) at moderate intensity with both legs. *P < 0.05 young vs. older men (main effect); # P < 0.05 pre vs. post EX (main effect); $ P < 0.05 same leg pre vs. post EX. Data are shown as mean values + SEM.
Muscle PDC activity in the young was higher (main effect, P < 0.05) than in the older men (Fig. 6 A). Furthermore, there was a negative correlation (r 2 = −0.40, P < 0.05) between the AUC for lactate release (Fig. 2 D) and ∆PDC activity pre‐ and postexercise (Fig. 6 B).
Figure 6. Pyruvate dehydrogenase complex (PDC) specific activity (A) and correlation between ∆PDC activity and area under the curve (AUC) for leg lactate release (B; Fig. 2 D) in young and older men after 2 weeks of unilateral immobilization with the other leg serving as a control .
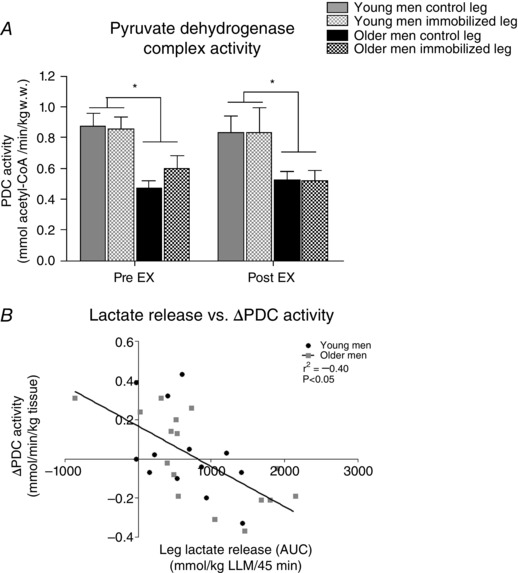
Values were measured pre and post 45 min isolated knee‐extensor exercise (EX) at moderate intensity with both legs. *P < 0.05 young vs. older men (main effect). Data are shown as mean values + SEM.
Calculations of energy consumption: indirect calorimetry and substrate utilization
The relative glucose and lipid oxidation calculated by indirect calorimetry did not differ between groups or legs [(glucose/lipid): young men, 72 ± 5%/28 ± 5% and 75 ± 5%/25 ± 5%; and older men, 70 ± 6%/30 ± 6% and 72 ± 6%/28 ± 6% for the control and immobilized leg, respectively]. The young men had an equal contribution of FA derived exogenously (control leg, 44 ± 7% and immobilized leg, 33 ± 6%) and endogenously in the leg (control leg, 56 ± 7% and immobilized leg, 67 ± 6%), whereas the older men had a larger exogenous contribution (control leg, 85 ± 6% and immobilized leg, 79 ± 4%) compared with the endogenous contribution (control leg, 15 ± 6% and immobilized leg, 21 ± 4%).
Discussion
Our main finding was that older men primarily mobilized exogenous FA, whereas the young men had greater use of endogenous fat stores during exercise at moderate intensity. This notion is based on the difference in net leg glycerol release between groups. The proposed difference in fat mobilization between young and older men may have been accounted for by the higher ATGL protein content measured in the young than in the older men, which would facilitate increased lipolysis rates in the muscle of the young men. In addition, higher AMPKβ2 and AMPKγ3 subunit protein contents were observed in young compared with older men, which supports the contention of augmented AMPK expression with age (Drummond et al. 2008; Mortensen et al. 2009). In contrast to our hypothesis and despite the muscle adaptations to the leg immobilization (i.e. decreased HAD activity, ATGL, AMPKβ1 and AMPKγ3 protein content) and the age difference (i.e. greater ATGL and ACC2 content and PDC activity in the young than the older men), there was no change in the relative carbohydrate and fat utilization (RQ) in response to immobilization or age. Overall, we demonstrate that both inactivity and age are associated with IMTG accumulation, but that this occurs only in part through the same mechanisms. Thus, age‐related lipid accumulation may occur through decreased lipolytic capacity and an increased exogenous mobilization of FA, leaving the IMTG for accumulation, whereas inactivity‐related lipid accumulation may be associated with decreased lipolytic capacity and mitochondrial FA oxidative capacity.
