Abstract
Purpose
Data on optimal adjuvant therapy after complete resection of small-cell lung cancer (SCLC) are limited, and in particular, there have been no studies evaluating the role of adjuvant chemotherapy, with or without prophylactic cranial irradiation, relative to no adjuvant therapy for stage T1-2N0M0 SCLC. This National Cancer Data Base analysis was performed to determine the potential benefits of adjuvant chemotherapy with and without prophylactic cranial irradiation in patients who undergo complete resection for early-stage small-cell lung cancer.
Patients and Methods
Overall survival of patients with pathologic T1-2N0M0 SCLC who underwent complete resection in the National Cancer Data Base from 2003 to 2011, stratified by adjuvant therapy regimen, was evaluated using Kaplan-Meier and Cox proportional hazards analysis. Patients treated with induction therapy and those who died within 30 days of surgery were excluded from analysis.
Results
Of 1,574 patients who had pT1-2N0M0 SCLC during the study period, 954 patients (61%) underwent complete R0 resection with a 5-year survival of 47%. Adjuvant therapy was administered to 59% of patients (n = 566), including chemotherapy alone (n = 354), chemoradiation (n = 190, including 99 patients who underwent cranial irradiation), and radiation alone (n = 22). Compared with surgery alone, adjuvant chemotherapy with or without radiation was associated with significantly improved survival. In addition, multivariable Cox modeling demonstrated that treatment with adjuvant chemotherapy (hazard ratio [HR], 0.78; 95% CI, 0.63 to 0.95) or chemotherapy with radiation directed at the brain (HR, 0.52; 95% CI, 0.36 to 0.75) was associated with improved survival when compared with no adjuvant therapy.
Conclusion
Patients with pT1-2N0M0 SCLC treated with surgical resection alone have worse outcomes than those who undergo resection with adjuvant chemotherapy alone or chemotherapy with cranial irradiation.
INTRODUCTION
Over the past 20 years, a number of studies have demonstrated 5-year survival rates of approximately 40% to 60% for patients undergoing surgery for stage I small-cell lung cancer (SCLC),1-5 and surgery with adjuvant chemotherapy is now recommended in the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of patients with clinical stage T1-2N0M0 SCLC.6 However, this recommendation comes from limited data; the role of adjuvant therapy for this patient population is not yet well characterized.
Currently, there are only a few single-arm phase II studies evaluating the outcomes of patients who have undergone surgery with adjuvant therapy for limited-stage SCLC.7-10 In addition, there have been no studies, either prospective or retrospective, evaluating the role of adjuvant chemotherapy, with or without radiation, relative to no adjuvant therapy for stage T1-2N0M0 SCLC.6 Furthermore, the NCCN recommendation of prophylactic cranial irradiation (PCI) for patients who have undergone a complete resection with adjuvant chemotherapy6 is based on data from trials evaluating the impact of chemotherapy and radiation (without surgery) on patients with SCLC.11 To date, there have not been any studies evaluating the impact of surgery followed by adjuvant chemotherapy and PCI for T1-2N0M0 SCLC.
This study was performed to evaluate the role of adjuvant therapy after surgical resection for T1-2N0M0 SCLC using the National Cancer Data Base (NCDB). Our objective was to elucidate the potential benefits of adjuvant chemotherapy with and without PCI in patients who undergo complete resection for T1-2N0M0 SCLC.
PATIENTS AND METHODS
National Cancer Data Base
The NCDB, which is a joint project of the American College of Surgeons Commission on Cancer and the American Cancer Society, includes approximately 70% of all newly diagnosed cancers nationwide and contains data collected from more than 1,500 cancer program registries in the United States and Puerto Rico.12 Clinical staging information is directly recorded in the NCDB using American Joint Committee on Cancer 6th and 7th edition TNM classifications for the years of study inclusion (2003 to 2011).13
Study Design
This NCDB study was approved by the Duke University Institutional Review Board. From a de-identified NCDB participant user file, all patients in the NCDB diagnosed with pathologic T1-2N0M0 SCLC from January 1, 2003, to December 31, 2011, were identified using International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) histology and topography codes. Using the ICD-O-3, classification of SCLC, we included tumor histology codes of 8041/3, 8042/3, 8043/3, 8044/3, and 8045/3, which corresponded to small-cell carcinoma (not otherwise specified), oat cell carcinoma, small-cell carcinoma (fusiform cell), small-cell carcinoma (intermediate cell), and combined small-cell carcinoma, respectively. The WHO defines combined SCLC as small-cell carcinoma combined with any non–small-cell histologic type, including adenocarcinoma, squamous cell carcinoma, and large-cell neuroendocrine carcinoma.14 The study period was chosen primarily for the following two reasons: the NCDB reports data on the Charlson/Deyo comorbidity condition (CDCC) score only for patients diagnosed in 2003 and later, and survival data were available for patients diagnosed up to 2011 at the time of analysis.
Methods of follow-up have been described previously (eg, reports from physician follow-up, program inpatient or outpatient services, death certificates).15 To minimize confounding, the cohort was limited to patients who were initially diagnosed with a single malignancy of SCLC. Patients who died within 30 days of surgery were excluded from analysis to minimize selection bias because these patients were unlikely to receive adjuvant therapy. Patients who were not diagnosed or treated at the reporting facility were excluded because the Commission on Cancer does not require follow-up for these patients. Additional exclusion criteria included incomplete resection, missing data on facility type, patients who received induction chemotherapy treatment, patients who were treated with palliative intent, and patients who received intraoperative radiation or had missing data on the start date of postoperative radiation. The primary outcome was overall survival (OS).
