Abstract
The beneficial effects of exercise have been well recognized for over half a century. Dr Jeremy Morris's pioneering studies in the fifties showed a striking difference in cardiovascular disease between the drivers and conductors on the double‐decker buses in London. These studies sparked off a vast amount of research on the effects of exercise in health, and the general consensus is that exercise contributes to improved outcomes and treatment for several diseases including osteoporosis, diabetes, depression and atherosclerosis. Evidence of the beneficial effects of exercise is reviewed here. One way of highlighting the impact of exercise on disease is to consider it from the perspective of good practice. However, the intensity, duration, frequency (dosage) and counter indications of the exercise should be taken into consideration to individually tailor the exercise programme. An important case of the beneficial effect of exercise is that of ageing. Ageing is characterized by a loss of homeostatic mechanisms, on many occasions leading to the development of frailty, and hence frailty is one of the major geriatric syndromes and exercise is very useful to mitigate, or at least delay, it. Since exercise is so effective in reducing frailty, we would like to propose that exercise be considered as a supplement to other treatments. People all over the world have been taking nutritional supplements in the hopes of improving their health. We would like to think of exercise as a physiological supplement not only for treating diseases, but also for improving healthy ageing.
Introduction: the London bus study on exercise and health
The favourable effects of exercise on health are now well accepted (Pedersen & Saltin, 2006). The situation, however, was very different in 1953 when one of the most important studies on exercise was first published: this was the London bus study performed by a London epidemiologist, Dr Jeremy Morris (Morris et al. 1953). Dr Morris analysed data on cardiovascular disease and its relationship with physical activity, showing a remarkable difference in cardiovascular disease between drivers of the famous double‐decker buses and the conductors. The conductors were at approximately half the risk of dying of cardiovascular disease when compared with the drivers. Dr Morris and his team observed the conductors’ behaviour in their daily activity and found that they would typically climb approximately 500–700 steps per day. This is a substantial amount of physical activity.
Even though stress may be a confounding factor, the all‐important publication of Morris and co‐workers in The Lancet in 1953 became a critical landmark in fostering interest in the field of physical exercise and its beneficial effects (Morris et al. 1953).
The hypothesis that physical activity promotes health and longevity is not new. In Graeco‐Roman times Hippocrates, and later Galen, recognised the need for promoting and prescribing exercise for health‐related benefits (Vina et al. 2012) but they had no scientific evidence. Dr Morris performed systematic research that brought to light the whole field of physical activity epidemiology. Subsequently, many other investigations have shown that exercise is good for health. Further on in this review we will describe how exercise can be considered as a treatment for diabetes, osteoporosis, depression and several other diseases (Vina et al. 2012).
We recently suggested that exercise is a powerful intervention that acts as a drug (Vina et al. 2012). Our views on this point will also be discussed.
Exercise and lifespan: primary and secondary ageing
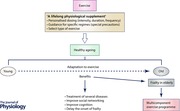
John O. Holloszy described two types of ageing: the first is primary ageing, which is ‘an inevitable deterioration of cellular structure and function independent of disease and environment’ (Holloszy, 2000). Slowing down primary ageing increases maximal lifespan in many species. There is no intervention able to reverse primary ageing in humans (Booth et al. 2011). Genetic factors are thought to be responsible for maximal longevity of any given species. To date, hundreds of genes connected with ageing and longevity have been identified (Sinclair & Guarente, 2006). Most of these genes perform functions that maintain cells, such as repairing damage to DNA or regulating antioxidant levels (Stewart, 2014). Individually, genes have a relatively small impact on lifespan, but together they account for 25% of our longevity (Stewart, 2014) (see Fig. 1).
Figure 1. Factors that are involved in the chances of living to old age .
Exercise is an environmental factor that increases lifespan and healthspan by decreasing the risk of secondary ageing and premature death. The longer‐term changes in the organism related to the adaptation to exercise in older persons is the delay in the onset of frailty (Fiatarone et al. 1994; Binder et al. 2002; Timonen et al. 2002; Colcombe & Kramer, 2003; Booth et al. 2011; Fairhall et al. 2012; Langlois et al. 2013; Nyberg et al. 2013; Gine‐Garriga et al. 2014; Manor et al. 2014; Cesari et al. 2015).
Secondary ageing is ‘caused by diseases and environmental factors such as smoking and exposure to ultraviolet radiation’ (Holloszy, 2000). Secondary ageing alters life expectancy (average length of life in a population), but not maximum lifespan. More than 70% of people over 65 have two or more chronic conditions, such as diabetes, arthritis, cancer, stroke, or heart disease (Hung et al. 2011; Fontana et al. 2014). Delaying one age‐related disease probably staves off others (Fontana et al. 2014). This aspect of ageing may be influenced through a lifetime of physical activity (Stewart, 2014) (see Fig. 1).
However the characteristics of exercise (intensity, duration, frequency, type of exercise) for extending lifespan are far from clear. Moreover several interspecies differences have been reported (Garcia‐Valles et al. 2013). Exercise has been associated with a slowing of age‐specific mortality in rats, and with increased median lifespan (Holloszy et al. 1985). However, contradictory results have been reported in mice (Samorajski et al. 1985; Navarro et al. 2004). Lifelong spontaneous exercise does not change either average or maximal lifespan in mice (Garcia‐Valles et al. 2013). These discrepancies may be explained by different experimental conditions, such as type of exercise or age of the animal at the onset of intervention, among others (Garcia‐Valles et al. 2013).
