Abstract
Purpose
To characterize the patterns of medication intake in healthy, reproductive-age women not using hormonal contraception.
Methods
259 healthy, premenopausal women (18–44 years of age) enrolled in the BioCycle Study (2005–2007), were followed over two menstrual cycles. Women were excluded if they were currently using oral contraceptives or other chronic medications. Over-the-counter and prescription medication use among participants was evaluated daily throughout the study via a diary assessing type of medication, dosage, units, and frequency. Medications were categorized as allergy, antibiotics, central nervous system (CNS), cold and cough, gastrointestinal (GI), musculoskeletal, and pain medication based on primary active ingredient. Medication use within each category was assessed across standardized 28-day cycles to evaluate differences in use across cycle phases (i.e. early, mid, late).
Results
Medication use was reported by 73% of participants. The most and least frequently used medications, respectively, were pain (69%) and musculoskeletal medications (1%). Pain, CNS, and antibiotic medication use varied significantly across the cycle, with pain and CNS medication more frequently reported during menses and antibiotics more frequently during the luteal phase. Allergy, cold and cough, GI, and musculoskeletal medication use did not vary across the cycle.
Conclusions
Patterns of medication use among reproductive-age women vary across the menstrual cycle for certain types of medications, particularly in pain (e.g. Ibuprofen), antibiotics (e,g, Amoxicillin), and CNS (e.g. Adderall) medications. Future studies involving use of these types of medication in premenopausal women may need to consider the relationship of their use to the menstrual cycle.
Keywords: medication use, over-the-counter, prescription, menstrual cycle
INTRODUCTION
More than 80% of the U.S. adult population report taking at least one medication in a week, and 25% report five or more.1 Furthermore, 82% of women aged 18–44 report using medication compared to 68% of men in the same age group, identifying a significant gender difference in prevalence of medication use.1 Other studies also report higher medication use in women than men and indicate a higher analgesic drug use among young women between 20 and 29 years attributable to use for menstrual discomfort.2 Indeed, our previous work reveals greater use of pain medication in reproductive aged women around the time of menses when compared to other phases of the menstrual cycle.3
Around half of pregnancies in the U.S. are not specifically planned, and accordingly, many public health recommendations apply to all women of reproductive age (e.g. quitting smoking and using folic acid supplements) to ensure sustained reproductive health for all women and their children.4,5 However, there is very little data available regarding patterns of daily medication use across the menstrual cycle in healthy, reproductive age women, particularly in women who are not actively attempting pregnancy but who are at risk of becoming pregnant because of lack of contraception use and variable sexual activity. Furthermore, recent changes (effective June 2015) in FDA regulation of medication labeling has eradicated pregnancy categories A, B, C, D, and X from all human prescription drug and biological product labeling, and also added a labeling category “Females and Males of Reproductive Potential” to address pregnancy testing, contraception, and infertility.6 These changes reflect both the complexity of the limited available information to clearly delineate potential medication risks in pregnancy to mother and fetus, as well as a realization of the importance of women who may become pregnant, but may not be planning a pregnancy. This study is the first study to thoroughly assess detailed daily capture of medication use over two menstrual cycles among women of reproductive age not using hormonal contraception. Characterizing patterns of medication use in reproductive age women may give women’s health providers insight into typical behaviors to ultimately improve women’s overall reproductive health.7,8 Therefore, the purpose of this study was to describe both prevalence and patterns of medication use including pain, CNS, cold and cough, GI, allergy, antibiotics, and musculoskeletal medications, in relation to the menstrual cycle among 259 healthy, reproductive age women not consuming chronic medications, including oral contraception, but who are not actively trying to become pregnant.
MATERIALS AND METHODS
Participants and study design
The BioCycle Study included 259 healthy, reproductive age women between 18 and 44 years from the western New York state region. Data were collected at the University at Buffalo in New York. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent. Details of study design, materials, and methods of the BioCycle study are reported elsewhere.9
Briefly, women with a self-reported body mass index (BMI) between 18 and 35 kg/m2 at baseline were enrolled and followed prospectively between 2005 and 2007 for one (n=9) or two (n=250) complete menstrual cycles.9 Cycles were captured consecutively, except for in 27 women who completed two non-consecutive cycles. Exclusion criteria included current use of oral contraceptive or other hormone supplement use in the past three months; hormonal contraceptive medication (i.e., Depo-Provera, Norplant, intrauterine device (IUD)) in the past 12 months; unwillingness to stop regular intake of vitamin and/or mineral supplements, or chronic use of certain medications including lipid lowering drugs, anti-hypertensive medications and/or aspirin; pregnancy or breastfeeding in the past six months; attempting to conceive in the past six months; or recent history of infections or diagnosis of chronic conditions, including menstrual or ovulatory disorders. Specifically, laparoscopy-confirmed endometriosis, current uterine fibroids or removal of a fibroid in the last 12 months, history of polycystic ovary syndrome, and women who, at any point in time, sought treatment for infertility were excluded.
Data collection
Participants provided fasting blood and urine samples up to 8 times for each menstrual cycle (all participants completed at least 5 clinic visits/cycle; 94% completed at least 7 visits/cycle). Study visits were scheduled to correspond to specific phases of the menstrual cycle using fertility monitors (Clearblue Easy Fertility Monitor, Inverness Medical, Waltham, MA, USA),10,11 including early menstruation, mid follicular phase, 3 days around expected ovulation (LH surge), and the early, mid and late luteal phase.