Leg , RQ and relative substrate utilization
We observed lower blood flow in the older men during exercise. This is very likely to be the result of an age‐related decline in regulatory pathways of vasodilatation by mechanisms such as reduced endothelium‐dependent vasodilatation (nitric oxide; Taddei et al. 2001), increased endothelin‐1 activity (Donato et al. 2009) and/or overactive sympathetic vasoconstriction (Mortensen et al. 2012). Furthermore, we observed an age‐related difference in leg O2 uptake during the exercise bout, with the young men having higher leg . This observation was unexpected because the groups were exercising at the same intensity and did not differ in lactate release. Nevertheless, the data indicate that exercise efficiency ( per watt) was higher in the older men compared with the young men.
We hypothesized that age and immobilization would increase the relative glucose oxidation and hence lead to an increased RQ during a subsequent exercise bout at moderate intensity. However, we found no differences in RQ between the immobilized and control leg during acute one‐legged exercise in young or older men. The immobilization protocol was sufficient to induce changes in muscle metabolism [e.g. increased relative workload, decreased citrate sythase (CS), mitochondrial respiration (Gram et al. 2014) and HAD activity, a lower AMPKβ2 (in the young men only), AMPKγ3, ATGL and FABPpm protein content and a higher ACCSer221 phosphorylation after acute exercise]. Hence, the metabolic changes induced by immobilization were either insufficient to induce a measurable change in RQ and/or the workload was not sufficiently high to elicit a difference. We cannot rule out the possibility that a difference in substrate utilization for young and older men might become detectable at a higher exercise intensity. However, based on pilot studies we found that higher intensities were not feasible for the participants. Moreover, the relevance of using the present exercise intensity may have clinical and physiological significance because it elucidates limitations in substrate mobilization during an intensity that mimics an everyday work intensity.
The RQ decreased in the older men at the 45 min time point in both legs during the exercise compared with 15 and 30 min, which indicates a shift towards a higher contribution from fat oxidation. This effect of age is not readily explained. We did see, although this was not significant, that lactate release was maintained in the old but lowered in the young men from 30 to 45 min, and it is possible that a subtle pH increase in muscle may have increased the fat oxidation in the older men. The older men had a lower resting RQ and, given that the blood flow was lower during exercise, this may have attenuated a higher fat oxidation also observed during exercise (Rosenkilde et al. 2010).
Lipid mobilization during exercise
We hypothesized that immobilization and age would change the source of FA mobilized during exercise to rely more on exogenous FA. Leg FA uptake did indeed increase during exercise, although this was greater in older men than in young men. In addition, in response to exercise, the skeletal muscle of young men showed net leg glycerol release, whereas in contrast, exercise induced net leg glycerol uptake in older men.
Suppression of adipose tissue lipolysis with nicotinic acid has been shown to be associated with increased IMTG utilization in young men (Watt et al. 2004), leading the authors to suggest that plasma FA availability is a regulator of IMTG utilization (O'Neill et al. 2004; Watt et al. 2004). However, in the present study there was no difference in the delivery of FA and glycerol throughout the exercise, and the observed difference can thus not be explained by substrate availability during exercise. Nevertheless, we have previously reported that older men have higher fasting plasma FA and visceral adipose tissue (Nørregaard et al. 2015). Hence, increased exogenous FA utilization may be an age‐related adaptation to chronic high resting FA availability. Fatty acid infusion has been shown to lead to IMTG accumulation (Schenk et al. 2005), and high plasma FA is known to inhibit IMTG utilization (O'Neill et al. 2004; Watt et al. 2004). Hence, the higher resting fasting plasma FA in the older men may have contributed to induce the observed higher IMTG. Accumulation of IMTG is related to insulin resistance, although not necessarily causal, and it is thus likely that the IMTG accumulation in the older men will lead to insulin resistance at some point (Unger, 2002; Moro et al. 2008). We have previously reported that young and older men did not differ in capillarization, muscle fibre type distribution or muscle fibre size (Vigelsø et al. 2015 b), and therefore, the muscle morphology cannot explain the higher FA uptake and ATGL protein content in the young men. Interestingly, ATGL has been proposed as the major lipase in skeletal muscle during contraction (Alsted et al. 2013), and this indicates that young men have a higher intramuscular lipolytic capacity, and thus a higher endogenous FA supply capacity, which may explain the higher leg glycerol release during exercise.
