Abstract
Our objective was to determine if consuming table grapes reduces adiposity and its metabolic consequences and alters gut microbiota in mice fed a high fat (HF), butter-rich diet. C57BL/6J mice were fed a low fat (LF) diet or HF diet with 3% or 5% grapes for 11 weeks. Total body and inguinal fat were moderately, but significantly reduced in mice fed both levels of grapes compared to their controls. Mice fed 5% grapes had lower liver weights and triglyceride levels, and decreased expression of glycerol-3-phosphate acyltransferase (Gpat1) compared to the 5% controls. Mice fed 3% grapes had lower hepatic mRNA levels of peroxisome proliferator-activated receptor gamma 2, sterol-CoA desaturase 1, fatty-acid binding protein 4, and Gpat1 compared to the 3% controls. Although grape feeding had only a minor impact on markers of inflammation or lipogenesis in adipose tissue or intestine, 3% grapes decreased the intestinal abundance of sulfidogenic Desulfobacter spp., and the Bilophila wadsworthia-specific dissimilatory sulfite reductase gene (dsrA-Bw), and tended to increase the abundance of the beneficial bacterium Akkermansia muciniphila compared to controls. Additionally, Bifidobacterium, Lactobacillus, Allobaculum, and several other genera correlated negatively with adiposity. Allobaculum in particular was increased in the LF and 3% grapes groups compared to the HF-fed controls. Notably, grape feeding attenuated the HF-induced impairment in epithelial localization of the intestinal tight junction protein zonula occludens. Collectively, these data indicate that some of the adverse health consequences of consuming a HF diet rich in saturated fat can be attenuated by table grape consumption.
Keywords: grapes, microbiota, intestines, obesity, steatosis
1. Introduction
Currently, 36% of adults and 17% of youth in the United States have been diagnosed as obese [1]. Obesity is also a global health condition affecting more than 10% of adults worldwide [2]. Of growing concern is the association between the rise in obesity and chronic inflammatory conditions such as type 2 diabetes, hypertension, and cardiovascular disease [3]. Physiologically, obesity is the result of an expansion of white adipose tissue (WAT) which typically elicits inflammatory signals involved in the recruitment of macrophages and other immune cells into the WAT. This results in an increase in circulating and tissue levels of proinflammatory cytokines, chemokines, and adipokine [4]. While increased tissue levels of these proinflammatory agents perpetuates the inflammatory cycle, those released systemically impair glucose disposal and lipid metabolism that contribute to the development of metabolic diseases. However, the exact mechanisms which initiate WAT inflammation resulting from diet-induced obesity remain unclear.
Gut microbes have received much attention due to their potential involvement in the development of obesity [5], chronic inflammation [6–8], and insulin resistance [9]. Diets high in fat, particularly those rich in saturated fatty acids found in milk fat [10], have been implicated in the reduction of gut barrier-protecting bacteria as well as an increase in the abundance of deleterious bacteria including sulfidogenic bacteria [reviewed in 11]. A correlation exists between the extent of these changes in microbiota and the degree of obesity and insulin resistance in test subjects [12]. Furthermore, the main byproduct of sulfidogenic bacteria like Bilophila wadsworthia and other members of the Desulfovibrionaceae family is hydrogen sulfide, a proinflammatory and genotoxic gas that is positively correlated with development of ulcerative colitis, gut inflammation, irritable bowel syndrome, and colon cancer [10, 13, 14].
In contrast, changes to the intestinal microbiome can have beneficial effects. For instance, research with animal models has demonstrated that elimination of gut microbes [5–9, 11, 12, 15] or inoculation with specific pre- (e.g., non-digestible sugars, fiber, or polyphenols) or probiotics (e.g., Lactobacillus acidophilus, Bifidobacterium spp., Akkermansia muciniphila, Clostridium trybutyricum) [reviewed in 11, 16–19] can attenuate obesity or metabolic dysfunction. Polyphenols found in fruits and vegetables [20–22] are of particular interest as they are poorly absorbed in the upper gastrointestinal tract, and thus persist in the distal small intestine, cecum, and colon [23], where they may influence microbial taxa and their metabolites [24]. In addition, the anti-inflammatory, anti-oxidant, or anti-microbial actions of polyphenols have been reported to positively influence gut microbes and inflammation [reviewed in 25].
Grapes are rich in polyphenols including anthocyanins [reviewed in 25], and thus may have beneficial effects on intestinal or systemic inflammation. The anti-inflammatory and anti-oxidant properties of California table grapes have been demonstrated in rats supplemented with nine human servings (3%, w/w) of powdered table grapes for 18 weeks, resulting in lowered blood pressure, improved cardiac function, and reduced systemic inflammation and oxidative damage [26]. These effects correlated with increased cardiac peroxisome proliferator-activated receptor (PPAR)α/γ activity and decreased nuclear factor kappa B (NF-κB) activity, along with reduced cytokine concentrations [27]. Similarly, table grape-mediated reductions in atherosclerotic lesion areas were associated with increased serum antioxidant status and decreased macrophage-mediated oxidation of low density lipoproteins (LDL) [28]. Additionally, when women were supplemented with powdered California table grapes (36 g/d) their plasma lipid profile improved and plasma (e.g., tumor necrosis factor alpha, TNFα) or urine (e.g., prostaglandin F2α) markers of inflammation and oxidative stress were reduced [29]. Consistent with these data, we previously demonstrated that C57BL/6J mice fed a high fat (HF) diet (i.e., 60% kcals from lard) supplemented with powdered California table grapes (3%, w/w), improved glucose tolerance at 5 weeks, and decreased markers of inflammation ≈20–50% in serum and WAT at 18 weeks without affecting body fat levels or food intake [30].
Collectively, these findings indicate that: (i) gut microbiota are influenced by diets abundant in saturated fats such as butter fat in a manner that impacts gut inflammation and barrier function, and systemic inflammation; (ii) non-digestible carbohydrates and polyphenols positively impact adverse outcomes caused by HF-feeding; and (iii) grape consumption can attenuate inflammation and oxidative stress in several animal models and in humans. However, little is known about how table grapes decrease chronic inflammation associated with saturated fat-feeding and obesity. Therefore, the objective of this study was to examine the extent to which consuming table grapes reduces adiposity, hepatic steatosis, markers of inflammation or lipid metabolism, or impacts gut microbiota in mice fed a HF, butter-rich diet.
2. Materials and Methods
2.1. Animals
Four-week old, male C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and acclimated on a standard chow diet for 1 week. Mice were housed in pairs, maintained at a temperature of 22°C with 50% humidity, and exposed to a 12 h light/12 h dark cycle. Mice received food and water ad libitum and measures of body weight and food intake were conducted once and twice per week, respectively. All experimental procedures were performed under ethical standards and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Greensboro.
2.2. Diets
Animals were randomly assigned to one of five dietary treatments (n=10 per treatment group) as follows: low fat (LF; 10% of energy from fat), high fat (HF; 34% of energy from fat) plus 3% powdered grapes (w/w; HF-3G), high fat plus 3% sugar (w/w; HF-3S), high fat plus 5% powdered grapes (HF-5G) and high fat plus 5% sugar (HF-5S). The HF diets consisted of approximately 3% energy from soybean oil and 31% energy from butter. Thus, the HF diets were rich in fat, especially saturated fat (i.e. 20.3% of energy), and mimicked the average calories from fat in an American-type diet (i.e., 34% of energy). The lyophilized (i.e., powdered) table grapes, kindly provided by the California Table Grape Commission consisted of a mixture of red, green, and black seeded and seedless grapes. The 3% and 5% dietary levels of grapes were comparable to 9 and 15 human servings (1 serving is equivalent to 3/4 cup of whole grapes) of grapes, respectively. The HF-3S and HF-5S diets consisted of a mixture of fructose and glucose to control for the natural sugar content of the powdered grape diets. Diets were prepared by Research Diets (New Brunswick, NJ, USA) and stored at −20°C until use. Detailed composition of the diets is provided in Supplemental Table 1.
2.3. Intraperitoneal glucose tolerance tests (GTT)s and fasting insulin levels
Intraperitoneal (i.p.) GTTs were performed on weeks 3, 6, and 9 on non-anesthetized mice. Mice were fasted for 8 h and given an i.p. injection of glucose (i.e., 20% solution at 1 g/kg body weight). Blood from the tail vein collected at baseline and 5, 15, 30, 60, and 120 minutes post-i.p. glucose injection was used to quantify glucose levels using a Bayer Contour blood glucose monitor and strips (Bayer Healthcare, Tarrytown, NY, USA). Plasma insulin levels were detected using an ultrasensitive mouse insulin kit (Crystal Chem, Inc, Downers Grove, IL). The homeostasis model assessment method (HOMA) for insulin resistance (IR) was used employing the following formula: [fasting insulin concentration (ng/ml) × 24 × fasting glucose concentration (mg/dl)]/405 [16].
