Abstract
Introduction
Emotional problems are amongst the most critical concerns to be intentionally handled to enhance the wellbeing and development of children.
Objective
To determine the predictors of socio-emotional development of Egyptian infants related to infant feeding practices, aspects of infant and maternal health and socioeconomic status.
Subjects and Methods
A cross-sectional comparative study included 322 breast fed, 240 bottle fed and 93 mixed fed infants, from 6–24 months of age, who were enrolled in the Well–Baby Clinic of the National Research Centre and from pediatric outpatient facilities in urban Cairo. Assessment of socio-emotional development was performed using Bayley Scales of Infant and Toddler Development (Bayley III). Detailed maternal and infant history was recorded. Levels of serum zinc, copper, iron, vitamin B12 and complete blood count (CBC) were assessed in a subsample of 193 infants.
Results
The risk of having below average socio-emotional composite score was nearly two and half times among formula-fed infants than among breast-fed infants. By binary logistical regression analysis, predictors of below average socio-emotional score were a lower serum zinc value, being formula fed during the first half-year and introduction of complementary food before the age of six months (p< 0.05).
Conclusion
Exclusive breastfeeding and to a lesser extent mixed feeding during the first half year is correlated with above average socio-emotional development. Maternal education and zinc status were also determinants of better infant mental health. Our endeavors ought to be directed towards integrated interventions addressing multiple risks to children’s development.
Introduction
Socio-emotional development during infancy and early childhood has been described as “the emerging capacity of the child to experience, control, and express feelings; form close and secure interpersonal connections; investigate the environment and learn, all in the setting of family, society and cultural anticipations” [1, 2].
As infants develop, emotional expressions become better-organized, partly due to caregivers’ supportive backing. Throughout the first two years, infants become progressively capable of understanding their own and others’, emotions, engaging in purposed communication and sharing deliberately with others [3, 4, 5].
Saarni et al. [6] Described four phases of the development of emotional communication between the infant and an adult. Phase 1 (prenatal to 6 weeks) describes the infant’s valenced reactions to emotion signals. Phase 2 (6 weeks to 9 months) focuses on the pre-referential communication, where the infant can engage in synchronous dyadic interaction with the caregiver. Phase 3 (9 months to 18 months) covers the development of referential emotion communication, behavioral regulation (i.e., where the expressive behaviors of the child are affected by the other’s emotional expressions). Phase 4 (18 months/2 years) is marked by the development of what is called self-conscious emotion (e.g., shame, guilt, pride).
Mental health disorders in infants and toddlers might be reflected as physical symptoms, overall delayed development, inconsolable crying, sleep problems, aggressive or impulsive behaviour and paralyzing fears [7]. Early childhood adversity or untreated mental health disorders can hinder children’s socio-emotional development aggravating early learning, social relationships, and deep-rooted wellbeing [8, 9]. Some early mental health disorders have lasting effects and may appear to be precursors of mental health problems in later life, including withdrawal, sleeplessness, or lack of appetite due to depression, anxiety, and traumatic stress reactions [10]. In Egypt, it was estimated that about 14% of the total burden of ailment were psychological well-being connected conditions [11].
Numerous variables may affect a child’s socio-emotional development [12]. Development relies on accessibility of sound nourishment [13], healthy environment [14], kind and thoughtful cooperation via parental figures, opportunities for learning and standard training and group support [15]. These elements have synergistic effects to promote proper child development [13].
Nutrition
Adequate nutrition (adequate energy, protein, fatty acids, and micronutrients) is essential to fuelling early childhood's rapid brain growth. The well-nourished child is better able to interact with the caregivers and environment in a way that provides the experiences necessary for optimal brain development [16].
Undernutrition impairs physical growth, physical activity, and motor development, which may, in turn, influence brain development through two pathways. The first pathway is through caregiver behavior and the second is through child exploration of the environment. First, caregivers may not provide age-appropriate stimulation for small for age or malnourished children, which could result in altered brain development. Also, undernourished children may be frequently ill and therefore fussy, irritable, and withdrawn, leading caregivers to treat them more negatively [17]. Reduced activity due to undernutrition may limit the child's exploration of the environment and initiation of caregiver interactions, which could also lead to poor brain development. [18].
