Abstract
Purpose
Patients treated with cranial radiation therapy (RT) are at risk for sensorineural hearing loss (SNHL). Although SNHL is often characterized as a delayed consequence of anticancer therapy, longitudinal reports of SNHL in childhood cancer survivors treated with contemporary RT are limited. We report the incidence, onset, severity, and long-term trajectory of SNHL among children receiving RT. Potential risk factors for SNHL were also identified.
Patients and Methods
Serial audiologic testing was conducted on 235 pediatric patients who were treated with conformal or intensity-modulated RT as part of an institutional phase II trial for localized primary brain tumors, including craniopharyngioma, ependymoma, and juvenile pilocytic astrocytoma. All but one patient had measurable cochlear radiation dose (CRD) greater than 0 Gy. The median follow-up from RT initiation to latest audiogram was 9 years with a median of 11 post-RT audiograms per patient. Audiograms were classified by the Chang Ototoxicity Grading Scale. Progression was defined by an increase in Chang grade from SNHL onset to the most recent evaluation.
Results
At last evaluation, SNHL was prevalent in 14% of patients: 2.1% had mild and 11.9% had significant SNHL requiring hearing aids. Median time from RT to SNHL onset was 3.6 years (range, 0.4 to 13.2 years). Among 29 patients with follow-up evaluations after SNHL onset, 65.5% experienced continued decline in hearing sensitivity in either ear and 34.5% had no change. Younger age at RT initiation (hazard ratio [HR], 2.32; 95% CI, 1.21 to 4.46), higher CRD (HR, 1.07; 95% CI, 1.03 to 1.11), and cerebrospinal fluid shunting (HR, 2.02; 95% CI, 1.07 to 3.78) were associated with SNHL.
Conclusion
SNHL is a late effect of RT that likely worsens over time. Long-term audiologic follow-up for a minimum of 10 years post-RT is recommended.
INTRODUCTION
Pediatric brain tumors are treated by surgical resection, chemotherapy, and/or radiation therapy (RT). Although contemporary multimodality therapy is typically effective, sensorineural hearing loss (SNHL) is a common adverse effect. Cranial RT is less ototoxic than platinum-based chemotherapy (i.e., cisplatin), but is still associated with high risk for SNHL,1-3 and even more so when combined with cisplatin.4,5 Risk and severity of SNHL increases when a higher radiation dose is delivered to the temporal bone, where otologic structures reside.1-3,6 Damage to any part of the auditory mechanism can cause hearing loss,7 and the reported incidence of RT-induced SNHL varies (from 0% to 54%) across studies.8
RT-induced histopathologic changes to the inner ear cause SNHL. In animal models and humans, RT-induced damage to the cochlear hair cells and supporting cells,9,10 vascular degeneration,11 and deterioration of the basilar membrane, spiral ligament, stria vascularis,10 spiral ganglion, and cochlear nerve9,10 have been reported. RT-induced SNHL typically manifests several years after RT,1,3,4,12 preferentially affects higher frequencies,3,12,13 and can be progressive.4 Damage to the auditory nerve and central pathways has also been reported.14
Risk for SNHL in adults receiving RT is well documented,5,7,8,15 whereas reports in pediatric patients or childhood cancer survivors are limited. The objectives of this study were to report the incidence, onset, and severity of SNHL; document the trajectory of SNHL progression; and identify potential risk factors associated with RT-induced SNHL in children.
PATIENTS AND METHODS
Patients
Eligible patients included 361 children enrolled in a phase II trial of conformal RT for localized brain tumors from 1997 through 2010 who received prospective longitudinal audiologic evaluations. Patients received no RT before enrollment. The trial was approved by the Human Subjects Institutional Review Board at St Jude Children’s Research Hospital, and informed consent was obtained from all patients/guardians. Patients were treated with photons, using forward-planned conformal RT or inversely planned intensity-modulated RT. Target volumes for RT varied according to tumor type. During this study, the gross tumor volume included the residual tumor or postoperative tumor bed; the clinical target volume margin included subclinical microscopic disease and was anatomically confined, varying from 10 to 5 mm beyond the gross tumor volume. The planning target volume margin varied from 5 to 3 mm and included a geometric expansion surrounding the clinical target volume. The prescribed dose to the planning target volume was 54 Gy (craniopharyngioma and low-grade glioma) or 54 to 59.4 Gy (ependymoma). Fractionation was 1.8 Gy/day administered in five fractions per week. Each cochlea was contoured within the temporal bone on computed tomography images without additional margin (Appendix Fig A1, online only). Because of the small size, mean dose was calculated to represent the cochlear radiation dose (CRD). During treatment planning, efforts were made to spare the cochlea without jeopardizing tumor coverage. Patients with the following conditions were excluded from this analysis: exposure to platinum-based chemotherapy (n = 55), permanent SNHL in at least one ear prior to RT (n = 36), permanent conductive hearing loss (n = 4), and insufficient audiologic data (i.e., fewer than two evaluations or incomplete testing (n = 31).
Audiologic Methods
Procedures for evaluating hearing varied based on patient age, health status, cognitive and developmental abilities, and cooperation. Otoscopy and tympanometry were used to determine the condition of the external ear canal, tympanic membrane, and middle-ear space. Pure-tone air-conduction thresholds were evaluated in a sound-treated booth at 0.25, 0.5, 1, 2, 3, 4, 6, and 8 kHz in decibel hearing level (dBHL) for most patients. Pure-tone bone-conduction thresholds were assessed at 0.25, 0.5, 1, 2, 3, and 4 kHz dBHL to establish type of hearing loss (i.e., conductive, sensorineural, or mixed). Although behavioral audiometric testing in a sound-treated booth is the gold standard, assessments that did not require active participation were necessary for some patients: tone-burst auditory brainstem response (ABR), auditory steady-state response (ASSR), and/or distortion-product otoacoustic emissions. These tests were used in patients who were young, had poor health, cognitive or developmental delay, or lack of cooperation.
