Abstract
Background
A susceptibility to metabolic diseases is associated with abdominal adipose tissue distribution and varies between ethnic groups. The distribution of abdominal adipose tissue at birth may give insights into whether ethnicity-associated variations in metabolic risk originate partly in utero.
Objective
We assessed the influence of ethnicity on abdominal adipose tissue compartments in Asian neonates in the Growing Up in Singapore Toward Healthy Outcomes mother-offspring cohort.
Design
MRI was performed at ≤2 wk after birth in 333 neonates born at ≥34 wk of gestation and with birth weights ≥2000 g. Abdominal superficial subcutaneous tissue (sSAT), deep subcutaneous tissue (dSAT), and internal adipose tissue (IAT) compartment volumes (absolute and as a percentage of the total abdominal volume) were quantified.
Results
In multivariate analyses that were controlled for sex, age, and parity, the absolute and percentage of dSAT and the percentage of sSAT (but not absolute sSAT) were greater, whereas absolute IAT (but not the percentage of IAT) was lower, in Indian neonates than in Chinese neonates. Compared with Chinese neonates, Malay neonates had greater percentages of sSAT and dSAT but similar percentages of IAT. Marginal structural model analyses largely confirmed the results on the basis of volume percentages with controlled direct effects of ethnicity on abdominal adipose tissue; dSAT was significantly greater (1.45 mL; 95% CI: 0.49, 2.41 mL, P = 0.003) in non-Chinese (Indian or Malay) neonates than in Chinese neonates. However, ethnic differences in sSAT and IAT were NS [3.06 mL (95% CI: −0.27, 6.39 mL; P = 0.0712) for sSAT and −1.30 mL (95% CI: −2.64, 0.04 mL; P = 0.057) for IAT in non-Chinese compared with Chinese neonates, respectively].
Conclusions
Indian and Malay neonates have a greater dSAT volume than do Chinese neonates. This finding supports the notion that in utero influences may contribute to higher cardiometabolic risk observed in Indian and Malay persons in our population. If such differences persist in the longitudinal tracking of adipose tissue growth, these differences may contribute to the ethnic disparities in risks of cardiometabolic diseases.
Keywords: abdominal adipose tissue compartments, Asian neonates, MRI, ethnic differences, metabolic risk, birth cohort study
Introduction
Over the past 2 decades, research on the developmental origins of health and disease has suggested that a susceptibility to metabolic diseases may originate early in life (1, 2). The maternal in utero environment appears to influence metabolic health by altering glucose metabolism and body composition (2, 3). The quantity and distribution of adipose tissue at birth reflect in utero environmental influences and may differ in ethnic groups (4).
In adults, adipose tissue compartments, particularly in the abdomen, are associated with risks of metabolic and cardiovascular diseases (5, 6). Although most studies have shown strong associations between visceral adipose tissue and insulin resistance and metabolic diseases, deep subcutaneous adipose tissue has increasingly been recognized to be metabolically relevant, similar to the importance of visceral adipose tissue, both in diabetic and nondiabetic individuals. Abdominal deep subcutaneous tissue (dSAT)19 was recently reported to be strongly related to insulin resistance and cardiovascular outcomes in patients with type 2 diabetes (7–9). dSAT is distinctly separated from superficial subcutaneous adipose tissue by Scarpa’s fascia (10–12); it differs morphologically and biologically from abdominal superficial subcutaneous tissue (sSAT) by its association with a more-proinflammatory, -lipogenic, and -lipolytic profile (13, 14).
Ethnic differences in adiposity between South Asian and European adults have been reported in many studies. Compared with Europeans, South Asians are more insulin resistant (15–19) despite their lower BMI. Studies in Singapore have shown that ethnic Indian (South Asian) adults have greater insulin resistance, larger waist circumferences, and higher risks of type 2 diabetes mellitus and cardiovascular disease than do ethnic Malays and Chinese (20–22). Increased adiposity and hyperinsulinemia, which are suggestive of an increased susceptibility to metabolic diseases in adulthood, have been observed even in South Asian school-age children (23, 24) and infants (25, 26). However, information has been sparse on how early in life such ethnic differences in adiposity emerge, especially with respect to regional adipose tissue distribution.
Previous studies have generally relied on skinfold thicknesses and air displacement plethysmography using PEA POD Infant Body Composition System version 3.1.0 (Cosmed) as measures of body composition. In one of the few studies that used MRI to quantify adipose tissue compartments, Modi et al. (27) reported that, in 69 neonates from Pune, India, and London, the Indian neonates had greater regional adiposity in all 3 abdominal adipose tissue compartments [AATCs; i.e., sSAT, dSAT, and abdominal internal adipose tissue (IAT)] than that of their European counterparts. We hypothesized that the quantity and distribution of AATCs at birth would vary in Asian ethnic groups. We tested this hypothesis in Asian neonates in the GUSTO (Growing Up in Singapore Toward Healthy Outcomes) birth cohort (28).
