Abstract
Background
Heterozygous familial hypercholesterolemia (FH) affects up to 1 in 200 individuals in the US, but atherosclerotic cardiovascular disease (ASCVD) outcomes of FH in the general US population have not been described. We therefore sought to evaluate long-term coronary heart disease (CHD) and total ASCVD risks in US adults with an FH phenotype.
Methods
Using individual pooled data from 6 large US epidemiologic cohorts, we stratified participants by low-density lipoprotein cholesterol (LDL-C) level at index ages from 20 to 79 years. For the primary analysis, LDL-C levels ≥190 and <130 mg/dL defined the FH phenotype and referent, respectively. Sensitivity analyses evaluated the effects of varying the FH phenotype definition. We used Cox regression models to assess covariate-adjusted associations of the FH phenotype with 30-year hazards for CHD (CHD death or nonfatal MI) and total ASCVD (CHD or stroke).
Results
We included 68,565 baseline person-exams; 3,850 (5.6%) had the FH phenotype by the primary definition. Follow-up across index ages ranged from 78,985 to 308,378 person-years. After covariate adjustment, the FH phenotype was associated with substantially elevated 30-year CHD risk, with hazard ratios up to 5.0 (95% CI 1.1–21.7). Across index ages, CHD risk was accelerated in those with the FH phenotype, by 10–20 years in men and 20–30 years in women. Similar patterns of results were found for total ASCVD risk, with hazard ratios up to 4.1 (95% CI 1.2–13.4). Alternative FH phenotype definitions incorporating family history, more stringent age-based LDL-C thresholds, or alternative lipid fractions decreased the FH phenotype prevalence to as low as 0.2–0.4% without materially affecting CHD risk estimates (hazard ratios up to 8.0, 95% CI 1.0–61.6).
Conclusions
In the general US population, the long-term ASCVD burden related to phenotypic FH, defined by LDL-C ≥190 mg/dL, is likely substantial. Our finding of CHD risk acceleration may aid efforts in risk communication.
Keywords: coronary disease, epidemiology, hypercholesterolemia
INTRODUCTION
Heterozygous familial hypercholesterolemia (FH) affects up to 1.5 million individuals in the US, with an estimated prevalence of 1 in 250 to 1 in 200.1–3 Importantly, for those who are recognized and treated, statin use is associated with significantly decreased atherosclerotic cardiovascular disease (ASCVD) morbidity and mortality.4–7 However, it is estimated that <1% of FH cases in the US have been diagnosed,7 and suboptimal treatment is common worldwide.2,8,9 The burden of ASCVD associated with undiagnosed or undertreated FH in the general US population is unknown.
Available ASCVD risk calculators specifically exclude FH patients, since participants with FH are uncommon in the community-based samples of asymptomatic, middle-aged individuals commonly used for derivation of risk equations, and the equations therefore cannot account for the lifetime exposure to extreme low-density lipoprotein cholesterol (LDL-C) levels that FH patients experience.10 Several prior reports have estimated ASCVD risk in untreated FH,4,7,11–18 but various methodological factors limit their application when considering FH patients in the general US population.19 These studies have largely utilized non-US samples4,7,12–17 from referral-based clinic populations4,7,14,15 or a few kindred,11–13 sometimes including those with prevalent ASCVD,7,14–18 and small outcome numbers have not allowed adjustment for known ASCVD risk factors. At present, US population-based data on long-term outcomes in FH are lacking, and a recent American Heart Association statement called for research that would more clearly define the natural history of this condition.20
We therefore sought to quantify the long-term (up to 30-year) risks for coronary heart disease (CHD) and total ASCVD associated with an FH phenotype in the general US population, including younger and older adults. We defined the FH phenotype primarily by LDL-C levels ≥190 mg/dL, consistent with diagnostic criteria that maximize sensitivity.14,20–22 We pooled data from multiple large, population-based studies to ensure sufficient sample size for stable risk estimates. We hypothesized that (1) compared to US adults with average LDL-C levels, those with an FH phenotype would have significantly increased hazards for CHD, even after adjustment for other risk factors, and (2) the hazards for CHD associated with the FH phenotype would be greatest at the youngest ages.
METHODS
Study Participants
Participants for this study were drawn from the Cardiovascular Lifetime Risk Pooling Project, which pools individual-level data from up to 22 large, population-based longitudinal cohort studies with at least 10 years of follow up for cardiovascular disease (CVD) events. Details of the inclusion of cohorts and pooling and harmonization of data are presented elsewhere23,24 and summarized in Supplemental Table 1. Datasets from the Pooling Project that were included in the present analysis were required to have at least 1 baseline examination at which participants underwent direct measurement of serum lipids in addition to physiologic and anthropometric variables. The following 6 cohorts were therefore selected: Framingham Heart Study (after 1968, when full lipid profile assessment began),25 Framingham Offspring Study,26 Cardiovascular Health Study,27 Atherosclerosis Risk in Communities study (ARIC),28 Coronary Artery Risk Development in Young Adults study (CARDIA),29 and National Health and Nutrition Examination Survey III Mortality study (NHANES III)30,31 (Supplemental Table 1). Participants were excluded from the analysis if they had preexisting CVD (myocardial infarction [MI], stroke, or heart failure) prior to the initial included examination or if they were missing serum lipid data. For the primary analysis, participants using cholesterol medication at the index age were included, but a sensitivity analysis evaluated the effect of excluding this group. This project was approved by the Institutional Review Board at Northwestern University.
Lipid and Covariate Measurements
Detailed protocols for lipid measurement as well as demographic and traditional risk factor assessment have been published for each cohort.25–30 Serum cholesterol levels including total cholesterol (TC), triglycerides, and high-density lipoprotein cholesterol (HDL-C) were measured directly. LDL-C was calculated using the Friedewald equation for individuals with triglycerides <400 mg/dL.32 Non-HDL cholesterol (non-HDL-C) was calculated as the difference between TC and HDL-C. Age, sex, race, smoking status, diabetes status, hypertension treatment, cholesterol treatment, and family history of CVD were determined by self-report. For these data, answers other than “yes” were assumed to be “no.” Diabetes was also considered present if fasting blood sugar was ≥126 mg/dL or diabetes medication use was reported. Blood pressure was determined using the average of 2 to 3 measurements taken while seated using a mercury sphygmomanometer.
