Abstract
Among the toxic polypeptides secreted in the venom of sea anemones, actinoporins are the pore-forming toxins whose toxic activity relies on the formation of oligomeric pores within biological membranes. Intriguingly, actinoporins appear as multigene families that give rise to many protein isoforms in the same individual displaying high sequence identities but large functional differences. However, the evolutionary advantage of producing such similar isotoxins is not fully understood. Here, using sticholysins I and II (StnI and StnII) from the sea anemone Stichodactyla helianthus, it is shown that actinoporin isoforms can potentiate each other's activity. Through hemolysis and calcein releasing assays, it is revealed that mixtures of StnI and StnII are more lytic than equivalent preparations of the corresponding isolated isoforms. It is then proposed that this synergy is due to the assembly of heteropores because (i) StnI and StnII can be chemically cross-linked at the membrane and (ii) the affinity of sticholysin mixtures for the membrane is increased with respect to any of them acting in isolation, as revealed by isothermal titration calorimetry experiments. These results help us understand the multigene nature of actinoporins and may be extended to other families of toxins that require oligomerization to exert toxicity.
Keywords: erythrocyte, ion channel, lipid-protein interaction, oligomerization, protein cross-linking, toxin, equinatoxin, lysis, pore-forming-toxin, sticholysin
Introduction
Actinoporins, single polypeptide chains of around 175 amino acids, constitute a family of toxic proteins produced by different sea anemone species. They show basic isoelectric point values and are usually cysteineless (1–4). Actinoporins belong to a much larger group of widely distributed proteins, known as pore-forming toxins, whose toxic activity relies on the formation of pores within biological membranes (5–9). All pore-forming toxins show a very similar dual behavior by which they remain mostly monomeric and stably folded in aqueous solution but become oligomeric integral proteins when encountering membranes (2–4, 10–23).
The incorporation of actinoporins into the membrane largely depends on lipid bilayer composition and membrane physicochemical state (18, 24–29). Both factors influence the conformational changes occurring during the transition from the water media to the inserted states of the protein (30, 31). Thus, high affinity recognition of sphingomyelin (SM)3 is crucial for specific attachment to a membrane, but the subsequent effects observed also depend on the physical properties derived from its particular composition and not only from its SM content (23, 32). In fact, although still controversial, the presence of cholesterol and the coexistence of different phases in the membrane seem to be important factors, if not for binding then at least for the final formation of the pore (23, 28, 29, 32–36).
Actinoporins have been isolated from more than 20 different sea anemone species (1, 3, 37–41) in agreement with their rather ubiquitous distribution within the Actinaria order (1). They display high sequence identities (between 60 and 80%) and appear as multigene families, giving rise to many protein isoforms within the same individual (42–46). Despite the small number of amino acid changes between them, actinoporin isoforms usually result in substantial functional differences in terms of solubility and lytic activity (38, 45, 47–51), as exemplified by StnI and StnII, produced by Stichodactyla helianthus, and also two of the best characterized actinoporins (3, 4, 20, 22, 24, 47–50, 52).
The reason why a single anemone produces several isoforms of actinoporins in its venom is still not fully understood. One possible explanation would be to expand the range of prey susceptible of being attacked (53). Such a strategy would extend and modulate the range of action of sea anemones. It has even been proposed an analogy with immunoglobulins, which suggests that sea anemone tentacles could produce many actinoporin isoforms because they would represent the embryo of a rudimentary defense system (45). However, so far the possibility that these different isoforms show synergistic activity has not been explored. This possibility is interesting because it would lead to more efficient venoms. The results presented here not only prove that StnI and StnII potentiate their lytic activity when they act together but also indicate that they can establish functional heteropores, suggesting that actinoporins have a more deeply regulated physiological mode of action than previously believed.
Experimental Procedures
Materials
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol (Chol), and porcine brain SM were obtained from Avanti Polar Lipids. Disuccinimidyl suberate (DSS) was purchased from Pierce (Thermo Scientific). The preparation of the cDNA coding for StnI, StnII, and the six His-tagged version of StnII (6HStnII), as well as the production and purification of the three different proteins, has been described before (30, 50, 54). Homogeneity of all protein samples used was analyzed by 0.1% (w/v) SDS-12–15% PAGE (w/v) performed under standard conditions (55) and amino acid analysis after acid hydrolysis of the proteins (5.7 m HCl, 24 h, 110 °C). These amino acid analyses were performed on a Biochrom 20 automatic analyzer (GE Healthcare). All protein batches used were also previously characterized in terms of recording their far-UV circular dichroism (CD) spectra on a Jasco 715 spectropolarimeter, also as described (20, 21, 56, 57).
Hemolysis
Hemolysis assays were performed in 96-multiwell plates as described previously (30, 50). Briefly, erythrocytes from heparinized sheep blood were washed in 10 mm Tris buffer, pH 7.4, containing 145 mm NaCl, to a final A655 of 0.5 when mixing equal volumes of the cell suspension and buffer. The hemolysis was followed as a decrease in A655 after addition of the erythrocyte suspension to different final concentrations of protein. An Expert 96 microplate reader (Asys Hitech, GmbH, Eugendorf, Austria) was employed to measure A655. The value obtained with 0.1% (w/v) Na2CO3 was considered as 100% hemolysis.
Lipid Vesicle Preparation
DOPC/SM/Chol (1:1:1) phospholipid vesicles were prepared as described previously (20, 23, 58). A phospholipid (0.1–1.0 mg) solution in 2:1 (v/v) chloroform/methanol was dried under a flow of nitrogen, and the dry film obtained was used to prepare a lipid dispersion by adding 0.5–2.0 ml of Tris-NaCl (10 mm Tris-HCl, pH 7.4, 140 mm NaCl), briefly vortex mixing, and incubating for 1 h at 37 °C. This suspension of multilamellar vesicles was further subjected to five cycles of extrusion at 37 °C through polycarbonate filters (100-nm pore size) to obtain a homogeneous population of unilamellar vesicles.
Calcein Leakage Assays
Calcein-entrapped DOPC/SM/Chol (1:1:1) large unilamellar vesicles (LUVs) were prepared as described (23) by extrusion through 100-nm filters (Nucleopore, Whatman) at 37 °C. Briefly, the desired lipids were mixed and dried under a stream of nitrogen. The lipids were re-dissolved in chloroform and dried again before removal of any traces of remaining solvent in vacuum for 60 min. Prior to extrusion, the dry lipid films were hydrated for 1 h at 37 °C in Tris buffer (10 mm Tris, 140 mm NaCl, 0.5 mm EDTA, pH 7.4), containing 100 mm calcein. The total lipid concentration was 1.25 mm. LUVs were separated from non-entrapped calcein by gel filtration on Sephacryl S200HR. These LUVs were used for permeabilization studies within 24 h. Phospholipid concentration was determined from measurement of phosphorus (59) after elution of vesicles during isolation. The concentrations of LUV phospholipids and protein during calcein leakage experiments were about 7.5 μm and 1–80 nm, respectively. Emission at 550 nm was followed at 23 °C as a function of time (excitation at 480 nm). Fluorescence emission was measured with an SLM Aminco 8000 spectrofluorimeter. To ensure that no major spontaneous leakage occurred, the emission was measured for each sample during 5 min before addition of toxin. A steady signal level, indicating intact vesicles, was observed for all samples. Maximum calcein release was determined upon LUV disintegration induced by 10% Triton X-100.