2.4. Body fat measurements via Dual X-Ray Absorptiometry (DEXA)
Percent body fat was measured using DEXA on a GE Lunar Prodigy Advanced System (GE Healthcare, Milwaukee, WI) at weeks 5 and 10. During the measurement, mice were lightly anesthetized with isoflurane using a SomnoSuite Small Animal Anesthesia System with Integrated Digital Vaporizer isoflurane system. Measurements were taken in duplicate to reduce the possibility of error and values expressed are an average of the two measurements.
2.5. Tissue collection
After 11 weeks of dietary intervention, mice were fasted for 8 h and euthanized via isoflurane-induced anesthesia followed by decapitation. Plasma was collected at the time of harvest. Four WAT depots were collected; epididymal, mesenteric, inguinal, and retroperitoneal. Additionally, livers were harvested and intestinal mucosa and digesta were collected from the duodenum, jejunum, ileum, cecum, and proximal and distal colon. Weights of the WAT depots and liver were recorded and all collected samples were immediately snap-frozen in liquid nitrogen and stored at −80°C until analysis.
2.6. Liver and serum triglyceride (TG) levels and Oil-Red-O staining
Liver TG content was measured as previously described [31]. Plasma TG content was determined using a commercial assay from Thermo Scientific and was conducted following the manufacturer’s protocol (Infinity TG assay #TR22421 and TG standards #TG22923; Norcross, GA). For Oil-Red-O staining, frozen liver tissues were cut at 5 μm, and mounted on slides. The sections were fixed with 10% formalin for 10 minutes and then the slides were washed with deionized water for 5 minutes. Fixed tissues were then rinsed with 60% isopropanol for 5 minutes. To prepare Oil-Red-O stock, 0.5 g of Oil-Red-O (Sigma-Aldrich, O0625) was mixed with 100 mL of isopropanol. To prepare Oil-Red-O working solution, 30 mL of Oil-Red-O stock was mixed with 20 mL of distilled water and filtered using a 0.24 μm vacuum filter. Samples were submerged in the working solution for 15 minutes, briefly rinsed with 60% isopropanol, and then rinsed with deionized water for 30 seconds before imaging.
2.7. RNA extraction and qPCR
Adipose and intestinal samples were homogenized in QIAsol reagent and total RNA was extracted using QIAgen mini lipid kit obtained from Qiagen (Valencia, CA). For hepatic samples, a QIAgen mini universal kit from Qiagen was used. The quality and concentration of RNA were examined using absorbance at 260 nm and integrity determined using the absorbance ratio of 260/280 on a Nanodrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE). Complementary DNA was created by reverse transcription using 1 ug of RNA and a high capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to manufacturer’s protocols. qPCR was performed in a 7500 FAST Real Time PCR system (Applied Biosystems). The expression of different genes related to inflammation, lipogenesis, and lipolysis in WAT depots and liver were measured using Taqman Gene expression assays purchased from Applied Biosystems. TATA-binding protein (Tbp) was the endogenous reference gene utilized for all assays and fold differences in gene expression were calculated as 2−ΔΔCt.
2.8. Immunoblotting
Immunoblotting was conducted as previously described [31] using primary antibodies at 1:1000 dilutions for stearoyl-CoA desaturase-1 (#2283S; SCD1, Cell Signaling), carnitine palmitoyltransferase 1A (#12252S; CPT1a, Cell Signaling), PPARγ (#2443S; Cell Signaling Technology, Inc., Danvers, MA), β-actin (#4967; Cell Signaling), PPARα, (#sc9000; Santa Cruz Biotechnology Inc., Santa Cruz, CA), glycerol-3-phosphate acyltransferase (#sc382257; GPAM, Santa Cruz), and sterol regulatory element-binding protein 1C (#sc366; SREBP1c, Santa Cruz). Adipocyte fatty acid binding protein (aP2) was kindly provided by Dr. David Bernlohr (U. of Minnesota) and used at a 1:10,000 dilution. Horseradish peroxidase-conjugated secondary antibodies were probed for 2 h at room temperate at 1:1000 dilutions. Blots were exposed to a chemiluminescence reagent and X-ray films were developed using a SRX-101A Konica Minolta film developer.
2.9. Barrier function in ileum
The localization of the tight junction protein zonula occludens-1 (ZO-1) was determined in ileal mucosa samples that had been embedded in Tissue-Tek Cyro-OCT compound, sliced in 5 um sections, fixed in methanol at −20° C, and incubated with a polyclonal rabbit anti-ZO-1 antibody as previously described [32]. Three to four samples of each group were analyzed by Image J software. Each photo was corrected by its own background and then adjusted by DAPI.
2.10. PCR amplification of 16S rRNA and functional gene targets
Genomic DNA extraction and qPCR experiments were conducted as previously described [32]. 16S rRNA gene-specific primers were used to target specific bacterial genera; A. muciniphila, B. wadsworthia (F-AAGTCCTTCGGGGCGAGTAA) (R-ATCCTCTCAGACCGGCTAC), Desulfovibrio spp. (DSV), Desulfobulbus spp. (DBB), Desulfobacter spp. (DSB), and Desulfotomaculum spp. (DFM) [32]. The B. wadsworthia-specific dissimilatory sulfite reductase (dsrA-Bw) [20,32], which encodes an enzyme that catalyzes a step in the reduction of sulfite to hydrogen sulfide, was targeted to measure the abundance of this member of the Desulfovibrionaceae family. Four functional genes utilized by Fusobacterium nucleatum in the fermentation of cysteine were measured; i.e., two homologues of L-cysteine desulfhydrase (FN0625 and FN1220), Cystathionine-β synthase (FN1055), and L-methionine-γ-lyase (FN1419). Standard curves were constructed using cloned 16S rRNA and functional genes or amplified PCR product.
2.11. Sequencing of 16s rRNA gene using Illumina Mi-Seq platform
To assess bacterial community structure of cecal mucosa, primers specific for 16S rRNA V4–V5 region (Forward: 338F: 5′-GTGCCAGCMGCCGCGGTAA-3′ and Reverse: 806R: 5′-GGACTACHVGGGTWTCTAAT-3′) that contained Illumina 3′ adapter sequences as well as a 12-bp barcode were used. Sequences were generated by an Illumina MiSeq DNA platform at Argonne National Laboratory and analyzed by the program Quantitative Insights Into Microbial Ecology (QIIME) [33]. Operational Taxonomic Units (OTUs) were picked at 97% sequence identity using open reference OTU picking against the Greengenes database (05/13 release) [34]. OTUs were quality filtered based on default parameters set in the open-reference OTU command in QIIME and sequences were rarified to an equal sampling depth of 15000 reads per sample. Representative sequences were aligned via PyNAST [33], taxonomy assigned using the RDP Classifier [35], and a phylogenetic tree was built using FastTree [36]. Beta Diversity was represented by measuring UniFrac distances calculated using both weighted and unweighted algorithms and visualized with PCoA plots generated in Emperor (Supplemental Fig 1). Prior to statistical analyses, OTUs occurring in less than 50% of samples were filtered from the OTU table. Significant changes in OTU abundance were assessed using Kruskal Wallis test (FDR correction p ≤ 0.05). Multivariate statistical tests performed included ADONIS, ANOSIM, and PERMANOVA tests [33]. Spearman correlations and Principal Component Analysis (PCA) were run using MATLAB software.
2.12. Statistical analysis
Data were analyzed using a one-way ANOVA and Student’s t test to compute individual pairwise comparisons of means (p<0.05). We also used Bonferroni’s posthoc test to perform specific comparisons where appropriate. Analyses were conducted using the JMP software program version 10.0 for Windows (SAS, Cary, NC). Relative abundances generated from 16s rRNA sequencing were analyzed in GraphPad Prism Version 6 using ANOVA followed by Dunn’s test for multiple comparisons. Data are expressed as means + S.E.M.
3. Results
3.1. Grape consumption decreases body fat
Body weight gains and energy intakes were greater in all HF-fed mice compared to the LF control mice (Table 1). Mice fed powdered grapes (HF-3G, HF-5G) had similar body weights and energy intakes compared to their HF-sugar controls (HF-3S, HF-5S; Table 1). Mice fed the HF-sugar control diets had greater body fat percentage and total WAT depot weights compared to the LF controls (Fig. 1). Mice fed the HF-3G diet had lower percent body fat at week 5 and mice fed powdered grapes at both levels had lower body fat percentages and inguinal fat depot weights at week 10 compared to their respective HF-sugar controls (Fig. 1). The HF-3G diet had lower total WAT depot weights compared to HF-3S controls (Fig. 1).
Table 1.
The effect of diet composition on total body weight gain, total food intake, food conversion efficiency, and total caloric intake
| Diets | BWG n=9–10 | FI n=5 | FCE n=5 | Kcal n=5 |
|---|---|---|---|---|
| LF | 7.0 ± 0.6b | 399 ± 8b | 28.6 ± 1.2a | 1534 ± 26b |
| HF-3G | 13.1 ± 0.7a | 465 ± 7a | 17.8 ± 0.8b | 2051 ± 26a |
| HF-3S | 11.5 ± 0.7a | 424 ± 20ab | 18.7 ± 0.9b | 1874 ± 80a |
| HF-5G | 11.3 ± 1.1a | 409 ± 12ab | 18.4 ± 1.1b | 1799 ± 45ab |
| HF-5S | 12.6 ± 1.0a | 438 ± 25ab | 17.8 ± 1.5b | 1957 ± 124a |
Mean ± SEM without a common lower case letter in a column differ (p<0.05) using one-way ANOVA and Student’s t-test. BWG= total body weight gain (g). FI= total food intake per cage (g). FCE= food conversion effeciency per cage. Kcal= total caloric intake per cage (kcals).