FH Phenotype and Referent Definitions
We selected definitions of the FH phenotype that align with commonly used criteria to identify possible FH,14,21,22 as well as the simplified diagnostic criteria for FH proposed in the recent American Heart Association statement: LDL-C ≥190 mg/dL plus a first-degree relative with similar degree of hypercholesterolemia or with premature CHD.20 For the primary analysis, we defined the FH phenotype simply as LDL-C ≥190 mg/dL to maximize sensitivity for the condition, recognizing the limitations of self-reported family history data9,33 as well as challenges in harmonizing family history data between cohorts (see Supplemental Table 1). In a sensitivity analysis, we defined it as LDL-C ≥190 mg/dL plus a family history of CVD, i.e., participant-reported history of CHD or stroke in either parent. In another sensitivity analysis, we restricted the definition further to require family history of premature CVD, i.e., participant-reported first CHD incident or stroke at ≤55 or ≤60 years of age in the father or mother, respectively. These family history data were available only for CARDIA and ARIC participants (Supplemental Table 1); thus prevalence estimates involving family history include only these two cohorts. The referent group for these analyses was defined by LDL-C <130 mg/dL; this level is near recent US population means34 and historically was a target for primary prevention. The third (middle) group included all participants who met criteria for neither the FH phenotype nor referent group (i.e., LDL-C 130–189 mg/dL for the primary analysis; and LDL-C 130–189 mg/dL or negative/missing family history with LDL-C ≥190 mg/dL for the sensitivity analyses).
Additional sensitivity analyses were performed with varying definitions of the FH phenotype also used by expert panels, including Make Early Diagnosis to Prevent Early Death (MEDPED),35 the Simon Broome Register,14 and the National Lipid Association.21 For the MEDPED analysis, age-based thresholds for the general population defined the FH phenotype for all participants: ≥220 mg/dL for age 20–29 years, ≥240 mg/dL for age 30–39 years, and ≥260 mg/dL for age 40–79 years; the referent was defined by LDL-C <130 mg/dL. The MEDPED criteria are expected to maximize specificity for molecular FH while sacrificing sensitivity (98% and 54% in the derivation sample, respectively).35 For the TC-based analysis, the FH phenotype was defined by TC ≥290 mg/dL, with the additional requirement for positive family history in a further analysis;14 the referent was TC <200 mg/dL. For the non-HDL-C-based analysis, the FH phenotype was defined by a composite of non-HDL-C ≥220 mg/dL or LDL-C ≥190 mg/dL, with the additional requirement for positive family history in a further analysis;21 the referent was defined by non-HDL-C <220 mg/dL and LDL-C <130 mg/dL.
Outcome Ascertainment
CHD events included CHD death and nonfatal MI. ASCVD events additionally included ischemic stroke. Events were ascertained according to each cohort’s specific protocol. With the exception of NHANES III, all studies adjudicated fatal and nonfatal events using standardized clinical criteria.27,28,36,37 NHANES III ascertained CHD death and ASCVD death using ICD-9 or -10 codes primarily from death certificate data through linkage to the National Death Index; nonfatal events were not ascertained.31 Participants in NHANES III contributed 4.3% to 19.5% of the total baseline person-exams at each index age (19.5%, 16.2%, 8.2%, 4.3%, 6.6%, and 10.0% for index ages 20–29, 30–39, 40–49, 50–59, 60–69, and 70–79 years, respectively).
Statistical Analyses
To understand the effects of the FH phenotype on CVD outcomes across the life course, we examined 30-year risk from different index (baseline) ages: 20–29, 30–39, 40–49, 50–59, 60–69, and 70–79 years. Analyses were by person-exam (using the earliest study examination within the index age range) and were independent for each index age; thus a given participant could contribute baseline and follow-up data for multiple index ages if he or she remained free of CVD and had lipids re-measured at the later ages. We stratified person-exams into the three groups (FH phenotype, referent, and middle group) according to lipid levels (and family history). Available follow-up data was collected from the index exam until the occurrence of a first CVD event, death, or completion of 30 years. The date of last available follow-up data ranged from 1999 to 2010 for individual cohorts (Supplemental Table 1).
We made comparisons across the three lipid groups for each index age. We compared baseline characteristics using general linear regression models for continuous variables and chi-square tests for categorical variables. We assessed risk for the primary outcome of CHD death or nonfatal MI and secondary outcomes of CHD death alone and total ASCVD (including fatal/nonfatal stroke). CHD, rather than ASCVD, was chosen as the primary outcome since the data relating LDL-C to stroke are not consistent.38 We calculated unadjusted event rates per 1000 person-years of follow-up for men and women separately. We used Cox proportional hazards regression to assess the association of the FH phenotype with outcome events, adjusting for cohort as well as for sex, age, race, body mass index, systolic blood pressure, hypertension treatment, cholesterol treatment, diabetes mellitus status, smoking status, and HDL-C level; we also generated Cox adjusted 30-year event-free survival curves. We tested proportional hazards assumptions and found them to be appropriate. We examined the interactions of sex as well as race (African American/non-African American) with LDL-C level on the outcome and found no significant interactions; we therefore present pooled, sex- and race-adjusted results, but we also performed Cox models separately by sex and race in order to examine whether patterns of association differed qualitatively. Finally, we calculated the proportion of incident CHD for the total cohort that would occur among those with the FH phenotype, independent of other CVD risk factors: we multiplied the complement of the 30-year Cox adjusted survival probabilities (i.e., the event probabilities) by the calculated prevalence of each LDL-C group, summed these to generate a weighted estimate of total event probability for the cohort, and divided the individual group CHD event estimates (i.e., the product of prevalence and 30-year event probability) by this total event probability estimate to give the proportion of CHD occurring in that LDL-C group. For all comparisons, a two-tailed p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Inc., Cary, NC), and the PROC PHREG procedure was used to compute Cox Proportional hazard regression models.
