Abstract
Vibrio vulnificus is a marine bacterium that causes human infections resulting in high mortality. This pathogen harbors five quorum-regulatory RNAs (Qrr1–5) that affect the expression of pathogenicity genes by modulating the expression of the master regulator SmcR. The qrr genes are activated by phosphorylated LuxO to different degrees; qrr2 is strongly activated; qrr3 and qrr5 are moderately activated, and qrr1 and qrr4 are marginally activated and are the only two that do not respond to cell density-dependent regulation. Qrrs function redundantly to inhibit SmcR at low cell density and fully repress when all five are activated. In this study, we found that iron inhibits qrr expression in three distinct ways. First, the iron-ferric uptake regulator (Fur) complex directly binds to qrr promoter regions, inhibiting LuxO activation by competing with LuxO for cis-acting DNA elements. Second, qrr transcription is repressed by iron independently of Fur. Third, LuxO expression is repressed by iron independently of Fur. We also found that, under iron-limiting conditions, the five Qrrs functioned additively, not redundantly, to repress SmcR, suggesting that cells lacking iron enter a high cell density mode earlier and could thereby modulate expression of virulence factors sooner. This study suggests that iron and quorum sensing, along with their cognate regulatory circuits, are linked together in the coordinated expression of virulence factors.
Keywords: bacterial signal transduction, host-pathogen interaction, iron, quorum sensing, small interfering RNA (siRNA), virulence factor, Fur, Qrrs, Vibrio vulnificus, smcR
Introduction
Vibrio vulnificus is a Gram-negative marine bacteria that causes septicemia and wound infection and is acquired either through a wound or through the gastrointestinal tract upon consumption of contaminated raw fish or water (1). Numerous virulence factors have been identified for this pathogen, including cytolysin detected in serum and skin lesions of infected mice, hemolysin, phospholipase, capsular polysaccharide, insulin-degrading enzyme, and metalloproteases (2–9). Repeats toxin (RTX), encoded by rtxA1, is important both in vitro and in vivo for survival during infection (10). Vulnibactin, a catechol siderophore, is essential for scavenging iron from human transferrin and therefore important for virulence (11, 12).
Bacterial pathogens experience a variety of stresses from natural or host environments during host infection, such as nutrient limitation, temperature changes, osmotic stress, and oxidative stress (13). Pathogenic bacteria have evolved sophisticated mechanisms through which to control gene expression under these differing environments by sensing relevant environmental factors and swiftly adapting to improve survival and pathogenicity.
It is well known that iron plays an important role in regulating virulence factors in pathogenic bacteria (14). In V. vulnificus, iron is necessary for growth and increased host mortality in vivo (15), and scavenging host iron is vitally important for its pathogenicity (16). Bacteria produce small molecules called siderophores that specifically bind Fe(III), ensuring iron acquisition from iron-scarce environments such as that in a host (14). V. vulnificus produces both hydroxamate- and phenolate-type siderophores (17). Mutants with impaired catechol (phenolate) siderophore production are less virulent when compared with wild type V. vulnificus (11), and vulnibactin is essential for utilization of transferrin- and lactoferrin-bound iron in vivo (18). Ferric uptake regulator (Fur)2 is the major iron-responsive transcriptional regulator in Gram-negative bacteria (19). In the presence of iron, Fur acts as a dimer to bind the consensus 19-bp palindromic Fur box (5′-GATAATGATAATCATTATC-3′) present in the promoter regions of target genes, and it represses transcription by inhibiting the binding of RNA polymerase (20). The Fur-iron complex regulates a series of genes, including those for siderophore synthesis and iron acquisition (21). In V. vulnificus, Fur is a 149-amino acid protein known to repress siderophore biosynthesis and utilization as well as heme utilization (11, 22, 23). Fur also directly regulates the expression of virulence factors such as VvhA in V. vulnificus (24).
Bacterial cell density is another factor that affects a broad range of cellular activities, including virulence. Regulation in response to cell density is accomplished through the quorum-sensing pathway, which monitors diffusible signal molecules that accumulate at high cell density, and subsequently modulates genes associated with survival and virulence (25). The quorum-sensing pathway in V. vulnificus is similar to that of Vibrio harveyi and Vibrio cholerae. V. vulnificus harbors a homolog of V. harveyi LuxS, which is an enzyme that synthesizes the autoinducer-2 signaling molecule (26, 27). However, in a well studied V. vulnificus strain, MO6-24/O, whose genome has been completely sequenced (GenBankTM accession number CP002469.1 for chromosome I and CP002470.1 for chromosome II) (28), there are no genes for the biosynthesis of either autoinducer-1 or cholera autoinducer-1. Homologs of LuxPQ, the cognate receptor for autoinducer-2 in V. harveyi, and LuxU and LuxO, which are involved in a phospho-relay, were identified in V. vulnificus (29–33). The autoinducer signal converges on LuxO, a nitrogen regulatory protein (NtrC) homolog, which in turn regulates the master regulator SmcR (34–36).
Involvement of small RNA molecules called quorum regulatory RNAs (Qrrs) in quorum sensing has been well documented (37). In V. harveyi and V. cholerae, Qrrs are transcribed at low cell density in a σ54-dependent manner and repress expression of the master regulators LuxR and HapR by pairing with untranslated regions of the coding genes (37–40). Bioinformatics analysis suggests the existence of five Qrrs in V. vulnificus (37). Qrrs are highly conserved at the nucleotide level among Vibrio species but vary in number and mechanism. V. harveyi has five Qrrs that function additively on LuxR expression (38). V. cholerae has four Qrrs that function redundantly on HapR (37). V. fischeri has only one Qrr that fully represses LitR (41). Considering the conservation of quorum-sensing pathways among Vibrio species (33), it is hypothesized that Qrrs in V. vulnificus repress the expression of SmcR, a homolog of V. harveyi LuxR. In V. vulnificus, the quorum-sensing master regulator SmcR is responsible for regulating expression of various virulence factors, and mutations in SmcR significantly attenuate the cytotoxicity of V. vulnificus (42). At high cell density, SmcR inhibits the expression of vvhA, which encodes hemolysin (27, 36), but it up-regulates the expression vvpE, which encodes elastase (43). SmcR also inhibits the transcription of rtxA1, a major virulence factor in V. vulnificus (44), and it inhibits vulnibactin synthesis by binding to the promoter region of vvsAB (23).
In this work, we show that Qrrs in V. vulnificus are also regulated by quorum-sensing signaling via LuxO and modulate the expression of virulence factors via SmcR. Furthermore, we observed that Qrrs are responsive to iron concentration and that both quorum sensing and iron sensing converge at Qrrs to coordinately control virulence factors.
Experimental Procedures
Bacterial Strains, Plasmids, and Culture Conditions
Strains and plasmids used in this study are listed in Table 1. For Escherichia coli strains, Luria-Bertani (LB) medium was used for culture at 37 °C. For V. vulnificus, LBS medium (LB medium supplemented with 1.5% NaCl) was used for culture at 30 °C. All media components were purchased from Difco (Sparks, MD), and antibiotics were purchased from Sigma. For iron-limiting conditions, the iron chelator 2,2′-dipyridyl was used at a concentration of 200 μm.
TABLE 1.
Strains and plasmids used in this study
| Bacterial strains and plasmids | Derivation/relevant characteristics | Ref. or source |
|---|---|---|
| E. coli | ||
| DH5α | λ− φ80dlacZΔM15Δ (lacZYA-argF)U169 recA1 endA1 hsdR17 (rK−mK−) supE44 thi-1 gyrA relA1 | Our collection |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| S17-1 | C600::RP4 2-(Tc::Mu) (Km::Tn7) thi pro hsdR hsdM+ recA | 70 |
| S17-1λpir | λpir lysogen of S17-1 | 70 |
| V. vulnificus | ||
| MO6-24/O | Clinical isolate; virulent | Our collection |
| HS031 (ΔsmcR) | Derivative of MO6-24/O, with a deletion in smcR | 43 |
| HLM101 (Δfur) | Derivative of MO6-24/O, with a deletion in fur | 71 |
| luxOD47E | Derivative of MO6-24/O, with LuxO Asp-47 to Glu | This study |
| KPM201 (ΔluxO) | Derivative of MO6-24/O, luxO::nptI | 36 |
| MIBE301 | Derivative of MO6-24/O luxO::nptI, with a deletion in smcR (This strain is also called as ΔluxOΔsmcR) | 23 |
| ΔluxOΔfur | Derivative of MO6-24/O luxO::nptI, with a deletion in fur | This study |
| Δqrr1 | Derivative of MO6-24/O, with a deletion in qrr1 | This study |
| Δqrr2 | Derivative of MO6-24/O, with a deletion in qrr2 | This study |
| Δqrr3 | Derivative of MO6-24/O, with a deletion in qrr3 | This study |
| Δqrr4 | Derivative of MO6-24/O, with a deletion in qrr4 | This study |
| Δqrr5 | Derivative of MO6-24/O, with a deletion in qrr5 | This study |
| Δqrr14 | Derivative of MO6-24/O, with deletion in qrr1 and qrr4 | This study |
| Δqrr134 | Derivative of MO6-24/O, with deletion in qrr1, qrr2, and qrr4 | This study |
| Δqrr1345 | Derivative of MO6-24/O, with deletion in qrr1, qrr2, qrr3, and qrr4 | This study |
| Δqrr1–5 | Derivative of MO6-24/O, with deletion in qrr1, qrr2, qrr3, qrr4, and qrr5 | This study |
| Δqrr1–5ΔsmcR | Derivative of Δqrr1–5 with deletion in smcR | This study |
| Plasmids | ||
| pASK-IBA-7 | Expression vector, Apr | IBA |
| pASK-IBA-Fur | pASK-IBA7 with V. vulnificus fur | 23 |
| pASK-IBA-LuxO | pASK-IBA7 with V. vulnificus luxO | This study |
| pHK0011 | pRK415, a promoterless luxAB, Tcr | 47 |
| pHK-qrr1 | pHK0011 with qrr1 promoter fused to luxAB | This study |
| pHK-qrr2 | pHK0011 with qrr2 promoter fused to luxAB | This study |
| pHK-qrr3 | pHK0011 with qrr3 promoter fused to luxAB | This study |
| pHK-qrr4 | pHK0011 with qrr4 promoter fused to luxAB | This study |
| pHK-qrr5 | pHK0011 with qrr5 promoter fused to luxAB | This study |
| pHK-luxO | pHK0011 with luxO promoter fused to luxAB | This study |
| pRK415 | IncP ori, broad host range vector; oriT of RP4, Tcr | 73 |
| pRK415-qrr1 | pRK415 with V. vulnificus qrr1 | This study |
| pRK415-qrr2 | pRK415 with V. vulnificus qrr2 | This study |
| pRK415-qrr3 | pRK415 with V. vulnificus qrr3 | This study |
| pRK415-qrr4 | pRK415 with V. vulnificus qrr4 | This study |
| pRK415-qrr5 | pRK415 with V. vulnificus qrr5 | This study |
| pDM4 | Suicide vector, oriR6K, Cmr | 74 |
| pDM4-SMCRKO | pDM4 with upstream and downstream sequence of smcR | 23 |
| pGEM®-T Easy | Cloning vector, Apr | Promega |
| pBBR1-MCS4 | Broad host range expression vector; Apr | 72 |
| pLuxO47E | pBBR1-MCS4 with luxOD47E | This study |
Construction of ΔluxOΔfur and LuxOD47E Mutants, Deletions in qrrs, and Δqrr1–5ΔsmcR
To construct ΔluxOΔfur, the primers fur-KF1 and fur-KR1 (Table 2) were used for amplification of the upstream region of fur, and the primers fur-KF2 and fur-KR2 were used for the downstream region of fur. S17-1 λpir harboring pDM4-fur, containing the fur upstream and downstream sequences, was constructed for conjugation with V. vulnificus ΔluxO. For construction of the V. vulnificus mutant luxOD47E, megaprimer PCR (45) was performed using the primers LuxO-F, LuxO-D47E, and LuxO-R (Table 2). The resulting 1424-bp PCR product was ligated into the pGEM-T Easy vector, and the generation of the mutation was confirmed by nucleotide sequencing. After digestion with ApaI and XbaI, the resulting DNA fragment was cloned into pDM4 to construct pDM4-luxOD47E, followed by mobilization from S17-1λpir to MO6-24/O via conjugation. A double crossover was performed as described above, and the subsequent mutation was confirmed by DNA sequencing. For construction of Δqrr1, a deletion in the qrr1 gene and the qrr1 upstream region was amplified by PCR using the primers qrr1-KF1 and qrr1-KR1, and the qrr1 downstream region was amplified by PCR with the primers qrr1-KF2 and qrr1-KR2 (Table 2). The PCR products were ligated and cloned into the pre-digested suicide vector pDM4. The resulting plasmid was mobilized from S17-1 λpir to V. vulnificus by conjugation. A double crossover was selected on LB containing 10% sucrose. Colonies that grew in sucrose but were sensitive to chloramphenicol were selected, and the mutation was confirmed by PCR and sequencing. Construction of Δqrr2, Δqrr3, Δqrr4, Δqrr5, Δqrr14 (double mutant), Δqrr134 (triple mutant), Δqrr1345 (quadruple mutant), and Δqrr1–5 (quintuple mutant) was performed in a similar manner. For qrr2 mutants, the primers qrr2-KF1, qrr2-KR1, qrr2-KF2, and qrr2-KR2 were used. For qrr3 mutants, the primers qrr3-KF1, qrr3-KR1, qrr3-KF2, and qrr3-KR2 were used. For qrr4 mutants, the primers qrr4-KF1, qrr4-KR1, qrr4-KF2, and qrr4-KR2 were used. For qrr5 mutants, the primers qrr5-KF1, qrr5-KR1, qrr5-KF2, and qrr5-KR2 were used. For construction of Δqrr1–5ΔsmcR, plasmid pDM4-SMCRKO was employed (23).
