Abstract
Purpose
Factors associated with early mortality after surgery and treatment with adjuvant chemotherapy in colon cancer are poorly understood. We aimed to characterize the determinants of early mortality in a large cohort of colon cancer trial participants.
Methods
A pooled analysis of 37,568 patients in 25 randomized trials of adjuvant systemic therapy was conducted. Multivariable logistic regression models with several definitions of early mortality (30, 60, and 90 days, and 6 months) were constructed, adjusting for clinically and statistically significant variables. A nomogram for 6-month mortality was developed and validated.
Results
Median age among patients was 61 years, patient demographics included 54% men and 90% White, 29% and 71% had stage II and III disease, respectively, and 79%, 20%, and 1% had an Eastern Cooperative Oncology Group performance status (PS) of 0, 1, and ≥ 2, respectively. Early mortality was low: 0.3% at 30 days, 0.6% at 60 days, 0.8% at 90 days, and 1.4% at 6 months. Of those patients who died by 6 months post–random assignment, 40% had documented disease recurrence prior to death. Early disease recurrence was associated with a markedly increased risk of death during the first 6 months post-treatment (hazard ratio, 82.6; 95%CI, 66.9 to 102.1). In prognostic analyses, advanced age, male sex, poorer PS, increasing ratio of positive to examined lymph nodes, earlier decade of enrollment, and higher tumor stage and grade predicted a greater likelihood of early mortality, whereas treatment received was not strongly predictive. A multivariable model for 6-month mortality showed strong optimism-adjusted discrimination (concordance index, 0.73) and calibration.
Conclusion
Early mortality was infrequent but more prevalent in patients with advanced age and a PS of ≥ 2, underscoring the need to carefully consider the risk-to-benefit ratio when making treatment decisions in these subgroups.
INTRODUCTION
Randomized controlled trials in oncology are the gold standard for developing novel cancer therapeutics, determining their optimal delivery, and evaluating their efficacy. Currently, over 11,000 clinical trials listed by the National Cancer Institute are accepting participants; however, only 2% to 4% of patients with cancer elect to participate in clinical studies.1-3 Low enrollment rates slow the progress of trials and may result in delayed study completion or even study closure.4,5 Likewise, recruitment of patients who are at substantial risk of significant treatment toxicity or early mortality compromises trial integrity, poses greater harm than benefit to participants, and complicates interpretation of trial results.1-3 Thus, the selection of appropriate participants for clinical trials is important, but the process can be challenging.
Recognizing the value of enriching patient selection for phase I trial entry, Olmos et al6 collected data from 2,182 patients who were treated in phase I trials across 14 European institutions. The authors derived eight independent prognostic factors for 90-day mortality with the intention of improving future phase I trial eligibility criteria.6 Additional research groups have devised similar systems, such as the Royal Marsden Hospital score,7,8 to better identify patients for whom prognosis is sufficiently poor and for whom the harms of trial participation outweigh any potential benefits.
Prior analyses exploring this issue were focused on phase I trials or were hindered by interpractice heterogeneity as well as limited sample sizes.9-11 The majority of present-day clinical trials of early-stage and metastatic cancers generally restrict enrollment to patients with an expected life expectancy of at least 3 to 6 months12; however, this criterion can be vague and largely dependent on physician clinical judgement. In many cancers for which disease trajectory and overall survival can be highly variable, an ability to objectively and reliably predict early death could inform both patient selection and future trial design. By using the large Adjuvant Colon Cancer Endpoints (ACCENT) database, our primary aims were to define prognostic factors and to develop a nomogram that can be used to guide risk-to-benefit assessments among patients being considered for phase III adjuvant colon cancer clinical trials.
METHODS
Description of the Database
The ACCENT database contains patient-level information on more than 35,000 patients participating in 25 adjuvant phase III studies since 1977 (Appendix Table A1, online only).13-15 Across these trials, median length of follow-up among surviving patients is 7.5 years. In this analysis, the primary outcome was early mortality, defined as death resulting from any cause by 30 days, 60 days, 90 days, and 6 months post–random assignment. At the 6-month time point, recurrences were also examined to distinguish deaths as a result of recurrent disease from deaths resulting from other causes, such as potential treatment complications.
Statistical Methods
Descriptive statistics for patient characteristics and mortality rates at each time point were summarized. Death rates were described by treatment across all time points and by recurrence status among those patients who died by 6 months. The impact of preceding disease recurrence on the likelihood of 6-month survival was examined by including recurrence as a time-dependent covariate in a Cox proportional hazards regression model that was right-censored at 6 months and adjusted for other patient characteristics.
Baseline patient factors that were evaluated for prognostic associations with early mortality included age, sex, race (white, black, Asian, other), performance status (PS; 0, 1, ≥ 2), disease stage (II and III), body mass index, tumor stage (T1 to T4), tumor grade (1, 2, ≥ 3), lymph node ratio (LNR; ratio of positive lymph nodes to nodes examined), primary tumor location (single left, single right, single transverse/flexures, any multiple), decade randomly assigned (1970s, 1980s, 1990s, 2000s), and treatment (surgery alone, fluorouracil plus leucovorin [5-FU + LV] variations, 5-FU + LV plus oxaliplatin, 5-FU + LV plus irinotecan). Missing baseline data were imputed via multiple imputation; specifically, bootstrapped regression with predictive mean matching was used to maintain a level of variability in the imputed data similar to that which exists in the available data.16
Univariable regression models were fitted to identify significant predictors of early mortality at each time point, for which significance required both P < 0.05 and clinically meaningful effects (odds ratios [OR]). In these models, continuous variables were modeled with restricted cubic splines16 and tested for possible nonlinearity of the effects on the log-odds scale. Where significant nonlinearity was found, effects were plotted on the probability scale with 95% confidence bands for visual inspection of the shape of effect, and spline modeling was subsequently used in multivariable models; otherwise, standard linear modeling was used. Two-way interactions between significant univariable contributors were also tested, for which both P < 0.01 for the interaction effect and clinically differentiable effects across factor levels indicated significance. Multivariable models were constructed from statistically and clinically significant variables and interaction effects for each time point. Variables that no longer contributed statistically or clinically meaningful effects in multivariable models were excluded. Disease recurrence was also excluded from consideration in these prognostic models, as recurrence status is unknown at the time adjuvant treatment decisions are made.