Lipid uptake and lipolytic proteins
The FABPpm (Kiens et al. 1997, 2004) and ATGL (Alsted et al. 2009; Louche et al. 2013; Vigelsø et al. 2016) have previously been shown to increase with endurance training. The present study is the first to show that these proteins decrease with immobilization for 2 weeks. Furthermore, it is also novel that the protein content of ATGL was higher in young than in older men.
AMPK and regulation of β‐oxidation
It is a new finding that the protein contents of ACC2 and AMPKβ2 subunit were higher in young than in older men. However, our observation of decreased AMPKγ3 protein in the immobilized legs of both the young and the older men is in contrast to two studies that used different models of inactivity. Mortensen et al. (2014) observed increased AMPKγ3 protein content after 9 days of bed rest, whereas Kostovski et al. (2013) observed increased AMPKγ3 in individuals with long‐term compared with recent spinal injury.
In skeletal muscle, AMPK may partly regulate fatty acid β‐oxidation through the inhibition of ACC2, thereby decreasing carnitine palmitoyltransferase 1‐mediated FA uptake in the mitochondria (Stephens et al. 2002). Little is known about this pathway in relation to immobilization and age. In the present study, exercise increased the phosphorylation of ACC2Ser221 in the immobilized legs of both groups. Paradoxically, this implies that the acute exercise in the immobilized leg increased mitochondrial FA supply and oxidation, which was not the case. We speculate that this may be related to the higher relative workload in the immobilized leg or that the ACC2 pathway possibly compensates for a decreased contribution of other pathways affected by the immobilization. The latter contention is supported by the observation that muscle FA oxidation can occur without changes in malonyl‐CoA concentrations (Odland et al. 1996, 1998; Dean et al. 2000). If the concentration of malonyl‐CoA is not essential for switching between glucose and fat oxidation in skeletal muscle, it seems unlikely that AMPK‐mediated ACC2 inhibition plays a vital role in the regulation of FA uptake by the mitochondria. Support for this notion is provided in a recent study by Fentz et al. (2015) in AMPKα knockout mice, where the authors suggested that the AMPKα subunit exerts additional indirect effects on FA utilization during exercise through regulation of FABPpm content.
Pyruvate dehydrogenase complex activity and lactate release
To our knowledge, the present study provides the first evidence that muscle mitochondrial PDC activation status at rest is lower in older men than in young men, and this remained unchanged after exercise. Although these observations would be intuitively expected given that PDC activation status is related to the aerobic/mitochondrial capacity (Constantin‐Teodosiu, 2013), this is in contrast to the recent srudy by Wall et al. (2015), who reported that muscle PDC activation increased in older men, but not in young men, after 5 days of leg immobilization. Additionally, another important finding of the present study was that leg lactate release (AUC) during exercise was negatively associated with the change in PDC activation during exercise (Fig. 6 A and B).
Indeed, PDC activity is the rate‐limiting step in glucose oxidation, and therefore, at least in part, controls muscle glucose and FA oxidation (Constantin‐Teodosiu et al. 1992; van Loon et al. 2001). The lower PDC activity in the older men, therefore, implies a lower capacity of muscle to oxidize glucose. However, this was not detected as a decreased glucose utilization during exercise. Moreover, the difference in PDC activity could not be related to differences in the mitochondrial content or function, as previously suggested (Constantin‐Teodosiu, 2013). We have previously published evidence that mitochondrial content (measured as CS activity, voltage‐dependent ion channel, mitochondrial complex protein content and mitochondrial respiratory capacity) did not differ between the groups (Gram et al. 2014). Given that IMTG accumulation has been proposed to be an inhibitor of PDC activity (Gurd et al. 2008), the lower PDC activity in the older men could potentially be accounted for by the greater levels of IMTG.