RESULTS
Participant Characteristics
Baseline characteristics of the FH phenotype and referent groups, stratified by index age, are shown in Table 1 and Supplemental Table 2. (Supplemental Table 3 shows baseline characteristics separately by sex.) Using the primary definition, the FH phenotype (LDL-C ≥190 mg/dL) group contributed 3,850 baseline person-exams and comprised 1.4% to 7.9% of the overall cohort at each index age; the prevalence most closely approached the reported FH prevalence of 0.4–0.5% at the youngest index ages but was substantially higher for older index ages. Across the index ages of the FH phenotype group, mean LDL-C level was 210 to 214 mg/dL and family history of CVD was reported in 33 to 46 percent. Compared to the referent group with LDL-C <130 mg/dL, those with the FH phenotype had a higher burden of other CVD risk factors on average (Table 1, Supplemental Table 2). There were low levels of cholesterol treatment at baseline, ranging from 0% to 7.9% for the FH phenotype group (significantly higher than for the referent group only at index ages 30–39 and 40–49 years).
Table 1.
Baseline characteristics of the familial hypercholesterolemia phenotype (LDL-C ≥190 mg/dL) and referent (LDL-C <130 mg/dL) groups, by index age.*
| Index Age and LDL Cholesterol Category | ||||||
|---|---|---|---|---|---|---|
| Index Age, Years | 20–29 | 30–39 | 40–49 | |||
| Category of baseline LDL cholesterol, mg/dL |
<130 (referent) |
≥190 (FH phenotype) |
<130 (referent) |
≥190 (FH phenotype) |
<130 (referent) |
≥190 (FH phenotype) |
| Baseline person-exams | 5,607 (76%) | 106 (1.4%) | 6,001 (70%) | 214 (2.5%) | 7,534 (59%) | 591 (4.6%) |
| Follow-up, person-years | 135,950 | 2,534 | 127,398 | 4,676 | 123,942 | 11,270 |
| Female | 3,120 (56%) | 57 (54%) | 3,581 (60%) | 70 (33%)† | 4,697 (62%) | 249 (42%)† |
| African-American | 2,133 (38%) | 48 (45%) | 1,976 (33%) | 63 (29%) | 2,124 (28%) | 154 (26%) |
| Total cholesterol, mg/dL | 165 (24) | 283 (31)† | 169 (24) | 282 (31)† | 176 (25) | 289 (34)† |
| LDL cholesterol, mg/dL | 96 (20) | 213 (38)† | 98 (20) | 210 (24)† | 100 (21) | 213 (28)† |
| HDL cholesterol, mg/dL | 53 (14) | 49 (13)† | 53 (15) | 45 (11)† | 54 (17) | 46 (13)† |
| Non-HDL cholesterol, mg/dL | 112 (23) | 234 (30)† | 116 (24) | 236 (29)† | 122 (26) | 243 (32)† |
| Systolic blood pressure, mm Hg | 111 (11) | 114 (12)† | 111 (13) | 118 (16)† | 116 (16) | 123 (18)† |
| Diastolic blood pressure, mm Hg | 69 (9) | 72 (11)† | 72 (10) | 77 (11)† | 75 (11) | 79 (12)† |
| Body mass index, kg/m2 | 24.2 (4.7) | 26.2 (5.0)† | 25.9 (5.8) | 27.7 (4.9)† | 27.5 (6.3) | 28.5 (4.9)† |
| Smoking | 2,000 (36%) | 46 (43%) | 2,100 (35%) | 112 (53%)† | 2,211 (29%) | 259 (44%)† |
| Diabetes mellitus | 28 (0.5%) | 1 (0.9%) | 92 (1.5%) | 5 (2.3%) | 405 (5.4%) | 35 (5.9%) |
| Family history of CVD‡ | 800 (23%) | 21 (35%)† | 844 (25%) | 26 (33%) | 1,418 (30%) | 119 (39%)† |
| Hypertension treatment at baseline | 69 (1.2%) | 3 (2.8%) | 112 (1.9%) | 10 (4.7%)† | 891 (11.8%) | 102 (17.3%)† |
| Cholesterol treatment at baseline | 2 (0.1%) | 0 | 6 (0.1%) | 4 (2.3%)† | 172 (2.3%) | 26 (4.4%)† |
Supplemental Table 2 shows baseline characteristics for index ages 50–79 years.
p<0.05 for the comparison between participants at same index age with LDL-C ≥190 vs. <130 mg/dL.
Estimate among cohorts with family history data available (see Supplemental Table 1).
Continuous data presented as mean (SD), categorical data presented as N(%).
LDL, low-density lipoprotein; LDL-C, LDL cholesterol; FH, familial hypercholesterolemia; CVD, cardiovascular disease; HDL, high-density lipoprotein.
Long-term CHD Risk Associated with the FH Phenotype, Defined by LDL-C ≥190 mg/dL
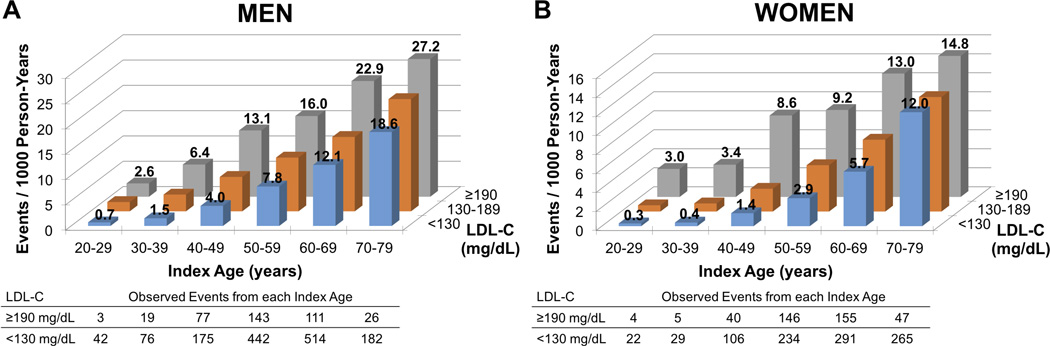
Figure 1 shows the unadjusted event rates and underlying observed event numbers for men and women in the FH phenotype and referent groups across index ages. Unadjusted event rates were similar for men and women with the FH phenotype for the index age 20–29 years, but for older index ages the rates in men were almost double those in women. Conversely, age-based acceleration of CHD risk in comparison to the referent group was greater for women than for men. For men with the FH phenotype (Figure 1A), the event rates for each index age were generally equivalent to those for men with average LDL-C (i.e., <130 mg/dL) who were 10 to 20 years older, indicating that CHD risk was accelerated by 10 to 20 years. For example, the event rate for 20–29-year-old men with the FH phenotype was 2.6 per 1000 person-years, which was equivalent to the rate for men between 30–39 (1.5/1000 person-years) and 40–49 years (4.0/1000 person-years) with average LDL-C. A similar pattern was evident for women with the FH phenotype (Figure 1B), but for women, CHD risk was accelerated by 20 to 30 years.
