Abstract
Purpose
Differential diagnostic evaluation associated with a drug may bias effect estimates due to an increased detection of preclinical outcomes. Persistent cough is a common side effect with angiotensin-converting enzyme inhibitors (ACEI) and we hypothesized that ACEI initiators would undergo more diagnostic evaluations, potentially leading to diagnosis of preclinical lung cancer. We compared the incidence of cough-related diagnostic evaluations and lung cancer among ACEI versus angiotensin receptor blockers (ARB) initiators.
Methods
Using a 20% sample of Medicare claims 2007–2012, we identified initiators of ACEI or ARB, age 66–99 years. Incidence of diagnostic evaluation and lung cancer were compared using adjusted Cox models. Monthly probabilities of workup were compared using proportion differences.
Results
There were 342,611 and 108,116 ACEI and ARB initiators, respectively. Monthly probability of chest X-rays ranged from minimum 4.7% to maximum 21.2% in the 6 months pre and post-initiation. Differences in incidence of diagnostic procedures in the 6 months after initiation were only minimal (chest X-rays hazard ratio (HR) = 1.12; 95% CI: 1.10–1.14), chest-MRI (0.86, 95% CI: 0.74–0.99), CT-scans (1.09, 95% CI: 0.99–1.18) or bronchoscopies (1.03, 95% CI: 0.83–1.29)). Proportion differences for chest X-rays peaked in the month pre-initiation (8.4%, 95% CI: 8.1–8.6) but negligible thereafter. There was no difference in the incidence of lung cancer among ACEI versus ARB initiators (HR=0.99, 95% CI: 0.84–1.16).
Conclusion
Results indicate minimal differential chest workup after ACEI vs ARB initiation and no difference in lung cancer incidence, but suggest differential workup in the month before the first recorded prescription. The latter may reflect drug use before the first observed pharmacy claim or increased workup before initiation of ACEI therapy.
Keywords: diagnostic evaluation, detection bias, antihypertensive drugs, ACE inhibitors
Introduction
In cohort studies, detection bias occurs when the exposure influences or triggers the search for the outcome.1 For example, if a drug is known to be associated with side effects, patients on the drug may be subjected to increased diagnostic evaluation to monitor those specific side effects relative to non-users or users of a comparator drug. Differential diagnostic evaluation may lead to increased or earlier discovery of a possibly more serious outcome in those using the drug, therefore introducing a spuriously higher incidence of the outcome above and beyond any potential causal effect of the drug on the outcome.
We sought to investigate differential diagnostic evaluation in a study comparing the initiation of two antihypertensives - angiotensin converting enzyme inhibitor (ACEI) and angiotensin II receptor blockers (ARB) which are both recommended initial treatment of hypertension according to the Joint National Committee 7 (JNC7) guidelines.2 Persistent dry cough is a common side effect associated with ACEI drugs, reported in 5–35% of the patients treated with ACEI.3,4 The onset of cough may range from a few hours after the initial dose to weeks or months after starting therapy and is not dose-dependent.4–6 If discontinuation of ACEI is required because of cough, an ARB is commonly substituted. The incidence of cough with ARB is similar compared to placebo and less than ACEI in patients intolerant to previous ACEI therapy.7–9
Given an increased incidence of cough with ACEI, patients starting ACEI may undergo more diagnostic evaluation for cough (chest X-rays, chest CT-scan, chest-MRI, bronchoscopy) compared to patients starting ARB. An increase in diagnostic evaluations for cough may lead to earlier/increased detection of preclinical lung cancer and therefore a spuriously higher incidence of lung cancer with ACEI versus ARB early after initiation. There is mixed evidence about lung cancer risk with ACEI versus ARB based on two epidemiologic studies, with one study finding a null association and another a slightly increased risk.10,11 An observed increased incidence of diagnostic evaluation followed by a higher short term incidence of lung cancer in ACEI versus ARB initiators could be suggestive of detection bias. While plausible, to our knowledge, limited data exist quantifying differential diagnostic evaluations and thus we compared common cough-related diagnostic procedures between ACEI and ARB initiators in order to assess the potential for differential diagnostic evaluation. We first investigated the monthly probability of diagnostic evaluation and hypothesized that the probability with ACEI and ARB would be similar before drug initiation, would be higher among ACEI initiators shortly after drug initiation, and taper off thereafter. Next we compared the overall incidence of diagnostic evaluation with ACEI and ARB in the 6 months after drug initiation and hypothesized that the incidence would be higher in ACEI versus ARB initiators. Finally we compared the incidence of lung cancer among initiators of ACEI versus ARB.
