Abstract
Insulin resistance plays a key role in the development of type 2 diabetes. Skeletal muscle is the major storage site for glucose following a meal and as such has a key role in maintenance of blood glucose concentrations. Insulin resistance is characterised by impaired insulin‐mediated glucose disposal in skeletal muscle. Multiple mechanisms can contribute to development of muscle insulin resistance and our research has demonstrated an important role for loss of microvascular function within skeletal muscle. We have shown that insulin can enhance blood flow to the microvasculature in muscle thus improving the access of glucose and insulin to the myocytes to augment glucose disposal. Obesity, insulin resistance and ageing are all associated with impaired microvascular responses to insulin in skeletal muscle. Impairments in insulin‐mediated microvascular perfusion in muscle can directly cause insulin resistance, and this event can occur early in the aetiology of this condition. Understanding the mechanisms involved in the loss of microvascular function in muscle has the potential to identify novel treatment strategies to prevent or delay progression of insulin resistance and type 2 diabetes.
Abbreviations
- Akt
protein kinase B
- eNOS
endothelial nitric oxide synthase
- GLUT4
glucose transporter 4
- IRS1
insulin receptor substrate 1
- IRS2
insulin receptor substrate 2
- MAPK
mitogen‐activated protein kinase
- NO
nitric oxide
- PI3K
phosphoinositide 3‐kinase
- TNFα
tumour necrosis factor α
- VEGF
vascular endothelial growth factor
Introduction
Skeletal muscle is responsible for up to 80% of insulin‐mediated glucose uptake in the post‐prandial state (Thiebaud et al. 1982). Exchange of nutrients between blood (or plasma) and tissue depends on (i) the permeability of the microvasculature, (ii) the surface area of the microvasculature, and (iii) the rate of blood flow through these vessels (Renkin, 1968). Here we review the literature demonstrating that control of the microvasculature in skeletal muscle is an important response for insulin and its action to enhance muscle glucose uptake.
Insulin enhances glucose uptake by muscle in three ways (Fig. 1). Firstly, insulin binds to receptors on the vascular endothelium activating processes leading to increased microvascular blood flow thus enhancing delivery of both insulin and glucose to the myocyte. Secondly, insulin transport from the vasculature to the interstitial space occurs by an insulin receptor‐dependent process. Thirdly, insulin binds to receptors on myocytes and increases glucose transporter 4 (GLUT4) translocation to the cell surface membrane, enhancing glucose uptake. All of these responses are impaired in insulin resistance and type 2 diabetes resulting in diminished glucose disposal by muscle. Here we review the literature (summarised in Table 1) demonstrating that loss of microvascular insulin action not only accompanies muscle insulin resistance and ageing, but can occur prior to the development of myocyte insulin resistance.
Figure 1. Effects of ageing and insulin resistance on glucose and insulin exchange between muscle compartments .
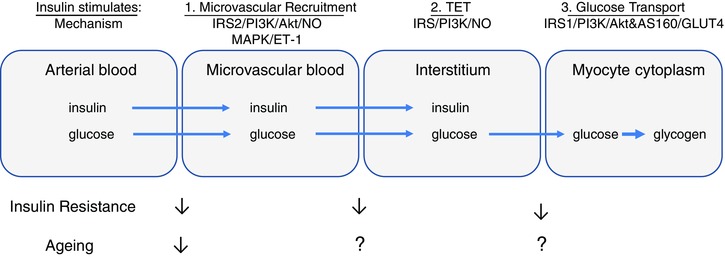
In this model there are a number of rate‐limiting transport steps for glucose and/or insulin between compartments: (1) the extent of microvascular recruitment; (2) trans‐endothelial transport (TET); and (3) sarcolemmal transport. The appearance of insulin and glucose in the microvascular compartment is limited by poor microvascular blood flow in both ageing and insulin‐resistant states. This may reflect the widespread impairment of NO‐dependent signalling in larger vessels in obesity and insulin resistance. Recent evidence suggests that insulin action at the myocyte is limited by reduced insulin‐stimulated TET in insulin resistance. Once at the myocyte glucose transport into the cytoplasm is also limited by reduced GLUT4 translocation across the sarcolemma in insulin‐resistant animals and humans. The effect of ageing on step 1 has only been partially investigated. Effects of ageing on steps 2 and 3 are either not known or have conflicting evidence. Clearly these are areas that require further investigation.
Table 1.