Patients completed hearing assessments pre-RT (baseline), every 6 months for 5 years post-RT, and annually thereafter for at least 5 years. Audiologic testing was performed by a certified, licensed audiologist. Audiometric data were assigned a grade based on the Chang Ototoxicity Grading Scale16 (Table 1), which uses absolute-hearing threshold levels highly correlated with recommendations for audiologic intervention (i.e., hearing aids, personal frequency-modulation systems). Grading was based on bone-conduction thresholds or air-conduction thresholds with a normal tympanogram. SNHL was defined as Chang grade 1a or higher. Progressive SNHL was defined as any increase in Chang grade in either ear from SNHL onset to latest evaluation.
Table 1.
Severity of Sensorineural Hearing Loss at Onset and at Last Evaluation by the Chang Ototoxicity Grading Scale (N = 235)
| Grade | Criteria | Onset, No. (%) | Last Evaluation, No. (%) |
|---|---|---|---|
| 0 | ≤ 20 dB at 1, 2, and 4 kHz | 202 (86.0) | 202 (86.0) |
| 1a | ≥ 40 dB at 6-12 kHz | 10 (4.3) | 3 (1.3) |
| 1b | > 20 and < 40 dB at 4 kHz | 2 (0.9) | 1 (0.4) |
| 2a | ≥ 40 dB at ≥ 4 kHz | 3 (1.3) | 1 (0.4) |
| 2b | > 20 and < 40 dB at < 4 kHz | 12 (5.1) | 9 (3.8) |
| 3 | ≥ 40 dB at ≥ 2 kHz | 3 (1.3) | 6 (2.6) |
| 4 | ≥ 40 dB at ≥ 1 kHz | 3 (1.3) | 13 (5.5) |
NOTE. Sensorineural hearing threshold in decibel (dB) was assessed by bone conduction or air conduction with normal tympanogram. The worse ear was used when a patient had an asymmetric Chang grade.
Statistical Analysis
Summary statistics were obtained to describe patient characteristics. The Wilcoxon-Mann-Whitney test was used to examine if age at RT initiation differed between patients with SNHL and those with normal hearing. Spearman rank correlations were used to evaluate associations between age at RT initiation and the CRD to each ear. The Fisher’s exact test was used to investigate the association between age (< 3 years v ≥ 3 years) and diagnosis (infratentorial ependymoma versus others). A paired t test was used to examine the difference in CRDs between ears.
Multivariable logistic regression with repeated measures was used to investigate potential risk factors for SNHL. Kaplan-Meier methods were used to describe time to SNHL and time to progression after SNHL diagnosis. Cox proportional hazards models with repeated measures were used to investigate potential risk factors associated with time to SNHL. A backward selection approach was used to identify final models. Potential explanatory variables included age at RT initiation (< 3 years v ≥ 3 years), CRD (Gy), sex, the presence of a CSF shunt, number of surgeries (≤ 1 v > 1), and tumor location (supratentorial versus infratentorial). A significance level of 0.05 was used throughout without adjusting for multiplicity. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2 (R Core Development Team; http://www.r-project.org)
RESULTS
Patient Characteristics
Among 235 evaluable patients, the most common diagnoses were ependymoma, craniopharyngioma, and juvenile pilocytic astrocytoma (Table 2). All but one patient had a measurable CRD (> 0 Gy) in at least one ear. One patient received no CRD to either cochlea and had normal hearing. Median age at RT initiation was 7.2 years (range, 1.0 to 24.4 years), and median CRDs to the left and right ears were 29.5 Gy (range, 0.0 to 61.7 Gy) and 28.8 Gy (range, 0.0 to 63.9 Gy), respectively. The median follow-up from RT initiation to latest audiogram was 9 years (range, 0.8 to 16.0 years), with a median of 11 post-RT audiograms per patient (range, 1 to 19). Of the 235 evaluable patients, 49 (21%) received initial ABR/ASSR evaluations with subsequent conventional audiometric evaluations; 1 (0.4%) had distortion-product otoacoustic emissions performed at baseline with subsequent conventional audiometric evaluations, and 2 (0.9%) received an ABR/ASSR evaluation at baseline and subsequent follow-up.
Table 2.
Patient Characteristics (N = 235)
| Characteristic | No. (%) | Median | Range | Interquartile Range |
|---|---|---|---|---|
| Sex | ||||
| Male | 119 (50.6) | |||
| Female | 116 (49.4) | |||
| Race | ||||
| White | 194 (82.6) | |||
| Nonwhite | 41 (17.4) | |||
| Age at RT initiation (years) | 7.2 | 1.0-24.4 | 3.9-12.3 | |
| < 3 | 43 (18.3) | |||
| ≥ 3 | 192 (81.7) | |||
| Age at latest audiogram (years) | 17.0 | 2.1-36.3 | 12.6-21.1 | |
| Time from RT initiation to latest audiogram (years) | 9.0 | 0.8-16.0 | 6.0-11.1 | |
| Post-RT audiograms, No. | 11 | 1-19 | 7-14 | |
| Shunt status | ||||
| Yes | 76 (32.3) | |||
| No | 159 (67.7) | |||
| Surgeries, No. | ||||
| ≤ 1 | 157 (66.8) | |||
| > 1 | 78 (33.2) | |||
| CRD (Gy) | ||||
| Left ear | 29.5 | 0.0-61.7 | 17.0-44.7 | |
| Right ear | 28.8 | 0.0-63.9 | 15.8-42.9 | |
| Tumor location | ||||
| Infratentorial | 86 (36.6) | |||
| Supratentorial | 149 (63.4) | |||
| Histologic diagnosis | ||||
| Ependymoma | 92 (39.1) | |||
| Craniopharyngioma | 73 (31.1) | |||
| Juvenile pilocytic astrocytoma | 37 (15.7) | |||
| Anaplastic astrocytoma | 7 (3.0) | |||
| Optic pathway glioma | 5 (2.1) | |||
| Low-grade astrocytoma | 3 (1.3) | |||
| Ganglioglioma | 3 (1.3) | |||
| Oligodendroglioma | 3 (1.3) | |||
| Glioblastoma multiforme | 2 (0.9) | |||
| Other astroglial tumors* | 10 (4.1) |
Abbreviations: CRD, cochlear radiation dose; RT, radiation therapy.