Methods
We conducted a new-user active-comparator cohort study12 using a 20% random sample of Medicare claims from 2007–2012. This sample includes beneficiaries with fee-for-service Part A, B and D enrollment in at least one month from January 1, 2007 to December 31, 2012. Medicare is the largest public health insurance program in the US and contains information about demographics and enrollment, diagnoses, procedures and prescription drugs.13,14
From this data source, we identified new-users of ACEI and ARB, 66–99 years of age, who had no dispensed prescriptions of either ACEI or ARB in the preceding 12 months. Initiation was defined as the first prescription of the above drugs, with the date of initiation as the index date. Eligible patients were required to be continuously enrolled for at least 12 months in Parts A, B and D before the index date. Since prevalent cancers may affect the incidence of diagnostic evaluation or lung cancer, we excluded patients with any evidence of prevalent cancer, cancer treatment or cancer related follow-up examinations identified using International Classification of Diseases Ninth Revision (ICD-9-CM), Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding System (HCPCS) codes (supplemental table 1).
The outcomes for aim 1 were diagnostic evaluation procedures commonly used for persistent cough (chest X-rays, chest MRIs, chest CT-scans and bronchoscopies) defined using CPT or ICD-9-CM procedure codes (supplemental table 2) from inpatient and outpatient claims. We compared the monthly probability (number of patients with at least one procedure in that month/total patients in the cohort) of cough-related diagnostic procedures for ACEI versus ARB initiators using age, sex and race-adjusted proportion (probability) differences and 95% confidence intervals in the 6 months pre and post initiation, estimated using additive binomial regression models. For this analysis, we required patients to be continuously enrolled for at least 6 months after the index date. We also fit an adjusted Cox model to compare the incidence of diagnostic evaluation in the 6 months after initiation without requiring continuous enrollment after the index date. Patients were followed from their index prescription to the earliest of the following events: occurrence of a cough-related diagnostic procedure, switching/discontinuation or augmentation of therapy, death, end of enrollment, December 31, 2012, or 6 months after index date.
Next we compared the incidence of lung cancer among ACEI and ARB initiators using adjusted Cox models. For this analysis, we further restricted the study cohort to initiators who filled a second script of the same drug class within 180 days of the index date. Follow-up started at the second prescription date and ended at the earliest of lung cancer, switching/discontinuation/augmentation, death, end of enrollment or December 31, 2012. Lung cancer was defined as at least two inpatient or outpatient claims with ICD-9-CM codes 162.xx within two months, a definition with high specificity (minimizes false positives, yields unbiased relative risk estimates) in a Medicare population.15
We adjusted for confounders using propensity scores (PS) estimated using demographic characteristics, baseline diagnostic evaluation and healthcare utilization, comorbidities and use of other medications including other antihypertensives measured before the index date.16–18 We implemented the PS using weights that led to “standardization” of covariates in the ARB group to the covariate distribution observed in the ACEI group. Specifically, we assigned a weight of 1 for ACEI and a weight of (PS/(1-PS)) for ARB.19,20 This weighting creates a pseudo-population of ARB initiators with measured patient characteristics similar to those observed in ACEI initiators. This balance of patient characteristics allows us to estimate the unconfounded treatment effect in a population of patients similar to those actually initiating ACEI under the assumption of no unmeasured confounding.19,21 We computed weighted Kaplan-Meier plots to check the proportional hazards assumption and then fit weighted Cox proportional hazards models with treatment as the only independent variable to compare incidence of diagnostic evaluation and lung cancer among initiators of ACEI and ARB. We also examined the frequency and incidence of chest X-rays in the six months following the index date stratifying by the presence of baseline chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) – conditions for which X-rays are commonly indicated.
Results
Table 1 presents the baseline covariate distribution for the new-user cohorts. Compared with the ARB initiators, ACEI initiators were more likely to be male and white. Mean age was around 76 years in both groups. ACEI initiators were less likely to have connective tissue disease, diabetes complications, or have ECG, blood tests, influenza vaccinations, and lipid panels performed compared with the ARB initiators. After weighting, the distribution of all covariates in the weighted ARB pseudo-population was virtually identical to the distribution observed in ACEI initiators (standardized mean differences <5%). This balance of measured covariates effectively eliminates confounding by those variables, although some level of residual or unmeasured confounding may still exist.
Table 1.