Methods
GUSTO is a birth-cohort study that examines the developmental pathways to metabolic diseases in Singapore (28). The GUSTO study recruited pregnant women during their first trimester at the antenatal diagnostic clinics of 2 major public maternity units in Singapore (i.e., the National University Hospital and the KK Women’s and Children’s Hospital) between June 2009 and September 2010.
Subjects
To be recruited into the GUSTO study, mothers had to be ≥18 y of age and intend to deliver in one of the 2 main public maternity units (i.e., the KK Women’s and Children’s Hospital or the National University Hospital) and to reside in Singapore for the next 5 y. Subjects, partners, and their parents had to be of the homogenous ethnic background from one of the following major ethnic groups in Singapore: Chinese, Malay, or Indian/South Asian (the last group is termed Indian henceforth in this report). A principal components analysis of the genotype data for the newborns in our study confirmed that the 3 ethnic groups are well separated genetically (29).
We approached 1115 of 1162 GUSTO mothers who attended the 32–34-wk antenatal ultrasound scan. The flow diagram of this study is shown in Supplemental Figure 1. Only 478 mothers (43%) gave signed consent to have neonatal MRI scans taken of their neonates. Healthy neonates born at ≥34 wk of gestational age with birth weight ≥2000 g were eligible for MRI scanning. A total of 379 eligible healthy neonates underwent an MRI at ≤2 wk after birth (mean ± SD: 10 ± 3 d). Sixty-two neonates were ineligible for MRI as a result of requiring special care (n = 52), requiring neonatal intensive care (n = 7), and having been born at < 34 wk of gestation. The remaining 37 neonates were unable to undergo MRI because consent was revoked by parents or because of an inconvenience to come for an MRI scan (n = 30), and inability to get an MRI slot (n = 4), dropping out of the study (n = 2), or being unable to contact (n = 1). Forty-six data did not pass our initial quality control for the analysis. Therefore, complete data sets for this analysis were obtained in 333 neonates. Neonatal feeding was classified into 3 groups as follows: exclusive or predominant breastfeeding if the neonate received only breast milk and water; partial breastfeeding if the neonate received both breastfeeding and formula; and total formula feeding if the neonate was fully formula fed.
Ethics
This study was approved by the Institutional Review Board of the Singapore National Health care Group and the Centralized Institutional Review Board of SingHealth. Parents of the neonates gave written consent.
MRI
Nonsedated neonates were fed and swaddled, placed in an immobilization bag 5–10 min into their sleep, and positioned supine within an adult head coil. The abdomen was scanned from the diaphragm to the symphysis pubis. T1-weighted water-suppressed (WS) and non-WS axial fast-spin echo sequences were acquired by GE Signa HDxt 1.5 tesla magnetic resonance scanner (GE Healthcare) with the use of a 600-ms repetition time, a 7-ms echo time, a 22-cm field of view, 3 excitations, a 256 × 256 matrix interpolated to 512 × 512, a phase-encoding direction anterior-posterior, a 70% phase field of view, and an echo train length of 7- and 5-mm contiguous slices. Approximately 34–36 slices provided ∼18 cm coverage, which was sufficient to encompass the neonate’s abdomen. Pulse and oxygen saturation amounts of the neonate were monitored in the presence of a neonatologist. WS images were processed to yield quantitative values of abdominal adipose tissue volumes. Non-WS images were used to assist in the localization of anatomical structures if necessary.
Definition of AATCs
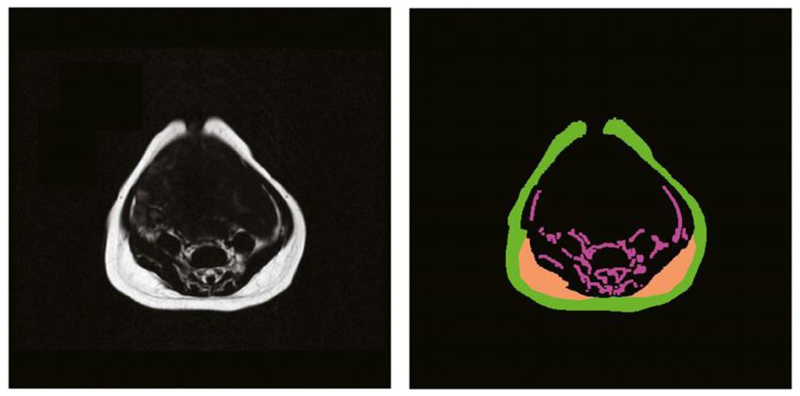
For consistency with previous publications, we defined the abdominal region for the analysis from the level of the diaphragm to the superior aspect of the sacrum (27, 30). The main outcome measure, abdominal adipose tissue, was categorized into the following 3 compartments: sSAT, dSAT, and IAT. sSAT had a clear anatomical outline that followed the contours of the abdominal image slices. dSAT that was located on the interior margin of the left and right posterior sSAT was distinctly separated from the sSAT by a fascial plane. IAT was the internal fat contained within the abdominal region (Figure 1). IAT includes intraperitoneal, retroperitoneal, intermuscular, as well as paravertebral and intraspinal fat within the abdominal region.
