Abstract
Purpose
Patients with cancer are more likely to file for bankruptcy than the general population, but the impact of severe financial distress on health outcomes among patients with cancer is not known.
Methods
We linked Western Washington SEER Cancer Registry records with federal bankruptcy records for the region. By using propensity score matching to account for differences in several demographic and clinical factors between patients who did and did not file for bankruptcy, we then fit Cox proportional hazards models to examine the relationship between bankruptcy filing and survival.
Results
Between 1995 and 2009, 231,596 persons were diagnosed with cancer. Patients who filed for bankruptcy (n = 4,728) were more likely to be younger, female, and nonwhite, to have local- or regional- (v distant-) stage disease at diagnosis, and have received treatment. After propensity score matching, 3,841 patients remained in each group (bankruptcy v no bankruptcy). In the matched sample, mean age was 53.0 years, 54% were men, mean income was $49,000, and majorities were white (86%), married (60%), and urban (91%) and had local- or regional-stage disease at diagnosis (84%). Both groups received similar initial treatments. The adjusted hazard ratio for mortality among patients with cancer who filed for bankruptcy versus those who did not was 1.79 (95% CI, 1.64 to 1.96). Hazard ratios varied by cancer type: colorectal, prostate, and thyroid cancers had the highest hazard ratios. Excluding patients with distant-stage disease from the models did not have an effect on results.
Conclusion
Severe financial distress requiring bankruptcy protection after cancer diagnosis appears to be a risk factor for mortality. Further research is needed to understand the process by which extreme financial distress influences survival after cancer diagnosis and to find strategies that could mitigate this risk.
INTRODUCTION
For the 1.5 million persons diagnosed with cancer each year, financial stress can be protracted and severe. At the extreme end of financial stress are those whose economic situation deteriorates to the point where they must seek protection from creditors. In a previous study, we found that patients are 2.5 times more likely to file for bankruptcy after a cancer diagnosis compared with individuals who have not been diagnosed with cancer.1
The term financial toxicity has been coined in reference to the growing recognition that high out-of-pocket expenditures during cancer treatment are putting many families into severe financial distress and, in some cases, leading to refusal of treatment or nonadherence to recommended treatments.2 Although some have called for clinicians to be more aware of the problem and to take proactive steps to reduce adverse financial impacts,3 clinicians are ill prepared to advise patients because they typically have little knowledge of their patients’ health insurance or general financial circumstances, and patients may be reluctant to discuss their financial concerns with providers. Altering the status quo would require substantial changes in doctor-patient discussions and may have broader implications for policies that affect out-of-pocket liabilities for patients with cancer. It is therefore reasonable to ask whether financial distress can lead to poorer outcomes, particularly survival, for patients with cancer.
Although other studies have shown a relationship between cancer diagnosis and financial distress, few have examined whether economic hardship leads to poorer outcomes. Accordingly, in this study, by using a population-based cancer registry linked with federal bankruptcy records for the region, we examined the mortality risk for patients with cancer who file for bankruptcy compared with patients who have cancer but do not file for bankruptcy.
METHODS
Study Population
Our study included individuals with cancers recorded in the Western Washington Cancer Surveillance System (CSS). CSS is population-based cancer registry that is part of the National Cancer Institute’s SEER program. We included persons with all cancers except nonmelanoma skin cancer diagnosed between January 1, 1995, and December 31, 2009. We excluded persons younger than age 21 years at the time of cancer diagnosis, those with another malignancy diagnosed before the study period, those with in situ cancers at diagnosis, or those whose cancers were diagnosed at time of death. The remaining individuals were linked with records of the US Bankruptcy Court, Western District of Washington (USBC-WDW). The court serves the 13 counties in the CSS region and has complete electronic case files dating from June 1991. The bankruptcy database includes information about the filing such as bankruptcy chapter, number of creditors, and assets and liabilities at the time of bankruptcy. We included only persons filing for either Chapter 7 or Chapter 13 bankruptcy through December 31, 2009. Debtors filing under Chapter 7 typically retain only exempt assets (nonexempt assets are liquidated for the benefit of creditors) in exchange for the discharge of some of their debt, whereas those filing under Chapter 13 retain ownership of most of their assets and typically repay creditors over 3 to 5 years. The majority of personal bankruptcies filed in the United States are under Chapter 7.
SEER-CSS records were linked to USBC-WDW bankruptcy records by using a probabilistic algorithm that included name, sex, address of residence, and the last four digits of the Social Security number. For patients who filed for bankruptcy multiple times, we included only the first filing after their cancer diagnosis. Deaths were determined from cancer registry records.