Baseline characteristics of ACEI and ARB initiators*: Medicare claims data 2007–2012
| ACEI (n = 342,611) |
ARB (n = 108,116) |
Weighted ARB group# | Absolute standardized difference& | |||
|---|---|---|---|---|---|---|
| N | % | N | % | % | % | |
| Age† at initiation Mean (SD) | 76.1 (7.6) | 76.2 (7.3) | 76.1 (10.3) | 0.0 | ||
| 66 to 75 years old | 182,309 | 53.2 | 56,112 | 51.9 | 53.2 | 0.1 |
| 76 to 85 years old | 113,142 | 33 | 38,051 | 35.2 | 33 | 0.0 |
| 86 to 99 years old | 47,160 | 13.8 | 13,953 | 12.9 | 13.7 | 0.1 |
| Male | 125,876 | 36.7 | 33,259 | 30.8 | 36.7 | 0.0 |
| white | 282,561 | 82.5 | 83,142 | 76.9 | 82.4 | 0.3 |
| black | 31,894 | 9.3 | 10,673 | 9.9 | 9.5 | 0.5 |
| other | 28,156 | 8.2 | 14,301 | 13.2 | 8.2 | 0.1 |
| Baseline comorbidities | ||||||
| Asthma | 27,739 | 8.1 | 11,214 | 10.4 | 8.4 | 1.1 |
| Angina | 14,176 | 4.1 | 5,055 | 4.7 | 4.4 | 1.4 |
| Bronchiectasis | 1,898 | 0.6 | 857 | 0.8 | 0.6 | 0.5 |
| Bronchitis | 33,484 | 9.8 | 10,819 | 10 | 10.3 | 1.9 |
| Heart failure | 53,378 | 15.6 | 15,947 | 14.8 | 16.3 | 2.0 |
| Connective tissue disease | 94,377 | 27.6 | 34,447 | 31.9 | 28.0 | 1.0 |
| Chronic obstructive pulmonary disease | 55,947 | 16.3 | 16,801 | 15.5 | 17.1 | 2.0 |
| Depression | 45,813 | 13.4 | 12,874 | 11.9 | 14.1 | 2.0 |
| Emphysema | 10,556 | 3.1 | 2,817 | 2.6 | 3.3 | 1.1 |
| Gastrointestinal disorders | 2,669 | 0.8 | 893 | 0.8 | 0.8 | 0.1 |
| Infection | 127,723 | 37.3 | 43,801 | 40.5 | 38 | 1.5 |
| Nephropathy | 7,332 | 2.1 | 3,250 | 3.0 | 2.3 | 1.4 |
| Neuropathy | 18,748 | 5.5 | 6,735 | 6.2 | 5.8 | 1.3 |
| Retinopathy | 15,063 | 4.4 | 5,632 | 5.2 | 4.5 | 0.6 |
| Stroke | 19,097 | 5.6 | 4,950 | 4.6 | 5.9 | 1.3 |
| Dementia | 38,104 | 11.1 | 8,894 | 8.2 | 11.6 | 1.4 |
| Hypotensive shock | 9,723 | 2.8 | 2,607 | 2.4 | 3.1 | 1.5 |
| Parkinson disease | 10,357 | 3.0 | 2,826 | 2.6 | 3.2 | 1.1 |
| Baseline medication use | ||||||
| Antihistamines | 34,775 | 10.2 | 12,363 | 11.4 | 10.3 | 0.5 |
| Cough suppressants | 1,310 | 0.4 | 486 | 0.5 | 0.4 | 0.0 |
| Beta blockers | 132,704 | 38.7 | 47,275 | 43.7 | 39.2 | 0.9 |
| Inhaled glucocorticoids | 23,415 | 6.8 | 8,335 | 7.7 | 7.1 | 0.9 |
| Oral glucocorticoids | 45,932 | 13.4 | 16,071 | 14.9 | 13.9 | 1.3 |
| Calcium channel blockers | 80,113 | 23.4 | 34,580 | 32.0 | 23.7 | 0.8 |
| Potassium sparing diuretics | 27,975 | 8.2 | 9,247 | 8.6 | 8.3 | 0.5 |
| Other diuretics | 5,654 | 1.7 | 2,014 | 1.9 | 1.7 | 0.7 |
| Thiazides | 66,891 | 19.5 | 21,798 | 20.2 | 19.6 | 0.2 |
| Tobacco useˆ | 30,055 | 8.8 | 7,514 | 7.0 | 9.3 | 1.7 |
| Baseline diagnostic work-up/general healthcare utilization | ||||||
| Skilled nursing facility | 22,529 | 6.6 | 5,035 | 4.7 | 7.1 | 1.9 |
| Stay in long term care facility | 7,793 | 2.3 | 1,733 | 1.6 | 2.5 | 1.4 |
| Bronchoscopy | 674 | 0.2 | 201 | 0.2 | 0.2 | 0.0 |
| ECG | 150,400 | 43.9 | 53,682 | 49.7 | 44.9 | 2.1 |
| Blood tests | 24,122 | 7.0 | 9,158 | 8.5 | 7.0 | 0 |
| Chest CT Scan | 7,381 | 2.2 | 2,054 | 1.9 | 2.3 | 1.0 |
| Chest MRI | 2,094 | 0.6 | 747 | 0.7 | 0.7 | 0.5 |
| Chest X-ray | 125,696 | 36.7 | 39,824 | 36.8 | 37.7 | 2.2 |
| Hip X-ray | 22,864 | 6.7 | 6,884 | 6.4 | 6.9 | 0.9 |
| Shoulder X-ray | 17,960 | 5.2 | 5,981 | 5.5 | 5.5 | 1.3 |
| Colonoscopy | 24,728 | 7.2 | 8,670 | 8.0 | 7.3 | 0.4 |
| Influenza vaccines | 158,095 | 46.1 | 53,869 | 49.8 | 46.0 | 0.4 |
| Lipid panel | 205,760 | 60.1 | 74,832 | 69.2 | 60.1 | 0.1 |
| Mammogram | 64,587 | 18.9 | 25,320 | 23.4 | 18.7 | 0.3 |
| Pap smear test | 17,750 | 5.2 | 7,570 | 7.0 | 5.1 | 0.2 |
| ED visits Mean (SD) | 1.0 (1.7) | 0.7 (1.5) | 1.0 (4.3) | 0.0 | ||
| Office visits Mean (SD) | 6.5 (6.3) | 8.2 (7.0) | 6.5 (12.2) | 0.0 | ||
| Number of drugs dispensed Mean (SD) | 7.6 (5.9) | 8.3 (6.0) | 7.6 (10.7) | 0.0 | ||
ACEI – angiotension converting enzyme inhibitors, ARB - angiotensin converting enzyme, CT - computed tomography, ECG - electrocardiogram, MRI - magnetic resonance imaging, ED - emergency department
Initiation defined as the first prescription of ACEI or ARB after a 12 month washout period. All covariates measured up to (index date - 30)th day.