Cross-linking Experiments
Cross-linking was performed essentially as described before (60). DSS was used as the cross-linking reagent in a reaction that was performed by adding a small aliquot of a concentrated freshly prepared cross-linker solution to the protein sample at the required concentrations. Protein (wild-type StnI and a His6-tagged version of StnII (6HStnII)) (50) and vesicle mixtures were prepared in 15 mm MOPS, pH 7.4, containing 50 mm NaCl, at the following final molar concentrations: 2.0 μm StnI, 0.5 μm 6HStnII, 0.1 μm (lipid concentration) DOPC/SM/Chol (1:1:1) phospholipid vesicles, and 0.04 μm DSS. The final concentration of each protein employed in this case was independent of the presence or not of the other actinoporin. A second set of cross-linking experiments was made with mixtures containing a constant concentration of 6HStnII and increasing amounts of StnI up to a StnI/6HStnII molar ratio of 95:5. The cross-linker was dissolved in dimethyl sulfoxide (DMSO). The protein/lipid reaction mixtures were incubated at 37 °C for 1 h, then the cross-linker was added and kept for 30 min at room temperature, and finally the mixtures were quenched for 15 min by adding an aliquot of the same buffer but containing 50 mm Lys. After addition of the corresponding electrophoresis loading buffer to each aliquot, they were boiled for 20 min in the presence of 0.5% (v/v) β-mercaptoethanol, and the cross-linked products were analyzed by SDS-PAGE following standard procedures (55). Western immunoblotting was used to detect 6HStnII using a mouse monoclonal anti-polyhistidine-peroxidase antibody from Sigma.
Protein Binding to Lipid Vesicles
Binding was measured using isothermal titration calorimetry (ITC) as described before (21, 30, 61), using a VP-ITC calorimeter (MicroCal). Briefly, protein solutions at 1.5–10.0 μm concentration were titrated by injection of 10- or 20-μl aliquots of lipid suspensions (phospholipid concentration, 0.85–5.00 mm). Binding isotherms were adjusted to a model where the protein binds to the membrane involving “n” lipid molecules (30).
Results
StnI and StnII Show Synergistic Hemolytic Activity
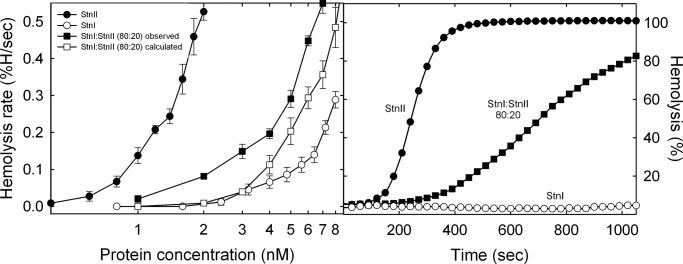
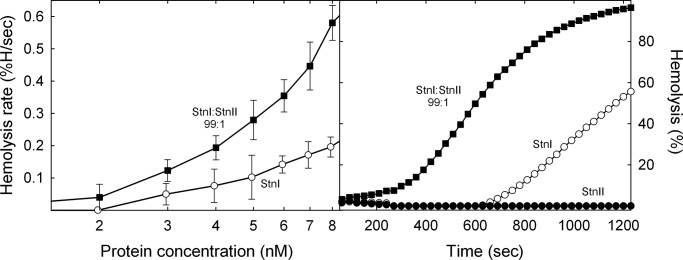
A hemolysis experiment was designed to study the potential formation of StnI/StnII heteropores and its functional consequences. With this idea, sheep erythrocyte hemolysis was assayed in the presence of isolated StnI or StnII at different concentrations or for a mixture of StnI/StnII at 80:20 constant molar ratio (Fig. 1). This experiment was so designed given the lower hemolytic activity of StnI. Inspection of results shown in Fig. 1 reveal how the mixture produced higher hemolysis rates than that one resulting from the arithmetical combination of the corresponding values obtained with the individual proteins.
FIGURE 1.
Left panel, maximum hemolytic rate values (expressed as percentage of hemolysis/s) are represented versus the logarithm of total protein concentration: StnI (white dots), StnII (black dots), and the StnI/StnII (80:20) mixture (black squares). The white squares line was obtained as the arithmetical addition of the rates obtained with the individual proteins for the real concentration of each one in the different mixtures employed. Results shown are the average of four independently performed experiments. Each of these experiments was made in duplicate. Error bars represent ±S.D. Right panel, as a representative example, the hemolytic activity curves of StnI (white dots), StnII (black dots), or a StnI/StnII (80:20) mixture (black squares), at a total protein concentration of 2 nm, are also shown.
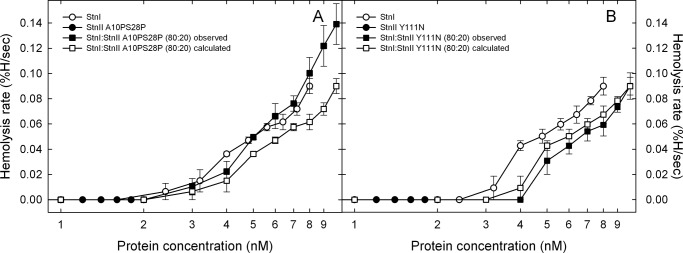
To evaluate the specificity of this observation, two different StnII mutants were employed as controls. First, the same experiment as that described in Fig. 1 was made using StnII A10PS28P instead of the wild-type protein. This mutant has been previously described as retaining its full membrane binding activity but showing a highly diminished pore-forming ability due to its inability to extend the needed α-helical stretch (30). As shown in Fig. 2A, even though the mutant was completely unable to lyse the erythrocytes within the full concentration range assayed, the mixture still produced higher hemolysis rates than those resulting from the arithmetical combination of the corresponding values obtained with the individual proteins (wild-type StnI and A10PS28P StnII at a 80:20 ratio).
FIGURE 2.
Maximum hemolytic rate values (expressed as percentage of hemolysis/s) are represented versus the logarithm of total protein concentration of individual and two different actinoporin mixtures: wild-type StnI and A10PS28P (A) or Y111N (B) StnII mutants. Both panels show the behavior of StnI (white dots), the StnII mutant (black dots), and the StnI/StnII mutant (80:20) mixture (black squares). The white squares line was obtained as the arithmetical addition of the rates obtained with the individual proteins for the real concentration of each one in the different mixtures employed. Results shown are the average of four independently performed experiments. Each of these experiments was made in duplicate. Error bars represent ±S.D.
In the second control experiment performed, the StnII mutant used was Y111N. In this StnII variant a key residue of the so-called phosphocholine-binding site has been replaced rendering a protein that cannot bind to the membrane and therefore shows a dramatically reduced hemolytic activity (30, 54). It can be seen how in this case (Fig. 2B) the synergistic effect of StnII is not observed. Overall, the three sets of experiments suggest not only the existence of synergistic action between both actinoporin isoforms, StnI and StnII, but also that this synergy seems to occur at the membrane binding step of the pore formation mechanism.
StnI and StnII Show Synergistic Lytic Activity toward Lipid Model Vesicles
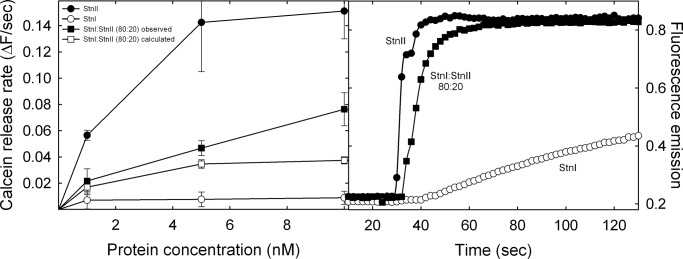
Erythrocytes are a rather complex model system to study protein-lipid interactions and assembly of pores at the membrane. Therefore, an experiment was designed to study leakage of calcein-containing DOPC/SM/Chol (1:1:1) phospholipid vesicles upon addition of different actinoporin concentrations. This type of vesicle represents one of the standard models most widely used to characterize StnI and StnII pore formation behavior (18, 20, 23, 28, 30, 54, 62). As can be observed in Fig. 3, the results obtained were similar to those corresponding to the hemolysis assays. The mixture of StnI and StnII produced higher calcein release rates than that resulting from the arithmetical combination of the corresponding values obtained with the individual proteins.