Figure 1.
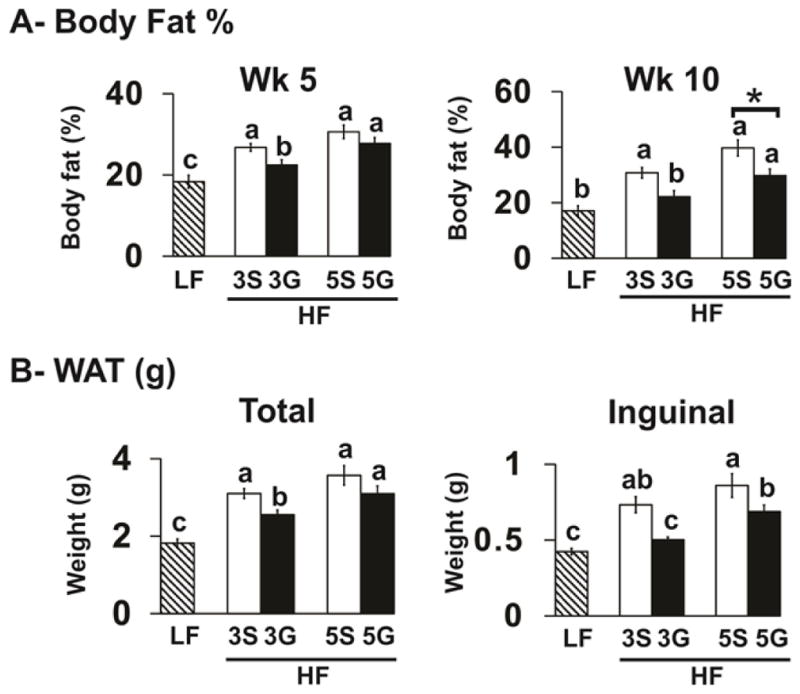
Adiposity indices of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. (A) Body fat percentages were measured at week 5 and week 10 using dual energy x-ray absorptiometry (DEXA). (B) At week 11, epididymal, inguinal, retroperitoneal, and mesenteric white adipose tissue (WAT) depots were excised and weighed. The weights of the epididymal, inguinal, retroperitoneal, and mesenteric depots were measured, and their sum labelled total WAT. Means ± SEM without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. Means ± SEM (n=9–10) sharing the symbol “*” differ using the Bonferroni’s adjustment (p<0.01). 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
3.2. GTTs, fasting glucose, insulin, and TG levels, and HOMA-IR scores unaffected by grapes
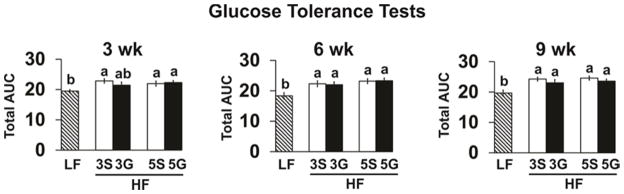
To assess the impact of grape consumption on insulin resistance and glucose sensitivity, GTTs were conducted at weeks 3, 6, and 10, and fasting plasma glucose and insulin and HOMA-IR were measured at week 11. Mice consuming the HF-sugar control diets had impaired GTTs at all three time points compared to the LF controls (Fig. 2). However, consuming grapes did not significantly improve GTT, fasting insulin, glucose, serum TG levels, or HOMA-IR scores.
Figure 2.
Glucose tolerance tests (GTT)s of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. At weeks 3, 6, and 9, GTTs were conducted on mice fasted for 8 h and injected i.p. with a 20% glucose solution. Data are expressed as total area under the curve (AUC) for the GTTs. Means ± SEM (n=9–10) without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
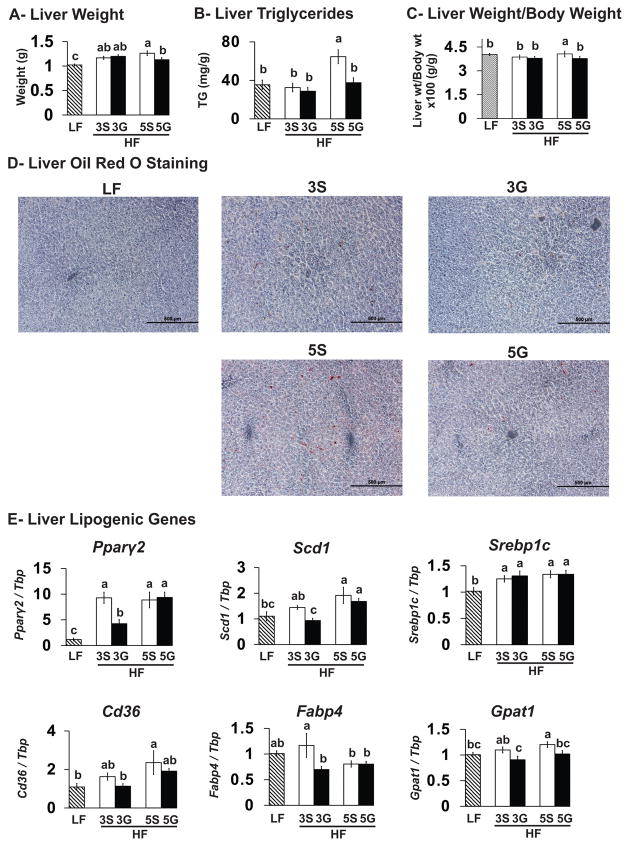
3.3. Grape consumption lowers hepatic TG levels, and the expression of several lipogenic genes
Liver tissues were analyzed to assess the impact of grape consumption on liver TG levels and markers of lipogenesis and fatty acid oxidation. Mice consuming the HF-sugar control diets had greater liver weights, TG levels (HF-5S only), visual lipid staining, and mRNA levels of the lipogenic genes Pparγ2, Scd1 (HF-5S only), Srebp1c, cluster of differentiation 36 (Cd36; HF-5S only), and glycerol-3-phosphate acyltransferase 1 (Gpat1; HF-5S only) compared to the LF-fed mice (Fig. 3). Mice fed the HF-3G diet had decreased mRNA levels of Pparγ2, Scd1, fatty acid binding protein 4 (Fabp4), and Gpat1 compared to the HF-3S controls. Mice fed the HF-5G diet had decreased TG levels and mRNA levels of Gpat1 compared to the HF-5S controls. None of the genes associated with fatty acid oxidation (e.g., Pparα, Cpt1a, acyl-CoA oxidase 1 (Acox1)) were impacted by grape feeding. Grape consumption did not impact the protein levels of Pparγ, Scd1, Pparα, Fabp, Cpt1a, or Gpam (data not shown) or influence the ratio of liver weight to body weight..
Figure 3.
Liver weights (A), liver triglyceride levels (B), ratio of liver weight to body weight (BW) (C), Oil red-O staining of liver, and the expression of several lipogenic genes in liver (E) of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. qPCR was conducted to measure mRNA abundance of genes associated with hepatic lipogenesis. Means ± SEM (n=9–10) without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
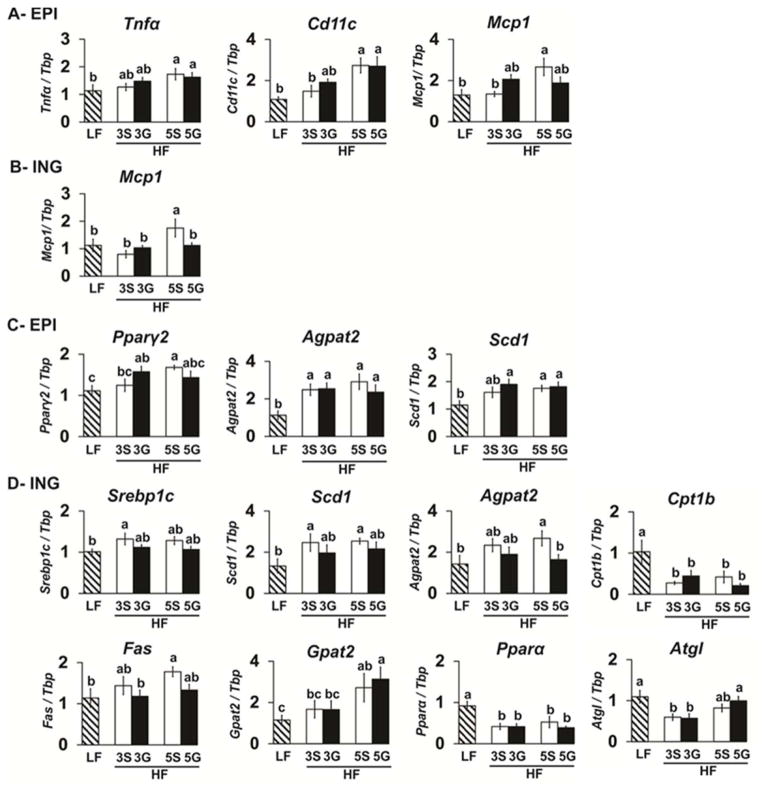
3.4. Grape consumption differentially impacts WAT genes associated with inflammation and lipid metabolism
To determine whether insulin resistance caused by HF-feeding was due to WAT inflammation, inguinal (subcutaneous), epididymal (visceral), and mesenteric (visceral) WAT mRNA levels for several proinflammatory genes (i.e., cluster of differentiation 11c (Cd11c), epidermal growth factor-like module containing mucin-like hormone receptor 1 (Erm1; F4/80 human orthologue), monocyte chemoattractant protein 1 (Mcp1), Tnfα, Toll-like receptor 4 (Tlr4), and interleukin 6 (Il6) were measured. In epididymal WAT, mice fed the HF-sugar controls had increased mRNA levels of Tnfα (HF-5S), Cd11c (HF-5S), Il6, and Mcp1 (HF-5S) compared to the LF controls (Fig. 4A). Grape feeding did not impact the expression of these genes. In inguinal depot WAT, only mice fed the HF-5S diet exhibited increased inflammatory gene expression (i.e., Mcp1), which was decreased in mice fed the HF-5G diet (Fig. 4B). In mesenteric WAT, inflammatory gene expression was not increased by HF-feeding.