The study design was approved by the Fred Hutchinson Institutional Review Board. Judge Karen Overstreet (then Chief Judge of the USBC-WDW, 2005) issued a letter of support on February 1, 2008, permitting linkage of the CSS and USBC-WDW databases.
Analyses
For all cancers, we calculated the cumulative incidence of bankruptcy conditional on survival until the time point of interest, treating death before bankruptcy as a competing event. Only the first bankruptcy filing after diagnosis was counted; subsequent filings were not included in the analysis. Details of our approach to determining bankruptcy incidence among patients with cancer are available in Ramsey et al.1
To assess the association between bankruptcy filing and mortality, we used Cox regression models on a propensity score matched sample. Propensity score matching was used to balance the distributions of observed baseline characteristics between patients with cancer who filed for bankruptcy and those who did not. This approach has been shown to reduce the effect of selection bias in observational studies.4,5
We obtained propensity scores within each cancer type by using baseline variables and logistic regression to model the odds of filing for bankruptcy. The baseline variables included sex, race, marital status, urban versus rural residence, income level on the basis of the individual’s home ZIP code, year of diagnosis, age at diagnosis, stage at diagnosis, and initial treatment modality (surgery, chemotherapy, radiation therapy, hormone therapy). By using the logistic regression model, we calculated the predicted probability of filing (the propensity score) for each patient. We then matched pairs of patients from the group that filed for bankruptcy and the group that did not file for bankruptcy by using their propensity scores and a caliper equal to one-quarter of the standard deviation of the logit of the propensity score. We examined the balance between baseline covariates before and after propensity score matching by using standardized differences.6
In the propensity score matched sample, we fit Cox models regressing survival on bankruptcy filing status with a robust variance estimator to account for clustering as a result of pair matching and adjusting for the propensity score. The predictor of interest was a time-dependent covariate indicating that the individual had filed for bankruptcy. We also included a time-dependent covariate to reflect the time of enactment of the Bankruptcy Abuse Prevention and Consumer Protection Act of 2005. This act, the largest revision of bankruptcy law since 1978, had a profound albeit temporary increase in the number of bankruptcy filings. We fit one model for all cancers and a separate model for each cancer type. In all models, we used age as the time scale to account for left truncation using age at diagnosis as the start time.7
To address the potential issue of reverse causality in which bankruptcy may have followed major disease progression (ie, cases in which bankruptcy was declared to protect an inheritance), and because the prognosis of advanced cancers is poor regardless of patient financial circumstances and unpredictably responsive to therapy, we conducted a sensitivity analysis limited to patients diagnosed when the disease was in early stage and who declared bankruptcy within 1 year of diagnosis so that they would still likely have been in early stage at the time of filing. We fit the same Cox models as mentioned before but limiting to patients diagnosed with local- or regional-stage cancers and censoring patients who declared for bankruptcy late (more than 1 year after diagnosis at the time of bankruptcy).
In a separate sensitivity analysis, we implemented propensity score matching that accounted for the time-dependent exposure. Instead of simultaneously matching all patients who were ever observed to file for bankruptcy to patients who were not, we used a sequential matching algorithm in which each patient who filed for bankruptcy was matched to a patient who was alive and still at risk for filing for bankruptcy at that time.8
Finally, we carried out a sensitivity analysis to investigate the susceptibility of our results to unmeasured confounding, that is, factors that may influence both the risk for bankruptcy and the risk for death. To do so, we assumed the existence of an unmeasured confounder that was moderately associated with bankruptcy. We then investigated what level of association with mortality would have rendered our results statistically nonsignificant and reversed the estimated association between bankruptcy and mortality in the model with all cancers had the confounder been included in the analysis.9 All analyses were conducted by using R statistical software, version 3.2.1.
RESULTS
Between 1995 and 2009, there were 231,596 persons recorded in SEER who were diagnosed with cancer and who met study criteria for inclusion. During that same time period, 4,728 of those individuals filed for bankruptcy protection (3,909 under Chapter 7 and 819 under Chapter 13).