Key findings on effects of insulin resistance and ageing on skeletal muscle microvascular responses
| Species | Experimental model | Muscle microvascular perfusion | References | |
|---|---|---|---|---|
| Healthy | Rat | Standard chow | ↑ Insulin | (Dawson et al. 2002; Vincent et al. 2002, 2003 a, 2004; St‐Pierre et al. 2010; Premilovac et al. 2013) |
| Mouse | Standard chow | ↑ Insulin | (Kubota et al. 2011) | |
| Human | BMI < 30 kg m–2, normal fasting plasma glucose and insulin levels | ↑ Insulin ↑ Mixed meal | (Coggins et al. 2001; Clerk et al. 2006; Vincent et al. 2006; Keske et al. 2009) | |
| Insulin resistant | Rat | High fat diet | ↔ Insulin | (St‐Pierre et al. 2010; Premilovac et al. 2013) |
| Rat | High salt diet | ↔ Insulin | (Premilovac et al. 2014) | |
| Rat | Zucker obese | ↔ Insulin | (Wallis et al. 2002) | |
| Rat | Acute infusion of agents to cause insulin resistance (vasoconstrictors, elevated FFA, TNFα) | ↔ Insulin | (Rattigan et al. 1999; Youd et al. 2000; Clerk et al. 2002; Ross et al. 2007) | |
| Mouse | High fat diet | ↔ Insulin | (Kubota et al. 2011) | |
| Mouse | IRS2 KO | ↔ Insulin | (Kubota et al. 2011) | |
| Mouse | VEGF KO | ↔ Glucose uptake during insulin infusion | (Bonner et al. 2013) | |
| Human | BMI > 30 kg m–2 | ↔ Insulin | (Clerk et al. 2006; Keske et al. 2009) | |
| Insulin resistant defined by insulin clamp or elevated insulin levels during mixed meal challenge | ↔ Mixed meal | |||
| Ageing | Human | 29 ± 2 years | ↑ Mixed meal delayed ↑ Mixed meal | (Vincent et al. 2006; Keske et al. 2009) |
| 41 ± 4 years | ||||
| Human | 32 ± 2 years | ↑ Insulin | (Timmerman et al. 2010 a,b) | |
| 71 ± 2 years | ↔ Insulin | |||
| Human | 21 ± 1 years | ↑ Essential amino acid meal | (Mitchell et al. 2013) | |
| 70 ± 2 years | ||||
| ↔ Essential amino acid meal |
Ages are means ± SEM. BMI, body mass index; KO, knockout. ↑ stimulation; ↔ no change.
Vascular actions of insulin in muscle
In skeletal muscle vasculature (and prior to insulin reaching the myocyte) insulin binds to receptors on the vascular endothelium causing vasodilatation through the production of nitric oxide (NO) (Vincent et al. 2003 b). Quon and colleagues determined that upon binding to insulin receptors on endothelial cells, insulin activates the insulin receptor substrate 1 (IRS1)/phosphoinositide 3‐kinase (PI3K)/phosphoinosi‐tide‐dependent kinase‐1/protein kinase B (Akt)/endoth‐elial nitric oxide synthase (eNOS) signalling molecules leading to NO production (Montagnani et al. 2001, 2002 a,b). This endothelial insulin signalling pathway shares common steps with the myocyte insulin signalling pathway that leads to GLUT4 translocation and glucose uptake (Vincent et al. 2003 b). The similarity between these two pathways suggests a common function and highlights the important link between the vascular and metabolic actions of insulin.
Insulin‐stimulated NO production induces vasodilatation thus increasing total blood flow to muscle in vivo. Baron and colleagues were among the first to champion the notion that insulin acts to increase total muscle blood flow to facilitate access of glucose and insulin to myocytes (Baron, 1994; Steinberg et al. 1994). However, the importance of this to the metabolic actions of insulin was initially controversial as the change in total blood flow to muscle was not always observed when physiological doses (typically seen after a meal) of insulin were used (Raitakari et al. 1996). Given this inconsistency we hypothesised that physiological doses of insulin may selectively increase nutritive microvascular blood flow in muscle. This may occur regardless of changes in total blood flow by redistributing flow to enhance perfusion of capillaries intimately associated with myocytes. This increase in capillary blood flow to myocytes increases the surface area available for nutrient exchange (hence ‘nutritive’) thus enabling enhanced glucose disposal by the myocytes.