Social Factors
Children’s families, schools, neighborhoods, peers and culture all play a role in emotional development. Although most psychologists agree that the family context has a major impact on children’s social and emotional development, the mechanisms through which context impacts development are less clear [19]. Research has recognized many risk factors that affect the family context and emotional development; such as poverty, stress, low parental education, dangerous neighbourhoods [20], domestic violence [21], and number of siblings. Morris et al., [22] suggested that the family context affects the development of emotional regulation through three important ways. Firstly, children learn about emotional regulation through observational learning, modelling and social referencing. Secondly, parenting practices specifically related to emotion and emotion management, which differs by cultural variation. Thirdly, the emotional climate of the family as parenting style, the attachment relationship, family expressiveness and the marital relationship. An emotionally arousing family climate may lead to increased hypothalamic-pituitary-adrenal (HPA) axis reactivity, which, over the long-term can lead to atrophy in structures in the prefrontal cortex that play a role in emotion regulation [23]. The overall amount of emotion in the family, particularly negative emotionality, may actually induce negative emotions in early infancy and beyond [24].
Maternal and Infant Well-being
Attachment relationships, an essential feature of socio-emotional development in infancy, are impacted by the emotional lives of caregivers and infants [25,26]. Maternal physical and mental health have a significant impact on the in utero environment and, thus, on fetal development and the health of the child later in life [27]. Maternal diabetes increases the risk of fetal anomalies, macrosomia, subsequent birth injury and hypoglycemia, all of which can negatively affect developmental outcomes in the infant. Hypertension, alone or combined with a renal or autoimmune disorder, can cause placental insufficiency and inadequate fetal growth. Infant factors include chromosomal disorders [28], prematurity [29], intra-uterine growth restriction, sensory or regulatory problems and chronic or congenital health problems [30, 31]. These risk factors will impact the type of care needed by the infant, how his caregivers respond to him, and his capacity for normal physical and mental development [32].
The objective of the present study was to assess various factors that may positively or adversely affect the socio-emotional development including, infant feeding practices, aspects of infant and maternal health and socioeconomic status in a sample of Egyptian infants.
It was previously presumed that only psychosocial risk factors possessed significant predictive capability for socioemotional development. This study assumes that socio-emotional development of infants would be equally affected by different factors among which is type of feeding in addition to other factors such as micronutrient adequacy, demographic factors (as maternal education and socioeconomic status), and host factors (as child order of birth).
Based on these assumptions, we hypothesized that children’s socioemotional development is significantly associated with breast feeding, level of maternal education and birth order.
Subjects and Methods
Study design
This research is a cross sectional comparative study. It was done over a period of three years starting from August 2010 till August 2013. The research focused on three groups of infants aged 6–24 months classified according to their mode of feeding during the initial six months of life into three groups: breastfed, non- breast fed (consuming other milk including fresh, tinned, and powdered milk from cows or other animals) and mixed-fed infants (both breast and artificially fed). Studied infants were enrolled in the Well–Baby Clinic of the National Research Centre or from pediatric outpatient facilities in urban Cairo.
Sample Size Calculations
The number of infants per each group was calculated according to the national prevalence of infants who are consuming other milk including fresh, tinned, and powdered milk from cows or other animals, which were calculated according to Egypt Demographic Health Survey (EDHS).
EDHS is responsible for collecting accurate representative data about health and population at a national level. El-Zanaty and associates conduct it on behalf of the Ministry of Health and Population in Egypt at irregular intervals according to the problem required to be investigated. The national prevalence of infants who are consuming other milk was studied by EDHS, 2005 [33] in which the estimated prevalence was as follows:
among breast fed infants: 42.9% aged > 6—< 12 months of age, 52.4% aged 12—< 18 months of age, 49.2% aged > 18—< 24 months of age
among non-breast fed infants: 75.1% aged > 6—< 12 months of age, 72.9% aged 12—< 18 months of age, 65.5% aged > 18—< 24 months of age
Subjects
The sample size was ascertained utilizing epi Info-Statcalc version 7 [34]. The aggregate number of infants who were submitted for developmental assessment was 655 infants who were chosen as follows:
322 predominately breast-fed.
240 non breast-fed (who were consuming other milk including fresh, tinned, and powdered milk from cows or other animals)
93 mixed fed (who were consuming artificial milk plus breast milk)
The power of this study to calculate the required sample size was set at 80% denoting probability of finding a difference when a difference really exists.
