Abstract
Sepsis is one of the leading causes of death worldwide. Although the prevailing theory for the sepsis syndrome is a condition of uncontrolled inflammation in response to infection, sepsis is increasingly being recognized as an immunosuppressive state known as endotoxin tolerance. We found sialylation of cell surface was significantly increased on LPS-induced tolerant cells; knockdown of Neu1 in macrophage cell line RAW 264.7 cells resulted in enhanced LPS-induced tolerance, whereas overexpression of Neu1 or treatment with sialidase abrogated LPS-induced tolerance, as defined by measuring TNF-α levels in the culture supernatants. We also found that the expression of Siglec-1 (a member of sialic acid-binding Ig (I)-like lectin family members, the predominant sialic acid-binding proteins on cell surface) was specifically up-regulated in endotoxin tolerant cells and the induction of Siglec-1 suppresses the innate immune response by promoting TGF-β1 production. The enhanced TGF-β1 production by Siglec-1 was significantly attenuated by spleen tyrosine kinase (Syk) inhibitor. Knockdown of siglec-1 in RAW 264.7 cells resulted in inhibiting the production of TGF-β1 by ubiquitin-dependent degradation of Syk. Mechanistically, Siglec-1 associates with adaptor protein DNAX-activation protein of 12 kDa (DAP12) and transduces a signal to Syk to control the production of TGF-β1 in endotoxin tolerance. Thus, Siglec-1 plays an important role in the development of endotoxin tolerance and targeted manipulation of this process could lead to a new therapeutic opportunity for patients with sepsis.
Keywords: endotoxin, immunosuppression, neuraminidase, sepsis, tolerance, sialylation, siglec-1
Introduction
Sepsis is generally defined as a systemic inflammatory response syndrome in response to infection. Sepsis can be potentially life threatening; of more than 1 million Americans who are diagnosed with severe sepsis every year, between 28 and 50% will die from this disease (1, 2). It is well-recognized that patients with sepsis are often immune suppressed, a state of reduced responsiveness to endotoxin known as endotoxin tolerance, deaths in this immunosuppressive phase are typically due to failure to control the secondary infections (3–5). The molecular mechanisms underlying this endotoxin tolerance phenomenon is poorly understood.
Sialic acids are a family of nine-carbon acidic monosaccharides and are involved in immune response, such as host-pathogen recognition, migration, and antigen presentation. Mounting experimental evidence suggests that the presence of sialic acid residue act as a marker of self in the immune system, as such residues are absent from most microbes (6). Indeed, host cells can be lysed by immune cytotoxic effector mechanisms after extensive desialylation, an observation that demonstrates the significant role played by cell surface sialic acids in the self-recognition process (7). In addition, normal human serum contains natural antibodies to sialidase-treated red blood cells (7–9), lymphocytes (10), and thymocytes (7). Recently, Meesmann et al. showed that desialylation acted as an “eat me” signal and caused an enhanced uptake of apoptotic cells (11). Increases of sialylation contribute to the tolerant phenotype in CD4+ T cells (12), dendritic cells, and regulatory T cells (13). The sialylation level of the cells is mainly depended on the activity of the two enzymes, sialyltransferases response for adding sialic acid residues to the glycolipids or glycoproteins and sialidase response for removing sialic acid residues from the glycolipids or glycoproteins. However, whether the two enzymes contribute to the development of endotoxin tolerance in macrophages is still unknown, and needs to be investigated.
Siglecs are membrane-bound lectins containing an N-terminal Ig V-set domain followed by 2–17 Ig C-2 domain (14, 15), most of them are expressed on immune cells and have immunosuppressive properties, which negatively regulate immune response (14–16). It has been shown that Siglecs play an important role in the internalization of sialic acid-expressing pathogens (17–19), in controlling allergic asthma (20, 21), and in self-tolerance (22). Previously, we found that Siglec G/10-CD24 interaction selectively represses the NF-κB-driven inflammatory response to danger-associated molecular patterns (DAMPs),2 but not pathogen-associated molecular patterns (PAMPs) (23, 24). Recently, we reported extensive and direct interactions between Siglecs and Toll-like receptors (TLRs) and dendritic cells from Siglece-deficient mice demonstrated increased responses to all TLR ligands tested (25). However, whether Siglecs contribute to the development of endotoxin tolerance is still unknown, and further experimental investigation is needed to delineate this and identify effective targets for sepsis therapy.
In the present study, we found that sialylation of cell surface was significantly increased on LPS-induced tolerant cells and specific up-regulation of Siglec-1 contributes to suppress the immune response by promoting the production of TGF-β1 during endotoxin tolerance, which might be an important regulatory mechanism for controlling immune response in LPS-induced tolerance.
Experimental Procedures
Reagents
Anti-Siglec-1 antibodies (catalogue no. MAB5610, lot no. CEIE0113111) and anti-Siglec-1-PE (cat. no. FAB5610, lot no. ABSE0114041) were purchased from R&D Systems (Minneapolis, MN). Anti-ST3Gal6 antibody (cat. no. ab106527, lot no. GR266217-1) was purchased from Abcam. Anti-mouse CD11c (cat. no. 550261, lot no. 5016523), CD11b (cat. no. 553310, lot no. 4314772), B220 (cat. no. 552771, lot no. 4324784), PE Mouse Anti-human CD14 (cat. no. 555398, lot no. 5156850), and PE mouse anti-human CD11c (cat. no. 560999, lot no. 6014759), antibodies were purchased from BD Bioscience (San Jose, CA). Anti-Neu1 (cat. no. sc-32936, lot no. B2813), anti-Neu3 (cat. no. sc-134931, lot no. L1712), Syk (cat. no. sc-1077, lot no. B1815), JNK (cat. no. sc-7345, lot no. G2814), P-JNK (cat. no. sc-6254, lot no. C1915), P38 (cat. no. sc-535, lot no. H1015), P-P38 (cat. no. sc-17852-R, lot no. H6015), Erk (cat. no. sc-94, lot no. H0615), P-ERK (cat. no. sc-7383, lot no. F2613), β-actin (cat. no. sc-1615, lot no. C2015), antibodies, streptavidin-HRP (cat. no. 21130, lot no. PJ208901), and horseradish peroxidase-conjugated anti-mouse (cat. no. sc-2005, lot no. I114), anti-goat (cat. no. sc-2056, lot no. E1314), or anti-rabbit (cat. no. sc-2004, lot no. H1015/I2314) secondary antibodies, and Syk siRNA (cat. no. sc-44328, lot no. J2214) and control siRNA (cat. no. sc-37007) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ubiquitin mouse monoclonal antibody (FK2) (cat. no. ST1200, lot no. D00165221) and lipopolysaccharide (LPS, from Escherichia coli 0111:B4) (cat. no. 437627) were from EMD Millipore, Merck KGaA, Darmstadt, Germany. Biotinylated Maackia Amurensis Lectin II (MAL II) (cat. no. B-1265, lot no. ZA1020) and biotinylated Sambucus Nigra Lectin (SNA, EBL) (cat. no. B-1305, lot no. Z1002) were purchased from Vector Laboratories (Burlingame, CA). Neuraminidase (sialidase) from Vibrio cholerae (cat. no. 11080725001) was purchased from Sigma. MG132 (cat. no. 3175-v, lot no. 640311) was purchased from peptide institute, Inc, Osaka, Japan. Healthy human peripheral blood was obtained from LONZA (cat. no. CC-2702). RAW 264.7 cells were obtained from ATCC and cultured in Dulbecco's modified Eagle's medium (ThermoFisher Scientific, Waltham, MA) supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 100 μg/ml penicillin and streptomycin. Syk inhibitor piceatannol (cat. no. sc-200610), SP600125 (JNK inhibitor) (cat. no. sc-200635), SB203580 (P38 inhibitor) (cat. no. sc-3533), ERK inhibitor (cat. no. sc-221593) were purchased from Santa Cruz Biotechnology.
Cell Culture and Lentivirus Infection
D2SC/1 dendritic cells were obtained from Dr. Peter G. Stock and Sang-Mo Kang (University of California, San Francisco, CA) with the permission from Dr. Paola Ricciardi-Castagnoli (University of Perugia, Italy), and maintained in Dulbeco's minimal essential medium supplied with 10% heat-inactivated fetal calf serum and 1% penicillin and streptomycin. The lentiviral vectors expressing Neu1 shRNAs were from ThermoFisher Scientific. The lentiviral vectors expressing Siglec-1 shRNAs were from Sigma. Puromycin (cat. no. sc-108071B) was purchased from Santa Cruz Biotechnology. Stable clones were obtained after selection with puromycin (2.5 μg/ml) for 2 weeks after infection.
Measurement of TGF-β1
Total TGF-β1 in cell culture supernatants was measured after converting latent TGF-β1 to active TGF-β1 by acidification (10-min incubation at room temperature with 0.2 volume of 1 n HCl for cell culture supernatants, followed by neutralization by adding the same volume of 1.2 n NaOH in 0.5 m HEPES) with a 2-antibody ELISA assay specific for the activated form of TGF-β1 (R&D Systems, cat. no. DY1679–05, lot no. 32710).