Figure 1.
Original abdominal water-suppressed MRI (left) and the results of the segmentation of abdominal adipose tissue compartments (right). Each compartment is filled with a different color as follows: green denotes superficial subcutaneous tissue, orange denotes the right and left deep subcutaneous tissue, and magenta denotes the internal adipose tissue.
Quantification of AATC volume
WS images simplified the assessment of MR signals from adipose tissue by suppressing the signals from nearby nonfat anatomical structures. Images were processed with the use of in-house semiautomated quantitative analysis software (MATLAB 7.13; The MathWorks Inc.) on the basis of morphologic image-analysis operations. A watershed transformation of local signal SDs yielded an initial segmentation of subcutaneous and internal adipose tissue compartments from the background. This initial step was not completely accurate in all slices because of image artifacts (e.g., partial voluming or the presence of unsuppressed feed). Therefore, sSAT and IAT segmentations were optimized by manually reassigning or removing automatically assigned voxel groups on the basis of the anatomical judgment of the analysts. dSAT was manually separated from sSAT and IAT by drawing 2 regions of interest at the right and left posterior aspects of the abdominal wall, which followed the contours of the separating fascial plane (Figure 1). AATC volumes were generated by multiplying the number of respective segmented voxels within each image slice by the voxel dimensions (typically 0.4 × 0.4 × 5.0 mm) except for voxels located at tissue boundaries, which were tallied as one-half of a voxel. This process recognized that boundary voxels are strictly shared between the enclosed and surrounding tissues or spaces and also ensured that tallied volumes were independent of image voxel size. The abdominal adipose tissue volume for each compartment was derived from the sum of the volumes in each slice from the level of diaphragm to the superior aspect of the sacrum. The total abdominal volume (TAV) was calculated as the volume enclosed by the outermost sSAT boundaries. When open (at the umbilicus; Figure 1), this boundary was closed with a convex hull operation. Abdominal compartment volumes were also expressed as percentages of TAV.
Reproducibility of image analysis
All MRI images were analyzed by a physician and an experienced magnetic resonance physicist, both of whom received intensive and ongoing training by a radiologist. Both the physician and physicist were blinded to all subject information including ethnicity. Mean interobserver coefficients of variation were 1.57 for sSAT, 3.23 for dSAT, and 2.06 for IAT. Mean intraobserver coefficients of variation were 0.88 for sSAT, 2.12 for dSAT, and 3.98 for IAT.
Statistics
Ethnicity was categorized as Chinese, Malay, or Indian. We used 2 models of multiple linear regression to assess the influence of ethnicity on AATCs. We controlled for neonatal factors that, on the basis of prior knowledge from the literature, are associated with neonatal adiposity: sex, age on MRI day, and parity. Girls are known to have greater adiposity than that of boys even at birth (31–33). Increasing parity is associated with increasing neonatal adiposity in Asians as well as in Western populations (34, 35). Gestational age and postnatal age have also been shown to be associated with increasing weight and adiposity (33, 35). Because MRI was performed at 2–18 postnatal days, we included postnatal age as a covariate. There has been some evidence that breastfed neonates may show somewhat different early neonatal weight-loss and -regain patterns (36, 37). However, gestational age and types of neonatal feeding were not adjusted in the models because these variables could be causal intermediates between ethnicity and abdominal adipose tissue volumes. However, we also performed 2 sensitivity analyses; the first analysis was conducted by restricting the analysis to neonates born between 37–41 completed weeks of gestation, and the second analysis was conducted by including types of neonatal feeding as a covariate. In addition, we did not adjust for birth weight in the model because birth weight is likely a causal pathway between ethnicity and adipose tissue volumes. To account for the lower birth weight of Indian and Malay (compared with Chinese) neonates, we carried out a second multiple linear regression model in which the AATC volume was expressed as a percentage of the TAV. Finally, we also used marginal structural models on the basis of inverse-probability weighting for birth weight (by centering on the sample mean birth weight) (38) and other covariates to estimate the controlled direct effect of ethnicity, which was dichotomized as non-Chinese (Indian or Malay) compared with Chinese, on the adipose tissue compartments (i.e., the pathway independent of the effect of ethnicity on birth weight). The marginal structural model analyses were carried out with the use of SAS version 9.3 software (SAS Institute). All other statistical analyses were performed with the use of SPSS Statistics for Windows software (version 21.0; IBM Corp.).