World Health Organization I & II, central neurocytoma, choroid plexus carcinoma, malignant glial neuronal, malignant neurocytoma, neurocytoma, pilomyxoid astrocytoma, and pleomorphic xanthroastrocytoma.
SNHL Prevalence and Severity
At last evaluation, 33 patients (14%) had SNHL (four patients had a conductive overlay but were included in the SNHL group based on bone-conduction thresholds). Thirteen had bilateral SNHL, and 20 had unilateral SNHL. Five (2.1%) had mild SNHL (grades 1a to 2a), and 28 (11.9%) had significant SNHL (grade ≥ 2b, requiring hearing aids) at last evaluation (Table 1). All 33 patients received a CRD greater than 0 Gy to both ears.
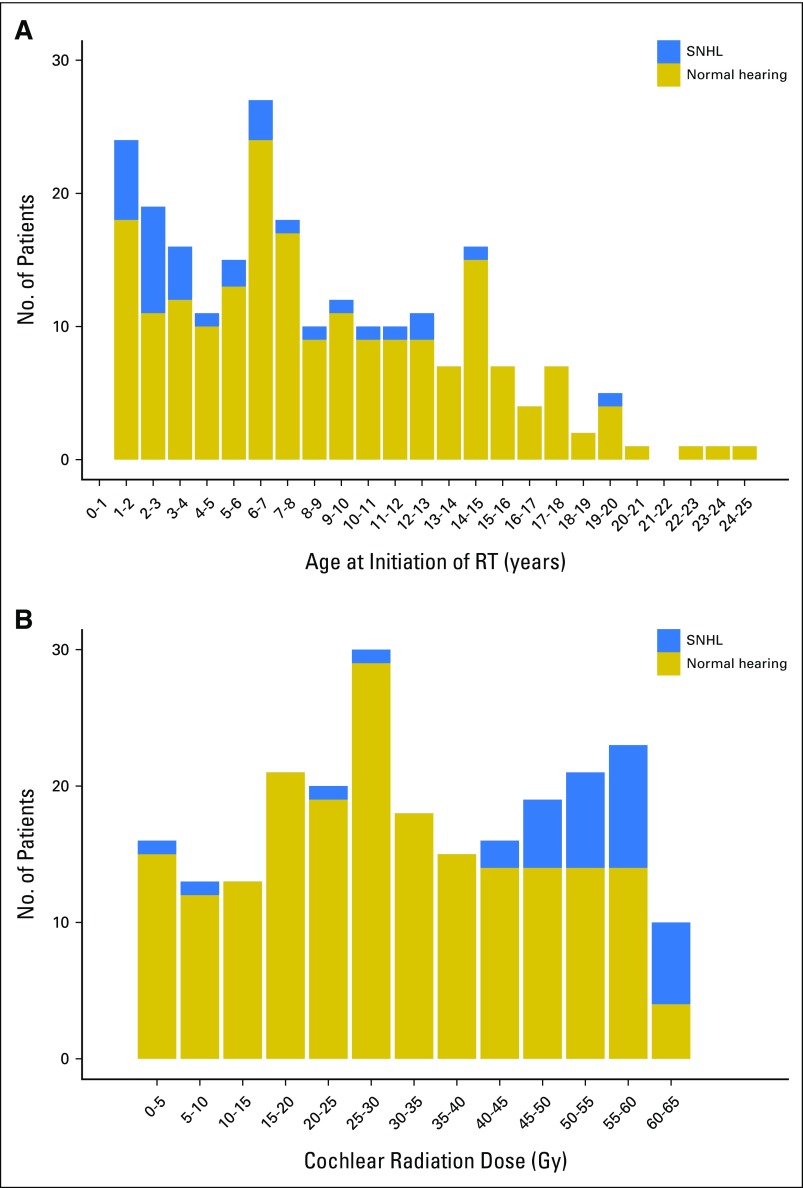
Patients with SNHL received RT at much younger ages than those with normal hearing (median age, 3.2 v 7.8 years; P < .001; Fig 1A). Patients with SNHL received a higher CRD than those with normal hearing (median dose, 54.0 v 29.0 Gy; P < .001; Fig 1B). Age at RT was inversely correlated with CRD to the left and right ears (Spearman r = −0.25 and −0.23, respectively; P < .001), indicating that younger patients received higher CRDs. The majority of younger patients (age < 3 years) had infratentorial ependymoma (81.4%; Fisher’s exact test P < .001). Left and right ear CRDs were correlated (Spearman r = 0.79; P < .001), with no difference between left and right ears (P = .41). Of the 20 patients with unilateral SNHL, 19 had SNHL in the ear that received a higher CRD (Appendix Fig A2).