Our research group, together with our collaborators at the University of Virginia, USA, have developed two techniques for assessing microvascular blood flow in vivo. The first technique relies on stoichiometric metabolism of exogenously infused 1‐methylxanthine to 1‐methylurate by microvascular xanthine oxidase (Rattigan et al. 1997, 1999; Youd et al. 2000; Vincent et al. 2002; Wallis et al. 2002; Zhang et al. 2004; St‐Pierre et al. 2010). The second technique is an adaptation of an ultrasound imaging technique (contrast‐enhanced ultrasound, CEU) to skeletal muscle (Coggins et al. 2001; Dawson et al. 2002; Vincent et al. 2002, 2003 a, 2004, 2006; Clerk et al. 2006, 2007; Keske et al. 2009). Using both techniques we have demonstrated that physiological doses of insulin increase microvascular blood flow and that this increase is associated with enhanced glucose uptake by muscle (Rattigan et al. 1997; Dawson et al. 2002; Vincent et al. 2002, 2003 a, 2004, 2006; Clerk et al. 2006; Keske et al. 2009). This microvascular insulin response has been observed in both humans and experimental animals (Rattigan et al. 1997; Coggins et al. 2001; Dawson et al. 2002; Vincent et al. 2002, 2003 a, 2004, 2006; Clerk et al. 2006; Keske et al. 2009) and we have shown that it is independent of insulin's macrovascular actions in muscle (Dawson et al. 2002; Vincent et al. 2002, 2004; Zhang et al. 2004). Additionally, we have shown that insulin increases microvascular blood flow earlier (within 10–15 min of infusion) than its effect to increase total blood flow to muscle (60–90 min of infusion) (Vincent et al. 2004). We have also observed that low doses of insulin, which do not elicit an increase in total blood flow to muscle, can still increase microvascular blood flow (Vincent et al. 2004; Zhang et al. 2004).
Systemic (Vincent et al. 2003 a, 2004) or local hindleg (Bradley et al. 2013) infusion of a NO synthase inhibitor blocks most, if not all, of the insulin‐mediated microvascular blood flow in muscle and inhibits ∼40% of muscle glucose uptake. Therefore, insulin‐mediated increases in microvascular blood flow in muscle are, at least in part, NO dependent.
Although it has been argued by others (Poole et al. 2013) that these measures may not actually represent capillary blood flow it is without doubt that the changes in microvascular perfusion that these techniques detect contribute to the insulin‐mediated glucose uptake in muscle.
Impaired microvascular blood flow and insulin resistance
The above findings suggest that increased microvascular perfusion by insulin is an important physiological response, and if so, this may be impaired in insulin‐resistant states. By manipulating insulin's vascular response using vasoconstrictors such as α‐methylserot‐onin (Rattigan et al. 1999) and endothelin‐1 (Ross et al. 2007) we have shown that insulin‐stimulated microvascular blood flow can be inhibited, resulting in impaired insulin‐mediated glucose uptake in vivo. This loss of vascular insulin function is also apparent during acute infusions of factors known to be elevated in various insulin‐resistant states, such as tumour necrosis factor α (TNFα) (Youd et al. 2000) and free fatty acids (elevated by infusion of Intralipid and heparin) (Clerk et al. 2002).
The importance of insulin's microvascular actions is further evidenced in chronic animal models of insulin resistance, including the high fat‐fed (St‐Pierre et al. 2010), Zucker obese (Wallis et al. 2002) and Zucker diabetic fatty (Clerk et al. 2007) rats. These animal models all display reduced microvascular responsiveness during insulin infusion and exhibit impairment of insulin‐mediated muscle glucose uptake. In humans, we have also shown that insulin infusion (Clerk et al. 2006) or the ingestion of a mixed meal (Keske et al. 2009) act to similarly increase microvascular blood flow and that this response is blunted in obese insulin‐resistant subjects (Clerk et al. 2006; Keske et al. 2009). Together, these studies highlight the important link between microvascular and metabolic actions of insulin in muscle and indicate that loss of microvascular insulin sensitivity may contribute to development or worsening of insulin resistance in skeletal muscle.
Microvascular‐derived insulin resistance
The mechanisms of skeletal muscle insulin resistance are multifactorial and there has been much focus on the myocyte per se as the main defect. Most animal models of insulin resistance (such as those described above) develop myocyte insulin resistance in addition to microvascular insulin resistance. Thus it has been difficult to distinguish the importance of microvascular versus myocyte insulin resistance. However, recent evidence has emerged that defects in the microvasculature can be an independent event leading to development of muscle insulin resistance (Kubota et al. 2011; Bonner et al. 2013; Premilovac et al. 2013, 2014).
