Abstract
The regulation of skeletal muscle blood flow and oxygen delivery to contracting skeletal muscle is complex and involves the mechanical effects of muscle contraction; local metabolic, red blood cell and endothelium‐derived substances; and the sympathetic nervous system (SNS). With advancing age in humans, skeletal muscle blood flow is typically reduced during dynamic exercise and this is due to a lower vascular conductance, which could ultimately contribute to age‐associated reductions in aerobic exercise capacity, a primary predictor of mortality in both healthy and diseased ageing populations. Recent findings have highlighted the contribution of endothelium‐derived substances to blood flow control in contracting muscle of older adults. With advancing age, impaired nitric oxide availability due to scavenging by reactive oxygen species, in conjunction with elevated vasoconstrictor signalling via endothelin‐1, reduces the local vasodilatory response to muscle contraction. Additionally, ageing impairs the ability of contracting skeletal muscle to blunt sympathetic vasoconstriction (i.e. ‘functional sympatholysis’), which is critical for the proper regulation of tissue blood flow distribution and oxygen delivery, and could further reduce skeletal muscle perfusion during high intensity and/or large muscle mass exercise in older adults. We propose that initiation of endothelium‐dependent hyperpolarization is the underlying signalling event necessary to properly modulate sympathetic vasoconstriction in contracting muscle, and that age‐associated impairments in red blood cell adenosine triphosphate release and stimulation of endothelium‐dependent vasodilatation may explain impairments in both local vasodilatation and functional sympatholysis with advancing age in humans.

Abbreviations
- ACh
acetylcholine
- ATP
adenosine triphosphate
- EDH
endothelium‐derived hyperpolarization
- EET
eicosatrenoic acid
- ET‐1
endothelin‐1
- FVC
forearm vascular conductance
- LBNP
lower body negative pressure
- MVC
maximum voluntary contraction
- NO
nitric oxide
- NOS
nitric oxide synthase
- PE
phenylephrine
- PG
prostaglandin
- SNS
sympathetic nervous system
The onset of dynamic exercise constitutes a major haemodynamic stress that is met with highly coordinated cardiovascular adjustments in order to ensure adequate oxygen delivery to contracting skeletal muscle. Within skeletal muscle, regulation of blood flow and oxygen delivery results from the integration of a number of stimuli including the mechanical effects of contraction, local metabolic and endothelium‐derived substances, vasoactive factors associated with erythrocytes (red blood cells), and the sympathetic nervous system (SNS). During dynamic exercise, contracting skeletal muscle has the vasodilatory capacity to increase blood flow by nearly 100‐fold (Richardson et al. 1993), which when extrapolated to whole‐body exercise, greatly exceeds the pumping capacity of the heart. Therefore, increased sympathetic nervous system activity, resulting in elevated cardiac output and peripheral vasoconstrictor signalling mediated primarily via release of noradrenaline (NA) from sympathetic nerve endings, which binds to α1‐ and α2‐adrenoceptors on resistance vessel smooth muscle cells, is necessary to achieve appropriate regulation of mean arterial pressure in the face of profound metabolic vasodilatation. The elevation in peripheral vasoconstriction during high intensity, whole‐body exercise is essential to redistribute cardiac output away from inactive and splanchnic tissues and towards active skeletal muscle. However, within the vasculature of contracting skeletal muscle, specific signalling results in blunting of the vasoconstrictor response in order to ensure adequate tissue oxygen delivery. This unique ability of contracting skeletal muscle to blunt sympathetically mediated vasoconstriction is commonly referred to as ‘functional sympatholysis’ (Remensnyder et al. 1962), and proper integration of these local vasodilator and neural vasoconstrictor signals within contracting skeletal muscle is imperative for normal blood flow control during exercise.
Skeletal muscle blood flow and oxygen delivery are strong predictors of aerobic exercise capacity. Primary (healthy) ageing is associated with a progressive decline in aerobic exercise capacity, and disease states that increase in prevalence with advancing age, such as congestive heart failure and diabetes, often are associated with a further reduction in exercise capacity and tolerance (Holloszy & Kohrt, 1995). This reduction in aerobic capacity is an independent predictor of cardiovascular disease morbidity and mortality, as well as reductions in physical functional capacity and overall quality of life. Thus, understanding the age‐associated changes that occur in the control of skeletal muscle blood flow and tissue oxygen delivery is of great clinical significance. Although the authors recognize and appreciate studies conducted in various experimental animal models, the focus of this review will be on our understanding of vascular control in contracting skeletal muscle of ageing humans.
Skeletal muscle blood flow during exercise in healthy older adults
Healthy human ageing is associated with a number of maladaptive changes within the cardiovascular system that result in impaired aerobic exercise capacity, eventually leading to reductions in functional independence and overall quality of life. Among these changes is impaired regulation of skeletal muscle blood flow. In young healthy humans, increases in blood flow are observed immediately upon the release of a single contraction. This rapid and transient increase in blood flow peaks within approximately five cardiac cycles and is graded with contraction intensity (Tschakovsky et al. 2004). While initially attributed to a muscle pump effect, it is now well established that the transient increase in blood flow is primarily due to a local vasodilatory response that serves as a feedforward mechanism for exercise hyperaemia (Crecelius et al. 2013 a). In older adults, impairments in rapid onset vasodilatation can be observed as early as the first cardiac cycle after initiation of a single muscle contraction and persist throughout the response across mild and moderate contraction intensities (Carlson et al. 2008; Casey & Joyner, 2012). Impairments in local blood flow control are also thought to contribute to slowed oxygen uptake kinetics during the onset of dynamic exercise in older individuals (DeLorey et al. 2004). To date, there have been relatively few studies investigating the mechanisms that contribute to impaired rapid onset vasodilatation and transient blood flow responses in older humans (Kirby et al. 2009; Casey & Joyner, 2012). The major part of our understanding regarding the mechanistic age‐associated changes in vascular control is derived from studies utilizing steady‐state submaximal exercise and therefore will be the primary focus of this review (Abstract figure).