A nomogram was constructed from the final multivariable model for mortality by 6 months. As measures of internal calibration, the concordance index, which is equivalent to the area under the receiver-operator characteristic curve, and a nonparametric smoothed calibration plot of actual versus predicted outcomes were reported.17 External validation of the nomogram was performed using data from 3,227 patients enrolled in the clinical trial N0147,18 for which average nomogram-predicted and actual early mortality rates were examined, both overall and within patient subgroups. In both univariable and multivariable models, statistically significant categorical effects, standard errors, and ORs were computed. All analyses were performed using R software (The R Project for Statistical Computing; The R Foundation, Vienna, Austria).5
RESULTS
Descriptive Statistics
A total of 37,568 patients from 25 ACCENT trials were analyzed. Baseline patient demographics and disease characteristics are summarized in Table 1. Early mortality rates were 0.3% at 30 days, 0.6% at 60 days, 0.8% at 90 days, and 1.4% at 6 months. The proportions of patients alive and dead at each time point are presented by treatment in Table 2. Patients treated with surgery alone exhibited the highest early mortality rates over time, from 0.6% at 30 days to 2.0% by 6 months. Adjuvant chemotherapy with 5-FU + LV alone or 5-FU + LV plus irinotecan were associated with mortality rates from 0.3% and 0.4% at 30 days to 1.5% and 1.3% by 6 months, respectively. The lowest early mortality rates across time points occurred among patients treated with 5-FU + LV plus oxaliplatin and ranged from 0.2% at 30 days to 1.2% at 6 months.
Table 1.
Demographics and Disease Characteristics of Patients Used for the Early Mortality Analyses
| Variable | Value |
|---|---|
| Age, years | |
| Mean (SD) | 50 (11) |
| Median (IQR) | 61 (53-68) |
| Sex, No. (%) | |
| Male | 20,389 (54) |
| Female | 17,179 (46) |
| Race, No. (%) | |
| White | 28,679 (90) |
| Black | 1,785 (6) |
| Asian | 601 (2) |
| Other | 633 (2) |
| Missing | 5,870 (16) |
| Performance status, No. (%) | |
| 0 | 25,108 (79) |
| 1 | 6,275 (20) |
| ≥ 2 | 300 (1) |
| Missing | 5,179 (14) |
| Body mass index | |
| Mean (SD) | 26 (5) |
| Median (IQR) | 26 (23-29) |
| Missing, No. (%) | 7,786 (21) |
| Stage, No. (%) | |
| II | 10,780 (29) |
| III | 26,788 (71) |
| Tumor stage | |
| T1 | 803 (2) |
| T2 | 3,518 (11) |
| T3 | 24,603 (76) |
| T4 | 3,465 (11) |
| Missing | 5,179 (14) |
| Tumor grade | |
| 1 | 2,915 (14) |
| 2 | 14,339 (67) |
| ≥ 3 | 4,048 (19) |
| Missing | 16,266 (43) |
| Node ratio | |
| Mean (SD) | 0.21 (0.26) |
| Median (IQR) | 0.12 (0.00-0.33) |
| Missing, No. (%) | 9,420 (25) |
| Decade enrolled, No. (%) | |
| 1970s-1980s | 5,669 (15) |
| 1990s | 17,940 (48) |
| 2000s | 13,955 (37) |
| Missing | 4 (0) |
| Treatment, No. (%) | |
| Surgery | 2,362 (6) |
| 5-FU + LV | 27,121 (72) |
| 5-FU + LV plus irinotecan | 2,203 (6) |
| 5-FU + LV plus oxaliplatin | 5,882 (16) |
| Death by 30 days, No. (%) | |
| Yes | 109 (0.3) |
| No | 37,356 (99.7) |
| Missing | 103 (0.3) |
| Death by 60 days, No. (%) | |
| Yes | 215 (0.6) |
| No | 37,223 (99.4) |
| Missing | 130 (0.3) |
| Death by 90 days, No. (%) | |
| Yes | 281 (0.8) |
| No | 37,133 (99.2) |
| Missing | 154 (0.4) |
| Death by 6 months, No. (%) | |
| Yes | 540 (1.4) |
| No | 36,825 (98.6) |
| Missing | 203 (0.5) |
| Recurrence by 30 days, No. (%) | |
| Yes | 113 (0.3) |
| No | 37,086 (99.7) |
| Missing | 369 (1.0) |
| Recurrence by 60 days, No. (%) | |
| Yes | 226 (0.6) |
| No | 36,877 (99.4) |
| Missing | 465 (1.2) |
| Recurrence by 90 days, No. (%) | |
| Yes | 384 (1.0) |
| No | 36,651 (99.0) |
| Missing | 533 (1.4) |
| Recurrence by 6 months, No. (%) | |
| Yes | 1,232 (3.3) |
| No | 35,637 (96.7) |
| Missing | 699 (1.9) |
Abbreviations: 5-FU + LV, fluorouracil plus leucovorin; IQR, interquartile range; SD, standard deviation.
Table 2.