AMPKα1 and protein synthesis
Although it was not our primary hypothesis, it is a noteworthy finding that AMPKα1 specific activity was higher in older than in young men (Fig. 5 A), despite no difference in AMPKα1 protein content. A high activity of AMPKα1 has been suggested to inhibit the mammalian target of rapamycin complex 1 (mTORC1) pathway, thereby inhibiting protein synthesis in skeletal muscle (Mounier et al. 2009, 2011), and to be important for lipid metabolism (Fentz et al. 2015). Hence, high AMPKα1 activity in older men could contribute to a lower contraction‐induced protein synthesis by mTORC1, which has been linked, albeit in rodents, to sarcopenia (Parkington et al. 2004; Thomson & Gordon, 2006).
Ectopic IMTG accumulation with age and inactivity
In agreement with others, we observed that age was associated with IMTG accumulation. However, in contrast to a recent study using 5 days of one‐leg immobilization (Wall et al. 2015), we observed IMTG accumulation with our immobilization intervention. The latter finding is likely to result from the duration of the intervention. It is likely that over time the IMTG accumulation observed in older men and in the immobilized leg will lead to insulin resistance (Unger, 2002; Moro et al. 2008). The age‐related IMTG accumulation may have arisen from the shift in the source of FA for oxidation and the inactivity‐related IMTG accumulation from a decline in ATGL and FA oxidative capacity.
Limitations
It is a limitation that we did not use tracers, such that we could have distinguished between sources of glycerol release, i.e. glycerol coming from blood lipoproteins (very low‐density lipoproteins) and plasma triglycerides or IMTG. However, the primary aim was to investigate the overall difference in substrate utilization measured by indirect calorimetry and to elucidate the source of FA mobilization (exogenous or endogenous) during a bout of moderate‐intensity exercise undertaken by young and older men. Equally, it is important to note that glycerol is both released and taken up by tissues (van Hall et al. 2002; Stallknecht et al. 2004; Helge et al. 2007), and the net glycerol release is probably underestimating total glycerol release. However, the catheters were inserted in the anterograde direction and, therefore, the measurements of glycerol release may be slightly overestimated and FA uptake slightly underestimated owing to contamination of venous blood from circumflexea ilium superficialis vein (van Hall et al. 1999). However, this contribution is minor (van Hall et al. 1999) and as this was done systematically, the contamination is likely to have affected both groups and legs equally. Finally, the protein content obtained by Western blotting does not provide information on intramuscular compartmentalization, functionality or activity (Prats et al. 2011).
Conclusions
Using an efficient immobilization protocol, we found that immobilization and age difference did not affect relative substrate metabolism during a bout of exercise of moderate intensity. This occurred despite impairments in muscle metabolism (lower PDC activity, AMPKβ2 and AMPKγ3 protein content) with age and despite a clear effect of the immobilization (i.e. lower muscle HAD activity, lower ATGL protein, lower AMPKα1, β1 and γ3 protein, higher ACCSer221 phosphorylation and higher lactate release during exercise in the young and older men with immobilization). Thus, our data support the notion that AMPK is impaired with both immobilization and age. Furthermore, the higher lipolytic capacity in the young men, as suggested by their higher ATGL protein content than in the older men, probably contributed to the increased endogenous FA utilization and higher glycerol release during exercise. In agreement with others, we report that age and inactivity are associated with IMTG accumulation. However, our findings indicate that age and immobilization lead to IMTG accumulation only in part through the same mechanisms. Thus, the age‐related IMTG accumulation may be related to a shift in the source of mobilized FA (i.e. primary reliance on exogenous FA recruited from the circulation), whereas the immobilization‐related IMTG accumulation was related to the muscle protein level.
Additional information
Competing interests
None declared.