Figure 1. Unadjusted rates of coronary heart disease death or nonfatal myocardial infarction per 1000 person-years, and underlying observed event numbers, in (A) men and (B) women.
Unadjusted event rates are graphed by index age and LDL-C category; LDL-C ≥190 mg/dL represents the FH phenotype and LDL-C <130 mg/dL represents the referent. For men (A), event rates in the FH phenotype group at any given age are equivalent to those for men in the referent group who are 10 to 20 years older, indicating acceleration of CHD risk by 10 to 20 years. For women (B), CHD risk is accelerated by 20 to 30 years. The tables show observed event numbers for the FH phenotype and referent groups, by sex and index age.
LDL-C, low-density lipoprotein cholesterol, FH, familial hypercholesterolemia.
Table 2 (left columns) shows the hazards for CHD death or nonfatal MI associated with having the FH phenotype compared with an average LDL-C level, after adjustment for cohort, sex, and baseline covariates. The hazards for CHD in the FH phenotype group were age-dependent, with up to five-fold increased risk for the index age 20–29 years, lower relative hazards at older ages, and a nonsignificant increase in risk for age 70–79 years. Exclusion of participants taking cholesterol medication at baseline did not significantly affect the findings (results not shown). There was no significant interaction between sex and LDL-C level on the hazard ratios for the FH phenotype, but at younger index ages, hazard ratio point estimates were higher for women than for men (Table 2, right columns). There was also no significant interaction between race (African American/non-African American) and LDL-C level on the hazard ratios, and hazard ratios were comparable between race groups (Supplemental Table 4).
Table 2.
Overall and sex-specific familial hypercholesterolemia phenotype (defined by LDL-C ≥190 mg/dL) prevalence and coronary heart disease death or nonfatal myocardial infarction at each index age.
| Overall | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index Age (years) |
Observed Events |
Adjusted* Hazard Ratio† (95% CI) |
FH Phenotype Prevalence (%) |
Adjusted* Proportion of all CHD Events‡ (%) |
Observed Events |
Adjusted* Hazard Ratio† (95% CI) |
FH Phenotype Prevalence (%) |
Adjusted* Proportion of all CHD Events‡ (%) |
Observed Events |
Adjusted* Hazard Ratio† (95% CI) |
FH Phenotype Prevalence (%) |
Adjusted* Proportion of all CHD Events‡ (%) |
| 20–29 | 7 | 5.0§ (1.1–21.7) |
1.4 | 5.1 | 3 | 2.7§ (0.8–8.9) |
1.5 | 3.3 | 4 | 7.8§ (2.6–23.3) |
1.4 | 8.1 |
| 30–39 | 24 | 3.1 (1.8–5.5) |
2.5 | 6.6 | 19 | 3.6 (2.1–6.1) |
3.7 | 8.5 | 5 | 6.8§ (3.3–19.9) |
1.5 | 6.4 |
| 40–49 | 117 | 2.9 (2.3–3.6) |
4.6 | 10.0 | 77 | 2.8 (2.1–3.7) |
6.0 | 11.5 | 40 | 3.5 (2.4–5.3) |
3.5 | 8.8 |
| 50–59 | 289 | 2.0 (1.7–2.3) |
7.8 | 11.6 | 143 | 2.1 (1.7–2.5) |
6.8 | 9.6 | 146 | 2.2 (1.8–2.8) |
8.6 | 13.5 |
| 60–69 | 266 | 1.9 (1.7–2.2) |
7.9 | 10.6 | 111 | 2.0 (1.6–2.5) |
5.6 | 7.3 | 155 | 2.0 (1.6–2.5) |
9.9 | 13.7 |
| 70–79 | 73 | 1.3 (1.0–1.7)‖ |
5.1 | 5.8 | 26 | 1.7 (1.1–2.6) |
3.2 | 4.0 | 47 | 1.1 (0.8–1.5) |
6.6 | 6.9 |
Adjusted for: age, race, body mass index, diabetes mellitus, smoking, systolic blood pressure, hypertension treatment, and cohort; data for the overall analysis have also been adjusted for sex, HDL-C, and cholesterol treatment.
Referent is (sex-specific) participants with LDL-C <130 mg/dL.
Proportion of total index age cohort 30-year CHD events projected to occur in the FH phenotype group.
Estimates may be unstable due to small numbers of events.
p>0.05
LDL-C, low-density lipoprotein cholesterol; FH, familial hypercholesterolemia; CI, confidence interval; CHD, coronary heart disease; HDL-C, high-density lipoprotein cholesterol.