Number of Deaths and Survivors and Death Rate at Each Time Point by Treatment
| Variable | 30 Days | 60 Days | 90 Days | 6 Months |
|---|---|---|---|---|
| No. of deaths | ||||
| Overall | 109 | 215 | 281 | 540 |
| Surgery | 13 | 20 | 23 | 46 |
| 5-FU + LV | 76 | 149 | 200 | 393 |
| 5-FU + LV plus irinotecan | 9 | 16 | 17 | 29 |
| 5-FU + LV plus oxaliplatin | 11 | 30 | 41 | 72 |
| No. of survivors | ||||
| Overall | 37,356 | 37,223 | 37,133 | 36,825 |
| Surgery | 2,347 | 2,340 | 2,336 | 2,313 |
| 5-FU + LV | 26,973 | 26,887 | 26,826 | 26,611 |
| 5-FU + LV plus irinotecan | 2,193 | 2,182 | 2,181 | 2,169 |
| 5-FU + LV plus oxaliplatin | 5,843 | 5,814 | 5,790 | 5,732 |
| Death rate, % | ||||
| Overall | 0.29 | 0.57 | 0.75 | 1.4 |
| Surgery | 0.55 | 0.85 | 0.98 | 2.0 |
| 5-FU + LV | 0.28 | 0.55 | 0.74 | 1.5 |
| 5-FU + LV plus irinotecan | 0.41 | 0.73 | 0.77 | 1.3 |
| 5-FU + LV plus oxaliplatin | 0.19 | 0.51 | 0.70 | 1.2 |
Abbreviation: 5-FU + LV, fluorouracil plus leucovorin.
Impact of Recurrence on Mortality
Among patients who died by 6 months post–random assignment, 40.1% had documented disease recurrence prior to death, 50.2% died without recurrence, and 9.3% had insufficient information to analyze recurrence status at the time of death. These rates differed according to stage of disease. Among patients with stage II disease who died by 6 months, 22.3% had disease recurrence prior to death, 74.1% died without disease, and 3.6% had insufficient recurrence information at the time of death. In contrast, among patients with stage III disease who died by 6 months, 44.6% had disease recurrence prior to death, 43.9% did not have recurrence, and 11.4% had inadequate information on recurrence. Of note, early recurrence was associated with an 82-fold increased risk of death during the first 6 months post-treatment in a Cox proportional hazards regression model that was adjusted for patient age, PS, grade, LNR, and decade of enrollment (hazard ratio, 82.6; 95% CI, 66.9 to 102.1). A series of two-variable models containing both recurrence and a patient or disease characteristic (eg, recurrence and age) showed statistically unchanged ORs and significance levels for patient factors after adjustment, indicating the relative independence and collective importance of these contributors to patients’ early mortality.
Univariable Analyses
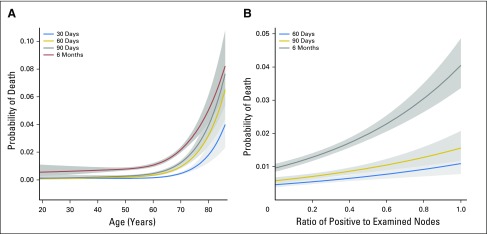
Age was correlated with early mortality at every time point (P < .001), and this association was found to increase with age in a nonlinear fashion (Fig 1A). Another strong predictor of early mortality was PS, where worse PS was highly associated with early mortality at all time points (P < .001 for 30, 60, and 90 days, and 6 months; 6 month PS 1 v PS 0 OR, 1.57; PS ≥ 2 v PS 0 OR, 3.65). Tumor grade was similarly associated, with higher risk of early mortality for higher-grade tumors (30 days P = .002; 60 days P < .001; 90 days P < .001; 6 months P < .001; grade 2 v 1 OR, 1.39; grade ≥ 3 v 1 OR, 3.52). T stage was also correlated with early mortality, although this relationship was not uniform over time and the risk did not increase monotonically (30 days P = .01; 60 days P = .009; 90 days P = .06; 6 months P < .001; T2 v T1 OR, 2.06; T3 v T1 OR, 1.85; T4 and T1 OR, 2.88). Early mortality was also greater in patients with stage III than stage II disease at 60 days (P = .008; OR, 1.57) and remained higher at 90 days (P = .014; OR, 1.43) and at 6 months (P < .001; OR, 1.55). A higher LNR was correlated with higher early mortality at 60 days (P < .001), 90 days (P < .001), and 6 months (P < .001), and this effect was nonlinear on the log-odds scale (Fig 1B). Decade of enrollment was not a significant predictor of early mortality until 6 months (P = .001; 1990s v 1970s to 1980s OR, 0.93; 2000s v 1970s to 1980s OR, 0.67). Male sex was associated with increased mortality at 30 days (P = .038; OR, 1.51), but it was not significantly associated at later time points. Early mortality rates were not associated with treatment, race, body mass index, or the number or location of primary tumors.
Fig 1.
Continuous effects of (A) age at 30, 60, and 90 days, and 6 months; (B) lymph node ratio at 60 and 90 days and 6 months. Shaded gray regions are 95% CIs.
Interaction Analyses
Among all possible two-way variable interactions at each time point, the only statistically significant and clinically meaningful interaction that occurred was between age and tumor grade at 6 months. Specifically, the risk of early mortality increased with both age and grade, with worse tumor grade having a stronger impact on early mortality with advanced age (P = .001).
Multivariable Analyses
The patient characteristics found to contribute both statistically and clinically to early mortality in univariable models, in addition to those deemed to be of sufficient clinical interest despite lack of statistical significance (decade of random assignment and treatment), were carried forward and evaluated in multivariable models for each time point. A two-way interaction between age and grade was further considered at 6 months. Final models retaining statistically and clinically relevant terms are shown in Table 3.
Table 3.