The ideal ‘dose’ of exercise for improving longevity in humans is also uncertain, but data support an inverse association between regular exercise and mortality. In longitudinal studies, physically active men and women have an approximately 30% lower risk of death compared with inactive people (Schnohr et al. 2015). No upper threshold for physical activity has ever been recommended (Pate et al. 1995). In fact, in studies performed on top‐level athletes, participation in endurance competitive sports increases life expectancy (Hartley & Llewellyn, 1939; Prout, 1972). Moreover, Karvonen and co‐workers found that Finnish champion skiers lived 2.8–4.3 years longer than the general male population in Finland (Karvonen et al. 1974). We tested the effect of strenuous exercise, performed by well‐trained humans, on their longevity. We measured average and maximal lifespan in cyclists who had taken part in the Tour de France between the years 1932 and 1964 and compared them with those of the average population in those years. Only cyclists born in Belgium, France and Italy were included in our study. The results were striking: we found an 11% increase in average longevity in Tour de France participants when compared with the general population (Sanchis‐Gomar et al. 2011). These results have been confirmed recently with the observation of a significant 41% lower mortality rate among French elite cyclists from the Tour de France, compared with the general male population (Marijon et al. 2013). Evidence from human studies supports the notion that regular, vigorous aerobic exercise might be a useful tool, with a dose–response effect, to improve the overall health status and longevity of the general population (Ruiz et al. 2010; Teramoto & Bungum, 2010). However, the controversy regarding the potential adverse effects of regular strenuous physical exercise continues (Benito et al. 2011; Schnohr et al. 2013). Schnohr and co‐workers have found that moderate‐intensity joggers have lower mortality rates that sedentary people or high intensity joggers. In other words the relationship between intensity of jogging and mortality follows a U‐shaped curve (Schnohr et al. 2015). Thus, the ideal ‘dose’ of exercise needed to improve longevity is not a simple linear relationship. Genetic aspects as well as lifestyle factors (smoking, diet and alcohol consumption) may be important in interpreting studies aimed at determining the effect of exercise training on longevity.
It is also relevant that training‐induced adaptations vary considerably between individuals. In the HERITAGE Family Study it was shown that there are two groups of persons in relation to the different health parameters associated with exercise: responders and non‐responders (Bouchard & Rankinen, 2001). Several previous studies have shown that a proportion of subjects demonstrate little or no improvements in , maximal work rate, submaximal exercise heart rate, submaximal respiratory exchange ratio or insulin sensitivity despite regular endurance training (Prud'homme et al. 1984; Skinner et al. 2001; Boule et al. 2005; Hautala et al. 2006; Scharhag‐Rosenberger et al. 2012). The degree to which improves in older people with training is also variable (Kohrt et al. 1991). Timmons et al. (2010) identified 11 single‐nucleotide polymorphisms that together explained 23% of the interindividual variance in training‐induced changes, corresponding to about 50% of the estimated genetic variance for .
From increases in longevity to healthy ageing: the concept of frailty
A vast increase in average lifespan of different populations has occurred worldwide in the 20th century, especially in developed countries. Indeed, the average lifespan in Spain in 1900 was around 30–35 years and in 2000 it was approximately 83–85 years (Garcia‐Valles et al. 2013). This enormous increase in average longevity has occurred due to the development of public health systems (prevention of disease), nutrition and clinical medicine.
In the last three decades, an enormous effort has been put into understanding the biological bases of ageing (for a recent review see Lopez‐Otin et al. 2013), and many laboratories have endeavoured to increase longevity in animals by genetic manipulation as well as by ‘lifestyle improvements’ (Matheu et al. 2007; Borras et al. 2011). The concept of longevity‐associated genes was coined around 20 years ago to describe genes that when upregulated in an animal resulted in an increase in lifespan (for a review see Sinclair & Guarente, 2006). Antioxidant genes, p53, telomerase and the IGF receptor are all proteins that are encoded by longevity‐associated genes whose modulation has resulted in significant increases in lifespan in animals (Vina et al. 2013 a). However, in the last 10–15 years, emphasis has changed from increasing average and even maximal longevity to promoting healthy ageing (Garcia‐Valles et al. 2013; Vina et al. 2013 b). The idea is to provide guidelines for the population to reach a lifespan that is closer to the maximum while also improving health and happiness.
As lifespan increases so the concept of frailty has emerged. Age‐associated frailty is a clinical syndrome that leads to a lowering of biological reserves, due to poor regulation of physiological systems, that results in an individual being at risk, especially when facing minor levels of stress. It leads to poorer outcomes in terms of disability, hospitalisation and early death (Fried et al. 2001; Ingles et al. 2014; Rodriguez‐Manas & Fried, 2014). The prevalence of frailty in the elderly approximates 15%.
There are two important characteristics of frailty: firstly if left unaddressed it will evolve into disability and eventually death, and secondly, if treated the onset of frailty can be delayed. Notably, analyses by the European Union have shown that by the year 2020, approximately half the population over 70 years of age will be at high risk of disability. Prevention of disability is obviously a major medical and social concern (Rodriguez‐Manas & Fried, 2014). Exercise is one of the most important interventions to delay the onset of frailty (Casas Herrero et al. 2015).
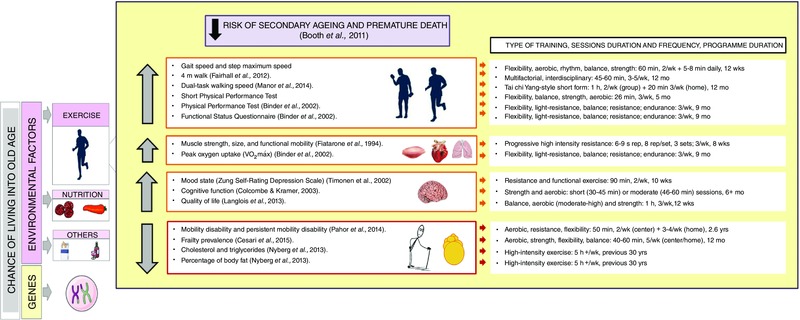
The number of studies analysing the effects of exercise on frailty is still relatively small (Binder et al. 2002; Chin et al. 2008; Daniels et al. 2008; Theou et al. 2011; Chou et al. 2012; de Vries et al. 2012; Clemson et al. 2012; Freiberger et al. 2012; Cadore et al. 2013). The general consensus is that exercise is the most successful way to delay the onset of frailty (see Fig. 1). Of the various types, multicomponent exercise is the most effective. This intervention includes resistance, balance, endurance and coordination training (Cadore et al. 2014). However, at present we lack a multicomponent exercise programme that can be stratified to protect against frailty‐associated problems and also social and cognitive aspects to enhance the quality of life in older individuals (Rodriguez‐Manas & Fried, 2014).