TABLE 2.
Primers and sequences used in the study
| Primer | Sequence (from 5′ to 3′) |
|---|---|
| For construction of ΔluxOΔfur | |
| fur-KF1 | TCTAGACGTTAAAGAGAAAATACTGC |
| fur-KR1 | GGATCCAAGACCAGCATCCTTTAGCGC |
| fur-KF2 | GGATCCAATCCAGACGCACATAAACG |
| fur-KR2 | CTCGAGTCAGAGACTTTGGGTGTTAAC |
| For construction of luxOD47E | |
| LuxO-F | GGGCCCTTGAAGCGTAATATCAAAGATATT |
| LuxO-D47E | CATTCCAGATCTTATTCTGCTGGAATTACGTCTAC |
| LuxO-R | TCTAGATCTTGTACCTCTTTCCAGG |
| For construction of qrr deletions | |
| qrr1-KF1 | CTCGAGATAAATGGCTTGTCTCCAC |
| qrr1-KR1 | GAATTCACTTTCGTTTTTGCATTGTT |
| qrr1-KF2 | GAATTCAGCCAATAGTGAAATGACTG |
| qrr1-KR2 | AGATCTAACTGAATACTTGCATG |
| qrr2-KF1 | AGATCTGAGCAGCAGAATAAAGCTGCC |
| qrr2-KR1 | CCGAATTCAAGGGTCGAGAAGTATTATGCA |
| qrr2-KF2 | GGAATTCGATTTGGGAGATGGCGCTCAA |
| qrr2-KR2 | AGATCTCATGAAATCTCACGAAAAACA |
| qrr3-KF1 | AGATCTGTCACCATTGGTCTGTTTGAA |
| qrr3-KR1 | CCTCTAGAAAATTCCGCGTTTTTACCATG |
| qrr3-KF2 | CCTCTAGACCGATCTAACTTCCCTACGAA |
| qrr3-KR2 | AGATCTCAGGGTTATCGTGATAAATGA |
| qrr4-KF1 | AGATCTATCGATCGCCAGTTGATTGAG |
| qrr4-KR1 | GGAATTCCGTATCAAATCGACGTATTTA |
| qrr4-KF2 | GGAATTCAGAACATTTGGCATAACAGCT |
| qrr4-KR2 | AGATCTGCCATCTGTGTGCTCACGATG |
| qrr5-KF1 | AGATCTGCCATCTGTGTGCTCACGATA |
| qrr5-KR1 | CGAATTCCCAACCCTATTATTTGCTACA |
| qrr5-KF2 | GGAATTCTCAATGTCACCCAAATGGTT |
| qrr5-KR2 | AGATCTAAAGCAGCACCTGCGATCACA |
| For primer extension | |
| qrr1-PE | AACAGTACTTCACTATTGGCATC |
| qrr2-PE | TTATGTTGAGTGAACAATGGTA |
| qrr3-PE | GCTTTTACATGTGACAAATCA |
| qrr4-PE | GTATATATGTGTGAACAAGTCATA |
| qrr5-PE | GCTGTATATATTTGTGAACAATCAG |
| For construction of transcriptional reporter fusions to luxO and qrr genes | |
| PluxO-F | GGGGTACCGAAATCCCGCAAGCAGAAA |
| PluxO-R | TTTCTAGAACGCCCAGTACCCACGATA |
| Pqrr1-F | GGGGTACCTTCTACCATCAGCAAGTAGCG |
| Pqrr1-R | GCTCTAGAAACAACGTCAGTTGGCTAGGT |
| Pqrr2-F | GGGGTACCCCATCGCTAAACCTTTTAAG |
| Pqrr2-R | GCTCTAGAGTATTCACTAACAACGTCAG |
| Pqrr3-F | GGGGTACCGGTTTCTTTGCCTTTCTTGGC |
| Pqrr3-R | GCTCTAGACTAGGTGACCCTCGGCTTAA |
| Pqrr4-F | GGGGTACCCCGGCTAAGAAAATGGAAATC |
| Pqrr4-R | GCTCTAGACCTCGGCTTAATAAGGGTCAC |
| Pqrr5-F | GGGGTACCTCAACACTAGAGGAAGGGCG |
| Pqrr5-R | GCTCTAGATCACTAACAACGTCAGTTGGCT |
| For Qrr complementation and pluxO D47E construction | |
| Cqrr1-F | TTTCTAGATGGCATGAGATAGGAGCGATAGA |
| Cqrr1-R | GGGGTACCAGAAGCGTGATGAAATTGAAAAG |
| Cqrr2-F | TTTCTAGAGACATAACGCTCCCTGCCTTC |
| Cqrr2-R | GGGGTACCTCGGATTTTTCTTTCCACACC |
| Cqrr3-F | TTTCTAGAAACCACACTGACATCACACTCC |
| Cqrr3-R | GGGGTACCTTTTTGAATAATGAATCTCTCG |
| Cqrr4-F | TTTCTAGAAATCGGATTTATATCAAGCGTTT |
| Cqrr4-R | GGGGTACCTCGTCTTATTTGTTCTCGTGGCG |
| Cqrr5-F | TTTCTAGAAATTTACCCTGGGATAGAGCAGT |
| Cqrr5-R | GGGGTACCTGAAATACCTCATCACAAACAAG |
| CLuxO-F | GGGGTACCGGCTAGATTATGCAACAAATAACG |
| CLuxO-R | TTTCTAGAATTTCCTCAGTCAACGAGGC |
| For qPCR | |
| vvpE-F | TTTACGCTACTTCGACCAACCCTC |
| vvpE-R | ATCTCAAAACCCTTACGCACATTC |
| vvhA-F | CGCAGAATGAGAACAAAAACTACCA |
| vvhA-R | ATCAAACACCAAGGTCTTCGAGTAG |
| rpsL-F | AGGAGCACTCGGTTGTTCTTATC |
| rpsL-R | GACCTTGTTTACGGTTGTTCACG |
| For strep-LuxO purification | |
| LuxO-Strep-F | CCGGAATTCATGCAACAAATAACGACAACG |
| LuxO-Strep-R | CCCTCGAGGATTTAGTAATTCCATTATGC |
| For gel shift assay | |
| Eqrr1-F | GTACCCACGATATTGATATCG |
| Eqrr1-R | GCCATTGTTATTTGCAAAATGC |
| Eqrr2-F | CGAGTATACCCAGATTCATGTC |
| Eqrr2-R | CTGAATATCCCATATATTAACTC |
| Eqrr3-F | TGACTTTATCACCCCAAACCAC |
| Eqrr3-R | CACCATGGTTTCTGTGATATTG |
| Eqrr4-F | GAGTGAGATCACGCGCATGATAG |
| Eqrr4-R | CACGAATTCCGTATCAAATCGAC |
| Eqrr5-F | GCTGGAAACGTTGAAAGAAGTC |
| Eqrr5-R | GCTACATTTTTAACCAAAATGC |
| ErpsL-F | TGAATCGCGACTAAGCACCAATAT |
| ErpsL-R | GTGAAAAATCTAATCCCCAACCAC |
Primer Extension to Determine qrr Transcriptional Start Sites
The transcriptional start sites of the five qrrs were determined using the PrimeScriptTM first strand cDNA synthesis kit (Takara, Ohtsu, Japan) and primers complementary to each qrr (Table 2). RNA was purified as described previously (46). Total RNA was extracted from wild type MO6-24/O cultured in LBS broth and harvested at log phase using the RNeasy mini kit (Qiagen, Valencia, CA). Reverse transcription reactions were performed at 42 °C for 1 h and then inactivated at 70 °C. The same primers were used to generate the sequencing ladder using the TopTM DNA sequencing kit (Bioneer, Seoul, Korea). The generated cDNAs were separated on a 6% denaturing polyacrylamide gel alongside the corresponding sequencing ladders and analyzed with a Fuji BAS 1500 Image Analyzer (Fujifilm, Tokyo, Japan).
Construction of the Transcriptional Reporter Fusions luxO-luxAB and qrr-luxAB and Measurement of Luciferase Activity
DNA fragments containing the promoter regions of luxO and each of the five qrr genes were amplified using the primers listed in Table 2. The resulting PCR products were digested using KpnI and XbaI and ligated into the transcription reporter plasmid pHK0011 (47), generating pHK-pluxO, pHK-pqrr1, pHK-pqrr2, pHK-pqrr3, pHK-pqrr4, and pHK-pqrr5. These constructs were subsequently mobilized from S17-1 into V. vulnificus wild type MO6-24/O, ΔluxO, LuxOD46E, Δfur, and ΔluxOΔfur. n-Decyl-aldehyde was added to 10 μl of each culture diluted in 500 μl of phosphate-buffered saline (PBS) (final concentration, 0.06% (v/v)). Luminescence was measured using a luminometer (Lumat LB 9507, Berthold Technologies, Bad Wildbad, Germany). Relative light units (light units/A600), which are light units normalized to cell densities, represent the transcription levels of corresponding genes.
Complementation of Δqrr1–5 and Construction of pLuxOD47E
DNA fragments containing each qrr were amplified using the following primers: Cqrr1-F and Cqrr1-R for qrr1; Cqrr2-F and Cqrr2-R for qrr2; Cqrr3-F and Cqrr3-R for qrr3;Cqrr4-F and Cqrr4-R for qrr4; and Cqrr5-F and Cqrr5-R for qrr5 (Table 2). The PCR products were digested with XbaI and KpnI and ligated into pRK415 to obtain pRK415-qrr1, pRK415-qrr2, pRK415-qrr3, pRK415-qrr4, and pRK415-qrr5. These constructs were mobilized to V. vulnificus Δqrr1–5 from S17-1 by conjugation. Using V. vulnificus mutant luxOD47E genomic DNA as a template, the luxO-coding region was amplified using the primers CLuxO-F and CLuxO-R (Table 2) and ligated into pBBR1-MCS4, generating pLuxOD47E. The resulting plasmid was mobilized from S17-1 to V. vulnificus ΔluxO and ΔluxOΔfur, harboring pHK-qrr1.
