Abstract
Background
Cancer survivors are a growing population, due in large part to the aging of the baby boomer generation and the related ‘silver tsunami’ facing the US health system. Understanding the impact of a graying nation on cancer prevalence and co-morbidity burden is critical in informing efforts to design and implement quality cancer care for this population.
Methods
Incidence and survival data from 1975–2011 were obtained from the Surveillance, Epidemiology and End Results (SEER) Program to estimate current cancer prevalence. SEER-Medicare claims data were used to estimate co-morbidity burden. Prevalence projections were made using US Census Bureau data and the Prevalence Incidence Approach Model, assuming constant future incidence and survival trends but dynamic projections of the US population.
Results
In 2016, there were an estimated 15.5 million cancer survivors living in the US, 62 percent of whom were 65 years or older. The prevalent population is projected to grow to 26.1 million by 2040, and include 73 percent of survivors who are 65 years and older. Co-morbidity burden was highest in the oldest survivors (those ≥85 years) and worst among lung cancer survivors.
Conclusions
Older adults, who often present with complex health needs, now constitute the majority of cancer survivors and will continue to dominate the survivor population over the next 24 years.
Impact
The oldest adults (i.e., those >75 years) should be priority populations in a pressing cancer control and prevention research agenda that includes expanding criteria for clinical trials to recruit more elderly participants and developing relevant supportive care interventions.
Introduction
Cancer survivors are a rapidly growing population, fueled in large part by the aging United States (US) population (1). Current prevalence figures already indicate that more than half of all cancer survivors are older than 65 years of age, and that the graying of the baby boomer generation will cause the numbers of older survivors to continue to rise dramatically (2, 3). This demographic shift in the composition of the survivor population will pose challenges to the design and delivery of quality cancer care (4). Older adults commonly have pre-existing co-morbid conditions which can be exacerbated by cancer-related toxicities that may persist after completion of primary treatment or appear years later (1, 5). One large population-based study estimated that older adults experienced an average of five co-morbid conditions, two of which were reported to have developed after a cancer diagnosis (6). It is increasingly recognized that older survivors have complex healthcare needs, but these are poorly understood (7). Importantly, little is known about the burden of cancer and co-morbidity among the oldest adults (i.e., especially those aged 85 years and older) compared to other older adults, and how this burden may change over time (8).
In assessing the needs of older survivors, it is important to consider the increasingly common experience of cancer as a chronic disease (9). With advances in early detection and the effectiveness of cancer treatments, long-term survivorship is becoming a reality for most of those diagnosed with cancer. Currently, an estimated 64 percent of all survivors have lived five years beyond diagnosis and 40 percent have lived 10 years beyond diagnosis (3, 10). This combination of attributes in the cancer survivor profile – advanced age, longevity and multiple health conditions – may foreshadow a “silver tsunami” (11) of cancer survivors whose health needs we are unprepared to meet (5, 12).
The primary purpose of this report is to provide current estimates of co-morbidity prevalence at diagnosis and projections of future cancer prevalence that reflect the aging of the US population under the assumptions of current cancer control efforts. This report will focus on cancer survivors 65 years and older to inform research and care targeting this growing, heterogeneous and often medically complex population. The specific aims are to: 1) assess the effect of an aging population on cancer prevalence among older adults from 1975 to present, and provide prevalence projections from 2016 to 2040; 2) update current prevalence of cancer distributed by gender and age; and 3) compare current co-morbidity burden among older adults with and without a cancer history across age-related subgroups using medical claims linked to cancer registry data.
Methods and Materials
Prevalence Projections: Method Overview
We projected future US cancer prevalence from 2016 to 2040 using the Prevalence Incidence Approach Model (PIAMOD) (13–15). This statistical method provides estimates and projections of cancer prevalence from cancer incidence, cancer survival, and mortality for all causes of death. The method uses a mathematical equation that relates prevalence to incidence and mortality. The input data required by PIAMOD are the estimated number of US cancer incidence cases, US population, US deaths (all causes) and modeled cancer survival. The PIAMOD method has been used previously to provide projections of cancer prevalence through 2020 (14).
Data Sources for Prevalence Projections
Population estimates from 1975 through 2013 were obtained from the US Census Bureau. We used a demographic method to obtain smoothed single age population estimates from age-related subgroups (85–89, 90–94 and 95–99 years) by calendar year, sex and race. US population projections from 2013 through 2040 were obtained from the 2014 National Projections (16). These projections are based on the 2010 Census and on assumptions about future births, deaths, and net international migration and are available for individuals ages 1–99 years and 100 years and older.
SEER Incidence
The number of US incidence cancer cases from 1975 through 2012 (17) is estimated using SEER data from the 9 initial registries which include the states of Connecticut, Hawaii, Iowa, New Mexico, Utah and four metropolitan areas: Atlanta, Detroit, San Francisco-Oakland, and Seattle, representing approximately 11 percent of the US population (18). Only the first malignant cancer is included in incidence rates so that individual observations are unique (i.e., counted only once) in prevalence estimates.
US Incidence
The number of US cancer cases was calculated by applying the yearly SEER-9 rates to the respective US population by sex, race (white, black and other), year (1975–2012) and age (0 to 99). Projections of US cancer cases from 2013 through 2040 were calculated by applying the (2008–2012) average SEER-9 5-year cancer incidence rates to the US population projections by sex, year, and age, thus assuming flat incidence (i.e., no changes) from 2013 through 2040.