For biochemical tests, the assessment would require 185 infants aged 6 to less than 24 months with the above stated criteria (who are consuming other milk including fresh, tinned, and powdered milk from cows or other animals). This number was estimated for calculating a true difference of 15% (denoting the probability of finding a difference when no difference existed i.e. margin of error: ± 0.15). This was categorized as 105 among breast fed infants vs. 80 among non-breast fed infants [33, 34]. Accordingly, every third woman in each group was asked to provide 5 ml of her infant's blood to be tested for serum zinc, copper, iron, vitamin B12 and CBC. Those who approved to be tested were assessed in a subsample of 193 infants (133 out of 322 breast fed infants and 60 out of 333 mixed and artificially fed infants) representing 41.3% and 18% of the total sampled infants respectively.
Inclusion criteria
Infants were enrolled if they were over 6 months and less than 24 months of age, belonging to the middle socioeconomic status and their guardians consented to participate in the study.
Exclusion criteria
Infants were excluded if they demonstrated any obvious congenital anomalies, features of genetic diseases, any metabolic or neurological problems, or if their mothers had a current or past history of mental illness.
Methods and procedures
A specific questionnaire was intended for evaluation of:
Socio-demographic variables: including the order of child, maternal age, living conditions, family income, maternal and paternal education and occupation.
Maternal medical history: including history of chronic diseases before or during pregnancy.
Perinatal history: including gestational age, type of labor, history of delivery problems, and admission to neonatal intensive care unit.
Infant nutritional history: including type of feeding during the initial six months and the time of introduction of complementary food
Physical examination and assessment of growth
Physical examination and assessment of growth was performed for each participant.
Assessment of infant development
Bayley Scales of Infant and Toddler Development (Bayley III), developed by Nancy Bayley in 2006 [35], was utilized to assess development of infants and toddlers between the age scopes of 1 month to 42 months. The Bayley scales are described as being the most widely used developmental assessment scheme [36]. Bayley-III covers five developmental domains. Cognitive, motor and language are administered with the child; interaction, social-emotional and adaptive behavior are administered with parent questionnaires. Domain subtests can be administered individually. The social–emotional scale includes items that assess the child's mastery of functional emotional skills, such as self-regulation and enthusiasm in the world: communicating needs; captivating others and setting up relationships; utilizing emotions in an interactive manner; and using emotion signals or gestures to solve problems.
Scoring of Bayley-III has been simplified from previous versions. Scoring for every item is either 1 (credit) or 0 (no credit). Scores available include raw scores, scaled scores, composite scores, percentile ranks and confidence intervals.
The measure with a series of developmental play tasks took between 45–60 minutes to administer. Raw scores of successfully completed items were converted to scaled scores and to composite scores. The scores obtained by toddlers were used to determine their performance compared with norms taken from typically developing children. The composite scores are scaled to a metric with a mean of 100 and a SD of 15, and a range from 40–160. The norm-referenced average is from 85–115.
According to the reference average social-emotional composite score (85–115), infants of this study were ordered into two groups: below average group (with socio-emotional composite score< 85) and average and above average group (with socio-emotional composite score > 85).
Validity studies comparing the Bayley–III with other tests do not show indications of Bayley–III standard score inflation. Bayley–III composite and subtest standard scores met theoretical expectations and are consistent with the results of the Wechsler preschool and primary scale of intelligence for children—Third edition (WPPSI–III), the Preschool language scale–Fourth edition (PLS–4) [37], and the Peabody Developmental Motor Scales, Second Edition (PDMS–2) [38].
The BSID-III has established reliability. Internal consistency was assessed using a split-half reliability method and shows reliability coefficients for the subscales and composite scores that range from 0.86 to 0.93 [35].
Biochemical assessment
Blood specimens were collected after a fasting period of 4 h for infants. Trace element-free syringes, stainless steel needles, and special trace element tubes (Becton-Dickinson) were used.
The serum samples were separated after 10 min of centrifugation. Serum samples were diluted at a 1:6 ratio with bi-distilled water. Serum Fe, Zn and Cu concentrations were measured using an atomic absorption flame emission spectrophotometer (Varian AA 100, Australia) (213.9 nm and 324.8, respectively) [39, 40]. A standard curve was established using a commercial Zn and Cu reference (Merck KGaA Darmstadt, Germany). The coefficient of variation of the measurements was always below 5%.
Serum vitamin B12 was assessed using the Bayer Centaur chemiluminescence method (Bayer Diagnostics, Glen¢eld, Auckland, New Zealand).