Statistical Analysis
Patients were grouped according to type of adjuvant therapy administered (surgery alone, chemotherapy, chemotherapy with radiation to lung, chemotherapy with radiation to brain, radiation to lung, and radiation to brain). Comparisons of patient characteristics between patients who underwent surgery alone and patients who received adjuvant therapy were performed using the Wilcoxon rank sum test for continuous variables and Pearson’s χ2 test for discrete variables.
Adjuvant chemotherapy was defined as chemotherapy administered within 5 months of surgery. In previous studies of adjuvant therapy and lung cancer, adjuvant chemotherapy has been defined as chemotherapy given within 3 to 4 months after surgery16,17; we chose 5 months as the time interval to reflect the real-world, nonclinical trial setting where patients may experience delays between surgery and initiation of adjuvant chemotherapy as a result of postoperative recovery from complications, delays in referral and/or consultation, and patient preference.17
We used the variable “radiation treatment volume” in the NCDB to identify the anatomic target of the radiation therapy; this variable reflected what was thought by the reporting radiation oncologist to be “the most clinically significant regional radiation therapy delivered to the patient during the first course of treatment.”18 Adjuvant radiation was defined as radiation administered within 8 months of surgery; 8 months was the time interval chosen because early-stage SCLC treatment typically involves four cycles (3 months) of chemotherapy,19 which is typically given within 3 months after surgery, and if PCI is given, it is typically given within approximately 1.5 to 2 months after chemotherapy.20 We did not extend the time interval beyond 8 months to minimize the possibility that any radiation treatment to the brain was given for recurrence as opposed to prophylaxis. Differences in median survival and 5-year survival were evaluated by the Kaplan-Meier product-limit approach and the log-rank test.
A Cox proportional hazards regression model was used to further evaluate OS among the patient population. All variables that were chosen for inclusion in the Cox model were determined a priori to be clinically significant. Variables included in the Cox model included the following: type of adjuvant therapy administered (surgery alone, chemotherapy, chemotherapy with radiation to lung, chemotherapy with radiation to brain, radiation to lung, and radiation to brain), type of operation (wedge resection, segmentectomy, lobectomy, or pneumonectomy), year of diagnosis, age, sex, race (white, black, or other), patient census tract median household income (< $38,000, $38,000 to $47,999, $48,000 to $62,999, or ≥ $63,000), urban versus nonurban area for patient residence, treatment facility type (community cancer program, comprehensive community cancer program, or academic/research program), distance between patient’s residence and treatment facility, CDCC score (0, 1, or 2+), and tumor size. Patients with missing data on type of operation were excluded in the Cox model. An exploratory analysis was also performed to evaluate the impact of timing of PCI on OS (Appendix Table A1, online only).
All statistical analyses were performed using Stata/MP software, version 13.1 for Mac (StataCorp, College Station, TX). A two-sided P = .05 was used to define significance.
RESULTS
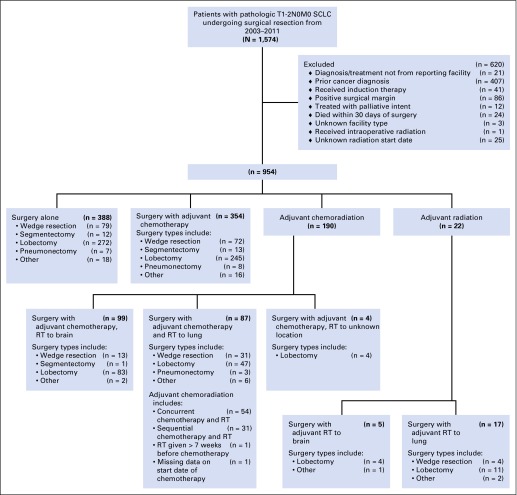
Between 2003 and 2011, 1,574 patients underwent surgical resection for pathologic T1-2N0M0 SCLC. Among these, 954 patients (63.1%) met study inclusion criteria (Fig 1). Of these 954 patients, adjuvant therapy after surgery was administered to 566 patients (59.3%). Adjuvant chemotherapy without radiation was administered to 354 patients (37.1%), and adjuvant chemoradiation was administered to 190 patients (19.9%). Within the adjuvant chemoradiation group, 99 patients (52.1%) received radiotherapy directed at the brain, and 87 patients (45.8%) received radiotherapy directed at the lung. Four patients (2.1%) in the chemoradiation group had other or unknown location of radiotherapy. Radiation therapy alone was administered to 22 patients (2.3%), with five patients receiving PCI and 17 patients receiving thoracic radiation.
Fig 1.
CONSORT diagram showing schema of study patient selection. RT, radiation therapy; SCLC, small-cell lung cancer.
Table 1 lists the preoperative and demographic characteristics of the patient cohort. The majority of patients had clinical T1N0 disease, had no comorbidities, and were treated at a comprehensive community cancer program. Appendix Table A2 (online only) lists the median time intervals between treatments for each of the adjuvant therapy groups.