Diet‐induced insulin resistance models
In our laboratory we have recently characterised two dietary animal models of insulin resistance (Premilovac et al. 2013, 2014). Both of these models support the concept that microvascular insulin resistance develops before, and contributes to, reduced muscle glucose uptake in vivo. The first of these models is the moderately raised dietary fat model in which dietary fat in rats is increased by 2‐fold (from 5% to 9% w/w), rather than the more common 5‐ to 7‐fold increase employed in many studies (Kraegen et al. 1986; St‐Pierre et al. 2010; Turner et al. 2013). After 4 weeks of moderate fat feeding, these animals develop whole body, muscle and microvascular insulin resistance in vivo, but retain normal increases in femoral artery blood flow in response to insulin (Premilovac et al. 2013). When we assessed myocyte insulin sensitivity ex vivo using the constant‐flow pump‐perfused hindleg technique (in which insulin and glucose are delivered to the myocyte in the absence of vascular actions of insulin) we found that insulin‐mediated myocyte glucose uptake was similar between 5 and 9% fat‐fed animals (Premilovac et al. 2013). Therefore, the moderate fat‐fed model provides evidence that the reduction in insulin‐stimulated muscle glucose disposal in vivo can be driven by impairment of microvascular actions of insulin without loss of macrovascular or myocyte insulin responsiveness. This is the first piece of evidence that suggests defects in microvascular insulin action are an early, independent event that directly contributes to fat‐induced muscle insulin resistance.
The second animal model we have characterised pertinent to this discussion is the high salt‐fed rat model (Premilovac et al. 2014). After 4 weeks of high salt feeding (8.0% NaCl w/w) these animals develop whole body, skeletal muscle and microvascular insulin resistance in vivo when compared to normal salt‐fed rats (0.3% NaCl w/w). In contrast to the moderate fat model above, high salt‐fed rats exhibit reduced basal femoral artery blood flow compared to control diet animals. Despite this reduction, insulin stimulated a comparable percentage increase in femoral artery blood flow in both normal and high salt‐fed rats. Assessing myocyte insulin sensitivity in this model using the constant‐flow perfused hindleg preparation, we found no difference in muscle glucose uptake between high and normal salt‐fed rats. Together, these data indicate that myocyte insulin sensitivity is normal in high salt‐fed animals and that impairment of microvascular insulin responsiveness is sufficient to induce muscle insulin resistance in vivo.
Therefore, we have identified two distinct dietary models that both develop skeletal muscle insulin resistance in vivo as a consequence of impaired microvascular insulin action. These data position the loss of normal microvascular function as an early driver in the development of muscle insulin resistance and provide a possible early therapeutic target for prevention of insulin resistance within skeletal muscle.
Knockout mouse models
Other researchers have develo‐ped knockout mouse models that provide important links between vascular and metabolic actions of insulin. Kubota and colleagues demonstrated that endothelial insulin receptor substrate 2 (IRS2) knockout mice (which have normal levels of IRS2 in skeletal muscle, liver and white adipose tissue) had normal liver insulin sensitivity, but whole body and muscle insulin resistance in vivo (Kubota et al. 2011). This mouse displayed microvascular insulin resistance, and impaired insulin‐mediated muscle glucose uptake in vivo, and this was associated with reduced acti‐vation of both Akt and eNOS in endothelial cells. When muscles from these animals were isolated and incubated in vitro with insulin (where delivery occurs by diffusion rather than via the microvasculature), insulin‐mediated myocyte glucose uptake was not different to control animals. These data suggest that impaired insulin signalling in endothelial cells reduces insulin‐mediated muscle glucose uptake by decreasing insulin‐mediated microvascular blood flow in skeletal muscle. These data implicate reduced insulin‐mediated endothelial NO production (via reduced eNOS activation) as a contributor to reduced insulin‐mediated microvascular blood flow and muscle glucose uptake.
The degree of capillarisation of skeletal muscle is another important factor that can influence insulin‐mediated glucose uptake by myocytes. Muscle‐specific vascular endothelial growth factor (VEGF) knockout animals have reduced capillary density in skeletal muscle and display whole body and muscle insulin resistance in vivo (Bonner et al. 2013). However, in vitro (muscle incubation) assessment of insulin‐mediated glucose uptake revealed no difference compared with muscle from wild‐type animals. Additionally, studies involving human subjects with reduced capillary density have also yielded results indicating reduced insulin‐mediated muscle glucose uptake in these individuals (Gavin et al. 2005). Thus, even in the presence of normal myocyte insulin sensitivity, reduced delivery of glucose and insulin to the myocyte through decreased capillary number is sufficient to significantly reduce insulin‐stimulated muscle glucose uptake in vivo.