absolute standardized difference (%) used to compare covariate balance between ACEI group and weighted ARB group.
Weighting standardized to the treated (ACEI group)
Age range: 66 to 99 years for all groups
Tobacco use defined as baseline diagnosis or CPT claim for tobacco use or Rx claim for Varenicline or Zyban.
There were 342,611 and 108,611 eligible initiators of ACEI and ARB, respectively, during the study period. Of these, 295,604 ACEI initiators and 93,284 ARB initiators were continuously enrolled for at least 6 months post-index. The monthly probability of having a chest X-ray ranged (minimum to maximum) from 4.8% to 21.2% in ACEI and 4.8% to 12.2% in the ARB group (supplemental table 3). The proportion difference comparing ACEI and ARB groups was the highest in the month before the index date (Proportion difference 8.37, 95% CI: 8.12–8.63) and decreased in the following months (figure 1). We also examined the distribution of days when chest X-rays were conducted in the month before initiation and found the median to be 8 days before initiation among ACEI initiators and 10 days before initiation among ARB initiators. The monthly proportion differences for other diagnostic procedures were more or less constant in the 6 months before and after the index date (supplemental table 3).
Figure 1. Age, sex, and race adjusted monthly proportion (risk) differences for chest X-rays comparing ACEI versus ARB initiators: 2007 – 2012.
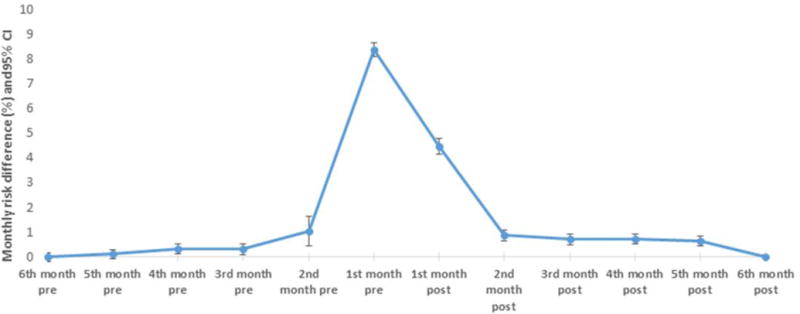
ACEI – angiotension converting enzyme inhibitors, ARB - angiotensin converting enzyme
CI – Confidence intervals
Index date – Date of the first recorded prescription (initiation date)
Pre – period before initiation
Post – period after initiation
The first month post-index also contains the index date (date of drug initiation)
Table 2 presents the event rates per 100,000 person-months, median time-to-event, and the crude and adjusted (weighted) hazard ratios for the incidence of diagnostic evaluation in the 6 months after the index date. A total of 90,951 (26.5%) ACEI initiators and 24,123 (22.3%) ARB initiators had at least one chest X-ray in the 6 months after drug initiation. The weighted HR comparing incidence of chest X-rays among ACEI and ARB initiators was 1.12 (95% CI: 1.10–1.14). The corresponding HRs for chest MRI (0.86, 95% CI: 0.74–0.99), chest CT-scans (1.09, 95% CI: 0.99–1.18), and bronchoscopies (1.03, 95% CI: 0.83–1.29) were all close to 1 suggesting minimal increased hazard of diagnostic evaluation with ACEI vs ARBs in the 6 months after drug initiation. Based on 902 lung cancers among 238,439 ACEI initiators and 261 lung cancers among 72,626 ARB initiators over 0.7 median years of follow-up, no increased risk of lung cancer was found with ACEI versus ARB (HR=0.99; 95% CI: 0.84–1.16; table 3).