In 1974, Wahren et al. were the first group to report age‐associated impairments in blood flow during submaximal graded cycle exercise in humans. In the decades following, findings from this initial study have been corroborated by most (Proctor et al. 1998, 2003 a; Poole et al. 2003; Lawrenson et al. 2003; Donato et al. 2006; Kirby et al. 2009) but not all (Proctor et al. 2003 b; Donato et al. 2006; Parker et al. 2008) investigations on the topic. In subsequent investigations utilizing graded cycle exercise, reduced leg blood flow was observed during steady‐state submaximal exercise in both sedentary and endurance trained older men (Proctor et al. 1998; Poole et al. 2003), as well as in healthy, non‐endurance trained women (Proctor et al. 2003 a). Additionally, leg blood flow is impaired at maximal exercise in both men and women; however, this could be partially due to age related declines in maximal cardiac output (Proctor et al. 2004). In order to minimize the potential confounding influence of central limitations associated with ageing, many studies have utilized small muscle mass exercise such as isolated (single) knee extensor and forearm exercise to investigate age‐related changes in local blood flow regulation. Within the forearm and single knee extensor models, most (Poole et al. 2003; Donato et al. 2006; Kirby et al. 2009; Mortensen et al. 2012) but not all (Donato et al. 2006; Parker et al. 2008) studies also report attenuated steady‐state exercise blood flow with age. While not exclusively, these impairments can exist at both absolute and relative work rates (Lawrenson et al. 2003; Donato et al. 2006), and independent of age‐associated declines in muscle mass (Proctor et al. 1998; Kirby et al. 2009). However, studies from various groups have identified both training status and sex as important modifiers of the‐age associated decline in blood flow during small muscle mass exercise (Beere et al. 1999; Parker et al. 2008; Mortensen et al. 2012).
The vast majority of experimental data indicate that during submaximal dynamic exercise in both arm and leg, there is an age‐associated attenuation in skeletal muscle blood flow that is typically due to impaired vascular conductance. Even in studies reporting maintained exercise blood flow in older subjects (Magnusson et al. 1994; Proctor et al. 2003 b; Donato et al. 2006; Parker et al. 2008), the presence of elevated mean arterial pressure points to a state of increased peripheral resistance during exercise in the majority of studies to date. Elevated resistance within contracting skeletal muscle of older individuals may arise from increased vascular stiffness (i.e. reduced compliance), impaired local vasodilatory or vasoconstrictor signalling, and/or elevated sympathetic vasoconstrictor tone. Understanding age‐associated changes in vascular architecture, as well as both vasodilatory and vasoconstrictor signalling, and further how these signals interact to control blood flow, is critical to identify potential therapeutic strategies to improve blood flow regulation and oxygen delivery in aged individuals. Along these lines, the vascular endothelium has been identified as an important site for the integration of both vasodilatory and vasoconstrictor signalling (Kerr et al. 2012) and may underlie many of the changes in local blood flow control associated with age.
Local control of muscle blood flow with age: role of endothelium‐derived substances
The endothelium produces a number of vasodilatory substances including nitric oxide (NO), prostaglandins (PGs), and eicosatrenoic acids (EETs) that may contribute to exercise hyperaemia (Clifford & Hellsten, 2004). It is important to note that findings from specific studies attempting to elucidate the contributions of these pathways may differ due to the timing of pharmacological inhibitor infusions (prior to exercise onset versus during exercise once steady‐state hyperaemia is achieved), local versus systemic effects of inhibitors, the exercising muscle studied (forearm versus knee extensor), and the exercise modality; a complete summary of these findings is beyond the scope of this review. In humans, the most widely studied of these endothelial vasodilatory pathways is NO. To date, in young healthy subjects, the overwhelming majority of studies identify a non‐obligatory role for NO in mediating exercise hyperaemia when quantified as an absolute change from rest to steady‐state exercise (Radegran & Saltin, 1999; Bradley et al. 1999; Frandsenn et al. 2001; Schrage et al. 2004; Heinonen et al. 2011). However, studies from Schrage et al. (2004) demonstrate ∼20% decrease in forearm blood flow and vascular conductance when nitric oxide synthase (NOS) inhibition is performed after steady‐state exercise hyperaemia is achieved, indicating that NO contributes to vascular tone during dynamic exercise in humans. These data are consistent with those obtained in a more recent study by Wray et al. (2011) demonstrating that NO contributes to exercise hyperaemia during handgrip exercise at higher work rates.
While an independent role of NO in exercise hyperaemia remains controversial, it is clear that there is significant cross‐talk between NO and PGs in the regulation of vascular tone. In this context, while neither NO nor PGs consistently contribute to exercise hyperaemia independently in either the leg or forearm, muscle blood flow and vascular conductance can be attenuated by ∼15–30% when their production is inhibited in combination (Boushel et al. 2002; Mortensen et al. 2007, 2009 b; Heinonen et al. 2011). However, it should be noted that other studies have demonstrated relatively normal hyperaemic responses to exercise during combined NO and PG inhibition (Crecelius et al. 2011 b, 2014, 2013 b). Similar interactions have been reported for NO and EET production in humans (Hillig et al. 2003) as well as an antagonistic interaction between NO and endothelin‐1 (ET‐1), a potent endothelium‐derived vasoconstrictor (Goligorsky et al. 1994; Westby et al. 2011). Altogether, in young heathy individuals it appears that the endothelium produces a number of vasodilatory compounds that act in a redundant and potentially synergistic manner to promote exercise hyperaemia, even when the presence of a single vasodilatory pathway is experimentally removed (Joyner & Wilkins, 2007).
The hallmark of cardiovascular ageing is progressive endothelial dysfunction (Taddei et al. 1995) typically identified as impaired responsiveness to endothelium‐dependent vasodilatory stimuli such as intra‐arterial infusion of acetylcholine (ACh) or shear stress (flow‐mediated dilatation). Classically, endothelial dysfunction is characterized by impaired NO bioavailability secondary to elevated reactive oxygen species and inflammation. In older individuals, it has been hypothesized that endothelial dysfunction underlies impaired exercise hyperaemia via reduced local dilatory signalling during exercise. Schrage et al. (2007) were the first to test this hypothesis and demonstrated that the contribution of NO to forearm exercise hyperaemia in older adults was ∼45% lower than their younger counterparts. In addition, the vasodilatory role for prostaglandins (albeit transient) observed in young individuals was absent in older individuals (Schrage et al. 2004, 2007). This was the first study to suggest in humans that the role of local endothelium‐derived substances in blood flow control was reduced with age during exercise. Subsequently, the observation that NO has a reduced contribution to exercise hyperaemia in older individuals was confirmed by other groups utilizing handgrip exercise (Crecelius et al. 2010; Trinity et al. 2013). In accordance with the hypothesis that reactive oxygen species scavenge NO and thus reduce exercise hyperaemia, our group demonstrated that intra‐arterial infusion of the anti‐oxidant ascorbic acid during exercise in older individuals restores blood flow to levels observed in younger individuals (Kirby et al. 2009), and a follow‐up study demonstrated that ascorbic acid‐mediated improvements in exercise hyperaemia were due to improved NO availability (Crecelius et al. 2010). Together, these studies support the notion that with age impaired NO bioavailability, due to increased scavenging by reactive oxygen species or potentially reductions in the NOS cofactor tetrahydrobiopterin, contributes to the age‐associated impairment in exercise hyperaemia in the human forearm.