Trans‐endothelial transport of insulin to the interstitial space
The movement of insulin from the vasculature to the interstitial space is another potential rate‐limiting step for insulin's metabolic actions in skeletal muscle (Fig. 1). The concentration of insulin in the interstitium (measured by lymphatic sampling or microdialysis) is substantially lower (∼50%) than the concentration in plasma (Yang et al. 1989, 1992; Sjöstrand et al. 1999). The time‐course for insulin action to augment muscle glucose disposal is delayed in insulin‐resistant and type 2 diabetics during a euglycaemic hyperinsulinaemic clamp (Nolan et al. 1997). Thus, these data are consistent with a delay in the transit of insulin from the vasculature into the interstitium in insulin‐resistant and type 2 diabetic individuals.
Barrett et al. have demonstrated that trans‐endothelial transport of insulin into the endothelium (cell culture) is insulin receptor mediated (Barrett et al. 2009; Barrett & Liu, 2013). The uptake of insulin into endothelial cells is dependent on various insulin signalling cascades (PI3K and MAPK) and the activation of eNOS (Wang et al. 2008). Cytokines such as TNFα and interleukin 6, which are elevated during states of insulin resistance, impair insulin uptake into the endothelium (Wang et al. 2011). Thus, these observations suggest a mechanism linking insulin resistance and impaired or delayed insulin delivery to the interstitial space in contact with myocytes.
Ageing and microvascular actions in muscle
Ageing is associated with an altered response to both vasodilators and vasoconstrictors (Celermajer et al. 1994; Seals et al. 2006; Barrett‐O'Keefe et al. 2013) suggesting a loss of normal function of the vascular system as age increases. In a young healthy cohort of subjects (29 ± 2 years (mean ± SEM)) we have demonstrated that a mixed meal challenge, which increases plasma insulin concentrations approximately 10‐fold, significantly increases microvascular blood flow in muscle by 60 min (Vincent et al. 2006). In contrast, when the same mixed meal was given to an older cohort (41 ± 4 years), microvascular blood flow remained unchanged at 60 min, but was significantly stimulated by 120 min post‐meal (Keske et al. 2009). However, these studies were performed independently of each other and therefore only provide indirect evidence of an association between ageing and reduced (or temporally delayed) insulin sensitivity in the microvasculature. Timmerman and colleagues have reported that insulin‐mediated microvascular responses to local infusion of insulin to one leg is markedly impaired in older (71 ± 2 years) versus younger (32 ± 2 years) people (Timmerman et al. 2010 a,b). However, similarly, these studies were performed independently of each other, and therefore only provide indirect evidence. Taken together these studies indicate that as age increases, the microvascular responsiveness to insulin decreases. Other investigators have looked more closely at this association.
Age‐related loss of microvascular responsiveness was recently confirmed by Mitchell and colleagues (Mitchell et al. 2013). Participants were given an essential amino acid meal, which raised plasma insulin concentrations by approximately 3‐fold. This amino acid meal stimulated an increase in microvascular blood flow in muscle by 45 min in the younger (21 ± 1 years) cohort and this effect was completely absent in the older (70 ± 1 years) cohort. Others have reported reductions in resting microvascular blood volume in older (67 ± 2 years) versus younger (30 ± 2 years) people (Durham et al. 2010). This study also showed that 60 min after a bout of exercise both younger and older participants had elevated microvascular blood flow; however, the increase was significantly smaller in the older participants (Durham et al. 2010). Whilst the mechanisms that lead to increased blood flow probably differ between insulin and post‐exercise, the attenuated microvascular blood flow response seen in the older participants indicates a loss of microvascular responsiveness in these individuals.
Evidence for the effect of ageing on insulin resistance in terms of glucose uptake is conflicting, with some investigators reporting no changes in whole body or muscle insulin‐mediated glucose uptake in both humans (Lind et al. 2001; Rasmussen et al. 2006; Chevalier et al. 2011) and experimental animals (Schulman et al. 2007), while others have shown impairment with age (Escriva et al. 1997; Luzi et al. 2001; Bhashyam et al. 2007). Likewise, myocyte insulin‐mediated glucose uptake in vitro during ageing is also equivocal (Frøsig et al. 2013; Ropelle et al. 2013). Therefore it is unclear whether ageing per se causes insulin resistance (reduced glucose uptake) directly, or whether it is due to other risk factors such as a sedentary lifestyle, increased adiposity or hypertension that often coexist with ageing (Hildrum et al. 2007). Clearly this is an area that requires further investigation.