Table 1.
Preoperative and Demographic Characteristics
| Characteristic | Patient Cohort (N = 954) | Surgery Alone (n = 388) | Adjuvant Therapy (n = 566) | P |
|---|---|---|---|---|
| Age, years | < .01 | |||
| Mean (SD) | 66.8 (8.9) | 68.3 (9.3) | 65.8 (8.4) | |
| Median (IQR) | 67 (62.0-73.0) | 69 (62.5-75.0) | 66 (61.0-72.0) | |
| Sex, No. (%) | .40 | |||
| Male | 422 (44.2) | 178 (45.9) | 244 (43.1) | |
| Female | 532 (55.8) | 210 (54.1) | 322 (56.9) | |
| Race, No. (%) | .27 | |||
| White | 873 (91.5) | 349 (90.0) | 524 (92.6) | |
| Black | 57 (6.0) | 29 (7.5) | 28 (5.0) | |
| Other | 24 (2.5) | 10 (2.6) | 14 (2.5) | |
| CDCC score, No. (%) | .27 | |||
| 0 | 459 (48.1) | 183 (47.2) | 276 (48.8) | |
| 1 | 356 (37.3) | 155 (40.0) | 201 (35.5) | |
| 2+ | 139 (14.6) | 50 (12.9) | 89 (15.7) | |
| Year of diagnosis, median (IQR) | 2006 (2004-2008) | 2006 (2004-2008) | 2006 (2005-2008) | .05 |
| Insurance type, No. (%) | .04 | |||
| Uninsured | 13 (1.4) | < 10 | < 10 | |
| Private | 305 (32.0) | 105 (27.1) | 200 (35.3) | |
| Medicare/aid | 619 (64.9) | 270 (69.6) | 349 (61.7) | |
| Other government | 11 (1.2) | < 10 | < 10 | |
| Unknown | < 10 | < 10 | < 10 | |
| Facility type, No. (%) | .18 | |||
| Community cancer program | 96 (10.1) | 35 (9.0) | 61 (10.8) | |
| Comprehensive community cancer program | 550 (57.7) | 215 (55.4) | 335 (59.2) | |
| Academic/research program | 308 (32.3) | 138 (35.6) | 170 (30.0) | |
| Clinical T status, No. (%) | .46 | |||
| T0 | < 10 | < 10 | 0 | |
| T1 | 396 (41.5) | 162 (41.8) | 234 (41.3) | |
| T2 | 124 (13.0) | 53 (13.7) | 71 (12.5) | |
| T3 | < 10 | 0 | < 10 | |
| T4 | < 10 | 0 | < 10 | |
| Unknown | 430 (45.1) | 171 (44.1) | 259 (45.8) | |
| Clinical N status, No. (%) | .39 | |||
| N0 | 504 (52.8) | 213 (54.9) | 291 (51.4) | |
| N1 | 17 (1.8) | < 10 | < 10 | |
| N2 | 10 (1.0) | < 10 | < 10 | |
| N3 | < 10 | < 10 | < 10 | |
| Unknown | 421 (44.1) | 163 (42.0) | 258 (45.6) | |
| Clinical M status, No. (%) | .98 | |||
| M0 | 949 (99.5) | 386 (99.5) | 563 (99.5) | |
| M1 | < 10 | < 10 | < 10 |
Abbreviations: CDCC, Charlson/Deyo comorbidity condition; IQR, interquartile range; SD, standard deviation.
The perioperative and postoperative data of the patient cohort are listed in Table 2. The majority of patients had pathologic T1 disease. The rate of readmission within 30 days of surgery was 5.0%, and the median length of hospital stay was 5 days. The majority of patients underwent lobectomy (n = 66 [70%]). Although the NCDB does not contain data on specific adjuvant chemotherapy regimens, it specifies whether the chemotherapy administered was single agent or multiagent. Of the 354 patients who received adjuvant chemotherapy without radiation, 313 (88.4%) received multiagent chemotherapy and six (1.7%) received single-agent chemotherapy, with the type and number of agents unknown for 35 patients (9.9%). Of the 190 patients who received adjuvant chemoradiation, 178 (93.7%) received multiagent chemotherapy and two (1.1%) received single-agent chemotherapy, with the type and number of agents unknown for 10 patients (5.3%).
Table 2.