Western Blot Analysis of SmcR
For analysis of SmcR expression, overnight cultures of V. vulnificus were subcultured into fresh LBS broth. Cells were harvested at log phase (A600 of ∼0.6) and stationary phase (A600 >2.0) and washed with PBS. Cells were lysed by ultrasonication, and the total protein concentration was assessed using the Lowry method (48). Next, 10 μg of protein was resolved by SDS-PAGE on a 12% polyacrylamide gel. After transfer to a Whatman Protran BA 83 nitrocellulose membrane (GE Healthcare UK Ltd., Buckinghamshire, UK), proteins were treated with anti-SmcR antibody (49) for 1 h at room temperature. Goat anti-rat IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a secondary antibody. SmcR expression was visualized using Western blotting Luminol Reagent (Santa Cruz Biotechnology).
Quantitative Real Time PCR
To analyze Qrr-regulated vvpE and vvhA expression in V. vulnificus, overnight cultures of V. vulnificus were subcultured into fresh LBS broth. Samples were harvested at log phase (A600 of ∼0.6) and stationary phase (A600 >2.0), and RNA was purified using the RNeasy mini kit (Qiagen). RNA concentration was determined using a Biophotometer (Eppendorf, Hamburg, Germany). Reverse transcription was performed using the PrimeScriptTM RT (Takara, Ohtsu, Japan) reagent kit. Quantitative PCR was performed in a 96-well PCR plate using SYBR® Primex Ex TaqTM and the ABI PRISM 7500 real time PCR system (Applied Biosystems, Carlsbad, CA). Primers are listed in Table 2. rpsL was used as an endogenous control. Relative RNA expression was analyzed by 7500 SDS software (Applied Biosystems, Carlsbad, CA).
Purification of Strep-LuxO and Strep-Fur
A DNA fragment containing the luxO-coding region was amplified by PCR using the primers LuxO-Strep-F and LuxO-Strep-R (Table 2) and subcloned into pASK-IBA-7 (IBA, Göttingen, Germany), which results in a Strep-tag II at the N terminus of LuxO. The resulting plasmid, pASK-IBA-LuxO, was transformed into E. coli BL21 (DE3) (Novagen, Madison, WI). The Strep-LuxO fusion protein was induced using 200 ng/ml anhydrotetracycline and purified using Strep-Tactin-Sepharose (IBA) according to the manufacturer's instructions. Fur was cloned into the expression vector pASK-IBA7 (22) to construct pASK-IBA-Fur and expressed and purified in the same way as LuxO. LuxO and Fur protein purity were assessed by performing SDS-PAGE, and protein concentration was assessed using a Lowry assay (48).
Electrophoresis Mobility Shift Assay
To prepare probes for the electrophoresis mobility shift assay (EMSA), DNA fragments containing the promoter regions of each qrr gene or rpsL were amplified by PCR using the primers ErpsL-F and ErpsL-R, Eqrr1-F and -R for qrr1, Eqrr2-F and -R for qrr2, Eqrr3-F and -R for qrr3, Eqrr4-F and -R for qrr4, and Eqrr5-F and -R for qrr5 (Table 2). The products were subsequently labeled with [γ-32P]ATP using T4 polynucleotide kinase (New England Biolabs, Beverly, MA). Fur binding to the upstream region of each qrr gene was performed in a 20-μl reaction containing Fur binding buffer (10 mm HEPES, 100 mm KCl, 10 μg/ml dI-dC and 10% glycerol, pH 7.5, with the supplementation of 25 μm MnSO4 or 1 mm EDTA). Ten ng of each DNA probe was incubated with increasing amounts of purified Fur protein (23). After incubation at 30 °C for 30 min, 4 μl of sucrose dye (0.25% bromphenol blue, 0.25% xylene cyanol, and 40% sucrose) was added to the reaction. Samples were separated by 5% neutral PAGE. DNA was visualized using the BAS 1500 imaging system (Fujifilm, Tokyo, Japan). Binding between LuxO and the upstream region of each qrr gene was performed in a 20-μl volume reaction containing LuxO binding buffer (10 mm Tris, pH 7.5, 300 mm NaCl, 1 mm MgSO4, 1 mm DTT, 10% glycerol, and 10 μg/ml dI-dC). Ten ng of each probe was incubated with increasing amounts of LuxO (0, 25, 50, 100 nm, 200, 400, and 800 nm). For the EMSA competition study between LuxO and Fur, LuxO (800 nm) and Fur (1 μm or 2 μm) were added separately or together to the binding mix containing 10 ng of each qrr probe. Binding reactions were performed in LuxO binding buffer with a supplementation of 100 μm MnSO4.
DNase I Footprinting Assay
To identify the Fur-binding sequences of the qrr genes, DNA fragments containing the promoter region of each qrr gene were amplified by PCR using primers listed in Table 2 (Eqrr1-F and qrr1-PE for qrr1, Eqrr2-F and qrr2-PE for qrr2, Eqrr3-F and qrr3-PE for qrr3, Eqrr4-F and qrr4-PE for qrr4, and Eqrr5-F and qrr5-PE for qrr5). Eqrr1-F, Eqrr2-F, Eqrr3-F, Eqrr4-F, and Eqrr5-F were pre-labeled with [γ-32P]ATP using T4 PNK (New England Biolabs). Each probe (200 ng) was incubated with increasing concentrations of Fur in a 50-μl reaction (10 mm Tris, pH 7.5, 300 mm NaCl, 1 mm MgSO4, 1 mm DTT, 100 μm MnSO4, 10% glycerol) at 30 °C for 30 min. After the addition of 50 μl MgCl2·CaCl2 solution (10 mm MgCl2, 5 mm CaCl2), samples were treated with 0.12 units of RQ1 RNase-free DNase I (Promega, Madison, WI) for 2 min. The DNase reaction was terminated with 90 μl of stop solution (200 mm NaCl, 30 mm EDTA, 1% SDS). The digested DNA was harvested by ethanol precipitation and dissolved in 10 μl of formamide loading dye (98% formamide, 0.025% bromphenol blue, 0.025% xylene cyanol FF) and separated on a 6% polyacrylamide-urea gel alongside the sequencing ladder generated by the same labeled primer.
Results
Identification of Five Quorum-regulatory RNAs in V. vulnificus
Qrrs were first identified in V. cholerae and V. harveyi (37), and prior to our study, these small RNAs had not been examined in V. vulnificus. Through a homologous sequence search using bioinformatic tools, the following five Qrrs were found in V. vulnificus: qrr1, located between VVMO6_RS10095 (encoding LuxO) and VVMO6_RS10100 (encoding exonuclease ABC subunit B) on chromosome I; qrr2, located between VVMO6_RS15230 (encoding a membrane protein) and VVMO6_RS15235 (encoding a hypothetical protein) on chromosome II; qrr3, located between VVMO6_RS20600 (the magnesium transporter gene mgtE) and VVMO6_RS20605 (encoding an AraC family transcriptional regulator) on chromosome II; qrr4, located between VVMO6_RS17015 (encoding a methyl-accepting chemotaxis protein) and VVMO6_RS17020 (encoding a transcriptional regulator) on chromosome II; and qrr5, located between VVMO6_RS17415 (encoding a hypothetical protein) and VVMO6_RS17420 (encoding a membrane-associated phospholipid phosphatase) on chromosome II.
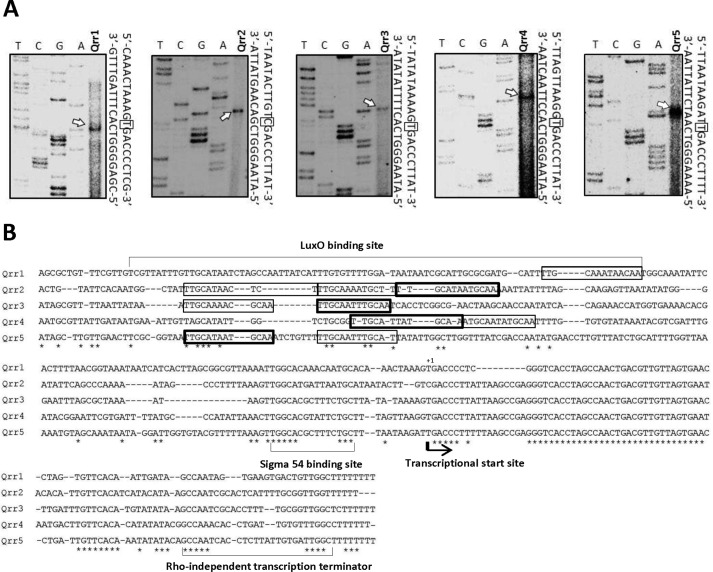
To confirm that these five Qrrs are indeed expressed in V. vulnificus and to identify transcriptional start sites, we performed primer extension experiments using total RNA extracted from wild type V. vulnificus cells grown to exponential phase (Fig. 1A).
FIGURE 1.
Identification of qrr transcriptional start sites in V. vulnificus. A, qrr transcriptional start sites were identified through primer extension experiments. T, C, G, and A represent the sequencing ladders. Transcriptional start sites are indicated with arrows on the gel images, and are boxed in the sequence to the right of each image. B, alignment of the qrr coding regions and upstream regions was performed using ClustalW. The transcriptional start sites are denoted with arrows, and the putative σ54-binding sites and Rho-independent terminator sequences are underlined. Putative LuxO-binding sites perfectly matched to the consensus sequences are denoted with thick-line boxes, and those partially matched with the consensus sequence are denoted with thin-line boxes.
Phosphorylated LuxO Directly Activates qrr Genes
The luxO gene in V. vulnificus (NCBI accession number ABG81424) shows 90 and 85% identity to that of V. harveyi (NCBI accession number P0C5S5) and V. cholerae (NCBI accession number NP_230666), respectively. In V. harveyi and V. cholerae, Qrrs are expressed in a cell density-dependent manner. At low cell density, phosphorylated LuxO acts as an enhancer to activate the σ54-initiated transcription of qrr genes. Consequently, master regulators such as LuxR in V. harveyi are repressed (37, 38). However, at high cell density, LuxO is dephosphorylated and no longer activates transcription of qrr genes, which also relieves master regulator repression.
