Abstract
Background
Up to 12% of patients with laryngeal dystonia report familial history of dystonia, pointing to involvement of genetic factors; however, its genetic causes remain unknown.
Methods
Using Sanger sequencing, we screened 57 patients with isolated laryngeal dystonia for mutations in known dystonia genes TOR1A (DYT1), THAP1 (DYT6), TUBB4A (DYT4) and GNAL (DYT25). Using functional MRI, we explored the influence of the identified mutation on brain activation during symptomatic task production.
Results
We identified one patient with laryngeal dystonia who was a GNAL mutation career. Compared to 26 patients without known mutations, the GNAL carrier had increased activity in the fronto-parietal cortex and decreased activity in the cerebellum.
Conclusions
Our data show that GNAL mutation may represent one of the rare causative genetic factors of isolated laryngeal dystonia. Exploratory evidence of distinct neural abnormalities in the GNAL carrier may suggest the presence of divergent pathophysiological cascades underlying this disorder.
Keywords: dystonia, spasmodic dysphonia, genetic factors, neuroimaging
Introduction
Isolated laryngeal dystonia (LD), or spasmodic dysphonia, is a focal adult-onset dystonia primarily affecting speech production. LD is characterized by involuntary spasm-inducing voice breaks with strained and strangled voice quality in the adductor form (ADLD) and excessive breathiness in the abductor form (ABLD). Despite well-described clinical symptoms, the underlying causes of this disorder remain unknown, as challenges associated with traditional genetic studies have hindered the identification of LD-specific causative genes. On the other hand, familial history of dystonia in about 12% of LD patients 1, 2 points to the contribution of genetic risk factors. Laryngeal involvement has also been reported in the cohorts of patients with generalized or segmental dystonias who are carriers of DYT1, DYT4, DYT6 and, most recently, DYT25 mutations 3-5. However, it is rare that any of these known gene mutations result in isolated LD.
In this study, we sought to investigate the contribution of DYT1, DYT4, DYT6 and DYT25 mutations as possible genetic causes of isolated sporadic and hereditary LD using Sanger sequencing of the corresponding coding regions. Because genes have direct influence on brain organization 6, we conducted an exploratory study to examine brain activation in an identified mutation carrier compared to healthy controls as well as to sporadic and familial LD cases using functional MRI during symptomatic speech and syllable production.
Materials and Methods
A total of 57 patients with isolated LD were recruited for genetic screening of known dystonia genes TOR1A (DYT1), THAP1 (DYT6), TUBB4A (DYT4) and GNAL (DYT25), which were previously found in dystonias with laryngeal involvement. Forty-one patients (23 ADLD/18 ABLD) were sporadic and 16 patients (12 ADLD/4 ABLD) had family history of LD and/or other primary dystonias (see demographic details in Table 1). All patients except 1 ABLD (African-American/Jewish) and 1 ADLD (African-American) were of a Caucasian descent. None had any present or past history of other neurological, psychiatric, or laryngeal problems. All patients had isolated LD, which was confirmed by fiberoptic nasolaryngoscopy, without involvement of any other body regions. The average age of onset was 40.9±12.3 (mean±standard deviation) in all sporadic LD patients and 40.9±13.1 in all familial LD patients (see further demographic details per clinical phenotype in Table 1).
Table 1.
Demographics of participants
| Type of LD | Gender |
Age at exam
(mean ± s.d.) |
Age at onset
(mean ± s.d.) |
Ethnicity | |
|---|---|---|---|---|---|
| All (n = 41) | 33 F/8 M | 57.6±10.6 | 40.9±12.3 | 39C/1AA/1AA-J | |
| Sporadic LD | ADLD (n = 23) |
16 F/7 M | 57.9±10.4/59.7±9.2 | 41.2±12.6/41.9±13.1 | 22C/1AA |
| ABLD (n = 18) |
17 F/1 M | 58.8±9.4/70 | 41.5±12.4/58 | 17C/1AA-J | |
|
| |||||
| All (n = 16) | 13 F/3 M | 61.2±11.1 | 40.9±13.1 | 16C | |
| Familial LD | ADLD (n = 12) |
10 F/2 M | 56.9±10.7/58.6±10.4 | 40.6/13.3±16.8 | 12C |
| ABLD (n = 4) |
3F/1M | 58.7±10.9/63 | 40.9±15.6/31 | 4C | |
|
| |||||
| Controls | All (n = 12) | 18F/9M | 53.9±9.4 | N/A | 12C |
|
| |||||
| Handedness | All: Right on Edinburgh Inventory | ||||
|
| |||||
| Language | All: Monolingual English speakers | ||||
|
| |||||
|
Cognitive
status |
All: Mini-Mental State Examination ≥ 27 points | ||||
The average age (± standard deviation) Healthy controls – no history of neurological, psychiatric or laryngeal problems. LD – laryngeal dystonia; ADLD – adductor laryngeal dystonia; ABLD – abductor laryngeal dystonia; C – Caucasian; AA – African-American: J – Jewish; F – female; M – male; N/A – not applicable; s.d. – standard deviation.