In contrast to observations in the forearm, infusion of an antioxidant (N‐acetylcysteine) did not improve exercise blood flow in the leg of older individuals despite improving markers of NO availability (Nyberg et al. 2012). These findings were attributed to differences between the leg and forearm in their reliance on NO to mediate exercise hyperaemia. Indeed, the vasodilatory pathways mediating exercise hyperaemia in the arm and leg may be different (Wray & Richardson, 2006). However, it is also worthy to note that in the investigation by Nyberg et al. in the leg, N‐acetylcysteine administered intravenously, had profound systemic effects such as lowered blood (perfusion) pressure, and the mechanisms of action of this antioxidant may differ from that of ascorbic acid, all of which may impact conclusions regarding local regulation of blood flow. Studies utilizing local intra‐arterial infusion of ascorbic acid or the antioxidant cocktail (composed of ascorbic acid, vitamin E, and α‐lipoic acid) are needed to more directly understand the role oxidative stress in age‐associated impairments of local blood flow control in the leg.
In addition to the loss of endothelium‐derived vasodilatory signalling with age, recent work from Wray et al. suggests that the endothelium‐derived vasoconstrictor ET‐1 may contribute to age‐associated impairments in muscle blood flow control during exercise (Barrett‐O'Keefe et al. 2014). In young individuals, ET‐1 release was elevated during exercise and was shown to actively restrain blood flow during graded knee extensor exercise (Barrett‐O'Keefe et al. 2013). With age, multiple groups have identified elevated ET‐1 signalling as a contributor to lower resting skeletal muscle blood flow and vascular tone (Van Guilder et al. 2007; Thijssen et al. 2007), and Wray et al. extended these findings to impairments in exercise hyperaemia with advancing age. Specifically, they observed that ET‐1‐mediated restraint of exercise blood flow was augmented in older adults and that blockade of endothelin subtype A receptors improved exercise hyperaemia and lowered blood pressure during exercise to levels observed in young adults. Given the interaction between NO and ET‐1 in the vasculature, it remains unclear whether impaired NO bioavailability with age influences ET‐1‐mediated vasoconstriction or production during exercise in older adults and this awaits further study. Taken together, the collective evidence indicates that loss of endothelial vasodilatory substances (NO, PGs) in conjunction with elevated ET‐1 vasoconstrictor signalling contributes to impaired local blood flow control with advancing age.
Impaired functional sympatholysis with advancing age
Initial investigations into the mechanisms of impaired blood flow with age demonstrated that ageing is associated with more than twofold greater noradrenaline spillover during cycle exercise (Taylor et al. 1992; Proctor et al. 1998), and this is superimposed on the elevated basal muscle sympathetic nerve activity observed in older adults (Ng et al. 1993; Davy et al. 1998; Dinenno et al. 1999). This finding implicated age‐associated changes in sympathetic regulation of the vasculature as a primary contributor to impaired blood flow in older adults. The first study to directly investigate sympathetic responsiveness in contracting muscle during dynamic exercise in older subjects was undertaken by Koch et al. (2003). Utilizing the cold pressor test to elevate sympathetic outflow during moderate intensity cycle ergometer exercise, they observed a greater decrease in leg vascular conductance in older men relative to younger control subjects. This study was the first to indicate that despite an attenuated responsiveness to α‐adrenoceptor agonists during resting conditions (Dinenno et al. 2002), older subjects may display greater responsiveness to sympathetic vasoconstriction during exercise. These findings have been replicated experimentally via use of lower body negative pressure (LBNP) to evoke sympathoexcitation, local intra‐arterial tyramine infusions to evoke endogenous NA release, as well as infusions of direct α1‐ and α2‐adrenoceptor agonists, during both handgrip (Fadel et al. 2004; Dinenno et al. 2005; Kirby et al. 2011) and knee extensor exercise (Mortensen et al. 2012). To date, studies on the effect of age on the modulation of sympathetic vasoconstriction in active muscle clearly demonstrate impairments in this regulation in both men and women, across multiple muscle beds, at both absolute and relative exercise intensities, utilizing diverse methods of sympathetic/α‐adrenoceptor stimulation (Fig. 1) (Koch et al. 2003; Fadel et al. 2004; Dinenno et al. 2005; Wray et al. 2009; Kirby et al. 2011; Mortensen et al. 2012).
Figure 1. Age‐associated impairments in functional sympatholysis .
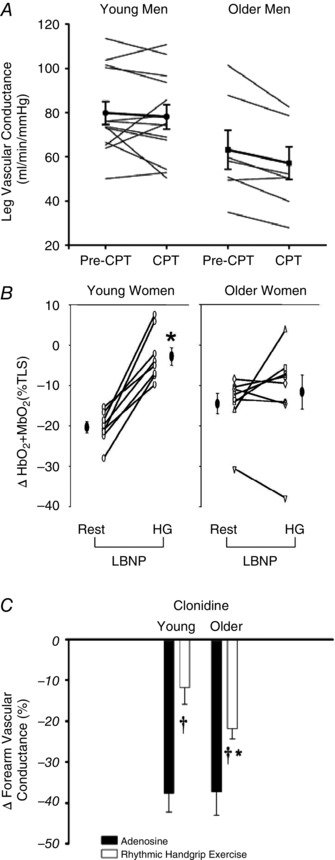
A, sympathetic vasoconstrictor responses to cold pressor test (CPT) during moderate intensity cycle exercise were greater in older relative to young men (%ΔFVC: −14 ± 3% vs. −2 ± 4%, respectively; P < 0.05; FVC: forearm vascular conductance). B, similarly, vasoconstrictor responses (assessed as decreases in skeletal muscle oxygenation) during sympathetic stimulation via lower body negative pressure (LBNP) were not blunted during moderate intensity handgrip exercise compared with resting conditions in older women, and were greater during exercise compared with young women (*P < 0.05 vs. Rest within condition). C, postjunctional α2‐adrenoceptor stimulation (via intra‐arterial clonidine) reduced vascular conductance similarly in resting skeletal muscle of young and older men during infusion of the vasodilator adenosine (filled bars). In contrast, vasoconstrictor responses during moderate intensity handgrip exercise were greater in older men relative to young men (open bars) († P < 0.05 vs. Adenosine within condition; *P < 0.05 vs. Young). The collective data demonstrate impaired modulation of sympathetic α‐adrenoceptor vasoconstriction in contracting muscle of older adults. From Koch et al. (2003), Fadel et al. (2004) and Dinenno et al. (2005).