Hematological parameters, such as hemoglobin (Hb), hematocrit (Ht), red blood cell count (RBC), and the red cell indices, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red cell distribution width (RDW) were performed by an automated cell counter from a tube of well-mixed EDTA-anticoagulated.
Hemoglobin concentration < 11 g/dl, was used as cutoff point for the diagnosis of anemia [41]. It was considered that iron deficiency existed, when serum iron <45 ug/dL [42]. Other nutritional deficiencies, were assessed according to the following cutoff values; vitamin B12 <203 pg/mL, [43] zinc <65 ug/dL [44] and copper < 63.7 ug/dL. [45]
Ethical considerations
The study complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects [46]. The Research and Ethical Committee of the National Research Centre cleared the study protocol. Informed consent was obtained from all participants involved in the study and the information obtained at the individual level was kept strictly confidential.
Statistical methods
All test data was manipulated and analyzed by using SPSS software program version 18.0. Qualitative data were presented by number and percent. Comparisons of groups of children having average and below average socio emotional scores were done. Data was compared using Chi square and p value was calculated to determine the statistically significant difference between the two groups. Odds ratio (OR) and 95% confidence interval (CI) were also computed [OR = (a/c)/(b/d)]. [47].
The difference between the two groups was considered statistically significant when p<0.05, and considered highly statistically significant when p<0.01. Binary Logistic regression was performed to ascertain the effects of the attained significant nutritional and social variables on the likelihood that infants have a below average socio-emotional composite score. Type of feeding, time of introduction of complementary food and level of serum zinc were considered as predictors, while other significant variables as child order of birth, level of maternal education and status of maternal employment were considered as covariates. Coding of variables followed simple coding method. The dependent variable (socio-emotional composite score) was coded on a dichotomous scale into two groups: having a below average score versus having an average or above score.
All independent variables whether continuous or nominal, were transformed into categorical variables and coded as two groups (0 and 1) except type of feeding. The reference categories were: introduction of complementary food after six months, child order ≤3, high mother education, working mother, normal serum zinc level (each was coded as 1) and breast feeding (coded as 2). Other categories: introduction of complementary food before six months, child order >3, low mother education, housewife mother, subnormal serum zinc level (each was coded as 0) were anticipated as predictors of below average socioemotional score. The categorical variable (type of feeding) included 3 groups; bottle fed infants were compared to breast fed and mixed fed infants were compared also to breast fed (reference category).
Results
Subjects were chosen and classified by sort of feeding into 3 groups: group (1) included 322 infants predominately breast-fed whose mean socio-emotional composite score was 102.81± 31.08, group (2) included 240 artificially fed infants whose mean score was 100.63± 36.72, and group (3) consisted of 93 mixed-fed infants, their mean score was 97.40 ± 33.90. No significant statistical difference (p> 0.05) could be detected between breast, formula, and mixed fed infants mean composite scores of the socio-emotional domain.
Nutritional factors
As shown in Fig 1, formula-fed infants were equally distributed as regard socio-emotional domain between below average class (50%) and average or above average classes (50%). This distribution was significantly different from that of the breast fed (29.8% vs. 70.2%) and mixed-fed infants (35.5% vs. 64.5%). The risk of having below average socio-emotional composite score in formula-fed infants was nearly two and half times higher than in breast fed infants (OR = 2.35, P<0.001). In the meantime, formula-fed infants had the risk of getting below average socio-emotional composite score nearly twice as much as mixed fed infants (OR = 1.82) with significant p value <0.05. Early introduction of complementary food (CF) before six months of age conveyed a significant risk of getting a below average socio-emotional composite score which was one and half times more than infants starting CF after six months of age (OR = 1.5, P <0.05). All details with respect to the impact of infant feeding practices on the socio-emotional composite score are demonstrated in Table 1.
Fig 1. Percentage distribution of infants in each feeding group according to the reference average socio-emotional composite score in a group of 655 Egyptian infants and children aged 6–24 months old.