FIGURE 3.
Left panel, calcein release of maximal rates (expressed as normalized fluorescence intensity increment/s) are represented versus the total protein concentration: StnI (white dots), StnII (black dots), and the StnI/StnII (80:20) mixture (black squares). The white squares line was obtained as the arithmetical addition of the rates obtained with the individual proteins taking into account the real concentration of each one of them in the different mixtures employed. Results shown are the average of three independently performed experiments. Each of these experiments was made in duplicate. Error bars represent ± S.D. Right panel, as a representative example, the calcein leakage traces of StnI (white dots), StnII (black dots), or an StnI/StnII (80:20) mixture (black squares), at a total protein concentration of 5 nm, are also shown.
StnI and StnII Can Be Cross-linked in the Presence of Lipid Membranes
The results obtained from both sets of activity experiments, hemolysis and calcein release assays, show that StnI and StnII display synergistic activity. One explanation for this synergy would be the formation of active StnI/StnII heteropores. If this were the case, molecules from both proteins studied should be in close enough proximity as to be cross-linked using a short bifunctional reagent such as DSS (spacer arm, 1.1 nm), which reacts against exposed primary amines. Consequently, mixtures of StnI and StnII were incubated in the presence or absence of DOPC/SM/Chol (1:1:1) phospholipid vesicles and then DSS was added, following a standard protocol (63). The resulting mixture of proteins was analyzed by means of SDS-PAGE followed by Western blotting and immunodetection. For this purpose, instead of the wild-type protein, a His6-tagged version of StnII (6HStnII) was employed. This protein has been described to retain the general features and molecular mechanism of wild-type StnII (50, 64). This 6HStnII variant shows two advantages for cross-linking experiments. First, the presence of the N-terminal poly(His) tag shifts its electrophoretic mobility to the point where it can be unequivocally distinguished from wild-type StnI (50). Second, it can be identified by an anti-poly-His antibody without cross-reactivity from StnI (18). Therefore, the results presented in blots of Figs. 4 and 5 reveal only the presence of 6HStnII, independently of the amount of StnI present.
FIGURE 4.

Immunoblotting detection of 6HStnII previously incubated in the presence, or not, of wild-type StnI, DOPC/SM/Chol (1:1:1) phospholipid vesicles, and/or DSS, as indicated. Proteins were detected using a mouse monoclonal anti-polyhistidine-peroxidase antibody. The amount of 6HStnII loaded was 2.5 pmol. The StnI:6HStnII molar ratio employed was 80:20 in all instances shown. Molecular weight standards (EZ-RUNTM pre-stained Rec Protein Ladder) were also loaded, and the corresponding molecular masses are indicated in kDa at the left margin.
FIGURE 5.
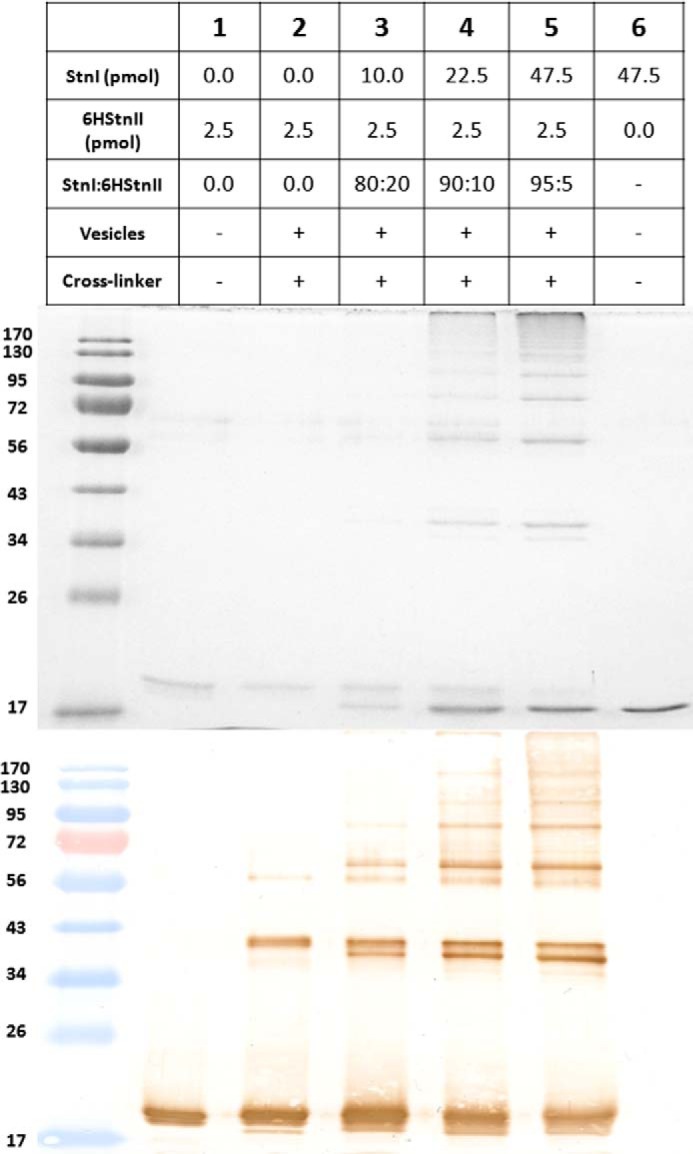
Coomassie Blue-stained gel (upper panel) and the corresponding immunoblotting detection (lower panel) of 6HStnII titrated with increasing amounts of StnI are shown. The proteins, and also the mixtures assayed, were incubated in the presence, or not, of wild-type StnI, DOPC/SM/Chol (1:1:1) phospholipid vesicles, and/or DSS, as indicated. Proteins were detected using a mouse monoclonal anti-polyhistidine-peroxidase antibody. The amount of 6HStnII loaded was 2.5 pmol in all instances shown. The StnI/6HStnII molar ratio employed is also indicated. Molecular weight standards (EZ-RUNTM pre-stained Rec Protein Ladder) were also loaded, and the corresponding molecular masses are indicated in kDa at the left margin.
In the absence of vesicles, only 6HStnII is detected in Fig. 4, lanes 1 and 2, despite the presence of a 4-fold higher concentration of StnI or the previous addition, or not, of the cross-linking agent. Fig. 4, lanes 3 and 4, shows the same set of results, cross-linked or not, but this time after incubation of both proteins with DOPC/SM/Chol (1:1:1) vesicles. Thus, again, in the absence of DSS, no other band but that one corresponding to monomeric 6HStnII was observed (Fig. 4, lane 3). However, when the cross-linker was present, new bands of different electrophoretic mobility were evident (Fig. 4, lane 4). At least two of these bands were not observed if 6HStnII (Fig. 4, lane 5) or StnI (lane 6) were the only proteins present. Therefore, they can only correspond to hetero-oligomers made of 6HStnII and wild-type StnI, the only situation that would explain their immunodetection by the anti-poly(His) antibody together with their singular electrophoretic mobility. It has been well determined that the presence of the six His tags at the N-terminal end of sticholysins has a deep impact on their electrophoretic mobility (50).