Significant efforts are being made worldwide to establish a suitable exercise programme. Another key question is whether personalised exercise and nutrition, in particular foods with high protein, mineral and vitamin content, can act synergistically to delay the onset of frailty.
In the context of this review focused on the benefits of exercise on healthy ageing, it is noteworthy that the LIFE Study shows that exercise reduces the incidence of major disability in ageing. Importantly, we propose that exercise should be viewed in terms not only of delaying the onset of frailty but also of contributing to cognition and social networking (Pahor et al. 2014).
Responses and adaptations to exercise in ageing
Hawley and co‐workers recently provided evidence that the capacity of humans to exploit endurance and thermoregulation enabled early East Africans to succeed as game hunters (Hawley et al. 2014).
Short‐term responses to exercise refer to changes in parameters in an organism that occur almost immediately after initiating exercise, whole‐body oxygen uptake increasing about 20‐fold during maximal exercise, heart rate increasing up to 200 beats min–1 and ventilation increasing up to maximum of around 150 L min–1 (Saltin & Astrand, 1967; Hawley et al. 2014). Whole‐body metabolism responds to exercise by increasing muscle glucose uptake, glycogenolysis, and ATP turnover (Monleon et al. 2014). Skeletal muscle is central in the response to exercise with dramatic changes in metabolism including active glycolysis, turning muscle into a tissue that produces large amounts of lactate, increasing glucose and oxygen utilisation, and CO2 production (Hawley et al. 2014). In addition to conventional metabolites a large number of myokines with profound signalling consequences are also released from muscle (Febbraio & Pedersen, 2005).
Adaptation to exercise refers to longer‐term changes in the organism to prepare it for subsequent exercise. We have recently measured characteristics of the adaptation to exercise in older sedentary and active individuals aged 60–65 years and compared them with individuals aged approximately 20–25 years. We found an important change in body composition between young and old subjects; the younger had 17.7% of body fat whereas individuals aged 60 with the same height and body weight had significantly higher body fat of 26% (Nyberg et al. 2013). Notably, if older individuals had performed endurance exercise (5 h per week for the past 30 years), they maintained their percentage of body fat at even lower levels than the young sedentary individuals, i.e. 15%. The of young individuals was 3.6 L min–1, whilst values were 50% lower in old sedentary individuals compared with young individuals. In contrast, the in active old individuals was as high as in the young cohort. Cholesterol, which tends to increase with age (the value in the young group being 3.8 mm and in the old sedentary group, 5.3 mm) tended to be lower in old active (4.9 mm). Plasma triglyceride levels were 0.8 mm in the young group, 1.5 mm in the old sedentary group, but 1.0 mm in the old active group. Thus, individuals who perform exercise for long periods in their lives have a more ‘healthy’ phenotype than those who do not exercise. As illustrated in Fig. 1, this healthy phenotype will lower the risk of secondary ageing and premature death.
To sum up, older persons lose both their capacity to respond to exercise and their capacity to adapt to it (Vina et al. 2013 b). In our opinion, adapting to exercise is more relevant in terms of health than responding to it. It is very important to point out that if an active lifestyle is maintained over long periods during one's life, one maintains a healthier, ‘younger’ phenotype. The experiments we have just reported indicate that exercise can be considered as an intervention to improve life and healthy ageing (see below) (Vina et al. 2012) and therefore, since we are dealing with the long‐term practice of exercise, one can say that it can be considered as a physiological supplement to improve life and healthy ageing (see below).
Special precautions needed in the prescription of exercise at old age
Older individuals are living with a significant burden of chronic disease, impairments in vision, cognition and hearing, and reduced physiological reserve (Vina et al. 2013 b). They have an increased susceptibility to age‐related diseases, such as cancer, cardiovascular and neurodegenerative diseases, and insulin‐independent diabetes. In diabetic patients (both types I and II) exercise should be postponed if blood glucose is >2.5 g L−1 together with ketonuria and >3.0 g L−1 even without ketonuria, in both cases before it is corrected (Caspersen et al. 2012) (see Fig. 2).
Figure 2. Special precautions needed in the prescription of exercise at old age .
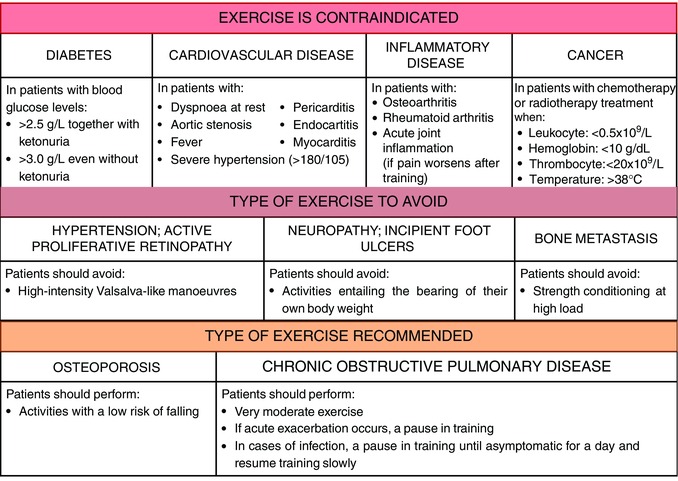
Exercise is absolutely contraindicated in some conditions of the age‐related diseases while, in another cases, a particular type of exercise should be avoided or, conversely, is recommended.