Perioperative and Postoperative Characteristics
| Characteristic | Patient Cohort (N = 954) | Surgery Alone (n = 388) | Adjuvant Therapy (n = 566) | P |
|---|---|---|---|---|
| Pathologic T status, No. (%) | .89 | |||
| T1 | 659 (69.1) | 269 (69.3) | 390 (68.9) | |
| T2 | 295 (30.9) | 119 (30.7) | 176 (31.1) | |
| Pathologic N0 status, No. (%) | 954 (100.0) | 388 (100.0) | 566 (100.0) | NA |
| Pathologic M0 status, No. (%) | 954 (100.0) | 388 (100.0) | 566 (100.0) | NA |
| Type of operation | .98 | |||
| Wedge resection | 199 (20.9) | 79 (20.4) | 120 (21.2) | |
| Segmentectomy | 26 (2.7) | 12 (3.1) | 14 (2.5) | |
| Lobectomy | 666 (69.8) | 272 (70.1) | 394 (69.6) | |
| Pneumonectomy | 18 (1.9) | < 10 | 11 (1.9) | |
| Other | 45 (4.7) | 18 (4.6) | 27 (4.8) | |
| Regional LNs examined | .24 | |||
| No. of patients with LN examined | 863 | 357 | 506 | |
| Median LNs (IQR) | 7 (4.0-13.0) | 7 (4.0-12.0) | 7 (4.0-14.0) | |
| Pathologic tumor size, cm, mean (SD) | 2.4 (1.6) | 2.4 (1.3) | 2.4 (1.8) | .24 |
| Histology, No. (%) | .70 | |||
| Small-cell carcinoma, not otherwise specified | 723 (75.8) | 289 (74.5) | 434 (76.7) | |
| Oat cell carcinoma | 17 (1.8) | < 10 | 10 (1.8) | |
| Small-cell carcinoma, fusiform cell | < 10 | 0 | < 10 | |
| Small-cell carcinoma, intermediate cell | 32 (3.4) | 14 (3.6) | 18 (3.2) | |
| Combined small-cell carcinoma | 180 (18.9) | 78 (20.1) | 102 (18.0) | |
| Readmission in 30 days, No. (%) | 46 (4.8) | 21 (5.4) | 25 (4.4) | .48 |
| Hospital length of stay, days from surgery | .04 | |||
| No. of patients with available data | 859 | 366 | 493 | |
| Median (IQR) | 6 (4-9) | 4 (6-9) | 4 (6-8) |
Abbreviations: IQR, interquartile range; LN, lymph node; NA, not applicable; SD, standard deviation.
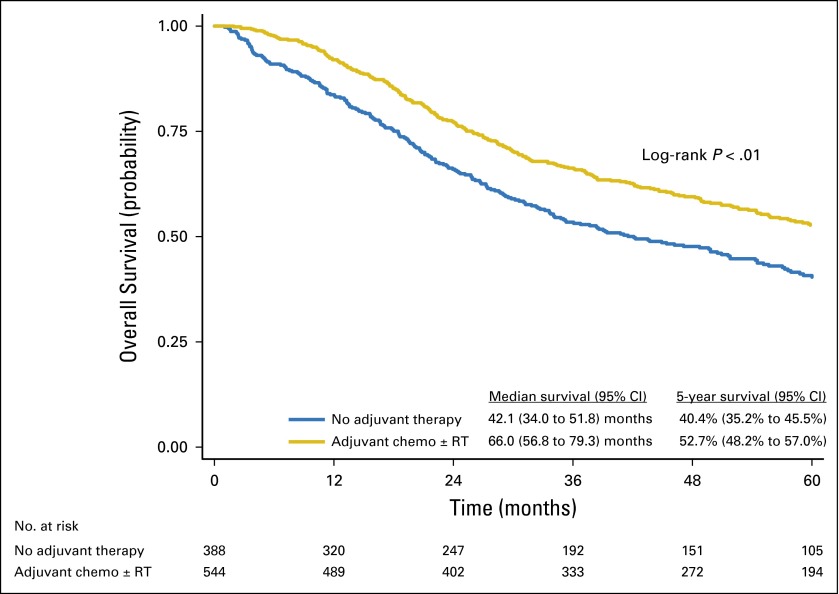
The median follow-up time for the entire cohort was 43 months (interquartile range, 20 to 68 months). Kaplan-Meier analysis demonstrated a median survival time of 55.6 months (95% CI, 49.1 to 62.7 months) and a 5-year survival rate of 47.4% (95% CI, 44.0% to 50.7%) for the entire cohort. Treatment with adjuvant chemotherapy with or without radiation, compared with no adjuvant therapy, was associated with a significant increase in median OS (66.0 months [95% CI, 56.8 to 79.3 months] v 42.1 months [95% CI, 34.0 to 51.8 months], respectively) and 5-year OS (52.7% [95% CI, 48.2% to 57.0%] v 40.4% [95% CI, 35.2% to 45.5%], respectively; log-rank P < .01; Fig 2).
Fig 2.
Overall survival of patients with pT1-2N0M0 small-cell lung cancer, stratified by no adjuvant therapy versus adjuvant chemotherapy (chemo) with or without radiation therapy (RT).