Experimental Animal Models
All mice were used at 6–8 weeks of age. All animal procedures were approved by the Animal Care and Use Committee of University of Tennessee Health Science Center. Wild-type C57BL/6J mice were purchased from Jackson Laboratory.
Quantitative Real-time PCR Analysis
Neu1–4, sialyltransferases and Siglecs expression were measured by real-time polymerase chain reaction. Samples were run in triplicate, and the relative expression was determined by normalizing expression of each target to the endogenous reference, hypoxanthine phosphoribosyltransferase (Hprt) transcripts or GAPDH. Real-time PCR primers used for mouse genes were as follows: Siglec1 sense, CTAGCAACACATTGGGCAAC; Siglec1 antisense, CCAGTACAGTGGCCTTAGCA; Siglec2 sense, GTTCCTGGTCACCCAGAAGT; Siglec2 antisense, TGGGACCTCCCTCTCTCTC; Siglec3 sense, AAGACCATGGAACCAACCTC; Siglec3 antisense, TTTCCCTGGACCAACTCTTC; Siglec4 sense, TCCAACCTTCTGTGTTAGCG; Siglec4 antisense, AGTGGCCTTTCAACCAAGTC; Siglece sense, GGTTGACTGACTGGACTGACT; Siglece antisense, AATCTTTGCGTCTGTCGGTTC; Siglecf sense, TACGAAAGGCAACCATCTTG; Siglecf antisense, AGATGACCCTGGATTGGGTA; Siglecg sense, GTCCCAGACTTGCATGAGAATC; Siglecg antisense, GACCCAGCTCAGTGTAGCA; Siglech sense, CTGGAGCTGGTGTGACTGTT; Siglech antisense, TTTCCCTGGACCAACTCTTC; Siglec15 sense, CCACGATCGCTATGAGAGTC; Siglec15 antisense, ACCGAGATGTTGACGATCC; ST6Gal1 sense, ACCATCCGCCTAGTGAACTC; ST6Gal1 antisense, CTTCTGATACCACTGCGGAA; ST6Gal2 sense, ACTTGAAGCAATGGCGACAAC; ST6Gal2 antisense, GCTTGCCCTGTAGAGGCAG; ST6GalNAc1 sense, TGACTGTGTTGGCATTGCTCT; ST6GalNAc1 antisense, CTCCTGTTTCTTCAGGTCCTTTG; ST6GalNAc2 sense, CCTCATGCTGTACTCCTCGG; ST6GalNAc2 antisense, CGGTGGTTTGGGGTCAAAGA; ST6GalNAc3 sense, CCACGAGCATTCTTTGACCC; ST6GalNAc3 antisense, CCAGGGACAGCGAGTGTTG; ST6GalNAc4 sense, AGCCTCTTATCCGAGAACTGT; ST6GalNAc4 antisense, GGCCTGAACCCAGCATCTG; ST6GalNAc5 sense, TTCAGGACCCGATGAATGTA; ST6GalNAc5 antisense, TCTGGTTTCCAGTCTGGTTG; ST6GalNAc6 sense, AACAGTGCCAACGAGGTCTTC; ST6GalNAc6 antisense, CTTGTTGCCGAGGATAGGGAA; ST3Gal1 sense, GGAGAGAATGTCAACATGGTCC; ST3Gal1 antisense, GTGTGAGTGATGGTGCCTGT; ST3Gal2 sense, TTCGGGTGTGGTTCCTCTCTA; ST3Gal2 antisense, CGCTGTAGTCCTGAATAGCCT; ST3Gal3 sense, AAGCTGGACTCTAAACTGCCT; ST3Gal3 antisense, TGCTGGCTTGGAGAACCTG; ST3Gal4 sense, ACCAGCAAATCTCACTGGAAG; ST3Gal4 antisense, CCCTGGAAGCATGGCTCTTTC; ST3Gal5 sense, CCCGAACCCAGCACAAGAT; ST3Gal5 antisense, ACTCCAAATGCAACCAACGTG; ST3Gal6 sense ACTGTGGGGAACAAATGGCTA; ST3Gal6 antisense, GGACATGGCAGCAACCTTT; ST8Sia1 sense, GCTACCCGTAGGAGCCAGT; ST8Sia1 antisense, CAGCACCCCTTGCACAATCT; ST8Sia2 sense, AGGCAGAGGTACAATCAGATCA; ST8Sia2 antisense, GAGAGAGCGTCTGGTTGTGTC; ST8Sia3 sense, AGTGTGCTAGGGCTGGTCAT; ST8Sia3 antisense, TGGCGTACTTGGGAGTGGT; ST8Sia4 sense, ATGCGCTCAATTAGAAAACGGT; ST8Sia4 antisense, CGATGAGTTGCGTCTCTTGGT; ST8Sia5 sense, AGATTTGTTGGGGAATCGAACTT; ST8Sia5 antisense, GCTGTCATTAAAGAGCCCAGTC; ST8Sia6 sense, TCCTGCGTATGCTCTGGTG; ST8Sia6 antisense, CTGTTCCTGGTGCGTGGTA; Neu1 sense, ATGTGACCTTCGACCCTGAG; Neu1 antisense, TCCTTCTGCCAGGATGTACC; Neu3 sense, TGCGTGTTCAGTCAAGCC; Neu3 antisense, GCAGTAGAGCACAGGGTTAC; GAPDH sense, CATGGCCTTCCGTGTTCCTA; GAPDH antisense, CCTGCTTCACCACCTTCTTGAT. Real-time PCR primers used for human genes were as follows: Siglec1 sense, AATCAAAGGCATCATTTTAGGGATA; Siglec1 antisense, CCATCAATGACCCCTTCATTG; GAPDH sense, AACGGGAAGCTTGTCATCAATGGA; GAPDH antisense, GCATCAGCAGAGGGGGCAGAG.
Construct of Plasmids
To generate a construct expressing mouse Neu1, cDNA for Neu1 was amplified by RT-PCR and subcloned into expression vector pCDNA6 (Life Technologies). To generate a construct expressing mouse Siglec-1, cDNA for Siglec-1 was amplified by PCR using MGC premier mouse cDNA clone BC141336 as template (TransOMIC technologies, AL) and subcloned into expression vector pCDNA6 (Life Technologies). Siglec-1 mutant (R122A) was made by using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Inc) with the primers (acctacaacttcgcctttgagatcagt, actgatctcaaaggcgaagttgtaggt). All constructs were verified by restriction enzyme digestion and DNA sequencing.
Flow Cytometry
Spleen cells from PBS or LPS treated WT mice, or culture cells were washed in Flow Cytometry staining buffer (1× PBS, 2% BSA), and incubated for 1 h on ice with different directly conjugated-antibodies in flow cytometry staining buffer. The intensity of cell-bound antibodies was analyzed on LSRFortess Flow Cytometer or Guava easyCyteTM System (EMD Millipore, Merck KGaA, Darmstadt, Germany).
Immunoprecipitation and Immunoblotting
RAW 264.7 cell lysates were prepared in lysis buffer (20 mm Tris-HCl, 150 mm NaCl, 1% Triton X-100, pH 7.6, including protease inhibitors, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride), sonicated, centrifuged at 13,000 rpm for 5 min and then diluted in IP buffer (20 mm Tris-HCl, 150 mm NaCl, pH 7.6, including the protease inhibitors as described above). Samples were pre-cleared with 60 μl of protein A-conjugated agarose beads (Upstate, Lake Placid, NY) for 1 h at 4 °C, and then incubated with corresponding antibodies. Immunoprecipitates were washed four times with IP buffer and resuspended in SDS sample buffer for Western blot analysis.
Measurement of Inflammatory Cytokines
Blood or cell culture supernatants were obtained at indicated times and cytokines in the serum or cell culture supernatants were determined using cytokine bead array designed for mouse inflammatory cytokines (BD Biosciences, cat. no. 552364) or human inflammatory cytokine kit (BD Biosciences, cat. no. 551811).
Neuraminidase Activity Assay
Sialidase activity was measured using 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid sodium salt hydrate (4-MU-NANA, catalogue no. sc-222055)(Santa Cruz Biotechnology) as the substrate. RAW 264.7 cells were harvested and suspended in 100 μl of lysis buffer (20 mm Tris-HCl, 1% Triton X-100, 150 mm NaCl, pH 7.6), sonicated and centrifuged at 13,000 rpm for 5 min. For one reaction, 5 μl of the supernatant was incubated with 4-MU-NANA (final concentration, 15 μm) for 30 min at 37 °C in 50 μl of reaction buffer (50 mm sodium phosphate, pH 5.0). The reaction was terminated by adding 600 μl stop buffer (0.25 m glycine-NaOH, pH 10.4) and then fluorescence intensity was measured with a Synergy HTX Multi-Mode Reader (EMD Millipore, Merck KGaA, Darmstadt, Germany) (excitation at 360 nm; emission at 460 nm). For determination of the cell surface sialidase activity, we used the same method as above except we used the intact cells instead of cell lysates.
Statistical Analysis
All statistical analyses were performed by the Student's t test. *, p < 0.05; **, p < 0.01; ***, p <0.001; n.s., not significant.