Conclusions
Obesity and declining vascular function in ageing frequently accompanies insulin resistance. Prior to the discovery of insulin's important microvascular responses in muscle it was unclear whether vascular dysfunction had any bearing on the development of insulin resistance. It is now clear that vascular dysfunction is likely to contribute substantially to muscle insulin resistance, particularly in the early stages of insulin resistance and type 2 diabetes. Although vascular dysfunction has been reported with ageing there is a need for further studies in aged populations to determine whether the commonly observed loss of insulin sensitivity in this population is also due to loss of the insulin‐mediated microvascular actions in muscle. A further key question that remains is to establish whether correcting this microvascular defect (i.e. restoring insulin‐mediated microvascular responses) is an effective therapeutic option for the treatment of more advanced type 2 diabetic states, either alone or in combination with existing therapies that address skeletal myocyte and/or liver insulin resistance.
Additional information
Competing interests
There are no competing interests.
Funding
This work was funded by various grants awarded from the National Health and Medical Research Council of Australia, Australian Research Council, Heart Foundation of Australia, Diabetes Australia, and the US National Institutes of Health.
Biography
Michelle A. Keske is a senior research fellow at the Menzies Institute for Medical Research, University of Tasmania. Her research has shown that (i) microvascular function in muscle plays an important role in blood glucose regulation, and (ii) microvascular dysfunction in muscle can cause insulin resistance. Her current research focuses on interventions to prevent or reverse insulin resistance by regulating microvascular blood flow within muscle. Stephen Rattigan is a professor and Deputy Director at the Menzies Institute for Medical Research. His research interests have focused on the control of skeletal muscle metabolism. Collaborative studies with investigators in the USA, Denmark and The Netherlands have elucidated the important role that the vascular system plays in regulating muscle glucose uptake. He has pioneered novel methods to investigate microvascular blood flow in vivo in experimental animals and humans.

This review was presented at the symposium Impact of physical activity, ageing, obesity and metabolic syndrome on muscle microvascular perfusion and endothelial metabolism, which took place at Physiology 2014, the annual meeting of The Physiological Society, London, UK on 1 July 2014.
References
- Baron AD (1994). Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab 267, E187–E202. [DOI] [PubMed] [Google Scholar]
- Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W & Liu Z (2009). The vascular actions of insulin control its delivery to muscle and regulate the rate‐limiting step in skeletal muscle insulin action. Diabetologia 52, 752–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EJ & Liu Z (2013). The endothelial cell: an ‘early responder’ in the development of insulin resistance. Rev Endocr Metab Disord 14, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett‐O'Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS & Wray DW (2013). Angiotensin II potentiates α‐adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124, 413–422. [DOI] [PubMed] [Google Scholar]
- Bhashyam S, Parikh P, Bolukoglu H, Shannon AH, Porter JH, Shen YT & Shannon RP (2007). Aging is associated with myocardial insulin resistance and mitochondrial dysfu‐nction. Am J Physiol Heart Circ Physiol 293, H3063–H3071. [DOI] [PubMed] [Google Scholar]
- Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP & Wasserman DH (2013). Muscle‐specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes 62, 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EA, Richards SM, Keske MA & Rattigan S (2013). Local NOS inhibition impairs vascular and metabolic actions of insulin in rat hindleg muscle in vivo. Am J Physiol Endocrinol Metab 305, E745–E750. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J & Deanfield JE (1994). Aging is associated with endothelial dysfunction in healthy men years before the age‐related decline in women. J Am Coll Cardiol 24, 471–476. [DOI] [PubMed] [Google Scholar]
- Chevalier S, Goulet ED, Burgos SA, Wykes LJ & Morais JA (2011). Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci 66, 681–688. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Rattigan S & Clark MG (2002). Lipid infusion impairs physiologic insulin‐mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51, 1138–1145. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Barrett E, Lankford MF & Lindner JR (2007). Skeletal muscle capillary responses to insulin are abnormal in late‐stage diabetes and are restored by angiotensin converting enzyme inhibition. Am J Physiol Endocrinol Metab 293, E1804–E1809. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR & Barrett EJ (2006). Obesity blunts insulin‐mediated microvascular recruitment in human forearm muscle. Diabetes 55, 1436–1442. [DOI] [PubMed] [Google Scholar]
- Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S & Barrett E (2001). Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50, 2682–2690. [DOI] [PubMed] [Google Scholar]
- Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong‐Poi H & Lindner JR (2002). Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282, E714–E720. [DOI] [PubMed] [Google Scholar]
- Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon‐Jones D, Sanford AP, Hickner RC, Grady JJ & Sheffield‐Moore M (2010). Age‐related anabolic resistance after endurance‐type exercise in healthy humans. FASEB J 24, 4117–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva F, Agote M, Rubio E, Molero JC, Pascual‐Leone AM, Andres A, Satrustegui J & Carrascosa JM (1997). In vivo insulin‐dependent glucose uptake of specific tissues is decreased during aging of mature Wistar rats. Endocrinology 138, 49–54. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Jensen TE, Jeppesen J, Pehmøller C, Treebak JT, Maarbjerg SJ, Kristensen JM, Sylow L, Alsted TJ, Schjerling P, Kiens B, Wojtaszewski JF & Richter EA (2013). AMPK and insulin action–responses to ageing and high fat diet. PLoS One 8, e62338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP, Stallings HW 3rd, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE & Hickner RC (2005). Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol (1985) 98, 315–321. [DOI] [PubMed] [Google Scholar]
- Hildrum B, Mykletun A, Hole T, Midthjell K & Dahl AA (2007). Age‐specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. BMC Public Health 7, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keske MA, Clerk LH, Price WJ, Jahn LA & Barrett EJ (2009). Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 32, 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Storlien LH, Burleigh KM & Chisholm DJ (1986). In vivo insulin resistance in individual peripheral tissues of the high fat fed rat: assessment by euglycaemic clamp plus deoxyglucose administration. Diabetologia 29, 192–198. [DOI] [PubMed] [Google Scholar]
- Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, Inoue M, Itoh S, Takamoto I, Sasako T, Kumagai K, Kawai T, Hashimoto S, Kobayashi T, Sato M, Tokuyama K, Nishimura S, Tsunoda M, Ide T, Murakami K, Yamazaki T, Ezaki O, Kawamura K, Masuda H, Moroi M, Sugi K, Oike Y, Shimokawa H, Yanagihara N, Tsutsui M, Terauchi Y, Tobe K, Nagai R, Kamata K, Inoue K, Kodama T, Ueki K & Kadowaki T (2011). Impaired insulin signaling in endothelial cells reduces insulin‐induced glucose uptake by skeletal muscle. Cell Metab 13, 294–307. [DOI] [PubMed] [Google Scholar]
- Lind L, Fugmann A, Millgard J, Berne C & Lithell H (2001). Ageing impairs insulin‐mediated vasodilatation but not forearm glucose uptake. Eur J Clin Invest 31, 860–864. [DOI] [PubMed] [Google Scholar]
- Luzi L, Giordano M, Caloni & Castellino P (2001). Effects of insulin and amino acids on leucine metabolism in young and middle‐aged humans. Eur J Nutr 40, 106–112. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Phillips BE, Williams JP, Rankin D, Smith K, Lund JN & Atherton PJ (2013). Development of a new Sonovue contrast‐enhanced ultrasound approach reveals temporal and age‐related features of muscle microvascular responses to feeding. Physiol Rep 1, e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani M, Chen H, Barr VA & Quon MJ (2001). Insulin‐stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276, 30392–30398. [DOI] [PubMed] [Google Scholar]
- Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ & Draznin B (2002. a). Inhibition of phosphatidylinositol 3‐kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277, 1794–1799. [DOI] [PubMed] [Google Scholar]
- Montagnani M, Ravichandran LV, Chen H, Esposito DL & Quon MJ (2002. b). Insulin receptor substrate‐1 and phosphoinositide‐dependent kinase‐1 are required for insulin‐stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16, 1931–1942. [DOI] [PubMed] [Google Scholar]
- Nolan JJ, Ludvik B, Baloga J, Reichart D & Olefsky JM (1997). Mechanisms of the kinetic defect in insulin action in obesity and NIDDM. Diabetes 46, 994–1000. [DOI] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Ferguson SK & Musch TI (2013). Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premilovac D, Bradley EA, Ng HL, Richards SM, Rattigan S & Keske MA (2013). Muscle insulin resistance resulting from impaired microvascular insulin sensitivity in Sprague Dawley rats. Cardiovasc Res 98, 28–36. [DOI] [PubMed] [Google Scholar]
- Premilovac D, Richards SM, Rattigan S & Keske MA (2014). A vascular mechanism for high‐sodium‐induced insulin resistance in rats. Diabetologia 57, 2586–2595. [DOI] [PubMed] [Google Scholar]