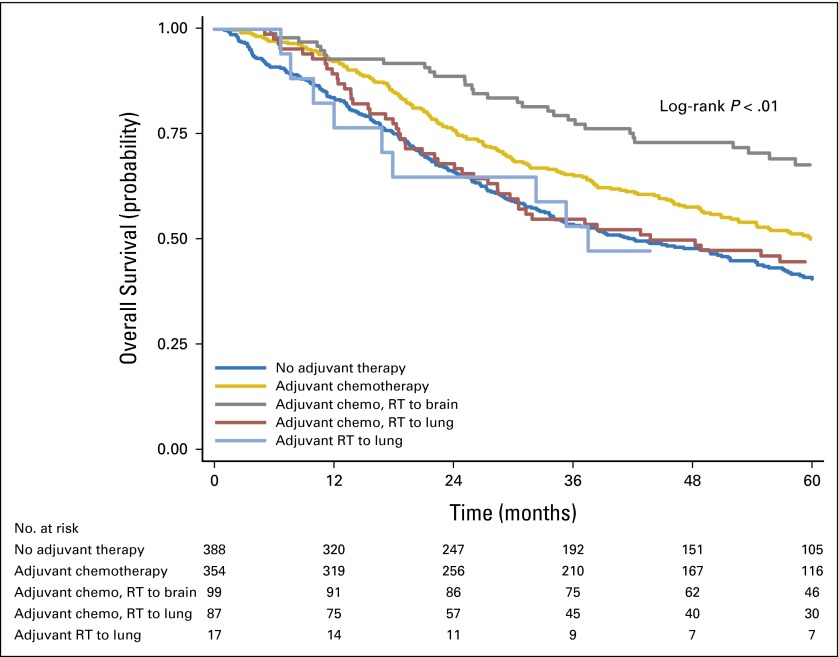
OS stratified by specific type of adjuvant therapy was assessed (Fig 3 and Appendix Table A3, online only). In univariable analysis, patients who received adjuvant chemotherapy alone had improved 5-year survival rates compared with patients who underwent surgery alone. Similarly, in univariable analysis, patients who received adjuvant chemotherapy with radiation to the brain had an improved 5-year survival rate compared with patients who underwent surgery alone. There were no significant differences in 5-year survival between patients who received adjuvant chemotherapy with radiation to the lung and patients who underwent surgery alone, or between patients who received adjuvant radiation to the lung and those who underwent surgery alone. After multivariable adjustment, the use of adjuvant chemotherapy alone and the use of adjuvant chemotherapy with radiation to the brain were significantly associated with improved survival rates (Table 3). In addition, in multivariable analysis, increasing age, tumor size, and CDCC score of 2+ were associated with worse survival, whereas use of lobectomy was associated with improved survival (Table 3).
Fig 3.
Overall survival of patients with pT1-2N0M0 small-cell lung cancer, stratified by type of adjuvant therapy and location of radiation therapy (RT). Chemo, chemotherapy.
Table 3.
Independent Predictors of Overall Survival After Cox Proportional Hazards Adjustment for Patients Who Have Undergone Complete Resection for pT1-2N0M0 SCLC
| Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (per year) | 1.04 | 1.03 to 1.05 | < .01 |
| Female v male | 0.87 | 0.72 to 1.05 | .15 |
| Race (ref = white) | |||
| Black | 0.75 | 0.49 to 1.16 | .19 |
| Other | 1.09 | 0.59 to 2.00 | .79 |
| Household income (ref < $38,000) | |||
| $38,000-$47,999 | 1.16 | 0.88 to 1.53 | .29 |
| $48,000-$62,999 | 0.85 | 0.64 to 1.14 | .28 |
| $63,000+ | 0.86 | 0.63 to 1.16 | .33 |
| Nonurban v urban | 1.10 | 0.85 to 1.42 | .47 |
| CDCC score (ref = 0) | |||
| 1 | 1.08 | 0.88 to 1.33 | .47 |
| 2+ | 1.54 | 1.20 to 1.99 | < .01 |
| Year of diagnosis (per year) | 0.99 | 0.94 to 1.04 | .59 |
| Tumor size (per cm) | 1.10 | 1.03 to 1.18 | < .01 |
| Facility type (ref = community) | |||
| Comprehensive | 1.01 | 0.71 to 1.44 | .95 |
| Academic/research | 1.08 | 0.74 to 1.56 | .70 |
| Distance from facility (per mile) | 1.00 | 1.00 to 1.00 | .32 |
| Type of operation (ref = wedge resection) | |||
| Segmentectomy | 0.77 | 0.46 to 1.30 | .33 |
| Lobectomy | 0.64 | 0.51 to 0.79 | < .01 |
| Pneumonectomy | 0.55 | 0.26 to 1.17 | .12 |
| Adjuvant therapy (ref = surgery alone) | |||
| Chemotherapy | 0.78 | 0.63 to 0.95 | .02 |
| Chemotherapy with radiation to brain | 0.52 | 0.36 to 0.75 | < .01 |
| Chemotherapy with radiation to lung | 0.88 | 0.63 to 1.23 | .45 |
| Radiation to brain | 1.46 | 0.46 to 4.64 | .52 |
| Radiation to lung | 0.83 | 0.42 to 1.64 | .59 |
Abbreviations: CDCC, Charlson/Deyo comorbidity condition; SCLC, small-cell lung cancer.
DISCUSSION
To our knowledge, this is the first population-based study to examine the role of adjuvant therapy for patients who underwent resection for pathologic stage T1-2N0M0 SCLC. In univariable analysis, adjuvant chemotherapy with or without radiation was associated with significantly improved OS when compared with no adjuvant therapy. In addition, multivariable Cox modeling demonstrated that adjuvant chemotherapy alone and adjuvant chemotherapy with PCI were associated with improved survival when compared with no adjuvant therapy. Adjuvant chemotherapy with thoracic irradiation and thoracic radiation alone were not associated with improved survival.