In addition to high compliance (94% completed at least seven in-person visits per cycle), all participants completed a daily diary, which recorded medication intake. Participants’ diaries were reviewed with study staff at each clinic visit, which included daily medication usage by type, dose, units, and number of times per day. Diary compliance was high with 89% of the participants completing ≥75% of their daily diaries. Medication was grouped into seven major categories based on indication: allergy (e.g. Zyrtec), antibiotics (e.g. Amoxicillin), CNS (e.g. Adderall), cold and cough, GI (e.g. Ciprofloxacin), musculoskeletal (e.g. Cyclobenzaprine), and pain (e.g. Ibuprofen). There were a total of 157 medication codes listed on the daily diaries, of which 96 could be categorized with the information provided into the seven categories presented here. Seventy six percent of all medication used on all days of the study included the 96 medication codes, whereas the remaining 24% of uncoded entries entailed predominantly vitamin/supplement use (of which regular use was a study exclusion criterion at enrollment), topical products (e.g. acne, wrinkle products), and various other categories of sporadic medication or other pharmaceutical product use not fitting into the seven categories presented in the current study (e.g. oral, topical, or inhaled corticosteroids; vaccinations; etc.). The Micromedex online database and manufacturer’s websites were used to identify each drug used by participants during the study. Combination drugs were assigned to a category based on the principal ingredient in the product.
Participants self-reported age, age at menarche, race, education, and smoking status through standard questionnaires at baseline, in addition to the Cohen Perceived Stress Scale (PSS) and the International Physical Activity Questionnaire.9,12,13 Information about alcohol consumption was obtained from the daily diaries of participants, averaged over the entire study, and categorized into low (0–0.5 drinks/day), moderate (0.5–1 drinks/day), or high (≥ 1 drinks/day) intake. Standing height and body weight were also measured at the beginning of the study by trained staff using standardized protocols to calculate BMI.
Statistical analysis
To evaluate patterns of medication use across the menstrual cycle, each cycle was standardized to 28 days: days were aligned in relation to the day of ovulation, which was estimated based on dates and levels of LH peak from the fertility monitor compared with the observed LH maximum value in serum and the day of progesterone rise.14 Participant-days consumed included the total number of days participants consumed a type of medication throughout the study. Descriptive statistics (Chi-square or Fisher exact tests for categorical variables and Student t test for continuous variables) were used to compare participant characteristics between the users and nonusers of medication overall and within the seven categories of medication across the study period of two cycles.
Average frequency of each category of medication use was calculated and compared between early cycle (standardized Day 1 [start of menses] to standardized Day 5 [average bleeding length]), which included menses), mid cycle (standardized Day 6 to standardized Day 13 [day of LH surge]) and late cycle (standardized day 14 [estimated day of ovulation] to standardized Day 28 [day prior to start of next menses]), as well as across seasons, using linear mixed models to account for repeated cycles within women. Pair-wise comparisons were made between early, mid, and late cycle phases using the Tukey method to account for multiple comparisons. Data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Participants’ characteristics overall and by type of medication use
The baseline characteristics of participants overall and within each of the seven categories of medication are shown in Table 1. Women who participated in the study were young (mean=27.3, SD=8.2), predominately white (n=154, 60%), educated (n=226, ~90% > high school education) and nonsmokers (n=249, 96%), with a mean (SD) BMI of 24.1 kg/m2 (3.9). Current age, age at menarche and BMI were not significantly different between users and nonusers of any medication type. Users of allergy, as well as cold & cough, medications were more frequently white and reported lower alcohol consumption compared to nonusers. Pain medication users were also more often white, but reported greater alcohol consumption than nonusers. Users of antibiotics had less post-secondary education, and women who reported using CNS medication were more likely to report high alcohol consumption. Lastly, women using GI medication were more likely to be smokers and have higher alcohol consumption. Few women used musculoskeletal medication (n=3) with no differences detected in characteristics of users compared to nonusers (data not shown).
Table 1.