Author contributions
Pyruvate dehydrogenase complex activity was measured by P.L.G. and D.C.‐T. at MRC/Arthritis Research UK Centre for Musculoskeletal Ageing Research, Arthritis Research UK Centre for Sport, Exercise and Osteoarthritis, School of Life Sciences, The Medical School, University of Nottingham. The AMPK and ACC data were measured by R.D., J.B.B. and J.F.P.W. at Section of Molecular Physiology, The August Krogh Centre, Department of Nutrition, Exercise and Sports, University of Copenhagen. All remaining data and experiments were performed at XLAB, Center for Healthy Aging, Department of Biomedical Sciences, University of Copenhagen by A.V., M.G., A.B.K., C.P., F.D. and J.W.H. A.V., F.D. and J.W.H. contributed to the conception and design of the experiments, the data collection, analysis, interpretation of the data and manuscript revision. M.G. and C.P. contributed to the design of the study and experiments, interpretation of data and manuscript revision. R.D., A.B.K., P.L.G., D.C.‐T., J.B.B. and J.F.P.W. contributed to the data collection and manuscript revision. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the UNIK (Investment Capital for University Research) research programme ‘Food, Fitness & Pharma for Health and Disease’ (Danish Ministry of Science, Technology and Innovation), The Nordea Foundation, A. P. Møller and Hustru Chastine Mc‐Kinney Møllers Foundation.
Acknowledgements
The technical assistance of Michael Taulo Lund, MD; Merethe Hansen, MD; Jesper Nørregaard, MD; Christina Neigaard Hansen, PhD; Regitze Kraunsøe; Katrine Qvist; and Jeppe Bach is gratefully acknowledged.
References
- Alibegovic AC, Hojbjerre L, Sonne MP, van Hall G, Alsted TJ, Kiens B, Stallknecht B, Dela F & Vaag A (2010). Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab 298, E555–E564. [DOI] [PubMed] [Google Scholar]
- Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R & Kiens B (2009). Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296, E445–E453. [DOI] [PubMed] [Google Scholar]
- Alsted TJ, Ploug T, Prats C, Serup AK, Høeg L, Schjerling P, Holm C, Zimmermann R, Fledelius C, Galbo H & Kiens B (2013). Contraction‐induced lipolysis is not impaired by inhibition of hormone‐sensitive lipase in skeletal muscle. J Physiol 591, 5141–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU (1974). Methods of Enzymatic Analysis. Academic Press, New York. [Google Scholar]
- Bergouignan A, Trudel G, Simon C, Chopard A, Schoeller DA, Momken I, Votruba SB, Desage M, Burdge GC, Gauquelin‐Koch G, Normand S & Blanc S (2009). Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 58, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk JB & Wojtaszewski JFP (2006). Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577, 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin‐Teodosiu D (2013). Regulation of muscle pyruvate dehydrogenase complex in insulin resistance: effects of exercise and dichloroacetate. Diabetes Metab J 37, 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin‐Teodosiu D, Cederblad G & Hultman E (1991). A sensitive radioisotopic assay of pyruvate dehydrogenase complex in human muscle tissue. Anal Biochem 198, 347–351. [DOI] [PubMed] [Google Scholar]
- Constantin‐Teodosiu D, Cederblad G & Hultman E (1992). PDC activity and acetyl group accumulation in skeletal muscle during prolonged exercise. J Appl Physiol (1985) 73, 2403–2407. [DOI] [PubMed] [Google Scholar]
- Cree MG, Newcomer BR, Katsanos CS, Sheffield‐Moore M, Chinkes D, Aarsland A, Urban R & Wolfe RR (2004). Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 89, 3864–3871. [DOI] [PubMed] [Google Scholar]