Figure 4.
The expression of markers of inflammation and lipid metabolism in epididymal and inguinal WAT of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. qPCR was conducted to measure mRNA abundance of genes associated with inflammation and lipid metabolism in epididymal (EPI; visceral) (A, C) and inguinal (ING; subcutaneous) (B, D) WAT depots. Means ± SEM (n=9–10) without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
To determine if the reduction in adiposity by grape consumption was due to alterations in the expression of genes associated with fat synthesis or oxidation, epididymal and inguinal WAT were analyzed for mRNA markers of: (i) lipogenesis (i.e., Pparγ2, Srebp1c, Scd1, acylglycerol-3-phosphate-O-acyltransferase 2 (Agpat2), fatty acid synthase (Fas), acetyl-CoA carboxylase (Acc), perilipin 1 (Plin1), Gpat1); (ii) lipolysis (i.e., adipose TG lipase (Atgl); and (iii) beta-oxidation (i.e., Acox1, Cpt1b, Pparα). In epididymal WAT, the mRNA levels of the lipogenic genes Pparγ2, Agpat2, and Scd1 were higher in the HF-5S group compared to the LF group, and similar to the HF-grape groups (Fig. 4C). In inguinal WAT, the mRNA levels of the lipogenic genes Srebp1c (HF-3S), Scd1, Agpat2 (HF-5S), Fas (HF-5S), and Gpat2 (HF-5S) were higher in the HF-sugar controls compared to the LF-controls (Fig. 4D). Mice fed the HF-5G diets exhibited lower mRNA expression of Agpat2 compared to HF-5S controls. The mRNA levels of the fatty-acid oxidizing genes Pparα and Cpt1b, and the lipolytic gene Atgl (HF-3S) were lower in the HF-sugar controls compared to the LF control, and similar to the HF-grape groups (Fig. 4D). The mRNA levels of Plin1, Acc, Acox1, uncoupling protein1, or cytochrome8b in epididymal or inguinal WAT were not significantly impacted in mice fed the grape diets (data not shown).
3.5. Minimal influence of HF-feeding and grape consumption on markers of intestinal inflammation and barrier function
Given the reported adverse effects of consuming saturated fats [reviewed in 11], particularly from milk fat [10], on intestinal health, and potential prebiotic impact of grapes, we measured the effects of our diets on markers of intestinal inflammation and barrier function. For inflammatory status, mRNA expression of Cd11c, Erm1, Mcp1, Tnfα, Tlr4, and Il6 was quantified in ileal and distal colonic mucosa and the activities of duodenal alkaline phosphatase and ileal myeloperoxidase were measured. Surprisingly, the only proinflammatory gene increased in the ileal mucosa of HF-sugar-fed mice was Tnfα, which was similar to the HF-5G group (data not shown). None of the proinflammatory genes measured in the colonic mucosa where increased by HF-sugar feeding or grape consumption (data not shown). Similarly, the activities of alkaline phosphatase and myeloperoxidase, enzymes associated with increased levels of lipopolysaccharide and neutrophils, respectively, were not influenced by HF-sugar feeding or grape consumption (data not shown).
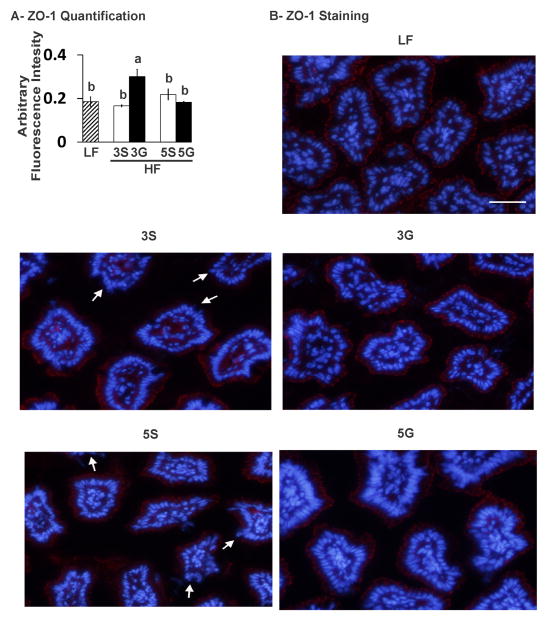
To assess intestinal barrier function, the mRNA levels of the tight junction proteins zonula occludens (Zo1), claudin-1, and occludin-1 and the localization of ZO-1 at the apical surface of the ileal epithelium were measured. Although the ileum mucosal gene expression of these tight junction proteins was not impacted by the diets (data not shown), the localization of ZO-1 was impaired in the HF-sugar control diets compared to the LF controls, and improved by grape feeding, particularly the HF-3G diet (Fig. 5).
Figure 5.
Ileal mucosa localization of the tight junction protein ZO-1 at the apical area of the ileum epithelium in C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. Localization of ZO-1 was visualized by immunostaining of ileum samples, and the staining was quantified (n=3–4). Arrows indicate impaired localization of ZO-1 at the apical area of the ileum epithelium. Means ± SEM without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
3.6. 16s rRNA sequencing of the gut microbiota reveals genera associated with body fat percentage and inguinal fat pad weight
Next, we measured the effects of our diets on the abundance of several mucosal sulfidogenic bacteria (i.e., DBB, DSB, DFM, DSV, and B. wadsworthia) or their gene products (i.e., B. wadsworthia specific dsrA-Bw, Fusobacterium nucleatum functional genes including two L-cysteine desulfohydrases (FN0625 and FN1220) cystathionine-β synthase (FN1055), and L-methionine-γ-lyase (FN1419)). Although HF-feeding did not increase the abundance of any of these genes associated with sulfur metabolism in ileal or colonic mucosa compared to LF control mice (data not shown), the abundance of DSB and dsrA-Bw was lower in ileal mucosa of mice consuming the HF-3G diet compared to mice consuming the HF-3S diet (Fig. 6A).
Figure 6.
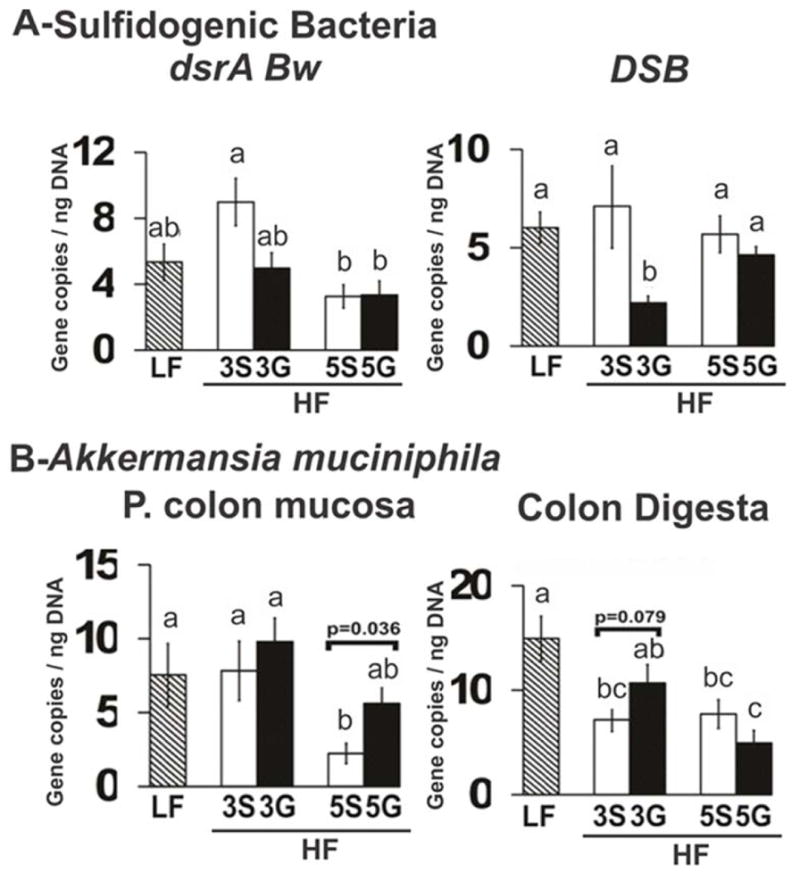
Abundance of sulfidogenic bacteria and A. muciniphilia in the intestinal mucosa or digesta of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. Abundance of B. wadsworthia-specific, functional gene target dissimilatory sulfate reductase (dsrA-Bw) and the targeted sulfidogenic bacterial genus Desulfobacter (DSB) species in the ileum mucosa (A). Abundance of A. muciniphilia in colonic mucosa and digesta and in cecal digesta (B). qPCR was conducted to measure abundance of 16S rRNA and functional genes. Means ± SEM (n=9–10) without a common lowercase letter differ (p<0.05) using one-way ANOVA and Student’s t test. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes. Means ± SEM (n=9–10) without a common lowercase letter differ (p<0.05).