Current NCCN guidelines recommend adjuvant chemotherapy after resection for T1-2N0M0 SCLC, but this recommendation is based on limited data.6 To date, there are only four single-arm prospective phase II trials evaluating the outcomes of patients receiving adjuvant therapy after surgery for SCLC. In the current study, the 5-year survival rates for patients who received adjuvant chemotherapy are comparable to those reported by these previous trials. Macchiarini et al7 reported the results of a prospective study of surgery plus adjuvant chemotherapy for patients with pathologic T1-3N0M0 SCLC, in which the 5-year survival rate was 36%. Karrer and Ulsperger8 reported a prospective trial of patients with T1-2N0MO SCLC who underwent surgery, followed by chemotherapy and PCI; the 4-year survival rate was 56% for patients with pathologic stage I disease. Rea et al9 observed a 5-year survival rate of 52% for patients with pathologic stage I SCLC who received adjuvant chemotherapy and radiotherapy after surgery. Tsuchiya et al10 reported a 5-year survival rate of 73% for patients with pathologic stage IA disease and 67% for patients with pathologic stage IB disease in a prospective trial evaluating the outcomes of surgery with adjuvant chemotherapy. The current study builds on these previous trials by comparing the outcomes of surgery with adjuvant chemotherapy to surgery alone. Our finding that adjuvant chemotherapy after resection for T1-2N0 SCLC is associated with improved survival provides further evidence to support the use of adjuvant chemotherapy as recommended by current NCCN guidelines.
In this study, a large proportion of patients in the adjuvant chemoradiation and adjuvant radiation groups received radiation that was directed at the brain. Because patients with metastatic disease were excluded from the cohort, these patients presumably received PCI. A notable finding is that patients who underwent surgery with adjuvant chemotherapy and presumed PCI had much better OS when compared with other groups. The sample sizes are small but suggest a survival benefit to PCI. The current NCCN recommendation to use PCI for all patients after the completion of adjuvant chemotherapy after complete resection6 is based on trials studying the impact of chemotherapy and radiation for patients who had not undergone surgery. To our knowledge, this is the first study to suggest a possible benefit to PCI after surgery and adjuvant chemotherapy and provides further evidence to support NCCN recommendations on PCI. Because previous data in the non–small-cell lung cancer literature have shown that thoracoscopy is associated with a higher adherence rate and fewer delayed or reduced doses of chemotherapy in patients receiving adjuvant chemotherapy,21 a minimally invasive resection for patients with T1-2N0 SCLC may improve the likelihood that patients receive adjuvant chemotherapy with PCI.
Our findings that adjuvant radiation alone or adjuvant chemotherapy with thoracic radiation was not associated with a survival benefit are consistent with previous analyses of the Surveillance, Epidemiology, and End Results (SEER) database.22-24 Of note, the major limitation of these studies is that the SEER database does not contain information on the anatomic target of radiation and the use of chemotherapy. It is also important to note that in both the current study and in the SEER studies, there may have been selection bias where patients who received only adjuvant radiation may have been too sick to undergo chemotherapy. However, we attempted to minimize this bias by including the CDCC score in the multivariable analysis. The role of adjuvant radiation in patients with SCLC who are not fit enough to undergo chemotherapy after resection warrants further study.
There are several limitations to this study. First, it is retrospective, and there is the possibility that confounding variables were not accounted for in the analysis. The NCDB does not contain information on the number of doses of chemotherapy administered, the specific chemotherapy agents, or toxicity. Similarly, we do not have information on the specific type of adjuvant radiation administered, although we do have information regarding the anatomic target for radiation. Because there are no data on recurrence, it is possible that adjuvant chemotherapy or radiation was administered to patients to treat a recurrence rather than the original malignancy. In an effort to minimize this possibility, for patients receiving adjuvant therapy, we only included patients who had adjuvant chemotherapy within 5 months of surgery and radiotherapy within 8 months of surgery. Our analysis may have been underpowered as a result of sample size limitations, and there may have been a possibility of a type II error. We do not know how many patients received radiation to both the lung and the brain because the NCDB records only what the reporting radiation oncologist determined was the most clinically significant radiation therapy delivered during the first course of treatment.18 Finally, there is a possibility that selection bias contributed to the much higher survival seen in patients who received adjuvant chemotherapy with radiation to the brain. For example, NCCN guidelines do not recommend that patients with poor performance status receive PCI, and patients who underwent adjuvant chemotherapy with radiation to the brain may have been healthier patients. We attempted to minimize this bias by including the CDCC score as a covariate in the multivariable model.
The findings of this NCDB analysis suggest that patients with pathologic stage T1-2N0M0 SCLC can benefit from both adjuvant chemotherapy and chemotherapy with PCI after surgery. Given the limitations of this study noted earlier, these findings should be evaluated further in randomized controlled trials.
Acknowledgment
We thank David Levy for his assistance with the manuscript. The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Data Base file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Appendix
Table A1.