Participant characteristics based on medication type used
| Total | Allergy | Antibiotic | CNS | Cold & Cough | GI | Pain | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Users | Non- users |
Users | Non- users |
Users | Non- users |
Users | Non- users |
Users | Non- users |
Users | Non- users |
||
| Number of women (%) |
259 | 32 (12) | 227 (88) | 18 (7) | 241 (93) | 8 (3) | 251 (97) | 37 (14) | 222 (86) | 21 (8) | 238 (92) | 179 (69) | 80 (31) |
| Age, yrs | 27.3 ± 8.2 |
29.6 ± 8.3 |
27.0 ± 8.3 |
28.8 ± 9.4 |
27.2 ± 8.1 |
29.6 ± 9.1 |
27.2 ± 8.2 |
27.1 ± 8.3 |
27.0 ± 8.2 |
28.4 ± 7.7 |
27.2 ± 8.3 |
27.8 ± 8.2 |
26.1 ± 8.1 |
| BMI, kg/m2 | 24.1 ± 3.9 |
24.7 ± 3.4 |
24.0 ± 3.9 |
24.0 ± 3.5 |
24.1 ± 3.9 |
23.5 ± 2.3 |
24.1 ± 3.9 |
24 ± 3.8 |
24.1 ± 3.9 |
25.7 ± 4.2 |
23.9 ± 3.8 |
24.4 ± 3.7 |
23.4 ± 4.1 |
| Age at menarche, yrs |
12.5 ± 1.2 |
12.3 ± 1.0 |
12.5 ± 1.3 |
12.6 ± 1.2 |
12.4 ± 1.2 |
12.5 ± 0.9 |
12.5 ± 1.3 |
12.4 ± 1.3 |
12.4 ± 1.3 |
12.5 ± 1.2 |
12.5 ± 1.3 |
12.4 ± 1.2 |
12.6 ± 1.4 |
| Race | |||||||||||||
| White | 154 (59) | 26 (81)* | 128 (56) | 12 (67) | 142 (59) | 5 (63) | 149 (59) | 29 (78)* | 125 (56) | 14 (67) | 140 (59) | 117 (65)* |
37 (46) |
| Black | 51 (20) | 5 (16) | 46 (20) | 4 (22) | 47 (20) | 2 (25) | 49 (20) | 6 (16) | 45 (20) | 5 (24) | 46 (19) | 30 (17) | 21 (26) |
| Other | 54 (21) | 1 (3) | 53 (23) | 2 (11) | 52 (22) | 1 (13) | 53 (21) | 2 (5) | 52 (23) | 2 (10) | 52 (22) | 32 (18) | 22 (28) |
| Post- secondary education |
226 (87) | 28 (88) | 198 (87) | 13 (72)* | 213 (88) | 7 (88) | 219 (87) | 35 (95) | 191 (86) | 18 (86) | 208 (87) | 156 (87) | 70 (88) |
| Nonsmoker | 249 (96) | 30 (94) | 219 (96) | 17 (94) | 232 (96) | 8 (100) | 241 (96) | 35 (95) | 214 (96) | 18 (86)* | 231 (97) | 173 (97) | 76 (95) |
| Current smoker |
10 (4) | 2 (6) | 8 (4) | 1 (6) | 9 (4) | 0 | 10 (4) | 2 (5) | 8 (4) | 3 (14) | 7 (3) | 6 (3) | 4 (5) |
| Physical activity |
|||||||||||||
| Low | 25 (10) | 3 (9) | 22 (10) | 1 (6) | 24 (10) | 0 | 25 (10) | 4 (11) | 21 (9) | 3 (14) | 22 (9) | 16 (9) | 9 (11) |
| Moderate | 92 (36) | 15 (47) | 77 (34) | 8 (44) | 84 (35) | 3 (38) | 89 (35) | 12 (32) | 80 (36) | 8 (38) | 84 (35) | 66 (37) | 26 (33) |
| High | 142 (55) | 14 (44) | 128 (56) | 9 (50) | 133 (55) | 5 (63) | 137 (55) | 21 (57) | 121 (55) | 10 (48) | 132 (55) | 97 (54) | 45 (56) |
| Alcohol consumption a |
|||||||||||||
| Low | 191 (74) | 21 (66)* | 170 (75) | 11 (61) | 180 (75) | 3 (38) | 188 (75) | 21 (57)* | 170 (77) | 7 (33) | 184 (77) | 122 (68)* |
69 (86) |
| Moderate | 34 (13) | 2 (6) | 32 (14) | 2 (11) | 32 (13) | 0 | 34 (14) | 8 (22) | 26 (12) | 6 (29) | 28 (12) | 27 (15) | 7 (9) |
| High | 34 (13) | 9 (28) | 25 (11) | 5 (28) | 29 (12) | 5 (63)* | 29 (12) | 8 (22) | 26 (12) | 8 (38)* | 26 (11) | 30 (17) | 4 (5) |
| Perceived Stressb |
|||||||||||||
| Low | 85 (33) | 7 (22) | 78 (34) | 8 (44) | 77 (32) | 3 (38) | 82 (33) | 10 (27) | 75 (34) | 9 (43) | 76 (32) | 54 (30) | 31 (39) |
| Moderate | 82 (32) | 13 (41) | 69 (30) | 2 (11) | 80 (33) | 3 (38) | 79 (31) | 12 (32) | 70 (32) | 5 (24) | 77 (32) | 66 (37)* | 16 (20) |
| High | 92 (36) | 12 (38) | 80 (35) | 8 (44) | 84 (35) | 2 (25) | 90 (36) | 15 (41) | 77 (35) | 7 (33) | 85 (36) | 59 (33) | 33 (41) |
Data are means ± SD or n (%).
Significantly different from non-user within same medication category (P<0.05).
Alcohol levels: low (≤ 0.5 drinks/day), moderate (0.5 to 1 drinks/day), high (≥ 1 drinks/day)
Stress levels were obtained from Perceived Stress Scale (PSS) and categorized into tertiles (low, moderate, and high stress).
CNS, central nervous system; GI, gastrointestinal
Medication use frequency by category
A total of 188 (73%) women reported any medication use. The most frequently used medication was pain medication with 68% of the participants (1100 participant-days consumed) reporting use of some type of pain medication, as previously reported.3 Cold and cough medications were the next most frequently used (14% of women, 112 participant-days consumed), followed by allergy (12%, 123 participant-days consumed), GI (8%, 50 participant-days consumed), antibiotics (7%, 111 participant-days consumed) and CNS (3%, 24 participant-days consumed). The least frequently used was musculoskeletal medication, which was used by only three participants for a total of ten person days. Use of certain medications, including pain, cold and cough, allergy, GI, and CNS varied across seasons. Of note, 43% of allergy medication use occurred during fall and 67% of cough & cold medication use was during fall and winter seasons (Table 2).
Table 2.