As an exploratory study to investigate possible neural correlates of gene mutations in LD, 27 ADLD patients from the same cohort, including 13 sporadic ADLD, 13 familial ADLD and 1 mutation carrier ADLD (18 females/9 males; 58.9±9.6 years old), as well as 27 age- and gender-matched healthy controls (18 females/9 males; 53.9±9.4 years old) underwent brain functional MRI during symptomatic task production. The patients who received regular botulinum toxin injections for symptom management participated in the MRI study only if they were symptomatic, at least 3-4 months after their last injection. All subjects gave written, informed consent, which was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
Genomic DNA was isolated from blood following the Purgene procedure (Gentra Systems). PCR amplification across the GAG deletion region of the TOR1A gene was performed as previously described 7. All exons and flanking regions of the THAP1, TUBB4A and GNAL genes were sequenced as previously described 5, 8, 9. PCR products were enzymatically cleaned and sequenced by Sanger sequencing 8.
MRI data were acquired on a 3 Tesla Philips scanner with an 8-channel head coil. Whole-brain functional brain images were obtained using a gradient-weighted echo planar imaging (EPI) pulse sequence (TR = 2 s per volume and 10.6 s between volumes, TE = 30ms, FA = 90, FOV = 240 mm, voxel size = 3.75 × 3.75 mm, 36 slices with 4-mm slice thickness) and an event-related sparse-sampling experimental design during the production of LD-symptomatic sentences (e.g., “Are the olives large?”), syllables /i?i/ and resting baseline, as described earlier 10. A high-resolution T1-weighted image was obtained for anatomical reference using magnetization prepared rapid gradient echo (MPRAGE) sequence (TR = 7.5 ms, TE = 3.4 ms, TI = 819 ms, FA = 8 degrees, FOV = 210 mm, 172 slices with 1-mm slice thickness). Following standard image pre-processing and smoothing with 4-mm full-width at half-maximum Gaussian kernel, multiple linear regression was used to analyze task-related responses with a single regressor for the task convolved with a canonical hemodynamic response function and six motion parameters, including three translations along the XYZ axes and three rotations (pitch, roll, and yaw) as covariates of no interest. Statistical comparisons were performed using two-sample t-tests between all 27 ADLD patients and 27 healthy controls to confirm the presence of brain abnormalities as reported earlier 10-12, and between one ADLD mutation carrier and 26 ADLD patients without known mutations to explore the pattern of abnormalities in the mutation carrier. The statistical significance was set at a family-wise error (FWE)-corrected p ≤ 0.05.