The physiological importance of impaired functional sympatholysis in the regulation of muscle blood flow during exercise with advancing age was highlighted in a recent study by Mortensen et al. (2012). In this study, the investigators observed that lifelong physical activity preserves functional sympatholysis in older individuals relative to their sedentary counterparts (Mortensen et al. 2012 b). More importantly, during dynamic knee extensor exercise at an absolute work rate, older sedentary individuals demonstrating impaired sympatholysis had lower , elevated blood lactate and higher arterial pressure, despite similar levels of bulk blood flow to the active limb when compared to older active individuals with intact sympatholysis. Therefore, despite no difference in the magnitude of the local vasodilatory response to an absolute workload in older sedentary individuals compared with age‐matched trained adults, the inability to appropriately redistribute blood flow and oxygen delivery during sympathetic stimulation resulted in significant metabolic dysregulation. This suggests that proper regulation of exercising muscle blood flow entails more than just a balance between vasodilatation and vasoconstriction; rather, the proper vasodilatory and sympatholytic signals must be present to appropriately redistribute blood flow within skeletal muscle. In this context, not all vasodilatory substances and/or pathways are equal in their ability to blunt sympathetic vasoconstriction (more discussion below), and this is an important distinction when considering this basic physiological phenomenon of functional sympatholysis, and moreover, the effect of age on the regulation of blood flow.
While the previous studies clearly demonstrate that ageing is associated with heightened sympathetic activity and vasoconstrictor responsiveness in active muscle during exercise, they were not designed to investigate whether the sympathetic nervous system exhibits greater ‘restraint’ of skeletal muscle blood flow during exercise in older adults. Richards et al. (2014) conducted the first investigation of sympathetic restraint during submaximal steady‐state exercise across a range of handgrip exercise intensities (∼15–70% of maximum work rate) in ageing humans. The primary finding of this study was that sympathetic vasoconstriction does not restrain forearm skeletal muscle blood flow and vascular conductance during exercise in young or older adults, thus suggesting that elevated sympathetic restraint does not contribute mechanistically to age‐associated impairments in skeletal muscle haemodynamics during handgrip exercise. It is important to note here that this small muscle mass exercise evoked rather modest increases in heart rate and mean arterial pressure (i.e. minimal sympathetic activation), and thus whether these findings can be replicated during larger muscle mass exercise of the lower extremities, such as knee extensor exercise or cycling that are likely to evoke greater sympathoexcitation, remains to be determined. An additional consideration is the inability of this study to determine the spatial distribution of blood flow within the active forearm muscle, as use of Doppler ultrasound can only provide information regarding bulk flow to the forearm vasculature. Thus, it also remains unclear how α‐adrenergic blockade affects distribution between active and inactive tissues within the forearm in young and older adults. In older physically active (i.e. not sedentary) adults, use of positron emission tomography revealed a more homogeneous blood flow pattern within the leg during sustained (non‐rhythmic) isometric knee extensor exercise relative to young subjects, suggesting an impaired ability to redistribute blood flow effectively within the exercising muscle (Rudroff et al. 2014). However, more studies are needed to determine whether tonic α‐adrenoceptor activation impacts muscle blood flow regulation with age, and further, how the distribution of blood flow and oxygen delivery is impacted in young and older adults during sympathetic activation across a wide range of exercise intensities and muscle mass recruited during dynamic exercise.
Current understanding of functional sympatholysis in young healthy humans
Investigations into the potential pathways contributing to sympatholysis in young healthy humans thus far have been equivocal. Considering the well documented role of NO in modulating resting vascular tone in humans (Vallance et al. 1989; Haynes et al. 1993; Seddon et al. 2008), in combination with data obtained in experimental animals (Thomas & Victor, 1998), much focus has centred on a potential role for NO in mediating functional sympatholysis. In healthy humans, Chavoshan et al. (2002) utilizing systemic doses of N G‐nitro‐l‐arginine methyl ester (l‐NAME) to block NO production, LBNP to elevate sympathetic outflow during handgrip exercise, and near‐infrared spectroscopy as an indirect measure of tissue perfusion, provided evidence to support a role for NO in functional sympatholysis. In contrast, studies from Dinenno et al. (2004) and more recently from Crecelius et al. (2015 a) demonstrate that local NOS inhibition (via intra‐arterial N G‐monomethyl‐l‐arginine (l‐NMMA) or l‐NAME) does not impact the ability of contracting muscle to blunt vasoconstrictor responses to tyramine (endogenous NA release) or selective α1‐ and α2‐adrenoceptor agonists. Further, intra‐arterial infusion of sodium nitroprusside, a direct NO donor, fails to blunt vasoconstrictor responses to tyramine, as well as α1‐ and α2‐adrenoceptor agonists (Tschakovsky et al. 2002; Rosenmeier et al. 2003). These observations strongly suggest that NO is neither sufficient nor obligatory to observe functional sympatholysis in humans.
Recently, our laboratory embarked on perhaps the most comprehensive attempt to pharmacologically manipulate functional sympatholysis in humans to date. We assessed vasoconstrictor responses to intra‐arterial infusion of phenylephrine (α1‐agonist) during handgrip exercise and control adenosine infusion. These responses were assessed before and after combined blockade of NO (l‐NMMA) and PGs (ketorolac), as well as pathways involved in smooth muscle hyperpolarization: inwardly rectifying potassium (KIR) channels (low dose barium chloride) and Na+/K+‐ATPase (ouabain). Contrary to our hypothesis, despite combined inhibition of these vasodilatory pathways clearly augmenting α1‐mediated vasoconstriction in resting muscle, there was absolutely no effect on α1‐mediated vasoconstriction in contracting muscle (Crecelius et al. 2015 b) (Fig. 2). It is important to point out here that the combination of these pharmacological inhibitors has been shown to reduce exercise hyperaemia by approximately ∼30% (Crecelius et al. 2014) and attenuate reactive hyperaemia by close to 90% (Crecelius et al. 2013 c). Thus, considering that this complex pharmacological approach substantially reduces the hyperaemic responses during these stimuli, it is rather impressive that no effect on functional sympatholysis was observed. This is also consistent with our posit that not all vasodilating substances and/or pathways are necessarily sympatholytic. Taken together, the mechanisms that contribute to functional sympatholysis in contracting skeletal muscle of humans still remain to be elucidated.
Figure 2. Lack of an obligatory role for NO, PGs, KIR channels, or Na+/K+‐ATPase in mediating functional sympatholysis in healthy humans .