Results
There were no significant differences in characteristics (birth weight, gestational age, parity, and mother prepregnancy BMI) of neonates who had neonatal MRI and neonates who did not undergo MRI.
Neonatal MRI scans were completed for 333 neonates as follows: 180 boys (54.1%), 153 girls (45.9%); 146 Chinese neonates (43.8%), 126 Malay neonates (37.8%), and 61 Indian neonates (18.3%). Table 1 summarizes the demographic and clinical data for the 3 ethnic groups. sSAT, dSAT, and IAT volumes were similar in neonates of primiparous mothers and in neonates of multiparous mothers [mean ± SD: 76.5 ± 20.6 compared with 78.9 ± 22.6 mL (P = 0.348), 13.1 ± 5.4 compared with 13.5 ± 5.7 mL (P = 0.482), and 22.8 ± 6.8 compared with 22.7± 8.1 mL (P = 0.942), respectively]. Neonates who were exclusively or predominantly breastfed had AATC volumes similar to those of neonates who received partial or total formula feeding [mean ± SD: 80.2 ± 19.4 compared with 81.3 ± 25.7 mL (P = 0.794), 13.5 ± 4.9 compared with 14.2 ± 7.2 mL (P = 0.517), and 24.8 ± 7.7 compared with 22.8 ± 9.2 mL (P = 0.189) for sSAT, dSAT, and IAT, respectively].
Table 1.
Characteristics of neonates who had MRI during the neonatal period in the GUSTO cohort study (n = 333)1
| Chinese | Malay | Indian | P | |
|---|---|---|---|---|
| Subjects, n | 146 | 126 | 61 | — |
| Birth weight, g | 3149 ± 4572 | 3122 ± 405 | 3061 ± 429 | 0.411 |
| Gestational age, wk | 38.9 ± 1.2 | 38.5 ± 1.3 | 38.8 ± 1.1 | 0.018 |
| Age on MRI day, d | 10 ± 3 | 10 ± 3 | 9 6 3 | 0.156 |
| Mother’s age, y | 31 ± 5 | 29 ± 6 | 29 ± 5 | 0.004 |
| Mother’s prepregnancy BMI, kg/m2 | 21.6 ± 3.7 | 24.5 ± 5.7 | 24.5 ± 5.2 | <0.001 |
| Sex, n (%) | 0.677 | |||
| M | 76 (52.1) | 72 (57.1) | 32 (52.5) | |
| Parity (n = 328), n (%) | 0.334 | |||
| Primiparous | 63 (44.1) | 46 (37.1) | 21 (52.5) | |
| Types of neonatal feeding (n = 320), n (%) | 0.206 | |||
| Exclusive/predominant breastfeeding | 24 (16.9) | 13 (10.7) | 11 (19.6) | |
| Partial/formula feeding | 118 (83.1) | 109 (89.3) | 45 (80.4) |
P values were determined with the use of ANOVA omnibus F tests for continuous variables and the chi-square test for categorical variables. GUSTO, Growing Up in Singapore Toward Healthy Outcomes.
Mean ± SD (all such values for continuous variables).
In multivariate analyses that were adjusted for ethnicity, age on MRI day, and parity, both sSAT and dSAT were significantly greater in female than in male neonates [7.94 mL (95% CI: 3.29, 12.58 mL; P = 0.001) and 1.94 mL (95% CI: 0.74, 3.15 mL; P = 0.002), respectively]. However, IAT was similar (0.16 mL; 95% CI: −1.74, 1.41 mL; P = 0.840) in male and female neonates. These associations between sex and each AATC volume were similar within each ethnic group.