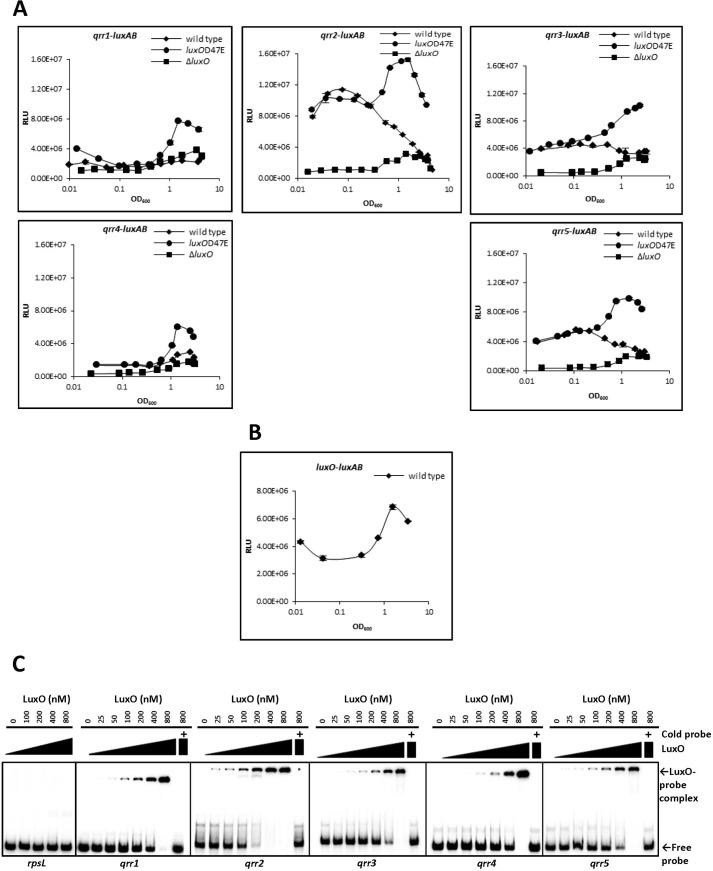
Sequences in the promoter region of Qrr-encoding genes were analyzed using ClustalW2 to locate regulatory protein-binding sites (50). The consensus LuxO-binding sequence (5′-TTGCAW3TGCAA-3′) found in V. cholerae (37) is present in the promoter region of all five V. vulnificus qrr genes (Fig. 1B), suggesting that LuxO regulates qrr expression as it does in V. cholerae. In V. harveyi, LuxO with an Asp to Glu mutation at residue 47 (luxOD47E) mimics phosphorylated LuxO and constitutively activates Qrr expression (31). To investigate whether Qrrs in V. vulnificus are expressed in a cell density-dependent manner through LuxO phosphorylation, we constructed a luxOD47E mutant and compared qrr expression in wild type V. vulnificus, a luxO mutant (ΔluxO), and luxOD47E using transcriptional fusions to each of the five qrr genes (qrr(1–5)-luxAB) (Fig. 2A). We observed a common expression pattern for each of the five qrr genes in all three phenotypic backgrounds. At low cell densities, expression levels in the luxOD47E strain were similar to wild type. However, at high cell densities, expression levels were higher in the luxOD47E strain than in wild type. Expression of all qrr genes was low at all growth stages in ΔluxO, suggesting that LuxO is required for the activation of Qrr expression. Differences in the pattern and magnitude of expression between each of the qrr genes in wild type cells were observed. The qrr1 and qrr4 genes showed a relatively low level of expression regardless of growth stage, although LuxO did appear to be required for expression. The expression level of qrr2 was highest, and yet it still decreased at high cell density. The expression patterns of qrr3 and qrr5 were similar to those of qrr2 but with approximately half the magnitude. In general, in the luxOD47E mutant, expression levels increased with increasing cell density. Measurements of LuxO levels at various growth stages revealed that LuxO expression was low at low cell density and increased at higher cell density (Fig. 2B).
FIGURE 2.
LuxO activates qrr transcription. A, expression of qrr genes in wild type, luxOD47E, and ΔluxO cells; B, expression of luxO in wild type cells. Expression levels of the five qrr genes and luxO were quantitatively measured using the luxAB reporter gene fusion at various growth stages as described under “Experimental Procedures.” Data shown are averages of experiments done in technical triplicate, and error bars denote the standard deviations. C, binding of purified LuxO to DNA upstream of each of the qrr genes as demonstrated by electrophoresis mobility shift assay. A 32P-labeled DNA fragment (10 ng), including the promoter region of each qrr gene, was incubated with purified LuxO at the following concentrations: 0, 25, 50, 100, 200, 400, and 800 nm. Unlabeled probe (300 ng) was used in a competition experiment and is shown at the far right of each gel image. The promoter region of rpsL (30S ribosomal protein S12) was employed as a negative control. The position of the free probe and the LuxO-probe complex are indicated by arrows. These results are representative of three independent experiments. RLU, relative light units.
Next, we used electrophoresis mobility shift assays to analyze direct interactions between LuxO and the promoter regions of each of the qrr genes, which were predicted to have LuxO-binding sites (Fig. 2C). We found that DNA fragments for all five qrr promoters were bound by LuxO. The strongest binding appeared to be between LuxO and qrr2, where as little as 200 nm LuxO was enough to shift a significant amount of the qrr2 fragment into a bound complex (see qrr2, 5th lane in Fig. 2C). By comparison, binding of LuxO to qrr3 and qrr5 was not as strong (see 6th lane for each in Fig. 2C), and binding to qrr1 and qrr4 was the weakest.
Qrrs Repress Expression of SmcR
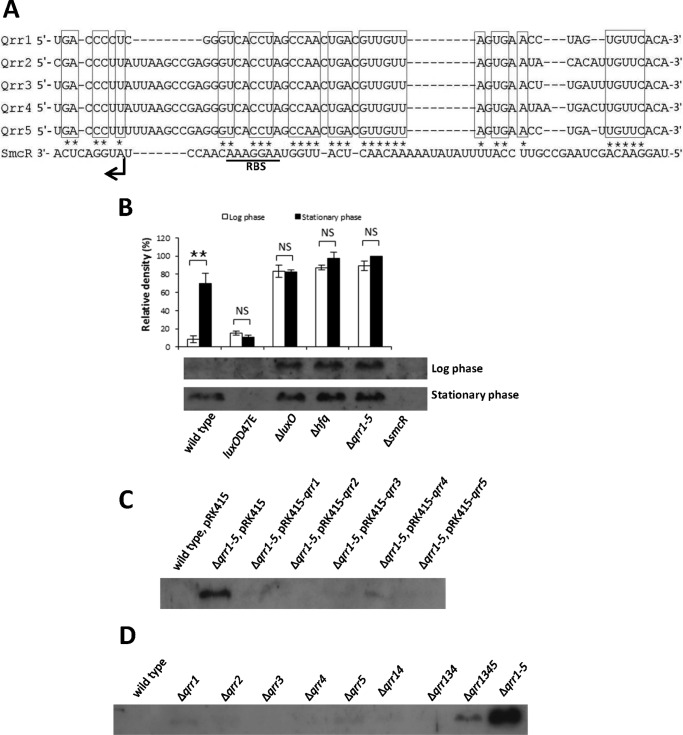
The quorum-sensing master regulator SmcR in V. vulnificus is homologous to LuxR in V. harveyi and HapR in V. cholerae (42, 51, 52). Qrrs in V. cholerae and V. harveyi inhibit the translation of their respective master regulator by binding to the 5′-untranslated region (UTRs) of each (37, 38). Alignment of the smcR 5′-UTR with the Qrr sequences of V. vulnificus suggested that Qrrs can form a hybrid structure with the region overlapping the ribosomal binding site (RBS) (Fig. 3A) suggesting that Qrrs may also inhibit SmcR translation in this species. To test this, we examined SmcR expression in wild type V. vulnificus and in Δqrr1–5 strains through Western blotting of protein extracts from cells grown to both log phase and stationary phase (Fig. 3B). In wild type, SmcR expression was barely detectable at low cell density but was significantly higher in cells that had reached stationary phase. In Δqrr1–5, SmcR was expressed abundantly in both log and stationary phases. These results suggest that Qrrs control SmcR expression through repression at low cell density but not at high cell density. We also examined SmcR expression in an hfq deletion mutant. The hfq gene encodes an sRNA chaperone required for Qrr function in V. harveyi and V. cholerae (37). Results for the Δhfq strain were similar to those for Δqrr1–5 suggesting that SmcR inhibition by Qrrs requires Hfq. SmcR expression in ΔluxO cells was similar to that of Δqrr1–5, suggesting low qrr expression when LuxO is absent (Fig. 2A). SmcR expression was not detected in luxOD47E cells. Qrr2, Qrr3, and Qrr5 were abundantly expressed in luxOD47E cells at both low and high cell density (Fig. 2A), again suggesting that LuxO is required for qrr expression.
FIGURE 3.
Qrrs repress expression of SmcR redundantly in V. vulnificus. A, nucleotide sequences of five Qrrs that potentially base pair with the 5′-untranslated region (UTR) of SmcR. Putative pairing sequences are boxed in the Qrr sequences and are marked with asterisks in the 5′-UTR of SmcR. The initiation codon and ribosome-binding site of SmcR are noted. B, repression of SmcR by Qrrs at log phase in V. vulnificus. Wild type, ΔsmcR, Δqrr1–5, ΔluxO, Δhfq, luxOD47E, and Δqrr1–5 cells were harvested at both log phase (A600 0.6–0.7) and stationary phase (A600 >2.0), and 10 μg of lysate was subjected to Western blotting using an antibody against SmcR. The upper panel represents the relative densities of the bands shown in the lower panel. Band intensities were quantified using MultiGauge version 3.0 software (Fujifilm, Tokyo, Japan). Values are averages normalized to the intensity of the Δqrr1–5 (stationary phase) sample from biological experiments done in triplicate. **, p < 0.005; NS, not significant in Student's t test with p > 0.05. C, regulation of SmcR by individual Qrrs. Wild type V. vulnificus cells harboring pRK415, Δqrr1–5 harboring pRK415, and Δqrr1–5 harboring pRK415-qrr1 through pRK415-qrr5 were harvested at log phase (A600 ∼0.6). Ten μg of lysate was subjected to Western blotting using an antibody against SmcR. This result is representative of three independent experiments. D, SmcR expression in cell extracts from qrr deletion strains was measured by Western blot hybridization. Wild type V. vulnificus MO6-24/O, Δqrr1, Δqrr2, Δqrr3, Δqrr4, Δqrr5, Δqrr14, Δqrr134, Δqrr1345, and Δqrr1–5 were harvested at log phase (A600 ∼0.6). Ten μg of lysate was subjected to Western blotting using an antibody against SmcR. This result is representative of three independent experiments.
To test whether each of the Qrrs could inhibit SmcR expression, we performed complementation experiments by returning each qrr gene back into the Δqrr1–5 mutant individually on pRK415 vector constructs (53). SmcR expression was determined by Western blotting of cell extracts obtained at log phase (Fig. 3C). Introduction of any one of the five qrr genes significantly restored inhibition of SmcR levels, similar to what was observed in wild type cell extracts. To determine whether the regulation of SmcR by the Qrrs is redundant or additive, we then constructed strains containing individual qrr mutations and measured SmcR expression levels in cells grown to log phase (Fig. 3D). None of the five single mutants significantly affected SmcR expression. We then measured SmcR expression in mutant strains containing combinations of qrr genes: Δqrr14, Δqrr134, Δqrr1345, and Δqrr1–5. Expression of SmcR in the double or triple mutants was no different from wild type and was only slightly increased in the quadruple mutant Δqrr1345. Only the quadruple mutant Δqrr1–5 showed full expression of SmcR. These results suggest that Qrrs in V. vulnificus act redundantly in SmcR regulation and that full derepression of SmcR requires depletion of all five Qrrs.
Qrrs Regulate Expression of the Virulence Factors VvpE and VvhA via SmcR
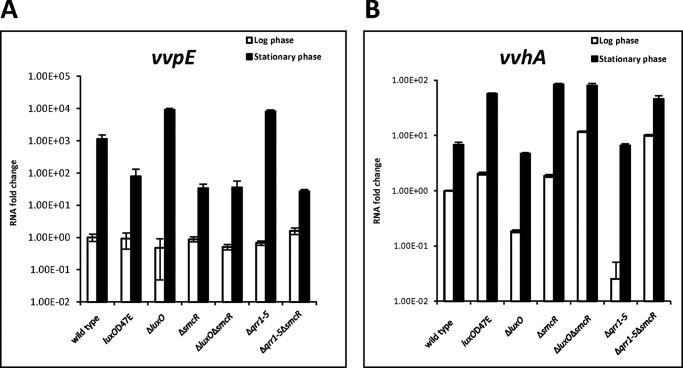
SmcR directly activates vvpE, a gene encoding the virulence factor metalloprotease, in V. vulnificus (43). Because Qrrs affect SmcR levels, we predicted that they would also affect vvpE expression. Consistent with a previous report in which vvpE was shown to have an RpoS-dependent promoter, vvpE expression was higher at stationary phase than at log phase (Fig. 4A). When compared with wild type cells, vvpE expression in cells at stationary phase was ∼15-fold lower in luxOD47E, where SmcR expression is repressed, and ∼30-fold lower in ΔsmcR and Δqrr1–5ΔsmcR. In ΔluxO and Δqrr1–5 strains, where SmcR expression is promoted, there was ∼8-fold higher vvpE expression than in wild type. These results suggest that Qrrs inhibits vvpE expression in V. vulnificus, very likely by affecting the levels of SmcR.