- Cree MG, Paddon‐Jones D, Newcomer BR, Ronsen O, Aarsland A, Wolfe RR & Ferrando A (2010). Twenty‐eight‐day bed rest with hypercortisolemia induces peripheral insulin resistance and increases intramuscular triglycerides. Metabolism 59, 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA & Ruderman N (2000). Exercise diminishes the activity of acetyl‐CoA carboxylase in human muscle. Diabetes 49, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K & Seals DR (2009). Vascular endothelial dysfunction with aging: endothelin‐1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 297, H425–H432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield‐Moore M, Volpi E & Rasmussen BB (2008). Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104, 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jørgensen SB, Lynch GS, Kemp BE & Steinberg GR (2008). AMPK‐independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586, 5819–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijnde BO, Derave W, Wojtaszewski JF, Richter EA & Hespel P (2005). AMP kinase expression and activity in human skeletal muscle: effects of immobilization, retraining, and creatine supplementation. J Appl Physiol 98, 1228–1233. [DOI] [PubMed] [Google Scholar]
- Eriksen L, Grønbæk M, Helge JW, Tolstrup JS & Curtis T (2011). The Danish Health Examination Survey 2007–2008 (DANHES 2007–2008). Scand J Public Health 39, 203–211. [DOI] [PubMed] [Google Scholar]
- Fentz J, Kjøbsted R, Birk JB, Jordy AB, Jeppesen J, Thorsen K, Schjerling P, Kiens B, Jessen N, Viollet B & Wojtaszewski JFP (2015). AMPKα is critical for enhancing skeletal muscle fatty acid utilization during in vivo exercise in mice. FASEB J 29, 1725–1738. [DOI] [PubMed] [Google Scholar]
- Fogelholm M (2013). New Nordic Nutrition Recommendations are here. Food Nutr Res 57, doi: 10.3402/fnr.v57i0.22903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram M, Vigelsø A, Yokota T, Hansen CN, Helge JW, Hey‐Mogensen M & Dela F (2014). Two weeks of one‐leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol 58, 269–278. [DOI] [PubMed] [Google Scholar]
- Gram M, A Vigelsø, Yokota T, Helge JW, Dela F & Hey‐Mogensen M (2015). Skeletal muscle mitochondrial H2O2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol 593, 4011–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd BJ, Peters SJ, Heigenhauser GJ, LeBlanc PJ, Doherty TJ, Paterson DH & Kowalchuk JM (2008). O2 uptake kinetics, pyruvate dehydrogenase activity, and muscle deoxygenation in young and older adults during the transition to moderate‐intensity exercise. Am J Physiol Regul Integr Comp Physiol 294, R577–R584. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2011). Energy sensing by the AMP‐activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc 70, 92–99. [DOI] [PubMed] [Google Scholar]
- Helge JW, Stallknecht B, Richter EA, Galbo H & Kiens B (2007). Muscle metabolism during graded quadriceps exercise in man. J Physiol 581, 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J (1977). Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol 270, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TE, Wojtaszewski JFP & Richter EA (2009). AMP‐activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Physiol (Oxf) 196, 155–174. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE & Wallis GA (2005). Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int J Sports Med 26 Suppl 1, S28–S37. [DOI] [PubMed] [Google Scholar]
- Jørgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA & Wojtaszewski JFP (2004). The α2‐5′AMP‐activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes 53, 3074–3081. [DOI] [PubMed] [Google Scholar]
- Kiens B, Kristiansen S, Jensen P, Richter EA & Turcotte LP (1997). Membrane associated fatty acid binding protein (FABPpm) in human skeletal muscle is increased by endurance training. Biochem Biophys Res Commun 231, 463–465. [DOI] [PubMed] [Google Scholar]
- Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J & Nielsen JN (2004). Lipid‐binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol 97, 1209–1218. [DOI] [PubMed] [Google Scholar]
- Klein DK, Pilegaard H, Treebak JT, Jensen TE, Viollet B, Schjerling P & Wojtaszewski JFP (2007). Lack of AMPKα2 enhances pyruvate dehydrogenase activity during exercise. Am J Physiol Endocrinol Metab 293, E1242–E1249. [DOI] [PubMed] [Google Scholar]
- Kostovski E, Boon H, Hjeltnes N, Lundell LS, Ahlsén M, Chibalin AV, Krook A, Iversen PO & Widegren U (2013). Altered content of AMP‐activated protein kinase isoforms in skeletal muscle from spinal cord injured subjects. Am J Physiol Endocrinol Metab 305, E1071–E1080. [DOI] [PubMed] [Google Scholar]