Results
Sialylation of Cell Surface Was Significantly Increased on LPS-induced Tolerant Cells
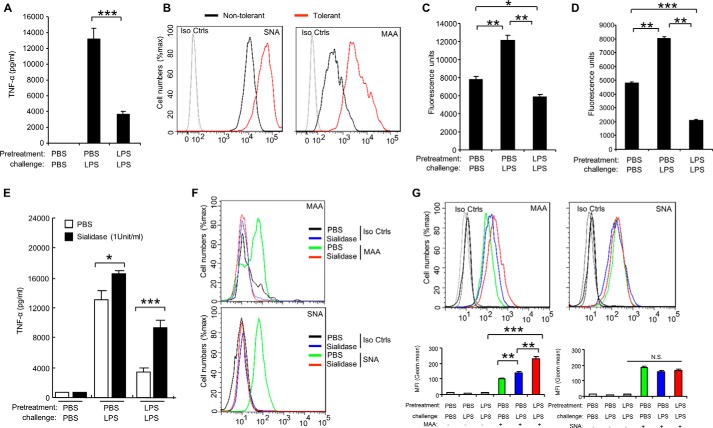
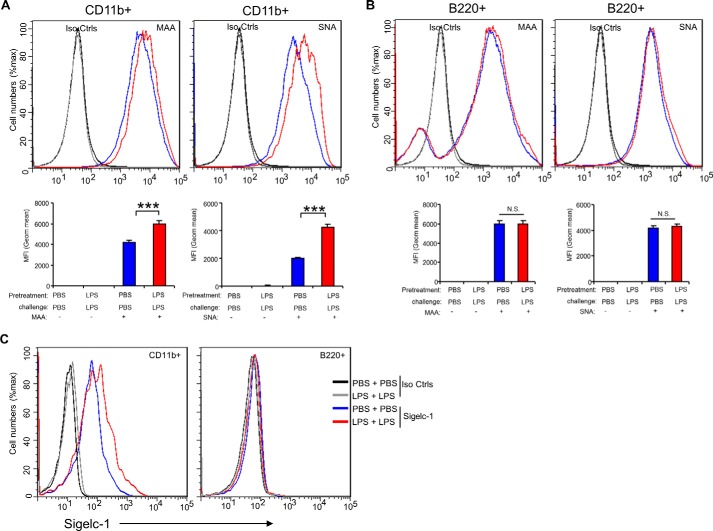
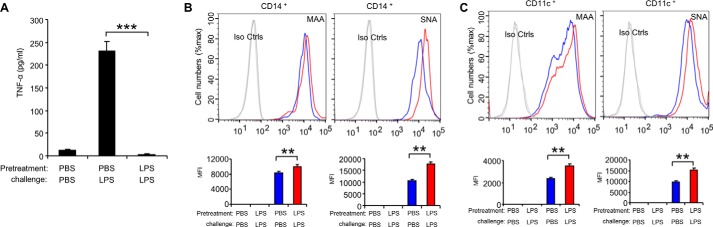
It was reported that sialylation regulates T cell response in the tolerant state (12, 13), however the function of sialylation in LPS-tolerant macrophages has not yet been analyzed. For induction of endotoxin tolerance, we pretreated the macrophage RAW 264.7 cells with 100 ng/ml LPS for 24 h, washed three times with PBS, and then challenged with 1 μg/ml LPS for 24 h. TNF-α is stably down-regulated in all tolerized models and is thus considered to be the most reliable marker of endotoxin tolerance (26–28). Therefore, here the levels of TNF-α protein are used as readout of endotoxin tolerance. TNF-α in the culture supernatants was measure using the CBA Beads kit as previously described (23–25). As shown in Fig. 1A, the secretion of TNF-α was significantly decreased in response to LPS challenge in cells pretreated with LPS compared with the cells pretreated with PBS. This is consistent with previous reports that TNF-α protein significantly increased after LPS stimulation in macrophage cell line RAW 264.7 cells, and these cells were then unable to response to second dose of LPS (27, 28). Interestingly, both α2,3- and α2,6-sialylation on the cell surface were significantly increased on tolerant RAW 264.7 cells in comparison to the PBS treatment only, non-tolerant cells (Fig. 1B). In addition to RAW 264.7 cells, we found that α2,3-sialylation on the cell surface was increased in response to LPS treatment in the mouse dendritic cell line D2SC/1 cells (Fig. 1G). Both α2,3-, and α2,6-sialylation were increased on mouse spleen CD11b+ cells (Fig. 2A) but neither was significantly altered in mouse B220+ cells (Fig. 2B) from LPS-tolerant wild-type mice compared with that from non-tolerant mice. We also found that both α2,3-, and α2,6-sialylation were significantly increased in human peripheral blood CD14+ cells (Fig. 3B) and CD11c + (Fig. 3C) cells after induction of endotoxin. The induction of endotoxin was confirmed by measuring TNF-α in the cell culture supernatants (Fig. 3A). Corresponding with increased sialylation of cell surface, sialidase activity was significantly decreased both in total cell lysates (Fig. 1C) and on cell surface (Fig. 1D) in tolerant RAW 264.7 cells compared with non-tolerant cells. Furthermore, RAW 264.7 cells are resistant to LPS-induced tolerance after sialidase treatment (Fig. 1E). The decrease of cell surface sialylation after sialidase treatment was confirmed by flow cytometric analysis (Fig. 1F). Taken together, these data suggest that sialylation of cell surface was significantly increased after induction of endotoxin tolerance in innate immune cells.
FIGURE 1.
Increase of α2,3-, and α2,6-sialylation on tolerant innate immune cells. RAW 264.7 cells were pretreated for 24 h with PBS or 100 ng/ml LPS, washed three times with PBS, and challenged for 24 h with PBS or 1 μg/ml LPS. The cells treated with PBS only as non-tolerant cells, the cells pretreated and challenged with LPS as tolerant cells. A, TNF-α in the cell culture supernatants was analyzed with cytokine bead array. B, flow cytometric analysis of sialylation on non-tolerant or tolerant RAW 264.7 cells. C and D, significantly decreased sialidase activity on tolerant RAW 264.7 cells. C, sialidase activity in the cell lysates was detected with 4-MU-NANA after induction of endotoxin tolerance. D, sialidase activity on the cell surface was detected with 4-MU-NANA after induction of endotoxin tolerance. E, loss of endotoxin tolerance in RAW 264.7 cells after sialidase treatment. RAW 264.7 cells were pretreated for 24 h with PBS or 100 ng/ml LPS, washed three times with PBS, and challenged for 24 h with PBS or 1 μg/ml LPS with or without sialidase. TNF-α in the cell culture supernatants was analyzed with cytokine bead array. F, loss sialylation on RAW 264.7 cells surface after sialidase treatment. Cells were treated as in D, and then stained with MAA or SNA. G, increase of α2,3 on tolerant dendritic cell line D2SC/1 cells. D2SC/1 cells were treated in A, and then stained with MAA or SNA. Histograms shown on top panels are FACS profiles. The bar graphs bottom panels represent geometric means S.D. of fluorescence intensity (n = 3). All experiments were repeated at least two or three times. Data are shown as mean ± S.D.
FIGURE 2.
Increased expression of α2,3 and α2,6 sialylation and Siglec-1 on spleen cells from endotoxin tolerant mice. C56BL/6J wild-type mice 6–8 weeks of age were intraperitoneally administrated LPS (4 μg/kg body weight). After 24 h, the mice were challenged with LPS (4 mg/kg body weight) for 4 h, and then spleen cells were collected and sialylation on the CD11b+ (A) and B220+ (B) cell surface was analyzed by flow cytometry. Histograms shown on top panels are FACS profiles. The bar graph bottom panels represent geometric means S.D. of fluorescence intensity (n = 3). Cell surface expression of Siglec-1 on the B220+ and CD11b+ cell surface was analyzed by flow cytometry (C). Data are representative of those from two independent experiments involving 3 mice per group.
FIGURE 3.
Increase of α2,3- and α2,6-sialylation on tolerant human peripheral blood cells. Human peripheral blood cells were pretreated for 24 h with PBS or 1 μg/ml LPS, washed three times with PBS, and challenged for 24 h with PBS or 1 μg/ml LPS.TNF-α in the cell culture supernatants was analyzed with cytokine bead array (A). Sialylation on the CD14+ (B) and CD11c+ (C) cell surface was analyzed by flow cytometry. Histograms shown on top panels are FACS profiles. The bar graph bottom panels represent geometric means S.D. of fluorescence intensity (n = 3). Data presented in this figure have been repeated once.