FIGURE 4.
Qrr-dependent regulation of virulence factor expression. A, expression levels of vvpE in various strains as measured by quantitative RT-PCR. RNA from wild type V. vulnificus, luxOD47E, ΔluxO, ΔsmcR, ΔluxOΔsmcR, Δqrr1–5, and ΔqrrΔsmcR was purified from cells at log phase (A600 ∼0.6) and stationary phase (A600 >2.0). RNA fold changes represent the vvpE expression level normalized to wild type. B, expression levels of vvhA in various strains as measured by quantitative RT-PCR. Values are averages from biological experiments done in triplicate. Error bars indicate the standard deviations.
Hemolysin, encoded by vvhA, is another important virulence factor in V. vulnificus. This gene was shown to be repressed by SmcR through the action of the transcription factor HlyU (44). To confirm that Qrrs affect vvhA expression via SmcR, we assessed vvhA expression in qrr mutants (Fig. 4B). At log phase, ΔluxO and Δqrr1–5, in which SmcR is derepressed, respectively, showed 5- and 50-fold lower expression of VvhA compared with wild type, whereas luxOD47E, ΔluxOΔsmcR, ΔsmcR, and Δqrr1–5ΔsmcR showed higher vvhA expression compared with wild type. At stationary phase, wild type, ΔluxO, and Δqrr1–5, which had similar levels of SmcR expression (Fig. 3B), also had similar levels of VvhA expression, whereas luxOD47E, ΔsmcR, ΔluxOΔsmcR, and Δqrr1–5ΔsmcR showed ∼10-fold higher expression of vvhA as compared with wild type. These results suggest that Qrrs activate vvhA through SmcR. It is noteworthy that vvhA expression in stationary phase is higher than that in log phase, independent of SmcR, which suggests that an additional unknown factor is involved in vvhA regulation. Taken together, our data suggest that quorum-sensing signals are transduced to Qrrs, which regulate SmcR to modulate the expression of virulence factors in V. vulnificus, similar to what has been observed for V. harveyi and V. cholerae.
Iron Represses Qrr Expression in V. vulnificus through Both Fur-dependent and Fur-independent Ways
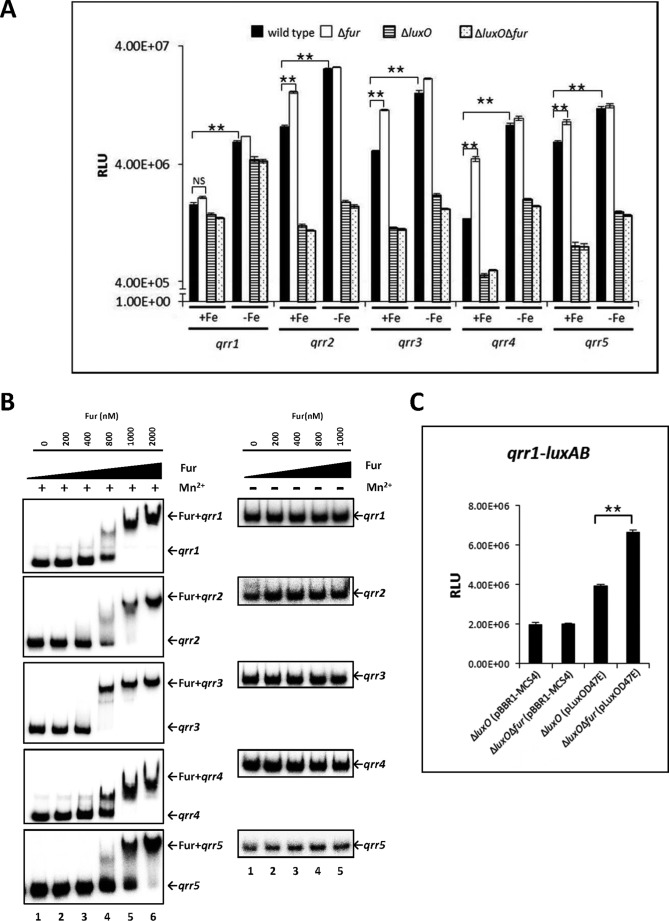
Iron is scarce in the natural environment and in the host. The LuxU-LuxO-SmcR signal transduction pathway may be regulated not only by the availability of autoinducer molecules as an indication of cell density, but also by other environmental factors, among which iron is particularly important. We showed that the iron-Fur complex represses the expression of SmcR by directly binding to the promoter region of this gene (49). From this, we hypothesized that qrr expression might also be affected by iron. To test this, we examined qrr expression under both iron-rich and iron-limiting conditions. V. vulnificus strains containing each individual qrr gene transcriptionally fused to the luxAB reporter were grown in rich medium with or without the iron chelator 2,2′-dipyridyl and quantitatively assessed for qrr expression. Depletion of iron led to a significant up-regulation of all five qrr genes in wild type cells (Fig. 5A). When this experiment was performed using cells grown in AB minimal medium with or without the supplementation of FeSO4, iron repression was also observed (data not shown). We therefore concluded that the presence of iron represses qrr expression.
FIGURE 5.
qrr transcription regulated by iron in V. vulnificus. A, regulation of qrr transcription by iron and Fur. Luciferase activity represents levels of luxAB-transcriptional reporter fusions to qrr1, qrr2, qrr3, qrr4, and qrr5 in wild type V. vulnificus, Δfur, ΔluxO, and ΔfurΔluxO harboring each of the plasmids pHK-qrr1, pHK-qrr2, pHK-qrr3, pHK-qrr4, and pHK-qrr5. Bacteria were cultured with or without 200 μm of the iron chelator 2,2′-dipyridyl, which was supplemented when cells were at an A600 of ∼0.2. Cell density and luminescence were measured at log phase (A600 of ∼0.6), as described under “Experimental Procedures.” Relative light units (RLU) represent light units normalized to cell density (luminescence/A600). Values are averages from three biological experiments, and error bars denote standard deviations. B, binding of Fur to the promoter regions of qrr genes as determined by electrophoresis mobility shift assay. Ten ng of DNA probes, including qrr promoter regions, were incubated with increasing concentrations of Fur in the presence (left panel) or absence (right panel) of 100 μm MnSO4. Lanes 1–6 represent Fur concentrations of 0, 200, 400, and 800 nm and 1 and 2 μm, respectively. C, Fur significantly represses transcription of qrr1 in the presence of overexpressed luxOD47E. ΔluxO and ΔfurΔluxO harboring pHK-qrr1 and pLuxOD47E or pBBR1-MCS4 were cultured until log phase. Values are averages from three independent experiments, and error bars denote standard deviations (A600 of ∼0.6). Expression of qrr1 was measured as described above (**, p < 0.005; NS, not significant in Student's t test with p > 0.05).
Fur is a global transcriptional regulator involved in the iron response, and it directly binds to the promoter regions of target genes when iron is present (54). We hypothesized that iron-dependent Qrr repression is elicited by Fur. To test this, Qrr expression was compared in wild type and Δfur cells under iron-rich and iron-limiting conditions (Fig. 5A). Under iron-rich conditions, expression levels of qrr2, qrr3, qrr4, and qrr5 were ∼2.0, 2.2, 3.2, and 1.5 times higher, respectively, in Δfur as compared with wild type. No significant difference was observed for qrr1 (Fig. 5A). Introduction of a wild type copy of fur into the Δfur strain restored qrr repression (data not shown). Expression of each of the qrr genes in the Δfur strain was further increased when the iron chelator was added. Notably, qrr expression levels were not significantly different between wild type and Δfur in the presence of the chelator. Taken together, these results suggest that Fur represses qrr2-5 in the presence of iron but that there is also an iron-regulatory mechanism that represses the five qrr genes independently of Fur.
As Qrr expression is dependent on LuxO, we explored the possibility that luxO plays a role in iron-dependent regulation of qrr genes by assessing qrr expression in a ΔluxO mutant and in a ΔluxOΔfur double mutant. As expected, qrr expression in these two mutants was lower than in wild type. However, there was no significant difference in qrr expression between the ΔluxO and ΔluxOΔfur strains (Fig. 5A). Under iron-limiting conditions, qrr expression in these mutants was significantly increased, suggesting that, even without LuxO, qrr expression was further decreased by iron, independent of Fur.
Fur Binds Directly to the Promoter Regions of All Five qrr Genes
To test our assumption that Fur affects qrr expression by binding the promoter region and inhibiting transcription of these genes, we performed EMSA using 32P-labeled DNA fragments of the qrr promoter regions and purified Fur protein (Fig. 5B). Purified Fur and the qrr probes were incubated in the presence of either 25 μm Mn2+ (instead of Fe2+) or 1 mm EDTA. We observed that Fur could bind to all five qrr promoters in the presence of Mn2+ in a density-dependent manner, suggesting that Fur acts as a repressor of the qrr genes. However, Fur affinity was lost when Mn2+ was not present.
Although Fur binds to the upstream region of qrr1, expression of this gene was not significantly different in wild type versus Δfur strains (Fig. 5A). This discrepancy led us to hypothesize that qrr1 expression is not high enough (see Fig. 2A) to clearly show Fur-mediated repression. To test this possibility, we employed pLuxOD47E, which constitutively expresses active LuxO (Table 1), in a ΔluxO background. When LuxOD47E was supplied in trans, qrr1 expression levels were two times the levels in ΔluxO alone (Fig. 5C). These results suggest that, under our experimental conditions, qrr1 expression is low due to a low affinity for LuxO. Comparing qrr1 expression in ΔluxOΔfur + LuxOD47E with that in ΔluxO + LuxOD47E showed that expression was higher in the absence of Fur. We concluded that qrr1 is indeed repressed by iron in a Fur-dependent manner, but this repression was not detectable in wild type cells due to a low level of qrr1 expression.
Fur Competes with LuxO in Binding to qrr Promoters
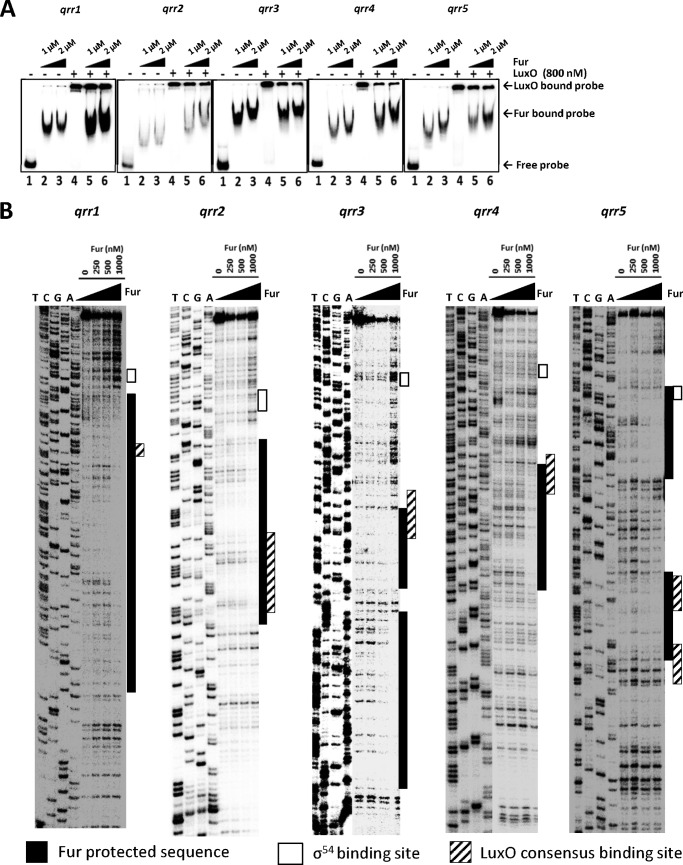
The above results suggested that both Fur and LuxO bind to regions upstream of the qrr genes and exert opposite effects on qrr expression. Furthermore, repression of qrr genes by Fur in the presence of iron was only seen upon LuxO activation (Fig. 5, A and C). We therefore hypothesized that Fur-binding sites overlap with LuxO-binding sites in qrr promoters, leading to competition for binding by the two regulatory proteins. To test this hypothesis, we performed an EMSA competition experiment between LuxO and Fur in the presence of divalent ions (Fig. 6A). Increasing Fur concentrations led to the formation of more Fur-qrr complexes and fewer LuxO-qrr complexes. These results suggest that the Fur-iron complex effectively competes for binding with LuxO, thereby inhibiting qrr expression.