Cancer Survival
Relative survival for patients diagnosed with malignant cancer between 1973 and 2011 in SEER-9 was calculated from SEER*Stat software (19) by sex, period of diagnosis (1973–1975, 1976–1978…2009–2011), and age at diagnosis (15–44, 45–54, 55–64, 65–74, 75–84 and 85–99). A parametric mixture cure model was fit to the SEER relative survival (20, 21). The model was stratified by age group with a trend parameter for year of diagnosis (15). Because of data variability and difficulties in modeling relative survival for ages 85 and older (models did not converge or provided unrealistic estimates), we assumed that cancer survival for patients diagnosed at ages 85–99 years was the same as for patients diagnosed at ages 75–84 years. To project prevalence, we assumed that cancer survival for patients diagnosed in 2012 or later was the same as the modeled survival for patients diagnosed in 2009–2011.
The number of US deaths (all causes) was provided by the National Center for Health Statistics from SEER*Stat software (19) using sex, age and year at death from 1975 to 2012. Projections of the number of US deaths from 2013 through 2040 was calculated by applying the 5-year average US mortality rates (2008–2012) to US population projections by sex, year and age groups.
Prevalence Projection Method and Assumptions
The cancer prevalence projections for years 2013 and beyond are based on dynamic projections of the US population and flat incidence and survival, as observed in the last years of available data (2008–2012). These projections can be interpreted as the effect of the growth and aging of the US population based on current cancer control technologies. The PIAMOD method provided prevalence estimates for ages 0 to 99. In order to include cancer survivors ages 100 years and older, we assumed that cancer prevalence of people ages 100 and older was the same as for people ages 95–99. We estimated the number of cancer survivors 100 years and older by multiplying the proportion of survivors between ages 95–99 and applying to estimates and projections of the population of adults 100 years and older.
Procedures for Calculating Co-morbidity Burden
Claims from the November 2014 SEER-Medicare linkage data and from a 5 percent random sample of Medicare beneficiaries residing in the SEER areas free of cancer were used to describe co-morbidity burden (18). The cancer cohort consisted of Medicare beneficiaries 66 to 90 years of age residing in the SEER 11 cancer registry areas diagnosed with cancer between 2007 and 2011. Non-cancer controls were randomly selected from the 5 percent sample of cancer-free beneficiaries in the SEER areas for each calendar year and were frequency-matched to cancer cases by sex and age. Co-morbid conditions for cancer survivors were identified in the year before the date of cancer diagnosis. For individuals without cancer, their co-morbid conditions were identified in the year before their birthday in the matched calendar year. Cases and controls were required to have continuous Part A and Part B fee-for-service Medicare coverage during the whole year. The 5 percent cohort of cancer-free Medicare beneficiaries consisted of multiple records per individual.
We ascertained 16 co-morbid conditions described by Charlson et al (22) using similar procedures developed by Klabunde et al. (23, 24). The conditions included: acute myocardial infarction (MI), myocardial infarction-history, acquired immune deficiency syndrome (AIDS), cerebrovascular disease (CVD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes, diabetes with complications, liver disease (mild), liver disease (moderate/severe), paralysis (hemiplegia or paraplegia), peripheral vascular disease, renal disease, rheumatologic disease, and ulcer. Similar conditions, including liver disease (mild) and liver disease (moderate/severe) as well as diabetes and diabetes with complications, were grouped together. Because the focus was on studying co-morbid conditions in cancer patients, solid cancers, leukemias and lymphomas were excluded. Conditions were identified from physician and hospital claims using the International Classification of Diseases, Ninth Revision, Clinical Modification and fourth edition of Common Procedural Terminology codes. Conditions in the physician claims, but not in hospital claims, were required to appear more than once in a period greater than 30 days.
Instead of using a simple count of conditions to estimate burden, weights for each condition were estimated using a Cox proportional hazard model to represent the impact of the condition on non-cancer survival (25). For each individual, a co-morbidity score was calculated as the weighted sum of their identified co-morbid conditions by the condition weight.
The severity of co-morbidity burden was considered by defining 3 groups based on co-morbidity weights and clinical implications (25, 26). The groups were: 1) no reported co-morbidities; 2) moderate co-morbidity, which included conditions that usually do not require physicians to adjust cancer treatment (such as acute MI, history of MI, ulcers, rheumatologic disease, diabetes, peripheral vascular disease, cerebrovascular disease and paralysis); and, 3) severe co-morbidity burden, which refers to severe illnesses that frequently lead to organ failure or systemic dysfunction and always require adjustment of cancer treatment. The severe conditions included chronic obstructive pulmonary disease, liver dysfunction, chronic renal failure, dementia, congestive heart failure, and acquired immunodeficiency syndrome as well as people with multiple co-morbidities who may require special considerations for cancer treatment. It should be noted that common conditions such as hypertension or arthritis have not been included in this study, which is consistent with Charlson’s index.