A Critical Role for Neu1 in Endotoxin Tolerance
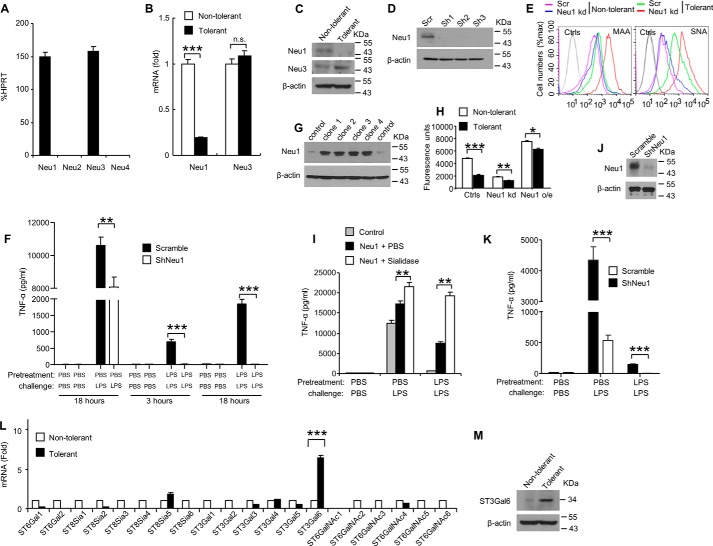
Because sialidase activity was significantly decreased in tolerant RAW 264.7 cells (Fig. 1, C and D), we evaluated the contribution of endogenous sialidases in these cells. Mammals have four sialidases, Neu1–4 (29). Real-time PCR analysis indicated that RAW 264.7 cells express Neu1 and Neu3, but not Neu2 or Neu4 (Fig. 4A). Therefore, we next investigated whether the actions of Neu1 or Neu3 contributes to the decrease of the sialidase activity in tolerant RAW 264.7 cells. Real-time PCR and Western blot analysis indicated that both Neu1 mRNA and Neu1 protein were significantly decreased in tolerant RAW 264.7 cells, whereas levels of Neu3 mRNA and protein did not change between the tolerant and no-tolerant (PBS treated only) cells (Fig. 4, B and C). Thus, the decrease in Neu1 protein levels corresponds to the observed decrease of sialidase activity in endotoxin tolerant RAW 264.7 cells. To investigate the possible function of Neu1 in regulating LPS-induced tolerance, we tested three independent Neu1 shRNA-silenced RAW 264.7 cell lines for their response to endotoxin tolerance. Knockdown efficiency of Neu1 was confirmed by Western blot analysis (Fig. 4D). Cell surface sialidase activity was significantly decreased (Fig. 4H) and both α2,3-, and α2,6-sialylation on cell surface were significantly increased after knockdown of Neu1 (Fig. 4E). Sialylation was increased higher on Neu1 knockdown cells than scrambled-controls after induction of tolerance by LPS (Fig. 4E). We found that the production of TNF-α was further significantly decreased in Neu1-depleted Raw 264.7 cells when challenged with LPS after LPS pretreatment, compared with the shRNA scrambled control (Fig. 4F), suggesting that Neu1 knockdown resulted in enhanced LPS-induced tolerance both at the 3 and 18 h time points. In addition to RAW 264.7 cells, knockdown of Neu1 in D2SC/1 cells (Fig. 4J) also resulted in deceased TNF-α secretion, indicating an enhanced LPS-induced tolerance (Fig. 4K). Next, we established RAW 264.7 cell clones stably over-expressing Neu1 (Fig. 4G), cell surface sialidase activity on these cells was significantly increased (Fig. 4H), and found that overexpression of Neu1 overcame endotoxin tolerance by increasing the TNF-α production, and sialidase treatment of the clones stably overexpressing Neu1 further inhibited LPS-induced tolerance (Fig. 4I). In addition, similar results were observed with different stable clones (data not shown). Although cell surface sialidase activity was further decreased after induction of tolerance by LPS, the sialidase activity on the cells stably overexpressing Neu1 was significantly higher than that on the controls or Neu1 knockdown cells (Fig. 4H). Collectively, these data suggest that a critical role for Neu1 in response to LPS-induced tolerance.
FIGURE 4.
A critical role for Neu1 in endotoxin tolerance. A, expression of Neu1–4 mRNA in RAW 264.7 cells was determined by RT-PCR. Data shown are mean ± S.D. transcript levels, expressed as % of the housekeeping gene HPRT. B, expression of Neu1 and Neu3 mRNA in non-tolerant and tolerant RAW 264.7 cells was determined by real-time PCR. C, immunoblot analysis of Neu1and Neu3 in non-tolerant and tolerant RAW 264.7 cells. Lysates from non-tolerant and tolerant RAW 264.7 cells were subjected to Western blot analysis using anti-Neu1, anti-Neu3, and β -actin antibodies. D, immunoblot analysis of Neu1 in RAW 264.7 cell clones stably overexpressing ShRNA for Neu1. Lysates from different neu1 shRNA knockdown cell clones were subjected to Western blot analysis using anti-Neu1 antibody. E, flow cytometric analysis of sialylation on non-tolerant or tolerant RAW 264.7 cells. Neu1 kd, Neu1 knockdown. F, silencing Neu1 by lentivirus shRNA enhanced the endotoxin tolerance. RAW 264.7 cells were infected with Neu1 shRNAs. Stable clones were obtained after selecting with puromycin for 2 weeks. Cells were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged with 1 μg/ml LPS for the indicated time. TNF-α in the cell culture supernatants was analyzed with cytokine bead array. G–I, characterization of RAW 264.7 cells clones stably overexpressing Neu1. G, different cell clones lysates were subjected to Western blot analysis using anti-Neu1 antibody. H, significantly decreased sialidase activity on tolerant RAW 264.7 cells. Sialidase activity on the cell surface was detected with 4-MU-NANA after induction of endotoxin tolerance. Ctrls, controls; Neu1 kd, Neu1 knockdown; Neu1 o/e, Neu1 overexpression. I, RAW 264.7 cell clones stably overexpressing Neu1 were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged for 16 h with 1 μg/ml LPS. TNF-α in the cell culture supernatants was analyzed with cytokine bead array. J, immunoblot analysis of Neu1 in D2SC/1 cell clones stably overexpressing ShRNA for Neu1. Lysates from neu1 shRNA knockdown cells were subjected to Western blot analysis using anti-Neu1 antibody. K, TNF-α in the D2SC/1 cell culture supernatants was analyzed with cytokine bead array. L, evaluation of the expression of sialytransferases in non-tolerant or tolerant RAW 264.7 cells by real-time PCR using specific primer sets. M, immunoblot analysis of ST3Gal6 in non-tolerant and tolerant RAW 264.7 cells. Lysates from non-tolerant and tolerant RAW 264.7 cells were subjected to Western blot analysis using anti-ST3Gal6 and β-actin antibodies. All experiments were repeated two or three times. Data are shown as mean ± S.D.
The sialylation level of the cells is mainly depended on the activity of the two enzymes, sialyltransferase and sialidase, which response for adding or removing sialic acid residues to or from glycolipids or glycoproteins. In addition to the decreased expression of the sialidase Neu1, we also found that both mRNA expression (Fig. 4L) and protein level (Fig. 4M) of the sialyltransferase ST3Gal6 were specifically increased on tolerant RAW 264.7 cells, ST3Gal6 acts to add sialic acid to the terminal portions of the glycolipids or glycoproteins via α-2,3 linkages (30).
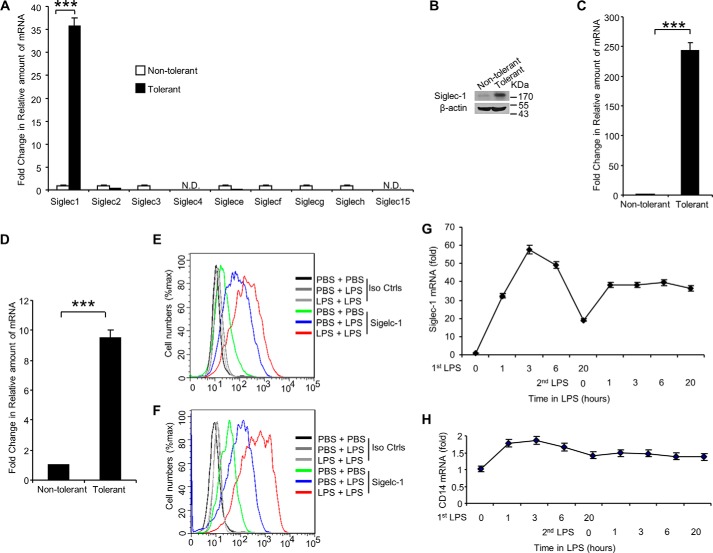
Siglec-1 Expression Significantly Up-regulated during LPS-induced Tolerance
Since it has been shown that Siglecs are predominantly bound to sialic acids on cell surface proteins (31), we investigated the expression of Siglecs in LPS-induced tolerant RAW 264.7 cells. As shown in Fig. 5A, induction of endotoxin tolerance in RAW 264.7 cells led to increased Siglec-1 mRNA expression by 35-fold compared with the control, non-tolerant cells. For all other Siglecs measured, we found decreased mRNA expression in tolerant cells when compared with non-tolerant cells (Fig. 5A). Increased expression of Siglec-1 protein in tolerant RAW 264.7 cells was also confirmed by Western blot analysis (Fig. 5B) and flow cytometry analysis (Fig. 5E). In addition, significantly increased expression of Siglec-1 at both in the transcriptional (Fig. 5C) and translational levels (Fig. 5F) was also found in LPS-induced tolerant D2SC/1 cells and in LPS-induced tolerant human peripheral blood cells (Fig. 5D). We also found that pretreatment with LPS followed by LPS challenge resulted in increased Siglec1 on surface of mouse spleen CD11b+ cells (Fig. 2C). In contrast, the same treatment of LPS did not result in increased Siglec-1 protein on the surface of mouse spleen B220+ cells (Fig. 2C). Next, we investigated the expression of Siglec-1 during LPS-induced tolerance in RAW 264.7 cells. As shown in Fig. 5G, Siglec-1 was increased during first LPS stimulation and maintained high levels during LPS tolerance; in contrast, CD14 expression was mainly unchanged (Fig. 5H). Collectively, these results indicate that induction of endotoxin tolerance in vitro and in vivo leads to increased and sustained expression of Siglec-1.