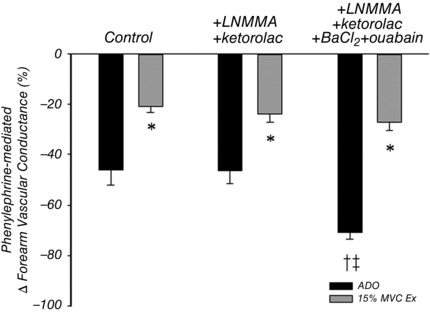
In young adults, vasoconstrictor responses to phenylephrine (PE; α1‐adrenoceptor agonist) in resting tissue during infusion of adenosine (ADO) as a high flow control condition and moderate intensity handgrip exercise (15% maximum voluntary contraction (MVC)). Combined inhibition of nitric oxide (NO; l‐NMMA), prostaglandins (PGs; ketorolac), inwardly rectifying potassium channels (KIR channels; BaCl2), and Na+/K+‐ATPase (oubain) had no effect on the exercise‐induced attenuation of vasoconstriction in response to PE (*P < 0.05 vs. ADO within condition; † P < 0.05 vs. Control; ‡ P < 0.05 vs. l‐NMMA+ketorolac). From Crecelius et al. (2015 b).
In addition to NO and PGs, studies conducted primarily in various animal models have identified endothelium‐derived hyperpolarization (EDH) as an important modulator of vascular tone in a variety of vascular beds (Behringer & Segal, 2012). In response to endothelium‐dependent vasodilators such as ACh, intracellular Ca2+ signals evoke potassium efflux through Ca2+ activated K+ channels and subsequent hyperpolarization of the endothelial cells. Because the endothelium and vascular smooth muscle cells are electrically coupled via gap junctions located in myoendothelial projections, hyperpolarization originating in the endothelium can be transmitted directly to smooth muscle cells resulting in vasodilatation (Dora et al. 2003; Griffith, 2004). In addition to direct electrical coupling locally to vascular smooth muscle, EDH can spread to adjacent endothelial cells and conduct vasodilatory signals upstream to more proximal feed arteries, thereby facilitating the redistribution of blood flow within a tissue. This is in contrast to NO donors which have been shown to cause robust vasodilatation locally, without initiating a conducted vasodilatory response (Kurjiaka & Segal, 1995 a). Interestingly, conducted dilatation in response to ACh applied to resistance vessels has been shown to blunt α‐adrenergic vasoconstriction in hamster skeletal muscle arterioles; conversely, sympathetically induced vasoconstriction can limit conducted vasodilatation (Kurjiaka & Segal, 1995 b). Similar to ACh, the endothelium‐dependent vasodilator adenosine triphosphate (ATP) has been shown to cause robust EDH and conducted vasodilatation in resistance vessels (McCullough et al. 1997; Winter & Dora, 2007). While a physiological role for ACh in mediating the haemodynamic response to exercise in humans remains controversial (Joyner & Halliwill, 2000), ATP is thought to play an important role in the matching of oxygen delivery to demand during physiological stressors such as hypoxia and exercise (Ellsworth, 2004; Crecelius et al. 2015 a).
To the best of our knowledge, the only vasodilator that has been shown to be independently sympatholytic in humans is ATP. Rosenmeier et al. (2008) were the first to demonstrate that local intra‐arterial infusion of ATP significantly blunts tyramine‐induced vasoconstriction in the leg, similar to what is observed during moderate knee extensor exercise. Our laboratory subsequently demonstrated that this unique ability of ATP to blunt sympathetic vasoconstriction involves both post‐junctional α1‐ and α2‐adrenoceptors, and importantly, that this was graded with ATP concentration such that low levels of ATP did not impact α1‐mediated vasoconstriction whereas increasing ATP concentration progressively limited α1‐mediated vasoconstriction (Kirby et al. 2008). These observations are quite similar to the intensity‐dependent nature of functional sympatholysis in contracting skeletal muscle (Tschakovsky et al. 2002). Further connecting ATP and exercise hyperaemia, venous plasma ATP levels draining active muscle increase in proportion to exercise intensity (Gonzalez‐Alonso, 2002; Kirby et al. 2012) and are likely to arise from red blood cells as they become deoxygenated and mechanically deformed when traversing the microcirculation of contracting skeletal muscle (Crecelius et al. 2013 b; Kirby et al. 2013). In humans, endothelium‐dependent ATP‐mediated vasodilatation appears to rely modestly on NO and PG synthesis (Mortensen et al. 2009 a; Crecelius et al. 2011 a); however, our laboratory has demonstrated that the primary pathway underlying ATP‐mediated vasodilatation involves activation of KIR channels (Crecelius et al. 2012) and inhibition of these channels significantly reduces skeletal muscle blood flow during exercise (Crecelius et al. 2014). Collectively, these findings point to a potentially important role for ATP in coordinating exercise hyperaemia in young healthy humans.
Potential mechanisms underlying impaired sympatholysis with age
In the context of ageing and endothelial dysfunction, it would seem logical that the responsiveness to ATP would decline as is observed with other endothelium‐dependent vasodilators such as ACh. However, within the forearm circulation, vasodilatation to exogenous ATP is not attenuated in older individuals (Kirby et al. 2011), fitting with a relatively minimal reliance of ATP on NO to produce vasodilatation in the forearm. In contrast, ATP‐mediated vasodilatation in the leg has been shown to be significantly attenuated in older adults (Mortensen et al. 2012). Perhaps most intriguing, despite the divergent effect of age on the normal vasodilatory response to ATP in the forearm and the leg, the ability of exogenous ATP to blunt sympathetic vasoconstriction is maintained in both limbs of older adults (Fig. 3). Indeed, in studies originally performed in our laboratory (Kirby et al. 2011) and subsequently by Mortensen et al. (2012), exogenous ATP retained the ability to blunt vasoconstriction despite the presence of impaired NO bioavailability and endothelial dysfunction (impaired ACh‐mediated vasodilatation). These observations highlight a few key points. First, these data further demonstrate that sympatholysis occurs independent of NO bioavailability in both young and older adults. Second, and perhaps more importantly, these findings suggest that classic endothelial dysfunction characterized by reduced NO bioavailability may not be the cause of impaired sympatholysis with advancing age. Rather, the endothelium seems to be capable of responding to sympatholytic stimuli (e.g. intravascular ATP), indicating that the lack of a functional signal may be the primary culprit in the age impairment underlying this phenomenon. To this end, our laboratory has recently demonstrated that intravascular [ATP] draining active skeletal muscle of older adults does not increase during exercise, whereas a progressive increase is observed with graded exercise intensity in young healthy adults. This impairment may be related to attenuated red blood cell‐mediated ATP release in response to deoxygenation (Kirby et al. 2012). Thus, given the dual nature of ATP eliciting vasodilatation (both local and conducted) as well as blunting sympathetic vasoconstriction, impairments in regulation of intravascular ATP could potentially explain attenuated local vasodilatation and impaired functional sympatholysis with age. Figure 4 depicts our working hypothesis regarding the vascular signalling that underlies functional sympatholysis in young healthy humans and how this is impaired with advancing age.
Figure 3. Modulation of α1‐mediated vasoconstriction during exercise and ATP infusions in young and older adults .