Baseline characteristics for the original study sample and the propensity score matched sample are provided in Table 1. In the original sample, there are systematic differences between patients with cancer who filed for bankruptcy compared with those who did not. Patients who filed were more likely to be younger, female, nonwhite, have local- or regional- (v distant-) stage disease at diagnosis (using SEER staging criteria), and have received treatment. After propensity score matching, 7,682 patients remained in the analysis, with 3,841 patients in each group. In the propensity score matched sample (Table 1), the two groups were similar with respect to baseline measures. Both groups in the propensity score matched sample consisted of patients who were diagnosed at a mean age of 53 years (standard deviation, 14.7 years), with more men than women in the sample (54% v 46%). A majority of the patients were white (86%), married (60%), and lived in urban residences (91%). Most patients were diagnosed with local-stage (59%) or regional-stage (25%) cancer with a smaller proportion (14%) diagnosed with distant-stage cancer. Both groups were balanced with respect to the first treatment modality received. Mean income (by ZIP code) in both groups was $49,000 (standard deviation, $12,000).
Table 1.
Demographic and Cancer-Related Factors by Bankruptcy Status in the Original Cohort and the Propensity Score Matched Sample, Puget Sound SEER, 1995-2009
| Variable | Original Cohort | Propensity Score Matched Sample | ||||
|---|---|---|---|---|---|---|
| Bankruptcy (n = 4,728) No. (%) | No Bankruptcy (n = 226,875) No. (%) | Standardized Difference | Bankruptcy (n = 3,841) No. (%) | No Bankruptcy (n = 3,841) No. (%) | Standardized Difference | |
| Demographics | ||||||
| Age at diagnosis (years ± standard deviation) | 52.6 ± 13.5 | 63.9 ± 14.6 | 0.803 | 52.9 ± 14.7 | 52.9 ± 13.5 | 0.005 |
| Male | 2,113 (45) | 114,807 (51) | 0.117 | 1,758 (54) | 1,750 (54) | 0.004 |
| White race | 4,049 (86) | 200,858 (89) | 0.104 | 3298 (86) | 3312 (86) | 0.011 |
| Married | 2,751 (58) | 131,099 (58) | 0.010 | 2279 (59) | 2299 (60) | 0.011 |
| Residence | ||||||
| Urban | 3,740 (79) | 187,994 (83) | 0.032 | 3508 (91) | 3488 (91) | 0.018 |
| Large rural | 203 (4) | 11,389 (5) | 0.023 | 194 (5) | 208 (5) | 0.016 |