FIGURE 5.
LPS-induced tolerance up-regulates Siglec-1 expression. Endotoxin tolerance was induced by pretreating RAW 264.7 cells or D2SC/1 cells for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged for 16 h with 1 μg/ml LPS. A, evaluation of Siglec expression in non-tolerant or tolerant RAW 264.7 cells by real-time PCR using Siglec-primer sets. B, immunoblot analysis of siglec-1 on tolerant RAW 264.7 cells. Evaluation of Siglec-1 expression in non-tolerant or tolerant D2SC/1 cells (C) or human peripheral blood cells (D) by real-time PCR. Flow cytometric analysis cell surface expression of Siglec-1 on RAW 264.7 cells (E), or D2SC/1 cells (F) after induction of endotoxin tolerance. G, induction of endotoxin tolerance leads to increased and maintained expression of Siglec-1. Expression of Siglec-1 mRNA in non-tolerant and tolerant RAW 264.7 cells was determined by Real-time PCR. H, induction of endotoxin tolerance does not affect the expression of CD14. Expression of CD14 mRNA in non-tolerant and tolerant RAW 264.7 cells was determined by Real-time PCR. All experiments were repeated three times. Data are shown as mean ± S.D.
A Critical Role for Siglec-1 in Endotoxin Tolerance
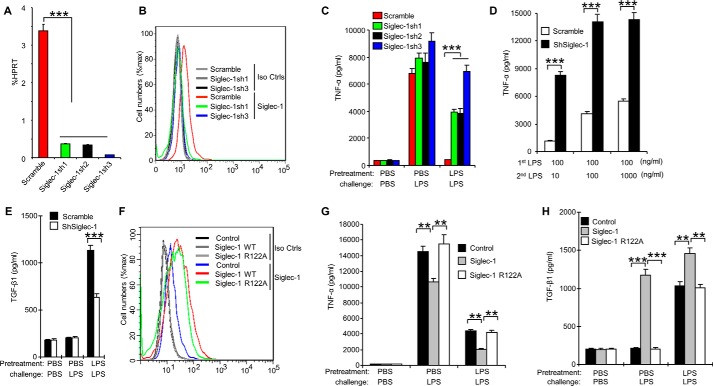
To determine whether Siglec-1 regulates the development of endotoxin tolerance, we tested three independent Siglec-1 shRNA-silenced RAW 264.7 cell lines for their response to endotoxin tolerance. As shown in Fig. 6, A and B, the three shRNAs silenced Siglec-1 in RAW 264.7 cells with different efficiencies: Siglec-1 was only partially suppressed by Sh1 (89.21% ± 5.35) and Sh2 (89.91% ± 4.49), while almost completely silenced by Sh3 (97.71% ± 2.93). These cell lines were treated with LPS as shown in Fig. 1 to induce endotoxin tolerance, and TNF-α in the culture supernatants was analyzed with CBA Beads kit. Corresponding with the silencing efficiencies, the production of TNF-α was more significantly increased by Sh3 than by Sh1 and Sh2, when compared with the scrambled control (Fig. 6C). Further, we found that Siglec-1 shRNA-silenced RAW 264.7 cell lines overcame endotoxin tolerance in a LPS dose-dependent manner by increasing the TNF-α production (Fig. 6D). Moreover, we established RAW 264.7 cell clones stably over-expressing Siglec-1 (Fig. 6F), and found that overexpression of Siglec-1 inhibited endotoxin tolerance (Fig. 6G); in contrast, overexpression of siglec-1 mutant (R122A, the amino acid arginine locates in the V-set domain and is critical for the sialic acid binding (32)) did not show inhibition activity (Fig. 6G).
FIGURE 6.
Siglec-1 promotes endotoxin tolerance by enhancing TGF-β1 secretion. RAW 264.7 cells were transduced with lentiviral vector carrying scrambled shRNA or Siglec-1 shRNA. After transduction and selection with puromycin, stable clones expressing the shRNA were isolated and expanded under the selection of 2 μg/ml puromycin. A, real-time PCR analysis of Siglec-1 mRNA level. B, cell surface expression of Siglec-1 on RAW 264.7 was analyzed by flow cytometry. C–E, characterization of RAW 264.7 cells clones stably overexpressing shRNA for Siglec-1. 2 × 105 RAW 264.7 cells were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged with 1 μg/ml LPS for 6 h (C) or with indicated concentration of LPS for 18 h (D). The culture supernatants were subsequently collected and analyzed for TNF-α (C, D) production as described in Ref. 23–25 and TGF-β1 (E) production by ELISA. F–H, overexpression of Siglec-1 promotes TGF-β1 secretion. F, flow cytometric analysis of Siglec-1 in RAW 264.7 cell clones stably overexpressing Siglec-1. RAW 264.7 cell clones stably overexpressing Siglec-1 were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged for 16 h with 1 μg/ml LPS. TNF-α in the cell culture supernatants was analyzed with cytokine bead array (G). TGF-β1 in the cell culture supernatants was assessed by ELISA (H). Data presented in this figure have been reproduced at least two times. Data are shown as mean ± S.D.
It was reported that the expression of TGF-β1 significantly increased in endotoxin-tolerized human peritoneal macrophages (33), mouse macrophages (34), mouse dendritic cells (35), and human blood from sepsis patients (36). TGF-β1 is also a major immunosuppressive cytokine and plays a vital role in the development of endotoxin tolerance (34, 37). Thus, we next investigated whether Siglec-1 is involved in the regulation of TGF-β1 production during the development of endotoxin tolerance. Very interestingly, we found TGF-β1 secretion from tolerant RAW 264.7 cell clones stably over-expressing shRNA for Siglec-1 was significantly decreased in response to LPS challenge (Fig. 6E). TGF-β1 secretion from tolerant RAW 264.7 cell clones stably over-expressing Siglec-1 was significantly increased, but not from tolerant RAW 264.7 cell clones stably overexpressing mutant Siglec-1(R122A) (Fig. 6H). Taken together, the data presented in this section demonstrate Siglec-1 regulates endotoxin tolerance by inhibiting production of TNF-α and inducing production of TGF-β1 in RAW 264.7 cells.
Down-regulation of Siglec-1 Promotes Degradation of Syk by Increasing Its Ubiquitination
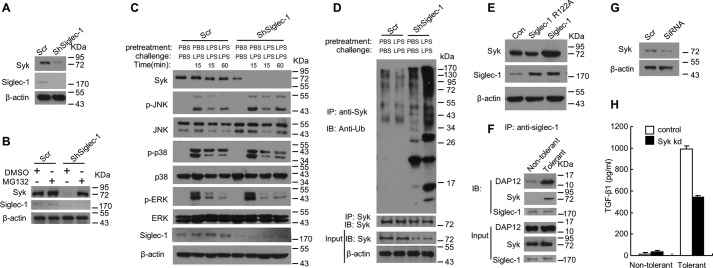
We next sought to determine the molecular mechanism by which Siglec-1 inhibits the development of endotoxin tolerance. Syk, a 72-kDa non-receptor tyrosine kinase, is most highly expressed by hematopoietic cells and plays a crucial role in signal transduction via its two SRC homology 2 (SH2) domains (38, 39). Recently it was shown that Syk plays an important role in TGF-β1 production both in monocytes/macrophages (40) and epithelial cells (41). It was shown that the expression of Syk is negatively regulated by the E3 ubiquitin ligase Casitas B-lineage lymphoma (CBl), leading to ubiquitylation and degradation of SYK (42–44). We found that knockdown of Siglec-1 in RAW 264.7 cells resulted in decreased Syk protein level compared with that in scrambled-controls (Fig. 7A), and resulted in a much lower level of endogenous Syk protein level after induction of endotoxin tolerance (Fig. 7C). Although phosphorylation of JNK, p38, and ERK was found to be decreased in Siglec-1 knockdown cells as well as in the scrambled controls in the time-course study, the very similar pattern of phosphorylation of these proteins in both experimental groups indicates that knockdown Siglec-1 did not affect the Map kinase pathway during the induction of tolerance by LPS (Fig. 7C). In addition, we found that inhibiting proteasome activity by MG132 increased the level of Syk in Siglec-1 knockdown RAW 264.7 cells (Fig. 7B). To test whether Siglec-1 regulates Syk protein level through regulation of its ubiquitination, lysates from tolerant or non-tolerant Raw 264.7 cells stably expression of shRNA for Siglec-1 or scramble were subjected to immunoprecipitation with anti-Syk antibodies. Immunoprecipitates were resolved by 10% SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-ubiquitination antibody. As shown in Fig. 7D, ubiquitinated-Syk strongly accumulated in Siglec-1 knockdown RAW 264.7 cells after induction of endotoxin tolerance, suggesting that knockdown of Siglec-1 resulted in enhanced ubiquitination of Syk. Next we examined whether Siglec-1 influences Syk protein stability by introducing an expression vector for Siglec-1 into RAW 264.7 cells. Overexpression of Siglec-1 in RAW 264.7 cells increased Syk protein level (Fig. 7E), indicating that Siglec-1 enhanced the steady-state levels of endogenous Syk.