Frequency of medication use among consumers within each category
| Medication Categories |
Days consumed n (%)* |
Number of users n (%) |
Number of days medication used mean ± SD (range) |
Number of days medication used median (IQR) |
Days consumed by season Spring/Summer/ Fall/Winter N |
Days consumed by season Spring/Summer/ Fall/Winter % |
|---|---|---|---|---|---|---|
| Allergy | 127 (0.87) | 32 (12) | 4 ± 6.6 (1, 30) | 2 (1, 3) | 22/37/53/15 | 17/29/42/12* |
| Antibiotics | 111 (0.76) | 18 (7.0) | 6.2 ± 2.5 (2, 12) | 6 (5, 8) | 17/34/26/34 | 15/31/23/31 |
| CNS | 24 (0.16) | 8 (3.0) | 3 ± 3.3 (1, 11) | 2 (1, 3) | 12/3/7/2 | 50/13/29/8* |
| Cold and Cough | 114 (0.78) | 37 (14) | 3.1 ± 2.5 (1, 11) | 2 (1, 5) | 20/19/36/39 | 17/17/32/34* |
| GI | 51 (0.35) | 21 (8) | 2.4 ± 2.3 (1, 11) | 1 (1, 3) | 17/4/14/16 | 33/8/27/32* |
| Musculoskeletal | 10 (0.07) | 3 (1.2) | 3.3 ± 3.2 (1, 7) | 2 (1, 7) | 0/7/1/2 | 0/70/10/20 |
| Pain Medication | 864 (5.89) | 179 (69) | 4.8 ± 4.2 (1, 27) | 4 (2, 6) | 181/217/182/284 | 21/25/21/33* |
CNS, central nervous system; GI, gastrointestinal; IQR, interquartile range
Denominator for days consumed=the mean of cycle length × the number of total cycle = 28.8 × 509 = 14659.
P<0.05, difference across season.
Medication use frequency by active ingredient
Table 3 illustrates the subtypes of medications consumed including information on active ingredients of multiple drug formulations. Category of medication was determined according to the primary active ingredient. Forty-nine different types of medications were included. Medications that were consumed most frequently were ibuprofen, multi-symptom medication, acetaminophen, Dayquil, and naproxen. Ibuprofen (i.e. Advil and Motrin) was consumed across 491 participant days, followed by multi-symptom medication (i.e. Midol, Pamprin, and Excedrin) across 190 participant days, acetaminophen (i.e. Tylenol) across 163 participant days, and both Dayquil and naproxen (i.e. Aleve) across 80 participant days. Among those participants who consumed these active ingredients, the average number of days consumed over the course of the study was 4.5 days for ibuprofen, 2.9 days for multi-symptom medication, 2.5 days for acetaminophen, 2.6 days for Dayquil, and 3.5 days for naproxen.
Table 3.
Medication use by subcategories of medication types
| Primary medication category |
Medication Subcategory* | Active Ingredients | Person-Days consumed n |
Number of days medication used mean ± SD (range) |
Number of days medication used median (IQR) |
|---|---|---|---|---|---|
| Allergy | Cetirizine (Zyrtec) | Cetirizine HCl | 15 | 2.5 ± 2.7 (1, 8) | 1.5 (1, 2) |
| Allergy | Clemastine (Tavist) | Clemastine Fumarate | 3 | 3 (3, 3) | 3 (3, 3) |
| Allergy | Diphenhydramine (Benadryl) | Diphenhydramine Hydrochloride | 28 | 2.0 ± 1.3 (1,5) | 1.5 (1, 3) |
| Allergy | Fexofenadine (Allegra) | Fexofenadine Hydrochloride | 32 | 16 ± 19.8 (1, 30) | 16 (2, 30) |
| Allergy | Loratadine (Claritin) | Loratadine | 47 | 4.3 ± 7.0 (1, 25) | 2 (1, 4) |
| Allergy | Tylenol Allergy Sinus | Chlorpheniramine Maleate Acetaminophen Pseudoephedrine HCl |
2 | 1 (1, 1) | 1 (1, 1) |
| Antibiotics | Amoxicillin | Amoxicillin | 36 | 5.1 ± 3.0 (1, 9) | 5 (2, 8) |
| Antibiotics | Augmentin (Amoxicillin Clavulanate) | Amoxicillin | 3 | 3.0 (3, 3) | 3 (3, 3) |
| Antibiotics | Azithromycin (Zithromax) | Azithromycin | 21 | 5.3 ± 1.5 (5, 6) | 5 (5, 5.5) |
| Antibiotics | Cephalexin | Cephalexin | 3 | 3 (3, 3) | 3 (3, 3) |
| Antibiotics | Ciprofloxacin | Ciprofloxacin | 3 | 3 (3, 3) | 3 (3, 3) |
| Antibiotics | Erythromycin | Erythromycin | 7 | 3.5 + 3.5 (1,6) | 3.5 (1, 6) |
| Antibiotics | Metronidazole (Flagyl) | Metronidazole | 11 | 5.5 + 3.5 (3, 8) | 5.5 (3, 8) |
| Antibiotics | Nitrofurantoin (Macrobid) | Nitrofurantoin | 16 | 8 (8, 8) | 8 (8, 8) |
| Antibiotics | Sulfamethoxazole | Sulfamethoxazole | 3 | 3 (3, 3) | 3 (3, 3) |
| Antibiotics | Valacyclovir (Valtrex) | Valacyclovir HCl | 8 | 8 (8, 8) | 8 (8, 8) |
| Cold and cough | Benzonatate | Benzonatate | 5 | 5 (5, 5) | 5 (5, 5) |
| Cold and cough | Dextromethorphan | Dextromethorphan | 1 | 1 (1, 1) | 1 (1, 1) |
| Cold and cough | Guaifenesin/Guaifenesin PSE | Guaifenesin | 3 | 1.5 ± 0.7 (1, 2) | 1.5 (1, 2) |
| Cold and cough | Nyquil | Dextromethorphan hydrobromide Acetaminophen Doxylamine succinate |
18 | 2.0 + 1.7 (1, 5) | 1 (1, 2) |
| Cold and cough | Other Cold & Cough | Variable | 6 | 1.5 + 1.0 (1, 3) | 1 (1, 2) |
| Cold and cough | Phenylephrine | Phenylephrine HCl | 8 | 2.7 ± 1.5 (1, 4) | 3 (1, 4) |
| Cold and cough | Pseudoephedrine | Pseudoephedrine HCl | 54 | 2.8 ± 2.2 (1, 7) | 2 (1, 5) |