Although the heart significantly benefits from physical activity, the incidence of heart failure increases with age, largely due to the development of heart failure risk factors such as hypertension and coronary artery disease. Thus, exercise should be prescribed with caution to these individuals (Vigen et al. 2012; Vina et al. 2012). Dyspnoea at rest, aortic stenosis, pericarditis, myocarditis, endocarditis, fever and severe hypertension are all contraindications of exercise (Pedersen & Saltin, 2006). In patients with hypertension and active proliferative retinopathy, high‐intensity training or training involving Valsalva‐like manoeuvres should be avoided. Patients with neuropathy and incipient foot ulcers should refrain from activities entailing the bearing of the patient's own body weight. Age‐related endothelial dysfunction and increased arterial stiffness contribute to the increased prevalence of hypertension, particularly systolic hypertension, among the elderly (Pimenta & Oparil, 2012). Hypertensive patients with a blood pressure higher than 180/105 should begin pharmacotherapy before regular physical activity is initiated (Pescatello et al. 2004) (see Fig. 2).
The beneficial effects of exercise to the lungs are also well established. There are no absolute contraindications to very moderate exercise in chronic obstructive pulmonary disease patients (Pedersen & Saltin, 2006). However, acute exacerbations of the disease are associated with increased mortality and hospitalization, especially in older individuals (Abbatecola et al. 2011). As in asthma patients, a pause in training is recommended when an acute exacerbation occurs. In cases of infection, a pause in training is recommended until the patient has been asymptomatic for a day, whereafter training can be slowly resumed (Pedersen & Saltin, 2006) (see Fig. 2).
There are conditions, such as osteoarthritis and rheumatoid arthritis, in which exercise is contraindicated and leads to acute phases of the disease. Exercise is also contraindicated in cases of acute joint inflammation if pain worsens after training (Pedersen & Saltin, 2006).
With increasing age, there is a significant reduction in bone formation. Age‐related bone loss is also seen in men (Demontiero et al. 2012). The training of patients with osteoporosis should include activities with a low risk of falling (Pedersen & Saltin, 2006).
Cancer in older persons is an increasingly common problem, due to the progressive prolongation of the life‐expectancy of the Western population (Carreca et al. 2005). Exercise is contraindicated in cancer patients being treated with chemotherapy or radiotherapy when a leukocyte concentration falls below 0.5 × 109/L, haemoglobin below 10 g dL−1, thrombocyte concentration below 20 × 109/L, and temperature above 38°C. Patients with bone metastases should not perform strength conditioning at high load (Pedersen & Saltin, 2006).
Cell signalling pathways in exercise and ageing
Exercise causes vast changes in many physiological and metabolic parameters in the organism to meet the increase in energy demands associated with it. The most obvious is the increase in oxygen consumption that increases from approximately 200 ml min–1 in a person of 70 kg in weight to more than 10‐fold in an untrained adult (Hawley et al. 2014). This obviously requires significant changes in cell metabolism. Important changes in cell metabolites during exercise include the ATP/ADP ratio, lower creatine phosphate, an increase in fatty acid oxidation, an increase in reactive oxygen species, a decrease in the pH and changes in calcium (Hood, 2001; Gomez‐Cabrera et al. 2015) (see Fig. 3). These changes led us to consider the major enzymes that contribute to alterations and adaptations in energy metabolism in the cell. AMP‐activated protein kinase (AMPK) is an obvious candidate as it is activated by increasing levels of AMP, but this is not the only enzyme that responds to exercise (Canto et al. 2009). Calcineurin and calmodulin‐dependent kinases are also involved and we observed that mitogen activated protein kinases (MAPKs) like ERK1/2 and p38 are also important (Gomez‐Cabrera et al. 2005). Finally, the mammalian target of rapamycin (mTOR) is involved in the final steps in the adaptation of muscles to increasing demands of energy, especially to increase muscle protein synthesis associated with strength training (Drummond et al. 2009). The insulin‐like growth factor (IGF) related pathway is critical to increase protein synthesis after exercise (Coffey & Hawley, 2007). It involves PI3K, AKT and mTOR, leading to an activation of p70S6K (Liu et al. 2002). The AMPK signals for a lower energy state in the cell and inhibits mTOR, telling the cell that protein synthesis should not be activated in endurance exercise (Atherton et al. 2005). However, MP‐activated protein Kinase (AMPK) is essential to increase the rate of peroxisome proliferator‐activated receptor γ coactivator 1α (PGC‐1α) synthesis. PGC‐1α (which is also activated by p38 MAP kinase) is an important co‐factor to activate the biosynthesis of mitochondria (Puigserver et al. 1998; Gomez‐Cabrera et al. 2008). So we are faced with a two‐sided adaptation. When anaerobic (strength) training activates IGF‐1, the mTOR pathway is activated and muscle protein synthesis is stimulated through mRNA translation and ribosomal biogenesis. When, however, the energy state of the cell is lowered by aerobic exercise, AMPK is activated, mTOR is inhibited and PGC‐1α becomes active leading to mitochondrial biogenesis (Hawley et al. 2014).
Figure 3. AMP‐activated protein kinase (AMPK), calcineurin‐ and calmodulin‐dependent kinases and mitogen activated protein kinases (MAPKs) like p38 are related to changes in cell metabolism during muscle contraction .