To show further evidence of the assembly of StnI and 6HStnII into cross-linkable heteropores, the titration experiment shown in Fig. 5 was performed. Again, under these conditions and in the absence of vesicles, only 6HStnII was detected in Fig. 5, lane 1. However, when cross-linker and vesicles were present, 6HStnII cross-linked oligomers could be detected (Fig. 5, lane 2). In fact, the observed distribution pattern is very similar to the one reported previously for equinatoxin II under almost identical conditions (25, 65). As shown in Fig. 5, lanes 2–5, upon increasing the StnI/6HStnII ratio, the appearance of the bands corresponding to cross-linked proteins is increasingly evident altogether with a decrease of the monomeric 6HStnII species. This second set of results not only confirms the presence of cross-linkable heteropores on the bilayer but also its marked protein concentration and StnI/6HStnII ratio dependence. The presence of other hetero-oligomers of lower electrophoretic mobility is also detected but, given the inherent influence of the DSS reaction on band electrophoretic mobility and sharpness (60), it is not possible to assign specific stoichiometries. We also used Coomassie staining to analyze the results of cross-linking. Compared with the Western blotting results, we found that the lower mobility heterooligomer bands at around 40 kDa that become apparent at higher StnI/6HStnII ratios have a lower reactivity against the anti-His antibody, in agreement with the lower content of His tags in the heterooligomers. In summary, this set of experiments allowed us to conclude that, in the presence of membranes, actinoporins StnI and StnII are close enough as to be cross-linked by a short cross-linking agent, suggesting that heteropores of StnI and StnII are formed.
Mixtures of StnI and StnII Show Increased Membrane Affinity
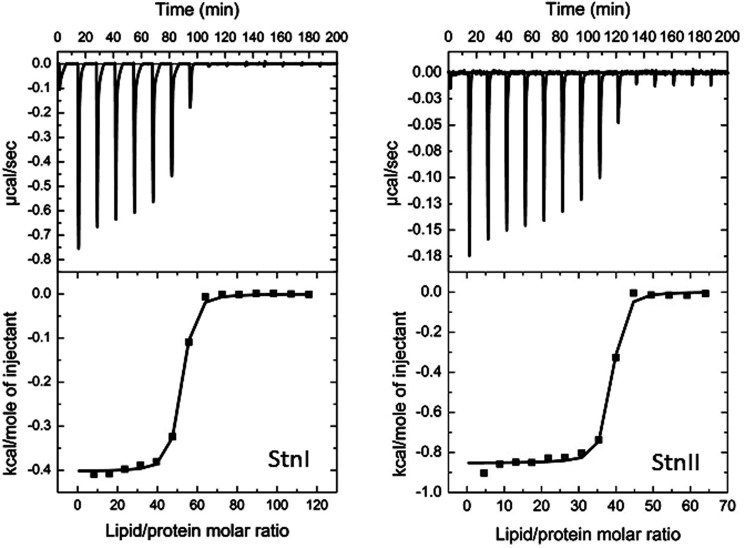
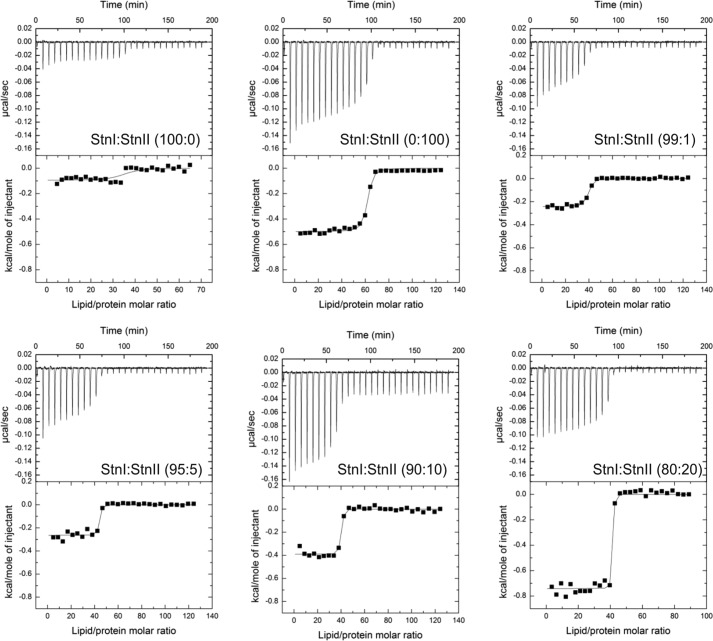
The actinoporins pore formation mechanism has been thoroughly studied although some of its details are still the subject of dispute (17, 32, 66–70). In a simplified version of this mechanism, two different steps can be distinguished. First, the protein binds to the membrane, and second, it assembles into a functional oligomeric pore (18, 30). Within the context of this simplified picture, it can be assumed that binding assays by ITC measure the affinity of the proteins to the membrane (30). As observed in Fig. 6, the affinity of StnI for DOPC/SM/Chol (1:1:1) phospholipid vesicles is lower than that corresponding to StnII (Table 1). In terms of relative membrane binding affinity, StnII binding to the vesicles is 4-fold higher, a result that is good enough by itself to explain the long known differences in terms of hemolytic and calcein leakage activities between StnI and StnII (26, 71).
FIGURE 6.
Binding of StnI and StnII to DOPC/SM/Chol (1:1:1) vesicles studied by ITC. Reactant concentrations were those ones shown in Table 1. Binding isotherms were adjusted to a model in which protein membrane binding involves the participation of “n” lipid molecules (30). The c values (c = Ka × P0) for the graphs shown are within the range 1–1000.
TABLE 1.
Binding of StnI, StnII, or different StnI/StnII mixtures to DOPC/SM/Chol (1:1:1) vesicles studied by ITC
Thermodynamic parameters for protein mixtures StnI/StnII (95:5), StnI/StnII (90:10), and StnI/StnII (80:20) (Fig. 7) should be considered only as estimations and are shown only for trend consistency purposes because binding affinities were so high that keeping the c values within range involved dilutions below the recommended detection limits of the instrument. Therefore, the corresponding c values of the three mixtures containing the higher proportion of StnII are higher than the recommended ones. Results shown are the average of at least three separate experiments.
| StnI/StnII (100:0) | StnI/StnII (0:100)a | StnI/StnII (99:1) | StnI/StnII (95:5) | StnI/StnII (90:10) | StnI/StnII (80:20) | |
|---|---|---|---|---|---|---|
| n | 49 ± 1 | 39 ± 4 | 38 ± 1 | 42 ± 1 | 38 ± 1 | 47 ± 9 |
| Ka (m−1) × 10−8 | 0.41 ± 0.03 | 1.70 ± 0.90 | 2.84 ± 0.91 | 33.90 ± 41.90 | 23.20 ± 19.90 | 237.00 ± 29.30 |
| ΔG (kcal/mol) | −8.21 ± 0.03 | −9.10 ± 0.50 | −10.90 ± 0.48 | −10.89 ± 0.48 | −10.73 ± 0.37 | −11.54 ± 1.29 |
| ΔH (kcal/mol) | −20.9 ± 2.1 | −44.0 ± 3.0 | −9.1 ± 0.1 | −11.1 ± 0.1 | −14.0 ± 0.1 | −27.3 ± 3.2 |
| ΔS (cal/mol·K) | −42.84 ± 6.78 | −115 ± 9.00 | −1.2 ± 0.73 | −0.8 ± 2.0 | −14.0 ± 1.7 | −52.9 ± 6.4 |
| [Protein] | 10.0 μm | 1.5 μm | 1.5 μm | 1.5 μm | 1.5 μm | 1.0 μm |
| c = Ka × [Protein] | 410 | 255 | 426 | 5090 | 3480 | 23700 |
| RBMb | 0.19 | 1.00 | 1.71 | 5.50 | 4.20 | 115.7 |
To explore whether improved binding could contribute to the synergistic lytic activity shown by sticholysins, we performed ITC binding experiments in which a total actinoporin concentration was fixed, but different StnI/StnII molar ratios were assayed (Fig. 7). Quite surprisingly, the binding affinity of mixtures of StnI and StnII was always higher than for any of the two actinoporins acting in isolation. This effect was already detected in the presence of trace amounts (1.0%) of StnII and correlated with increased relative membrane binding (Fig. 7 and Table 1). It is difficult to conceive how the binding affinity could increase without direct interaction between StnI and StnII. Hence, the ITC experiments lend additional support to the hypothesis that StnI and StnII can assemble into functional heteropores, leading to synergistic lytic activity.