Empirical Probabilities of Early Mortality and Multivariable Model-Based (Adjusted) Factor ORs, 95% CIs, and P Values for Early Mortality at Each Time Point
| Variable | 30 Days | 60 Days | 90 Days | 6 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | OR (95% CI) | P | Rate | OR (95% CI) | P | Rate | OR (95% CI) | P | Rate | OR (95% CI) | P | |
| Age, years | < .001 | < .001 | < .001 | < .001 | ||||||||
| 40 | 0.0011 | 0.71 (0.33 to 1.49) | 0.0006 | 0.43 (0.22 to 0.83) | 0.0009 | 0.43 (0.25 to 0.76) | 0.0042 | 0.65 (0.47 to 0.90) | ||||
| 50 | 0.0011 | 0.72 (0.47 to 1.10) | 0.0016 | 0.59 (0.42 to 0.83) | 0.0022 | 0.60 (0.45 to 0.79) | 0.0074 | 0.74 (0.60 to 0.91) | ||||
| 60 | 0.0015 | — | 0.0040 | — | 0.0054 | — | 0.0128 | — | ||||
| 70 | 0.0046 | 2.58 (1.88 to 3.54) | 0.0099 | 2.52 (2.01 to 3.15) | 0.0128 | 2.46 (2.02 to 2.98) | 0.0218 | 1.87 (1.57 to 2.24) | ||||
| 80 | 0.0178 | 8.61 (5.34 to 13.9) | 0.0251 | 7.58 (5.35 to 10.7) | 0.0309 | 7.17 (5.27 to 9.75) | 0.0374 | 4.05 (3.11 to 5.28) | ||||
| PS | < .001 | < .001 | < .001 | .0015 | ||||||||
| 0 | 0.0020 | — | 0.0044 | — | 0.0061 | — | 0.0127 | — | ||||
| 1 | 0.0058 | 2.33 (1.56 to 3.47) | 0.0098 | 1.78 (1.33 to 2.38) | 0.0114 | 1.47 (1.13 to 1.91) | 0.0198 | 1.30 (1.07 to 1.58) | ||||
| ≥ 2 | 0.0158 | 4.32 (1.82 to 10.3) | 0.0316 | 4.11 (2.22 to 7.61) | 0.0342 | 3.18 (1.77 to 5.72) | 0.0449 | 2.07 (1.24 to 3.47) | ||||
| Grade | .0086 | .0041 | < .001 | < .001 | ||||||||
| 1 | 0.0021 | — | 0.0044 | — | 0.0050 | — | 0.0084 | — | ||||
| 2 | 0.0025 | 1.14 (0.60 to 2.16) | 0.0051 | 1.07 (0.69 to 1.68) | 0.0066 | 1.23 (0.81 to 1.87) | 0.0116 | 1.32 (0.96 to 1.82) | ||||
| ≥ 3 | 0.0050 | 2.10 (1.06 to 4.15) | 0.0092 | 1.75 (1.08 to 2.84) | 0.0127 | 2.14 (1.38 to 3.34) | 0.0289 | 2.85 (2.04 to 3.97) | ||||
| LNR | 1.22 (1.05 to 1.42) | .0099 | 1.27 (1.12 to 1.45) | < .001 | 1.50 (1.37 to 1.64) | < .001 | ||||||
| 0.00 | 0.0046 | 0.0058 | 0.0096 | |||||||||
| 0.50 | 0.0071 | 0.0095 | 0.0198 | |||||||||
| 1.00 | 0.0109 | 0.0156 | 0.0405 | |||||||||
| T stage | .0051 | |||||||||||
| T1 | 0.0074 | — | ||||||||||
| T2 | 0.0251 | 1.87 (0.85 to 4.11) | ||||||||||
| T3 | 0.0136 | 2.11 (0.99 to 4.49) | ||||||||||
| T4 | 0.0211 | 2.93 (1.34 to 6.38) | ||||||||||
| Decade | .0050 | |||||||||||
| < 1990s | 0.0171 | — | ||||||||||
| 1990s | 0.0159 | 0.85 (0.67 to 1.08) | ||||||||||
| 2000s | 0.0115 | 0.66 (0.51 to 0.86) | ||||||||||
NOTE. LNR is modeled as linear. Age is modeled as continuous and nonlinear, but spline-derived ORs versus age 60 are shown for selected ages along the continuum.
Abbreviations: —, reference group; < 1990s, enrolled prior to January 1, 1990; LNR, lymph node ratio; OR, odds ratio; PS, performance status.
In the final model for early mortality at 30 days, only age (P < .001), PS (P < .001), and tumor grade (P = .009) remained after removal of nonsignificant terms, with increased risk for advanced age, worse PS, and higher grade. The final models at 60 and 90 days also included age (both P < .001), PS (both P < .001), and grade (60 days P = .004; 90 days P < .001), but further included LNR (60 days P = .010; 90 days P < .001), where increased LNR corresponded to greater risk of early mortality. The final model for early mortality at 6 months included age (P < .001), PS (P = .002), tumor grade (P < .001), LNR (P < .001), T stage (P = .005), and decade of enrollment (P = .005), where increased T stage and earlier decade of enrollment were associated with higher risk of death. Of note, the two-way interaction between age and tumor grade considered at 6 months was not included in the final model, and treatment group was nonsignificant after adjustment and not significant at any time points.
Nomogram for 6-Month Mortality: Internal Validation
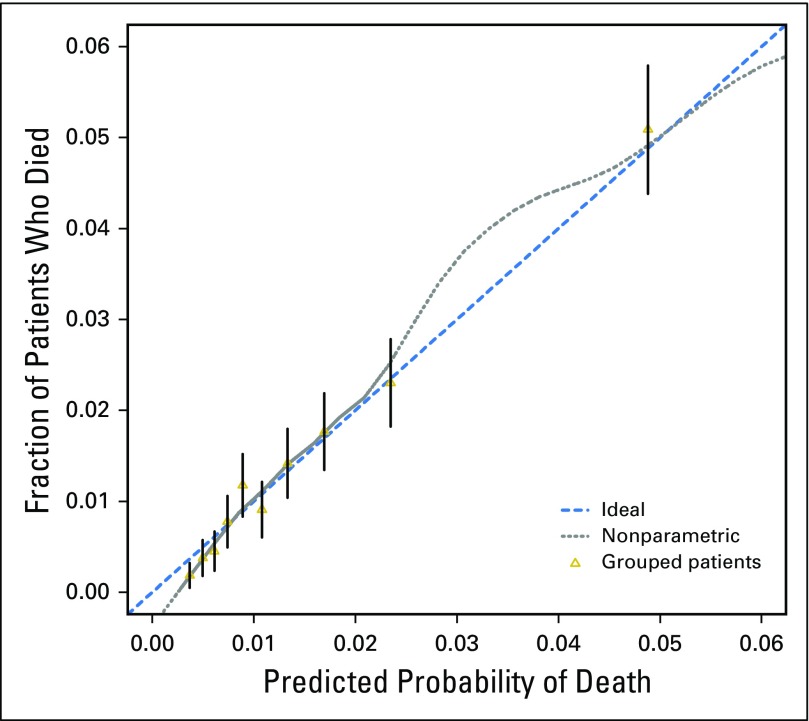
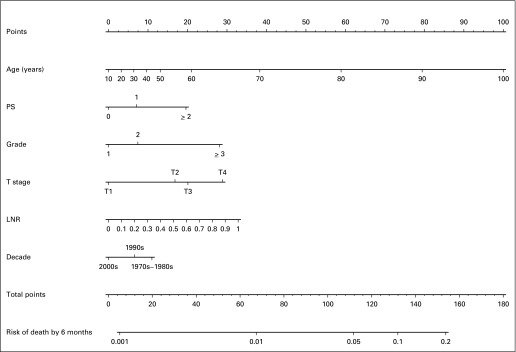
The final multivariable model for mortality at 6 months showed strong internal validity, with a discrimination concordance index of 0.732 indicating a 73.2% correct ordering of risk across pairs of patients, and good calibration of observed versus predicted outcomes as shown in Figure 2. A nomogram representation of the model is provided in Figure 3, where, for a specific patient, the predicted probability of 6-month mortality can be computed (Appendix, online only).