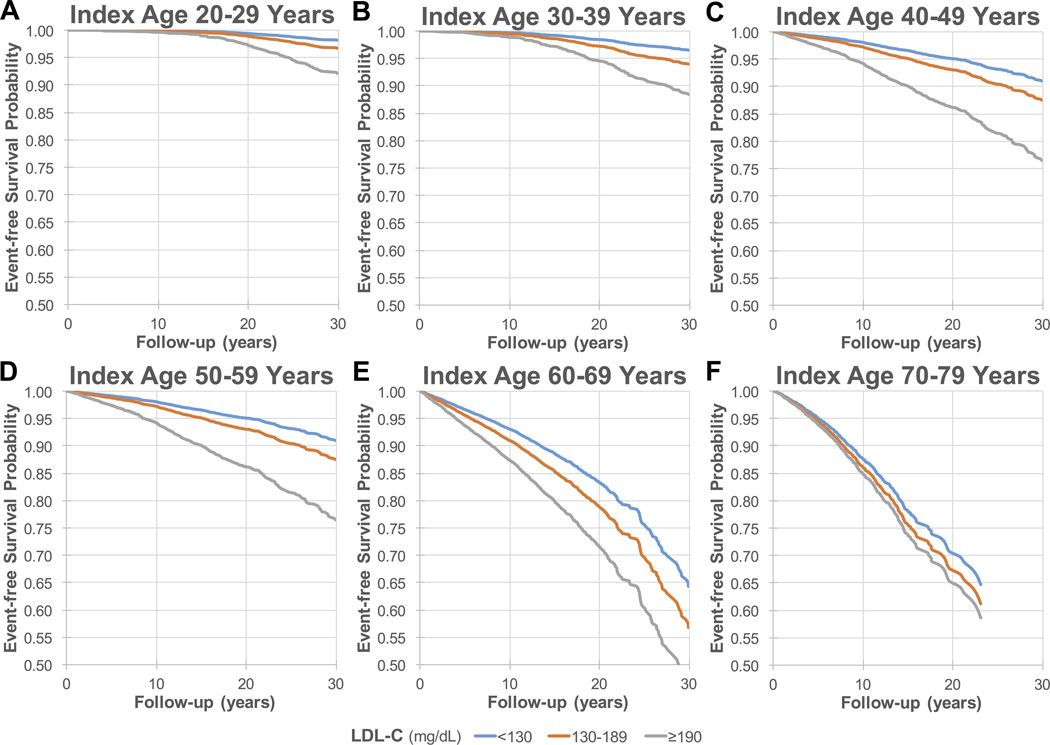
Figure 2 shows adjusted 30-year event-free survival curves for each index age. The survival curves for the FH phenotype group diverge progressively from those for the referent and middle groups, particularly for younger index ages.
Figure 2. Adjusted* 30-year survival free from coronary heart disease death or nonfatal myocardial infarction for index ages (A) 20–29, (B) 30–39, (C) 40–49, (D) 50–59, (E) 60–69, and (F) 70–79† years.
Over 30 years of follow-up, event-free survival is progressively worse for the FH phenotype group (LDL-C ≥190 mg/dL) than for the other two groups. Baseline mean (SD) LDL-C levels (mg/dL) for the LDL-C <130 (referent), 130–189, and ≥190 mg/dL (FH phenotype) groups were respectively: (A) 20–29 years: 96 (20), 148 (15), 213 (38); (B) 30–39 years: 98 (20), 150 (15), 210 (24); (C) 40–49 years: 100 (21), 152 (16), 213 (28); (D) 50–59 years: 100 (26), 154 (16), 214 (25); (E) 60–69 years: 101 (26), 154 (16), 213 (27); and (F) 70–79 years: 100 (25), 153 (16), 213 (22).
*Adjusted for: age, sex, race, body mass index, diabetes mellitus, smoking, systolic blood pressure, antihypertensive therapy, high-density lipoprotein cholesterol, cholesterol treatment, and cohort.
†Follow-up for index age 70–79-years is truncated at 23 years, due to insufficient sample size thereafter.
LDL-C, low-density lipoprotein cholesterol.
Secondary Analyses: Long-term Risk for CHD Death Alone or Total ASCVD Associated with the FH Phenotype, Defined by LDL-C ≥190 mg/dL
In the analyses focusing on the outcomes of CHD death alone (Supplemental Table 5) and total ASCVD (Supplemental Table 6), results approximated those for the primary outcome of CHD death or nonfatal MI. Hazard ratios were similar, and the width of the confidence intervals varied with the numbers of observed events.
Sensitivity Analyses: Varying Definitions of the FH Phenotype
Table 3 shows observed CHD event numbers and adjusted hazard ratios related to having the FH phenotype, defined in various ways. When family history was added to the LDL-C-based definition, the FH phenotype prevalence (0.5–3.3%) more closely approached the reported FH prevalence (0.4–0.5%), and adjusted hazard ratios for CHD events approximated those in the primary analysis, with slightly higher point estimates but overlapping confidence intervals (Table 3). When the FH phenotype definition was further restricted to require family history of premature CVD, the prevalence of this group declined further (0.1–0.8%), but again the pattern of CHD risk was unchanged (results not shown; too few events for stable risk estimates). When MEDPED LDL-C criteria were used to maximize specificity for molecular FH, the prevalence of the FH phenotype was 0.2–0.4 percent. CHD risk was qualitatively more striking in this analysis, with higher hazard ratio point estimates and statistical significance for even the oldest index age; however, confidence intervals were wide and overlapped those for the primary analysis (Table 3). When the FH phenotype was defined by alternative lipid fractions (TC or non-HDL-C), patterns were again unchanged (Table 3).
Table 3.
Observed outcome events and adjusted hazard ratios for coronary heart disease death or nonfatal myocardial infarction associated with having the familial hypercholesterolemia phenotype, using various definitions.
| Familial Hypercholesterolemia Phenotype Definition | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary definition: LDL-C ≥190 mg/dL |
LDL-C ≥190 mg/dL plus Family History |
MEDPED LDL-C Thresholds* |
TC ≥290 mg/dL | TC ≥290 mg/dL plus Family History |
Non-HDL-C ≥220 or LDL- C ≥190 mg/dL |
Non-HDL-C ≥220 or LDL- C ≥190 mg/dL plus Family History |
||||||||
| Index Age (years) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
Observed Events s |
Adjusted Hazard Ratio† (95% CI) |
Observed Events |
Adjusted Hazard Ratio† (95% CI) |
| 20–29 | 7 | 5.0‡ (1.1–21.7) |
2 | § | 2 | § | 3 | 8.0‡ (1.0–61.6)¶ |
0 | § | 7 | 2.6‡ (0.6–11.6) |
2 | § |
| 30–39 | 24 | 3.1 (1.8–5.5) |
4 | 5.7‡ (1.3–24.5) |
3 | 7.2‡ (1.0–52.7)‖ |
10 | 4.9‡ (2.2–10.7) |
2 | § | 31 | 3.0 (1.8–5.0) |
4 | 5.4‡ (1.3–23.0) |
| 40–49 | 117 | 2.9 (2.3–3.6) |
24 | 3.5 (2.3–5.4) |
8 | 6.0‡ (3.0–12.3) |
55 | 3.2 (2.4–4.4) |
10 | 3.6‡ (1.9–6.9) |
159 | 2.6 (2.1–3.2) |
32 | 3.8 (2.6–5.6) |
| 50–59 | 289 | 2.0 (1.7–2.3) |
77 | 2.1 (1.7–2.7) |
18 | 2.7 (1.7–4.3) |
185 | 2.3 (1.9–2.7) |
46 | 2.8 (2.0–3.8) |
406 | 1.9 (1.7–2.2) |
102 | 2.3 (1.8–2.8) |
| 60–69 | 266 | 1.9 (1.7–2.2) |
63 | 2.0 (1.5–2.6) |
12 | 2.0 (1.1–3.5) |
172 | 2.3 (1.9–2.7) |
33 | 3.0 (2.1–4.3) |
396 | 1.9 (1.6–2.1) |
91 | 2.3 (1.8–2.8) |
| 70–79 | 73 | 1.3 (1.0–1.7)‖ |
3 | 1.7‡ (0.5–5.2) |
6 | 2.9‡ (1.1–7.7) |
59 | 1.5 (1.1–2.1) |
2 | § | 112 | 1.2 (0.9–1.5) |
5 | 1.4‡ (0.6–3.4) |
Age-based: ≥220 mg/dL (20–29 years), ≥240 mg/dL (30–39 years), and ≥260 mg/dL (40–79 years).