| Cold and cough | Robitussin/Tussin CF | Dextromethorphan hydrobromide Guaifenesin Pseudoephredrine HCl (Tussin CF only) |
7 | 2.3 ± 0.6 (1, 3) | 2 (2, 3) |
| Cold and cough | Tylenol Cold/Flu/Sinus | Dextromethorphan hydrobromide Pseudoephedrine HCl Acetaminophen |
14 | 3.1 ± 0.03 (1, 6) | 2 (1, 2) |
| CNS | Adderall | Amphetamine Aspartate Amphetamine Sulfate Dextroamphetamine saccharate Dextroamphetamine sulfate |
13 | 4.3 + 5.8 (1, 11) | 1 (1, 11) |
| CNS | Axert (Almotriptan Malate) | Almotriptan Malate | 1 | 1 (1, 1) | 1 (1, 1) |
| CNS | Eletriptan (Relpax) | Eletriptan Hydrobromide | 5 | 2.5 ± 0.7 (1, 3) | 2.5 (2, 3) |
| CNS | Ephedra | Ephedrine | 2 | 2.0 ± (2, 2) | 2 (2, 2) |
| CNS | Sumatriptan (Imitrex) | Sumatriptan Succinate | 3 | 3 (3, 3) | 3 (3, 3) |
| GI | Anatacid (Rolaids/Tum/Alka-Seltzer) | Variable | 17 | 1.5 ± 0.7 (1, 3) | 1 (1, 2) |
| GI | Bismuth Subsalicylate (Pepto-Bismol) | Bismuth Subsalicylate | 7 | 1.4 ± 0.9 (1, 3) | 1 (1, 3) |
| GI | Docusate (Colace, Correctol) | Docusate Sodium | 6 | 3.0 ± 1.4 (1, 4) | 3 (2, 4) |
| GI | Famotidine (Pepcid) | Famotidine | 1 | 1 (1, 1) | 1 (1, 1) |
| GI | Hyoscyamine Sulfate (Levsin SL) | Hyoscyamine Sulfate | 4 | 4 (4, 4) | 4 (4, 4) |
| GI | Omeprazole (Prilosec) | Omeprazole | 1 | 1 (1, 1) | 1 (1, 1) |
| GI | Rabeprazole (AcipHex) | Rubeprazole | 11 | 11 (11, 11) | 11 (11, 11) |
| GI | Simethicone (Gas-X) | Simethicone | 3 | 3 (3, 3) | 3 (3, 3) |
| Musculoskeletal | Cyclobenzaprine (Flexeril) | Cyclobenzaprine HCl | 7 | 3.5 ± 3.5 (1, 6) | 3.5 (1, 6) |
| Musculoskeletal | Orphenadrine (Norflex) | Orphenadrine Citrate | 3 | 1.5 + 0.7 (1, 2) | 1.5 (1, 2) |
| Pain | Acetaminophen (Tylenol) | Acetaminophen Dextromethorphan HBr Guaifenesin Pseudoephedrine HCl |
158 | 2.4 ± 2.0 (1, 10) | 2 (1, 3) |
| Pain | Advil Cold & Sinus | Ibuprofen Pseudoephedrine HCl |
23 | 4.6 ± 6.5 (1, 16) | 1 (1, 4) |
| Pain | Aspirin | Aspirin | 26 | 2.0 ± 1.0 (1, 4) | 2 (1, 3) |
| Pain | Dayquil | Acetaminophen | 16 | 2.7 ± 2.3 (1, 7) | 2 (1, 3) |
| Pain | Ibuprofen (Advil, Motrin) | Ibuprofen | 491 | 4.2 ± 4.0 (1, 18) | 3 (1, 5.5) |
| Pain | Multi-symptom (Midol/Pamprin/Excedrin) |
Acetaminophen (additional ingredients variable) |
70 | 3.0 ± 2.4 (1, 11) | 2 (1, 4) |
| Pain | Naproxen (Aleve) | Naproxen sodium | 80 | 3.5 ± 3.0 (1, 12) | 3 (1, 5) |
| Pain | Prescription pain medication | Variable | 16 | 1.8 ± 1.4 (1,5) | 1 (1, 2) |
IQR, interquartile range
Medication subcategories were determined according to the primary active ingredient
Medication use across the menstrual cycle
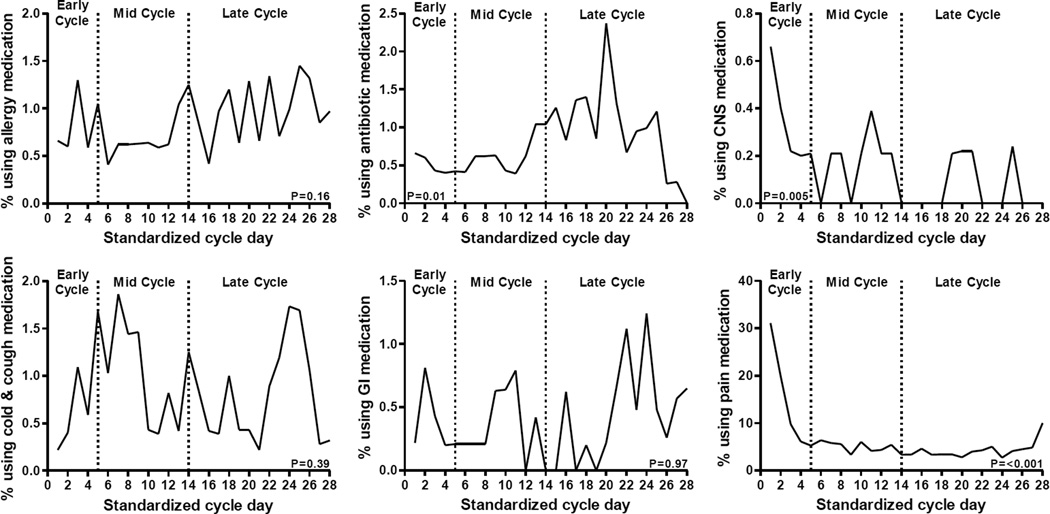
Variation in sporadic medication use across the menstrual cycle was not apparent for most categories of medication, including allergy, cold and cough, and gastrointestinal medications. However, antibiotic (p = 0.01), CNS (p = 0.005), and pain (p <0.001) medication use varied significantly across menstrual cycle phases (Figure 1). Pain medication was consumed most frequently during menses followed by a decline in use. We found that pain medication was used more in the early phase compared to either of the mid- or late cycle phases, as previously reported.3 CNS medication was also consumed most frequently during menses followed by a decline in use. We found a significant difference in mean CNS medication intake between early (i.e. menses; mean ± standard error, 0. 4 ± 0. 1 % of women) and late (i.e., luteal; 0. 1 ± 0. 1 %) phases of the cycle (p=0.004). Also, antibiotics were more frequently used during the luteal phase with a significant difference (p=0.039) in antibiotic use between the early (0.5 ± 0. 3 %) and late phase (1.0 ± 0.2 %) of the cycle. In this population, reported symptoms of illness from daily diaries indicated that among the 18 women reporting antibiotic use, 10 also reported cold/flu symptoms, 4 reported sinusitis/sinus infection, 4 reported ‘strep throat’ or sore throat, 2 reported urinary tract infection, 1 reported vaginal bacterial infection, 3 reported other infections (e.g. upper respiratory infection, mononucleosis, herpes), and 2 women had no reported symptoms.
Figure 1.
Medication use by category across standardized 28-day menstrual cycle
P-value represents global test of difference across cycle phases (early, mid, late).
(Ab)*Significantly different compared to early (P=0.039) cycle phase, based on Tukey-adjusted pairwise comparisons.
(CNS)† Significantly different compared to late (P=0.004) cycle phase, based on Tukey-adjusted pairwise comparisons.
(Pain)#Significantly different compared to mid (P<0.0001) and late (P<0.0001) cycle phase, based on Tukey-adjusted pairwise comparisons.