Fig 1.
(A) Number of patients with SNHL (Chang grade > 0) or normal hearing grouped by age at initiation of RT (years) (N = 235). (B) Number of patients with SNHL (Chang grade > 0) or normal hearing grouped by cochlear radiation dose (Gy), which refers to the higher cochlear dose that a patient received between the left and right ears (N = 235). RT, radiation therapy; SNHL, sensorineural hearing loss.
Risk Factors Associated With RT-Induced SNHL
In a multivariable model, age at RT and CRD were associated with higher odds of SNHL. The odds of developing SNHL were 2.39 times higher for patients younger than 3 years at RT initiation (95% CI, 1.01 to 5.63; P = .05), with every Gy increase in CRD estimated to increase the odds of SNHL by 7% (95% CI, 1.03 to 1.11; P < .001).
SNHL Onset and Progression
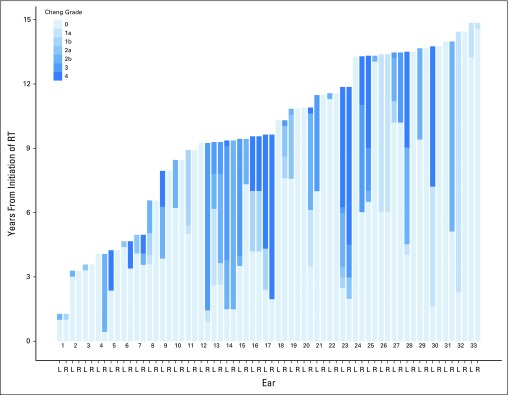
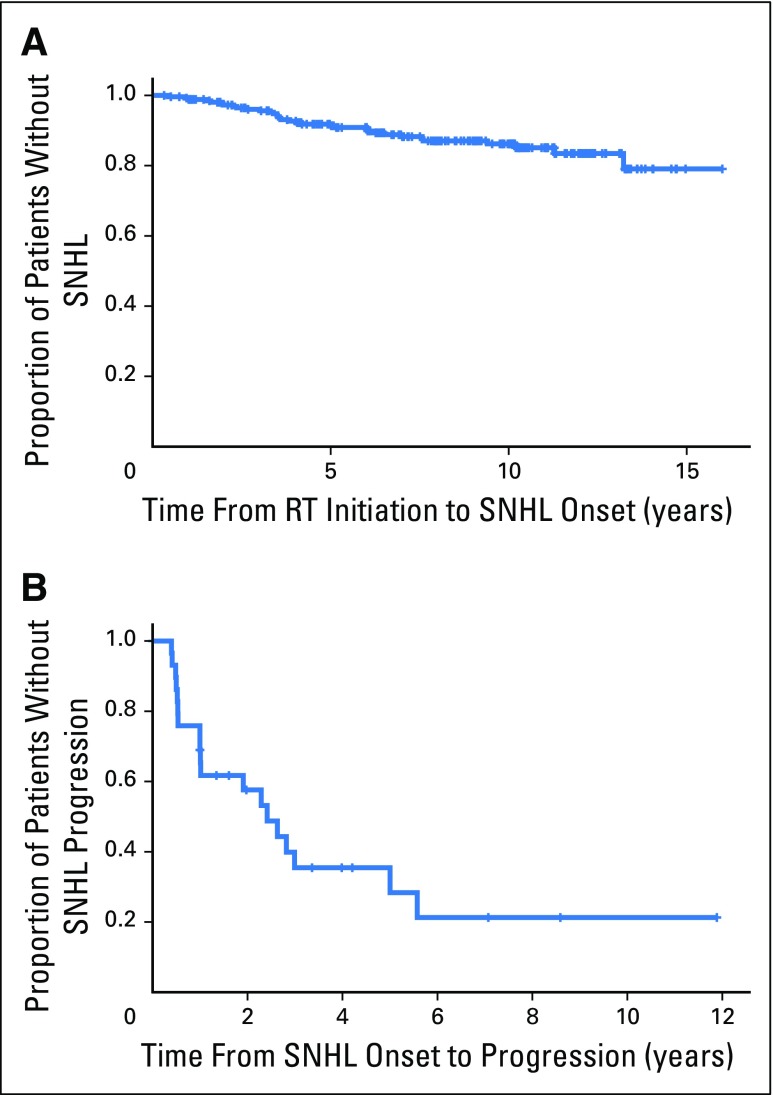
For each patient with SNHL, we calculated the time from RT initiation to SNHL onset (defined as Chang grade > 0 in either ear; Fig 2). For the 202 patients with normal hearing, the time variable was calculated from RT initiation to the latest audiologic evaluation. The median time to SNHL onset was 3.6 years (range, 0.4 to 13.2 years). Two patients experienced SNHL post-RT, one at 11.3 years and the other at 13.2 years. The error (± SE) estimated SNHL-free survival at 5 years post-RT was 91% ± 2.1% and at 10 years was 84% ± 3.7% (Fig 3A). The median follow-up for those who did not experience SNHL was 8.5 years (range, 0.8 to 16 years).
Fig 2.
Overview of time from RT initiation to sensorineural hearing loss (SNHL) onset (Chang grade > 0) and progression (an increase in Chang grade from SNHL onset to the most recent evaluation). All 33 patients with SNHL were sorted by duration of follow-up. Patients with shorter follow-up periods were placed on the left side of the plot, and those with longer follow-up were placed on the right side. For all patients and ears in the plot, an arbitrary 100 days were added to the last evaluation so that the Chang grade at last evaluation can be visualized. L, left; R, right; RT, radiation therapy.
Fig 3.