FIGURE 7.
Knockdown of siglec-1 reduces Syk protein levels and enhances ubiquitination of Syk in RAW 264.7 cells. A, immunoblot analysis was performed to measure Syk protein in RAW 264.7 cell clones stably overexpressing ShRNA for Siglec-1. B, Siglec-1 knockdown RAW 264.7 cells or scrambled controls were treated with 5 μm MG132 for 24 h. Cell lysates were made and immunoblotted with anti-Syk, anti-Siglec-1, and anti-β-actin antibodies. C, immunoblot analysis of the indicated molecules in lysates of RAW 264.7 cell clones stably overexpressing ShRNA for Siglec-1 stimulated with LPS or pretreated and challenged with LPS for the indicated time. D, silencing of Siglec-1 in RAW 264.7 cells enhances ubiquitination of Sky. RAW 264.7 cell clones stably overexpressing ShRNA for Siglec-1 were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged for 24 h with 1 μg/ml LPS. Cell lysates were made and immunoprecipitation was performed with anti-Syk antibodies and immunoblotted for ubiquitin. E, immunoblot analysis was performed to measure Syk protein in RAW 264.7 cell clones stably overexpressing Siglec-1. F, Siglec-1, DAP12 and Syk form a complex after induction of endotoxin tolerance. RAW 264.7 cells were tolerized for 24 h with 100 ng/ml LPS, washed three times with PBS, and then challenged for 24 h with 1 μg/ml LPS. Cell lysates were made and immunoprecipitation was performed with anti-Siglec-1 antibodies and immunoblotted for DAP12 and Syk. G and H, introduction of siRNA for Syk to Raw 264.7 cells significantly reduced the protein level of Syk and this led to suppression of TGF-β1 production. The siRNA was transfected into cells with FuGENE 6. After 24 h, the cells were pretreated for 24 h with PBS or 100 ng/ml LPS, washed three times with PBS, and challenged for 24 h with PBS or 1 μg/ml LPS. Immunoblot analysis was performed to measure Syk protein expression (G). TGF-β1 in the cell culture supernatants was assessed by ELISA (H). Data presented in this figure have been reproduced two times.
The adaptor protein DAP12 is expressed in macrophages and can interact with Syk via the ITAM domain to modify Toll-like receptor signaling to enhance the release of cytotoxic cytokines from macrophages (45, 46). Recently, it was reported that Siglec-1 is associated with DAP12 in VSV-infected macrophages to control the antiviral innate immune response (47). We next examined whether Siglec-1, DAP12, and Syk form a complex to regulate the production of TGF-β1 in RAW 264.7 cells after induction of endotoxin tolerance. Lysates from tolerant or non-tolerant Raw 264.7 cells were subjected to immunoprecipitation with anti-Siglec-1 antibody. Immunoprecipitates were resolved by 10% SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-DAP12 and Syk antibodies. As shown in Fig. 7F, Siglec-1, DAP12, and Syk form a complex after induction of endotoxin tolerance in tolerant RAW 264.7 cells, but not in the non-tolerant cells. Moreover, knockdown of Syk (Fig. 7G) in RAW 264.7 cells with SiRNA inhibited the production of TGF-1 in Raw 264.7 cells (Fig. 7H). Taken together, these results are consistent with the possibility that Siglec-1 has a significant role in modulating Syk levels through altering its state of ubiquitination to regulate the production of TGF-β1 in RAW 264.7 cells.
Endotoxin Tolerance Induced TGF-β1 Expression Is Attenuated by Suppression of Syk
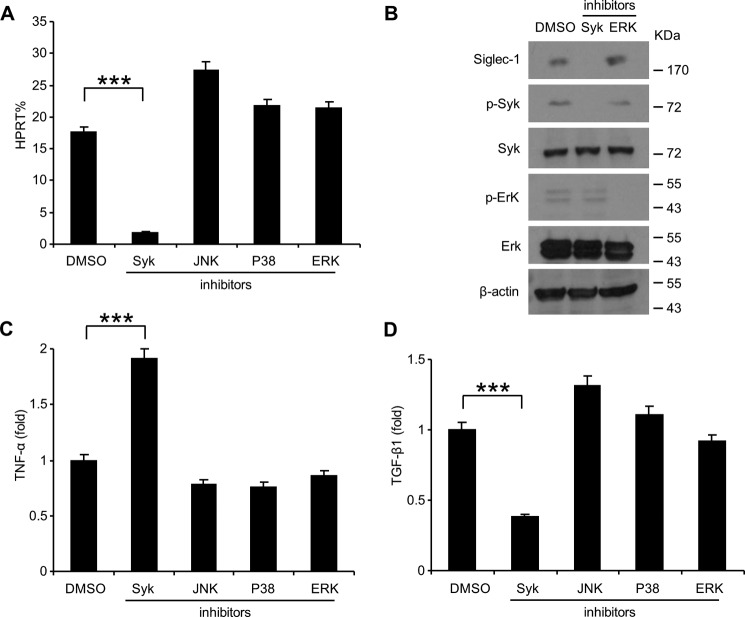
We next examined whether Syk is involved in the signal transduction pathway downstream of the Siglec-1-DAP12 complex by using the specific Syk inhibitor, piceatannol. Endotoxin tolerance was induced in RAW 264.7 cells by pretreatment with 100 ng/ml LPS for 24 h and then challenged with 1 μg/ml LPS for 24 h with the either 75 μm piceatannol, 10 μm SP600125 (JNK inhibitor), 10 μm SB203580 (P38 inhibitor), or 10 μm ERK inhibitor. As shown in Fig. 8A, Sigelc-1 expression was significantly decreased after treated with the Syk inhibitor piceatannol but not other inhibitors. The protein level of Siglec-1 was also significantly reduced by treatment with Syk inhibitor piceatannol but not by ERK inhibitor (Fig. 8B). The inhibitory efficiency of the Syk inhibitor piceatannol and ERK inhibitors was confirmed by Western blot analysis (Fig. 8B). TNF-α production was significantly increased after treated with the Syk inhibitor piceatannol but not other inhibitors (Fig. 8C). TGF-β1 secretion was significantly decreased by treating with the Syk inhibitor piceatannol but not other inhibitors (Fig. 8D), indicating that the specific inhibition of Syk can significantly block the production of TGF-β1.
FIGURE 8.
Endotoxin tolerance induced TGF-β1 expression is attenuated by suppression of Syk. RAW 264.7 cells were induced tolerance by pretreated with 100 ng/ml LPS for 24 h and then challenged with 1 μg/ml LPS for 24 h with the indicated inhibitors or DMSO as control. Sigelc-1 expression in these cells was determined by real-time PCR (A) and Western blot analysis (B), TNF-α in the cell culture supernatants was analyzed with cytokine bead array (C), and TGF-β1 in the cell culture supernatants was assessed by ELISA (D). Data presented in this figure have been repeated two times.
Discussion
Sepsis is still a leading cause of death in the intensive care unit. While sepsis syndrome is generally defined as a condition of uncontrolled inflammation in response to infection, however, the contribution of endotoxin tolerance is becoming increasingly recognized (48–50). While the treatments targeted for inflammatory cytokines and other inflammatory mediators such as nitric oxide have limited effect on phase III clinical trials (51, 52), treatments focused on reversing the immunosuppressive phase of sepsis hold great promise for efficacious sepsis therapy.
Sialic acids residues are broadly expressed and could act as a marker of self in the immune system (6). Sialylation level was mainly controlled by the two opposite process by adding or removing terminal sialic acid residues from the glycolipids or glycoproteins via sialyltransferases and sialidases. Desialylation played a crucial role in the recognition process (6) and acted as an “eat me” signal in the up-taking of apoptotic cells (11), while increase of sialylation contributed to the tolerant phenotype in CD4+ T cells (12) and regulatory T cells (13). Here, we found sialylation of cell surface was significantly increased on LPS-induced tolerant cells, and our data shows that this is attributable to the action of Neu1 down-regulation during the development of endotoxin tolerance. Knockdown of Neu1 was able to enhance tolerance and overexpression of Neu1 or treatment with sialidase abrogated tolerance. Furthermore, in addition to the decrease expression of the Neu1, we also found the expression of sialyltransferase ST3 Gal6 was specifically increased on tolerant RAW 264.7 cells, the action of which adds sialic acid to the terminal portions of the glycolipids or glycoproteins via α-2,3 linkages (30).