We also measured the abundance of A. muciniphila, a mucin-degrading bacteria associated with prebiotic-mediated reduction in obesity [37]. Although not statistically significant, mice consuming grapes tended to have increased levels of A. muciniphila in the colonic digesta (HF-3G) and proximal colonic mucosa (HF-5G) compared to their HF-sugar controls (Fig. 6B).
3.7. Sequencing of the 16s rRNA gene reveals alterations in microbial structure and relationships to adiposity
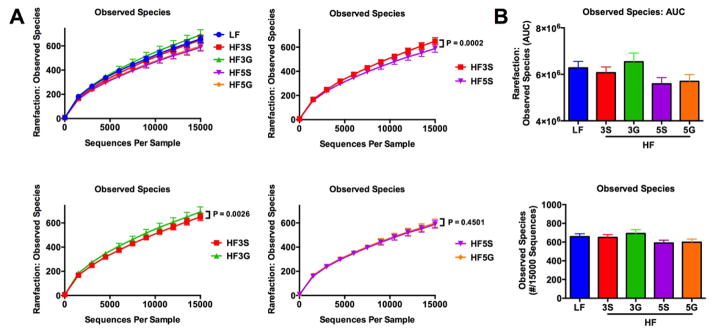
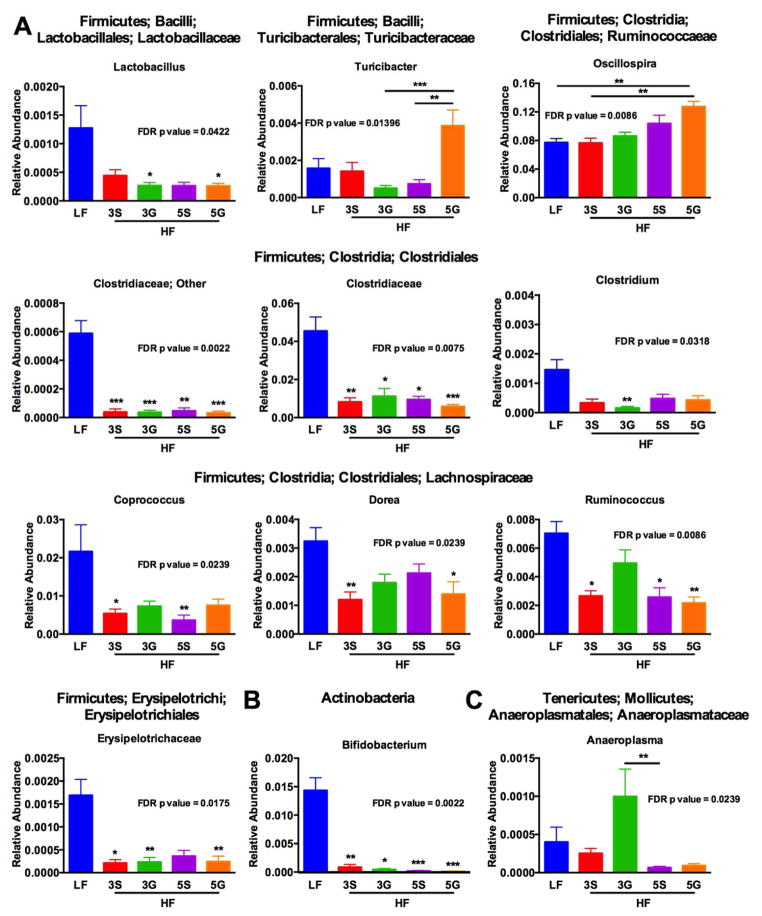
In order to better understand the impact of grape feeding on gut bacterial community structure, an untargeted approach was used by sequencing the 16s rRNA gene in cecal mucosal samples. Sequencing was performed on an Illumina MiSeq platform using primers targeting the V4–V5 region of the 16s rRNA gene. Data were analyzed using QIIME 1.8 Software [33]. Alpha Diversity analyses revealed that the HF-5G or HF-5S diets reduced observed species compared to HF-3G (p = 0.0002) or HF-3S diets (p< 0.0001), respectively (Fig. 7). Notably, the HF-3S diet reduced observed species compared to the HF-3G diet (p=0.0026), but there was no difference in observed species in HF-5G or HF-5S groups. These results suggest that elevated sugar content may result in decreased alpha diversity or membership of the gut microbiota. PCA of weighted UniFrac distances did not reveal obvious differences in beta diversity or community structure across diet groups, but there was clear separation between the LF group and HF groups based on unweighted UniFrac distances (Supplemental Fig. 1). To further interrogate differences between diet groups, multivariate statistical tests including ADONIS, ANOSIM, and PERMANOVA were conducted. Significant differences were found among unweighted and weighted UniFrac distances across diet groups (Table 2). Subsequently, OTUs that were not represented in up to 50% of the samples were removed to reduce noise from low abundance OTUs. Next, a Kruskal Wallis test was performed to determine significant differences in relative abundance of taxa between groups. Here, it was found that several bacterial taxa were significantly altered based on diet (Fig. 8). While many genera were reduced in the HF diets compared to the LF diet, some were selectively increased in HF-3G group such as Ruminoccocus and Anaeroplasma in the Firmicutes and Tenericutes phylum, respectively (Fig. 8).
Figure 7.
Observed bacterial taxa in cecal mucosa of C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. (A) Rarefaction curves of observed species are shown. Samples were rarified to 15,000 sequencing reads per sample. (B) Above - Area Under the Curve (AUC) of rarefaction curves shown for each diet group. Below – Observed species at 15,000 sequencing reads for each diet group. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes. Data are presented as means ± SEM (n=9–10) using one-way ANOVA and Dunn’s test for multiple comparisons.
Table 2.
Statistical Analyses of Unifrac Distances
| Weighted | R2 | P Value |
|---|---|---|
| Adonis | 0.12361 | 0.001 |
| Anosim | 0.3048 | 0.01 |
| Pseudo F Statistic | p value | |
| Permanova | 1.4105 | 0.001 |
| Unweighted | R2 | P Value |
| Adonis | 0.23825 | 0.001 |
| Anosim | 0.2609 | 0.01 |
| Pseudo F Statistic | p value | |
| Permanova | 3.1277 | 0.001 |
Figure 8.
Significantly altered relative abundances of microbial taxa (i.e., A- Firmicutes, B- Actinobacteria, and C- Tenericutes) found across C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. Taxa shown were significantly altered based on Kruskal Wallis test run using QIIME software following filtering of OTUs that were not present in 50% of samples. FDR corrected p values based on this analysis are shown. To conduct multiple comparisons relative abundances were analyzed via ANOVA followed by Dunn’s test for multiple comparisons. 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes. Data are presented as means ± SEM (n=9–10). Unless otherwise indicated, asterisks show significant differences of HF diets with or without grapes compared to LF control.