(A) Kaplan-Meier plot showing the probability of not experiencing SNHL (Chang grade > 0) after exposure to RT (N = 235). (B) Kaplan-Meier plot showing the probability of not experiencing progression of hearing loss after SNHL onset (n = 33). RT, radiation therapy; SNHL, sensorineural hearing loss.
The majority of patients with SNHL (97.9%) participated in a follow-up evaluation after SNHL onset; 19 (65.5%) experienced continued decline in hearing sensitivity, and 10 (34.5%) had no change. For patients with SNHL progression, the median time from SNHL onset to increased Chang grade was 1 year (range, 0.4 to 5.6 years). Hearing loss progressed within 3 years after onset in 17 patients and between 5 and 6 years in two patients. The estimated probability of no progression at 5 years after SNHL onset was 35% ± 11.6% (Fig 3B). Among 15 patients who had mild SNHL at onset, 14 had at least one follow-up evaluation; 10 (71.4%) progressed to significant SNHL requiring hearing aids.
Risk Factors Associated With Time to SNHL Onset
Based on a multivariable Cox model, younger age, higher CRD, and having a CSF shunt were associated with higher risk for SNHL (Table 3). The hazard of SNHL was 2.3 times (95% CI, 1.21 to 4.46 times; P = .01) higher in patients younger than 3 years at RT compared with those who were 3 years or older. Similarly, the hazard of SNHL increased with higher CRD (HR, 1.1; CI, 1.03 to 1.11; P < .001), and the hazard of SNHL in patients with a CSF shunt was 2.0 times higher than were those without a shunt (95% CI, 1.07 to 3.78 times; P = .03).
Table 3.
Univariable and Multivariable Cox Proportional Hazards Models for Time to Sensorineural Hearing Loss Onset
| Variable | Univariable Model | Multivariable Model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Continuous variable | ||||||
| Age at RT (years) | 0.87 | 0.78-0.98 | .0189 | 0.93 | 0.85-1.02 | .1153 |
| CRD (Gy) | 1.08 | 1.04-1.12 | < .001 | 1.07 | 1.04-1.11 | .002 |
| Shunt status (yes) | — | — | 2.00 | 1.06-3.75 | .0321 | |
| Categorical variable | ||||||
| Age at RT (< 3 years) | 3.95 | 1.96-7.93 | .001 | 2.32 | 1.21-4.46 | .0117 |
| CRD (Gy) | — | — | 1.07 | 1.03-1.11 | .003 | |
| Sex (male) | 1.31 | 0.65-2.65 | .4493 | — | — | |
| Shunt status (yes) | 2.27 | 1.13-4.56 | .0207 | 2.02 | 1.07-3.78 | .0290 |
| No. surgeries (> 1) | 1.21 | 0.58-2.52 | .6115 | — | — | |
| Tumor location (infratentorial) | 3.47 | 1.63-7.38 | .0012 | — | — | |
Abbreviations: —, not applicable; CRD, cochlear radiation dose; HR, hazard ratio; RT, radiation therapy.
DISCUSSION
We prospectively and longitudinally examined hearing sensitivity and associated risk factors for SNHL in pediatric patients treated with RT for brain tumors and found that RT is associated with clinically significant SNHL in the absence of ototoxic chemotherapy. Patients younger than 3 years at RT initiation, who have a CSF shunt, and who receive a higher CRD are at greater risk. SNHL typically manifests about 3.5 years post-RT and worsens over time. Because our study included frequent audiograms over long follow-up, we were able to identify delayed onset and progression of SNHL.
High-frequency SNHL can interfere with the acquisition of certain phonemes (primarily fricatives) that are crucial for normal development and comprehension of speech and language,17 especially if SNHL goes undetected and/or untreated. Even mild, atypical, or unilateral hearing loss can impede communication and academic achievement. Children with mild SNHL perform more poorly than their normal-hearing peers, and 37% repeat grades; the normative rate for grade repetition is 3%.18 One study reported that 35% of children with unilateral hearing loss failed at least one grade, and an additional 13% required supplementary educational resources.19 Long-term childhood cancer survivors with SNHL have declines in cognition,20 inferior academic performance,20,21 and overall poorer self-reported quality of life.21 Early detection and treatment of SNHL results in better outcomes for speech and language development, academic achievement, and social well-being.22-24
In the current study, we found 14% of children who received RT and no ototoxic chemotherapy suffered SNHL. Williams et al,12 however, observed a higher cumulative SNHL incidence (27.4%) in a retrospective review of 100 children with brain tumors treated with RT alone. This discrepancy is likely because the two studies used different criteria to define SNHL. In Williams’ study, SNHL was defined as a 20 dBHL or more decrease in either ear at 500, 1,000, or 2,000 Hz on a minimum of three audiograms, including one pre-RT baseline evaluation. Our study used the Chang Ototoxicity Grading Scale16 to calculate the incidence and severity of SNHL because it is based on absolute-hearing thresholds and has shown a strong correlation between ototoxicity grade and intervention/hearing aid recommendation, increasing the relevance and clinical utility of our findings for clinicians who treat these patients. Although the Chang scale was developed to assess platinum-induced ototoxicity, it is also appropriate for radiation-induced ototoxicity, given the emphasis the criteria place on higher frequencies (i.e., higher frequencies are more severely affected by platinum chemotherapy and RT) and particularly because it includes a criterion (grade 2b) that specifies SNHL at any frequency below 4,000 Hz, which captures milder degrees or atypical configurations (i.e., low or mid frequency) of SNHL that would not be captured by other criteria, such as the International Society of Pediatric Oncology ototoxicity grading scale25 (Data Supplement).