| Small rural | 99 (2) | 5,329 (2) | 0.009 | 90 (2) | 99 (3) | 0.015 |
| Isolated | 51 (1) | 3,147 (1) | 0.023 | 49 (1) | 46 (1) | 0.007 |
| Income (US dollars ± standard deviation)* | 49,000 ± 11,000 | 51,000 ± 13,000 | 0.106 | 49,000 ± 11,000 | 49,000 ± 12,000 | 0.034 |
| Cancer-related factors | ||||||
| Cancer site | ||||||
| Breast | 1,071 (23) | 36,904 (16) | 0.163 | 825 (21) | 825 (21) | 0.000 |
| Colorectal | 392 (8) | 21,080 (9) | 0.035 | 326 (8) | 326 (8) | 0.000 |
| Leukemia/lymphoma | 473 (10) | 23,008 (10) | 0.004 | 411 (11) | 411 (11) | 0.000 |
| Lung | 301 (6) | 28,863 (13) | 0.217 | 255 (7) | 255 (7) | 0.000 |
| Prostate | 627 (13) | 38,456 (17) | 0.103 | 514 (13) | 514 (13) | 0.000 |
| Melanoma | 324 (7) | 11,452 (5) | 0.077 | 260 (7) | 260 (7) | 0.000 |
| Thyroid | 240 (5) | 4,979 (2) | 0.155 | 205 (5) | 205 (5) | 0.000 |
| Uterine | 202 (4) | 6,939 (3) | 0.065 | 162 (4) | 162 (4) | 0.000 |
| Other† | 1,091 (23) | 55,194 (24) | 0.029 | 883 (23) | 883 (23) | 0.000 |
| Stage | ||||||
| Local | 2,794 (59) | 110,527 (49) | 0.211 | 2,258 (59) | 2,280 (59) | 0.012 |
| Regional | 1,184 (25) | 49,168 (22) | 0.081 | 952 (25) | 950 (25) | 0.001 |
| Distant | 597 (13) | 55,011 (24) | 0.303 | 523 (14) | 507 (13) | 0.012 |
| Unstaged | 146 (3) | 12,169 (5) | 0.113 | 108 (3) | 104 (3) | 0.006 |
| Year of diagnosis | ||||||
| 1995 | 401 (9) | 12,565 (6) | 0.116 | 0 (0) | 0 (0) | |
| 1996 | 429 (9) | 12,848 (6) | 0.131 | 215 (6) | 230 (6) | 0.017 |
| 1997 | 434 (9) | 13,295 (6) | 0.127 | 404 (11) | 388 (10) | 0.014 |
| 1998 | 406 (9) | 14,050 (6) | 0.092 | 392 (10) | 385 (10) | 0.006 |
| 1999 | 443 (9) | 14,555 (6) | 0.110 | 422 (11) | 468 (12) | 0.037 |
| 2000 | 436 (9) | 14,393 (6) | 0.108 | 415 (11) | 392 (10) | 0.020 |
| 2001 | 430 (9) | 14,738 (7) | 0.098 | 410 (11) | 407 (11) | 0.003 |
| 2002 | 387 (8) | 15,170 (7) | 0.058 | 357 (9) | 351 (9) | 0.005 |
| 2003 | 333 (7) | 15,190 (7) | 0.014 | 302 (8) | 314 (8) | 0.012 |
| 2004 | 306 (7) | 15,590 (7) | 0.016 | 284 (7) | 267 (7) | 0.017 |
| 2005 | 228 (5) | 16,013 (7) | 0.094 | 207 (5) | 213 (6) | 0.007 |
| 2006 | 173 (4) | 16,486 (7) | 0.159 | 164 (4) | 171 (4) | 0.009 |
| 2007 | 157 (3) | 17,291 (8) | 0.190 | 136 (4) | 123 (3) | 0.019 |