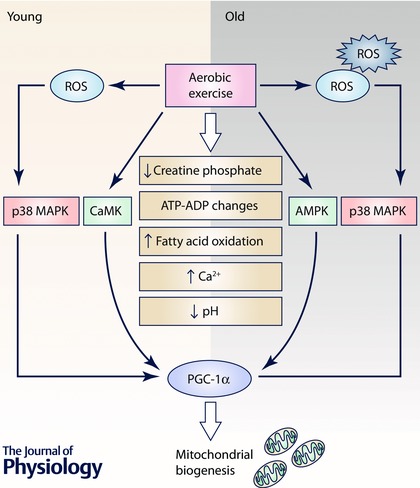
They are involved in the rate of activation of PGC‐1α, an important co‐factor present in mitochondrial biogenesis. In the muscles from old animals these physiological pathways may be disrupted because their cells are under constant oxidative stress that activates p38 continuously, leading to a lack of reactivity of PGC‐1α.
However, this is the case in young animals. We have observed that mitochondrial biogenesis in old animals closely resembles that of PGC‐1α knock‐out animals, i.e. its activation by aerobic exercise is very weak (Derbre et al. 2011). We studied this phenomenon in some detail and came to the conclusion that p38 (an essential activator of PGC‐1α) is continuously activated in old animals. It has to be said now that p38 is an indicator of oxidative stress and cells from old animals are under constant oxidative stress, as proposed by the free radical theory of ageing (Vasilaki et al. 2006; Vina et al. 2013 a). Thus, p38 activation, which is continuous, ceases to be a signal to activate PGC‐1α and the latter becomes non‐reactive to the former. This is a very important difference between the muscles of old and young animals. Figure 3 shows that mitochondrial biogenesis is inhibited in muscle of old animals and that this may be due to the non‐sensitivity of PGC‐1α to p38 activation.
Moreover, resistance exercise training studies typically show an attenuated muscle protein anabolic response in older compared with younger adults due to a dysregulation in the mTORC1 signalling pathway (Kosek et al. 2006; Slivka et al. 2008; Mayhew et al. 2009). As mentioned in the previous paragraph, one hypothesis that could explain this impairment is that older adults display an increase in markers of cellular stress that may affect the remodelling response following resistance exercise (Drummond et al. 2008).
The experiments we have just summarised give support to our modified free radical theory of ageing (Vina et al. 2013 a). We postulated that free radicals are not directly involved in damage associated with ageing, but rather in the impairment of cell signalling pathways. The altered mitochondrial biogenic pathway in ageing is an example supporting the role of free radicals by altering cellular signalling pathways in ageing.
Concluding remarks: exercise as a supplement for better ageing
We would now like to extend our previous view that exercise is a beneficial physiological stimulus to delay the onset of frailty to viewing exercise as a ‘supplement’.
Millions of people worldwide are, and have been, taking supplements for decades, especially nutritional supplements, to improve their health and in the specific area of exercise, to improve their physical condition. Among those who supported supplements were scientists of the stature of Linus Pauling (Pauling, 1986).
Scientific evidence has proved that supplementing with high doses of antioxidants is detrimental for human health (Bjelakovic et al. 2004, 2007) and we found that supplementing athletes with vitamin C hampers the efficiency of training (Gomez‐Cabrera et al. 2008). However, there is no doubt that exercise considered as a lifelong supplement is very good for one's health. Exercise, even if performed at the highest intensity by well‐trained people, is hypothesised to enhance longevity. We found that Tour de France cyclists display an average longevity 11% higher than the general population (Sanchis‐Gomar et al. 2011). As we stated at the beginning of this review, the London bus study proved for the first time that exercise is good for one's health. We would like to propose that exercise should be considered as a ‘physiological supplement’. The FDA defines a clear‐cut difference between a drug and supplement: a drug is something that has to be proved to be effective in order to be used in clinics; a supplement, on the other hand, is something that can be used unless it is proved to be unhealthy. We would like to propose that exercise should be considered as a physiological supplement for the general population to improve health. Ample scientific evidence supports treating exercise as a supplement with guidance for specific regimes of exercise to promote healthy ageing.
Additional information
Competing interests
None declared.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work is supported by grants SAF2013‐44663‐R, from the Spanish Ministry of Education and Science (MEC); ISCIII2012‐RED‐43‐029 from the ‘Red Tematica de investigacion cooperativa en envejecimiento y fragilidad’ (RETICEF); PROMETEO2014/056 from ‘Conselleria d'Educació, Cultura i Esport de la Generalitat Valenciana’; RS2012‐609 Intramural Grant from INCLIVA; and EU Funded CM1001 and FRAILOMIC‐HEALTH.2012.2.1.1‐2. The study has been co‐financed by FEDER funds from the European Union.
Acknowledgements
We thank Mrs Marilyn Noyes for her kind help in reviewing the manuscript.
Biographies
Mari Carmen Gomez‐ Cabrera has a permanent position at the Department of Physiology at the University of Valencia. Over the last 17 years she has contributed on different topics related to oxidative stress, cell signalling in exercise, and ageing. She has visited and collaborated with different research groups at the University of Southern California, University of Wisconsin and University of Liverpool. Jose Viña studied Medicine at the University of Valencia and did research work under the auspices of Prof Hans Krebs in Oxford. He obtained his PhD in 1976. He is a Professor of Physiology at the University of Valencia and leads a successful research group (FreshAge) working on different aspects of oxidative stress.
![]()
Leocadio Rodriguez‐Mañas is the Head of the Department of Geriatrics at Hospital Universitario de Getafe (Madrid), Professor of Geriatrics at the Universidad Europea de Madrid, Coordinator of the Spanish Collaborative Research Network on Aging and Frailty – RETICEF (Ministry of Science and Innovation), and Co‐director of the Toledo Study on Healthy Ageing. He is the principal Investigator in four research projects focused on frailty recently funded by the EU 7th Framework Programme. In addition he is leading two DG‐SANCO‐funded projects. Francisco José Tarazona‐Santabalbina studied Medicine at the University of Valencia, where he obtained his PhD in 2000. He works as a consultant at the Geriatric Medicine Department of the Hospital Universitario de la Ribera (Alzira, Valencia) and is associated professor at the Universidad Católica de Valencia San Vicente Mártir.