Fig 2.
Calibration of the final model for 6-month mortality.
Fig 3.
Nomogram for 6-month mortality. LNR, lymph node ratio; PS, performance status.
Nomogram for 6-Month Mortality: External Validation With N0147 Patients
Across the 3,227 patients from trial N0147 with fully available nomogram and outcome data, 36 patients (1.1%) died by 6 months, exactly matching the average nomogram-predicted probability of 1.1% for 6-month mortality across patients. Within all subgroups of N0147 patients differentiated by the variables contained in the nomogram, average nomogram-predicted rates of early mortality fell within the 95% CIs for actual early mortality rates, with one exception: among patients with T4 tumors, the average nomogram-predicted early mortality rate was 0.37% higher than the upper 95% CI of the actual rate (Appendix Table A2, online only).
The ability of this model to distinguish between low-risk and high-risk patients can be demonstrated by considering two hypothetical individuals who might be encountered in practice: Patient A is 50 years old with a PS of 0 and an LNR of 0, whereas Patient B is 75 years old with a PS of 1 and an LNR of 0.40. We further assume that both patients have T3 tumors for which they recently received treatment. Our model predicted that Patient A has a 0.37% chance of early death by 6 months (95% CI, 0.30% to 0.47%) whereas Patient B has a 2.9% chance of early death (95% CI, 2.3% to 3.7%); that is, the risk of early mortality for Patient B is predicted to be 7.7 times greater than the risk for Patient A.
DISCUSSION
In this pooled analysis of patient-level data from individuals with colon cancer enrolled in phase III trials of adjuvant systemic therapy, early mortality at various time points within the first 6 months of random assignment were low, ranging from 1.2% to 2.0%, depending on the treatment received. In the subgroup of patients that experienced early death by 6 months, a significant proportion occurred in individuals in whom there was a documented early recurrence. This finding persisted when recurrence was examined as a time-varying factor in the multivariable model for early mortality. For the under-represented subset of study participants that was either elderly or had a poor PS, risk of early death was particularly high. In an effort to facilitate early prognostication, a nomogram was developed and validated both internally and externally.
Our observation that mortality rates were low highlights the fact that current inclusion and exclusion criteria of clinical trials are effective in identifying eligible patients who may potentially benefit from study participation without experiencing excessive risk of serious harm. This can inform the treatment decision-making process, and may be particularly valuable for clinicians who are screening potential study participants and providing reassurance to patients and families who are considering study enrollment. Because trials are costly and resource intensive, this approach to patient selection further optimizes internal validity by minimizing unexpected attrition of patients as a result of toxicities or death that could otherwise compromise trial integrity, prompt slow accrual or study closure, or complicate the subsequent interpretation of results.
Of interest, mortality rates were lowest in the subset of patients that received adjuvant therapy, especially FOLFOX (infusional fluorouracil, leucovorin, and oxaliplatin; 1.2%), and highest in the group that underwent surgery alone without further postoperative therapy (2.0%). Contrary to the general assumption that systemic therapy exerts its effect mainly by reducing late recurrences, this observation suggests that chemotherapy may also prevent early recurrences, even within the first six months of treatment. Our current finding that nearly one half of the observed early deaths were preceded by a documented recurrence supports this hypothesis. Sargent et al19 previously showed that adjuvant therapy significantly reduces the risk of early recurrences. Our observations further underscore the safety and tolerability of modern adjuvant chemotherapy, such as FOLFOX, as evidenced by the fact that the short-term risk of death with adjuvant treatment is lower than with surgery alone or 5-FU therapy alone in this study.
Consistent with prior research, patients with colon cancer who are of advanced age or who have poor PS were under-represented in most contemporary trials of adjuvant systemic therapy that were included in this pooled analysis.20,21 Those patients who enrolled were more likely to experience early mortality, which demonstrated that many of these studies were not designed to accommodate the vulnerabilities of these demographic subgroups. In recent years, clinical trials specific to the elderly and to those with poor functional status have emerged, albeit infrequently. Studies such as AVEX and FOCUS2 limited enrollment to patients with metastatic colorectal cancer who were age 70 years and older and who had an ECOG PS of ≥ 2, respectively, and confirmed that it was feasible and effective to tailor studies to the needs of these under-represented patient populations.22-25 Both of these clinical trials were performed among metastatic patients, so similar efforts are needed in the adjuvant colon cancer setting.