Results
Projected Cancer Prevalence
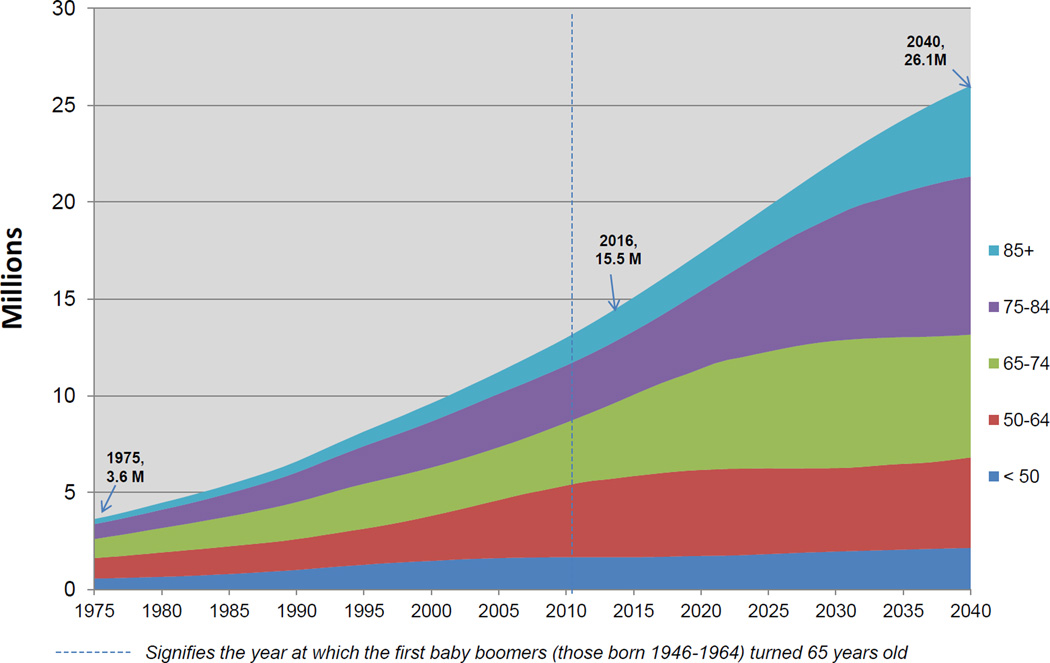
Between 2016 and 2040, the projected prevalence of cancer survivors will increase from an estimated 15.5 million (total population 324 million) to 26.1 million survivors (total population 380 million) across all age groups. In the prevalent population, it is expected that 73 percent of survivors will be age 65 and older in 2040, compared to 18 percent of survivors 50–64 years and just 8 percent of survivors who will be less than 50 years old at that time. This dramatic increase mirrors the pattern of increased incidence driven by an aging population, such that in 2040, the most prevalent groups of survivors will be those from older age groups. Specifically, in 2040, survivors ages 65–74 will account for 24 percent of all survivors, those 75–84 will account for 31 percent of all survivors and those 85 and older will represent 18 percent of survivors (Figure 1).
Figure 1.
Estimated cancer prevalence by age in the US population from 1975 (216 M) to 2040 (380 M)
Pairing these projections with historical trends since 1975, we observed a steep overall incline in prevalence, especially for older survivors. Among survivors 50–64 years, we estimated an almost 4-fold increase in projected prevalence from 1975 to 2040. However, the projected increase in prevalence was substantially larger in older age groups. Comparing 1975 prevalence estimates to projected prevalence estimates in 2040, we noted a 6-fold increase for those 65–74 years; a 10-fold increase for those ages 75–84 years and a 17-fold increase for those 85 years and older (Figure 1). These data foretell a steady rise in cancer prevalence among older age groups over the next 24 years.
Updated Prevalence of Cancer Distributed by Age and Gender
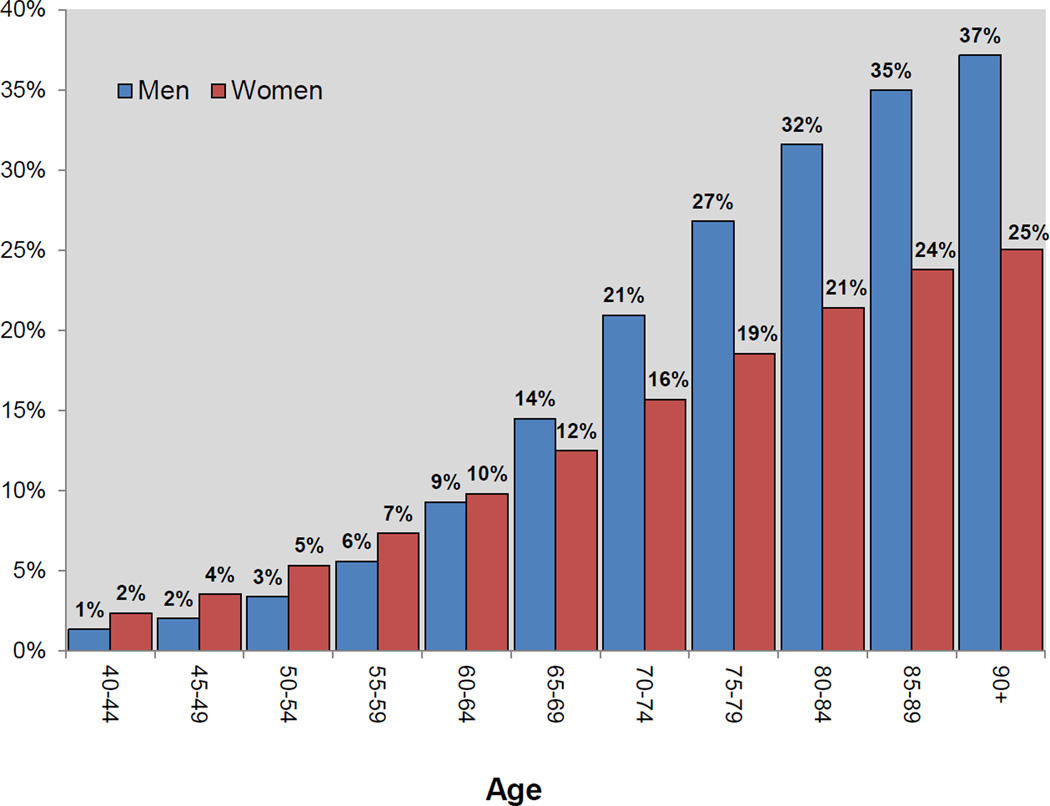
Examining prevalence across the US population (estimated at 323,995,680 in 2016), we observed that overall cancer prevalence was similar between men and women of all ages, but that cancer prevalence was higher among men than women in older age groups (Figure 2). In younger age groups, including those ages 55–59 and 60–64, the percent of male and female cancer survivors is similar, with men trailing slightly behind women (6–7 percent and 9–10 percent, respectively), but this shifts in adults older than age 65 years, wherein 14 percent of the nearly 8 million men ages 65–69 had been diagnosed with cancer, compared to 12 percent of 8.9 million women of the same age group. This differential becomes larger in older age groups, such that there is a higher percent of male than female survivors for: 70–74 year olds (5 percent more males than females); 75–79 year olds (8 percent more males than females); and 80–84 and 85–89 year olds (11 percent more male than female survivors). The greatest difference for cancer survivors by gender is for those 90 years and older, wherein 37 percent of the 765,660 men ages 90 years and older are cancer survivors, 12 percent more than females of the same age group, which included 1.7 million women (Figure 2).