The descriptive statistics of AATC volumes are shown in Table 2. No crude ethnic differences were observed in sSAT or dSAT absolute volumes, whereas IAT volumes were significantly smaller in Indian neonates than in Chinese neonates. However, volume percentages for sSAT and dSAT were significantly greater for Malay and Indian neonates, whereas volume percentages for IAT showed no ethnic differences. Table 3 shows the correlation between birth weight and AATC volumes in the 3 ethnic groups. All 3 AATC volumes were highly correlated with birth weight in all 3 ethnic groups (P < 0.001 for all correlations). Table 4 shows the multivariate analyses that were controlled for sex, age on MRI day, and parity. dSAT was greater in Indian neonates than in Chinese neonates in both absolute and percentage volumes. The difference in sSAT was significant only for the volume percentage, whereas IAT was significantly lower in Indian neonates than in Chinese neonates for only absolute volumes. Percentage volumes of sSAT and dSAT (not absolute volumes) were greater in Malay neonates than in Chinese neonates, whereas IAT volumes (both absolute and percentage) were similar in Malay neonates and Chinese neonates. The marginal structural model analyses largely confirmed the results on the basis of volume percentages with controlled direct effects of ethnicity on adipose tissue. dSAT was significantly greater (1.45 mL; 95% CI: 0.49, 2.41 mL; P = 0.003) in non-Chinese (Indian or Malay) neonates than in Chinese neonates. However, ethnic differences in sSAT and IAT were NS [3.06 mL (95% CI: −0.27, 6.39 mL; P = 0.071) and −1.30 mL (95% CI: −2.64, 0.04 mL; P = 0.057) in non-Chinese neonates and Chinese neonates, respectively].
Table 2.
Abdominal adipose tissue volumes and volume percentages in 3 ethnic groups: crude (unadjusted) results (n = 333)1
| Abdominal adipose tissue
compartment volumes, mL |
Abdominal adipose
tissue,2 % |
|||||
|---|---|---|---|---|---|---|
| sSAT | dSAT | IAT | sSAT | dSAT | IAT | |
| Chinese (n = 146) | 77.1 ± 21.5 | 12.8 ± 5.7 | 23.9 ± 8.3 | 9.4 ± 1.6 | 1.5 ± 0.6 | 2.9 ± 0.7 |
| P | Reference | Reference | Reference | Reference | Reference | Reference |
| Malay (n = 126) | 77.9 ± 21.1 | 13.6 ± 5.3 | 22.5 ± 7.1 | 9.9 ± 1.8 | 1.7 ± 0.6 | 2.8 ± 0.7 |
| P | 0.752 | 0.239 | 0.116 | 0.023 | 0.013 | 0.583 |
| Indian (n = 61) | 80.4 ± 23.8 | 14.3 ± 5.9 | 21.03 ± 6.5 | 10.4 ± 1.9 | 1.8 ± 0.6 | 2.7 ± 0.6 |
| P | 0.316 | 0.066 | 0.012 | < 0.001 | 0.001 | 0.106 |
All values are means ± SDs. P values were determined with the use of a linear regression model. dSAT, abdominal deep subcutaneous tissue; IAT, abdominal internal adipose tissue; sSAT, abdominal superficial subcutaneous tissue.
Derived from the ratio of adipose tissue volume of each compartment and the total abdominal volume.
Table 3.
Pearson’s correlations between abdominal adipose tissue compartment volumes and birth weight in 3 ethnic groups of neonates who had MRI (n = 333)1
| sSAT | dSAT | IAT | |
|---|---|---|---|
|
| |||
| Chinese (n = 146) | 0.69 | 0.63 | 0.66 |
| Malay (n = 126) | 0.77 | 0.65 | 0.54 |
| Indian (n = 61) | 0.79 | 0.69 | 0.65 |
|
| |||
All correlations were significant at P < 0.001. dSAT, abdominal deep subcutaneous tissue; IAT, abdominal internal adipose tissue; sSAT, abdominal superficial subcutaneous tissue.
Table 4.
Abdominal adipose tissue volumes and volume percentages: adjusted differences in Indian and Malay neonates compared with Chinese neonates (n = 333)1
| Abdominal adipose tissue volume,
mL |
Percentage abdominal adipose
tissue,2 % |
|||||
|---|---|---|---|---|---|---|
| sSAT | dSAT | IAT | sSAT | dSAT | IAT | |
| Chinese (n = 146) | Reference | Reference | Reference | Reference | Reference | Reference |
| Malay (n = 126) | 0.57 (−4.69, 5.81) | 0.85 (−0.51, 2.21) | −1.51 (−3.20, 0.28) | 0.50 (0.09, 0.92) | 0.19 (0.05, 0.33) | −0.04 (−0.20, 0.13) |
| P | 0.834 | 0.220 | 0.097 | 0.017 | 0.008 | 0.650 |
| Indian (n = 61) | 4.22 (−2.19, 10.63) | 1.78 (0.12, 3.44) | −2.40 (−4.57, 20.23) | 1.02 (0.52, 1.52) | 0.31 (0.14, 0.48) | 20.13 (−0.33, 0.07) |
| P | 0.196 | 0.036 | 0.031 | <0.001 | <0.001 | 0.201 |
All values are regression coefficients (adjusted differences); 95% CIs in parentheses. Values were determined with the use of a general linear model. The model was adjusted for sex, age on MRI day, and parity. P values were determined with the use of a multiple linear regression model. dSAT, abdominal deep subcutaneous tissue; IAT, abdominal internal adipose tissue; sSAT, abdominal superficial subcutaneous tissue.