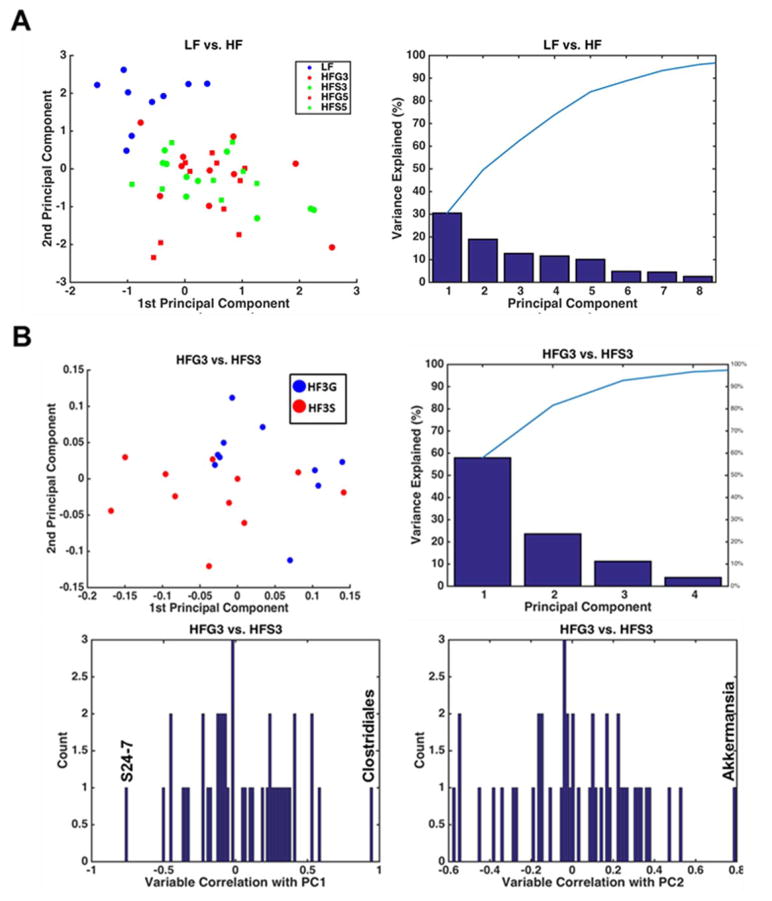
To assess differences between specific diet groups, PCA was conducted [38] and clear clustering was evident between the LF and HF groups (Fig. 9A) and between the HF-3G and HF-3S group (Fig. 9B). PCA between the LF and HF groups revealed that ~30% of the variance in relative bacterial abundance between the two groups is explained with Principal Component 1 (PC1). Principal component 2 (PC2) explained an additional 20% of the variance between groups and was most strongly correlated with Bacteroides and S24-7. PCA between the HF-3G and HF-3S groups revealed that ~60% of the variance in relative microbial abundance between the two groups was explained by PC1. PC1 was strongly correlated with S24-7 and Clostridiales (Fig. 9B). PC2 explained ~20% of the variance and exhibited a strong correlation with Akkermansia; however this was positively associated with the HF-3S group. These data indicate that most variance between the LF and HF-3G was explained by the abundance of Bacteroides in the LF group. Intriguingly, S24-7 also explained the variance between the HF-3G and HF-3S groups, as it was positively correlated with HF-3G compared to HF-3S. These data may indicate that S24-7 is associated with improved metabolic profile of the LF and HF-3G groups.
Figure 9.
The microbial relative abundances from C57BL/6J mice fed a low fat (LF) diet or high fat (HF), butter-rich diets with or without 3% or 5% powdered grapes for 10 weeks. (A) Top Left - PCA plot between low fat (LF) and HF + 3% grapes (HF3G) are shown. Top Right – Pareto plot showing percentage of variance explained by principal components. Below – Histogram showing individual genera that were negatively or positively correlated with LF and/or HF-3G diets. (B) Same as A, but showing PCA of HF-3G vs HF + 3% sugar (HF-3S) groups.
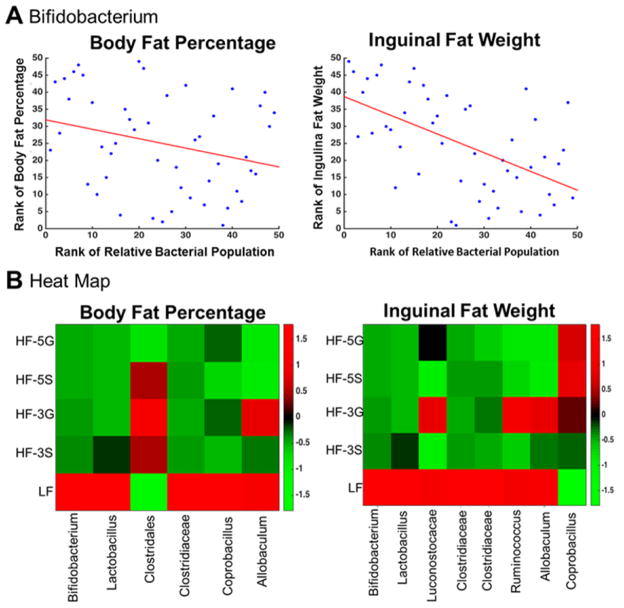
Lastly, Spearman Correlation analysis revealed significant negative correlations between body fat percentage and several genera (Fig. 10, Table 3) with the most profound being Bifidobacterium (p = 0.0001), a butyrate producer, and others including Lactobacillus (p = 0.0221) and Allobaculum (p = 0.034; Fig 10, Table 3). These particular genera were similarly correlated with inguinal fat pad weight. Clostridiales of the Firmicutes phylum was positively correlated with body fat percentage (p = 0.0065). Interestingly, a heatmap made from the taxa that were significantly correlated with body fat percentage, revealed that Allobaculum belonging to the Tenericutes phylum was increased in the LF and HF3G groups (Fig. 10). Little is known regarding the functional role of Allobaculum in the gut ecosystem and is currently being investigated for its potential association with a lean phenotype and improved metabolic health.
Figure 10.
(A) Bifidobacterium spp. relative abundance is negatively correlated with body fat percentage (r = −0.53, p = 0.001) and inguinal fat pad weight (r = −0.48, p = 0.004) as determined by Spearman Correlation Analysis. (B) Heatmap showing relative abundances of taxa that were significantly correlated with body fat percentage and inguinal fat pad weight. Increasing intensity of red color signifies higher abundance and increasing intensity of green color signifies lower relative abundance of taxa shown. LF, low fat; HF, high fat; 3S, HF diet containing 3% sugar; 3G, HF diet containing 3% grapes; 5S, HF diet containing 5% sugar; 5G, HF diet containing 5% grapes.
Table 3.
Taxa Associated with Body Fat
| Taxa Associated with Body Fat Percentage: (Phylum; Class; Order; Family; Genus; Species) | Correlation | P Value |
|---|---|---|
| Actinobacteria; Actinobacteria; Bifidobacteriales; Bifidobacteriaceae; Bifidobacterium | −0.53 | 0.0001 |
| Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus | −0.33 | 0.0221 |
| Firmicutes; Clostridia; Clostridiales; Other; Other | 0.39 | 0.0063 |
| Firmicutes; Clostridia; Clostridiales; Clostridiaceae; Other | −0.32 | 0.0243 |
| Firmicutes; Clostridia; Clostridiales; Lachnospiraceae; Coprococcus | −0.35 | 0.0147 |
| Firmicutes; Erysipelotrichi; Erysipelotrichales; Erysipelotrichaceae; Allobaculum | −0.30 | 0.0369 |
| Taxa Associated with Inguinal Fat Pad Weight: (Phylum; Class; Order; Family; Genus; Species) | Correlation | P Value |
| Actinobacteria; Actinobacteria; Bifidobacteriales; Bifidobacteriaceae; Bifidobacterium | −0.48 | 0.0004 |
| Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae; Lactobacillus | −0.39 | 0.0053 |
| Firmicutes; Bacilli; Lactobacillales; Leuconostocaceae; Leuconostoc | −0.29 | 0.0413 |
| Firmicutes; Clostridia; Clostridiales; Other; Other | 0.38 | 0.0065 |
| Firmicutes; Clostridia; Clostridiales; Clostridiaceae | −0.34 | 0.0177 |
| Firmicutes; Clostridia; Clostridiales; Lachnospiraceae; Ruminococcus | −0.35 | 0.0131 |
| Firmicutes; Erysipelotrichi; Erysipelotrichales; Erysipelotrichaceae; Allobaculum | −0.30 | 0.0391 |
| Firmicutes; Erysipelotrichi; Erysipelotrichales; Erysipelotrichaceae; Coprobacillus | 0.31 | 0.0297 |
| Tenericutes; Mollicutes; Anaeroplasmatales; Anaeroplasmataceae; Anaeroplasma | −0.30 | 0.0380 |
4. Discussion
Our data demonstrate that consuming table grapes (i.e., 3–5%, w/w; equivalent to 9–15 human servings) modestly, but significantly attenuates the accumulation of body and liver fat in mice fed a HF diet rich in butter compared to control mice. However, these lipid-lowering effects of grapes were not associated with significant improvements in glucose tolerance or markers of inflammation in intestinal mucosa or WAT. Notably, the impaired localization of the intestinal tight junction protein ZO-1 in HF-fed mice was improved by grape consumption. Abundance of Desulfobacter spp, and the B. wadsworthia-specific dissimilatory sulfite reductase gene were decreased by grape consumption (HF-3G), and the beneficial bacterium A. muciniphila tended to be in greater abundance in the colonic mucosa (HF-5G) or digesta (HF-3G) of grape fed mice compared to their respective controls. Additionally, via 16s rRNA sequencing analysis, significant differences across groups were observed in the relative abundance of S24-7 and Akkermansia, which were found to be negatively correlated with HF compared to the LF diet. Intriguingly, correlation analysis showed a strong negative correlation between Bifidobacterium and body fat percentage and inguinal fat pad weight. Also evident was an increase in Allobaculum in the LF and HF-3G groups. This bacterium has recently been associated with lean phenotypes in several reports [39–41]; however the mechanism establishing this link is unknown. Taken together, these data indicate that consumption of California table grapes moderately attenuates adiposity and steatosis that are positively correlated with a marker of intestinal integrity and changes in several species of gut microbes in mice fed a HF, butter-rich diet.