FIGURE 6.
Fur competes with LuxO for binding to regions upstream of qrr genes. A, electrophoresis mobility shift assay of binding competition between LuxO and Fur for qrr promoter regions. Ten ng of each qrr promoter was incubated with either LuxO, Fur, or both. Lanes 1–6 include each probe incubated with the following: lane 1, no protein; lane 2, 1 μm Fur; lane 3, 2 μm Fur; lane 4, 800 nm LuxO; lane 5, 800 nm LuxO with 1 μm Fur; lane 6, 800 nm LuxO with 2 μm Fur. Positions of probes bound and shifted by LuxO or Fur are indicated by arrows. This result is representative of two independent experiments. B, DNase I footprinting of Fur protein binding to each qrr. A 32P-labeled probe (200 ng) was incubated with increasing concentrations of Fur (0, 250 and 500 nm, and 1 μm). The nucleotide sequences protected by Fur (shaded boxes), the σ54-binding site (unshaded boxes), and the consensus LuxO-binding sites (hatched boxes) are indicated.
We identified the specific qrr nucleotide sequences bound by Fur using a DNase I footprinting assay (Fig. 6B). With respect to the transcription start site, the Fur-binding regions are located at −185 to −41 for qrr1, at −119 to −51 for qrr2, at −203 to −152 and -145 to −110 for qrr3, at −155 to −94 for qrr4, and at −153 to −121 and −82 to −16 for qrr5. All regions bound by Fur contain sequences homologous to the known Fur consensus binding box (5′-GATAATGATAATCATTATC-3′) (data not shown). Comparing the Fur binding regions to the LuxO consensus binding sequence, we observed that the binding sites for these two proteins overlap in all five of the qrr promoters. This result is consistent with the competition binding results shown in Fig. 6A. We conclude that Fur represses qrr transcription by physically interfering with the binding of LuxO.
LuxO Is Repressed by Iron in a Fur-independent Manner
Because LuxO activates qrr transcription, we hypothesized that iron might regulate luxO expression. To test this, we used a luxO-luxAB transcriptional fusion construct in both wild type and Δfur strains. We found that luxO expression was significantly induced in the presence of an iron chelator in both wild type and Δfur. Expression levels were not significantly different between wild type and Δfur, indicating that Fur itself exerts no effect on luxO expression (Fig. 7) and suggesting that iron inhibits luxO expression in a Fur-independent manner. Low expression of LuxO under iron-rich conditions might also lead to even lower levels of qrr transcription.
FIGURE 7.
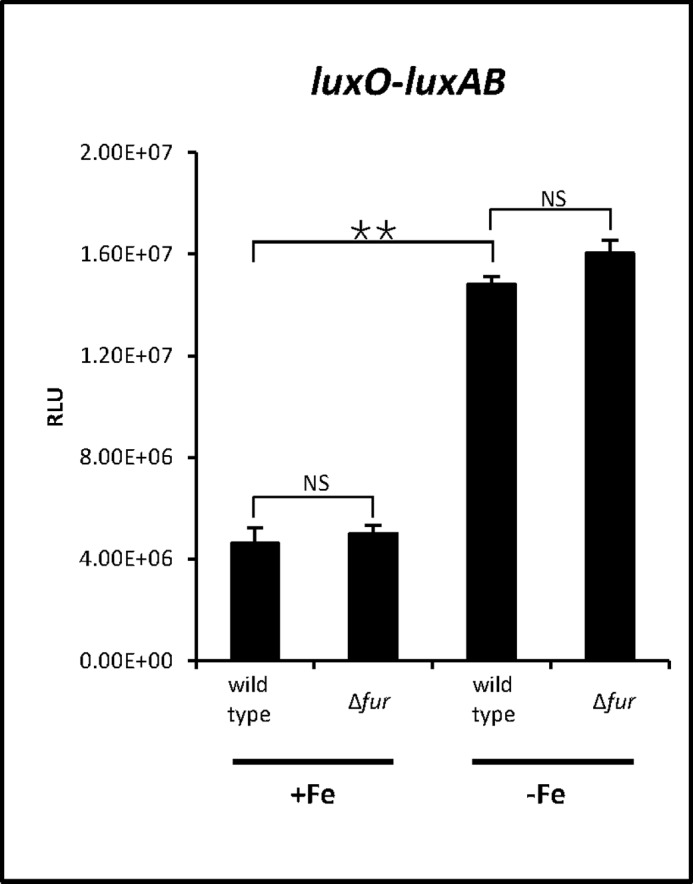
Effects of iron on luxO transcriptional levels. Luciferase activity representing transcriptional levels of luxO was measured in wild type V. vulnificus, and Δfur. Cells were cultured with or without 200 μm iron chelator 2,2′-dipyridyl, which was supplemented when cells were at an A600 of ∼0.2. Luminescence and cell density were measured at an A600 ∼0.6. Relative light units (RLU) represent light units normalized to cell density (luminescence/A600). Results are averages from three independent samples, and error bars denote standard deviations (**, p < 0.005; NS, not significant in Student's t test with p > 0.05).
Qrrs Function Additively to Repress SmcR under Iron-limiting Conditions
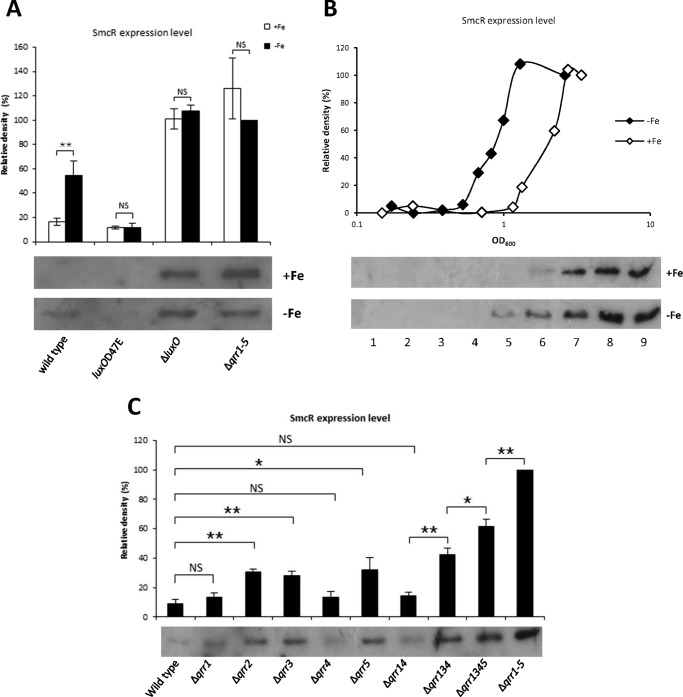
We assessed levels of SmcR expression under iron-limiting conditions in wild type, luxOD47E, ΔluxO, and Δqrr1–5 cells by Western blotting (Fig. 8A). In wild type cells grown under iron-rich conditions and harvested in log phase, SmcR was not detected. However, when iron was limiting, low levels of SmcR expression were observed. In luxOD47E mutants, SmcR was not detected under either condition. However, SmcR expression was observed in ΔluxO and Δqrr1–5 cells regardless of iron conditions. In a previous study, we showed that the Fur-iron complex directly inhibits smcR transcription (49). In this study, we confirmed that the transcriptional levels of smcR were lower under iron-rich conditions in all four strains (data not shown). Nevertheless, SmcR was not decreased by iron in ΔluxO and Δqrr1–5. It is possible that the iron-mediated transcriptional repression of smcR is not strong enough to affect protein levels, especially at high cell density when SmcR translation is no longer inhibited by Qrrs. Consequently, the prediction is that SmcR levels would be repressed by iron at low cell density but not affected at high cell density. To test this model, we measured SmcR levels at various growth stages in wild type cells. At low cell density, SmcR was not detectable regardless of iron levels (Fig. 8B). However, SmcR expression was induced at a much earlier growth phase under iron-limiting conditions than under iron-rich conditions, and it reached a similar level under both conditions when cells were at high density. This pattern was also observed for SmcR-directed VvpE expression in our previous study (49). This result suggests that the direct repressive effect of iron on smcR is not strong and that at stationary phase, when no Qrrs are expressed, smcR expression is fully derepressed making repression by Fur-iron negligible. When iron is limiting, SmcR is expressed at an earlier growth stage compared than under iron-rich conditions, making cells more sensitive to the effects of cell density. To investigate this further, we assessed the expression of smcR under iron-limiting conditions using qrr deletion strains (Fig. 8C). SmcR expression in Δqrr2, Δqrr3, and Δqrr5 was significantly derepressed as compared with wild type but was not significantly different in Δqrr1 and Δqrr4. SmcR expression in the double mutant Δqrr14 was barely different from wild type. This is in agreement with our observation that qrr1 and qrr4 are expressed at low levels and therefore cannot effectively inhibit expression of SmcR regardless of iron levels. In contrast, Δqrr2, Δqrr3, and Δqrr5 had much higher levels of SmcR expression, consistent with higher expression of qrr2, qrr3, and qrr5 in wild type cells (Fig. 2A). The other multiple mutant strains, Δqrr134, Δqrr1345, and Δqrr1–5, showed gradually increasing levels of smcR expression, suggesting that Qrrs function additively to repress smcR under iron-limiting conditions.
FIGURE 8.
Effect of iron on SmcR regulation. A, Western blot hybridization of SmcR in wild type, luxOD47E, Δqrr1–5, and ΔluxO under iron-rich and iron-limiting conditions. The iron chelator 2,2′-dipyridyl (200 μm), was supplemented when cells were at an A600 of ∼0.2, and cells were harvested at an A600 of ∼0.6. Ten μg of each lysate sample was loaded onto the gel. The upper panel represents the relative densities of bands in the hybridization shown in the lower panel. Values are averages normalized to the intensity of the Δqrr1–5 (without iron) sample from three biological experiments. Error bars denote standard deviations (**, p < 0.005; NS, not significant in Student's t test with p > 0.05). B, SmcR expression under iron-rich and iron-limiting conditions. Wild type V. vulnificus was cultured with or without 100 μm of 2,2′-dipyridyl supplemented at an A600 of ∼0.1. Cells were harvested for Western blotting at different growth stages as indicated, and 10 μg of each lysate were loaded onto the gel. Wild type cells without iron chelator were harvested at A600 readings of 0.148, 0.240, 0.502, 0.709, 1.156, 1.335, 2.22, 2.75, and 3.395 (lanes 1–9, respectively). Wild type cells supplemented with the iron chelator were harvested at A600 readings of 0.172, 0.242, 0.382, 0.527, 0.671, 0.822, 0.998, 1.296, and 2.62 (lanes 1–9, respectively). The upper panel represents the relative densities of bands shown in the lower panel. Values are averages normalized to the intensity of the sample in lane 9. C, expression of SmcR under iron-limiting conditions. V. vulnificus mutants, including Δqrr1, Δqrr2, Δqrr3, Δqrr4, Δqrr5, Δqrr14, Δqrr134, Δqrr1345, and Δqrr1–5, were cultured with 200 μm 2,2′-dipyridyl added at an A600 of ∼0.2. Cells were harvested for Western blotting at an A600 of ∼0.4, and 10 μg of each lysate was loaded onto the gel. The upper panel represents the relative densities of bands shown in the lower panel. Values are averages normalized to the intensity of the Δqrr1–5 sample from three biological experiments (**, p < 0.005; *, p < 0.01; NS, not significant in Student's t test with p > 0.05).