| 2008 | 116 (3) | 17,167 (8) | 0.236 | 100 (3) | 97 (3) | 0.005 |
| 2009 | 42 (1) | 17,524 (8) | 0.342 | 33 (1) | 35 (1) | 0.006 |
| Treatment | ||||||
| Surgery | 3,660 (78) | 135,893 (60) | 0.386 | 2,953 (77) | 2,985 (78) | 0.020 |
| Chemotherapy | 1,604 (34) | 67,569 (30) | 0.089 | 1,358 (35) | 1,374 (36) | 0.009 |
| Radiation | 1,889 (40) | 74,735 (33) | 0.147 | 1,588 (41) | 1,612 (42) | 0.013 |
| Hormones | 1,037 (22) | 39,059 (17) | 0.121 | 882 (23) | 893 (23) | 0.007 |
Median income from ZIP code of residence based on 2000 census data.
“Other” includes all remaining cancers except nonmelanoma skin cancers. Individual cancers in this category comprised less than 3% of all cancers in the sample.
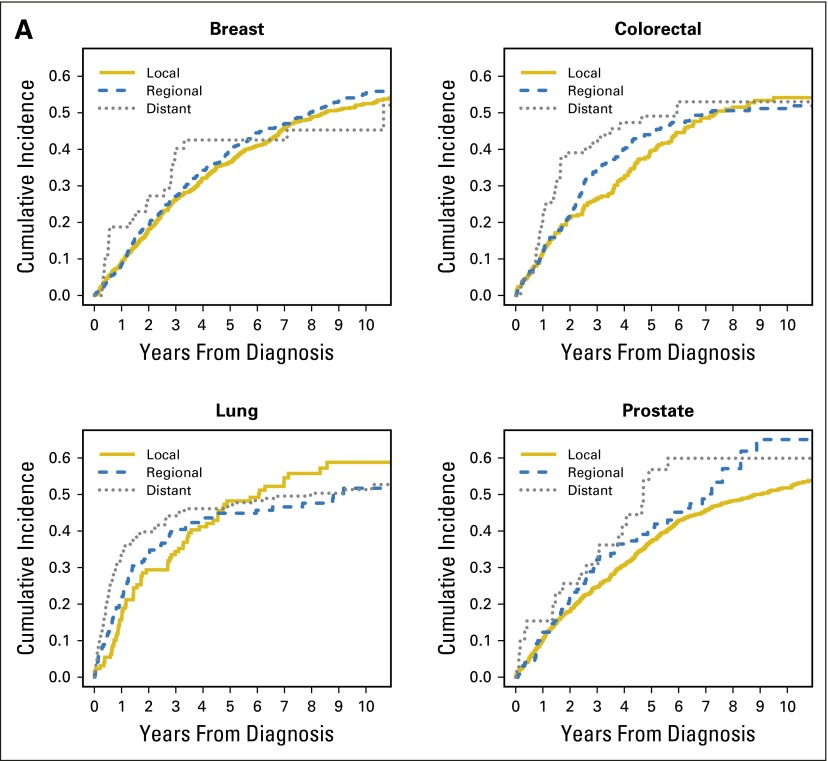
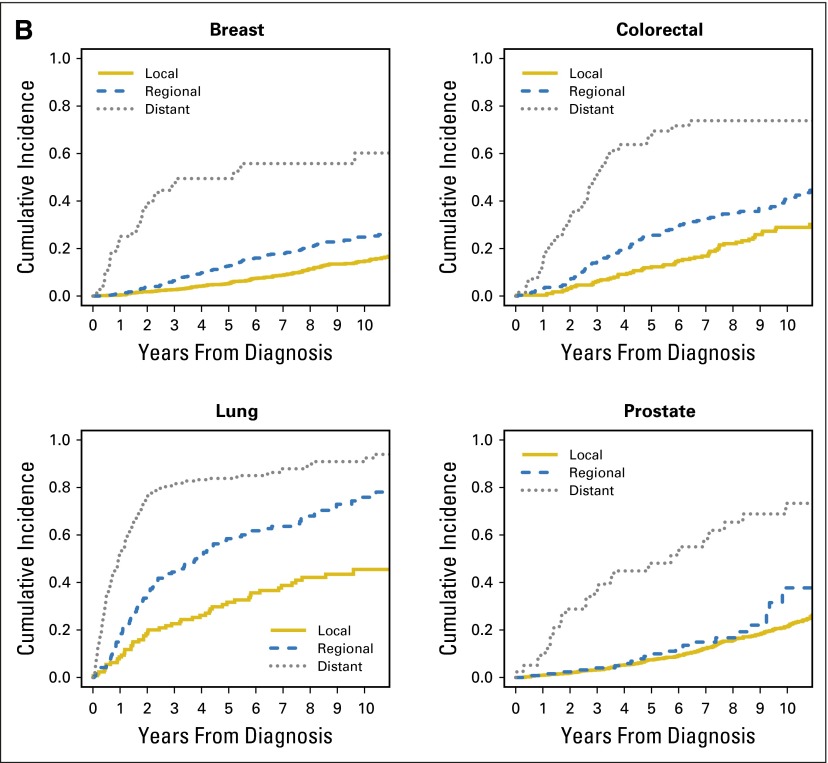
Table 2 displays the cumulative probabilities of bankruptcy conditional on survival and the survival probabilities of patients with cancer in the first 5 years after diagnosis. Figure 1 displays the cumulative incidence of bankruptcy and mortality for the four major cancers (breast, colorectal, lung, and prostate) stratified by stage at diagnosis. At 5 years from diagnosis, lung cancer had the highest cumulative incidence of bankruptcy and the poorest overall survival.
Table 2.
Cumulative Incidence of Bankruptcy and Overall Survival Probability in the First 5 Years After Cancer Diagnosis in the Propensity Score Matched Sample
| Cancer Type | No. of Years After Diagnosis | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 5 | ||||
| Bankruptcy (%) | Survival (%) | Bankruptcy (%) | Survival (%) | Bankruptcy (%) | Survival (%) | |
| Prostate (n = 1,028) | 11 | 99 | 19 | 97 | 38 | 91 |
| Breast (n = 1,650) | 9 | 99 | 19 | 97 | 38 | 91 |
| Lung (n = 510) | 25 | 70 | 35 | 52 | 47 | 38 |
| Leukemia/lymphoma (n = 822) | 12 | 92 | 21 | 87 | 41 | 78 |
| Colorectal (n = 652) | 12 | 97 | 23 | 92 | 42 | 76 |
| Melanoma (n = 520) | 11 | 99 | 20 | 97 | 41 | 93 |
| Uterine (n = 324) | 10 | 100 | 20 | 97 | 40 | 91 |
| Thyroid (n = 410) | 10 | 100 | 20 | 100 | 41 | 98 |
| Other (n = 1,766) | 14 | 90 | 24 | 83 | 42 | 73 |
Fig 1.
Cumulative incidence of (A) bankruptcy and (B) death, stratified by stage at diagnosis for the four major cancers (breast, colorectal, lung, and prostate) in the propensity score matched sample.