4.1. Influence of dietary polyphenols on adiposity, steatosis, and glucose tolerance
Many studies have demonstrated that consuming diets rich in calories from fat, particularly saturated fat, and sugars promote obesity and its metabolic complications [reviewed in 11]. The ability of foods rich in polyphenols, including grapes, to prevent obesity-mediated inflammation or related disorders has been demonstrated [reviewed in 25]. For example, grape seed procyanidin extract supplementation reduces body weight gain and WAT mass in hamsters fed a HF diet [42]. Additionally, mice fed a HF diet supplemented with muscadine grape phytochemicals rich in anthocyanins had decreased body weights, less lipid accumulation in the liver, and improved glucose tolerance compared to HF controls [43].
While several studies demonstrate anti-inflammatory and anti-obesity effects of grapes or their extracts, the mechanisms by which these effects occur are less clear. Anthocyanins are one of the most abundant phytochemicals in grapes [31] and therefore may be responsible for mediating the reductions in adiposity and steatosis. For example, anthocyanins purified from purple sweet potatoes attenuated hepatic lipid accumulation via the activation of adenosine monophosphate-activated protein kinase and decreased expression of SREBP1 and its downstream target genes in mice fed a HF diet [44]. In the present study, powdered grape supplementation at 3% and 5% modestly reduced body weight and fat gain and hepatic lipid accumulation, but surprisingly did not significantly affect the expression of Srebp1c and Fas. Although we discovered that the expression of several hepatic genes associated with lipogenesis (e.g., Pparγ, Scd1, Fabp4, Gpat1) in liver were dampened in mice consuming the HF-3G compared to the HF-3S controls, the amount of protein encoded by these genes was not lowered by grape feeding. However, we did not measure the enzymatic activity of these proteins, and thus do not know if they were impacted by grape consumption.
4.2. Relationship between dietary fat, sulfite-reducing bacteria, barrier function, and inflammation
Devkota et al. [10] demonstrated that feeding a milk-fat-based diet similar in amount and composition to the western diet (i.e., 37% kcals from fat) to IL-10 knockout mice decreased gut barrier-protecting bacteria and increased the sulfite-reducing bacterium B. Wadsworthia and intestinal markers of inflammation consistent with inflammatory bowel disease [10]. This outcome was associated with a pro-inflammatory T helper type immune response and increased colitis. Notably, taurine-conjugated bile salts caused these inflammatory responses, in part, due to their high sulfur content, which stimulates sulfite-reducing bacteria like B. wadsworthia. In a parallel 3 week study, these authors fed C57BL/6J mice the same HF diet [10]. Consistent with the IL-10 knockout mice, C57BL/6J mice fed the milk fat diet exhibited increased abundance of the sulfidogenic bacterium B. wadsworthia and the B. wadsworthia-specific dissimilatory sulfite reductase gene dsrA-Bw. Milk fat-fed C57BL/6J mice also had a higher abundance of Bacteriodetes and a lower abundance of Firmicutes compared to the LF-fed mice [10]. However, milk fat-fed C57BL/6J mice did not present overt colitis as did the IL-10 knockout mice [10]. In the present study, we did not observe any increases in the abundance of sulfidogenic bacteria-related genes in the HF-fed controls compared to the LF-fed mice, although dsrA-Bw and DSB were present in lower abundance in the ileum mucosa of HF-3G mice compared to the HF-3S control group. This decreased abundance of sulfidogenic bacteria correlated with improved localization of the ileal tight junction protein ZO-1. Although markers of intestinal inflammation were not significantly increased by the milk fat-based diet or decreased by grape feeding, the expression levels of several inflammatory genes in visceral epididymal WAT (i.e., Tnfα, Cd11c, Mcp1) and subcutaneous inguinal WAT (i.e., Mcp1) were increased by HF feeding (Fig. 4A), and HF-5G consumption tempered Mcp1 expression in inguinal WAT compared to its control HF-5S.
These results are comparable to data showing that HF-feeding selectively increases the abundance of a specific type of bacteria that are associated with intestinal inflammation [45]. Apolipoprotein A-I (apoA-I) knockout mice, which present impaired glucose tolerance and increased adiposity, and wild type mice were fed a very HF diet (i.e., ~60% kcals from lard) for 25 weeks. HF-fed mice had increased abundance of Desulfovibrionaceae, a family of sulfate/sulfite reducing bacteria that produce hydrogen sulfide, a genotoxic gas that causes barrier dysfunction and endotoxemia [46]. Consistent with these data, we previously showed that C57BL/6J mice fed a very HF diet (i.e., ~60% kcals from lard) for 20 weeks had increased levels of three types of sulfidogenic bacteria in colonic mucosa, impaired localization of ZO-1, and increased mRNA levels of markers of macrophage infiltration in intestinal mucosa and WAT compared to LF-fed mice [32]. Collectively, these data demonstrate that consuming diets enriched with lard or butter, two fat sources containing high levels of saturated fatty acids, increases the abundance of sulfidogenic bacteria associated with impairment of intestinal barrier function and contribute to systemic inflammation.
4.2. Relationship between dietary fat, A. muciniphila, barrier function, and inflammation
A. muciniphila is a commensal, mucin-degrading bacterium that plays a role in preventing the development of diet-induced obesity [37]. Under normal conditions, these bacteria represent 3–5% of the gut microbial population in humans. However, HF-feeding reduces the colonic abundance of these bacteria in mice 100-fold, and oligofructose prebiotic treatment prevents this loss [37]. Oligofructose-mediated increase in A. muciniphila was linked to decreased metabolic endotoxemia and markers of inflammation in WAT. Furthermore, supplementation of C57BL/6J mice fed a HF diet with A. muciniphila for 4 weeks lead to a decrease in adiposity, gut barrier dysfunction, metabolic endotoxemia, glucose intolerance, insulin resistance, and Cd11c expression in WAT, and improved mucus thickness along the epithelium compared to control mice [37]. Consistent with these data demonstrating that oligofructose prebiotic treatment attenuates a HF diet-induced reduction of A. muciniphila, our data indicate that supplementing HF butter-rich diets with 5% California table grapes modestly increases the abundance of A. muciniphila in proximal colon mucosa compared to the HF-sugar controls. This increase in A. muciniphila was positively associated with improved localization of ZO-1 in the apical area of the ileal epithelium compared to HF control mice. Furthermore, based on our 16S rRNA sequencing data of the cecal mucosa, abundance of the genus Akkermansia was positively associated with mice fed a LF diet, further supporting a relationship with a lean phenotype.
4.4. Limitations and unanswered questions
The current study did not achieve the anticipated increases in intestinal and systemic inflammation in young C57Bl/6J mice fed a HF diet rich in milk-fat as demonstrated in IL-10 knockout mice fed milk-fat [10] or in apo A-I knockout mice fed lard [44]. Perhaps feeding mice extremely HF diets (e.g., 60% kcals from lard) is necessary to instigate intestinal and systemic inflammation in relatively short term studies [11, 30]. The current study sought to provide a more physiological approach to achieve diet-induced obesity accompanied by systemic and intestinal inflammation. However, the use of this more physiological model (i.e., an amount of fat and sugar calories similar to the average American diet; 34% and 16%, respectively) for the time span of 11 weeks may not have been aggressive enough in this mouse model to elicit a robust inflammatory response; especially because mice were started on the diet at a relatively young age (e.g., 5-weeks of age). Moreover, there were differential effects between the two grape diets, depending on the outcome measured, that lack explanation by the authors. Furthermore, we do not know which compound(s) in table grapes reduced adiposity and steatosis. Therefore, future studies should examine the effect of different grape fractions (e.g., phytochemical, fiber, or sugar-rich fraction) or specific polyphenols on the development of obesity, steatosis, and inflammation using a fat composition that is more typical of the average American diet (i.e., a mixture of animal and vegetable fats consisting of beef tallow, lard, milk fat, shortening, and vegetable oils [47]), instead of mostly butter fat.
Supplementary Material
Acknowledgments
This work was supported by a grant from the California Table Grape Commission to H.R.G. and M.K.M. Lyophilized grape powder was kindly provided to M.K.M. by the California Table Grape Commission.