Derived from the ratio of adipose tissue volume of each compartment and the total abdominal volume.
A sensitivity analysis in which gestational age was restricted to neonates born between 37 and 41 completed weeks of gestation (Supplemental Table 1) showed the effect sizes of both absolute volumes, and percentage volumes of AATCs were similar to the main findings in Table 4. The effect sizes for dSAT absolute volumes between Indian and Chinese neonates were similar (in the full group: β = 1.78, P = 0.036; in neonates born at 37–41 completed weeks: β = 1.75, P = 0.043). A sensitivity analysis that included types of neonatal feeding as a covariate in the model for the full group did not change our findings significantly as shown in Supplemental Table 2.
Discussion
We observed significant differences in neonatal abdominal adiposity in the 3 Asian ethnic groups in Singapore. Despite having lower mean birth weights, Indian and Malay neonates had significantly greater dSAT volumes than did Chinese neonates even after adjusting for confounding factors that may have influenced adiposity.
Several studies have reported that Indian infants preserve their adiposity despite having a lower birth weight, waist circumference, and fat-free mass than do British infants (23–26, 39). However, most of those studies were based on skinfold thicknesses or air-displacement plethysmography as the measures of body composition. An exception was the study by Modi et al. (27), which used MRI to quantify regional abdominal adipose tissue volumes.
Although our findings that Indian neonates who have relatively greater sSAT and dSAT are consistent with those of Modi et al. (27), we did not observe differences in IAT in Chinese, Malay, and Indian neonates. Several explanations are possible. First, Modi et al. compared Indian infants from Pune, India, and European infants from London, whereas our study compared 3 Asian ethnic groups (Indian, Malay, and Chinese) in Singapore. Second, our Indian infants had lower birth weights than those of Chinese and Malay infants. The correlation between IAT and birth weight for Indian infants was strong (r = 0.65). The null controlled direct effect of Indian ethnicity on IAT in the marginal structural model (which removed the effect mediated through birth weight) suggested that the total effect was mediated by birth weight. Finally, technical differences in MRI methodologies may also explain the different results because the software algorithms used were different. In our in-house semiautomated software, sSAT and IAT were automatically generated and subsequently optimized by manually reassigning or removing automatically assigned voxel groups on the basis of the analysts’ anatomical judgements. dSAT was manually defined by the analysts. Therefore, the absolute volumes of the various AATCs we reported may not be directly comparable with those of Modi et al. (27).
Our study adds substantially to the information available on the quantity and distribution of abdominal adipose tissue in neonates. Few previous research studies have measured the regional or abdominal adipose tissue volume in neonates. To our knowledge, our study is one of the first trials to explore intra-abdominal adipose tissue distribution at birth. The sample sizes of previous studies that used MRI were much smaller (the largest sample size was 69) than ours (n = 333). To our knowledge, only our study and that of Modi et al. (27) measured dSAT in neonates. Our Indian neonates had higher dSAT than that of Chinese and Malay neonates. These differences may have long-term implications and may predispose adults of South Asian ethnicity to higher cardiometabolic risk.
Our study sheds light on the early manifestation of ethnic differences in abdominal adiposity, supporting the hypothesis that ethnic variation in adipose tissue distribution may in part originate in utero (i.e., is not solely a consequence of behavioral or lifestyle factors in childhood or adulthood). It is important to see if these differences persist in the later measures of abdominal adipose tissue by MRI, which are planned for the GUSTO cohort. However, because we required consent to be given by the parents for MRI of their neonates and, therefore, had MRI for a subset of 333 neonates of the cohort, it may be difficult to generalize the findings to those who did not have MRI, although there were no significant differences in the characteristics of neonates of these 2 groups.
In conclusion, we advise caution in generalizing our findings to the entire Singapore population and especially in other settings. Thus, additional confirmatory studies are required. The longitudinal tracking of adipose tissue growth over the life course in Asian and other ethnic groups should contribute to an understanding of the impact of ethnic variation in adiposity on subsequent risk of metabolic diseases.
Supplementary Material
2 Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://ajcn.nutrition.org.