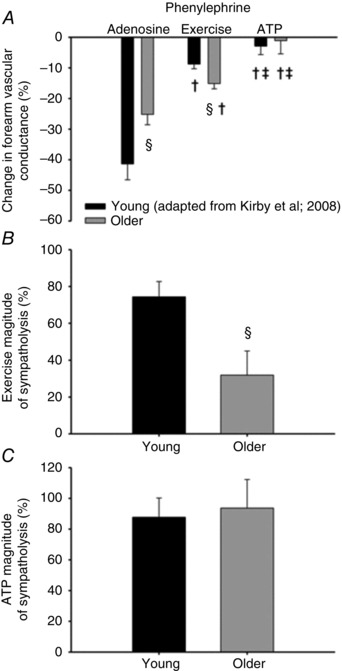
Vasoconstrictor responses to phenylephrine (PE; α1‐adrenoceptor agonist) during passive vasodilatation with adenosine, moderate handgrip exercise (15% MVC), and passive vasodilatation with adenosine triphosphate (ATP). A, vasoconstrictor responsiveness in resting skeletal muscle during infusion of adenosine was attenuated in older adults, whereas vasoconstriction was greater in older adults during handgrip exercise. ATP blunted vasoconstriction similarly in both young and older adults. B and C, magnitude of sympatholysis, describing the ability of contracting skeletal muscle to blunt sympathetic vasoconstriction, is impaired in older adults during exercise (B), but maintained during exogenous ATP infusions (C). The percentage magnitude of sympatholysis was calculated as ((%FVC adenosine − %FVC exercise or ATP)/%FVC adenosine) × 100. (§ P < 0.05 vs. Young within condition; † P < 0.05 vs. Adenosine within age group; ‡ P < 0.05 vs. Exercise within age group). From Kirby et al. (2011).
Figure 4. Working hypothesis: contribution of ATP to functional sympatholysis in young and older adults .
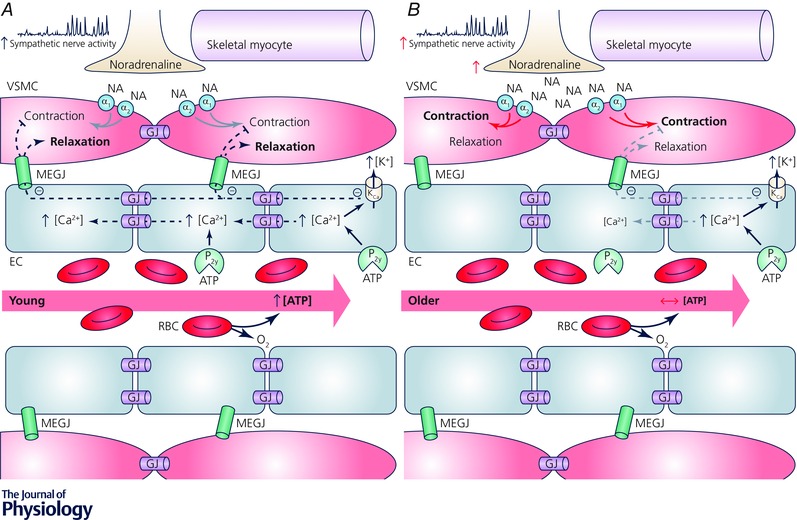
A, young, red blood cells (RBCs) traversing the microcirculation of contracting skeletal muscle encounter areas of high oxygen demand resulting in diffusion of oxygen to the active tissue, desaturation of haemoglobin, and subsequent release of the potent vasodilator adenosine triphosphate (ATP). Locally, ATP binds to purinergic receptors (P2y) located on the endothelial cell (EC), which elevates intracellular calcium, in turn activating iKCa and sKCa channels (calcium activated potassium channels, Kca) resulting in efflux of potassium (K+) and hyperpolarization of the endothelium. Endothelium‐derived hyperpolarization (EDH) conducts bi‐directionally, away from the local signal, along the endothelium through gap junctions (GJ) and spreads directly to the vascular smooth muscle cells (VSMC) through myoendothelial gap junctions (MEGJ). The effect of EDH on VSMC tone is twofold, causing vasodilatation as well as blunting sympathetic vasoconstriction, thus facilitating distribution of blood flow and oxygen to areas of high metabolic demand. B, older, while exogenous ATP is still capable of evoking vasodilatation and can blunt sympathetic vasoconstriction in older adults (denoted as small grey arrows), impaired endogenous ATP release from RBCs may result in reduced local and conducted vasodilatation, as well as an impaired ability to modulate sympathetic vasoconstriction in contracting muscle. These impairments may result in attenuated bulk blood flow delivery to, and inefficient distribution within, contracting skeletal muscle of older adults. Θ Indicates hyperpolarization.
Summary and future directions
With advancing age, the normal regulation of exercising muscle blood flow is impaired and may lead to metabolic dysregulation and exercise intolerance. The age‐associated impairments in vascular conductance are thought to be the result of (1) altered bioavailability of endothelium‐derived substances and (2) the impaired ability to blunt sympathetic vasoconstriction within active tissues. The attenuated local vasodilatory response can be explained in part by impaired NO and PG availability due to classic endothelial dysfunction and a shift towards the production of the endothelium‐derived vasoconstrictor ET‐1. While the ability of exercise to blunt sympathetically mediated vasoconstriction and redistribute bulk flow is also impaired with age, this is likely to reflect more than a simple imbalance between vasodilatory and vasoconstrictor signalling and we hypothesize this is due to a reduction in hyperpolarization of endothelial cells. Despite classic endothelial dysfunction (impaired NO bioavailability), exogenous ATP retains the ability to blunt sympathetic vasoconstriction. This finding suggests that a vasodilatory pathway independent of NO and PGs, such as EDH, is important for the proper regulation of vascular tone within contracting skeletal muscle. Further studies are needed to better understand the basic signalling mechanisms capable of modulating sympathetic tone in contracting muscle in humans, and specifically, how EDH may contribute to functional sympatholysis. Additionally, the finding that the vasculature of older individuals remains capable of responding to sympatholytic stimuli such as ATP suggests the loss of a functional signal as the primary contributor to impaired functional sympatholysis. In this context, the normal graded rise in plasma [ATP] during exercise observed in young subjects is impaired in older adults and may be related to impaired red blood cell ATP release in response to deoxygenation. Loss of ATP signalling during exercise could contribute to both attenuated local vasodilatory signalling and impaired functional sympatholysis. Future studies should be aimed at developing therapeutic strategies to improve red blood cell ATP release during exercise in ageing and disease populations as an approach to improve both local vasodilatory signalling and regulation of sympathetic tone. Finally, studies investigating blood flow distribution within active skeletal muscle are necessary, not only to more fully understand the age‐associated impairments in blood flow distribution, but also to properly assess the efficacy of therapeutic interventions in improving regional blood flow control with age and disease.