One of the distinguishing features of this study is the development of an easy-to-use, well-calibrated, and internally and externally valid nomogram that allows for the prediction of mortality at 6 months after study randomization. On the basis of results from the multivariable model, this nomogram incorporates parameters that are readily available at baseline. Such a tool can be valuable because patients considering enrollment in clinical trials must frequently consider many factors, including the likelihood of the treatment being effective, the time and cost involved, the potential for other alternatives, and the risk of toxicities or death. A quantitative assessment of the risk of death related to study treatments can be exceedingly difficult, especially when therapies tested in phase III trials are still considered experimental and highly variable. Therefore, clinicians often have little data on which to base their risk-to-benefit discussions with potential clinical trial patients. Although careful and diligent follow-up of study participants is already an established component of most clinical trial protocols, our nomogram can further augment care by identifying individuals for whom increased vigilance may be necessary.
Our findings should be interpreted in the context of several limitations. First, although we were able to classify deaths into those patients with documented cancer recurrences versus those without, we were unable to specifically determine the proportion of deaths attributable to treatment-related toxicities. Second, our findings are applicable only to patients with colon cancer who were enrolled in clinical trials of adjuvant systemic therapy, and the generalizability to other cancers, metastatic disease, and nontrial populations are unclear. However, these limitations should be weighed against the strengths of the study, which include the large cohort size, the inclusion of many clinical trials across different decades, and development of a validated nomogram that could prove useful in future clinical trials.
In summary, early mortality rates were low among patients with colon cancer who participated in adjuvant systemic therapy clinical trials. However, elderly patients and those with poor PS experienced worse early prognosis, lending importance to the emerging number of clinical trials that are specifically designed to represent these subpopulations. A nomogram on the basis of individual patient characteristics may be a potentially useful tool that can quantify the risk of early death and, thus, better inform discussions between clinicians and prospective trial participants as well as facilitate the informed consent process.
Appendix
The ACCENT (Adjuvant Colon Cancer Endpoints) Group consists of: D.J.S., E. Green, A.G., S.R.A., Q. Shi, and L.A.R. (Mayo Clinic, Rochester, MN); G.Y., M.J. O'Connell, and N. Wolmark (National Surgical Adjuvant Breast and Bowel Project Biostatistical and Operations Centers, Pittsburgh, PA); A.d.G. (CTD-INCa GERCOR, Assistance Publique des Hôpitaux de Paris, UPMC Paris VI, Paris, France); R. Gray and D.K. (Quick and Simple and Reliable Collaborative Group, Birmingham and Oxford, United Kingdom); D.G.H. (Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA); K.G. (Southwest Oncology Group Statistical Center, Seattle, WA); M. Buyse (International Drug Development Institute, Louvain-la-Neuve, Belgium); R.L. (Ospedali Riuniti, Bergamo, Italy); J.-F.S. (University of the Mediterranean, Marseilles, France); C.J.O. (National Cancer Institute of Canada Clinical Trials Group, Queens University, Kingston, Ontario, Canada); G.F. (University of Siena, Siena, Italy); P.J. Catalano (Eastern Cooperative Oncology Group Statistical Center, Boston, MA); C.D. Blanke (Oregon Health Sciences University, Portland, OR); T.A. (Hôpital Saint Antoine, Paris, France); R.M. Goldberg (Ohio State University Comprehensive Cancer Center, Columbus, OH); A. Benson (Northwestern University, Chicago, IL); C.T. (University of Bradford, West Yorkshire, United Kingdom); F. Sirzen (Roche, Basel, Switzerland); L. Cisar (Pfizer, New York, NY); E.V.C. (University Hospital Gasthuisberg, Gasthuisberg, Belgium); and L.B.S. (Memorial Sloan Kettering Cancer Center, New York, NY).
Instructions for Use of the Nomogram for 6-Month Mortality
Risk points associated with each variable are first obtained via vertical translation of the variable value of the patient (eg, tumor stage at T3) to the scale labeled Points in the nomogram, that is, a T3 tumor contributes 20 points to the 6-month mortality risk. Next, the points associated with each variable value for the patient are totaled across the variables. This total is then located on the scale Total Points and vertically mapped to obtain the prediction of interest (eg, 112 total points corresponds to a 6-month mortality rate of approximately 5%).
Table A1.
ACCENT Trials Used for Early Mortality Analyses
| Trial | Years | Treatment Arm | No. of Participants |
|---|---|---|---|
| NSABP C01 | 1977-1983 | Surgery alone v MOF | 724 |
| NSABP C02 | 1984-1988 | Surgery alone v PVI + 5-FU | 686 |
| NSABP C03 | 1987-1989 | MOF v FU + LV | 1,042 |
| NSABP C04 | 1989-1990 | 5-FU + LEV v 5-FU + LV v 5-FU + LV + LEV | 2,083 |
| NSABP C05 | 1991-1994 | 5-FU + LV v 5-FU + LV + IFN | 2,136 |
| NSABP C06 | 1997-1999 | IFU + LV v UFT + LV | 1,556 |
| NSABP C07 | 2000-2002 | 5-FU + LV v FOLFOX | 2,434 |
| NSABP C08 | 2004-2006 | mFOLFOX6 ± Bev | 2,612 |
| CALGB 89803 | 1999-2001 | 5-FU + LV v 5-FU + LV + IFL | 1,239 |
| FFCD | 1982-1990 | Surgery alone v 5-FU + LV | 256 |
| GERCOR | 1996-1999 | Bolus v infusional 5-FU + LV | 900 |
| GIVIO | 1989-1992 | Surgery alone v 5-FU + LV | 846 |
| INT-0035 | 1984-1987 | Surgery alone v 5-FU + LEV | 926 |
| INT-0089 | 1990-1992 | 5-FU + LEV v 5-FU + LV (HD or LD) v 5-FU + LV + LEV | 3,363 |
| MOSAIC | 1998-2001 | 5-FU + LV v FOLFOX | 2,241 |
| NCCTG-78-48-52 | 1978-1984 | Surgery alone v 5-FU + LEV | 247 |
| NCCTG-87-46-51 | 1988-1989 | Surgery alone v 5-FU + LV | 408 |
| NCCTG-89-46-51 | 1989-1991 | 5-FU + LV ± LEV for 6 or 12 months | 914 |
| NCCTG-91-46-53 | 1993-1998 | 5-FU + LV + HD or standard LEV | 878 |
| NCIC | 1987-1992 | Surgery alone v 5-FU + LV | 359 |
| PETACC-3 | 1999-2002 | 5-FU + LV (AIO or LVFU2) v ± IFL | 3,186 |
| QUASAR | 1994-1997 | 5-FU + LV (HD or LD) ± LEV | 3,507 |
| SWOG 9415 | 1995-1999 | Bolus v infusional 5-FU + LEV + LV | 939 |
| SIENA | 1984-1990 | Surgery alone v 5-FU + LV | 239 |
| XACT | 1998-2001 | 5-FU + LV v Cap | 1,983 |
| XELOXA | 2003-2004 | 5-FU + LV v XELOX | 1,864 |
| Complete ACCENT database | 1977-2004 | 37,568 |
Abbreviations: 5-FU, fluorouracil; AIO, folic acid, fluorouracil, and irinotecan; Bev, bevacizumab; CALGB, Cancer and Leukemia Group B; Cap, capecitabine; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; GERCOR, Groupe d’Etude et de Recherche Clinique en Oncologie et Radiothérapie; HD, high dose; IFL, irinotecan; IFN, interferon alfa-2a; INT, Intergroup; LD, low dose; LEV, levamisole; LV, leucovorin; LVFU2, semimonthly fluorouracil and leucovorin; mFOLFOX6, modified FOLFOX; MOF, semustine, vincristine, and fluorouracil; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; PVI, portal vein infusion; SWOG, Southwest Oncology Group; UFT, tegafur-uracil; XELOX, intravenous oxaliplatin plus oral capecitabine.