Results
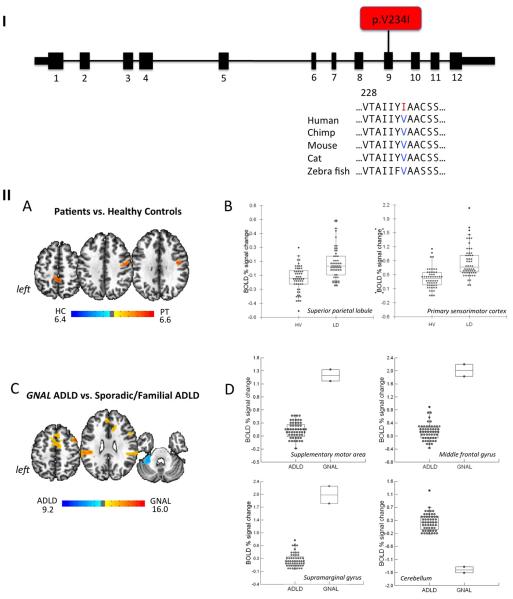
Among 57 LD patients, none were carriers of TOR1A (DYT1), THAP1 (DYT6) or TUBB4A (DYT4) mutations. However, one sporadic ADLD patient was a carrier of a novel coding variant in the GNAL (DYT25) gene. Clinically, this patient (Caucasian male, 37 y.o. at the time of initial evaluation with the onset of LD at 36 years) presented with isolated ADLD without past or present familial history of dystonia or any other movement disorders. At the four-year follow-up after the onset of disorder, the patient continued to exhibit isolated LD only, although a possibility of future spread of dystonia to other body regions cannot be ruled out. The mutation caused a G→A change in the coding region of the GNAL gene (at genomic position chr.18:11868562; hg19/GRCh37) resulting in an amino acid substitution, p.V234I (isoform NM_001142339) (Fig. 1-I). This variant is predicted by PolyPhen-2 13 to be probably damaging and by SIFT 14 to be deleterious. In addition, cross-species alignment using MutationTaster 15 (accessed on 6/11/2015) showed that this variant gives rise to an amino acid substitution in a region of GNAL that is highly conserved throughout evolution. This mutation is not present in any of the variant databases including dbSNP 142 (http://www.ncbi.nlm.nih.gov/SNP/), the Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA (URL: http://evs.gs.washington.edu/EVS/, accessed on 6/11/2015), or the Exome Aggregation Consortium (ExAC), Cambridge, MA (URL: http://exac.broadinstitute.org, accessed on 6/11/2015). This same variant was also present in his unaffected mother, confirming the reduced penetrance of GNAL mutations as shown previously 5, 16.
Figure 1. (I) Mutation identified in the GNAL gene in ADLD patient.
Schematic of the exon-intron structure of the short isoform of GNAL with a mutation shown in red. Protein sequence alignment of Gαolf across species is obtained from RefSeq database and aligned using MutationTester. Altered residue is colored in red. (II) Differences in brain activation during symptomatic voice production in the GNAL mutation carrier compared to healthy controls and patients with sporadic and familial LD. (A) Statistically significant differences in brain activation during symptomatic sentence and syllable production between all 27 LD patients, including the GNAL mutation carrier, sporadic and familial cases without known mutations, and 27 healthy controls are presented on a series of axial brains slices in the standard Talairach space (B) with the BOLD percent signal change in each individual shown on the bar charts at an FWE-correction of p ≤ 0.05. (C) Significant differences between one GNAL mutation carrier and 26 sporadic and familial LD patients are shown on a series of axial slices in the standard Talairach space with (D) the corresponding bar charts, depicting the individual levels of BOLD percent signal change during the production of symptomatic sentences and syllables at an FWE-correction p ≤ 0.05.
At the neural level, comparisons between healthy controls and all ADLD patients, including both sporadic and familial patients, as well as the GNAL carrier, showed typically increased brain activation during symptom production in the primary sensorimotor cortex and superior parietal lobule as reported previously 10, 11 (Fig. 1-IIA,B). However, when comparing the GNAL mutation carrier with a group of sporadic and familial LD patients (both groups without known dystonia mutations), significant activation increases were identified in the supplementary motor area (SMA), middle frontal gyrus (MFG) and supramarginal gyrus (SMG) that were distinctive of the GNAL mutation carrier, whereas the cerebellum was over-activate in sporadic/familial ADLD patients (Fig. 1-IIC). Mean BOLD percentage signal change in the significant clusters showed that the GNAL carrier resided significantly outside the range of values of all sporadic and familial ADLD patients (Fig. 1-IID).
Discussion
Mutations in GNAL (DYT25), the first gene identified in adult-onset dystonia 5, have been reported in approximately 0.4-1.7% of both sporadic and familial patients with predominantly cervical or cranio-cervical segmental dystonias 5, 16-23. Our finding of one GNAL carrier in 57 LD patients (1.8%) demonstrates that rare mutations in this gene do cause isolated LD, thus broadening the range of clinical phenotypes of dystonia associated with GNAL mutations. Our findings further indicate that gene mutations may underlie even sporadic presentations of dystonia due to reduced penetrance, thus stratification of patients into sporadic and familial cases remains somewhat arbitrary, pending the discovery of novel genetic factors contributing to this disorder. As none of patients from our cohort had coding mutations in TOR1A, THAP1, or TUBB4A genes, these mutations are perhaps either absent or as rare as 0.4% 24 in patients with isolated LD.