DISCUSSION
Among healthy, relatively young women not taking oral contraceptives, medication use was common, with nearly three-quarters of women using some medication within the approximate two months of study participation. The most frequently reported medications used were pain, cough and cold, and allergy medications. Additionally, use of CNS medications and antibiotics, in addition to pain medication use which has been previously discussed,3 may be related to menstrual cycle phase. Importantly, data of usual patterns and prevalence of medication use among this population is of special interest because of the potential risk for unplanned pregnancy, particularly given that approximately 50% of pregnancies in the U.S. are not specifically planned.4
These data comprehensively describe both prevalence and patterns of medication use in relation to the menstrual cycle among healthy, reproductive age women not consuming chronic medications, including oral contraception, but who are not actively trying to become pregnant. Such information may be helpful to health care providers discussing the use of OTC medications with women of reproductive age and particularly with women with greater potential to become pregnant (i.e., not using hormonal contraception).15 Close to 75% of the women in the study took some type of medication, with greater use of pain, cold and cough, and allergy medications compared to the other categories that were examined (i.e. antibiotics, CNS, GI, and musculoskeletal). Importantly, the most frequently used medications have potential for harm when used during pregnancy, with ibuprofen use associated with low birth weight and childhood asthma,16 and acetaminophen, which is generally regarded as safe in pregnancy, recently linked to attention-deficit/hyperactivity disorder behaviors in children.17
Such frequency of use likely reflects use for treating symptoms of illness (cough, cold and allergy medications) and menstrual pain (pain medication). Indeed, greater use of certain medications was observed during seasons when greater suffering from allergies (e.g. summer and fall) and colds (e.g. fall and winter) as observed here would be expected. These overall findings are consistent with previous work that cites these types of medication as those most frequently used by young women.15 In a U.S. telephone survey, >20% of women aged 18–44 years used pain medication (e.g. acetaminophen and ibuprofen) whereas 14% used some form of prescription only medication (e.g. ethinyl estradiol) at least once in a preceding week.1,18
Though use of other categories was less frequent overall, use of antibiotics and CNS categories of medication, in addition to pain medication, varied significantly across the menstrual cycle. Data from this study have previously shown that the greatest use of pain medication was around the time of menses.19
Likewise, there was increased use of CNS medication during menses whereas increased use of antibiotics was evident in the luteal phase of the menstrual cycle. Specifically, amoxicillin, a medication commonly used to treat streptococcal infections, was the most frequently used antibiotic medication. It is unclear why antibiotic use would be higher during the latter half of the cycle in women not taking oral contraceptives. Antibiotics are implicated in the effectiveness of hormonal contraceptives, as they can have interference with absorption of oral forms.7 For example, previous research indicates that use of rifampin, an antibiotic used to treat tuberculosis, impairs the effectiveness of OCs resulting in pregnancy in at least 30% of cases.20 However, understanding the reverse relationship, of menstrual cycle phase and reproductive hormones impacting infection requiring antibiotic use, is less studied. Furthermore, in developing countries, where there are limited controls on the sale of antimicrobials, women of childbearing age report using antibiotics to reduce menstrual symptoms including cramps, regulate heavy flow and prevent “infections” from feminine sanitary products.21 Particularly in recognition of misuse, awareness of antibiotic utilization patterns by women is important, given the known teratogenicity of certain common, and thus more widely available, antibiotics.22 Given that 35.1% of obstetric patients receive antimicrobial agents,8 and because of the limited number of participants using antibiotics in this study, a future study is needed to determine whether our finding of significant use of antibiotics during the luteal phase is a chance finding or possibly due to a greater susceptibility to infection during certain cycle phases.