Acknowledgments
We thank members of the GUSTO study group for their contributions, including Arijit Biswas, Choon Looi Bong, Birit FP Broekman, Shirong Cai, Jerry Kok Yen Chan, Cornelia Yin Ing Chee, Helen YH Chen, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Mary Foong-Fong Chong, Shang Chee Chong, Mei Chien Chua, Eric Andrew Finkelstein, Doris Fok, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D Holbrook, Chin-Ying Hsu, Hazel Inskip, Ivy Yee-Man Lau, Bee Wah Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Wei Pang, Anqi Qiu, Boon Long Quah, Mary Rauff, Salome A Rebello, Jenny L Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Leher Singh, Walter Stunkel, Lin Su, Kok Hian Tan, Oon Hoe Teoh, Hugo PS van Bever, Rob M van Dam, Inez Bik Yun Wong, PC Wong, and George Seow Heong Yeo.
1 Supported by the Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (Singapore-NMRC/TCR/004-NUS/2008). KMG is supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre and by the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition (grant 289346). This is a free access article, distributed under terms (http://www.nutrition.org/publications/guidelines-and-policies/license/) that permit unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Footnotes
Abbreviations used: AATC, abdominal adipose tissue compartment; dSAT, abdominal deep subcutaneous tissue; GUSTO, Growing Up in Singapore Toward Healthy Outcomes; IAT, abdominal internal adipose tissue; sSAT, abdominal superficial subcutaneous tissue; TAV, total abdominal volume; WS, water suppressed.
The authors’ responsibilities were as follows—MTT: conducted the research, image analysis and interpretation, and statistical analysis and wrote the manuscript; MVF and BS: contributed to the acquisition and image analysis; KMG, PDG, Y-SC, and Y-SL: designed the research, interpreted the data, and critically revised the manuscript for important intellectual content; JK, VSR, PA, AC, KN, and IBMA: contributed to the acquisition of the data; Y-HC, S-ES, and MSK: contributed to the statistical analysis and critical revision of the manuscript; FY and S-MS: contributed to the project conception and revision of the manuscript; and Y-SC: had primary responsibility for the final content of the manuscript. KMG, PDG, and Y-SC: have received reimbursement for speaking at conferences that were sponsored by companies selling nutritional products and are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle, and Danone.
The other authors declared no conflicts of interest.
References
- 1.Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21:199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Symonds ME, Mendez MA, Meltzer HM, Koletzko B, Godfrey K, Forsyth S, van der Beek EM. Early life nutritional programming of obesity: mother-child cohort studies. Ann Nutr Metab. 2013;62:137–45. doi: 10.1159/000345598. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey KM, Haugen G, Kiserud T, Inskip HM, Cooper C, Harvey NC, Crozier SR, Robinson SM, Davies L Southampton Women’s Survey Study Group. Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PLoS One. 2012;7:e41759. doi: 10.1371/journal.pone.0041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8. doi: 10.1152/ajpendo.2000.278.5.E941. [DOI] [PubMed] [Google Scholar]
- 8.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–7. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 10.Johnson D, Dixon AK, Abrahams PH. The abdominal subcutaneous tissue: computed tomographic, magnetic resonance, and anatomical observations. Clin Anat. 1996;9:19–24. doi: 10.1002/(SICI)1098-2353(1996)9:1<19::AID-CA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Lancerotto L, Stecco C, Macchi V, Porzionato A, Stecco A, De Caro R. Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat. 2011;33:835–42. doi: 10.1007/s00276-010-0772-8. [DOI] [PubMed] [Google Scholar]
- 12.Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, Liuzzi A, Di Blasio AM. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15:1933–43. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 13.Marinou K, Hodson L, Vasan SK, Fielding BA, Banerjee R, Brismar K, Koutsilieris M, Clark A, Neville MJ, Karpe F. Structural and functional properties of deep abdominal subcutaneous adipose tissue explain its association with insulin resistance and cardiovascular risk in men. Diabetes Care. 2014;37:821–9. doi: 10.2337/dc13-1353. [DOI] [PubMed] [Google Scholar]
- 14.Tordjman J, Divoux A, Prifti E, Poitou C, Pelloux V, Hugol D, Basdevant A, Bouillot JL, Chevallier JM, Bedossa P, et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol. 2012;56:1152–8. doi: 10.1016/j.jhep.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Chandalia M, Lin P, Seenivasan T, Livingston EH, Snell PG, Grundy SM, Abate N. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandalia M, Abate N, Garg A, Stray-Gundersen J, Grundy SM. Relationship between generalized and upper body obesity to insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:2329–35. doi: 10.1210/jcem.84.7.5817. [DOI] [PubMed] [Google Scholar]