GNAL encodes the stimulatory α subunit of the G protein, Golf, and has been linked to the mediation of odorant signaling in the olfactory epithelium 25, striatal dopaminergic signaling via coupling with D1 receptors of the direct pathway, and adenosine A2A receptors of the indirect pathway 26-31, as well as being co-localized with corticotropin-releasing hormone receptors in the cerebellar Purkinje cells 16. Relevant to dystonia pathophysiology, abnormal dopaminergic function and altered structural and functional organization of the cerebellum have been previously reported in isolated LD 10-12, 32-34. As dopamine is one of the main modulators of brain function during cognitive and executive processes, the effects of striatal dopaminergic abnormalities may be reflected in aberrant fronto-parietal cortical activity, leading to additional alterations at the preparatory and sensorimotor integrative stages of motor sequence execution in the GNAL mutation carrier compared to other LD patients. On the other hand, a similar level of abnormalities in the primary sensorimotor cortex appeared to be a shared feature of brain changes across all LD patients compared to healthy controls, whereas greater cerebellar alterations in sporadic and familial cases without known genetic causes compared to the GNAL mutation carrier are suggestive of the distinct contribution of this structure to the pathophysiology of different forms of dystonia. Although this fMRI study compared a single GNAL mutation carrier to a larger group of isolated LD cases with no known mutations, our results should be interpreted with caution as they offer only initial clues about the potential links between a particular pattern of brain activity and genetic status in LD and suggest that future studies of GNAL mutation carriers in LD and other forms of isolated dystonia are warranted.
Acknowledgements
We thank Nutan Sharma, MD, PhD, for patient referrals, Amanda Pechman, Heather Alexander, and Melissa Choy for data acquisition. The authors would like to thank the Scientific Computing Department at the Icahn School of Medicine at Mount Sinai (Biomedical Research Support Shared Instrumentation Grant (S10) from the National Institutes of Health, Project # 1S10OD018522-01), and the NHLBI GO Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
Funding sources: This work was supported by a grant R01DC01180 to KS from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Footnotes
Authors’ Roles
G. G. Putzel - Research project: Data analysis; Manuscript: Review and Critique.
T. Fuchs - Research project: Data analysis; Manuscript: Review and Critique.
G. Battistella - Research project: Data analysis; Manuscript: Review and Critique.
E. Rubien-Thomas - Research project: Subject screening, Data collection and analysis; Manuscript: Review and Critique.
S. J. Frucht - Research project: Subject neurological evaluation; Manuscript: Review and Critique.
A. Blitzer - Research project: Subject laryngological evaluation; Manuscript: Review and Critique.
L. J. Ozelius – Research project: Conception, Data analysis; Manuscript: Review and Critique.
K. Simonyan - Research project: Conception, Organization, Execution; Statistical analysis; Manuscript: Review and Critique.
Relevant conflicts of interest/financial disclosures
Gregory G. Putzel, PhD, Giovanni Battistella, PhD, Tania Fuchs, PhD, Estee Rubien-Thomas, BA, have nothing to disclose.
Steven J. Frucht, MD, has received consulting fees from Merz Pharmaceutical and Impax Laboratories, Inc., unrelated to the research in this article.
Andrew Blitzer, MD, DDS, serves as an editor for Laryngoscope and the Journal of the American Academy of Otolaryngology - Head and Neck Surgery; served on the scientific advisory boards for Allergan, Inc. and Revance Therapeutics; has received honoraria for activities with Myotech; has received research support from Allergan, Inc., Merz Pharma, and Revance Therapeutics; and has received royalty payments from Xomed/Medtronics, unrelated to this study.
Laurie J. Ozelius, PhD, is a current member of the scientific advisory boards of the National Spasmodic Dysphonia Association, the Benign Essential Blepharospasm Research Foundation and Tourette Syndrome Association, Inc., and a past member of the scientific advisory boards of the Bachmann-Strauss Dystonia and Parkinson Foundation and The Dystonia Medical Research Foundation. Dr. Ozelius receives royalty payments from Athena Diagnostics related to patents.
Kristina Simonyan, MD, PhD, is a current member of the medical and scientific advisory council of the Dystonia Medical Research Foundation.
Statement of the conflict: The authors report no conflict of interest.