- Larsen S, Danielsen JH, Søndergård SD, Søgaard D, Vigelsoe A, Dybboe R, Skaaby S, Dela F & Helge JW (2015). The effect of high‐intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand J Med Sci Sports 25, e59–e69. [DOI] [PubMed] [Google Scholar]
- Levadoux E, Morio B, Montaurier C, Puissant V, Boirie Y, Fellmann N, Picard B, Rousset P, Beaufrere B & Ritz P (2001). Reduced whole‐body fat oxidation in women and in the elderly. Int J Obes Relat Metab Disord 25, 39–44. [DOI] [PubMed] [Google Scholar]
- Louche K, Badin PM, Montastier E, Laurens C, Bourlier V, de Glisezinski I, Thalamas C, Viguerie N, Langin D & Moro C (2013). Endurance exercise training up‐regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. J Clin Endocrinol Metab 98, 4863–4871. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Johansson C, Lindgren K, Hjälm G, Barnes BR, Krook A, Zierath JR, Andersson L & Marklund S (2004). Expression profiling of the gamma‐subunit isoforms of AMP‐activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab 286, E194–E200. [DOI] [PubMed] [Google Scholar]
- Martin WH 3rd, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS & Holloszy JO (1993). Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol Endocrinol Metab 265, E708–E714. [DOI] [PubMed] [Google Scholar]
- Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J & Evans WJ (1989). Peripheral effects of endurance training in young and old subjects. J Appl Physiol 66, 2844–2849. [DOI] [PubMed] [Google Scholar]
- Moro C, Bajpeyi S & Smith SR (2008). Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294, E203–E213. [DOI] [PubMed] [Google Scholar]
- Mortensen B, Friedrichsen M, Andersen NR, Alibegovic AC, Højbjerre L, Sonne MP, Stallknecht B, Dela F, Wojtaszewski JFP & Vaag A (2014). Physical inactivity affects skeletal muscle insulin signaling in a birth weight‐dependent manner. J Diabetes Complications 28, 71–78. [DOI] [PubMed] [Google Scholar]
- Mortensen B, Poulsen P, Wegner L, Stender‐Petersen KL, Ribel‐Madsen R, Friedrichsen M, Birk JB, Vaag A & Wojtaszewski JFP (2009). Genetic and metabolic effects on skeletal muscle AMPK in young and older twins. Am J Physiol Endocrinol Metab 297, E956–E964. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K & Saltin B (2012). Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590, 6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Foretz M & Viollet B (2011). Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle 10, 2640–2646. [DOI] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M & Viollet B (2009). Important role for AMPKα1 in limiting skeletal muscle cell hypertrophy. FASEB J 23, 2264–2273. [DOI] [PubMed] [Google Scholar]
- Nørregaard J, Gram M, A Vigelsø, Wiuff C, Kuhlman AB, Helge JW & Dela F (2015). The effect of reduced physical activity and retraining on blood lipids and body composition in young and older adult men. J Aging Phys Act 23, 489–495. [DOI] [PubMed] [Google Scholar]
- O'Neill M, Watt MJ, Heigenhauser GJ & Spriet LL (2004). Effects of reduced free fatty acid availability on hormone‐sensitive lipase activity in human skeletal muscle during aerobic exercise. J Appl Physiol 97, 1938–1945. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Lopaschuk GD & Spriet LL (1996). Human skeletal muscle malonyl‐CoA at rest and during prolonged submaximal exercise. Am J Physiol Endocrinol Metab 270, E541–E544. [DOI] [PubMed] [Google Scholar]
- Odland LM, Howlett RA, Heigenhauser GJ, Hultman E & Spriet LL (1998). Skeletal muscle malonyl‐CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol Endocrinol Metab 274, E1080–E1085. [DOI] [PubMed] [Google Scholar]
- Parkington JD, LeBrasseur NK, Siebert AP & Fielding RA (2004). Contraction‐mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol 97, 243–248. [DOI] [PubMed] [Google Scholar]
- Peronnet F & Massicotte D (1991). Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16, 23–29. [PubMed] [Google Scholar]
- Prats C, Gómez‐Cabello A & Hansen AV (2011). Intracellular compartmentalization of skeletal muscle glycogen metabolism and insulin signalling. Exp Physiol 96, 385–390. [DOI] [PubMed] [Google Scholar]