- 17.McKeigue PM, Miller GJ, Marmot MG. Coronary heart disease in south Asians overseas: a review. J Clin Epidemiol. 1989;42:597–609. doi: 10.1016/0895-4356(89)90002-4. [DOI] [PubMed] [Google Scholar]
- 18.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 19.Bhopal R, Rahemtulla T, Sheikh A. Persistent high stroke mortality in Bangladeshi populations. BMJ. 2005;331:1096–7. doi: 10.1136/bmj.331.7525.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoo CM, Sairazi S, Taslim S, Gardner D, Wu Y, Lee J, van Dam RM, Shyong Tai E. Ethnicity modifies the relationships of insulin resistance, inflammation, and adiponectin with obesity in a multiethnic Asian population. Diabetes Care. 2011;34:1120–6. doi: 10.2337/dc10-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tai ES, Lim SC, Chew SK, Tan BY, Tan CE. Homeostasis model assessment in a population with mixed ethnicity: the 1992 Singapore National Health Survey. Diabetes Res Clin Pract. 2000;49:159–68. doi: 10.1016/s0168-8227(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 22.Liew CF, Seah ES, Yeo KP, Lee KO, Wise SD. Lean, nondiabetic Asian Indians have decreased insulin sensitivity and insulin clearance, and raised leptin compared to Caucasians and Chinese subjects. Int J Obes Relat Metab Disord. 2003;27:784–9. doi: 10.1038/sj.ijo.0802307. [DOI] [PubMed] [Google Scholar]
- 23.Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study) Int J Epidemiol. 2011;40:33–44. doi: 10.1093/ije/dyq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whincup PH, Gilg JA, Owen CG, Odoki K, Alberti KG, Cook DG. British South Asians aged 13-16 years have higher fasting glucose and insulin levels than Europeans. Diabetes Med. 2005;22:1275–7. doi: 10.1111/j.1464-5491.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 25.Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, Joglekar CV, Yudkin JS. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–80. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 26.Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 27.Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatr Res. 2009;65:584–7. doi: 10.1203/pdr.0b013e31819d98be. [DOI] [PubMed] [Google Scholar]
- 28.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong YS, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:140l–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 29.Teh AL, Pan H, Chen L, Ong ML, Dogra S, Wong J, MacIsaac JL, Mah SM, McEwen LM, Saw SM, et al. The effect of genotype and in utero environment on interindividual variation in neonate DNA methylomes. Genome Res. 2014;24:1064–74. doi: 10.1101/gr.171439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington TA, Thomas EL, Modi N, Frost G, Coutts GA, Bell JD. Fast and reproducible method for the direct quantitation of adipose tissue in newborn infants. Lipids. 2002;37:95–100. doi: 10.1007/s11745-002-0868-4. [DOI] [PubMed] [Google Scholar]
- 31.Fields DA, Krishnan S, Wisniewski AB. Sex differences in body composition early in life. Gend Med. 2009;6:369–75. doi: 10.1016/j.genm.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez G, Samper MP, Ventura P, Moreno LA, Olivares JL, Perez-Gonzalez JM. Gender differences in newborn subcutaneous fat distribution. Eur J Pediatr. 2004;163:457–61. doi: 10.1007/s00431-004-1468-z. [DOI] [PubMed] [Google Scholar]
- 33.Simon L, Borrego P, Darmaun D, Legrand A, Roze JC, Chauty-Frondas A. Effect of sex and gestational age on neonatal body composition. Br J Nutr. 2013;109:1105–8. doi: 10.1017/S0007114512002991. [DOI] [PubMed] [Google Scholar]
- 34.Joshi NP, Kulkarni SR, Yajnik CS, Joglekar CV, Rao S, Coyaji KJ, Lubree HG, Rege SS, Fall CH. Increasing maternal parity predicts neonatal adiposity: Pune Maternal Nutrition Study. Am J Obstet Gynecol. 2005;193:783–9. doi: 10.1016/j.ajog.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Catalano PM, Drago NM, Amini SB. Factors affecting fetal growth and body composition. Am J Obstet Gynecol. 1995;172:1459–63. doi: 10.1016/0002-9378(95)90478-6. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald PD, Ross SR, Grant L, Young D. Neonatal weight loss in breast and formula fed infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F472–6. doi: 10.1136/fn.88.6.F472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crossland DS, Richmond S, Hudson M, Smith K, Abu-Harb M. Weight change in the term baby in the first 2 weeks of life. Acta Paediatr. 2008;97:425–9. doi: 10.1111/j.1651-2227.2008.00685.x. [DOI] [PubMed] [Google Scholar]
- 38.VanderWeele TJ. Marginal structural models for the estimation of direct and indirect effects. Epidemiology. 2009;20:18–26. doi: 10.1097/EDE.0b013e31818f69ce. [DOI] [PubMed] [Google Scholar]
- 39.Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol. 2012;41:1409–18. doi: 10.1093/ije/dys139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.