Impact of Timing of PCI on Overall Survival
| Factor | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Age (per year) | 1.06 | 1.01 to 1.11 | .02 |
| Female v male | 0.90 | 0.44 to 1.86 | .79 |
| CDCC score (ref = 0) | |||
| 1 | 0.59 | 0.26 to 1.31 | .19 |
| 2+ | 2.07 | 0.83 to 5.15 | .12 |
| Year of diagnosis (per year) | 0.81 | 0.67 to 0.98 | .03 |
| Tumor size (per cm) | 1.20 | 0.90 to 1.60 | .23 |
| PCI given more than 113 days after start of chemotherapy v PCI given less than 113 days after start of chemotherapy | 1.72 | 0.84 to 3.53 | .14 |
NOTE. The National Cancer Data Base does not contain data on the end date of chemotherapy, but it does contain data on the start date of chemotherapy and the start date of radiation. For patients who received PCI, the median interval between start of chemotherapy and start of PCI was 113 days. Patients who received adjuvant chemotherapy with PCI were divided into two groups depending on whether they received PCI before (n = 51) or after (n = 48) 113 days after the start of chemotherapy. A Cox proportional hazards regression model was performed to assess the impact of PCI timing on overall survival. Variables included in the Cox model were as follows: age, sex, CDCC score (0, 1, or 2+), year of diagnosis, tumor size, and timing of PCI. PCI given more than 113 days after chemotherapy was associated with a trend toward worse survival compared with PCI given less than 113 days after the start of chemotherapy (hazard ratio, 1.72; 95% CI, 0.84 to 3.53; P = .14).
Abbreviations: CDCC, Charlson/Deyo comorbidity condition; PCI, prophylactic cranial irradiation.
Table A2.
Median Time Intervals Between Treatments
| Adjuvant Therapy Type and Interval Between Treatments | Interval Between Treatments (days), Median (IQR) |
|---|---|
| Chemotherapy | |
| Length of time between surgery and start date of chemotherapy | 40.0 (30-54) |
| Chemotherapy with radiation (overall) | |
| Length of time between surgery and start date of chemotherapy | 34.5 (26-46) |
| Length of time between surgery and start date of radiation | 130.0 (66-161) |
| Length of time between start date of chemotherapy and start date of radiation | 98.0 (21-124) |
| Chemotherapy with radiation to brain | |
| Length of time between surgery and start date of chemotherapy | 35.0 (26-48) |
| Length of time between surgery and start date of radiation | 154.0 (128-187) |
| Length of time between start date of chemotherapy and start date of radiation | 113.0 (98-142) |
| Chemotherapy with radiation to lung | |
| Length of time between surgery and start date of chemotherapy | 33.0 (25-45) |
| Length of time between surgery and start date of radiation | 65.0 (41-119) |
| Length of time between start date of chemotherapy and start date of radiation | 21.0 (0-91) |
| Radiation | |
| Length of time between surgery and start date of radiation | 101.0 (36-174) |
Abbreviation: IQR, interquartile range.
Table A3.
Overall Survival Estimates for Patients Who Have Undergone Complete Resection for pT1-2N0M0 SCLC, Stratified by Adjuvant Therapy
| Adjuvant Therapy | Events (deaths), No. | Median Survival, Months (95% CI) | 5-Year Survival, % (95% CI) |
|---|---|---|---|
| None | 243 of 388 | 42.1 (34.0 to 51.8) | 40.4 (35.2 to 45.5) |
| Chemotherapy | 188 of 354 | 59.8 (50.8 to 71.1) | 50.0 (44.2 to 55.3) |
| Chemotherapy with radiation to brain | 37 of 99 | 88.9 (72.5 to NA) | 67.6 (56.9 to 76.2) |
| Chemotherapy with radiation to lung | 52 of 87 | 43.8 (28.5 to 78.7) | 44.5 (33.6 to 54.9) |
| Concurrent chemoradiation | 30 of 54 | 56.8 (31.3 to 91.5) | 48.6 (34.3 to 61.5) |
| Sequential chemoradiation | 21 of 31 | 29.7 (18.7 to 95.1) | 38.2 (21.5 to 54.8) |
| Radiation to lung | 11 of 17 | 37.5 (19.0 to NA) | 47.1 (23.0 to 68.0) |
NOTE. Survival data for patients who received adjuvant radiation directed at the brain was not reported as a result of a small sample size (n < 10), in accordance with the National Cancer Data Base Participant Use File Data Use Agreement.
Abbreviations: NA, not available; SCLC, small-cell lung cancer.
Footnotes
See accompanying editorial on page 1027
Supported by the National Institutes of Health Cardiothoracic Surgical Trials Network (B.C.G. and M.G.H.), Grant No. 5U01HL088953-05, and the American College of Surgeons Resident Research Scholarship (C.-F.J.Y.).