FIGURE 7.
Binding of StnI, StnII, and different StnI/StnII mixtures (molar ratios as indicated) to DOPC/SM/Chol (1:1:1) vesicles studied by ITC. Reactant concentrations for the examples shown are P0 = 1.5 μm and L0 = 0.85 mm for all experiments, where P0 refers to the initial total protein concentration within the calorimeter cell and L0 is the lipid concentration within the dispensing auto-pipette. Binding isotherms were adjusted to a model in which protein membrane binding involves the participation of “n” lipid molecules (30). The c values (c = Ka × P0) for the graphs shown were in the 1–1000 range only for the StnI/StnII (0:100), StnI/StnII (99:1), and StnI/StnII (100:0). In the other three thermograms shown the binding affinities were so high that keeping the c values within range involved dilutions below the recommended detection limits of the instrument.
Finally, given the large effect on affinity of only traces of StnII in the mixtures, we studied whether this increase in binding affinity was directly correlated with an enhancement of function. As can be seen in Fig. 8, just 1.0% of StnII in the mixtures was enough to dramatically improve their hemolytic activity, as revealed by the hemolysis assays performed with different StnI/StnII (99:1) mixtures over a 1–10 nm concentration range (Fig. 8).
FIGURE 8.
Left panel, maximum hemolytic rate values (expressed as percentage of hemolysis/s) are represented versus the logarithm of total protein concentration of StnI (white dots) and an StnI/StnII (99:1) mixture (black squares). In these experiments the amount of StnII present in all the mixtures was so low that, when assayed in the absence of StnI, its hemolytic activity was undetectable in the time range measured. Results shown are the average of four independently performed experiments. Each of these experiments was made in duplicate. Error bars represent ±S.D. Right panel, as a representative example, the hemolytic activity curves of StnI at 3.96 nm (white dots), StnII at 0.04 nm (black dots), and the corresponding StnI/StnII (99:1) 4 nm mixture (black squares) are also shown.
Discussion
Sea anemones produce a wide variety of toxic compounds that are mainly stored in their nematocysts (72). Among them, actinoporins constitute a well studied family of toxic pore-forming proteins (3, 4, 70). It has long been known that individual sea anemone species produce many different actinoporin isoforms that, indeed, are very differently represented in terms of the amount present in their venomous secretions. In this regard, actinoporins represent a well established example of a multigenic protein family (38, 39, 43, 45, 46, 51, 73). They are not, however, the only example of toxic pore-forming multigene protein families (74), suggesting that the results shown now could be of larger significance and not only restricted to the actinoporin family. In fact, it has been very recently suggested that the pore responsible for damaging mitochondria during apoptosis could be made of hetero-oligomers of the Bax and Bak proteins (75). The natural biological function of this genetic multiplicity is indeed far from being understood. Regarding the actinoporin family, it has been proposed that the existence of multiple isoforms would broaden the range of possible prey for a given species (38, 39, 43, 45, 46, 51, 73). In this regard, actinoporins might be similar to immunoglobulins, which require a plethora of highly diverse genes to counteract foreign antigens (45).
Here, we propose a complementary hypothesis to explain the evolutionary advantage of multigenic actinoporins, formation of mixed functional pores. This mechanism would enable a much wider and finely tuned modulation of their toxicity and specificity. These results support the feasibility of this novel hypothesis.
StnI and StnII are two of the best well studied actinoporins and also constitute an optimum example of two almost identical isoforms (they share 91.0% of amino acid sequence identity) produced by the same sea anemone species but showing very different hemolytic activities (24, 47, 50, 76). Consequently, they were the model proteins chosen to study the possibility of molecularly different actinoporins assembling into the same functional pore structure. Indeed, the employment of independently produced and isolated recombinant protein species excludes artifacts produced by traces of cross-contamination in the experiments shown that were made with only one single protein component.
Actinoporin pore structure and stoichiometry are still highly controversial and far from being solved (22, 36, 66, 69, 70, 77–79). The latest actinoporin pore-like oligomeric structure published is a crystalline octameric ensemble of FraC (66). According to those results, the two side-chain residues showing more buried surface upon oligomerization would be FraC Val-60 and Trp-149 (66, 80). These residues have their corresponding counterparts in StnI (Ile-59 and Trp-149) and StnII (Ile-58 and Trp-146). Both amino acids could nicely perform an identical function in the StnI-StnII mixed oligomers. Therefore, from a molecular point of view there would not be impediments for the formation of StnI-StnII heteropores.
Synergistic activity of StnI and StnII in both hemolysis and calcein-release experiments suggested that both proteins are in fact able to interact within the same pore structure (Figs. 1–3). Cross-linking experiments further support this observation because, and only in the presence of lipid vesicles, both StnI and StnII could be cross-linked with a short cross-linking agent (Figs. 4 and 5). Finally, we have also shown that mixtures of StnI and StnII show increased affinity for lipid vesicles, in agreement with the fact that both isoforms interact in the process of membrane binding and pore formation (Fig. 7). ITC and hemolytic experiments also show that 1.0% of StnII dramatically enhances StnI binding to lipid model vesicles driving a dramatic improvement of hemolytic activity. Taking into account the only moderate effects observed in calcein-leakage experiments, and the hemolysis results obtained with the two different StnII mutants used as control (Fig. 2), it can be also suggested that synergy between StnI and StnII could mostly be due to a better interaction with the membrane, although the final step of pore formation would not be strongly affected.
As stated above, actinoporins represent multigene families. However, only two or three different isoforms are usually produced by the same sea anemone species in amounts large enough to be detected and purified. For example, up to 19 different cDNA sequences have been detected for S. helianthus, the sea anemone producing StnI and StnII (43). Thus, taking into account this discrepancy between the number of actinoporin encoding genes found in sea anemones and the amount and diversity of isotoxins made, it is tempting to speculate that the less represented isoforms might play a role in regulating and/or potentiating the activity and specificity of those ones produced in larger amounts. Maybe this is just a strategy to fulfill a much more modulated and specific cytotoxic action against potential prey and/or predators.
Conclusions
Overall, the results presented here show that two different actinoporins produced by the same sea anemone species act in synergy, most probably interacting to form functional pores made of distinct protein isoforms. As far as we know, this observation has not been reported before. This interaction has sound consequences in terms of the biological functionality of actinoporins and suggests that it could represent a more general strategy employed by other pore-forming proteins. According to the results reported now, it can be speculated that one of the reasons for actinoporins being multigene families is the possibility of interaction among different isotoxins to exert a much more modulated and/or potentiated action against their prey and/or predators. This possibility would translate into more versatile defense and/or attack responses in their natural environment.
Author Contributions
E. R. T., S. G. L., and J. A. C. conducted the experiments. E. R. T., S. G. L., J. A. C., J. L., J. G. G., and A. M. P. conceived and designed the experiments, analyzed and discussed the results, wrote and corrected the manuscript, and suggested modifications.
This work was supported by Grant BFU2012-32404 from the Spanish Ministerio de Ciencia e Innovación (to A. M. P.), a Formación de Personal Universitario fellowship (to S. G. L.), a Universidad Complutense de Madrid collaboration fellowship (to E. R. T.), and Ramón y Cajal Award RYC-2014-16604) (to J. A. C.). The authors declare that they have no conflicts of interest with the contents of this article.
- SM
- sphingomyelin
- 6HStnII
- sticholysin II tagged with six histidine residues at the N terminus
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- DSS
- disuccinimidyl suberate
- ITC
- isothermal titration calorimetry
- LUV
- large unilamellar vesicle
- Stn
- sticholysin
- Chol
- cholesterol.