Additional information
Competing interests
No conflicts of interest, financial or otherwise, are declared by the author.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported, in part, by National Heart, Lung, and Blood Institute grants HL095573, HL119337, HL102720 and HL087952.
Biographies
Christopher M. Hearon Jr received his undergraduate degree from Texas Tech University (2010) and his master's degree from the University of Colorado Boulder (2012). He is presently a PhD candidate training under Frank Dinenno in the Human Cardiovascular Physiology Laboratory at Colorado State University. His research focuses on the signalling mechanisms regulating peripheral vascular tone with a special interest in the role of the sympathetic nervous system in both ageing and pathophysiological conditions.
Frank A. Dinenno is the Director of the Human Cardiovascular Physiology Research Laboratory at Colorado State University. His research interests include understanding basic vascular signalling mechanisms in human subjects, how these operate to regulate tissue blood flow and oxygen delivery, and how ageing and disease impact vascular function and lead to reductions in physical functional capacity and elevations in cardiovascular disease risk. His undergraduate degree (1996) was obtained from the University of Arizona, his master's (1998) and doctoral degrees (2000) from the University of Colorado Boulder, and he trained as a postdoctoral research fellow at the Mayo Clinic and Foundation in Rochester, MN (2000–2003). He has been funded by the NIH since 2000 to perform human physiology research.

This report was presented at The Journal of Physiology Symposium on Limitations of skeletal muscle oxygen supply in ageing, which took place at the themed Meeting of The Physiological Society ‘Ageing and Degeneration: A physiological perspective’, Edinburgh, UK on 10–11 April 2015. It was commissioned by the Editorial Board and reflects the views of the authors.
References
- Barrett‐O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS & Wray DW (2013). Taming the “sleeping giant”: the role of endothelin‐1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol 304, H162–H169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett‐O'Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS & Wray DW (2014). Endothelin‐A‐mediated vasoconstriction during exercise with advancing age. J Gerontol A Biol Sci Med Sci 70, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere PA, Russell SD, Morey MC, Kitzman DW & Higginbotham MB (1999). Aerobic exercise training can reverse age‐related peripheral circulatory changes in healthy older men. Circulation 100, 1085–1094. [DOI] [PubMed] [Google Scholar]
- Behringer EJ & Segal SS (2012). Spreading the signal for vasodilatation: implications for skeletal muscle blood flow control and the effects of ageing. J Physiol 590, 6277–6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M & Kjaer M (2002). Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol 543, 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Kingwell BA & McConell GK (1999). Nitric oxide synthase inhibition reduces leg glucose uptake but not blood flow during dynamic exercise in humans. Diabetes 48, 1815–1821. [DOI] [PubMed] [Google Scholar]
- Carlson RE, Kirby BS, Voyles WF & Dinenno FA (2008). Evidence for impaired skeletal muscle contraction‐induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294, H1963–H1970. [DOI] [PubMed] [Google Scholar]
- Casey DP & Joyner MJ (2012). Influence of α‐adrenergic vasoconstriction on the blunted skeletal muscle contraction‐induced rapid vasodilation with aging. J Appl Physiol 113, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG & Thomas GD (2002). Nitric oxide‐dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS & Hellsten Y (2004). Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97, 393–403. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS & Dinenno FA (2015. a). Intravascular ATP and the regulation of blood flow and oxygen delivery in humans. Exerc Sport Sci Rev 43, 5–13. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Hearon CM, Luckasen GJ, Larson DG & Dinenno FA (2015. b). Contracting human skeletal muscle maintains the ability to blunt α1‐adrenergic vasoconstriction during KIR channel and Na+/K+‐ATPase inhibition. J Physiol 593, 2735–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG & Dinenno FA (2012). ATP‐mediated vasodilatation occurs via activation of inwardly rectifying potassium channels in humans. J Physiol 590, 5349–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG & Dinenno FA (2013. a). Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305, H29–H40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC & Dinenno FA (2013. b). Mechanical effects of muscle contraction increase intravascular ATP draining quiescent and active skeletal muscle in humans. J Appl Physiol 114, 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ & Dinenno FA (2011. a). Mechanisms of ATP‐mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol 301, H1302–H1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF & Dinenno FA (2010). Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299, H1633–H1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Voyles WF & Dinenno FA (2011. b). Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589, 3671–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Luckasen GJ, Larson DG & Dinenno FA (2014). KIR channel activation contributes to onset and steady‐state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol 307, H782–H791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Richards JC, Luckasen GJ, Larson DG & Dinenno FA (2013. c). Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+‐ATPase in humans. Circ Res 113, 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Seals DR & Tanaka H (1998). Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32, 298–304. [DOI] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuk JM & Paterson DH (2004). Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate‐intensity cycling exercise. J Appl Physiol 97, 165–172. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM & Joyner MJ (2002). Aging and forearm postjunctional α‐adrenergic vasoconstriction in healthy men. Circulation 106, 1349–1354. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR & Tanaka H (1999). Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100, 164–170. [DOI] [PubMed] [Google Scholar]
- Dinenno FA & Joyner MJ (2004). Combined NO and PG inhibition augments α‐adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287, H2576–H2584. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S & Joyner MJ (2005). Impaired modulation of sympathetic α‐adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L & Richardson RS (2006). Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290, H272–H278. [DOI] [PubMed] [Google Scholar]
- Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE & Garland CJ (2003). Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res 40, 480–490. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML (2004). Red blood cell‐derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36, 35–41. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W & Thomas GD (2004). Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsenn U, Bangsbo J, Sander M, Höffner L, Betak A, Saltin B & Hellsten Y (2001). Exercise‐induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N G‐nitro‐l‐arginine methyl ester in humans. J Physiol 531, 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky MS, Tsukahara H, Magazine H, Andersen TT, Malik AB & Bahou WF (1994). Termination of endothelin signaling: role of nitric oxide. J Cell Physiol 158, 485–494. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J (2002). Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Griffith TM (2004). Endothelium‐dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol 141, 881–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WG, Noon JP, Walker BR & Webb DJ (1993). Inhibition of nitric oxide synthesis increases blood pressure in healthy humans. J Hypertens 11, 1375–1380. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Saltin B, Kemppainen J, Sipilä HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K & Hellsten Y (2011). Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol 300, H1510–H1517. [DOI] [PubMed] [Google Scholar]
- Hillig T, Krustrup P, Fleming I, Osada T, Saltin B & Hellsten Y (2003). Cytochrome P450 2C9 plays an important role in the regulation of exercise‐induced skeletal muscle blood flow and oxygen uptake in humans. J Physiol 546, 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO & Kohrt WM (1995). Exercise In Handbook of Physiology. Aging, pp. 633–666. American Physiological Society, Bethesda, MD, USA. [Google Scholar]