C.-F.J.Y. and D.Y.C. contributed equally to this work as co-first authors; M.F.B. and D.H.H. were co-senior authors.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Chi-Fu Jeffrey Yang, Paul J. Speicher
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Role of Adjuvant Therapy in a Population-Based Cohort of Patients With Early-Stage Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Chi-Fu Jeffrey Yang
No relationship to disclose
Derek Y. Chan
No relationship to disclose
Paul J. Speicher
No relationship to disclose
Brian C. Gulack
No relationship to disclose
Xiaofei Wang
No relationship to disclose
Matthew G. Hartwig
No relationship to disclose
Mark W. Onaitis
No relationship to disclose
Betty C. Tong
Consulting or Advisory Role: W.L. Gore & Associates
Thomas A. D'Amico
Consulting or Advisory Role: Scanlan
Mark F. Berry
No relationship to disclose
David H. Harpole
No relationship to disclose
REFERENCES
- 1.Inoue M, Miyoshi S, Yasumitsu T, et al. Surgical results for small cell lung cancer based on the new TNM staging system. Thoracic Surgery Study Group of Osaka University, Osaka, Japan. Ann Thorac Surg. 2000;70:1615–1619. doi: 10.1016/s0003-4975(00)01401-6. [DOI] [PubMed] [Google Scholar]
- 2.Rostad H, Naalsund A, Jacobsen R, et al. Small cell lung cancer in Norway: Should more patients have been offered surgical therapy? Eur J Cardiothorac Surg. 2004;26:782–786. doi: 10.1016/j.ejcts.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Brock MV, Hooker CM, Syphard JE, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: Its time has come. J Thorac Cardiovasc Surg. 2005;129:64–72. doi: 10.1016/j.jtcvs.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Lim E, Belcher E, Yap YK, et al. The role of surgery in the treatment of limited disease small cell lung cancer: Time to reevaluate. J Thorac Oncol. 2008;3:1267–1271. doi: 10.1097/JTO.0b013e318189a860. [DOI] [PubMed] [Google Scholar]
- 5.Schneider BJ, Saxena A, Downey RJ. Surgery for early-stage small cell lung cancer. J Natl Compr Canc Netw. 2011;9:1132–1139. doi: 10.6004/jnccn.2011.0094. [DOI] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer (version 2.2015). Fort Washington, PA, National Comprehensive Cancer Network, 2015. [DOI] [PMC free article] [PubMed]
- 7.Macchiarini P, Hardin M, Basolo F, et al. Surgery plus adjuvant chemotherapy for T1-3N0M0 small-cell lung cancer: Rationale for current approach. Am J Clin Oncol. 1991;14:218–224. doi: 10.1097/00000421-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Karrer K, Ulsperger E, for the ISC-Lung Cancer Study Group Surgery for cure followed by chemotherapy in small cell carcinoma of the lung. Acta Oncol. 1995;34:899–906. doi: 10.3109/02841869509127202. [DOI] [PubMed] [Google Scholar]
- 9.Rea F, Callegaro D, Favaretto A, et al. Long-term results of surgery and chemotherapy in small cell lung cancer. Eur J Cardiothorac Surg. 1998;14:398–402. doi: 10.1016/s1010-7940(98)00203-6. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya R, Suzuki K, Ichinose Y, et al. Phase II trial of postoperative adjuvant cisplatin and etoposide in patients with completely resected stage I-IIIa small cell lung cancer: The Japan Clinical Oncology Lung Cancer Study Group Trial (JCOG9101) J Thorac Cardiovasc Surg. 2005;129:977–983. doi: 10.1016/j.jtcvs.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 11.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic Cranial Irradiation Overview Collaborative Group Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 12.Sher DJ, Liptay MJ, Fidler MJ. Prevalence and predictors of neoadjuvant therapy for stage IIIA non-small cell lung cancer in the National Cancer Database: Importance of socioeconomic status and treating institution. Int J Radiat Oncol Biol Phys. 2014;89:303–312. doi: 10.1016/j.ijrobp.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual (ed 6) New York, NY, : Springer; 2002. doi: 10.1007/978-1-4757-3656-4. [Google Scholar]
- 14.Wagner PL, Kitabayashi N, Chen YT, et al. Combined small cell lung carcinomas: Genotypic and immunophenotypic analysis of the separate morphologic components. Am J Clin Pathol. 2009;131:376–382. doi: 10.1309/AJCPYNPFL56POZQY. [DOI] [PubMed] [Google Scholar]
- 15. American College of Surgeons Commission on Cancer: Cancer Program Standards: Ensuring Patient-Centered Care. Chicago, IL, American College of Surgeons, 2012. [Google Scholar]
- 16.Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): A randomized, double-blind, phase III trial. J Clin Oncol. 2015;33:4007–4014. doi: 10.1200/JCO.2015.61.8918. [DOI] [PubMed] [Google Scholar]
- 17.Booth CM, Shepherd FA, Peng Y, et al. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: A population-based study. Cancer. 2013;119:1243–1250. doi: 10.1002/cncr.27823. [DOI] [PubMed] [Google Scholar]
- 18. American College of Surgeons Commission on Cancer: The CoC/NCDB PUF Data Dictionary Items. http://ncdbpuf.facs.org/node/259?q=print-pdf-all.
- 19. Jett JR, Schild SE, Kesler KA, et al: Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed—American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143:e400S-e419S, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Le Péchoux C, Dunant A, Senan S, et al. Prophylactic Cranial Irradiation (PCI) Collaborative Group Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): A randomised clinical trial. Lancet Oncol. 2009;10:467–474. doi: 10.1016/S1470-2045(09)70101-9. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg. 2007;83:1245–1250. doi: 10.1016/j.athoracsur.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Yu JB, Decker RH, Detterbeck FC, et al. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010;5:215–219. doi: 10.1097/JTO.0b013e3181cd3208. [DOI] [PubMed] [Google Scholar]
- 23.Varlotto JM, Recht A, Flickinger JC, et al. Lobectomy leads to optimal survival in early-stage small cell lung cancer: A retrospective analysis. J Thorac Cardiovasc Surg. 2011;142:538–546. doi: 10.1016/j.jtcvs.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 24.Weksler B, Nason KS, Shende M, et al. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. 2012;94:889–893. doi: 10.1016/j.athoracsur.2012.01.015. [DOI] [PubMed] [Google Scholar]