Of the 33 patients with SNHL, the majority (n = 28) had significant SNHL (grade ≥ 2b) and required a hearing aid(s). Two other studies of SNHL in children who received RT but not ototoxic chemotherapy reported severe SNHL13 and mild-to-moderate SNHL.3 In both studies, SNHL was more severe for higher frequencies. High-frequency SNHL is typical for patients with post-RT SNHL,3,12,13 which is consistent with our findings. However, atypical SNHL patterns also occur: Nine of our patients had flat SNHL (similar loss across all frequencies); two had tent-shaped SNHL (loss in the low and high frequencies but normal in the mid- to high-frequency range), and two had U-shaped SNHL (loss in the mid-frequency range with better hearing in the low and high frequencies).
The onset of SNHL post-RT varies across studies, occurring as early as 3 months26 and as late as 13 years. The median time to SNHL in our study was 3.6 years. This finding is consistent with two previous studies in children who received RT; SNHL occurred at 18 to 36 months,13 and the mean time to onset was 49 months.12 Late onset of SNHL in children (i.e., after 5 years post-RT) was also documented in previous investigations.3,12
Previous studies of older children and adults receiving chemoradiation have shown progressive SNHL.1,26,27 Likewise, most patients with SNHL in our study experienced declining hearing during the first 3 years after SNHL onset. Nearly 75% of patients with mild SNHL at diagnosis experienced hearing deterioration and eventually required hearing aids.
Increased risk for post-RT SNHL in adults older than 50 years is well documented.1,5,8,28 To our knowledge, the association of age with post-RT SNHL in children has not been previously reported. Nevertheless, a lower mean CRD has been recommended for children (< 35 Gy v ≤ 45 Gy for adults)3,6,28,29 to minimize the risk of SNHL, suggesting that younger patients may be at higher risk for SNHL at RT doses tolerated by adults. In our study, SNHL was twice as likely to occur in patients younger than 3 years. This was not surprising, as young age at the time of platinum-based chemotherapy also increases a child’s risk for SNHL.30-33 Our study also indicated that infratentorial ependymoma occurred more frequently in younger patients and the prescribed tumor dose was higher for ependymoma; thus, younger patients were more likely to receive higher CRDs.
Our study indicates that higher CRD and SNHL are associated, and several authors have suggested a CRD threshold for pediatric patients. Fong et al13 reported delayed, severe SNHL in four children treated with 50 to 54 Gy and no chemotherapy. Merchant et al2 recommended an average CRD of 32 Gy, over 6 weeks, to minimize risk of SNHL in children. In a study of 78 children treated with RT alone, Hua et al3 suggested a CRD threshold of 35 to 45 Gy, with minimal risk of SNHL developing within 5 years post-RT at CRDs less than 35 Gy. A Memorial Sloan-Kettering Cancer Center study34 revealed a 6% rate of significant SNHL in 31 pediatric and adult patients treated with intensity-modulated RT and adjuvant chemotherapy. In that study, a mean CRD of 38.6 ± 3.1 Gy was delivered for patients receiving an 18-Gy dose of craniospinal irradiation (CSI) with a 54-Gy tumor-bed boost, 40.6 ± 4.7 Gy for those receiving a 23-Gy dose of CSI with a 55.8-Gy tumor-bed boost, and 49.1 ± 4.6 Gy for those receiving a 36- or 39.6-Gy dose of CSI with a 55.8-Gy tumor-bed boost; however, relatively short median follow-up (19 months) did not allow detection of late-onset SNHL.
We also found an association between CSF shunting and risk of SNHL post-RT in children. Guillaume et al35 demonstrated an independent association between CSF shunting and SNHL in children receiving treatment of medulloblastoma. Our data support those findings; subjects with a CSF shunt were twice as likely to have RT-induced SNHL. This is not surprising; SNHL is a well-known complication of shunt placement for hydrocephalus and other procedures resulting in loss of CSF.36-39 The etiology of SNHL after shunt placement is not fully understood; however, changes in CSF pressure may alter cochlear physiology.39,40 Excessive CSF drainage via a dilated cochlear aqueduct has been associated with SNHL.40 Because children have a patent cochlear aqueduct, they may be at greater risk for SNHL from shunt placements or other procedures that cause CSF pressure changes.40
Strengths of this study include a large sample size; radiation exposure; prospective design; long-term follow-up; high-quality, standardized treatment; and ototoxicity-monitoring protocols. The limitation of this study is that it included only patients who had adequate audiologic follow-up; SNHL data for patients who did not survive to participate, who had insufficient audiologic evaluations, or who were lost to follow-up are not available and may have differed from that of participants. Also, follow-up periods varied substantially, from 0.8 to 16 years. Some patients with brief follow-up may have had late-onset SNHL that was missed.
Hearing loss is a serious health concern, particularly for children. Thus, children who receive RT need long-term audiologic follow-up to help mitigate the negative consequences of hearing loss. We recommend audiologic follow-up every 6 months for the first 5 years post-RT and then annually thereafter for at least 5 additional years. In addition, prospective trials of advanced RT approaches should include long-term audiologic follow-up to determine whether hearing is preserved by such modalities.
Acknowledgment
We thank Angela McArthur, director of Scientific Editing at St Jude Children’s Research Hospital, for her invaluable assistance in editing this paper and to the patients and families who participated in this study.
Appendix
Fig A1.
Computed tomography image demonstrating how the cochlea was contoured within the temporal bone without additional margin.
Fig A2.