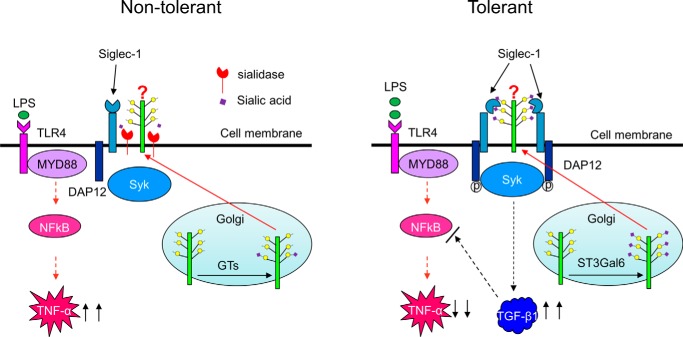
Siglecs are sialic acid-binding immunoglobin-like lectins. Most Siglecs inhibit immune responses via the recruitment of tyrosine phosphatases such as SHP1 and SHP2 by their cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIM) domain. Siglecs which lack an ITIM domain (such as Siglec-14, 15, and 16) associate with DAP12 via a positively charged amino acid in their transmembrane region to activate receptors through the recruitment of Syk (15, 40). Siglec-1, the first discovered member of Siglecs, has been shown to play an important role in sialylated pathogen uptake (17, 19, 53–55), antigen presentation (56, 57), lymphocyte proliferation (58), self-tolerance (59, 60) and antiviral immune response (47). However, the biological function of Siglec-1 in endotoxin tolerance is unknown. Here we found that Siglec-1 was specifically up-regulated in endotoxin tolerant cells and suppresses the innate immune response by promoting TGF-β1 production. In addition, overexpression of Siglec-1 inhibited endotoxin tolerance by promoting the secretion of TGF-β1; in contrast, overexpression of siglec-1 mutant (R122A) did not show inhibition activity, suggesting that the role of Siglec-1 in promoting the secretion of TGF-β1 requires the interaction between Siglec-1 and its endogenous ligand. Fig. 9 shows the working model for the regulation of sialylation and TGF-β1 production during LPS-induced tolerance.
FIGURE 9.
Model for the regulation of sialylation and TGF-β1 production during LPS-induced tolerance. Under non-tolerant conditions, proteins are partially sialylated by sialyltransferases (GTs), and sialic acids on the proteins are further removed by sialidase on the cell surface. After induction of endotoxin tolerance, proteins are heavily sialylated by the increased expression of ST3Gal6, and the interaction between the proteins and Siglec-1 enhances TGF-β1 production.
Ubiquitination is one of the most versatile post-translational modifications of proteins and plays critical roles in the regulation of innate immune response (61). Modification of Syk by the E3 ubiquitin ligase Casitas B-lineage lymphoma (CBl), leading to ubiquitylation and degradation of Syk (42–44). Interestingly, here we found that ubiquitinated-Syk strongly accumulated in Siglec-1 knockdown RAW 264.7 cells after induction of endotoxin tolerance, indicating the Siglec-1 regulates Syk protein levels through altering its state of ubiquitination in RAW 264.7 cells, although the E3 ubiquitin ligases contribute to Syk degradation still needs to be fully elucidated. TGF-β1, one of the major immunosuppressive cytokines, plays a critical role in the development of endotoxin tolerance (34, 37). Recently, it was shown that Syk regulates TGF-β1 production both in monocyte/macrophages (40) and epithelial cells (41). Since Siglec-1 lacks an ITIM domain, we found that Siglec-1 forms a complex with DAP12 and Syk in endotoxin-tolerant RAW 264.7 cells, suggesting that the interaction among Siglec-1, DAP12 and Syk could control the development of endotoxin tolerance by regulating the production of TGF-β1 in RAW 264.7 cells. However, how the complex regulates the production of TGF-β1 in endotoxin tolerance needs to be investigated in the future.
In summary, our data show that Siglec-1 is a critical regulator for the development of endotoxin tolerance by regulating innate immune response. Blockade of the Siglec-1 pathway or decrease Siglec-1 expression may reverse the immunosuppression in vivo. A better understanding of this pathway might shed new light on the pathogenesis of this disease and suggest possible therapeutic strategies for patients with sepsis.
Author Contributions
G. Y. C. designed the study and wrote the paper. Y. W. performed most experiments, analyzed data and interpreted results. C. L. and D. R. R. constructed vectors for expression of Siglec-1 and Siglec-1 mutant and analyzed the mutant phenotypes. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank Kim S. Lemessurier for helping to collect the flow data. We thank Dr. Amanda Preston for critical reading of the manuscript and editorial assistance.
This study was supported by Grant AI105727 from the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- DAMP
- danger-associated molecular pattern
- CBl
- E3 ubiquitin ligase Casitas B-lineage lymphoma
- DAP12
- DNAX-activation protein
- DC
- dendritic cell
- Hprt
- phosphoribosyltransferase
- ITIM
- immunoreceptor tyrosine-based inhibitory motif
- LPS
- lipopolysaccharide
- MAA
- fluorescein-Maackia amurensis lectin I
- PAMP
- pathogen-associated molecular pattern
- SH2 domain
- SRC homology 2 domain
- SNA
- fluorescein-Sambucus nigra (elderberry) bark lectin
- Syk
- spleen tyrosine kinase
- TLRs
- toll-like receptors.
References
- 1. Hall M. J., Williams S. N., DeFrances C. J., and Golosinskiy A. (2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief, 1–8 [PubMed] [Google Scholar]
- 2. Wood K. A., and Angus D. C. (2004) Pharmacoeconomic implications of new therapies in sepsis. Pharmacoeconomics 22, 895–906 [DOI] [PubMed] [Google Scholar]
- 3. Torgersen C., Moser P., Luckner G., Mayr V., Jochberger S., Hasibeder W. R., and Dünser M. W. (2009) Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth. Analg. 108, 1841–1847 [DOI] [PubMed] [Google Scholar]
- 4. Otto G. P., Sossdorf M., Claus R. A., Rödel J., Menge K., Reinhart K., Bauer M., and Riedemann N. C. (2011) The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 15, R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sundar K. M., and Sires M. (2013) Sepsis induced immunosuppression: Implications for secondary infections and complications. Indian J. Crit. Care Med. 17, 162–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen G. Y., Brown N. K., Zheng P., and Liu Y. (2014) Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology 24, 800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilatte Y., Tisserand E. M., Greffard A., Bignon J., and Lambré C. R. (1990) Anticarbohydrate autoantibodies to sialidase-treated erythrocytes and thymocytes in serum from patients with pulmonary sarcoidosis. Am. J. Med. 88, 486–492 [DOI] [PubMed] [Google Scholar]
- 8. Springer G. F. (1984) T and Tn, general carcinoma autoantigens. Science 224, 1198–1206 [DOI] [PubMed] [Google Scholar]
- 9. Vaith P., and Uhlenbruck G. (1978) The Thomsen agglutination phenomenon: a discovery revisited 50 years later. Zeitschrift fur Immunitatsforschung. Immunobiology 154, 1–15 [PubMed] [Google Scholar]
- 10. Rogentine G. N. Jr., and Plocinik B. A. (1974) Carbohydrate inhibition studies of the naturally occurring human antibody to neuraminidase-treated human lymphocytes. J. Immunol. 113, 848–858 [PubMed] [Google Scholar]
- 11. Meesmann H. M., Fehr E. M., Kierschke S., Herrmann M., Bilyy R., Heyder P., Blank N., Krienke S., Lorenz H. M., and Schiller M. (2010) Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J. Cell Sci. 123, 3347–3356 [DOI] [PubMed] [Google Scholar]
- 12. Brennan P. J., Saouaf S. J., Van Dyken S., Marth J. D., Li B., Bhandoola A., and Greene M. I. (2006) Sialylation regulates peripheral tolerance in CD4+ T cells. Int. Immunol. 18, 627–635 [DOI] [PubMed] [Google Scholar]
- 13. Jenner J., Kerst G., Handgretinger R., and Müller I. (2006) Increased α2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp. Hematol. 34, 1212–1218 [DOI] [PubMed] [Google Scholar]
- 14. Pillai S., Netravali I. A., Cariappa A., and Mattoo H. (2012) Siglecs and immune regulation. Annu. Rev. Immunol. 30, 357–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macauley M. S., Crocker P. R., and Paulson J. C. (2014) Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 14, 653–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crocker P. R., and Varki A. (2001) Siglecs, sialic acids and innate immunity. Trends Immunol. 22, 337–342 [DOI] [PubMed] [Google Scholar]
- 17. Yu X., Feizpour A., Ramirez N. G., Wu L., Akiyama H., Xu F., Gummuluru S., and Reinhard B. M. (2014) Glycosphingolipid-functionalized nanoparticles recapitulate CD169-dependent HIV-1 uptake and trafficking in dendritic cells. Nat. Commun. 5, 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tateno H., Li H., Schur M. J., Bovin N., Crocker P. R., Wakarchuk W. W., and Paulson J. C. (2007) Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol. Cell Biol. 27, 5699–5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones C., Virji M., and Crocker P. R. (2003) Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49, 1213–1225 [DOI] [PubMed] [Google Scholar]