References
- 1. Macek P. (1992) Polypeptide cytolytic toxins from sea anemones (Actiniaria). FEMS Microbiol. Immunol. 5, 121–129 [DOI] [PubMed] [Google Scholar]
- 2. Anderluh G., and Macek P. (2002) Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 40, 111–124 [DOI] [PubMed] [Google Scholar]
- 3. Alegre-Cebollada J., Oñaderra M., Gavilanes J. G., and del Pozo A. (2007) Sea anemone actinoporins: the transition from a folded soluble state to a functionally active membrane-bound oligomeric pore. Curr. Protein Pept. Sci. 8, 558–572 [DOI] [PubMed] [Google Scholar]
- 4. García-Ortega L., Alegre-Cebollada J., García-Linares S., Bruix M., Martínez-Del-Pozo A., and Gavilanes J. G. (2011) The behavior of sea anemone actinoporins at the water-membrane interface. Biochim. Biophys. Acta 1808, 2275–2288 [DOI] [PubMed] [Google Scholar]
- 5. Parker M. W., and Feil S. C. (2005) Pore-forming protein toxins: from structure to function. Prog. Biophys. Mol. Biol. 88, 91–142 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez M. R., Bischofberger M., Pernot L., van der Goot F. G., and Frêche B. (2008) Bacterial pore-forming toxins: the (w)hole story? Cell. Mol. Life Sci. 65, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bischofberger M., Iacovache I., and van der Goot F. G. (2012) Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12, 266–275 [DOI] [PubMed] [Google Scholar]
- 8. Iacovache I., van der Goot F. G., and Pernot L. (2008) Pore formation: an ancient yet complex form of attack. Biochim. Biophys. Acta 1778, 1611–1623 [DOI] [PubMed] [Google Scholar]
- 9. Gouaux E. (1997) Channel-forming toxins: tales of transformation. Curr. Opin. Struct. Biol. 7, 566–573 [DOI] [PubMed] [Google Scholar]
- 10. Anderluh G., Dalla Serra M., Viero G., Guella G., Macek P., and Menestrina G. (2003) Pore formation by equinatoxin II, a eukaryotic protein toxin, occurs by induction of nonlamellar lipid structures. J. Biol. Chem. 278, 45216–45223 [DOI] [PubMed] [Google Scholar]
- 11. Athanasiadis A., Anderluh G., Macek P., and Turk D. (2001) Crystal structure of the soluble form of equinatoxin II, a pore-forming toxin from the sea anemone Actinia equina. Structure 9, 341–346 [DOI] [PubMed] [Google Scholar]
- 12. Kristan K. C., Viero G., Dalla Serra M., Macek P., and Anderluh G. (2009) Molecular mechanism of pore formation by actinoporins. Toxicon 54, 1125–1134 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert R. J., Dalla Serra M., Froelich C. J., Wallace M. I., and Anderluh G. (2014) Membrane pore formation at protein-lipid interfaces. Trends Biochem. Sci. 39, 510–516 [DOI] [PubMed] [Google Scholar]
- 14. Hinds M. G., Zhang W., Anderluh G., Hansen P. E., and Norton R. S. (2002) Solution structure of the eukaryotic pore-forming cytolysin equinatoxin II: implications for pore formation. J. Mol. Biol. 315, 1219–1229 [DOI] [PubMed] [Google Scholar]
- 15. Marchioretto M., Podobnik M., Dalla Serra M., and Anderluh G. (2013) What planar lipid membranes tell us about the pore-forming activity of cholesterol-dependent cytolysins. Biophys. Chem. 182, 64–70 [DOI] [PubMed] [Google Scholar]
- 16. Rojko N., Cronin B., Danial J. S., Baker M. A., Anderluh G., and Wallace M. I. (2014) Imaging the lipid-phase-dependent pore formation of equinatoxin II in droplet interface bilayers. Biophys. J. 106, 1630–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rojko N., Kristan K. Č., Viero G., Žerovnik E., Maček P., Dalla Serra M., and Anderluh G. (2013) Membrane damage by an α-helical pore-forming protein, Equinatoxin II, proceeds through a succession of ordered steps. J. Biol. Chem. 288, 23704–23715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alegre-Cebollada J., Rodríguez-Crespo I., Gavilanes J. G., and del Pozo A. (2006) Detergent-resistant membranes are platforms for actinoporin pore-forming activity on intact cells. FEBS J. 273, 863–871 [DOI] [PubMed] [Google Scholar]
- 19. Castrillo I., Araujo N. A., Alegre-Cebollada J., Gavilanes J. G., Martínez-del-Pozo A., and Bruix M. (2010) Specific interactions of sticholysin I with model membranes: an NMR study. Proteins 78, 1959–1970 [DOI] [PubMed] [Google Scholar]
- 20. García-Linares S., Castrillo I., Bruix M., Menéndez M., Alegre-Cebollada J., Martínez-del-Pozo Á., and Gavilanes J. G. (2013) Three-dimensional structure of the actinoporin sticholysin I. Influence of long-distance effects on protein function. Arch. Biochem. Biophys. 532, 39–45 [DOI] [PubMed] [Google Scholar]
- 21. García-Linares S., Richmond R., García-Mayoral M. F., Bustamante N., Bruix M., Gavilanes J. G., and Martínez-Del-Pozo A. (2014) The sea anemone actinoporin (Arg-Gly-Asp) conserved motif is involved in maintaining the competent oligomerization state of these pore-forming toxins. FEBS J. 281, 1465–1478 [DOI] [PubMed] [Google Scholar]
- 22. Mancheño J. M., Martín-Benito J., Martínez-Ripoll M., Gavilanes J. G., and Hermoso J. A. (2003) Crystal and electron microscopy structures of sticholysin II actinoporin reveal insights into the mechanism of membrane pore formation. Structure 11, 1319–1328 [DOI] [PubMed] [Google Scholar]
- 23. Alm I., García-Linares S., Gavilanes J. G., Martínez-Del-Pozo Á., and Slotte J. P. (2015) Cholesterol stimulates and ceramide inhibits sticholysin II-induced pore formation in complex bilayer membranes. Biochim. Biophys. Acta 1848, 925–931 [DOI] [PubMed] [Google Scholar]
- 24. Valcarcel C. A., Dalla Serra M., Potrich C., Bernhart I., Tejuca M., Martinez D., Pazos F., Lanio M. E., and Menestrina G. (2001) Effects of lipid composition on membrane permeabilization by sticholysin I and II, two cytolysins of the sea anemone Stichodactyla helianthus. Biophys. J. 80, 2761–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belmonte G., Pederzolli C., Macek P., and Menestrina G. (1993) Pore formation by the sea anemone cytolysin equinatoxin-II in red blood cells and model lipid membranes. J. Membr. Biol. 131, 11–22 [DOI] [PubMed] [Google Scholar]
- 26. Tejuca M., Serra M. D., Ferreras M., Lanio M. E., and Menestrina G. (1996) Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 35, 14947–14957 [DOI] [PubMed] [Google Scholar]
- 27. Shin M. L., Michaels D. W., and Mayer M. M. (1979) Membrane damage by a toxin from the sea anemone Stoichactis helianthus. II. Effect of membrane lipid composition in a liposome system. Biochim. Biophys. Acta 555, 79–88 [DOI] [PubMed] [Google Scholar]
- 28. de los Rios V., Mancheño J. M., Lanio M. E., Oñaderra M., and Gavilanes J. G. (1998) Mechanism of the leakage induced on lipid model membranes by the hemolytic protein sticholysin II from the sea anemone Stichodactyla helianthus. Eur. J. Biochem. 252, 284–289 [DOI] [PubMed] [Google Scholar]