Figure 2.
Percent of cancer survivors in the US population (324 M) by gender and age, 2016
Comparison of Co-morbidity Burden of Older Adults with and without a Cancer History
In our assessment of survivors of all cancer sites across age groups, we noted some unexpected results (Table 1). For example, approximately 50 per cent of survivors 70–74 years experienced no co-morbidities (based on our procedures) prior to diagnosis. However, an increase in history of severe co-morbidity was observed with increasing age, rising from 27 percent in survivors 66–69 years (all cancer sites) to 47 percent in adults 85 years and older (all cancer sites).
Table 1.
Co-morbidity burden* by age and cancer site
| Non-cancer | All sites | Bladder | CRC | Female Breast |
Leukemia | Lung | Oral | Prostate | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 100,000 | 457,659 | 26,045 | 48,138 | 52,179 | 12,447 | 76,365 | 8,043 | 80,087 |
| 66–69 years | 20,180 | 92,362 | 4,036 | 7,361 | 11,407 | 1,893 | 14,558 | 1,918 | 22,196 |
| None | 66% | 56% | 52% | 56% | 65% | 56% | 39% | 56% | 66% |
| Moderate | 17% | 18% | 20% | 20% | 17% | 19% | 12% | 16% | 18% |
| High | 16% | 26% | 28% | 24% | 17% | 24% | 48% | 27% | 16% |
| 70–74 years | 24,699 | 113,029 | 5,740 | 10,036 | 12,870 | 2,512 | 18,982 | 1,998 | 25,315 |
| None | 61% | 50% | 47% | 51% | 59% | 51% | 34% | 52% | 61% |
| Moderate | 19% | 19% | 21% | 20% | 20% | 20% | 13% | 16% | 19% |
| High | 20% | 31% | 32% | 29% | 21% | 30% | 52% | 32% | 19% |
| 75–79 years | 21,555 | 98,644 | 5,848 | 10,077 | 10,951 | 2,623 | 17,781 | 1,592 | 17,185 |
| None | 54% | 45% | 43% | 46% | 54% | 45% | 32% | 46% | 55% |
| Moderate | 19% | 19% | 20% | 19% | 20% | 20% | 13% | 18% | 20% |
| High | 26% | 36% | 37% | 35% | 26% | 35% | 55% | 35% | 24% |
| 80–84 years | 17,409 | 79,676 | 5,339 | 9,630 | 8,824 | 2,528 | 14,180 | 1,241 | 9,362 |
| None | 48% | 40% | 38% | 42% | 49% | 42% | 30% | 43% | 49% |
| Moderate | 18% | 18% | 19% | 19% | 21% | 17% | 14% | 17% | 19% |
| High | 33% | 41% | 42% | 40% | 30% | 41% | 56% | 40% | 32% |
| 85+ years | 16,157 | 73,948 | 5,082 | 11,034 | 8,127 | 2,891 | 10,864 | 1,294 | 6,029 |
| None | 42% | 37% | 36% | 37% | 44% | 36% | 29% | 39% | 43% |
| Moderate | 17% | 17% | 17% | 17% | 19% | 15% | 13% | 17% | 16% |
| High | 42% | 47% | 47% | 46% | 37% | 49% | 57% | 44% | 41% |
Categories included co-morbidities described in the Charlson Index and weighted for effect on non-cancer mortality. None implies a “0” score for co-morbidities, though this may not reflect common conditions that are not currently included in the Index. Moderate co-morbidity included conditions that usually do not require physicians to adjust cancer treatment. Severe co-morbidity burden refers to severe illnesses that frequently lead to organ failure or systemic dysfunction and always require adjustment of cancer treatment for survivors. See methods for more detail.
We noted more similarity in co-morbidity burden related to age than cancer status, which has also been observed in other studies (27, 28). In the non-cancer cohort, an estimated 42 percent of those 85 years and older had serious co-morbidity burden, which was only slightly lower than the estimated prevalence of serious co-morbidity burden in survivors of all cancer sites (47 percent). The groups with the least burden were those 66–74 years, where up to 66 percent of adults without a cancer history scored zero for co-morbid conditions. But, up to 56 percent of cancer survivors 66–74 years also fell into this category, again suggesting the role of younger age in mitigating co-morbidity burden.