Andrea Salvador‐Pascual earned her bachelor degree in Physical Education in 2013, and her Master in Physiology in 2014. Currently she has a grant to develop her PhD that is focused in the study of the molecular pathways involved in sarcopenia and frailty and the role of some drugs and physical exercise in their prevention.
References
- Abbatecola AM, Fumagalli A, Bonardi D & Guffanti EE (2011). Practical management problems of chronic obstructive pulmonary disease in the elderly: acute exacerbations. Curr Opin Pulm Med 17 Suppl 1, S49–S54. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ & Wackerhage H (2005). Selective activation of AMPK‐PGC‐1alpha or PKB‐TSC2‐mTOR signaling can explain specific adaptive responses to endurance or resistance training‐like electrical muscle stimulation. FASEB J 19, 786–788. [DOI] [PubMed] [Google Scholar]
- Benito B, Gay‐Jordi G, Serrano‐Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S & Mont L (2011). Cardiac arrhythmogenic remodeling in a rat model of long‐term intensive exercise training. Circulation 123, 13–22. [DOI] [PubMed] [Google Scholar]
- Binder EF, Schechtman KB, Ehsani AA, Steger‐May K, Brown M, Sinacore DR, Yarasheski KE & Holloszy JO (2002). Effects of exercise training on frailty in community‐dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 50, 1921–1928. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG & Gluud C (2007). Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta‐analysis. JAMA 297, 842–857. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Simonetti RG & Gluud C (2004). Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta‐analysis. Lancet 364, 1219–1228. [DOI] [PubMed] [Google Scholar]
- Booth FW, Laye MJ & Roberts MD (2011). Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985) 111, 1497–1504. [DOI] [PubMed] [Google Scholar]
- Borras C, Monleon D, Lopez‐Grueso R, Gambini J, Orlando L, Pallardo FV, Santos E, Vina J & Font de Mora J (2011). RasGrf1 deficiency delays aging in mice. Aging (Albany NY) 3, 262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C & Rankinen T (2001). Individual differences in response to regular physical activity. Med Sci Sports Exerc 33, S446–S451; discussion S452–S443. [DOI] [PubMed] [Google Scholar]
- Boule NG, Weisnagel SJ, Lakka TA, Tremblay A, Bergman RN, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC & Bouchard C (2005). Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care 28, 108–114. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Casas‐Herrero A, Zambom‐Ferraresi F, Idoate F, Millor N, Gomez M, Rodriguez‐Manas L & Izquierdo M (2014). Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age (Dordr) 36, 773–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadore EL, Rodriguez‐Manas L, Sinclair A & Izquierdo M (2013). Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res 16, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart‐Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P & Auwerx J (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreca I, Balducci L & Extermann M (2005). Cancer in the older person. Cancer Treat Rev 31, 380–402. [DOI] [PubMed] [Google Scholar]
- Casas Herrero A, Cadore EL, Martinez Velilla N & Izquierdo Redin M (2015). [Physical exercise in the frail elderly: an update]. Rev Esp Geriatr Gerontol 50, 74–81. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Thomas GD, Boseman LA, Beckles GL & Albright AL (2012). Aging, diabetes, and the public health system in the United States. Am J Public Health 102, 1482–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Vellas B, Hsu FC, Newman AB, Doss H, King AC, Manini TM, Church T, Gill TM, Miller ME & Pahor M (2015). A physical activity intervention to treat the frailty syndrome in older persons‐results from the LIFE‐P study. J Gerontol A Biol Sci Med Sci 70, 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin APMJ, van Uffelen JG, Riphagen I & van Mechelen W (2008). The functional effects of physical exercise training in frail older people: a systematic review. Sports Med 38, 781–793. [DOI] [PubMed] [Google Scholar]
- Chou CH, Hwang CL & Wu YT (2012). Effect of exercise on physical function, daily living activities, and quality of life in the frail older adults: a meta‐analysis. Arch Phys Med Rehabil 93, 237–244. [DOI] [PubMed] [Google Scholar]
- Clemson L, Fiatarone Singh MA, Bundy A, Cumming RG, Manollaras K, O'Loughlin P & Black D (2012). Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ 345, e4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey VG & Hawley JA (2007). The molecular bases of training adaptation. Sports Med 37, 737–763. [DOI] [PubMed] [Google Scholar]
- Colcombe S & Kramer AF (2003). Fitness effects on the cognitive function of older adults: a meta‐analytic study. Psychol Sci 14, 125–130. [DOI] [PubMed] [Google Scholar]
- Daniels R, van Rossum E, de Witte L, Kempen GI & van den Heuvel W (2008). Interventions to prevent disability in frail community‐dwelling elderly: a systematic review. BMC Health Serv Res 8, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries NM, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Staal JB & Nijhuis‐van der Sanden MW (2012). Effects of physical exercise therapy on mobility, physical functioning, physical activity and quality of life in community‐dwelling older adults with impaired mobility, physical disability and/or multi‐morbidity: a meta‐analysis. Ageing Res Rev 11, 136–149. [DOI] [PubMed] [Google Scholar]