- Raitakari M, Nuutila P, Ruotsalainen U, Laine H, Teräs M, Iida H, Mäkimattila S, Utriainen T, Oikonen V, Sipilä H, Haaparanta M, Solin O, Wegelius U, Knuuti J & Yki‐Järvinen H (1996). Evidence for dissociation of insulin stimulation of blood flow and glucose uptake in human skeletal muscle: studies using [15O]H2O, [18F]fluoro‐2‐deoxy‐d‐glucose, and positron emission tomography. Diabetes 45, 1471–1477. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL & Volpi E (2006). Insulin resistance of muscle protein metabolism in aging. FASEB J 20, 768–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattigan S, Clark MG & Barrett EJ (1997). Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46, 1381–1388. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Clark MG & Barrett EJ (1999). Acute vasoconstriction‐induced insulin resistance in rat muscle in vivo. Diabetes 48, 564–569. [DOI] [PubMed] [Google Scholar]
- Renkin EM (1968). Transcapillary exchange in relation to capillary circulation. J Gen Physiol 52, 96–108. [PMC free article] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Cintra DE, da Silva AS, De Souza CT, Guadagnini D, Carvalho BM, Caricilli AM, Katashima CK, Carvalho‐Filho MA, Hirabara S, Curi R, Velloso LA, Saad MJ & Carvalheira JB (2013). Targeted disruption of inducible nitric oxide synthase protects against aging, S‐nitrosation, and insulin resistance in muscle of male mice. Diabetes 62, 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RM, Kolka CM, Rattigan S & Clark MG (2007). Acute blockade by endothelin‐1 of haemodynamic insulin action in rats. Diabetologia 50, 443–451. [DOI] [PubMed] [Google Scholar]
- Schulman IH, Zhou MS, Jaimes EA & Raij L (2007). Dissociation between metabolic and vascular insulin resistance in aging. Am J Physiol Heart Circ Physiol 293, H853–H859. [DOI] [PubMed] [Google Scholar]
- Seals DR, Moreau KL, Gates PE & Eskurza I (2006). Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41, 501–507. [DOI] [PubMed] [Google Scholar]
- Sjöstrand M, Holmäng A & Lönnroth P (1999). Measurement of interstitial insulin in human muscle. Am J Physiol Endocrinol Metab 276, E151–E154. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N & Baron AD (1994). Insulin‐mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 94, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St‐Pierre P, Genders AJ, Keske MA, Richards SM & Rattigan S (2010). Loss of insulin‐mediated microvascular perfusion in skeletal muscle is associated with the development of insulin resistance. Diabetes Obes Metab 12, 798–805. [DOI] [PubMed] [Google Scholar]
- Thiebaud D, Jacot E, DeFronzo RA, Maeder E, Jequier E & Felber JP (1982). The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 31, 957–963. [DOI] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Dreyer HC, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Sheffield‐Moore M, Rasmussen BB & Volpi E (2010. a). Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial‐dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab 95, 3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL, Lee JL, Fujita S, Dhanani S, Dreyer HC, Fry CS, Drummond MJ, Sheffield‐Moore M, Rasmussen BB & Volpi E (2010. b). Pharmacological vasodilation improves insulin‐stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 59, 2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee‐Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA & Bruce CR (2013). Distinct patterns of tissue‐specific lipid accumulation during the induction of insulin resistance in mice by high‐fat feeding. Diabetologia 56, 1638–1648. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Barrett EJ, Lindner JR, Clark MG & Rattigan S (2003. a). Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285, E123–E129. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S & Barrett EJ (2004). Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53, 1418–1423. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong‐Poi H & Barrett EJ (2006). Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290, E1191–E1197. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG & Barrett EJ (2002). Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51, 42–48. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Montagnani M & Quon MJ (2003. b). Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 3, 279–288. [DOI] [PubMed] [Google Scholar]
- Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD & Clark MG (2002). Insulin‐mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51, 3492–3498. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang AX & Barrett EJ (2011). Caveolin‐1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab 300, E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang AX, Liu Z & Barrett EJ (2008). Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 57, 540–547. [DOI] [PubMed] [Google Scholar]
- Yang YJ, Hope ID, Ader M & Bergman RN (1989). Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84, 1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YJ, Hope I, Ader M, Poulin RA & Bergman RN (1992). Dose‐response relationship between lymph insulin and glucose uptake reveals enhanced insulin sensitivity of peripheral tissues. Diabetes 41, 241–253. [DOI] [PubMed] [Google Scholar]
- Youd JM, Rattigan S & Clark MG (2000). Acute impairment of insulin‐mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF‐α. Diabetes 49, 1904–1909. [DOI] [PubMed] [Google Scholar]
- Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG & Barrett EJ (2004). Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53, 447–453. [DOI] [PubMed] [Google Scholar]