Focusing on specific cancer sites, we noted that lung cancer survivors consistently had the worst co-morbidity burden of any cancer site across age groups, with nearly 50 percent of lung cancer survivors ages 65–69 years experiencing severe co-morbidity burden and 57 percent of survivors 85 years or older experiencing severe co-morbidity burden. In comparison, breast and prostate cancer survivors had consistently lower co-morbidity burden than survivors of other cancer sites, which was generally more similar to the non-cancer cohort, an association that persisted in older age groups. For example, breast and prostate cancer survivors ages 65–69 years had lower burden of severe co-morbidity (17 percent and 16 percent, respectively) compared to 16% for non-cancer controls. This burden rose to 37 percent (breast cancer survivors) and 41 percent (prostate cancer survivors) in survivors 85 years and older, still lower than the estimates for non-cancer controls (42%) of that age group. These estimates were also notably lower than the observed co-morbidity burden among similarly-aged survivors of other cancer sites, including colorectal, oral, bladder and leukemia cancers (Table 1).
Among cancer survivors, the three most prevalent co-morbid conditions were congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) and diabetes (Table 2). Colorectal cancer survivors had the highest overall prevalence of CHF compared to other sites, with the oldest survivors having the highest burden (22 percent of those 85 years and older). Lung cancer survivors had the highest prevalence of COPD, more than 40 percent of lung cancer survivors 65–84 years experiencing this condition. Comparatively, female breast cancer survivors had the lowest prevalence of COPD, with 13 percent or less experiencing this condition in any age group. Diabetes was consistently the most common of the three conditions for survivors of all cancer sites, across all age groups. The range of those experiencing diabetes was 22 percent to 29 percent of survivors, with little variation between age groups (Table 2).
Table 2.
Percent of common co-morbidities in non-cancer controls and cancer survivors (by age and cancer site)*
| Age | Non-cancer (n=100,000) |
All sites(n=457,659) | Bladder | CRC | Female Breast |
Leukemia | Lung | Oral | Prostate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHF | % with condition |
(% without) |
% with condition |
(% without) |
% with condition |
% with condition |
% with condition |
% with condition |
% with condition |
% with condition |
% with condition |
| 66–69 years |
4% | (96%) | 6% | (94%) | 6% | 7% | 3% | 7% | 10% | 6% | 4% |
| 70–74 years |
6% | (94%) | 8% | (92%) | 9% | 10% | 5% | 9% | 12% | 7% | 5% |
| 75–79 years |
8% | (92%) | 11% | (89%) | 11% | 12% | 7% | 13% | 15% | 10% | 7% |
| 80–84 years |
11% | (89%) | 15% | (85%) | 14% | 16% | 10% | 17% | 17% | 13% | 11% |
| 85+ years |
17% | (83%) | 20% | (80%) | 19% | 22% | 16% | 23% | 23% | 19% | 17% |
| COPD | |||||||||||
| 66–69 years |
9% | (91%) | 17% | (83%) | 17% | 13% | 11% | 14% | 40% | 18% | 8% |
| 70–74 years |
10% | (90%) | 18% | (82%) | 18% | 15% | 12% | 15% | 43% | 20% | 10% |
| 75–79 years |
12% | (88%) | 21% | (79%) | 20% | 18% | 13% | 18% | 43% | 20% | 11% |
| 80–84 years |
14% | (86%) | 21% | (79%) | 20% | 18% | 13% | 19% | 41% | 19% | 14% |
| 85+ years |
14% | (86%) | 19% | (81%) | 18% | 17% | 12% | 18% | 36% | 18% | 14% |
| Diabetes | |||||||||||
| 66–69 years |
20% | (80%) | 25% | (75%) | 27% | 28% | 21% | 26% | 24% | 20% | 21% |
| 70–74 years |
23% | (77%) | 27% | (73%) | 28% | 30% | 24% | 26% | 26% | 23% | 23% |
| 75–79 years |
24% | (76%) | 28% | (72%) | 29% | 29% | 25% | 28% | 26% | 26% | 24% |
| 80–84 years |
23% | (77%) | 27% | (73%) | 28% | 28% | 24% | 25% | 25% | 24% | 24% |
| 85+ years |
20% | (80%) | 23% | (77%) | 23% | 23% | 21% | 21% | 22% | 22% | 21% |
CHF = Congestive Heart Failure COPD = Chronic Obstructive Pulmonary Disease CRC=Colorectal Cancer
For cancer survivors, co-morbidities were identified prior to receiving a cancer diagnosis. See methods for details.
Compared to non-cancer controls of similar age groups, a perceptibly larger proportion of survivors experienced CHF, COPD and diabetes. For example, among adults 66–84 years without cancer, 9–14% experienced COPD, compared to 17–21% of survivors of all sites ages 66–84 years. Although the difference between survivors and non-cancer controls was less pronounced when assessing presence of CHF and diabetes, a general pattern across age groups was still present which suggested slightly higher prevalence of these conditions among survivors of all sites compared to other adults without cancer history of similar ages (Table 2). However, it should be noted that breast/prostate cancer survivors were the exception to this pattern, presenting similar prevalence of CHF, COPD and diabetes as non-cancer controls.