Table 2.
Incidence of diagnostic evaluation procedures among ACEI and ARB initiators in the 6 months after initiation*: Medicare claims data 2007 – 2012
| Procedure | Drug class | Number of new-users | incident diagnostic procedure in the 6 months after index date | time to event in months interquartile range (median) | Total person-months# | Incidence (per 100,000 person months) |
Unadjusted HR (95%CI) |
Adjusted HR (95%CI) § |
|---|---|---|---|---|---|---|---|---|
| Chest X-ray | ||||||||
| ACEI | 342,611 | 90,951 | 2.0 – 6.0 (3.9) | 1,247,809 | 7,288.86 | 1.21 (1.20, 1.24) | 1.12 (1.10, 1.14) | |
| ARB | 108,116 | 24,123 | 2.0 – 6.0 (4.0) | 403,894 | 5,972.61 | 1.00 (reference) | 1.00 (reference) | |
| Chest MRI scans | ||||||||
| ACEI | 342,611 | 1,073 | 2.0 – 6.0 (5.2) | 1,483,911 | 72.31 | 0.87 (0.77, 0.97) | 0.86 (0.74, 0.99) | |
| ARB | 108,116 | 389 | 2.0 – 6.0 (5.1) | 465,705 | 83.53 | 1.00 (reference) | 1.00 (reference) | |
| Chest CT scan | ||||||||
| ACEI | 342,611 | 4,581 | 2.0 – 6.0 (5.1) | 1,475,666 | 310.44 | 1.21 (1.14, 1.29) | 1.09 (0.99, 1.18) | |
| ARB | 108,116 | 1,189 | 2.0 – 6.0 (5.0) | 463,766 | 256.38 | 1.00 (reference) | 1.00 (reference) | |
| Bronchoscopy | ||||||||
| ACEI | 342,611 | 627 | 2.0 – 6.0 (5.2) | 1,485,407 | 42.21 | 1.14 (0.96, 1.35) | 1.03 (0.83, 1.29) | |
| ARB | 108,116 | 173 | 2.0 – 6.0 (5.1) | 466,364 | 37.10 | 1.00 (reference) | 1.00 (reference) |
ACEI – angiotension converting enzyme inhibitors, ARB - angiotensin converting enzyme, CT - computed tomography, MRI - magnetic resonance imaging, HR – Hazard ratio, CI – confidence interval
Initiation defined as the first prescription of ACEI or ARB after a 12 month washout period.
As treated analysis approach used. Patients were followed up from drug initiation till the earliest of the following - outcome (diagnostic evaluation procedures), switching/discontinuation or augmentation of therapy, death, end of enrollment, December 31, 2012 or 6 months after index date.
Treatment discontinuation defined as no new prescription of a drug from the same drug class within (days-supply + 180 days grace period) after the last prescription. Switching defined as filling a prescription for a comparison drug without filling another prescription for the study drug within days-supply + 180 days grace period. Augmenting defined as adding a prescription of a comparison drug with another prescription of the study drug within days-supply + 180 days grace period.
Adjusted for variables in table 1 using weighted analysis (standardized to the ACEI group)
Table 3.
Incidence of lung cancer among ACEI and ARB initiators: Medicare claims data 2007–2012*
| Drug class | Number of new-users | Lung Cancer | time to event in years interquartile range (median) |
Total person-years# | Incidence (per 100,000 person years) |
Unadjusted HR (95% CI) |
Adjusted HR (95%CI)§ |
|---|---|---|---|---|---|---|---|
| ACEI | 238,439 | 902 | 0.4 – 1.5 (0.7) | 254,492 | 354.43 | 1.01 (0.88, 1.16) | 0.99 (0.84, 1.16) |
| ARB | 72,626 | 261 | 0.5 – 1.4 (0.8) | 74,116 | 352.15 | 1.00 (reference) | 1.00 (reference) |
ACEI – angiotension converting enzyme inhibitors, ARB - angiotensin converting enzyme, HR – Hazard ratio, CI: confidence interval
Initiation defined as the first prescription of ACEI or ARB after a 12 month washout period. To increase the chance that patients were actually on the drug, For this analysis patients were required to fill 2 prescriptions of the same drug class within 180 days of initiation.
As treated analysis approach used. Patients were followed up from the date of the second prescription till the earliest of the following - lung cancer, switching/discontinuation or augmentation of therapy, death, end of enrollment, December 31, 2012. Treatment discontinuation defined as no new prescription of a drug from the same drug class within (days-supply + 180 days grace period) after the last prescription. Switching defined as filling a prescription for a comparison drug without filling another prescription for the study drug within days-supply + 180 days grace period. Augmenting defined as adding a prescription of a comparison drug with another prescription of the study drug within days-supply + 180 days grace period.