- 29. Martínez D., Otero A., Alvarez C., Pazos F., Tejuca M., Lanio M. E., Gutiérrez-Aguirre I., Barlic A., Iloro I., Arrondo J. L., González-Mañas J. M., and Lissi E. (2007) Effect of sphingomyelin and cholesterol on the interaction of St II with lipidic interfaces. Toxicon 49, 68–81 [DOI] [PubMed] [Google Scholar]
- 30. Alegre-Cebollada J., Cunietti M., Herrero-Galán E., Gavilanes J. G., and Martínez-del-Pozo A. (2008) Calorimetric scrutiny of lipid binding by sticholysin II toxin mutants. J. Mol. Biol. 382, 920–930 [DOI] [PubMed] [Google Scholar]
- 31. Menestrina G., Cabiaux V., and Tejuca M. (1999) Secondary structure of sea anemone cytolysins in soluble and membrane bound form by infrared spectroscopy. Biochem. Biophys. Res. Commun. 254, 174–180 [DOI] [PubMed] [Google Scholar]
- 32. Pedrera L., Gomide A. B., Sánchez R. E., Ros U., Wilke N., Pazos F., Lanio M. E., Itri R., Fanani M. L., and Alvarez C. (2015) The presence of sterols favors sticholysin I-membrane association and pore formation regardless of their ability to form laterally segregated domains. Langmuir 31, 9911–9923 [DOI] [PubMed] [Google Scholar]
- 33. Bakrac B., and Anderluh G. (2010) Molecular mechanism of sphingomyelin-specific membrane binding and pore formation by actinoporins. Adv. Exp. Med. Biol. 677, 106–115 [PubMed] [Google Scholar]
- 34. Barlic A., Gutiérrez-Aguirre I., Caaveiro J. M., Cruz A., Ruiz-Argüello M. B., Pérez-Gil J., and González-Mañas J. M. (2004) Lipid phase coexistence favors membrane insertion of equinatoxin-II, a pore-forming toxin from Actinia equina. J. Biol. Chem. 279, 34209–34216 [DOI] [PubMed] [Google Scholar]
- 35. Caaveiro J. M., Echabe I., Gutiérrez-Aguirre I., Nieva J. L., Arrondo J. L., and González-Mañas J. M. (2001) Differential interaction of equinatoxin II with model membranes in response to lipid composition. Biophys. J. 80, 1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wacklin H. P., Bremec B. B., Moulin M., Rojko N., Haertlein M., Forsyth T., Anderluh G., and Norton R. S. (2016) Neutron reflection study of the interaction of the eukaryotic pore-forming actinoporin equinatoxin II with lipid membranes reveals intermediate states in pore formation. Biochim. Biophys. Acta 1858, 640–652 [DOI] [PubMed] [Google Scholar]
- 37. Bellomio A., Morante K., Barlic A., Gutiérrez-Aguirre I., Viguera A. R., and González-Mañas J. M. (2009) Purification, cloning and characterization of fragaceatoxin C, a novel actinoporin from the sea anemone Actinia fragacea. Toxicon 54, 869–880 [DOI] [PubMed] [Google Scholar]
- 38. Monastyrnaya M., Leychenko E., Isaeva M., Likhatskaya G., Zelepuga E., Kostina E., Trifonov E., Nurminski E., and Kozlovskaya E. (2010) Actinoporins from the sea anemones, tropical Radianthus macrodactylus and northern Oulactis orientalis: comparative analysis of structure-function relationships. Toxicon 56, 1299–1314 [DOI] [PubMed] [Google Scholar]
- 39. Monastyrnaya M. M., Zykova T. A., Apalikova O. V., Shwets T. V., and Kozlovskaya E. P. (2002) Biologically active polypeptides from the tropical sea anemone Radianthus macrodactylus. Toxicon 40, 1197–1217 [DOI] [PubMed] [Google Scholar]
- 40. Leichenko E. V., Monastirnaya M. M., Zelepuga E. A., Tkacheva E. S., Isaeva M. P., Likhatskaya G. N., Anastyuk S. D., and Kozlovskaya E. P. (2014) Hct-A is a new actinoporin family from the Heteractis crispa sea anemone. Acta Naturae 6, 89–98 [PMC free article] [PubMed] [Google Scholar]
- 41. Hu B., Guo W., Wang L. H., Wang J. G., Liu X. Y., and Jiao B. H. (2011) Purification and characterization of gigantoxin-4, a new actinoporin from the sea anemone Stichodactyla gigantea. Int. J. Biol. Sci. 7, 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anderluh G., Barlic A., Podlesek Z., Macek P., Pungercar J., Gubensek F., Zecchini M. L., Serra M. D., and Menestrina G. (1999) Cysteine-scanning mutagenesis of an eukaryotic pore-forming toxin from sea anemone: topology in lipid membranes. Eur. J. Biochem. 263, 128–136 [DOI] [PubMed] [Google Scholar]
- 43. de los Ríos V., Oñaderra M., Martínez-Ruiz A., Lacadena J., Mancheño J. M., Martínez del Pozo A., and Gavilanes J. G. (2000) Overproduction in Escherichia coli and purification of the hemolytic protein sticholysin II from the sea anemone Stichodactyla helianthus. Protein Expr. Purif. 18, 71–76 [DOI] [PubMed] [Google Scholar]
- 44. Turk T. (1991) Cytolytic toxins from sea anemones. J. Toxicol. Toxin Rev. 10, 223–262 [Google Scholar]
- 45. Wang Y., Yap L. L., Chua K. L., and Khoo H. E. (2008) A multigene family of Heteractis magnificalysins (HMgs). Toxicon 51, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 46. Anderluh G., Krizaj I., Strukelj B., Gubensek F., Macek P., and Pungercar J. (1999) Equinatoxins, pore-forming proteins from the sea anemone Actinia equina, belong to a multigene family. Toxicon 37, 1391–1401 [DOI] [PubMed] [Google Scholar]
- 47. Alvarez C., Mancheño J. M., Martínez D., Tejuca M., Pazos F., and Lanio M. E. (2009) Sticholysins, two pore-forming toxins produced by the Caribbean sea anemone Stichodactyla helianthus: their interaction with membranes. Toxicon 54, 1135–1147 [DOI] [PubMed] [Google Scholar]
- 48. del Monte-Martínez A., González-Bacerio J., Romero L., Aragón C., Martínez D., de Los Á Chávez M., Álvarez C., Lanio M. E., Guisán J. M., and Díaz J. (2014) Improved purification and enzymatic properties of a mixture of sticholysin I and II: isotoxins with hemolytic and phospholipase A activities from the sea anemone Stichodactyla helianthus. Protein Expr. Purif. 95, 57–66 [DOI] [PubMed] [Google Scholar]
- 49. Martínez D., Morera V., Alvarez C., Tejuca M., Pazos F., García Y., Raida M., Padrón G., and Eliana Lanio M. (2002) Identity between cytolysins purified from two morphos of the Caribbean sea anemone Stichodactyla helianthus. Toxicon 40, 1219–1221 [DOI] [PubMed] [Google Scholar]
- 50. Alegre-Cebollada J., Clementi G., Cunietti M., Porres C., Oñaderra M., Gavilanes J. G., and Pozo A. M. (2007) Silent mutations at the 5′-end of the cDNA of actinoporins from the sea anemone Stichodactyla helianthus allow their heterologous overproduction in Escherichia coli. J. Biotechnol. 127, 211–221 [DOI] [PubMed] [Google Scholar]
- 51. Uechi G., Toma H., Arakawa T., and Sato Y. (2010) Molecular characterization on the genome structure of hemolysin toxin isoforms isolated from sea anemone Actineria villosa and Phyllodiscus semoni. Toxicon 56, 1470–1476 [DOI] [PubMed] [Google Scholar]
- 52. Ros U., Pedrera L., Diaz D., Karam J. C., Sudbrack T. P., Valiente P. A., Martinez D., Cilli E. M., Pazos F., Itri R., Lanio M. E., Schreier S., and Ávarez C. (2011) The membranotropic activity of N-terminal peptides from the pore-forming proteins sticholysin I and II is modulated by hydrophobic and electrostatic interactions as well as lipid composition. J. Biosci. 36, 781–791 [DOI] [PubMed] [Google Scholar]
- 53. Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., and Cruz L. J. (1990) Diversity of Conus neuropeptides. Science 249, 257–263 [DOI] [PubMed] [Google Scholar]