Abbreviations
- Acox
acyl-CoA oxidase
- Agpat
acylglycerol-3-phosphate-O-acyltransferase
- aP2
adipose fatty acid binding protein
- Atgl
adipose triglyceride lipase
- AUC
area under the curve
- B. wadsworthia
Bilophila wadsworthia
- Cd36
cluster of differentiation 36
- Cd11c
cluster of differentiation 11c
- CPT
carnitine palmitoyltransferase
- DEXA
dual-energy X-ray
- DBB
Desulfobulbus spp
- DFM
Desulfotomaculum spp
- DSB
Desulfobacter spp
- dsrA-Bw
B. wadsworthia specific dissimilatory sulfite reductase
- DSV
Desulfovibrio spp
- EPI
epididymal
- Erm1 (F4/80 human orthologue)
epidermal growth factor-like module containing mucin-like hormone receptor 1
- Fas
fatty acid synthase
- Fabp4
fatty acid binding protein 4
- Gpat
glycerol-3-phosphate acyltransferase
- GTT
glucose tolerance test
- HF
high fat
- HOMA-IR
homeostasis model assessment method for insulin resistance
- ING
inguinal
- LDL
low density lipoprotein
- IL
interleukin
- LF
low fat
- MCP
monocyte chemoattractant protein
- NF-κB
nuclear factor kappa B
- OTU
operational taxonomic units
- PCA
primary component analysis
- PPAR
peroxisome proliferator activated receptor
- QIIME
quantitative insights into microbial ecology
- Scd1
stearoyl-CoA desaturase 1
- Srebp
sterol regulatory element binding protein
- Tbp
TATA-binding protein (TBP)
- TG
triglyceride
- Tlr4
toll-like receptor 4
- Tnf
tumor necrosis factor
- WAT
white adipose tissue
- ZO-1
zonula occludens
Appendix A. Supplementary data
Supplementary data (1 table, 1 color figure) to this article can be found online.
Footnotes
No conflicts of interest: Jessie Baldwin, Brian Collins, Patricia Wolf, Kristina Martinez, Wan Shen, Chia-Chi Chuang, Wei Zhong, Paula Cooney, H. Rex Gaskins, Chase Cockrell, Eugene Chang, and Michael McIntosh
References
- 1.Ogdan CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Obesity. http://www.who.int/topics/obesity/en/. Viewed. Jan. 20, 2015.
- 3.NIH. Obesity. http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0004552/. Viewed Jan. 20, 2015.
- 4.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18(10):1918–1925. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding S, Chi M, Scull M, Rigby R, Schwerbrock N, Magness S, Jobin C, Lund P. High fat diet: bacterial interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mice. PLOS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131(1):33. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani P, Amar J, Iglesias M, Poggi M, Knauf C, Bastelica D, Neyrinck N, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 8.Cani P, Bibiloni R, Knauf C, Waget A, Neyrinck A, Delzenne N, Barcelona R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 9.Delzenne N, Cani P. Gut microbiota and pathogenesis of insulin resistance. Cur Diab Rep. 2011;11:154–159. doi: 10.1007/s11892-011-0191-1. [DOI] [PubMed] [Google Scholar]
- 10.Devkota S, Wang Y, Musch M, Leone V, Fehlner-Peach Hl, Nadimpalli A, Antonopoulos D, Jabra B, Chang E. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen W, Gaskins HR, McIntosh M. Influence of dietary fat on intestinal microbes, inflammation, barrier function, and metabolic outcomes. J Nutr Biochem. 2014;25:270–280. doi: 10.1016/j.jnutbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics, and diet relevant to development of metabolic syndromes in mice. The ISME Journal. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 13.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowan FE, Docherty NG, Coffey JC, O’Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. Br J Surg. 2009;96(2):151–158. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- 15.Backhed F, Manchester J, Semenkovich C, Gordon J. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Nat Acad Sci. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delzenne N, Neyrinck A, Backhed F, Cani P. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nature Reviews Endocrin. 2011;7:639–646. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 17.Delzenne N, Cani P. Interaction between obesity and gut microbiota: relevance to nutrition. Ann Rev Nutr. 2011b;31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 18.Delzenne N, Neyrinck A, Cani P. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microbial Cell Factories. 2011;10:S10. doi: 10.1186/1475-2859-10-S1-S10. http://microbialcellfactories.com/content/10/S1/S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cani P, Neyrinck N, Fava F, Knauf C, Burcelin R, Tuophy K, et al. Selective increases of bifidobacteria in the gut microbiota improve high-fat-diet induced diabetes in mice through a mechanisms involving endotoxemia. Diabetolgia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 20.Keperman R, Bolca S, Roger L, Vaughan E. Novel approaches for analyzing gut microbes and dietary polyphenols: challenges and opportunities. Microbiology. 2010;156:3224–3231. doi: 10.1099/mic.0.042127-0. [DOI] [PubMed] [Google Scholar]
- 21.Neyrinck A, Van Hee V, Bindels L, Possemiers S, de Backer F, Cani P, et al. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolemia in high-fat-induced obese mice: potential implication of the gut microbiota. Br J Nutr. 2013;109:802–809. doi: 10.1017/S0007114512002206. [DOI] [PubMed] [Google Scholar]
- 22.Benn T, Kim B, Park YK, Wegner C, Harness E, Nam TG, Kim DO, Lee J, Lee JY. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J Nutr Biochem. 2014;25:1019–1025. doi: 10.1016/j.jnutbio.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Van Duynhoven J, Vaughan E, Jacobs D, Kemperman R, van Velzen E, Gross G, Roger L, Possemiers S, Smilde A, Dore J, Westerhuis J, Van de Wiele T. Metabolic fate of polyphenols in the human superorganism. Proc Nat Acad Sci. 2011;108:4531–4538. doi: 10.1073/pnas.1000098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selma M, Espin J, Tomas-Barberan F. Interactions between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 25.Chuang C, McIntosh M. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Ann Rev Nutr. 2011;31:155–176. doi: 10.1146/annurev-nutr-072610-145149. [DOI] [PubMed] [Google Scholar]
- 26.Seymour E, Singer A, Bennink M, Parikh R, Kirakosyan A, Kaufman P, Broiling S. Chronic intake of phytochemical-enriched diet reduces cardiac fibrosis and diastolic dysfunction caused by prolonged salt-sensitive hypertension. J Gerontol A Biol Sci Med Sci. 2008;63:1034–1042. doi: 10.1093/gerona/63.10.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour EM, Bennink MR, Watts SW, Bolling SF. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappaB activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55:1179–85. doi: 10.1161/HYPERTENSIONAHA.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuhrman B, Volkova N, Coleman R, Aviram M. Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient mice and reduce macrophage atherogenicity. J Nutr. 2005;135:722–728. doi: 10.1093/jn/135.4.722. [DOI] [PubMed] [Google Scholar]
- 29.Zern T, Wood R, Greene C, West K, et al. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–1917. doi: 10.1093/jn/135.8.1911. [DOI] [PubMed] [Google Scholar]
- 30.Chuang CC, Shen W, Chen H, Xie G, Jia W, Chung S, McIntosh M. Differential effects of grape powder and its extract on glucose tolerance and chronic inflammation in high fat-fed obese mice. J Agric Food Chem. 2012;60:12458–12468. doi: 10.1021/jf3028107. [DOI] [PubMed] [Google Scholar]
- 31.Shen W, Chuang CC, Martinez K, Reid T, Brown JM, Xi L, Hixson L, Hopkins R, Starnes J, McIntosh M. Conjugated Linoleic Acid Reduces Adiposity and Increases Markers of Browning and Inflammation in White Adipose Tissue of Mice. J Lipid Research. 2013;54:909–922. doi: 10.1194/jlr.M030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen W, Wolf PG, Carbonero F, Zhong W, Reid T, Gaskins HR, McIntosh MK. Intestinal and systemic inflammatory responses are positively associated with sulfidogenic bacteria abundance in high-fat-fed male C57BL/6J mice. J Nutr. 2014;144:1181–87. doi: 10.3945/jn.114.194332. [DOI] [PubMed] [Google Scholar]
- 33.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hogenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME J. 2012;6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price MN, Dehal PS, Arkin AP. FastTree 2 – Approximately Maximum-Likelihood Trees for Large Alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Nat Acad Sci. 2013;110(20):9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filtzmoser P, Hron K, Reimann C. Principal component analysis for compositional data with outliers. Environmetrics. 2009;20(6):621–632. [Google Scholar]
- 39.Zhang C, Li S, Yang L, Huang P, Li W, Wang S, Zhao G, Zhang M, Pang X, Yan Z, Liu Y, Zhao L. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nature Communications. 2013 doi: 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17(1):141–52. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. The ISME J. 2014;8(10):2116–30. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camari A, del Bas JM, Crescenti A, Arola L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes (Lond) 2013;37(4):576–83. doi: 10.1038/ijo.2012.75. [DOI] [PubMed] [Google Scholar]
- 43.Gourineni V, Shay NF, Chung S, Sandhu AK, Gu L. Muscadine Grape (Vitis rotundifolia) and Wine Phytochemicals Prevented Obesity-Associated Metabolic Complications in C57BL/6J Mice. J Agric Food Chem. 2012;60(31):7674–81. doi: 10.1021/jf3013663. [DOI] [PubMed] [Google Scholar]
- 44.Hwang YP, Choi JH, Han EH, Kim HG, Wee JH, Jung KO, Jung KH, Kwon KI, Jeong TC, Chung YC, Jeong HG. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr Res. 2011;31(12):896–906. doi: 10.1016/j.nutres.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics, and diet relevant to development of metabolic syndromes in mice. The ISME Journal. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 46.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Frontiers Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells HF, Buzby JC. Dietary Assessment of Major Trends in U.S. Food Consumption, 1970–2005. US Department of Agriculture and Economic Resources. 2008:10–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.