Adjusted for variables in table 1 using weighted analysis (standardized to the ACEI group)
Discussion
We hypothesized that the incidence of diagnostic evaluation for cough would be substantially higher in the months immediately following drug initiation among ACEI versus ARB initiators which could lead to detection bias in any study of cancers, specifically of lung cancer. Contrary to our hypothesis, we found only minimal differences in the diagnostic procedures in the 6 months after initiation of ACEI versus ARB based on time-to-event analyses. While small differences existed (12–14%), these may not be enough to account for a spuriously increased incidence of lung cancer with ACEI versus ARB that we wanted to explore.
Given the minimal differences in the incidence of diagnostic evaluations in ACEI and ARB patients after drug initiation, we expected no difference in the incidence of lung cancer with ACEI versus ARB, and that is what we observed. Thus, lung cancer incidence might be considered in this setting as a negative control outcome.22,23 Smoking is an important confounder of the relationship between ACEI vs ARB and lung cancer; but it is unmeasured in Medicare claims. However we adjusted for baseline claims for tobacco use and this and all other covariates were balanced both before and after weighting. The observation of a null association suggests minimal differences in smoking between ACEI and ARB initiators (and therefore no major concern for confounding by smoking) in this study, but this is beyond the scope of this paper.
Examination of monthly proportion differences comparing ACEI and ARB initiators showed some indication of differential chest X-rays in the days around the index date. Contrary to our expectation, however, the proportion difference was the highest in the month before initiation (as defined by the first record of a dispensed prescription) instead of post-initiation. Several possibilities could explain the peak in the proportion difference in the month before initiation. First, because ACEI are known to be associated with persistent cough, it is possible that more ACEI initiators were subject to X-rays to check the lungs before starting therapy. A second and possibly more plausible reason is that we are missing the true ‘initiation’ of drug therapy, i.e., it is possible that initiators defined by our algorithm may have been on drug therapy a few days or weeks before their first dispensed prescription was captured in claims.
While speculative, the observed difference prior to the first recorded drug dispensing could be explained if patients were given free drug samples by their physicians as observed in some other settings.24,25 However, both ACEI and ARB are widely available as inexpensive generics.26 In our cohort, >99% of ACEI and about 50% of the ARB prescriptions were for generic versions compatible with less sample use for ACEI. Missing the initial period of drug use could also be partly attributable to patients filling some prescriptions outside of the context of part D for example through dual eligibility with pharmacy benefit programs like the Veterans Affairs coverage or out-of-pocket payment particularly after the introduction of low-cost generic programs, although we do not have the relevant data to evaluate this possibility.27 This points to a potential limitation of the new-user design based on pharmacy claims which has implications for studying short term outcomes, drug safety and definition of baseline covariates potentially affected by treatment.24
One strength of our study is the use of an active comparator which is a therapeutic alternative to ACEI therapy. Use of an active comparator with the same indication as that of ACEI synchronized patients with respect to disease severity and baseline characteristics and limited confounding by these factors.28,29 Table 1 reflects the covariate balance achieved by our study design (crude) and remaining differences of measured covariates were greatly reduced by propensity score weighting. Given that many covariates were already balanced by using an active comparator new user design (even before propensity score implementation), unmeasured confounding might not be a major concern in our study, although it cannot be ruled out.
Compared to other procedures, the proportion of ACEI and ARB initiators with at least one chest X-ray in the 6 months post initiation was much higher (about 22–26%). On closer examination, we found that 98% of the chest X-rays were coded using CPT codes 71010 (Radiologic examination, chest; single view, frontal) and 71020 (Radiologic examination, chest, two views, frontal and lateral). A study by Levin et al examining the trends in utilization of cardiothoracic imaging procedures in Medicare fee-for-service beneficiaries found about 94 chest X-rays per 100 beneficiaries in 2005.30 We found a similar high rate of chest X-ray use (92 per 100 beneficiaries) in our entire 20% Medicare claims sample indicating that our data represent chest X-ray utilization well (data not presented).
We also examined the frequency of chest X-rays in our new-user cohort stratified by CHF and COPD, conditions for which chest X-rays are likely to be indicated. As expected, about 36–42% of the ACEI or ARB initiators with CHF or COPD at baseline had at least one incident chest X-ray in the 6 months after initiation while only a quarter of those without either of the two conditions had at least one claim for chest X-rays (supplemental table 4). The difference in monthly probability of chest X-rays peaked in the month just before drug initiation in all subgroups (data not shown). Finally, to provide contrast to the observation of a peak in chest X-rays in the month before initiation, we examined the monthly proportion differences of a ‘control’ diagnostic evaluation (hip X-rays) and found no substantial peak in the 6 months before and after initiation (supplemental table 5).