The pattern of greater CNS medication during the early phase of the cycle, particularly the time around menses, may be due to changes in hormone levels relating to premenstrual syndrome (PMS), in combination with high levels of stress or anxiety. Adderall, most commonly used to treat attention deficit hyperactivity disorder (ADHD), was the most frequently used CNS medication, followed by prescription formulations to treat migraines (e.g. eletriptan HBr, sumatriptan succinate). The finding of prescription psychostimulant drug use was surprising, as women with psychiatric conditions requiring medical therapy were excluded from study enrollment. Alternatively, the limited Adderall use observed here might be self-administered, recreational or performance-enhancing use. This speculation is further supported by the observed greater use of CNS drugs in spring (50% overall, including 92% of Adderall use) and fall (29%), seasons which encapsulate known periods (i.e., midterm and final exams) of greater illicit stimulant drug use in college students,23 in combination with this study being a University-based study site. CNS medication use increasing around menses in this study may correspond to findings in previous studies reporting abnormal CNS responses to normal hormone levels in some women.24,25 Specifically, while women with normal pituitary-gonadal function and PMS exhibited symptoms of PMS in response to normal hormonal changes during the menstrual cycle, susceptible women exhibited abnormal responses (e.g. worsening mood).25
There are several unique strengths and limitations of this study that should be noted. The monitoring of a large number of young, healthy women across two menstrual cycles was a major strength of the study. The exclusion criteria of the study reduced potential for bias from history of infertility, chronic disease, and chronic medication use that could have affected the hormone levels across the menstrual cycle or resulted in an increased risk of drug-to-drug interaction. However, there are several important limitations, including a small number of women reporting use of antibiotic and CNS medications (n=18 and n=8, respectively). This could lead to a misrepresentation of medication use in these categories. Also, data regarding indications for medication, particularly antibiotic, use were not included in the daily assessment of participants. Self-report of medication use in daily diaries may lead to misclassification, and there were missing diaries, which may contribute to underreporting of medication use prevalence. The generalizability of these findings to the U.S. female population is limited by the homogeneity of certain characteristics (e.g. smoking, education) in this cohort. Furthermore, patterns and prevalence of medication use may differ in other populations, as the present study excluded women actively trying to become pregnant, or using vitamins or hormonal contraception, strong indicators of specific planning of pregnancy intentions.
Among this healthy, relatively young population of women, use of medication was prevalent, as nearly three-quarters of women used some type of medication during the study. It is expected that as technology advances and development of new drugs continues, new prescription medication will be introduced, and older ones will become increasingly available as OTC medications.1 Therefore, in combination with these findings, ongoing information on medication use patterns in women of reproductive age and whether use of any of this medication might relate to normal biologic variation, e.g. the menstrual cycle is needed. Typical patterns of medication use varied across the menstrual cycle for certain types of medication in the present study, particularly pain, antibiotic, and CNS categories. Both pain and CNS medication use was more frequent during the early phase of the cycle, while an increase in antibiotic use occurred during the late (i.e. luteal) phase of the menstrual cycle, though use of CNS and antibiotic medications was relatively low. In addition, use of certain medications varied across season. Therefore, in studies involving these types of medications in reproductive aged women, timing related to menstrual cycle phase and season may need to be considered. Clinically, care providers of women of reproductive age should consider reproductive potential when counseling women on both prescription and OTC medication use.
Key Points.
Medication use across the menstrual cycle was prevalent (73% of participants) among this healthy, relatively young population of women.
Patterns of medication use varied across phases of the menstrual cycle for certain types of medication: antibiotics, pain, and CNS medications.
Pain and CNS medication use was highest during the early (i.e. menses) phase of the menstrual cycle.
Antibiotic use was highest during the late (i.e. luteal) phase of the menstrual cycle.
Menstrual cycle phase may need to be considered in future studies assessing use of these types of medications in reproductive-aged women.
Knowledge of usual patterns of medication use in reproductive-aged women will aid in protecting and improving women’s overall and reproductive health.