- 20. Jia Y., Yu H., Fernandes S. M., Wei Y., Gonzalez-Gil A., Motari M. G., Vajn K., Stevens W. W., Peters A. T., Bochner B. S., Kern R. C., Schleimer R. P., and Schnaar R. L. (2015) Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J. Allergy Clin. Immunol. 135, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. von Gunten S., Vogel M., Schaub A., Stadler B. M., Miescher S., Crocker P. R., and Simon H. U. (2007) Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J. Allergy Clin. Immunol. 119, 1005–1011 [DOI] [PubMed] [Google Scholar]
- 22. Bökers S., Urbat A., Daniel C., Amann K., Smith K. G., Espéli M., and Nitschke L. (2014) Siglec-G deficiency leads to more severe collagen-induced arthritis and earlier onset of lupus-like symptoms in MRL/lpr mice. J. Immunol. 192, 2994–3002 [DOI] [PubMed] [Google Scholar]
- 23. Chen G. Y., Tang J., Zheng P., and Liu Y. (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen G. Y., Chen X., King S., Cavassani K. A., Cheng J., Zheng X., Cao H., Yu H., Qu J., Fang D., Wu W., Bai X. F., Liu J. Q., Woodiga S. A., Chen C., Sun L., Hogaboam C. M., Kunkel S. L., Zheng P., and Liu Y. (2011) Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nat. Biotechnol. 29, 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen G. Y., Brown N. K., Wu W., Khedri Z., Yu H., Chen X., van de Vlekkert D., D'Azzo A., Zheng P., and Liu Y. (2014) Broad and direct interaction between TLR and Siglec families of pattern recognition receptors and its regulation by Neu1. eLife 3, e04066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nahid M. A., Satoh M., and Chan E. K. (2011) MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol. Immunol. 8, 388–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. West M. A., and Koons A. (2008) Endotoxin tolerance in sepsis: concentration-dependent augmentation or inhibition of LPS-stimulated macrophage TNF secretion by LPS pretreatment. J. Trauma 65, 893–898; discussion 898–900 [DOI] [PubMed] [Google Scholar]
- 28. Fan H., and Cook J. A. (2004) Molecular mechanisms of endotoxin tolerance. J. Endotoxin Res. 10, 71–84 [DOI] [PubMed] [Google Scholar]
- 29. Miyagi T., and Yamaguchi K. (2012) Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology 22, 880–896 [DOI] [PubMed] [Google Scholar]
- 30. Glavey S. V., Manier S., Natoni A., Sacco A., Moschetta M., Reagan M. R., Murillo L. S., Sahin I., Wu P., Mishima Y., Zhang Y., Zhang W., Zhang Y., Morgan G., Joshi L., Roccaro A. M., Ghobrial I. M., and O'Dwyer M. E. (2014) The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood 124, 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crocker P. R. (2002) Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 [DOI] [PubMed] [Google Scholar]
- 32. Crocker P. R., and Varki A. (2001) Siglecs in the immune system. Immunology 103, 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Randow F., Syrbe U., Meisel C., Krausch D., Zuckermann H., Platzer C., and Volk H. D. (1995) Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor β. J. Exp. Med. 181, 1887–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sly L. M., Rauh M. J., Kalesnikoff J., Song C. H., and Krystal G. (2004) LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21, 227–239 [DOI] [PubMed] [Google Scholar]
- 35. Morelli A. E., and Thomson A. W. (2007) Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621 [DOI] [PubMed] [Google Scholar]
- 36. Draisma A., Pickkers P., Bouw M. P., and van der Hoeven J. G. (2009) Development of endotoxin tolerance in humans in vivo. Crit. Care Med. 37, 1261–1267 [DOI] [PubMed] [Google Scholar]
- 37. Beutler B. (2004) SHIP, TGF-β, and endotoxin tolerance. Immunity 21, 134–135 [DOI] [PubMed] [Google Scholar]
- 38. Mócsai A., Ruland J., and Tybulewicz V. L. (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geahlen R. L. (2014) Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol. Sci. 35, 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takamiya R., Ohtsubo K., Takamatsu S., Taniguchi N., and Angata T. (2013) The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology 23, 178–187 [DOI] [PubMed] [Google Scholar]
- 41. Yang W. S., Chang J. W., Han N. J., Lee S. K., and Park S. K. (2012) Spleen tyrosine kinase mediates high glucose-induced transforming growth factor-beta1 up-regulation in proximal tubular epithelial cells. Exp. Cell Res. 318, 1867–1876 [DOI] [PubMed] [Google Scholar]
- 42. Lupher M. L. Jr., Rao N., Lill N. L., Andoniou C. E., Miyake S., Clark E. A., Druker B., and Band H. (1998) Cbl-mediated negative regulation of the Syk tyrosine kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J. Biol. Chem. 273, 35273–35281 [DOI] [PubMed] [Google Scholar]
- 43. Yankee T. M., Keshvara L. M., Sawasdikosol S., Harrison M. L., and Geahlen R. L. (1999) Inhibition of signaling through the B cell antigen receptor by the protooncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J. Immunol. 163, 5827–5835 [PubMed] [Google Scholar]
- 44. Rao N., Dodge I., and Band H. (2002) The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J. Leukocyte Biol. 71, 753–763 [PubMed] [Google Scholar]
- 45. Lanier L. L. (2009) DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 227, 150–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Humphrey M. B., Lanier L. L., and Nakamura M. C. (2005) Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol. Rev. 208, 50–65 [DOI] [PubMed] [Google Scholar]
- 47. Zheng Q., Hou J., Zhou Y., Yang Y., Xie B., and Cao X. (2015) Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res. 25, 1121–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang T. S., and Deng J. C. (2008) Molecular and cellular aspects of sepsis-induced immunosuppression. J. Mol. Med. 86, 495–506 [DOI] [PubMed] [Google Scholar]
- 49. Fink M. P., and Heard S. O. (1990) Laboratory models of sepsis and septic shock. J. Surg. Res. 49, 186–196 [DOI] [PubMed] [Google Scholar]
- 50. Volk H. D., Reinke P., Krausch D., Zuckermann H., Asadullah K., Müller J. M., Döcke W. D., and Kox W. J. (1996) Monocyte deactivation-rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 22, S474–481 [DOI] [PubMed] [Google Scholar]
- 51. Rittirsch D., Hoesel L. M., and Ward P. A. (2007) The disconnect between animal models of sepsis and human sepsis. J. Leukocyte Biol. 81, 137–143 [DOI] [PubMed] [Google Scholar]
- 52. Changsirivathanathamrong D., Wang Y., Rajbhandari D., Maghzal G. J., Mak W. M., Woolfe C., Duflou J., Gebski V., dos Remedios C. G., Celermajer D. S., and Stocker R. (2011) Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit. Care Med. 39, 2678–2683 [DOI] [PubMed] [Google Scholar]
- 53. Vanderheijden N., Delputte P. L., Favoreel H. W., Vandekerckhove J., Van Damme J., van Woensel P. A., and Nauwynck H. J. (2003) Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 77, 8207–8215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Monteiro V. G., Lobato C. S., Silva A. R., Medina D. V., de Oliveira M. A., Seabra S. H., de Souza W., and DaMatta R. A. (2005) Increased association of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasitol. Res. 97, 380–385 [DOI] [PubMed] [Google Scholar]
- 55. Heikema A. P., Bergman M. P., Richards H., Crocker P. R., Gilbert M., Samsom J. N., van Wamel W. J., Endtz H. P., and van Belkum A. (2010) Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect Immun. 78, 3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinez-Pomares L., and Gordon S. (2012) CD169+ macrophages at the crossroads of antigen presentation. Trends Immunol. 33, 66–70 [DOI] [PubMed] [Google Scholar]
- 57. Kawasaki N., Vela J. L., Nycholat C. M., Rademacher C., Khurana A., van Rooijen N., Crocker P. R., Kronenberg M., and Paulson J. C. (2013) Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc. Natl. Acad. Sci. U.S.A. 110, 7826–7831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu C., Rauch U., Korpos E., Song J., Loser K., Crocker P. R., and Sorokin L. M. (2009) Sialoadhesin-positive macrophages bind regulatory T cells, negatively controlling their expansion and autoimmune disease progression. J. Immunol. 182, 6508–6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miyake Y., Asano K., Kaise H., Uemura M., Nakayama M., and Tanaka M. (2007) Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J. Clin. Invest. 117, 2268–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McGaha T. L., Chen Y., Ravishankar B., van Rooijen N., and Karlsson M. C. (2011) Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 117, 5403–5412 [DOI] [PubMed] [Google Scholar]
- 61. Mohapatra B., Ahmad G., Nadeau S., Zutshi N., An W., Scheffe S., Dong L., Feng D., Goetz B., Arya P., Bailey T. A., Palermo N., Borgstahl G. E., Natarajan A., Raja S. M., Naramura M., Band V., and Band H. (2013) Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochim. Biophys. Acta 1833, 122–139 [DOI] [PMC free article] [PubMed] [Google Scholar]