Our study had some limitations. Our analyses of differences in monthly proportions of diagnostic testing were adjusted only for age, sex and race, and should therefore be interpreted with caution. We did not conduct analyses with time-varying covariates since some of the covariates (example, baseline diagnostic procedures) might be affected by treatment in this setting. Second, it is possible that some ‘new-users’ of ARB used ACEI prior to the 12 month washout period and discontinued ACEI because of cough. For such patients, the diagnostic evaluation before the initiation of ARB may seem elevated (due to the prior ACEI therapy). It seems unlikely, however, that the differences would be minimal throughout the six-month pre-index period and then peak at one month before initiation under this hypothesis. It is also possible that patients undergoing diagnostic work-up for persistent cough after receiving an ACEI sample would be less likely to fill a prescription for ACEI and are therefore missing from our study. The proportion of such patients is expected to be low, however, given the availability of several low-cost generic versions of ACEI drugs and the resultant low potential for sample use. Third, there are no validated algorithms to identify cough-related diagnostic evaluations, to our knowledge. However we used a comprehensive list of codes based on substantive knowledge and expert opinion. Fourth, our cohort of initiators of antihypertensive monotherapy should mainly consist of stage 1 hypertension patients (but this is empirically unverifiable due to absence of blood pressure or stage data). Further, patients were allowed to be on other antihypertensive drugs during the washout period and this information was used in the estimation of propensity scores. A sensitivity analysis excluding patients with use of any antihypertensive drug during washout period resulted in very similar estimates (supplemental table 6). To explore the potential for effect modification by hypertension stage, we compared same-day initiators of ACEI plus thiazide versus ARB plus thiazide (proxy for stage 2 hypertension) and found no difference in the incidence of chest X-rays in the 6 months post-index (supplemental table 7). Finally, our analyses were based on initiators of antihypertensives in the Medicare population and generalizability is therefore restricted to older adults on the Medicare fee-for-service plan or in health care settings with similar drug use or prescribing behaviors.
Conclusion
We found minimal differences in diagnostic evaluation in the 6 months after initiation and no difference in the incidence of lung cancer with ACEI versus ARB. There was some indication of differential chest X-ray workup in the days around the index date, however, and contrary to our expectation, the proportion difference was highest in the month before initiation but negligible thereafter. Our study provides some suggestion of potential drug use prior to the first recorded pharmacy claim as also observed in some other settings,24 or else some tendency for some physicians to assess lung function prior to prescribing ACEI. Results may differ in other settings, with different drugs and outcomes. In some scenarios, differential diagnostic evaluation may lead to considerable detection bias that uncovers more severe outcomes in addition to simply finding a greater number of cases. Analyses like the ones presented here will help researchers to evaluate the potential for bias in other data sources and drug-outcome relationships where diagnostic suspicion may be suspected.
Supplementary Material
Key bullet points.
Differential diagnostic evaluation associated with a drug, e.g., based on known side effects, may result in biased effect estimates in pharmacoepidemiologic studies due to an increased detection of preclinical conditions.
Persistent cough is a common side effect with angiotensin converting enzyme inhibitors (ACEI) and we hypothesized that ACEI initiators would have more cough-related diagnostic evaluation than initiators of angiotensin receptor blockers (ARB), potentially leading to diagnosis of preclinical lung cancer.
Using Medicare beneficiaries 66–99 years of age, who initiated monotherapy with ACEI or ARB between 2007 and 2012, this study found minimal differences in diagnostic evaluations among initiators of ACEI versus ARB in the 6 months after drug initiation and no difference in lung cancer incidence over median 0.7 years follow-up, adjusted for a number of baseline characteristics.
Contrary to our expectation, the difference in probabilities of having a chest X-ray comparing ACEI versus ARB groups was the highest in the month before initiation instead of post-initiation. This may be an indication of ACEI exposure prior to the first observed pharmacy claim as seen in some other settings, or a tendency of some physicians to assess the lungs prior to prescribing ACEI.
Assessment of diagnostic evaluations before and after the initiation of treatments compared will help researchers to evaluate the potential for bias in specific pharmacoepidemiologic studies.
Acknowledgments
Funding/grant number: This study was funded by a Gillings Innovation Laboratory (GIL) Award (GIL 200811.0010) from the UNC Gillings School of Global Public Health, a research grant from Merck & Co., Inc., and R01AG023178 from the National Institute on Aging at the NIH. The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health, the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR001111), the Cecil G. Sheps Center for Health Services Research, UNC, and the UNC School of Medicine.
Footnotes
Conflicts of interest:
M.G. is a doctoral student at the University of North Carolina, Chapel Hill. Cynthia J Girman was a former employee of Merck & Co., Inc, and owns stock in Merck and other biotech companies. Girman is now President of CERobs Consulting, LLC which provides consulting services for pharmaceutical companies. M.J.F. receives investigator-initiated research funding and support as Principal Investigator from the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI, R01 HL118255); as a Co-Investigator from the NIH National Institute on Aging (NIA, R01 AG023178), the NIH National Center for Advancing Translational Sciences (NCATS, 1UL1TR001111), AstraZeneca, and the Patient Centered Outcomes Research Institute (PCORI, 1IP2PI000075). Dr. Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company, though she receives salary support from the Center for Pharmacoepidemiology in the Department of Epidemiology, Gillings School of Global Public Health (current members: GlaxoSmithKline, UCB BioSciences, Merck). T.S. receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214), National Institutes of Health (NIH). He also receives salary support as Director of the Comparative Effectiveness Research (CER) Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, AstraZeneca) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Johnsen & Johnsen.