- Reihmane D, Hansen AV, Gram M, Kuhlman AB, Nørregaard J, Pedersen HP, Lund MT, Helge JW & Dela F (2013). Immobilization increases interleukin‐6, but not tumour necrosis factor‐α, release from the leg during exercise in humans. Exp Physiol 98, 778–783. [DOI] [PubMed] [Google Scholar]
- Richter EA & Ruderman NB (2009). AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J 418, 261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkilde M, Nordby P, Nielsen LB, Stallknecht BM & Helge JW (2010). Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. Int J Obes (Lond) 34, 871–877. [DOI] [PubMed] [Google Scholar]
- Sacchetti M, Saltin B, Olsen DB & van Hall G (2004). High triacylglycerol turnover rate in human skeletal muscle. J Physiol 561, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Cook JN, Kaufman AE & Horowitz JF (2005). Postexercise insulin sensitivity is not impaired after an overnight lipid infusion. Am J Physiol Endocrinol Metab 288, E519–E525. [DOI] [PubMed] [Google Scholar]
- Sial S, Coggan AR, Carroll R, Goodwin J & Klein S (1996). Fat and carbohydrate metabolism during exercise in elderly and young subjects. Am J Physiol Endocrinol Metab 271, E983–E989. [DOI] [PubMed] [Google Scholar]
- Siggaard‐Andersen O, Wimberley PD, Fogh‐Andersen N & Gøthgen IH (1988). [Blood oxygen parameters: uncompensated venous oxygen tension and cardiac compensation factors]. Ugeskr Laeger 150, 2899–2901. [PubMed] [Google Scholar]
- Stallknecht B, Kiens B, Helge JW, Richter EA & Galbo H (2004). Interstitial glycerol concentrations in human skeletal muscle and adipose tissue during graded exercise. Acta Physiol Scand 180, 367–377. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE & McConell GK (2002). Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab 282, E688–E694. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A & Salvetti A (2001). Age‐related reduction of NO availability and oxidative stress in humans. Hypertension 38, 274–279. [DOI] [PubMed] [Google Scholar]
- Thomson DM & Gordon SE (2006). Impaired overload‐induced muscle growth is associated with diminished translational signalling in aged rat fast‐twitch skeletal muscle. J Physiol 574, 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH (2002). Lipotoxic diseases. Annu Rev Med 53, 319–336. [DOI] [PubMed] [Google Scholar]
- van Hall G, González‐Alonso J, Sacchetti M & Saltin B (1999). Skeletal muscle substrate metabolism during exercise: methodological considerations. Proc Nutr Soc 58, 899–912. [DOI] [PubMed] [Google Scholar]
- van Hall G, Sacchetti M, Rådegran G & Saltin B (2002). Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol 543, 1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LJ, Greenhaff PL, Constantin‐Teodosiu D, Saris WH & Wagenmakers AJ (2001). The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536, 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigelsø A, Dybboe R, Hansen CN, Dela F, Helge JW & Guadalupe Grau A (2015. a). GAPDH and beta‐actin protein decreases with aging, making Stain‐Free technology a superior loading control in Western blotting of human skeletal muscle. J Appl Physiol 118, 386–394. [DOI] [PubMed] [Google Scholar]
- Vigelsø A, Gram M, Wiuff C, Andersen JL, Helge JW & Dela F (2015. b). Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass, and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J Rehabil Med 47, 552–660. [DOI] [PubMed] [Google Scholar]
- Vigelsø A, Gram M, Wiuff C, Hansen CN, Prats C, Dela F & Helge JW (2016). Effects of immobilization and aerobic training on proteins related to intramuscular substrate storage and metabolism in young and older men. Eur J Appl Physiol 116, 481–494. [DOI] [PubMed] [Google Scholar]
- Wall BT, Dirks ML, Snijders T, Stephens FB, Senden JM, Verscheijden ML & van Loon LJ (2015). Short‐term muscle disuse atrophy is not associated with increased intramuscular lipid deposition or a decline in the maximal activity of key mitochondrial enzymes in young and older males. Exp Gerontol 61, 76–83. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE & Febbraio MA (2004). Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287, E120–E127. [DOI] [PubMed] [Google Scholar]