Adjusted for: age, sex, race, body mass index, diabetes mellitus, smoking, systolic blood pressure, hypertension treatment, cholesterol treatment, and cohort; LDL-C and TC analyses also adjusted for HDL-C level. Referent groups are participants with: LDL-C <130 mg/dL (LDL-C analyses); TC <200 mg/dL (TC analysis); and LDL-C <130 and non-HDL-C <220 mg/dL (non-HDL-C analysis).
Estimates may be unstable due to small numbers of events.
Hazard ratio estimate not reported due to paucity of events.
p>0.05
p<0.05
LDL-C, low-density lipoprotein cholesterol; MEDPED, Make Early Diagnosis to Prevent Early Deaths; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; CI, confidence interval.
Population Impact: Proportion of Total Cohort CHD Related to the FH phenotype, Defined by LDL-C ≥190 mg/dL
Table 2 also shows the proportion of incident CHD for the total cohort at each index age projected to occur in those with the FH phenotype, independent of other CVD risk factors. For the combined group of men and women, that proportion is more than double the prevalence of the FH phenotype for younger index ages. The disproportionate CHD burden is more prominent for women than for men; for the index age 20–29 years, 8.1% of CHD events in women would occur in the 1.4% of women with the FH phenotype, while 3.3% of CHD events in men would occur in the 1.5% of men with the FH phenotype.
DISCUSSION
This analysis provides novel information about the risks of CHD and total ASCVD (including stroke) associated with having the FH phenotype in a broad US population-based sample. The principal finding is that the long-term CHD and ASCVD risks for US adults with the FH phenotype, defined simply by LDL-C ≥190 mg/dL, are substantially elevated (up to approximately 5-fold) compared to those with average levels of LDL-C, even after adjustment for other CVD risk factors. This increased risk is age-dependent, with the highest relative risks seen for younger index ages. Moreover, across the spectrum of baseline (index) ages, individuals with the FH phenotype experience an acceleration of CHD risk by 10 to 20 years in men and 20 to 30 years in women. The addition of family history or use of maximally specific LDL-C criteria or alternative lipid fractions to define the FH phenotype did not materially affect the pattern of results, although power was limited for some analyses.
Findings in Context
Prior reports have examined the natural history of FH in specific settings and found substantial, though variable, elevation of CHD and stroke risk, with similar risk patterns to those in the present analysis. For example, age-dependency of risk has previously been observed in FH patients, such as in the Simon Broome Register,14 just as it is observed in the general population38 (in which it probably reflects a higher genetic contribution to hypercholesterolemia that develops earlier in life). In the Register’s UK referral clinic patients, the overall risk for CHD death was 3.9 times that in the general population, but age-specific relative risk was 97 for ages 20 to 39 years while it was not increased for ages 60 years and greater. This age-dependency is likely related to both accumulation of risk in the referent group with aging as well as survivor bias, with survival of lower-risk FH patients to older ages as the higher-risk patients have succumbed at younger ages. Similarly, acceleration of risk in FH patients has been observed previously. Stone et al18 and Jensen et al12 demonstrated acceleration of risk for MI or CHD death by 14–20 years in men and women with FH compared to unaffected relatives. Finally, Stone et al also found greater relative risks for women than for men,18 a finding that was suggested by our analysis, though it did not reach statistical significance. Notably in both studies, the greater relative risks for women are observed despite higher absolute risks in men, and therefore are largely a result of the lower-risk referent group in women versus men.
The numeric risk estimates in this analysis do differ from those in some prior reports, but where marked differences exist, they can likely be explained by differences in study methodology. For example, the Simon Broome report was based on a referral population in which 32% of participants had prevalent CVD at registration, and no adjustment was made for other CVD risk factors.14 In contrast, the present analysis benefited from a large US population-based sample, including a substantial proportion of African Americans, and risk estimates were fully adjusted. These are important distinctions, since prior studies clearly indicate a wide range of CHD risk among those with FH, likely due to genetic factors as well as gene-environment interactions.9,11,13 Our findings therefore may provide unique information about the burden of disease for the broader group with the FH phenotype, including those who remain undiagnosed.
Limitations
Some limitations to our study design may have led to underestimation of the risk for CHD and ASCVD in molecularly or clinically diagnosed FH. First, because our aim was to estimate risk related to FH in the general population, we had to rely on a “phenotype” rather than genetic diagnosis. Although we employed criteria used clinically for diagnosis, we relied on a single LDL-C measurement, did not rule out secondary causes of hypercholesterolemia (e.g., thyroid disease, nephrosis), and had limited family history data. Therefore, we almost certainly included some individuals with polygenic or secondary LDL-C elevation, as evidenced by a higher-than-expected prevalence of the FH phenotype (defined by LDL-C ≥190 mg/dL), especially at older ages. Importantly, when we required positive family history or used the stringent MEDPED LDL-C criteria to define the FH phenotype, the risk estimates did not change significantly, although power was limited. Second, although our results were unchanged after excluding individuals using cholesterol-lowering medication at baseline, we could not identify nor account for initiation of therapy during follow-up. The vast majority of our data were collected prior to the widespread use of statins, but whatever statin use existed would lead to underestimation of risk for the natural history of FH. Third, one of the six included cohorts, NHANES III, recorded mortality outcomes only. To the extent that nonfatal MI or stroke occurred without subsequent ASCVD death during follow-up in this cohort, risk for the combined outcomes of CHD death or nonfatal MI and total ASCVD would be underestimated. However, given that the NHANES III cohort contributed a minority (4.3–19.5%) of person-exams, this effect is probably small. Finally, as with any population-level risk estimates, our data likely underestimate risk for certain individuals, such as the higher risk patients presenting to referral centers with significant personal or family histories.