Table A2.
External Validation of the Nomogram for 6-Month Mortality Using 3,227 Patients From Trial N0147
| Patient Group | Actual Rate (95% CI) | Average Prediction | No. of Participants |
|---|---|---|---|
| Overall | 0.0112 (0.0081 to 0.0154) | 0.0119 | 3,227 |
| Age, years | |||
| < 70 | 0.0072 (0.0044 to 0.0111) | 0.0094 | 2,765 |
| ≥ 70 | 0.0346 (0.0199 to 0.0556) | 0.0275 | 462 |
| PS | |||
| 0 | 0.0089 (0.0056 to 0.0134) | 0.0106 | 2,472 |
| 1 | 0.0177 (0.0095 to 0.0301) | 0.0161 | 735 |
| ≥ 2 | 0.0500 (0.0013 to 0.2487) | 0.0264 | 20 |
| Grade | |||
| 1 | 0.0073 (0.0015 to 0.0212) | 0.0069 | 410 |
| 2 | 0.0094 (0.0057 to 0.0146) | 0.0087 | 2,024 |
| ≥ 3 | 0.0177 (0.0097 to 0.0294) | 0.0229 | 793 |
| Lymph node ratio | |||
| < 0.50 | 0.0104 (0.0070 to 0.0149) | 0.0104 | 2,794 |
| ≥ 0.50 | 0.0162 (0.0065 to 0.0330) | 0.0222 | 433 |
| Tumor stage | |||
| T1 | 0.0075 (0.0002 to 0.0412) | 0.0034 | 133 |
| T2 | 0.0112 (0.0031 to 0.0284) | 0.0086 | 357 |
| T3 | 0.0127 (0.0086 to 0.0180) | 0.0119 | 2,367 |
| T4 | 0.0027 (0.0001 to 0.0150) | 0.0187 | 370 |
NOTE. Average predicted probabilities of 6-month mortality and actual 6-month mortality rates with exact binomial 95% CIs, presented overall and by patient subgroup defined by the nomogram. All average predictions fall within the exact 95% CIs for the actual rates, with the exception of tumor stage T4.
Abbreviation: PS, performance status.
Footnotes
See accompanying editorial on page 1170
Supported by the National Institutes of Health National Cancer Institute Grant No. CA 25224.
Written on behalf of the Adjuvant Colon Cancer Endpoints (ACCENT) Group.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Winson Y. Cheung, Lindsay A. Renfro, Daniel J. Sargent
Financial support: Daniel J. Sargent
Administrative support: Greg Yothers, Daniel J. Sargent
Provision of study materials or patients: Eric Van Cutsem
Collection and assembly of data: Winson Y. Cheung, Katherine A. Guthrie, Greg Yothers, Daniel J. Sargent
Data analysis and interpretation: Winson Y. Cheung, Lindsay A. Renfro, David Kerr, Aimery de Gramont, Leonard B. Saltz, Axel Grothey, Steven R. Alberts, Thierry Andre, Roberto Labianca, Guido Francini, Jean-Francois Seitz, Chris O'Callaghan, Chris Twelves, Eric Van Cutsem, Daniel G. Haller, Greg Yothers, Daniel J. Sargent
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Determinants of Early Mortality Among 37,568 Patients With Colon Cancer Who Participated in 25 Clinical Trials From the Adjuvant Colon Cancer Endpoints Database
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Winson Y. Cheung
No relationship to disclose
Lindsay A. Renfro
No relationship to disclose
David Kerr
Employment: Oxford Cancer Biomarkers
Leadership: Oxford Cancer Biomarkers
Stock or Other Ownership: Oxford Cancer Biomarkers, OxOnc
Consulting or Advisory Role: Amgen, Merck Serono
Speakers' Bureau: Fresenius Kabi
Research Funding: Roche
Aimery de Gramont
Honoraria: Roche
Consulting or Advisory Role: Roche, Sanofi
Leonard B. Saltz
Consulting or Advisory Role: Genentech, Eli Lilly, McNeil PPC (I), AbbVie, Johnson & Johnson (I)
Research Funding: Taiho Pharmaceutical (Inst)
Axel Grothey
Consulting or Advisory Role: Genentech (Inst), Bayer AG (Inst), Sanofi (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Amgen (Inst)
Research Funding: Genentech (Inst), Bayer AG (Inst), Pfizer (Inst), Eisai (Inst), Sanofi (Inst), Eli Lilly (Inst), Boston Biomedical (Inst)
Travel, Accommodations, Expenses: Genentech, Bayer AG, Bristol-Myers Squibb, Boston Biomedical, Amgen
Steven R. Alberts
No relationship to disclose
Thierry Andre
No relationship to disclose
Katherine A. Guthrie
No relationship to disclose
Roberto Labianca
No relationship to disclose
Guido Francini
No relationship to disclose
Jean-Francois Seitz
Consulting or Advisory Role: Roche, Sanofi, Eli Lilly
Speakers' Bureau: Bayer AG
Chris O'Callaghan
No relationship to disclose
Chris Twelves
Honoraria: Eisai
Consulting or Advisory Role: Eisai, Pfizer
Speakers' Bureau: Eisai
Research Funding: Astellas Pharma (Inst), Nektar (Inst), AstraZeneca (Inst), GW Pharma (Inst), Merck Serono (Inst)
Travel, Accommodations, Expenses: Eisai, Nektar
Eric Van Cutsem
No relationship to disclose
Daniel G. Haller
Consulting or Advisory Role: Genentech, Targovax, Halozyme
Speakers' Bureau: Celgene, Taiho Pharmaceutical
Greg Yothers
Employment: Mountainview Pediatrics (I)
Consulting or Advisory Role: Pharmacyclics
Daniel J. Sargent
Consulting or Advisory Role: AbbVie, Acerta Pharma, ARIAD, Astellas Pharma, AstraZeneca/MedImmune, Biothera, Celldex, Exelixis, Genentech, Incyte, Kyowa Hakko Kirin, Medivation, Merck, Merrimack, Nektar, Novartis, Pharmacyclics, Pique, Spiration, Xbiotech
Research Funding: Celgene (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Celgene