Prior presentation: Poster presentation, International Conference on Pharmacoepidemiology & Therapeutic Risk Management (ICPE), 2013
Authors’ Contributions: M.G., C.G., and T.S. participated in study conception and design. M.G., T.S., M.J.F., V.P. participated in the acquisition of the data. M.G., V.P., participated in the analysis and all authors contributed to the interpretation of the data. M.G., T.S. and C.G. wrote the first draft of the manuscript. All authors reviewed and provided comments on the manuscript. M.G. is the guarantor of this work; M.G. and V.P. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Delgado-Rodriguez M, Bias Llorca J. J Epidemiol Community Health. 2004;58(8):635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chobanian Aram V, Bakris George L, Black Henry R, Cushman William C, Green Lee A, Izzo Joseph L, Jones Daniel W, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough ACCP evidence-based clinical practice guidelines. CHEST Journal. 2006;129(1_suppl):169S–173S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- 4.Vegter S, Jong-van den Berg D, Lolkje T. Misdiagnosis and mistreatment of a common side-effect–angiotensin-converting enzyme inhibitor-induced cough. Br J Clin Pharmacol. 2010;69(2):200–203. doi: 10.1111/j.1365-2125.2009.03571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Os I, Bratland B, Dahlof B, Gisholt K, Syvertsen JO, Tretli S. Female preponderance for lisinopril-induced cough in hypertension. Am J Hypertens. 1994;7(11):1012–1015. doi: 10.1093/ajh/7.11.1012. DOI: 0895-7061(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 6.Overlack A. ACE inhibitor-induced cough and bronchospasm. Drug Safety. 1996;15(1):72–78. doi: 10.2165/00002018-199615010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors. American Journal of Cardiovascular Drugs. 2012;12(4):263–277. doi: 10.1007/BF03261835. [DOI] [PubMed] [Google Scholar]
- 8.Pylypchuk GB. ACE inhibitor- versus angiotensin II blocker-induced cough and angioedema. Ann Pharmacother. 1998;32(10):1060–1066. doi: 10.1345/aph.17388. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Hernandez R, Sosa-Canache B, Velasco M, Armas-Hernandez MJ, Armas-Padilla MC, Cammarata R. Angiotensin II receptor antagonists role in arterial hypertension. J Hum Hypertens. 2002;16(Suppl 1):S93–9. doi: 10.1038/sj.jhh.1001352. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK general practice research database. BMJ. 2012;344:e2697. doi: 10.1136/bmj.e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasternak B, Svanstrom H, Callreus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation. 2011;123(16):1729–1736. doi: 10.1161/CIRCULATIONAHA.110.007336. [DOI] [PubMed] [Google Scholar]
- 12.Ray WA. Evaluating medication effects outside of clinical trials: New-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 13.Medicare program - general information. https://www.cms.gov/Medicare/Medicare-General-Information/MedicareGenInfo/index.html (accessed November 10, 2015)
- 14.Brief summaries of Medicare & Medicaid. 2011 https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/SummaryMedicareMedicaid.html (accessed November 10, 2015)
- 15.Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 17.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 20.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. doi: 10.1093/aje/kwj047. DOI: kwj047. [DOI] [PubMed] [Google Scholar]
- 22.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–8. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchetgen Tchetgen E. The control outcome calibration approach for causal inference with unobserved confounding. Am J Epidemiol. 2014;179(5):633–40. doi: 10.1093/aje/kwt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Sturmer T, Brookhart MA. Evidence of sample use among new users of statins: Implications for pharmacoepidemiology. Med Care. 2014;52(9):773–780. doi: 10.1097/MLR.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Using ACE inhibitors to treat high blood pressure and heart disease comparing effectiveness, safety, and price. http://consumerhealthchoices.org/wp-content/uploads/2012/08/BBD-ACEI-Full.pdf. Accessed September 1, 2015.
- 27.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiol Drug Saf. 2013;22(8):899–906. doi: 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturmer T. Study design considerations. In: Velentgas P, Dreyer NA, Nourjah P, Smith S, Torchia MM, editors. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. 2013. pp. 21–34. [PubMed] [Google Scholar]
- 29.Schneeweiss S, Patrick AR, Sturmer T, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 Supl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent trends in utilization rates of noncardiac thoracic imaging: An example of how imaging growth might be controlled. Journal of the American College of Radiology. 2007;4(12):886–889. doi: 10.1016/j.jacr.2007.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