Discussion
Although the aging of the US population has long been recognized as a major contributor to the cancer burden (29, 30), this report provides a discrete and previously undescribed view of the cancer prevalence exclusively among age-related subgroups of older adults projected to 2040. Current US demographics will literally change the face of the survivor population in the decades to come. In a prior prevalence report (3), the population of US cancer survivors was estimated at 13.7 million (2012). In 2016, we estimate that there are 15.5 million US cancer survivors, a number that will continue to rise. Currently, we estimate that almost 40% of men age 90 and older are cancer survivors, out of a total population of 765,660 American men in that age group. Similarly, we estimate that 25% of women age 90 and older, out of a total population of 1,739,100, are cancer survivors. It can be inferred that higher cancer prevalence in elderly men is due to prostate cancer, which is typically diagnosed in men at older ages. Further, our projections portend a steady increase in cancer prevalence by age, wherein the oldest adults (i.e., 85 years and older) are expected to have the sharpest increase in prevalence looking from 1975 forward to 2040, compared to other age groups. Similar, but slightly less dramatic projected increases, will also occur among adults between 65–74 and 74–85 years, underscoring the need to focus on adults who are (or are soon to become) Medicare-eligible as priority populations for cancer prevention and control research.
Despite their numbers, older adults continue to be understudied, a point emphasized by the Institute of Medicine in addressing challenges in delivery quality cancer care (4). Cancer patients older than age 65 have been largely excluded from clinical trials (31), precluding opportunities to develop greater insight about the unique needs of older as well as long-term survivors. Recent studies have demonstrated variability of physical and psychosocial conditions for older adults as they exceed age 65, differences which may be amplified in cancer survivors (32–34). Clinicians are increasingly recognizing the benefits of a geriatric assessment framework, including opportunities for personalized medicine in geriatric oncology (8, 35). Greater participation by elderly survivors in clinical trials could provide more clarity in optimal dosing of chemotherapeutic agents, potentially reducing treatment-related toxicity for elderly cancer patients among other benefits for health and well-being into long-term survivorship (35).
Based on our data, more than 60 percent of survivors ages 85 and older had at least one co-morbid condition prior to a cancer diagnosis and between 37–49 percent had more severe co-morbidity burden, based on weighted scores. However, because we only included co-morbidities identified by Charlson et al, common conditions that affect the elderly (such as hypertension, arthritis, atrial fibrillation, chronic ischemic coronary artery disease and thromboembolic disease) are not reflected and our report of co-morbidity prevalence may in fact be an underestimate of actual co-morbidity burden in older survivors. Clinicians may also not code for all co-morbidities an older patient may experience, further increasing the likelihood of an underestimate of actual co-morbidity burden. Importantly, we did not see a large difference between co-morbidity burden in older adults of similar age groups when stratified on cancer history. However, we did observe that survivorship experiences with co-morbidity varied by cancer site, such that lung cancer survivors had the highest co-morbidity burden prior to diagnosis across age groups and breast/prostate cancer survivors had some of the lowest co-morbidity burden prior to diagnosis across age groups. We also observed small, but notable differences in CHF, COPD and diabetes, suggesting slightly higher prevalence of these conditions in cancer survivors of all sites compared to non-cancer controls, with the exception of breast/prostate cancer survivors, who were similar to non-cancer controls. Longitudinal assessment of co-morbidity burden (including differences by age and cancer site) would be informative in future research studies.
These findings have several implications. First, cancer survivors, like adults in the general population, are at risk for diseases associated with aging and lifestyle. Lifestyle counseling, including physical activity, may be important to reduce cancer-related symptoms, as symptom burden may impede an active lifestyle after treatment (36, 37). Additionally, evidence suggests that co-morbidities identified prior to a cancer diagnosis are important predictors of function and health status in the post-treatment period, potentially offering an opportunity to focus on rehabilitation to reduce these negative sequelae (27, 38). Further, our findings relative to co-morbidity patterns point to the need to consider more systematic screening for functional risk and broader delivery of supportive interventions, including rehabilitation, to vulnerable subgroups (such as lung cancer survivors) to help increase their ability to live through and beyond cancer treatment (39, 40). However, among the challenges in addressing the needs of the growing survivor population is the looming question of cost of care and caregiving capacity.
With anticipated shortages of oncology providers (4), and the complex health needs of older patients, finding efficient and effective ways to meet the medical surveillance needs of older survivors will become increasingly important (5, 7, 41, 42). The role of primary care providers may become even more critical in recognizing and managing late and long-term effects of treatment (43) as well as broader morbidity and mortality risk (44). At the same time, determining which survivors could be appropriately served in a primary care setting, versus a more oncology-driven model, are questions of particular interest (45, 46). Moving forward, research and education will be needed to facilitate care coordination between oncology and primary care providers.
As part of caregiving considerations, understanding the impact on family and other informal caregivers will also be required. Efforts to address the health and functional needs of informal caregivers (sometimes viewed as ‘secondary’ survivors) are critically needed as their numbers and burden of care (including financial burden) will likely increase in step with the prevalent cancer population (47, 48). In addition to increasing burden of care, informal caregivers may also become progressively more involved with medical decisions. In a recent report, the National Alliance on Caregiving estimated that 73 percent of caregivers over age 75 communicate with health care professionals on behalf of their care recipient, making them an important part of the medical team (49). Efforts will be needed to address the capacity of informal cancer caregivers to support care recipients as well as mitigate the potential adverse effects of caregiving demands on their own health and function (48, 50).
While this report provides timely insights on burden of cancer in older adults, we acknowledge several limitations of our analysis. Data from SEER cancer registries and Medicare claims only include data from specific regions that participate in the registry, and thus are not nationally representative. Further, to generate our prevalence estimates, we held incidence and survival constant. In reality, incidence has been declining slowly and steadily for most cancers, and survival, in turn, has increased. It is possible that these trends may balance each other (i.e., fewer cancer cases, but longer survival). A shift in either of these trends over the long term could result in our estimates being an over- or under-estimation of the true figures. However, the US Census Bureau provides the best available population projections to estimate the future number of cancer survivors in the US.