- Demontiero O, Vidal C & Duque G (2012). Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis 4, 61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbre F, Gomez‐Cabrera MC, Nascimento AL, Sanchis‐Gomar F, Martinez‐Bello VE, Tresguerres JA, Fuentes T, Gratas‐Delamarche A, Monsalve M & Vina J (2011). Age associated low mitochondrial biogenesis may be explained by lack of response of PGC‐1alpha to exercise training. Age (Dordr) 34, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield‐Moore M, Volpi E & Rasmussen BB (2008). Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985) 104, 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E & Rasmussen BB (2009). Rapamycin administration in humans blocks the contraction‐induced increase in skeletal muscle protein synthesis. J Physiol 587, 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K & Cameron ID (2012). Effect of a multifactorial interdisciplinary intervention on mobility‐related disability in frail older people: randomised controlled trial. BMC Med 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA & Pedersen BK (2005). Contraction‐induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 33, 114–119. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA & Evans WJ (1994). Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med 330, 1769–1775. [DOI] [PubMed] [Google Scholar]
- Fontana L, Kennedy BK, Longo VD, Seals D & Melov S (2014). Medical research: treat ageing. Nature 511, 405–407. [DOI] [PubMed] [Google Scholar]
- Freiberger E, Haberle L, Spirduso WW & Zijlstra GA (2012). Long‐term effects of three multicomponent exercise interventions on physical performance and fall‐related psychological outcomes in community‐dwelling older adults: a randomized controlled trial. J Am Geriatr Soc 60, 437–446. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G & McBurnie MA (2001). Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56, M146–M156. [DOI] [PubMed] [Google Scholar]
- Garcia‐Valles R, Gomez‐Cabrera MC, Rodriguez‐Mañas L, Garcia‐Garcia FJ, Diaz A, Noguera I, Olaso‐Gonzalez G & Viña J (2013). Life‐long spontaneous exercise does not prolong lifespan but improves health span in mice. Longev Healthspan 2, 14. doi: 10.1186/2046-2395-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gine‐Garriga M, Roque‐Figuls M, Coll‐Planas L, Sitja‐Rabert M & Salva A (2014). Physical exercise interventions for improving performance‐based measures of physical function in community‐dwelling, frail older adults: a systematic review and meta‐analysis. Arch Phys Med Rehabil 95, 753–769.e3. [DOI] [PubMed] [Google Scholar]
- Gomez‐Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL & Vina J (2005). Decreasing xanthine oxidase mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J & Vina J (2008). Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training‐induced adaptations in endurance performance. Am J Clin Nutr 87, 142–149. [DOI] [PubMed] [Google Scholar]
- Gomez‐Cabrera MC, Salvador‐Pascual A, Cabo H, Ferrando B & Vina J (2015). Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic Biol Med 86, 37–46. [DOI] [PubMed] [Google Scholar]
- Hartley PH & Llewellyn GF (1939). Longevity of oarsmen. Br Med J 1, 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala AJ, Kiviniemi AM, Makikallio TH, Kinnunen H, Nissila S, Huikuri HV & Tulppo MP (2006). Individual differences in the responses to endurance and resistance training. Eur J Appl Physiol 96, 535–542. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ & Zierath JR (2014). Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Holloszy JO (2000). The biology of aging. Mayo Clin Proc 75 Suppl, S3–S8; discussion S8–S9. [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M & Adams S (1985). Effect of voluntary exercise on longevity of rats. J Appl Physiol 59, 826–831. [DOI] [PubMed] [Google Scholar]
- Hood DA (2001). Invited Review: contractile activity‐induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90, 1137–1157. [DOI] [PubMed] [Google Scholar]
- Hung WW, Ross JS, Boockvar KS & Siu AL (2011). Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr 11, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles M, Gambini J, Carnicero JA, Garcia‐Garcia FJ, Rodriguez‐Manas L, Olaso‐Gonzalez G, Dromant M, Borras C & Vina J (2014). Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc 62, 1324–1328. [DOI] [PubMed] [Google Scholar]
- Karvonen MJ, Klemola H, Virkajarvi J & Kekkonen A (1974). Longevity of endurance skiers. Med Sci Sports 6, 49–51. [PubMed] [Google Scholar]
- Kohrt WM, Malley MT, Coggan AR, Spina RJ, Ogawa T, Ehsani AA, Bourey RE, Martin WH 3rd & Holloszy JO (1991). Effects of gender, age, and fitness level on response of VO2max to training in 60–71 yr olds. J Appl Physiol (1985) 71, 2004–2011. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM & Bamman MM (2006). Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol 101, 531–544. [DOI] [PubMed] [Google Scholar]
- Langlois F, Vu TT, Chasse K, Dupuis G, Kergoat MJ & Bherer L (2013). Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci 68, 400–404. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W & Barrett EJ (2002). Amino acids stimulate translation initiation and protein synthesis through an Akt‐independent pathway in human skeletal muscle. J Clin Endocrinol Metab 87, 5553–5558. [DOI] [PubMed] [Google Scholar]
- Lopez‐Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G (2013). The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor B, Lough M, Gagnon MM, Cupples A, Wayne PM & Lipsitz LA (2014). Functional benefits of tai chi training in senior housing facilities. J Am Geriatr Soc 62, 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijon E, Tafflet M, Antero‐Jacquemin J, El Helou N, Berthelot G, Celermajer DS, Bougouin W, Combes N, Hermine O, Empana JP, Rey G, Toussaint JF & Jouven X (2013). Mortality of French participants in the Tour de France (1947‐2012). Eur Heart J 34, 3145–3150. [DOI] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Klatt P, Flores I, Garcia‐Cao I, Borras C, Flores JM, Vina J, Blasco MA & Serrano M (2007). Delayed ageing through damage protection by the Arf/p53 pathway. Nature 448, 375–379. [DOI] [PubMed] [Google Scholar]