The association between bankruptcy filing and mortality varied widely across individual cancers (Table 3; full regression models available upon request). Mortality rates among patients with breast, lung, colorectal, or prostate cancer who filed for bankruptcy were significantly higher than for patients with those cancers who did not file for bankruptcy. The risk of mortality was almost twice as high among patients with prostate cancer who filed for bankruptcy compared with those who did not, and it was 2.5 times as high among patients with colorectal cancer who filed compared with those who did not. Restricting the analysis to early-stage cancers and bankruptcy filings to within 1 year of diagnosis showed similar results (Table 4). Sequential matching on the propensity score to account for the time-dependent exposure also showed similar results (available upon request).
Table 3.
Bankruptcy Impact on All-Cause Mortality in the Propensity Score Matched Sample
| Cancer Type | No. at Risk | No. of Deaths | HR | 95% CI | P |
|---|---|---|---|---|---|
| Overall | 17,021 | 2,026 | 1.79 | 1.64 to 1.96 | < .001 |
| Breast | 3,788 | 280 | 1.48 | 1.15 to 1.91 | .003 |
| Lung | 958 | 350 | 1.55 | 1.22 to 1.98 | < .001 |
| Melanoma | 1,197 | 51 | 1.50 | 0.83 to 2.72 | .179 |
| Thyroid | 952 | 23 | 1.71 | 0.69 to 4.27 | .249 |
| Prostate | 2,365 | 214 | 2.07 | 1.56 to 2.74 | < .001 |
| Leukemia/lymphoma | 1,792 | 254 | 1.22 | 0.93 to 1.61 | .146 |
| Uterine | 739 | 42 | 1.09 | 0.55 to 2.16 | .795 |
| Colorectal | 1,430 | 217 | 2.47 | 1.85 to 3.31 | < .001 |
| Other | 3,800 | 595 | 1.49 | 1.25 to 1.78 | < .001 |
Abbreviation: HR, hazard ratio.
Table 4.
Bankruptcy Impact on All-Cause Mortality Restricted to Local- and Regional-Stage Patients Who Filed for Bankruptcy Within 1 Year of Diagnosis in the Propensity Score Matched Sample
| Cancer Type | No. at Risk | No. of Deaths | HR | 95% CI | P |
|---|---|---|---|---|---|
| Overall | 10,567 | 993 | 1.86 | 1.58 to 2.20 | < .001 |
| Breast | 2,623 | 192 | 1.55 | 1.00 to 2.39 | .049 |
| Lung | 458 | 125 | 1.37 | 0.87 to 2.15 | .172 |
| Melanoma | 867 | 35 | 1.66 | 0.65 to 4.25 | .287 |
| Thyroid | 637 | 8 | NA | NA | NA |
| Prostate | 1,634 | 118 | 1.98 | 1.25 to 3.15 | .004 |
| Leukemia/lymphoma | 478 | 35 | 2.03 | 0.89 to 4.63 | .091 |
| Uterine | 534 | 30 | 0.54 | 0.14 to 2.11 | .374 |
| Colorectal | 938 | 112 | 2.73 | 1.66 to 4.48 | < .001 |
| Other | 2,398 | 338 | 1.63 | 1.22 to 2.17 | .001 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Finally, our results were found to not be very sensitive to unmeasured confounding. Specifically, in a sensitivity analysis, we assumed that an unmeasured confounder existed, and we estimated the conditions under which adjustment for the unmeasured confounder would have an impact on the results for the model with all cancers. We found that under moderate association between the unmeasured confounder and bankruptcy, the adjusted association between bankruptcy and mortality would remain positive and statistically significant as long as the association between the unmeasured confounder and mortality was also moderate (hazard ratio, ≤ 1.6).
DISCUSSION
Financial distress and insolvency are now recognized as unfortunate but common events that pose significant difficulties for patients with cancer and their families.10 By linking the Western Washington SEER Cancer Registry and federal bankruptcy records over 15 years, we were able to explore the question of whether those who filed for bankruptcy after a cancer diagnosis had a higher risk for death from any cause. We found a consistent, positive association between filing for bankruptcy and earlier mortality, suggesting that those who reach the point of financial insolvency after a cancer diagnosis have significantly poorer outcomes than those who do not.
Other studies have shown that financial hardship is independently associated with excess mortality risk. Evaluating data from the 1996 Health and Retirement Study, Tucker-Seeley et al11 found that older persons reporting one or more financial hardships had hazard ratios of 1.4 to 1.8 for mortality compared with those who did not report any financial hardships. Earlier studies also showed a general association between financial hardship and other adverse health outcomes.12-16 Our study appears to be unique in that we include verified information on a specific medical condition (cancer), bankruptcy, and mortality.