References
- 1.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108(10):1435–1441. doi: 10.1097/00005537-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015 doi: 10.1007/s00415-015-7751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker N. Hereditary whispering dysphonia. J Neurol Neurosurg Psychiatry. 1985;48(3):218–224. doi: 10.1136/jnnp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozelius LJ, Lubarr N, Bressman SB. Milestones in dystonia. Mov Disord. 2011;26(6):1106–1126. doi: 10.1002/mds.23775. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. 2013;45(1):88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Lindenberg A. Intermediate or brainless phenotypes for psychiatric research? Psychological medicine. 2010;40(7):1057–1062. doi: 10.1017/s0033291709991929. [DOI] [PubMed] [Google Scholar]
- 7.Ozelius LJ, Hewett JW, Page CE, et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17(1):40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs T, Gavarini S, Saunders-Pullman R, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41(3):286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 9.Lohmann K, Wilcox RA, Winkler S, et al. Whispering dysphonia (DYT4 dystonia) is caused by a mutation in the TUBB4 gene. Ann Neurol. 2013;73(4):537–545. doi: 10.1002/ana.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20(11):2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49(5):1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- 12.Haslinger B, Erhard P, Dresel C, Castrop F, Roettinger M, Ceballos-Baumann AO. "Silent event-related" fMRI reveals reduced sensorimotor activation in laryngeal dystonia. Neurology. 2005;65(10):1562–1569. doi: 10.1212/01.wnl.0000184478.59063.db. [DOI] [PubMed] [Google Scholar]
- 13.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. 2013 doi: 10.1002/0471142905.hg0720s76. Current protocols in human genetics / editorial board, Jonathan L Haines [et al] Chapter 7:Unit7 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic acids research. 2012;40(Web Server issue):W452–457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods. 2014;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 16.Vemula SR, Puschmann A, Xiao J, et al. Role of Galpha(olf) in familial and sporadic adult-onset primary dystonia. Human molecular genetics. 2013;22(12):2510–2519. doi: 10.1093/hmg/ddt102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegan J, Wittstock M, Westenberger A, et al. Novel GNAL mutations in two German patients with sporadic dystonia. Mov Disord. 2014;29(14):1833–1834. doi: 10.1002/mds.26066. [DOI] [PubMed] [Google Scholar]
- 18.Dobricic V, Kresojevic N, Westenberger A, et al. De novo mutation in the GNAL gene causing seemingly sporadic dystonia in a Serbian patient. Mov Disord. 2014;29(9):1190–1193. doi: 10.1002/mds.25876. [DOI] [PubMed] [Google Scholar]
- 19.Zech M, Gross N, Jochim A, et al. Rare sequence variants in ANO3 and GNAL in a primary torsion dystonia series and controls. Mov Disord. 2014;29(1):143–147. doi: 10.1002/mds.25715. [DOI] [PubMed] [Google Scholar]
- 20.Dufke C, Sturm M, Schroeder C, et al. Screening of mutations in GNAL in sporadic dystonia patients. Mov Disord. 2014;29(9):1193–1196. doi: 10.1002/mds.25794. [DOI] [PubMed] [Google Scholar]
- 21.Kumar KR, Lohmann K, Masuho I, et al. Mutations in GNAL: a novel cause of craniocervical dystonia. JAMA neurology. 2014;71(4):490–494. doi: 10.1001/jamaneurol.2013.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miao J, Wan XH, Sun Y, Feng JC, Cheng FB. Mutation screening of GNAL gene in patients with primary dystonia from Northeast China. Parkinsonism Relat Disord. 2013;19(10):910–912. doi: 10.1016/j.parkreldis.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Saunders-Pullman R, Fuchs T, >San Luciano M, et al. Heterogeneity in primary dystonia: lessons from THAP1, GNAL, and TOR1A in Amish-Mennonites. Mov Disord. 2014;29(6):812–818. doi: 10.1002/mds.25818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74(3):229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244(4906):790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 26.Drinnan SL, Hope BT, Snutch TP, Vincent SR. G(olf) in the basal ganglia. Molecular and cellular neurosciences. 1991;2(1):66–70. doi: 10.1016/1044-7431(91)90040-u. [DOI] [PubMed] [Google Scholar]
- 27.Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58(4):771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 28.Herve D, Levi-Strauss M, Marey-Semper I, et al. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13(5):2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herve D, Le Moine C, Corvol JC, et al. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci. 2001;21(12):4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem. 2001;76(5):1585–1588. doi: 10.1046/j.1471-4159.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 31.Herve D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. 2011;5:48. doi: 10.3389/fnana.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 2013;33(37):14705–14714. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22(2):417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simonyan K, Tovar-Moll F, Ostuni J, et al. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131(Pt 2):447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]