- Joyner MJ & Halliwill JR (2000). Neurogenic vasodilation in human skeletal muscle: possible role in contraction‐induced hyperaemia. Acta Physiol Scand 168, 481–488. [DOI] [PubMed] [Google Scholar]
- Joyner MJ & Wilkins BW (2007). Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583, 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CHT, Welsh DG & Plane F (2012). Endothelial feedback and the myoendothelial projection. Microcirculation 19, 416–422. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Richards JC & Dinenno FA (2013). Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol 98, 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF & Dinenno FA (2011). Modulation of postjunctional α‐adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589, 2641–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF & Dinenno FA (2012). Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111, 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Carlson RE & Dinenno FA (2008). Graded sympatholytic effect of exogenous ATP on postjunctional α‐adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586, 4305–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG & Dinenno FA (2009). Endothelium‐dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587, 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA & Proctor DN (2003). Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551, 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjiaka DT & Segal SS (1995. a). Conducted vasodilation elevates flow in arteriole networks of hamster striated muscle. Am J Physiol Heart Circ Physiol 269, H1723–H1728. [DOI] [PubMed] [Google Scholar]
- Kurjiaka DT & Segal SS (1995. b). Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res 76, 885–891. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P & Richardson RS (2003). Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285, H1023–H1031. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Isberg B & Saltin B (1994). Cardiovascular responses during one‐ and two‐legged exercise in middle‐aged men. Acta Physiol Scand 150, 353–362. [DOI] [PubMed] [Google Scholar]
- McCullough WT, Collins DM & Ellsworth ML (1997). Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272, H1886–H1891. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, González‐Alonso J, Bune LT, Saltin B, Pilegaard H & Hellsten Y (2009. a). ATP‐induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol 296, R1140–R1148. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, González‐Alonso J, Damsgaard R, Saltin B & Hellsten Y (2007). Inhibition of nitric oxide and prostaglandins, but not endothelial‐derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Thaning P, Saltin B & Hellsten Y (2009. b). Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53, 993–999. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K & Saltin B (2012). Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590, 6227–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG & Seals DR (1993). Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21, 498–503. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y & Mortensen SP (2012). Lifelong physical activity prevents an age‐related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590, 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD & Proctor DN (2008). Sex‐specific influence of aging on exercising leg blood flow. J Appl Physiol 104, 655–664. [DOI] [PubMed] [Google Scholar]
- Poole JG, Lawrenson L, Kim J, Brown C & Richardson RS (2003). Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284, H1251–H1259. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU & Leuenberger UA (2003. a). Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95, 1963–1970. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL & Leuenberger UA (2004). Leg blood flow and Vo 2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc 36, 623–631. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA & Leuenberger UA (2003. b). Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol 94, 1859–1869. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL & Joyner MJ (1998). Reduced leg blood flow during dynamic exercise in older endurance‐trained men. J Appl Physiol 85, 68–75. [DOI] [PubMed] [Google Scholar]
- Radegran G & Saltin B (1999). Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276, H1951–H1960. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH & Sarnoff SJ (1962). Functional sympatholysis during muscular activity: Observations on influence of carotid sinus on oxygen uptake. Circ Res 11, 370–380. [DOI] [PubMed] [Google Scholar]
- Richards JC, Luckasen GJ, Larson DG & Dinenno FA (2014). Role of α‐adrenergic vasoconstriction in regulating skeletal muscle blood flow and vascular conductance during forearm exercise in ageing humans. J Physiol 592, 4775–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Fritzlar SJ, Dinenno FA & Joyner MJ (2003). Exogenous NO administration and α‐adrenergic vasoconstriction in human limbs. J Appl Physiol 95, 2370–2374. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Yegutkin GG & González‐Alonso J (2008). Activation of ATP/UTP‐selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol 586, 4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Weissman JA, Bucci M, Seppänen M, Kaskinoro K, Heinonen I & Kalliokoski KK (2014). Positron emission tomography detects greater blood flow and less blood flow heterogeneity in the exercising skeletal muscles of old compared with young men during fatiguing contractions. J Physiol 592, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH & Joyner MJ (2007). Ageing reduces nitric‐oxide‐ and prostaglandin‐mediated vasodilatation in exercising humans. J Physiol 579, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ & Dinenno FA (2004). Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557, 599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon MD, Chowienczyk PJ, Brett SE, Casadei B & Shah AM (2008). Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation 117, 1991–1996. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I & Salvetti A (1995). Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91, 1981–1987. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG & Seals DR (1992). Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation 86, 1789–1799. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Rongen GA, van Dijk A, Smits P & Hopman MTE (2007). Enhanced endothelin‐1‐mediated leg vascular tone in healthy older subjects. J Appl Physiol 103, 852–857. [DOI] [PubMed] [Google Scholar]
- Thomas GD & Victor RG (1998). Nitric oxide mediates contraction‐induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinity JD, Wray DW, Witman MAH, Layec G, Barrett‐O'Keefe Z, Ives SJ, Conklin JD, Reese V & Richardson RS (2013). Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305, R893–R899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T & Dwyer EM (2004). Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96, 639–644. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z & Joyner MJ (2002). Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541, 623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Collier J & Moncada S (1989). Effects of endothelium‐derived nitric oxide on peripheral arteriolar tone in man. Lancet 2, 997–1000. [DOI] [PubMed] [Google Scholar]
- Van Guilder GP, Westby CM, Greiner JJ, Stauffer BL & DeSouza CA (2007). Endothelin‐1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 50, 403–409. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L & Pernow B (1974). Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33, 79–86. [DOI] [PubMed] [Google Scholar]
- Westby CM, Weil BR, Greiner JJ, Stauffer BL & DeSouza CA (2011). Endothelin‐1 vasoconstriction and the age‐related decline in endothelium‐dependent vasodilatation in men. Clin Sci (Lond) 120, 485–491. [DOI] [PubMed] [Google Scholar]
- Winter P & Dora KA (2007). Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries. J Physiol 582, 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Nishiyama SK & Richardson RS (2009). Role of α1‐adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296, H497–H504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW & Richardson RS (2006). Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc 38, 1804–1810. [DOI] [PubMed] [Google Scholar]
- Wray DW, Witman MAH, Ives SJ, McDaniel J, Fjeldstad AS, Trinity JD, Conklin JD, Supiano MA & Richardson RS (2011). Progressive handgrip exercise: evidence of nitric oxide‐dependent vasodilation and blood flow regulation in humans. Am J Physiol Heart Circ Physiol 300, H1101–H1107. [DOI] [PMC free article] [PubMed] [Google Scholar]