- 54. Alegre-Cebollada J., Lacadena V., Oñaderra M., Mancheño J. M., Gavilanes J. G., and del Pozo A. M. (2004) Phenotypic selection and characterization of randomly produced non-haemolytic mutants of the toxic sea anemone protein sticholysin II. FEBS Lett. 575, 14–18 [DOI] [PubMed] [Google Scholar]
- 55. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 56. Alegre-Cebollada J., Martínez del Pozo A., Gavilanes J. G., and Goormaghtigh E. (2007) Infrared spectroscopy study on the conformational changes leading to pore formation of the toxin sticholysin II. Biophys. J. 93, 3191–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pardo-Cea M. A., Castrillo I., Alegre-Cebollada J., Martínez-del-Pozo Á., Gavilanes J. G., and Bruix M. (2011) Intrinsic local disorder and a network of charge-charge interactions are key to actinoporin membrane disruption and cytotoxicity. FEBS J. 278, 2080–2089 [DOI] [PubMed] [Google Scholar]
- 58. Martínez-Ruiz A., García-Ortega L., Kao R., Lacadena J., Oñaderra M., Mancheño J. M., Davies J., Martínez del Pozo A., and Gavilanes J. G. (2001) RNase U2 and α-sarcin: A study of relationships. Methods Enzymol. 341, 335–351 [DOI] [PubMed] [Google Scholar]
- 59. Bartlett G. R. (1959) Colorimetric assay methods for free and phosphorylated glyceric acids. J. Biol. Chem. 234, 469–471 [PubMed] [Google Scholar]
- 60. Oñaderra M., Mancheño J. M., Lacadena J., de los Rios V., Martínez del Pozo A., and Gavilanes J. G. (1998) Oligomerization of the cytotoxin α-sarcin associated with phospholipid membranes. Mol. Membr. Biol. 15, 141–144 [DOI] [PubMed] [Google Scholar]
- 61. Maula T., Isaksson Y. J., García-Linares S., Niinivehmas S., Pentikäinen O. T., Kurita M., Yamaguchi S., Yamamoto T., Katsumura S., Gavilanes J. G., Martínez-del-Pozo A., and Slotte J. P. (2013) 2NH and 3OH are crucial structural requirements in sphingomyelin for sticholysin II binding and pore formation in bilayer membranes. Biochim. Biophys. Acta 1828, 1390–1395 [DOI] [PubMed] [Google Scholar]
- 62. García-Linares S., Alm I., Maula T., Gavilanes J. G., Slotte J. P., and Martínez-Del-Pozo Á. (2015) The effect of cholesterol on the long-range network of interactions established among sea anemone Sticholysin II residues at the water-membrane interface. Mar. Drugs 13, 1647–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de los Ríos V., Mancheño J. M., Martínez del Pozo A., Alfonso C., Rivas G., Oñaderra M., and Gavilanes J. G. (1999) Sticholysin II, a cytolysin from the sea anemone Stichodactyla helianthus, is a monomer-tetramer associating protein. FEBS Lett. 455, 27–30 [DOI] [PubMed] [Google Scholar]
- 64. Pazos I. F., Martínez D., Tejuca M., Valle A., del Pozo A., Alvarez C., Lanio M. E., and Lissi E. A. (2003) Comparison of pore-forming ability in membranes of a native and a recombinant variant of Sticholysin II from Stichodactyla helianthus. Toxicon 42, 571–578 [DOI] [PubMed] [Google Scholar]
- 65. Macek P., Belmonte G., Pederzolli C., and Menestrina G. (1994) Mechanism of action of equinatoxin II, a cytolysin from the sea anemone Actinia equina L. belonging to the family of actinoporins. Toxicology 87, 205–227 [DOI] [PubMed] [Google Scholar]
- 66. Tanaka K., Caaveiro J. M., Morante K., González-Mañas J. M., and Tsumoto K. (2015) Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 6, 6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cosentino K., Ros U., and García-Sáez A. J. (2016) Assembling the puzzle: oligomerization of α-pore forming proteins in membranes. Biochim. Biophys. Acta 1858, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ros U., and García-Sáez A. J. (2015) More than a pore: the interplay of pore-forming proteins and lipid membranes. J. Membr. Biol. 248, 545–561 [DOI] [PubMed] [Google Scholar]
- 69. Subburaj Y., Ros U., Hermann E., Tong R., and García-Sáez A. J. (2015) Toxicity of an α-pore-forming toxin depends on the assembly mechanism on the target membrane as revealed by single-molecule imaging. J. Biol. Chem. 290, 4856–4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rojko N., Dalla Serra M., Maček P., and Anderluh G. (2016) Pore formation by actinoporins, cytolysins from sea anemones. Biochim. Biophys. Acta 1858, 446–456 [DOI] [PubMed] [Google Scholar]
- 71. Martinez D., Campos A. M., Pazos F., Alvarez C., Lanio M. E., Casallanovo F., Schreier S., Salinas R. K., Vergara C., and Lissi E. (2001) Properties of St I and St II, two isotoxins isolated from Stichodactyla helianthus: a comparison. Toxicon 39, 1547–1560 [DOI] [PubMed] [Google Scholar]
- 72. Wong E. S., and Belov K. (2012) Venom evolution through gene duplications. Gene 496, 1–7 [DOI] [PubMed] [Google Scholar]
- 73. Valle A., Alvarado-Mesén J., Lanio M. E., Álvarez C., Barbosa J. A., and Pazos I. F. (2015) The multigene families of actinoporins (part I): isoforms and genetic structure. Toxicon 103, 176–187 [DOI] [PubMed] [Google Scholar]
- 74. Brinkman D. L., Konstantakopoulos N., McInerney B. V., Mulvenna J., Seymour J. E., Isbister G. K., and Hodgson W. C. (2014) Chironex fleckeri (box jellyfish) venom proteins: expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 289, 4798–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dewson G. (2016) Doughnuts, daisy chains and crescent moons: the quest for the elusive apoptotic pore. EMBO J. 35, 371–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ros U., Rodríguez-Vera W., Pedrera L., Valiente P. A., Cabezas S., Lanio M. E., García-Sáez A. J., and Alvarez C. (2015) Differences in activity of actinoporins are related with the hydrophobicity of their N terminus. Biochimie 116, 70–78 [DOI] [PubMed] [Google Scholar]
- 77. Martín-Benito J., Gavilanes F., de Los Ríos V., Mancheño J. M., Fernández J. J., and Gavilanes J. G. (2000) Two-dimensional crystallization on lipid monolayers and three-dimensional structure of sticholysin II, a cytolysin from the sea anemone Stichodactyla helianthus. Biophys. J. 78, 3186–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mechaly A. E., Bellomio A., Gil-Cartón D., Morante K., Valle M., González-Mañas J. M., and Guérin D. M. (2011) Structural insights into the oligomerization and architecture of eukaryotic membrane pore-forming toxins. Structure 19, 181–191 [DOI] [PubMed] [Google Scholar]
- 79. Baker M. A., Rojko N., Cronin B., Anderluh G., and Wallace M. I. (2014) Photobleaching reveals heterogeneous stoichiometry for equinatoxin II oligomers. Chembiochem 15, 2139–2145 [DOI] [PubMed] [Google Scholar]
- 80. Morante K., Caaveiro J. M., Viguera A. R., Tsumoto K., and González-Mañas J. M. (2015) Functional characterization of Val60, a key residue involved in the membrane-oligomerization of fragaceatoxin C, an actinoporin from Actinia fragacea. FEBS Lett. 589, 1840–1846 [DOI] [PubMed] [Google Scholar]