Discussion
In this study, we characterized five quorum-regulatory RNAs in V. vulnificus that control the expression of the master regulator SmcR. Based on the magnitude and pattern of expression, the five Qrrs could be separated into three groups, with differences that may be attributed to the varying affinity of LuxO for cis-acting elements in the upstream promoter region of each. qrr2 has three LuxO-binding sites, one of which is a perfect match to the canonical LuxO consensus binding sequence (Fig. 1B) (37). qrr3, qrr4, and qrr5 each have two binding sites, one of which is a perfect match to the consensus. qrr1 has only one binding site that differs from the consensus sequence by two nucleotides (Fig. 1B). These differences likely affect the binding affinity of LuxO for each qrr, which we observed in preliminary experiments using EMSA (Fig. 2B). Differences in expression between qrr genes have also been observed in V. cholerae and V. harveyi (37, 38).
It is not clear why V. vulnificus employs multiple Qrrs for the purpose of repressing SmcR. V. cholerae, V. harveyi, and Vibrio parahemeolyticus harbor four, five, and five Qrrs, respectively, to repress the LuxR-type regulator, so our observations are consistent with what has been found in these other Vibrio species (37, 38). A collection of Qrrs with redundant activities might help guarantee strong repression of SmcR when cells are at a low density. It is also possible that some or all of the Qrrs regulate targets other than SmcR. In V. harveyi and V. cholerae, aphA is activated by Qrrs at low cell density, and this gene product then regulates the expression of ∼300 additional genes, including numerous virulence factors (55). An aphA homolog was identified in V. vulnificus (56), and although expression of this gene was not directly affected by Qrrs, it was repressed by SmcR.3 We speculate that there are other as yet unidentified regulatory proteins that are activated at low cell density in V. vulnificus. Qrrs are expressed at low cell densities and derepressed under iron-limiting conditions, suggesting that they may play a role in the regulation of genes required under these conditions. Recent studies have shown that Qrrs also directly regulate target genes not involved in quorum sensing, such as the type VI secretion system, genes associated with biofilm formation, and numerous other recently discovered genes in V. cholerae (57–59). A recent study showed that even a single Qrr can transduce quorum-sensing signals through multiple mechanisms. In V. harveyi, Qrrs act through different mechanisms for different targets and employ unique base pairing regions to discriminate between targets (40, 60). The presence of a variety of non-conserved regions among the five V. vulnificus Qrrs suggests that each may also regulate specific target genes independently. If there are conditions under which repression of a particular qrr gene is necessary to properly manipulate regulation of one of these unique targets, the presence of the remaining Qrrs might suffice to transduce the quorum-sensing signal and regulate SmcR.
It is possible that some or all Qrrs are involved in transduction of non-quorum-sensing signals. The iron-dependent regulation of Qrrs and luxO shown in this study might represent the first example of such multiple roles for Qrrs. These results suggest that the presence of multiple Qrrs make it possible to simultaneously monitor multiple environmental signals and coordinately modulate various target genes to fine-tune gene expression and elicit efficient and effective responses under a given condition. Identifying the effects of other environmental conditions on Qrr expression may reveal more mechanisms by which these quorum-sensing signals are affected.
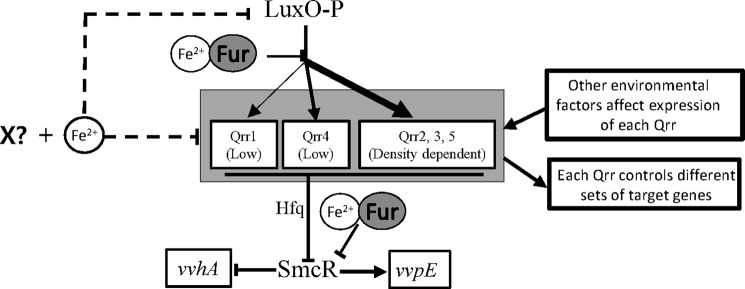
This study showed that iron affects Qrr expression in three different ways (Fig. 9). First, iron directly represses qrr transcription by antagonizing LuxO-mediated activation. Second, iron represses transcription of all five qrr genes independently of Fur through the action of an unknown factor (labeled X in Fig. 9). Third, transcription of LuxO is repressed by iron and an unknown factor independently of Fur thereby repressing qrr transcription. We determined that several factors involved in cell density, iron, and growth stage, including SmcR, IscR (61), and RpoS (62), are irrelevant to the observed iron-associated regulation (data not shown) and are therefore unlikely candidates for the unknown factor (X in Fig. 9). This unknown factor remains to be identified.
FIGURE 9.
Working model for the regulation of the quorum-sensing signaling pathway by cell density and iron in V. vulnificus. At low cell density, phosphorylated LuxO activates σ54-dependent transcription of the five qrr genes to different degrees and with different patterns. It is possible that the expression of each qrr gene is also affected by environmental factors other than cell density and that each Qrr has its own target genes to regulate. Qrr redundantly represses expression of SmcR with assistance from Hfq. SmcR is a global transcriptional regulator involved in regulation of various virulence factors, including activation of vvpE and repression of vvhA. The Fur-iron complex directly represses the expression of both Qrr and SmcR. Iron also inhibits the transcription of luxO and the five qrr genes, possibly through an unidentified factor labeled X.
Iron affects smcR expression both positively and negatively. In the presence of iron, luxO and qrr expression are repressed, resulting in up-regulation of smcR. Conversely, the iron-Fur complex represses smcR expression by directly binding to the promoter region (49). However, it appears that iron-Fur-mediated repression is not strong enough to effectively repress smcR expression at high cell density when the qrr genes are strongly inhibited by iron (Fig. 8). Therefore, the overall effect of iron is the up-regulation of smcR expression, leading to a greater cell response to quorum sensing, i.e. an earlier transition to high cell density mode in the absence of iron leads to activation of the quorum-sensing response. The biological role of the direct repression of smcR by the Fur-iron complex is likely modulation at high cell density, where Qrrs are not expressed and cannot be regulated by iron. In summary, by employing this dual regulatory system, cells can effectively turn on the quorum-sensing response in the presence of iron at low cell density and attenuate the response in the presence of iron at high cell density.
We further showed that Qrrs repress SmcR redundantly under iron-rich conditions (Fig. 5) but additively under iron-limiting conditions (Fig. 8D). It is possible that under iron-limiting conditions, smcR is no longer repressed by the Fur iron complex (49), meaning that stronger Qrr control is needed to keep SmcR repressed. Regardless of the molecular basis underlying the additive action of Qrrs under iron-limiting conditions, such a regulatory mode results in a faster transition from low cell density mode to the high cell density mode (Fig. 8, B and C). Iron availability is an important limitation for pathogenic microorganisms to overcome to survive and thrive in a host environment. Rapid transition to high cell density mode under these conditions may allow pathogens to more quickly express virulence factors and thereby acquire iron.
The transition from single-cell mode to quorum-sensing mode involves more than LuxO-P, Qrrs, and SmcR. Complicated feedback loops, dose compensation, and self-regulation are also involved in the precise timing of the switch between high and low cell density modes. In V. cholerae, four Qrrs compensate for each other to calibrate total Qrr activity through the Qrr-LuxO and HapR-Qrr feedback loops and ensure the timing of quorum sensing (39, 63). HapR self-repression and LuxR-Qrr feedback ensure the fast elimination of LuxR during the transition from high cell density to low cell density (63, 64). HapR and LuxO also constrain their expression through self-repression (64, 65). As all of these factors are affected by iron, these regulatory mechanisms need to be carefully studied under iron-limiting conditions.
Cell density is important for the survival and pathogenesis of bacteria as they encounter the differing conditions of a host environment, and iron and cell density influence one another by affecting the growth rate of cells (66–68). Alternatively, cell density might also directly affect the availability of iron. Therefore, cognate signal transduction systems for the quorum-sensing and iron-regulation pathways regulate both pathways and coordinate the regulation of virulence genes. In Pseudomonas aeruginosa, PrrF1 and PrrF2, small RNAs involved in iron-dependent regulation, activate quorum sensing by promoting the production of an autoinducer (69). In V. vulnificus, both SmcR and Fur repress the transcription of vvhA and the vulnibactin synthesis gene vvsAB (23). SmcR expression is directly repressed through binding of the Fur-iron complex, leading to an attenuated quorum-sensing response under iron-rich conditions (49). In summary, this study showed that cognate signal transduction pathways for iron and quorum sensing converge on Qrrs to control the expression of virulence factors and ensure optimal growth conditions for the pathogen while in the host.
Author Contributions
Y. W. and K. S. K. designed the study and wrote the paper. Y. W. and I. H. K. performed the experiments.
This work was supported by the National Research Foundation Grants NRF-2011-0018115 and NRF-2015M3C9A2054020 from the Korean Government. The authors declare that they have no conflict of interest with the contents of this article.
Y. Wen, I. H. Kim, and K.-S. Kim, unpublished results.
- Fur
- ferric uptake regulator
- Qrr
- quorum regulatory RNA.