Because it is difficult to anticipate future developments in cancer control technologies and their impact on survival and incidence trends, we produced future prevalence estimates based on projections assuming flat incidence and survival. These projections represent the effect of the growth and aging of the US population based on current cancer control strategies. Overall cancer incidence decreased for men 1.8 percent annually from 2007 to 2011 and incidence was stable for women from 1998 to 2011 (22). Cancer survival has been increasing for most cancer sites in the most recent years (5-year relative survival for patients diagnosed in 2002–2004 with any cancer was 67 percent compared to 69 percent for patients diagnosed in 2005–2011)(17). Compared to previous reports (1, 3), prevalence projections incorporating recent trends in survival and incidence are similar, assuming flat trends. Additionally, co-morbidity measures were based on a claims algorithm and Medicare data and do not include information about the role of co-morbidity on functional status, which may be helpful in interpreting the burden of illness and quality of life. Finally, while we were able to estimate current prevalence of co-morbidity in adult cancer survivors using data from the year prior to cancer diagnosis, few data are available to examine co-morbidity burden in long-term cancer survivors. This represents a significant gap in our current knowledge base.
Conclusions and Impact
This report clearly demonstrates a rising tide of cancer prevalence, stimulated by the maturation of the baby boomers, as well as marked heterogeneity in co-morbidity burden that is more pronounced in the oldest age groups. The overall message continues to be that cancer survivors are a diverse population, with needs that vary by age as well as cancer site and other factors. We hope, however, that the data presented here will lend urgency to previous calls to action (4, 51), particularly for the complex needs of older survivors. While a number of interventions have been developed to help survivors cope with cancer and thrive in the face of cancer-related symptoms, few have targeted older adults and this remains an area of critical need in survivorship science (52). Importantly, effective strategies must be implemented now to address the tsunami of aging cancer survivors which is rapidly approaching.
Acknowledgments
The authors would like to thank William Klein, Janet de Moor and other staff in the NCI Division of Cancer Control and Population Sciences for their assistance in the preparation of this report.
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors confirm there are no potential conflicts of interest.
References
- 1.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev. 2011 Oct;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey WH. Baby boomers and the new demographics of america's seniors. Generations. 2010;34:28–37. [Google Scholar]
- 3.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the united states: Prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013 Apr;22:561–570. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: Institute of Medicine; 2013. [PubMed] [Google Scholar]
- 5.Rowland JH, Bellizzi KM. Cancer survivorship issues: Life after treatment and implications for an aging population. Journal of Clinical Oncology. 2014 Aug 20;32:2662–2668. doi: 10.1200/JCO.2014.55.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach CR, Weaver KE, Aziz NM, Alfano CM, Bellizzi KM, Kent EE, et al. The complex health profile of long-term cancer survivors: Prevalence and predictors of comorbid conditions. Journal of Cancer Survivorship. 2014;9:239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 7.Bellizzi KM, Aziz NM, Rowland JH, Weaver K, Arora NK, Hamilton AS, et al. Double jeopardy? age, race, and HRQOL in older adults with cancer. Journal of cancer epidemiology. 2012;2012 doi: 10.1155/2012/478642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, et al. Improving the evidence base for treating older adults with cancer: American society of clinical oncology statement. Journal of Clinical Oncology. 2015 Jul 20; doi: 10.1200/JCO.2015.63.0319. [DOI] [PubMed] [Google Scholar]
- 9.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the united states: Age, health, and disability. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58:M82–M91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 10.Rowland JH, Yancik R. Cancer survivorship: The interface of aging, comorbidity, and quality care. Journal of the National Cancer Institute. 2006 Apr 19;98:504–505. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 11.Bartels SJ, Naslund JA. The underside of the silver tsunami — older adults and mental health care. N Engl J Med. 2013 Feb 07;368:493–496. doi: 10.1056/NEJMp1211456. 2015/10. [DOI] [PubMed] [Google Scholar]
- 12.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the united states: Burdens upon an aging, changing nation. Journal of Clinical Oncology. 2009 Jun 10;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 13.Verdecchia A, Angelis GD, Capocaccia R. Estimation and projections of cancer prevalence from cancer registry data. Stat Med. 2002;21:3511–3526. doi: 10.1002/sim.1304. [DOI] [PubMed] [Google Scholar]
- 14.Mariotto AB, Yabroff KR, Feuer EJ, De Angelis R, Brown M. Projecting the number of patients with colorectal carcinoma by phases of care in the US: 2000–2020. Cancer Causes & Control. 2006;17:1215–1226. doi: 10.1007/s10552-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 15.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the united states: 2010–2020. J Natl Cancer Inst. 2011 Jan 19;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colby SL, Ortman JM. Current Population Reports. Washington, DC: US Census Bureau; 2014. Projections of the size and composition of the U.S. population: 2014 to 2060. Report No.: P25-1143. [Google Scholar]
- 17.Howlader N, Noone A, Krapcho M. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; 2015. [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-medicare data: Content, research applications, and generalizability to the united states elderly population. Med Care. 2002:IV3–IV18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance research program, national cancer institute SEER*Stat software. ( www.seer.cancer.gov/seerstat) version 8.2.1.