Acknowledgments
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Contract Number: HHSN275200403394C). The authors thank Ya-Ling Lu, Ph.D. for her assistance in reviewing recent scientific literature and revising the final manuscript.
Abbreviations
- CNS
central nervous system
- GI
gastrointestinal
Footnotes
Conflicts of interest: The authors have no conflicts to disclose.
REFERENCES
- 1.Kaufman D, Kelly J, Rosenburg L, Anderson T, Mitchell A. Recent Patterns of Medication Use in the Ambulatory Adult Population of the United States. JAMA. 2002;287(3) doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 2.Furu K, Straume B, Thelle DS. Legal Drug Use in a General Population: Association with Gender, Morbidity, Health Care Utilization, and Lifestyle Characteristics. J Clin Epidemiology. 1997;50(3):341–349. doi: 10.1016/s0895-4356(96)00362-9. [DOI] [PubMed] [Google Scholar]
- 3.Matyas R, Mumford S, Schliep K, et al. Effects of over-the-counter analgesic use on reproductive hormones and ovulation in healthy, premenopausal women. Human Reproduction. 2015;30(5) doi: 10.1093/humrep/dev099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finer L, Zolna M. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception. 2011;84(5):478–485. doi: 10.1016/j.contraception.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finer L, Zolna M. Unintended Pregnancy in the United States. American Journal of Public Health. 2014;104(S1):S44–S48. doi: 10.2105/AJPH.2013.301416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA. Services DoHaH, editor. Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling. Federal Register. 2014;79:72063–72103. [PubMed] [Google Scholar]
- 7.Cleary B, Butt H, Strawbridge J, Gallagher P, Fahey T, DJ M. Medication use in early pregnancy-prevalence and determinants of use in a prospective cohort of women. Pharmacoepidemiology and Drug Safety: Wiley InterScience. 2010;19:408–417. doi: 10.1002/pds.1906. [DOI] [PubMed] [Google Scholar]
- 8.Glover D, Amonkar M, Rybeck B, Tracy T. Prescription, over-the-counter, and herbal medicine use in a rural, obstetric population. 2002 doi: 10.1067/mob.2003.223. [DOI] [PubMed] [Google Scholar]
- 9.Wactawski-Wende J, Schisterman E, Hovey K, et al. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Pediatric and Perinatal Epidemiology. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PP H, EF S, J W-W, JE R, AA F, KM H. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howards P, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig C, AL M, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Zhang C, Yeung E, et al. Age at Menarche and Metabolic Markers for Type 2 Diabetes in Premenopausal Women: The BioCycle Study. J Clin Endocrinol Metab. 2011;96(6):E1007–E1012. doi: 10.1210/jc.2010-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumford S, EF S, Gaskins A, et al. Realignment and multiple imputation of longitudinal data: an application to menstrual cycle data. Paediatr Perinat Epidemiol. 2011;25:448–459. doi: 10.1111/j.1365-3016.2011.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop A, Gardiner P, Shellhaas C, Menard M, McDiarmid M. The clinical content of preconception care: the use of medications and supplements among women of reproductive age. American Journal of Obstetrics and Gynecology. 2008;199(6):S367–S372. doi: 10.1016/j.ajog.2008.07.065. [DOI] [PubMed] [Google Scholar]
- 16.Nezvalová-Henriksen K, Spigset O, Nordeng H. Effects of ibuprofen, diclofenac, naproxen, and piroxicam on the course of pregnancy outcome: a prospective cohort study. BJOG: An International Journal of Obstetrics and Gynecology. 2013;120(8):948–959. doi: 10.1111/1471-0528.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieu Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatrics. 2014;168(4):313–320. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- 18.Curhan G, Bullock A, Hankinson S, Willett W, Speizer F, Stampfer M. Frequency of use of acetaminophen, nonsteroidal anti-inflammatory drugs, and aspirin in US women. Pharmacoepidemiology and Drug Safety. 2002;11:687–693. doi: 10.1002/pds.732. [DOI] [PubMed] [Google Scholar]
- 19.Matyas R, Mumford S, Schliep K, et al. Effects of over-the-counter analgesic use on reproductive hormones and ovulation in healthy, premenopausal women. Human Reproduction. 2015;30(7):1714–1723. doi: 10.1093/humrep/dev099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson B, Altman R, Nielsen N, Sterling M. Drug Interactions Between Oral Contraceptives and Antibiotics. The American College of Obstetricians and Gynecologists. 2001;98(5) doi: 10.1016/s0029-7844(01)01532-0. [DOI] [PubMed] [Google Scholar]
- 21.Sapkota A, Coker M, Goldstein R, et al. Self-medication with antibiotics for the treatment of menstrual symptoms in southwest Nigeria: a cross-sectional study. BMC Public Health. 2010;10(610):1471–2458. doi: 10.1186/1471-2458-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin K, Mitchell A, Yau W, Louik C, Hernández-Díaz S. Maternal exposure to amoxicillin and the risk of oral clefts. Epidemiology. 2012;23(5):699–705. doi: 10.1097/EDE.0b013e318258cb05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore D, Burgard D, Larson R, Ferm M. Psychostimulant use among college students during periods of high and low stress: An interdisciplinary approach utilizing both self-report and unobtrusive chemical sample data. Addictive Behaviors. 2014;39:987–993. doi: 10.1016/j.addbeh.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Pinkerton JV, Guico-Pabia CJ, Taylor HS. Menstrual cycle-related exacerbation of disease. Am J Obstet Gynecol. 2010;202(3):221–231. doi: 10.1016/j.ajog.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338(4):209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]