References
- 1. Kumamoto K. S., and Vukich D. J. (1998) Clinical infections of Vibrio vulnificus: a case report and review of the literature. J. Emerg. Med. 16, 61–66 [DOI] [PubMed] [Google Scholar]
- 2. Testa J., Daniel L. W., and Kreger A. S. (1984) Extracellular phospholipase A2 and lysophospholipase produced by Vibrio vulnificus. Infect. Immun. 45, 458–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kothary M. H., and Kreger A. S. (1987) Purification and characterization of an elastolytic protease of Vibrio vulnificus. J. Gen. Microbiol. 133, 1783–1791 [DOI] [PubMed] [Google Scholar]
- 4. Gray L. D., and Kreger A. S. (1989) Detection of Vibrio vulnificus cytolysin in V. vulnificus-infected mice. Toxicon 27, 459–464 [DOI] [PubMed] [Google Scholar]
- 5. Wright A. C., Simpson L. M., Oliver J. D., and Morris J. G. Jr. (1990) Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58, 1769–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S. E., Ryu P. Y., Kim S. Y., Kim Y. R., Koh J. T., Kim O. J., Chung S. S., Choy H. E., and Rhee J. H. (2004) Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem. Biophys. Res. Commun. 324, 86–91 [DOI] [PubMed] [Google Scholar]
- 7. Kim C. M., Park R. Y., Chun H. J., Kim S. Y., Rhee J. H., and Shin S. H. (2007) Vibrio vulnificus metalloprotease VvpE is essentially required for swarming. FEMS Microbiol. Lett. 269, 170–179 [DOI] [PubMed] [Google Scholar]
- 8. Lee M. A., Kim J. A., Shin M. Y., Lee J. K., Park S. J., and Lee K. H. (2015) VvpM induces human cell death via multifarious modes including necroptosis and autophagy. J. Microbiol. Biotechnol. 25, 302–306 [DOI] [PubMed] [Google Scholar]
- 9. Kim I. H., Kim I. J., Wen Y., Park N. Y., Park J., Lee K. W., Koh A., Lee J. H., Koo S. H., and Kim K. S. (2015) Vibrio vulnificus secretes an insulin-degrading enzyme that promotes bacterial proliferation in vivo. J. Biol. Chem. 290, 18708–18720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee B. C., Choi S. H., and Kim T. S. (2008) Vibrio vulnificus RTX toxin plays an important role in the apoptotic death of human intestinal epithelial cells exposed to Vibrio vulnificus. Microbes Infect. 10, 1504–1513 [DOI] [PubMed] [Google Scholar]
- 11. Litwin C. M., Rayback T. W., and Skinner J. (1996) Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 64, 2834–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim C.-M., Park R.-Y., Park J.-H., Sun H.-Y., Bai Y.-H., Ryu P.-Y., Kim S.-Y., Rhee J.-H., and Shin S.-H. (2006) Vibrio vulnificus vulnibactin, but not metalloprotease VvpE, is essentially required for iron-uptake from human holotransferrin. Biol. Pharm. Bull. 29, 911–918 [DOI] [PubMed] [Google Scholar]
- 13. Storz G., and Hengge R. (2010) in Bacterial Stress Responses (Storz G., and Hengge R., eds) American Society for Microbiology, Washington, D. C. [Google Scholar]
- 14. Ratledge C., and Dover L. G. (2000) Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54, 881–941 [DOI] [PubMed] [Google Scholar]
- 15. Wright A. C., Simpson L. M., and Oliver J. D. (1981) Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34, 503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris J. G. Jr., Wright A. C., Simpson L. M., Wood P. K., Johnson D. E., and Oliver J. D. (1987) Virulence of Vibrio vulnificus: association with utilization of transferrin-bound iron, and lack of correlation with levels of cytotoxin or protease production. FEMS Microbiol. Lett. 40, 55–59 [Google Scholar]
- 17. Simpson L. M., and Oliver J. D. (1983) Siderophore production by Vibrio vulnificus. Infect. Immun. 41, 644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okujo N., Akiyama T., Miyoshi S., Shinoda S., and Yamamoto S. (1996) Involvement of vulnibactin and exocellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol. Immunol. 40, 595–598 [DOI] [PubMed] [Google Scholar]
- 19. Hantke K. (1981) Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182, 288–292 [DOI] [PubMed] [Google Scholar]
- 20. Bagg A., and Neilands J. B. (1987) Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26, 5471–5477 [DOI] [PubMed] [Google Scholar]
- 21. Hantke K. (2001) Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4, 172–177 [DOI] [PubMed] [Google Scholar]
- 22. Webster A. C., and Litwin C. M. (2000) Cloning and characterization of vuuA, a gene encoding the Vibrio vulnificus ferric vulnibactin receptor. Infect. Immun. 68, 526–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen Y., Kim I. H., Son J.-S., Lee B.-H., and Kim K.-S. (2012) Iron and quorum sensing coordinately regulate the expression of vulnibactin biosynthesis in Vibrio vulnificus. J. Biol. Chem. 287, 26727–26739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee H.-J., Kim J.-A., Lee M.-A., Park S.-J., and Lee K.-H. (2013) Regulation of haemolysin (VvhA) production by ferric uptake regulator (Fur) in Vibrio vulnificus: repression of vvhA transcription by Fur and proteolysis of VvhA by Fur-repressive exoproteases. Mol. Microbiol. 88, 813–826 [DOI] [PubMed] [Google Scholar]
- 25. Miller M. B., and Bassler B. L. (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199 [DOI] [PubMed] [Google Scholar]
- 26. Chen X., Schauder S., Potier N., Van Dorsselaer A., Pelczer I., Bassler B. L., and Hughson F. M. (2002) Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549 [DOI] [PubMed] [Google Scholar]
- 27. Kim S. Y., Lee S. E., Kim Y. R., Kim C. M., Ryu P. Y., Choy H. E., Chung S. S., and Rhee J. H. (2003) Regulation of Vibrio vulnificus virulence by the LuxS quorum-sensing system. Mol. Microbiol. 48, 1647–1664 [DOI] [PubMed] [Google Scholar]
- 28. Park J. H., Cho Y.-J., Chun J., Seok Y.-J., Lee J. K., Kim K.-S., Lee K.-H., Park S.-J., and Choi S. H. (2011) Complete genome sequence of Vibrio vulnificus MO6-24/O. J. Bacteriol. 193, 2062–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bassler B. L., Wright M., and Silverman M. R. (1994) Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13, 273–286 [DOI] [PubMed] [Google Scholar]
- 30. Bassler B. L., Wright M., and Silverman M. R. (1994) Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol. Microbiol. 12, 403–412 [DOI] [PubMed] [Google Scholar]
- 31. Freeman J. A., and Bassler B. L. (1999) A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31, 665–677 [DOI] [PubMed] [Google Scholar]
- 32. Freeman J. A., and Bassler B. L. (1999) Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 181, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Milton D. L. (2006) Quorum sensing in Vibrios: complexity for diversification. Int. J. Med. Microbiol. 296, 61–71 [DOI] [PubMed] [Google Scholar]
- 34. Wyman C., Rombel I., North A. K., Bustamante C., and Kustu S. (1997) Unusual oligomerization required for activity of NtrC, a bacterial enhancer-binding protein. Science 275, 1658–1661 [DOI] [PubMed] [Google Scholar]
- 35. Lilley B. N., and Bassler B. L. (2000) Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36, 940–954 [DOI] [PubMed] [Google Scholar]
- 36. Roh J. B., Lee M. A., Lee H. J., Kim S. M., Cho Y., Kim Y. J., Seok Y. J., Park S. J., and Lee K. H. (2006) Transcriptional regulatory cascade for elastase production in Vibrio vulnificus: LuxO activates luxT expression and LuxT represses smcR expression. J. Biol. Chem. 281, 34775–34784 [DOI] [PubMed] [Google Scholar]
- 37. Lenz D. H., Mok K. C., Lilley B. N., Kulkarni R. V., Wingreen N. S., and Bassler B. L. (2004) The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118, 69–82 [DOI] [PubMed] [Google Scholar]
- 38. Tu K. C., and Bassler B. L. (2007) Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev. 21, 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Svenningsen S. L., Tu K. C., and Bassler B. L. (2009) Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. EMBO J. 28, 429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shao Y., and Bassler B. L. (2012) Quorum-sensing non-coding small RNAs use unique pairing regions to differentially control mRNA targets. Mol. Microbiol. 83, 599–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miyashiro T., Wollenberg M. S., Cao X., Oehlert D., and Ruby E. G. (2010) A single qrr gene is necessary and sufficient for LuxO-mediated regulation in Vibrio fischeri. Mol. Microbiol. 77, 1556–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee J. H., Rhee J. E., Park U., Ju H. M., Lee B. C., Kim T. S., Jeong H. S., and Choi S. H. (2007) Identification and functional analysis of Vibrio vulnificus SmcR, a novel global regulator. J. Microbiol. Biotechnol. 17, 325–334 [PubMed] [Google Scholar]
- 43. Jeong H. S., Lee M. H., Lee K. H., Park S. J., and Choi S. H. (2003) SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J. Biol. Chem. 278, 45072–45081 [DOI] [PubMed] [Google Scholar]
- 44. Shao C.-P., Lo H.-R., Lin J.-H., and Hor L.-I. (2011) Regulation of cytotoxicity by quorum-sensing signaling in Vibrio vulnificus is mediated by SmcR, a repressor of hlyU. J. Bacteriol. 193, 2557–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sambrook J., and Russell D. W. (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 46. Lee H. S., Lim J. G., Han K., Lee Y., and Choi S. H. (2015) Molecular analysis of promoter and intergenic region attenuator of the Vibrio vulnificus prx1ahpF operon. J. Microbiol. Biotechnol. 25, 1380–1389 [DOI] [PubMed] [Google Scholar]
- 47. Jeong H. S., Jeong K. C., Choi H. K., Park K.-J., Lee K.-H., Rhee J. H., and Choi S. H. (2001) Differential expression of Vibrio vulnificus elastase gene in a growth phase-dependent manner by two different types of promoters. J. Biol. Chem. 276, 13875–13880 [DOI] [PubMed] [Google Scholar]
- 48. Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 49. Kim I. H., Wen Y., Son J.-S., Lee K.-H., and Kim K.-S. (2013) The Fur-iron complex modulates expression of the quorum-sensing master regulator, SmcR, to control expression of virulence factors in Vibrio vulnificus. Infect. Immun. 81, 2888–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson J. D., Higgins D. G., and Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swartzman E., Silverman M., and Meighen E. A. (1992) The luxR gene product of Vibrio harveyi is a transcriptional activator of the lux promoter. J. Bacteriol. 174, 7490–7493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jobling M. G., and Holmes R. K. (1997) Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homolog of the Vibrio harveyi luxR gene. Mol. Microbiol. 26, 1023–1034 [DOI] [PubMed] [Google Scholar]
- 53. Mather M. W., McReynolds L. M., and Yu C. A. (1995) An enhanced broad-host-range vector for gram-negative bacteria: avoiding tetracycline phototoxicity during the growth of photosynthetic bacteria. Gene 156, 85–88 [DOI] [PubMed] [Google Scholar]
- 54. Escolar L., Pérez-Martín J., and de Lorenzo V. (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181, 6223–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rutherford S. T., van Kessel J. C., Shao Y., and Bassler B. L. (2011) AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 25, 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lim J. G., Park J. H., and Choi S. H. (2014) Low cell density regulator AphA upregulates the expression of Vibrio vulnificus iscR gene encoding the Fe-S cluster regulator IscR. J. Microbiol. 52, 413–421 [DOI] [PubMed] [Google Scholar]
- 57. Hammer B. K., and Bassler B. L. (2007) Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 104, 11145–11149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shao Y., Feng L., Rutherford S. T., Papenfort K., and Bassler B. L. (2013) Functional determinants of the quorum-sensing non-coding RNAs and their roles in target regulation. EMBO J. 32, 2158–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shao Y., and Bassler B. L. (2014) Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae. Mol. Microbiol. 92, 921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feng L., Rutherford S. T., Papenfort K., Bagert J. D., van Kessel J. C., Tirrell D. A., Wingreen N. S., and Bassler B. L. (2015) A qrr noncoding RNA deploys four different regulatory mechanisms to optimize quorum-sensing dynamics. Cell 160, 228–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lim J. G., and Choi S. H. (2014) IscR is a global regulator essential for pathogenesis of Vibrio vulnificus and induced by host cells. Infect. Immun. 82, 569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Battesti A., Majdalani N., and Gottesman S. (2011) The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65, 189–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Svenningsen S. L., Waters C. M., and Bassler B. L. (2008) A negative feedback loop involving small RNAs accelerates Vibrio cholerae's transition out of quorum-sensing mode. Genes Dev. 22, 226–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin W., Kovacikova G., and Skorupski K. (2005) Requirements for Vibrio cholerae HapR binding and transcriptional repression at the hapR promoter are distinct from those at the aphA promoter. J. Bacteriol. 187, 3013–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tu K. C., Long T., Svenningsen S. L., Wingreen N. S., and Bassler B. L. (2010) Negative feedback loops involving small regulatory RNAs precisely control the Vibrio harveyi quorum-sensing response. Mol. Cell 37, 567–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Touati D. (2000) Iron and oxidative stress in bacteria. Arch. Biochem. Biophys. 373, 1–6 [DOI] [PubMed] [Google Scholar]
- 67. Schaible U. E., and Kaufmann S. H. (2004) Iron and microbial infection. Nat. Rev. Microbiol. 2, 946–953 [DOI] [PubMed] [Google Scholar]
- 68. Waters C. M., and Bassler B. L. (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 [DOI] [PubMed] [Google Scholar]
- 69. Oglesby A. G., Farrow J. M. 3rd., Lee J.-H., Tomaras A. P., Greenberg E. P., Pesci E. C., and Vasil M. L. (2008) The influence of iron on Pseudomonas aeruginosa physiology a regulatory link between iron and quorum sensing. J. Biol. Chem. 283, 15558–15567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simon R., Priefer U., and Pühler A. (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1, 784–791 [Google Scholar]
- 71. Lee H. J., Park K. J., Lee A. Y., Park S. G., Park B. C., Lee K. H., and Park S. J. (2003) Regulation of fur expression by RpoS and fur in Vibrio vulnificus. J. Bacteriol. 185, 5891–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kovach M. E., Elzer P. H., Hill D. S., Robertson G. T., Farris M. A., Roop R. M. 2nd., and Peterson K. M. (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166, 175–176 [DOI] [PubMed] [Google Scholar]
- 73. Keen N. T., Tamaki S., Kobayashi D., and Trollinger D. (1988) Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70, 191–197 [DOI] [PubMed] [Google Scholar]
- 74. Milton D. L., O'Toole R., Horstedt P., and Wolf-Watz H. (1996) Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178, 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]