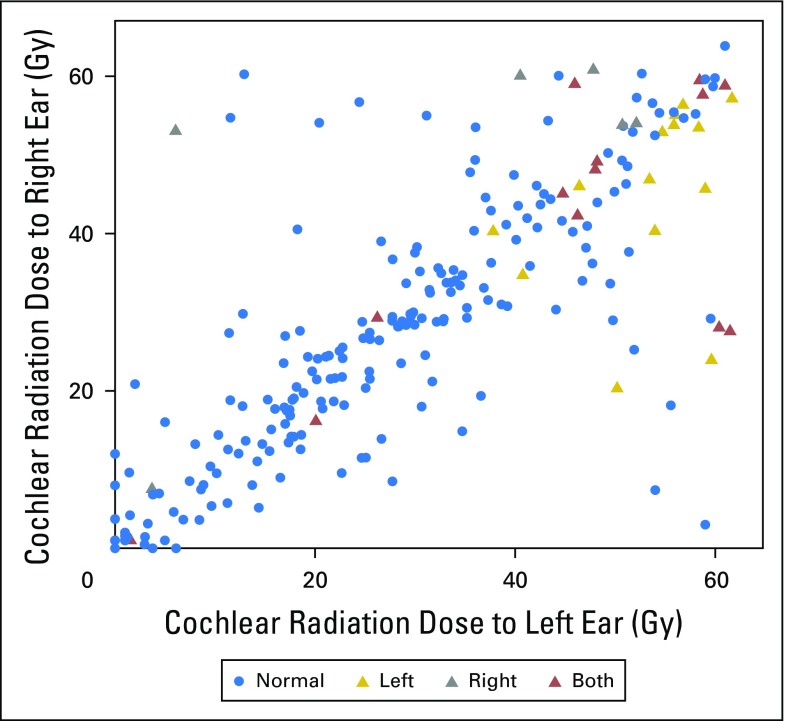
Scatter plot of cochlear radiation dose to the left and right ears of the 235 evaluable patients. Both, patients with bilateral sensorineural hearing loss (SNHL) (Chang grade > 0) (n = 13); left, patients with unilateral SNHL (Chang grade > 0) in left ear (n = 14); normal, patients with normal hearing (n = 202); right, patients with unilateral SNHL (Chang grade > 0) in right ear (n = 6).
Footnotes
Supported in part by research project Grant No. RPG-99-252-01-CCE from the American Cancer Society, and by the American Lebanese Syrian Associated Charities.
Presented in part at the European Symposium on Late Complications after Childhood Cancer, Edinburgh, U.K., September 15–16, 2014, and at the annual meeting of the American Academy of Audiology, San Antonio, TX, March 25–28, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Thomas E. Merchant
Collection and assembly of data: Johnnie K. Bass, Jie Huang, Arzu Onar-Thomas, Skye Jones, Stephanie White, Thomas E. Merchant
Data analysis and interpretation: Johnnie K. Bass, Chia-Ho Hua, Jie Huang, Arzu Onar-Thomas, Kirsten K. Ness, Shaum P. Bhagat, Kay W. Chang, Thomas E. Merchant
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hearing Loss in Patients Who Received Cranial Radiation Therapy for Childhood Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Johnnie K. Bass
No relationship to disclose
Chia-Ho Hua
No relationship to disclose
Jie Huang
No relationship to disclose
Arzu Onar-Thomas
No relationship to disclose
Kirsten K. Ness
No relationship to disclose
Skye Jones
No relationship to disclose
Stephanie White
No relationship to disclose
Shaum P. Bhagat
No relationship to disclose
Kay W. Chang
Consulting or Advisory Role: Otonomy
Thomas E. Merchant
Travel, Accommodations, Expenses: IBA
REFERENCES
- 1.Ho WK, Wei WI, Kwong DL, et al. Long-term sensorineural hearing deficit following radiotherapy in patients suffering from nasopharyngeal carcinoma: A prospective study. Head Neck. 1999;21:547–553. doi: 10.1002/(sici)1097-0347(199909)21:6<547::aid-hed8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Gould CJ, Xiong X, et al. Early neuro-otologic effects of three-dimensional irradiation in children with primary brain tumors. Int J Radiat Oncol Biol Phys. 2004;58:1194–1207. doi: 10.1016/j.ijrobp.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 4.Low WK, Toh ST, Wee J, et al. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: A single, blinded, randomized study. J Clin Oncol. 2006;24:1904–1909. doi: 10.1200/JCO.2005.05.0096. [DOI] [PubMed] [Google Scholar]
- 5.Bhandare N, Antonelli PJ, Morris CG, et al. Ototoxicity after radiotherapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 2007;67:469–479. doi: 10.1016/j.ijrobp.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Bhandare N, Jackson A, Eisbruch A, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76(3) Suppl:S50–S57. doi: 10.1016/j.ijrobp.2009.04.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jereczek-Fossa BA, Zarowski A, Milani F, et al. Radiotherapy-induced ear toxicity. Cancer Treat Rev. 2003;29:417–430. doi: 10.1016/s0305-7372(03)00066-5. [DOI] [PubMed] [Google Scholar]
- 8.Kwong DL, Wei WI, Sham JS, et al. Sensorineural hearing loss in patients treated for nasopharyngeal carcinoma: A prospective study of the effect of radiation and cisplatin treatment. Int J Radiat Oncol Biol Phys. 1996;36:281–289. doi: 10.1016/s0360-3016(96)00302-1. [DOI] [PubMed] [Google Scholar]
- 9.Bohne BA, Marks JE, Glasgow GP. Delayed effects of ionizing radiation on the ear. Laryngoscope. 1985;95:818–828. [PubMed] [Google Scholar]
- 10.Hoistad DL, Ondrey FG, Mutlu C, et al. Histopathology of human temporal bone after cis-platinum, radiation, or both. Otolaryngol Head Neck Surg. 1998;118:825–832. doi: 10.1016/S0194-5998(98)70276-1. [DOI] [PubMed] [Google Scholar]
- 11.Sikand A, Longridge N. CSF otorrhea complicating osteoradionecrosis of the temporal bone. J Otolaryngol. 1991;20:209–211. [PubMed] [Google Scholar]
- 12.Williams GB, Kun LE, Thompson JW, et al. Hearing loss as a late complication of radiotherapy in children with brain tumors. Ann Otol Rhinol Laryngol. 2005;114:328–331. doi: 10.1177/000348940511400413. [DOI] [PubMed] [Google Scholar]
- 13.Fong RS, Beste DJ, Murray KJ. Pediatric sensorineural hearing loss after temporal bone radiation. Am J Otol. 1995;16:793–796. [PubMed] [Google Scholar]
- 14.Gibb AG, Loh KS. The role of radiation in delayed hearing loss in nasopharyngeal carcinoma. J Laryngol Otol. 2000;114:139–144. doi: 10.1258/0022215001904905. [DOI] [PubMed] [Google Scholar]