- Mayhew DL, Kim JS, Cross JM, Ferrando AA & Bamman MM (2009). Translational signaling responses preceding resistance training‐mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107, 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monleon D, Garcia‐Valles R, Morales JM, Brioche T, Olaso‐Gonzalez G, Lopez‐Grueso R, Gomez‐Cabrera MC & Vina J (2014). Metabolomic analysis of long‐term spontaneous exercise in mice suggests increased lipolysis and altered glucose metabolism when animals are at rest. J Appl Physiol (1985) 117, 1110–1119. [DOI] [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PA, Roberts CG & Parks JW (1953). Coronary heart‐disease and physical activity of work. Lancet 265, 1053–1057 (contd 1111–1120). [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez‐Cepero JM & Boveris A (2004). Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286, R505–R511. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Cabo H, Gomez‐Cabrera MC, Vina J & Hellsten Y (2013). Roles of sedentary aging and lifelong physical activity in exchange of glutathione across exercising human skeletal muscle. Free Radic Biol Med 73, 166–173. [DOI] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM & Williamson JD (2014). Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. JAMA 311, 2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC et al (1995). Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 273, 402–407. [DOI] [PubMed] [Google Scholar]
- Pauling L (1986). How to Live Longer and Feel Better. Freeman & Co., San Francisco/Oregon State University Press, Corvallis. [Google Scholar]
- Pedersen BK & Saltin B (2006). Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16 Suppl 1, 3–63. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA & Ray CA (2004). American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 36, 533–553. [DOI] [PubMed] [Google Scholar]
- Pimenta E & Oparil S (2012). Management of hypertension in the elderly. Nat Rev Cardiol 9, 286–296. [DOI] [PubMed] [Google Scholar]
- Prout C (1972). Life expectancy of college oarsmen. JAMA 220, 1709–1711. [PubMed] [Google Scholar]
- Prud'homme D, Bouchard C, Leblanc C, Landry F & Fontaine E (1984). Sensitivity of maximal aerobic power to training is genotype‐dependent. Med Sci Sports Exerc 16, 489–493. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M & Spiegelman BM (1998). A cold‐inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Manas L & Fried LP (2014). Frailty in the clinical scenario. Lancet 385, e7–e9. [DOI] [PubMed] [Google Scholar]
- Ruiz JR, Moran M, Arenas J & Lucia A (2010). Strenuous endurance exercise improves life expectancy: it's in our genes. Br J Sports Med 45, 159–161. [DOI] [PubMed] [Google Scholar]
- Saltin B & Astrand PO (1967). Maximal oxygen uptake in athletes. J Appl Physiol 23, 353–358. [DOI] [PubMed] [Google Scholar]
- Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA & Dunlap WP (1985). Effect of exercise on longevity, body weight, locomotor performance, and passive‐avoidance memory of C57BL/6J mice. Neurobiol Aging 6, 17–24. [DOI] [PubMed] [Google Scholar]
- Sanchis‐Gomar F, Olaso‐Gonzalez G, Corella D, Gomez‐Cabrera MC & Vina J (2011). Increased average longevity among the “Tour de France” cyclists. Int J Sports Med 32, 644–647. [DOI] [PubMed] [Google Scholar]
- Scharhag‐Rosenberger F, Walitzek S, Kindermann W & Meyer T (2012). Differences in adaptations to 1 year of aerobic endurance training: individual patterns of nonresponse. Scand J Med Sci Sports 22, 113–118. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Marott JL, Lange P & Jensen GB (2013). Longevity in male and female joggers: the Copenhagen City Heart Study. Am J Epidemiol 177, 683–689. [DOI] [PubMed] [Google Scholar]
- Schnohr P, O'Keefe JH, Marott JL, Lange P & Jensen GB (2015). Dose of jogging and long‐term mortality: the Copenhagen City Heart Study. J Am Coll Cardiol 65, 411–419. [DOI] [PubMed] [Google Scholar]
- Sinclair DA & Guarente L (2006). Unlocking the secrets of longevity genes. Sci Am 294, 48–51, 54–47. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Jaskolski A, Jaskolska A, Krasnoff J, Gagnon J, Leon AS, Rao DC, Wilmore JH & Bouchard C (2001). Age, sex, race, initial fitness, and response to training: the HERITAGE Family Study. J Appl Physiol (1985) 90, 1770–1776. [DOI] [PubMed] [Google Scholar]
- Slivka D, Raue U, Hollon C, Minchev K & Trappe S (2008). Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol 295, R273–R280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L (2014). Gerontology: Will you still need me, will you still feed me? Nature 514, S14–S15. [DOI] [PubMed] [Google Scholar]
- Teramoto M & Bungum TJ (2010). Mortality and longevity of elite athletes. J Sci Med Sport 13, 410–416. [DOI] [PubMed] [Google Scholar]
- Theou O, Stathokostas L, Roland KP, Jakobi JM, Patterson C, Vandervoort AA & Jones GR (2011). The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res 2011, 569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Knudsen S, Rankinen T, Koch LG, Sarzynski M, Jensen T, Keller P, Scheele C, Vollaard NB, Nielsen S, Akerstrom T, MacDougald OA, Jansson E, Greenhaff PL, Tarnopolsky MA, van Loon LJ, Pedersen BK, Sundberg CJ, Wahlestedt C, Britton SL & Bouchard C (2010). Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol (1985) 108, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen L, Rantanen T, Timonen TE & Sulkava R (2002). Effects of a group‐based exercise program on the mood state of frail older women after discharge from hospital. Int J Geriatr Psychiatry 17, 1106–1111. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, McArdle F, Iwanejko LM & McArdle A (2006). Adaptive responses of mouse skeletal muscle to contractile activity: The effect of age. Mech Ageing Dev 127, 830–839. [DOI] [PubMed] [Google Scholar]
- Vigen R, Maddox TM & Allen LA (2012). Aging of the United States population: Impact on heart failure. Curr Heart Fail Rep 9, 369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Abdelaziz KM, Garcia‐Valles R & Gomez‐Cabrera MC (2013. a). The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal 19, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J, Borras C, Sanchis‐Gomar F, Martinez‐Bello VE, Olaso‐Gonzalez G, Gambini J, Ingles M & Gomez‐Cabrera MC (2013. b). Pharmacological properties of physical exercise in the elderly. Curr Pharm Des 20, 3019–3029. [DOI] [PubMed] [Google Scholar]
- Vina J, Sanchis‐Gomar F, Martinez‐Bello V & Gomez‐Cabrera M (2012). Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol 167, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