Implications
The present study has important public health implications in that it confirms the substantial elevation in ASCVD risk for adults with the FH phenotype in a US population-based sample. Notably, this is true even when using a simple threshold of LDL-C ≥190 mg/dL to identify the FH phenotype, which provides support for the simplified FH diagnostic criteria proposed in the recent American Heart Association statement.20 In combination with prior work suggesting the vast under-diagnosis of FH,2,9 our data indicate that there is likely to be an important long-term burden of ASCVD in phenotypic but unrecognized FH patients in the US. This is consistent with international reports that the prevalence of FH among young patients with MI may be many times higher than the prevalence of FH in the general population, from ~2–4% of patients <60 years of age3,39 to almost 20% in patients <45 years of age.39 In light of this amplification of FH prevalence among those with CHD, economic modeling in the UK has shown that identification and treatment of FH patients is not only cost-effective, but cost-saving.8 Several recent publications have emphasized the need for improved screening, awareness and care of FH in the US,2,8,10,20,40 and through the ongoing CASCADE-FH (Cascade Screening for Awareness and Detection of Familial Hypercholesterolemia) registry, the FH foundation is working to identify patterns and gaps in diagnosis and management.9 Our findings support the importance of this work.
Our data also have clinical implications for risk communication. Building on prior reports, we confirmed that FH patients not only have a higher risk for CHD but also have a shift in that risk toward younger ages. Clinician-patient discussions about guideline-supported therapies can be informed by this data, as in the following scenario: a 25-year-old woman with newly diagnosed FH can be informed that at her current age, if her cholesterol were to remain untreated, her risk of CHD death or nonfatal MI is comparable to that for a 55-year-old woman. Such an analogy, paired with counseling about how to improve risk, may motivate behavioral changes as well as adoption of and adherence to evidence-based medications.41
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
This is the first prospective evaluation of atherosclerotic cardiovascular disease (ASCVD) outcomes for adults with heterozygous familial hypercholesterolemia (FH) in the general US population. Using individual pooled data from 6 epidemiologic cohorts (68,565 baseline person-exams and 1.2 million person-years of follow-up), we confirmed substantially elevated long-term (up to 30-year) risks for coronary heart disease (CHD) and total ASCVD (including stroke) in US adults with an FH phenotype, with acceleration of CHD risk by up to 20 to 30 years.
The findings were independent of other risk factors and were consistent using various definitions of the FH phenotype.
What are the clinical implications?
Our results indicate that there is likely to be an important long-term burden of ASCVD in phenotypic but unrecognized FH patients in the US, and current efforts to identify patterns and gaps in diagnosis and management are well justified.
Our findings on risk acceleration also have implications for risk communication, as clinicians can use our data to provide patients with a vivid, personalized risk message in the discussion about guideline-supported therapies.
Acknowledgments
None.
FUNDING SOURCES: The Cardiovascular Lifetime Risk Pooling Project has been supported by R21 HL085375 from the National Heart, Lung, and Blood Institute, and by institutional funds from Northwestern University Feinberg School of Medicine.
Footnotes
DISCLOSURES: None.
REFERENCES
- 1.de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES) Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791. [DOI] [PubMed] [Google Scholar]
- 2.Knowles JW, O'Brien EC, Greendale K, Wilemon K, Genest J, Sperling LS, Neal WA, Rader DJ, Khoury MJ. Reducing the burden of disease and death from familial hypercholesterolemia. Am Heart J. 2014;168:807–811. doi: 10.1016/j.ahj.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do R, Stitziel NO, Won HH, Jorgensen AB, Duga S, Angelica Merlini P, Kiezun A, Farrall M, Goel A, Zuk O, Guella I, Asselta R, Lange LA, Peloso GM, Auer PL, Girelli D, Martinelli N, Farlow DN, DePristo MA, Roberts R, Stewart AF, Saleheen D, Danesh J, Epstein SE, Sivapalaratnam S, Hovingh GK, Kastelein JJ, Samani NJ, Schunkert H, Erdmann J, Shah SH, Kraus WE, Davies R, Nikpay M, Johansen CT, Wang J, Hegele RA, Hechter E, Marz W, Kleber ME, Huang J, Johnson AD, Li M, Burke GL, Gross M, Liu Y, Assimes TL, Heiss G, Lange EM, Folsom AR, Taylor HA, Olivieri O, Hamsten A, Clarke R, Reilly DF, Yin W, Rivas MA, Donnelly P, Rossouw JE, Psaty BM, Herrington DM, Wilson JG, Rich SS, Bamshad MJ, Tracy RP, Cupples LA, Rader DJ, Reilly MP, Spertus JA, Cresci S, Hartiala J, Tang WH, Hazen SL, Allayee H, Reiner AP, Carlson CS, Kooperberg C, Jackson RD, Boerwinkle E, Lander ES, Schwartz SM, Siscovick DS, McPherson R, Tybjaerg-Hansen A, Abecasis GR, Watkins H, Nickerson DA, Ardissino D, Sunyaev SR, O'Donnell CJ, Altshuler D, Gabriel S, Kathiresan S. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versmissen J, Oosterveer DM, Yazdanpanah M, Defesche JC, Basart DC, Liem AH, Heeringa J, Witteman JC, Lansberg PJ, Kastelein JJ, Sijbrands EJ. Efficacy of statins in familial hypercholesterolaemia. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scientific Steering Committee, Simon Broome Register Group. Mortality in treated heterozygous familial hypercholesterolaemia. Atherosclerosis. 1999;142:105–112. [PubMed] [Google Scholar]