Our methods address several issues that might bias retrospective database evaluations of the association between bankruptcy and survival. First, patients who filed for bankruptcy have to survive long enough to do so, whereas the group of patients who did not file for bankruptcy perhaps includes sicker patients who should have filed but died before they had the chance to go through the complicated process of filing for bankruptcy. These issues may be more prevalent in the distant-stage group, particularly over the short term (ie, < 3 years from diagnosis). To address this issue, we used propensity score matching to balance the proportion of patients in the bankruptcy and no bankruptcy groups by stage of cancer at diagnosis. Second, propensity score matching addresses the possibility that patients who are treated may be more likely to go bankrupt as a result of the associated expenses but are less likely to die because of the benefits afforded by treatment. Third, propensity score matching addresses the fact that patients who are diagnosed at younger ages are more likely to go bankrupt but less likely to die as a result of all causes.
Even with these adjustments, our analysis has important limitations. First, propensity matching cannot correct for unmeasured factors that may differ between the bankrupt and nonbankrupt groups that also influence survival. It is possible that those who go bankrupt have higher rates of behaviors that are also related to earlier mortality (eg, smoking). We considered this issue with sensitivity analysis and found that such unmeasured confounding would need to be somewhat severe to render our results statistically nonsignificant. Second, as noted earlier, we cannot identify a causal pathway between bankruptcy and early mortality given the limitations of our data set. Finally, although we did not have specific information on previous financial status, other variables such as age, sex, race, marital status, ZIP code–level income, and treatment received served as appropriate proxies for financial status.
Our results highlight the need for future studies that identify causal factors linking bankruptcy and excess mortality for patients with cancer. The following are possible explanations for this association: patients with cancer who filed for bankruptcy were less likely to complete or have access to follow-up treatment, patients with advanced-stage diagnoses filed for bankruptcy to protect their assets for heirs, and the primary motivator for bankruptcy was to reduce or eliminate collection activity. Failure to complete appropriate treatment could also be the result of refusal of prebankruptcy health care providers to continue providing care after the discharge of debts owed for prebankruptcy care. On the basis of our analysis, it seems unlikely that patients with terminal disease filed for bankruptcy to preserve their assets for their heirs or to alleviate their heirs from collection activities they knew would be forthcoming if they did not file for bankruptcy. When we excluded patients with advanced-stage diagnoses, the bankruptcy rate for the remaining patients was unchanged, suggesting that those with advanced-stage diagnoses did not file for bankruptcy at a higher rate than the remaining patients. Second, unless there is significant equity in a home to protect through filing for bankruptcy (the Washington State homestead exemption is $125,000), a debtor is typically left with only minimal exempt assets after filing for bankruptcy, leaving little for heirs. Thus, only the reduction in collection activity would remain as a possible factor, and for some patients, this could be a strong stress-relieving motivator.
Our results may have important policy implications. The impacts of financial insolvency on mortality observed for this study are similar to or exceed observed socioeconomic disparities in survival outcomes.17-19 If the risk of such severe financial distress after a cancer diagnosis can be reduced through intervention, it may confer an important benefit for the individuals who would otherwise face this problem. Bankruptcy has been shown to be more than 2.5 times more common in patients with cancer compared with those without cancer.1 In addition, because bankruptcy represents the extreme end of a spectrum of financial hardship, it is possible that levels of financial difficulty short of bankruptcy could also influence survival. Previous studies have shown an association between high out-of-pocket costs and nonadherence to chemotherapy, which may represent an early point on the trajectory toward bankruptcy and early mortality at which intervention could take place.20-22 Future studies that include information on the financial and insurance status of patients at the time of diagnosis and throughout their treatment will be needed to fully understand the relationship among cancer, financial difficulties, and bankruptcy. Also important is the impact of cancer on the patient’s ability to remain employed, because most health insurance is obtained through the workplace. These factors are particularly important in younger working-age populations in which employment, income, insurance status, and personal assets vary greatly. The new Affordable Care Act has given many more persons access to health insurance and thus may moderate the differences we observed in this study.
Because financial distress appears to have a significant negative impact on health outcomes, we believe that cancer care facilities and oncology practitioners may need to consider the financial health of their patients as a matter of course simultaneously with the initiation of therapy. Strategies that ensure access to and completion of recommended therapies should be an integral part of cancer care. Our results underscore the importance of considering the recommendation for and use of services that have limited evidence of substantial benefit and potential high out-of-pocket costs. Finally, the rapid rise in the cost of individual cancer therapies is raising out-of-pocket costs for patients with cancer, even in the face of expanding insurance coverage. Policies aimed at reducing financial exposure of patients, such as caps on patient out-of-pocket costs, expanding access to patient assistance programs, or limits on rate of rise in the price of treatments may be necessary to mitigate the negative health consequences that stem from the rapidly rising cost of cancer care.