- 15.Raaijmakers E, Engelen AM. Is sensorineural hearing loss a possible side effect of nasopharyngeal and parotid irradiation? A systematic review of the literature. Radiother Oncol. 2002;65:1–7. doi: 10.1016/s0167-8140(02)00211-6. [DOI] [PubMed] [Google Scholar]
- 16.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 17.Stelmachowicz PG, Pittman AL, Hoover BM, et al. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 18.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Bess FH, Tharpe AM. Unilateral hearing impairment in children. Pediatrics. 1984;74:206–216. [PubMed] [Google Scholar]
- 20.Schreiber JE, Gurney JG, Palmer SL, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro-oncol. 2014;16:1129–1136. doi: 10.1093/neuonc/nou006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurney JG, Tersak JM, Ness KK, et al. Children’s Oncology Group Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children’s Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinaga-Itano C, Sedey AL, Coulter DK, et al. Language of early- and later-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]
- 23.Yoshinaga-Itano C. Benefits of early intervention for children with hearing loss. Otolaryngol Clin North Am. 1999;32:1089–1102. doi: 10.1016/s0030-6665(05)70196-1. [DOI] [PubMed] [Google Scholar]
- 24.Downs MP, Yoshinaga-Itano C. The efficacy of early identification and intervention for children with hearing impairment. Pediatr Clin North Am. 1999;46:79–87. doi: 10.1016/s0031-3955(05)70082-1. [DOI] [PubMed] [Google Scholar]
- 25.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LF, Kuo WR, Ho KY, et al. A long-term study on hearing status in patients with nasopharyngeal carcinoma after radiotherapy. Otol Neurotol. 2004;25:168–173. doi: 10.1097/00129492-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Theunissen EAR, Zuur CL, Bosma SCJ, et al. Long-term hearing loss after chemoradiation in patients with head and neck cancer. Laryngoscope. 2014;124:2720–2725. doi: 10.1002/lary.24802. [DOI] [PubMed] [Google Scholar]
- 28.Pan CC, Eisbruch A, Lee JS, et al. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys. 2005;61:1393–1402. doi: 10.1016/j.ijrobp.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- 30.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: A practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Qaddoumi I, Bass JK, Wu J, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–1041. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurney JG, Bass JK, Onar-Thomas A, et al. Evaluation of amifostine for protection against cisplatin-induced serious hearing loss in children treated for average-risk or high-risk medulloblastoma. Neuro-oncol. 2014;16:848–855. doi: 10.1093/neuonc/not241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polkinghorn WR, Dunkel IJ, Souweidane MM, et al. Disease control and ototoxicity using intensity-modulated radiation therapy tumor-bed boost for medulloblastoma. Int J Radiat Oncol Biol Phys. 2011;81:e15–e20. doi: 10.1016/j.ijrobp.2010.11.081. [DOI] [PubMed] [Google Scholar]
- 35.Guillaume DJ, Knight K, Marquez C, et al. Cerebrospinal fluid shunting and hearing loss in patients treated for medulloblastoma. J Neurosurg Pediatr. 2012;9:421–427. doi: 10.3171/2011.12.PEDS11357. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y, Tomii M, Sawauchi S, et al. A case of hearing loss caused by overdrainage of cerebrospinal fluid after ventriculo-peritoneal shunting procedure[in Japanese] No Shinkei Geka. 1997;25:367–371. [PubMed] [Google Scholar]
- 37.Löppönen H, Sorri M, Serlo W, et al. Audiological findings of shunt-treated hydrocephalus in children. Int J Pediatr Otorhinolaryngol. 1989;18:21–30. doi: 10.1016/0165-5876(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 38.Stoeckli SJ, Böhmer A. Persistent bilateral hearing loss after shunt placement for hydrocephalus: Case report. J Neurosurg. 1999;90:773–775. doi: 10.3171/jns.1999.90.4.0773. [DOI] [PubMed] [Google Scholar]
- 39.van Veelen-Vincent MLC, Delwel EJ, Teeuw R, et al. Analysis of hearing loss after shunt placement in patients with normal-pressure hydrocephalus. J Neurosurg. 2001;95:432–434. doi: 10.3171/jns.2001.95.3.0432. [DOI] [PubMed] [Google Scholar]
- 40.Walsted A, Nielsen OA, Borum P. Hearing loss after neurosurgery: The influence of low cerebrospinal fluid pressure. J Laryngol Otol. 1994;108:637–641. doi: 10.1017/s0022215100127719. [DOI] [PubMed] [Google Scholar]