REFERENCES
- 1.Barrios CH, Werutsky G, Martinez-Mesa J. The global conduct of cancer clinical trials: Challenges and opportunities. Am Soc Clin Oncol Educ Book. 2015;35:e132–e139. doi: 10.14694/EdBook_AM.2015.35.e132. [DOI] [PubMed] [Google Scholar]
- 2.St Germain D, Denicoff AM, Dimond EP, et al. Use of the National Cancer Institute Community Cancer Centers Program screening and accrual log to address cancer clinical trial accrual. J Oncol Pract. 2014;10:e73–e80. doi: 10.1200/JOP.2013.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute-American Society of Clinical Oncology cancer trial accrual symposium: Summary and recommendations. J Oncol Pract. 2013;9:267–276. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters-Lawrence MH, Bell MC, Hsu LL, et al. Sickle Cell Disease Clinical Research Network (SCDCRN) Clinical trial implementation and recruitment: Lessons learned from the early closure of a randomized clinical trial. Contemp Clin Trials. 2012;33:291–297. doi: 10.1016/j.cct.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroen AT, Petroni GR, Wang H, et al. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin Trials. 2010;7:312–321. doi: 10.1177/1740774510374973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olmos D, A’hern RP, Marsoni S, et al. Patient selection for oncology phase I trials: A multi-institutional study of prognostic factors. J Clin Oncol. 2012;30:996–1004. doi: 10.1200/JCO.2010.34.5074. [DOI] [PubMed] [Google Scholar]
- 7.Garrido-Laguna I, Janku F, Vaklavas C, et al. Validation of the Royal Marsden Hospital prognostic score in patients treated in the phase I clinical trials program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 8.Wheler J, Tsimberidou AM, Hong D, et al. Survival of 1,181 patients in a phase I clinic: The MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. 2012;18:2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penel N, Delord JP, Bonneterre ME, et al. Development and validation of a model that predicts early death among cancer patients participating in phase I clinical trials investigating cytotoxics. Invest New Drugs. 2010;28:76–82. doi: 10.1007/s10637-009-9224-x. [DOI] [PubMed] [Google Scholar]
- 10.Penel N, Vanseymortier M, Bonneterre ME, et al. Prognostic factors among cancer patients with good performance status screened for phase I trials. Invest New Drugs. 2008;26:53–58. doi: 10.1007/s10637-007-9088-x. [DOI] [PubMed] [Google Scholar]
- 11.Bachelot T, Ray-Coquard I, Catimel G, et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Ann Oncol. 2000;11:151–156. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]
- 12.Ploquin A, Olmos D, Ferté C, et al. Life-expectancy of patients enrolled in phase I clinical trials: A systematic review of published prognostic models. Crit Rev Oncol Hematol. 2012;83:242–248. doi: 10.1016/j.critrevonc.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Sargent D, Shi Q, Yothers G, et al. Adjuvant Colon Cancer End-points (ACCENT) Group Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: Data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;47:990–996. doi: 10.1016/j.ejca.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargent DJ, Patiyil S, Yothers G, et al. ACCENT Group End points for colon cancer adjuvant trials: Observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 15.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 16.Harrell F, editor. (ed): Regression Modeling Strategies. New York, NY,: Springer-Verlag; 2010. (ed) [Google Scholar]
- 17.Steyerberg E, editor. (ed): Clinical Prediction Models. New York, NY,: Springer Science+Business Media; 2010. (ed) [Google Scholar]
- 18.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balducci L. Studying cancer treatment in the elderly patient population. Cancer Contr. 2014;21:215–220. doi: 10.1177/107327481402100306. [DOI] [PubMed] [Google Scholar]
- 21.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: Barriers and solutions. Cancer Contr. 2014;21:209–214. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham D, Lang I, Marcuello E, et al. AVEX study investigators Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 23.Seymour MT, Thompson LC, Wasan HS, et al. FOCUS2 Investigators. National Cancer Research Institute Colorectal Cancer Clinical Studies Group Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landre T, Uzzan B, Nicolas P, et al. Doublet chemotherapy vs. single-agent therapy with 5FU in elderly patients with metastatic colorectal cancer: A meta-analysis. Int J Colorectal Dis. 2015;30:1305–1310. doi: 10.1007/s00384-015-2296-5. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33:3826–3833. doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]