- 20.Yu B, Tiwari RC, Cronin KA, Feuer EJ. Cure fraction estimation from the mixture cure models for grouped survival data. Stat Med. 2004;23:1733–1747. doi: 10.1002/sim.1774. [DOI] [PubMed] [Google Scholar]
- 21.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the united states: 2010–2020. J Natl Cancer Inst. 2011 Jan 19;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Legler JM, Warren JL, Baldwin L, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Mariotto AB, Wang Z, Klabunde CN, Cho H, Das B, Feuer EJ. Life tables adjusted for comorbidity more accurately estimate noncancer survival for recently diagnosed cancer patients. J Clin Epidemiol. 2013;66:1376–1385. doi: 10.1016/j.jclinepi.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho H, Klabunde CN, Yabroff KR, Wang Z, Meekins A, Lansdorp-Vogelaar I, et al. Comorbidity-adjusted life expectancy: A new tool to inform recommendations for optimal screening strategies. Ann Intern Med. 2013;159:667–676. doi: 10.7326/0003-4819-159-10-201311190-00005. [DOI] [PubMed] [Google Scholar]
- 27.Garman KS, Pieper CF, Seo P, Cohen HJ. Function in elderly cancer survivors depends on comorbidities. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003 Dec 01;58:M1119–M1124. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: Other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013 Aug 1;178:339–349. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards BK, Howe HL, Ries LAG, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002 May 15;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 30.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005 Nov-Dec;:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005 May 1;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 32.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA: a cancer journal for clinicians. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 33.Puts MTE, Santos B, Hardt J, Monette J, Girre V, Atenafu EG, et al. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Annals of Oncology. 2014 Feb 01;25:307–315. doi: 10.1093/annonc/mdt386. [DOI] [PubMed] [Google Scholar]
- 34.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen MLG, Extermann M, et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. Journal of Clinical Oncology. 2014 Aug 20;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walko CM, McLeod HL. Personalizing medicine in geriatric oncology. Journal of Clinical Oncology. 2014 Aug 20;32:2581–2586. doi: 10.1200/JCO.2014.55.9047. [DOI] [PubMed] [Google Scholar]
- 36.Sprod LK, Mohile SG, Demark-Wahnefried W, Janelsins MC, Peppone LJ, Morrow GR, et al. Exercise and cancer treatment symptoms in 408 newly diagnosed older cancer patients. Journal of geriatric oncology. 2012;3:90–97. doi: 10.1016/j.jgo.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA, et al. Grasping the ‘teachable moment’: Time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psycho-Oncology. 2015;24:1250–1257. doi: 10.1002/pon.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurria A. Management of elderly patients with cancer. J Natl Compr Canc Netw. 2013 May;11:698–701. doi: 10.6004/jnccn.2013.0205. [DOI] [PubMed] [Google Scholar]
- 39.Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. Journal of Clinical Oncology. 2014 Aug 20;32:2627–2634. doi: 10.1200/JCO.2014.55.3065. [DOI] [PubMed] [Google Scholar]
- 40.Cohen HJ. Functional assessment and the cancer survivor: Something old, something new. Journal of the National Cancer Institute. 2010 Sep 22; doi: 10.1093/jnci/djq365. [DOI] [PubMed] [Google Scholar]
- 41.Hurria A, Mohile SG, Dale W. Research priorities in geriatric oncology: Addressing the needs of an aging population. J Natl Compr Canc Netw. 2012 Feb;10:286–288. doi: 10.6004/jnccn.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. Journal of Clinical Oncology. 2014 Aug 20;32:2611–2616. doi: 10.1200/JCO.2014.55.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nekhlyudov L, Aziz NM, Lerro C, Virgo KS. Oncologists' and primary care physicians' awareness of late and long-term effects of chemotherapy: Implications for care of the growing population of survivors. J Oncol Pract. 2014 Mar;10:e29–e36. doi: 10.1200/JOP.2013.001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howlader N, Mariotto AB, Woloshin S, Schwartz LM. Providing clinicians and patients with actual prognosis: Cancer in the context of competing causes of death. J Natl Cancer Inst Monogr. 2014 Nov;2014:255–264. doi: 10.1093/jncimonographs/lgu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chubak J, Tuzzio L, Hsu C, Alfano CM, Rabin BA, Hornbrook MC, et al. Providing care for cancer survivors in integrated health care delivery systems: Practices, challenges, and research opportunities. Journal of Oncology Practice. 2012 doi: 10.1200/JOP.2011.000312. JOP. 2011.000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin G, Berendsen A, Crawford SM, Dommett R, Earle C, Emery J, et al. The expanding role of primary care in cancer control. The Lancet Oncology. 2015;16:1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 47.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115:4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 48.Romito F, Goldzweig G, Cormio C, Hagedoorn M, Andersen BL. Informal caregiving for cancer patients. Cancer. 2013;119:2160–2169. doi: 10.1002/cncr.28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caregiving in the U.S. Bethesda, MD: National Caregiving Alliance; AARP; 2015. [Google Scholar]
- 50.Kent EE, Rowland JH, Northouse L, Litzelman K, Chou WS, Shelburne N, et al. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer. 2016 doi: 10.1002/cncr.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moy B, Flaig TW, Muss HB, Clark B, Tse W, Windham TC. Geriatric oncology for the 21st century: A call for action. J Oncol Pract. 2014 Jul;10:241–243. doi: 10.1200/JOP.2013.001333. [DOI] [PubMed] [Google Scholar]
- 52.Stanton AL, Ganz PA, Rowland JH, Meyerowitz BE, Krupnick JL, Sears SR. Promoting adjustment after treatment for cancer. Cancer. 2005;104:2608–2613. doi: 10.1002/cncr.21246. [DOI] [PubMed] [Google Scholar]