Acknowledgment
We thank Jeffrey Jarvik, MD, MPH, for his conceptual contributions.
Footnotes
Listen to the podcast by Dr Zafar at www.jco.org/podcasts
Supported by Grant No. RC1004135 from the National Center on Minority Health and Health Disparities, National Institutes of Health.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Scott D. Ramsey, Karen A. Overstreet, Veena Shankaran, Polly Newcomb
Collection and assembly of data: Aasthaa Bansal, Catherine R. Fedorenko, David K. Blough
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Financial insolvency as a risk factor for early mortality among patients with cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Scott D. Ramsey
No relationship to disclose
Aasthaa Bansal
No relationship to disclose
Catherine R. Fedorenko
No relationship to disclose
David K. Blough
No relationship to disclose
Karen A. Overstreet
No relationship to disclose
Veena Shankaran
No relationship to disclose
Polly Newcomb
No relationship to disclose
REFERENCES
- 1.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18:381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ubel PA, Abernethy AP, Zafar SY. Full disclosure: Out-of-pocket costs as side effects. N Engl J Med. 2013;369:1484–1486. doi: 10.1056/NEJMp1306826. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 5.Rosenbaum PR. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 6.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolkewitz M, Allignol A, Schumacher M, et al. Two pitfalls in survival analyses of time-dependent exposure: A case study in a cohort of Oscar nominees. Am Stat. 2010;64:205–211. [Google Scholar]
- 8.Lu B. Propensity score matching with time-dependent covariates. Biometrics. 2005;61:721–728. doi: 10.1111/j.1541-0420.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 9. Rosenbaum P: Observational Studies. New York, NY, Springer Science+Business Media, 2002 doi: 10.1007/978-1-4757-3692-2. [Google Scholar]
- 10.Shankaran V, Jolly S, Blough D, et al. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. J Clin Oncol. 2012;30:1608–1614. doi: 10.1200/JCO.2011.37.9511. [DOI] [PubMed] [Google Scholar]
- 11.Tucker-Seeley RD, Li Y, Subramanian SV, et al. Financial hardship and mortality among older adults using the 1996-2004 Health and Retirement Study. Ann Epidemiol. 2009;19:850–857. doi: 10.1016/j.annepidem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahnquist J, Fredlund P, Wamala SP. Is cumulative exposure to economic hardships more hazardous to women’s health than men’s? A 16-year follow-up study of the Swedish Survey of Living Conditions. J Epidemiol Community Health. 2007;61:331–336. doi: 10.1136/jech.2006.049395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrie JE, Martikainen P, Shipley MJ, et al. Self-reported economic difficulties and coronary events in men: Evidence from the Whitehall II study. Int J Epidemiol. 2005;34:640–648. doi: 10.1093/ije/dyi063. [DOI] [PubMed] [Google Scholar]
- 14.Georgiades A, Janszky I, Blom M, et al. Financial strain predicts recurrent events among women with coronary artery disease. Int J Cardiol. 2009;135:175–183. doi: 10.1016/j.ijcard.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 15.Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med. 1997;337:1889–1895. doi: 10.1056/NEJM199712253372606. [DOI] [PubMed] [Google Scholar]
- 16.Rosengren A, Hawken S, Ounpuu S, et al. INTERHEART investigators Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 17.Byers TE, Wolf HJ, Bauer KR, et al. Patterns of Care Study Group The impact of socioeconomic status on survival after cancer in the United States: Findings from the National Program of Cancer Registries Patterns of Care Study. Cancer. 2008;113:582–591. doi: 10.1002/cncr.23567. [DOI] [PubMed] [Google Scholar]
- 18.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 19.Pulte D, Redaniel MT, Brenner H, et al. Changes in survival by ethnicity of patients with cancer between 1992-1996 and 2002-2006: Is the discrepancy decreasing? Ann Oncol. 2012;23:2428–2434. doi: 10.1093/annonc/mds023. [DOI] [PubMed] [Google Scholar]
- 20.Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]
- 21.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaisaeng N, Harpe SE, Carroll NV. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm. 2014;20:669–675. doi: 10.18553/jmcp.2014.20.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]