- 6.Neil A, Cooper J, Betteridge J, Capps N, McDowell I, Durrington P, Seed M, Humphries SE. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkas F, Elisaf M, Milionis H. Statins decrease the risk of stroke in individuals with heterozygous familial hypercholesterolemia. Atherosclerosis. 2015;243:60–64. doi: 10.1016/j.atherosclerosis.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population. Eur Heart J. 2013;34:3478a–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.deGoma EM, Ahmad ZS, O'Brien EC, Kindt I, Shrader P, Newman CB, Pokharel Y, Baum SJ, Hemphill LC, Hudgins LC, Ahmed CD, Gidding SS, Duffy D, Neal W, Wilemon K, Roe MT, Rader DJ, Ballantyne CM, Linton MF, Duell PB, Shapiro MD, Moriarty PM, Knowles JW. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States. Circ Cardiovasc Genet. 2016 doi: 10.1161/CIRCGENETICS.116.001381. [published online March 24 2016]. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles JW, Stone NJ, Ballantyne CM. Familial hypercholesterolemia and the 2013 American College of Cardiology/American Heart Association guidelines. Am J Cardiol. 2015;116:481–484. doi: 10.1016/j.amjcard.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 11.Williams RR, Hasstedt SJ, Wilson DE, Ash KO, Yanowitz FF, Reiber GE, Kuida H. Evidence that men with familial hypercholesterolemia can avoid early coronary death. JAMA. 1986;255:219–224. [PubMed] [Google Scholar]
- 12.Jensen J, Blankenhorn DH, Kornerup V. Coronary disease in familial hypercholesterolemia. Circulation. 1967;36:77–82. doi: 10.1161/01.cir.36.1.77. [DOI] [PubMed] [Google Scholar]
- 13.Sijbrands EJ, Westendorp RG, Defesche JC, de Meier PH, Smelt AH, Kastelein JJ. Mortality over two centuries in large pedigree with familial hypercholesterolaemia. BMJ. 2001;322:1019–1023. doi: 10.1136/bmj.322.7293.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scientific Steering Committee, Simon Broome Register Group. Risk of fatal coronary heart disease in familial hypercholesterolaemia. BMJ. 1991;303:893–896. doi: 10.1136/bmj.303.6807.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slack J. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 1969;2:1380–1382. doi: 10.1016/s0140-6736(69)90930-1. [DOI] [PubMed] [Google Scholar]
- 16.Krogh HW, Mundal L, Holven KB, Retterstol K. Patients with familial hypercholesterolaemia are characterized by presence of cardiovascular disease at the time of death. Eur Heart J. 2016;37:1398–1405. doi: 10.1093/eurheartj/ehv602. [DOI] [PubMed] [Google Scholar]
- 17.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 18.Stone NJ, Levy RI, Fredrickson DS, Verter J. Coronary artery disease in 116 kindred with familial type II hyperlipoproteinemia. Circulation. 1974;49:476–488. doi: 10.1161/01.cir.49.3.476. [DOI] [PubMed] [Google Scholar]
- 19.Austin MA, Hutter CM, Zimmern RL, Humphries SE. Familial hypercholesterolemia and coronary heart disease. Am J Epidemiol. 2004;160:421–429. doi: 10.1093/aje/kwh237. [DOI] [PubMed] [Google Scholar]
- 20.Gidding SS, Ann Champagne M, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes-Virella M, Watts GF, Wierzbicki AS. The agenda for familial hypercholesterolemia. Circulation. 2015;132:2167–2192. doi: 10.1161/CIR.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. Familial hypercholesterolemias. J Clin Lipidol. 2011;5:S9–S17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Familial hypercholesterolemia. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 23.Wilkins JT, Karmali KN, Huffman MD, Allen NB, Ning H, Berry JD, Garside DB, Dyer A, Lloyd-Jones DM. Data resource profile: the Cardiovascular Disease Lifetime Risk Pooling Project. Int J Epidemiol. 2015;44:1557–1564. doi: 10.1093/ije/dyv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 26.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 27.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 28.The ARIC investigators. The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 29.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat. 1994;1:1–407. [PubMed] [Google Scholar]
- 31.The Third National Health and Nutrition Examination Survey (NHANES III) linked mortality file, mortality follow-up through 2006. Hyattsville, Maryland: National Center for Health Statistics; 2009. Office of Analysis and Epidemiology. [Google Scholar]
- 32.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 33.Murabito JM, Nam BH, D'Agostino RB, Sr, Lloyd-Jones DM, O'Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics--2016 update. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 35.Williams RR, Hunt SC, Schumacher MC, Hegele RA, Leppert MF, Ludwig EH, Hopkins PN. Diagnosing heterozygous familial hypercholesterolemia using new practical criteria validated by molecular genetics. Am J Cardiol. 1993;72:171–176. doi: 10.1016/0002-9149(93)90155-6. [DOI] [PubMed] [Google Scholar]
- 36.The CARDIA Endpoints Surveillance and Adjudication Committee. CARDIA endpoint events manual of operations. [Accessed March 1, 2016]; http://www.cardia.dopm.uab.edu/images/more/pdf/MM_endpoints_followup/MM_endpoints/CARDIA%20Endpoint%20Events%20MOO%20v2014-06-30.pdf. Published June 30, 2014. [Google Scholar]
- 37.Abbott R, McGee D. The Framingham Study. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. [Google Scholar]
- 38.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 39.Sniderman AD, Tsimikas S, Fazio S. The severe hypercholesterolemia phenotype. J Am Coll Cardiol. 2014;63:1935–1947. doi: 10.1016/j.jacc.2014.01.060. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg AC, Robinson JG, Cromwell WC, Ross JL, Ziajka PE. Future issues, public policy, and public awareness of familial hypercholesterolemias. J Clin Lipidol. 2011;5:S46–S51. doi: 10.1016/j.jacl.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Goldman RE, Parker DR, Eaton CB, Borkan JM, Gramling R, Cover RT, Ahern DK. Patients' perceptions of cholesterol, cardiovascular disease risk, and risk communication strategies. Ann Fam Med. 2006;4:205–212. doi: 10.1370/afm.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.