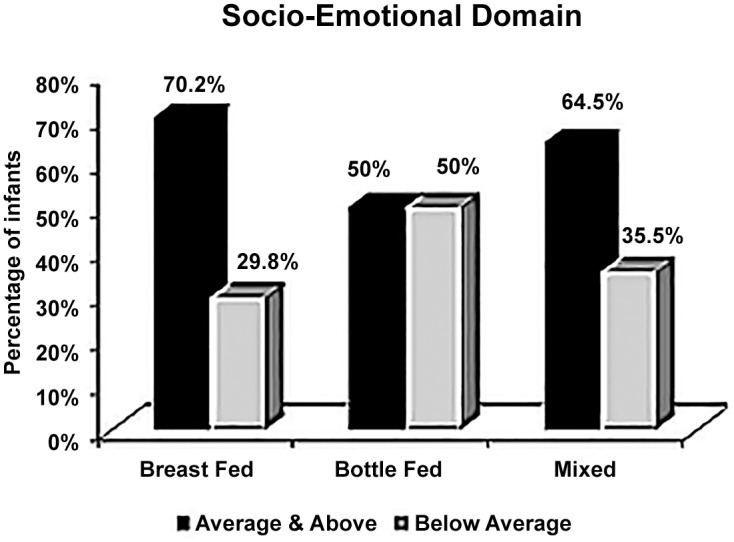
Table 1. Association of infant feeding practices with socio-emotional composite scores categories in a group of 655 Egyptian infants and children aged 6–24 months old.
| Parameter | n | Below Average (n = 249) | Average & above average (n = 406) | OR (95%CI) | df | X2 | P-value |
|---|---|---|---|---|---|---|---|
| Type of feeding | 2a | 15.898 a | <0.001 a | ||||
| formula fed | 240 | 120 (50%) | 120 (50%) | 2.35(1.64–3.39) 1 | 1 | 23.685 | <0.001* |
| Expected count (all) | 91.2 | 148.8 | |||||
| Expected count1 | 92.2 | 147.8 | |||||
| Expected count2 | 110.3 | 129.7 | |||||
| Mixed fed | 93 | 33 (35.5%) | 60 (64.5%) | 1.82(1.08–3.07) 2 | 1 | 5.687 | 0.017* |
| Expected count (all) | 35.4 | 57.6 | |||||
| Expected count2 | 42.7 | 50.3 | |||||
| Expected count3 | 28.9 | 64.1 | |||||
| Breast fed | 322 | 96 (29.8%) | 226 (70.2%) | 1.29(0.77–2.17) 3 | 1 | 1.083 | 0.298 |
| Expected count (all) | 122.4 | 199.6 | |||||
| Expected count1 | 123.8 | 198.2 | |||||
| Expected count3 | 100.1 | 221.9 | |||||
| Time of introduction of CF | |||||||
| Before six months | 201 | 90(44.8%) | 111(55.2%) | 1.5 (1.06–2.14) | 1 | 5.948 | 0.02* |
| Expected count | 76.4 | 124.6 | |||||
| After six months | 454 | 159(35.0%) | 295 (65.0%) | ||||
| Expected count | 172.6 | 281.4 |
CF: complementary food
* significant at p <0.05
a: Comparison between the three types of feeding; breast, bottle and mixed feeding revealed significant difference
1- Bottle fed vs. breastfed
2- Bottle fed vs. mixed fed
3- Mixed fed vs. breastfed
Social factors
Social variables that influenced the likelihood of having below average socio-emotional composite score in infants are shown in Table 2. These include child order < 3, low maternal education and no maternal occupation (i.e. being a housewife). Other social factors such as maternal age and father income did not demonstrate statistically significant risk of having a below average socio-emotional composite score.
Table 2. Association of socioeconomic variables with socio-emotional composite scores categories in a group of 655 Egyptian infants and children aged 6–24 months old.
| Parameter | n | Below average (n = 249) | Average &above (n = 406) | OR (95%CI) | df | X2 | P-value |
|---|---|---|---|---|---|---|---|
| Child's order | |||||||
| Child's order (>3) | 443 | 143(32.3%) | 300(67.7%) | 0.48(0.34–0.68) | 1 | 19.107 | <0.001* |
| Expected count | 168.4 | 274.6 | |||||
| Child's order (≤3) | 212 | 106(50%) | 106(50%) | ||||
| Expected count | 80.6 | 131.4 | |||||
| Maternal age | |||||||
| ≤25 years | 241 | 100(41.5%) | 141(58.5%) | 1.26(0.90–1.77) | 1 | 1.958 | 0.16 |
| Expected count | 91.6 | 149.4 | |||||
| >25years | 414 | 149(36%) | 265(64%) | ||||
| Expected count | 157.4 | 256.6 | |||||
| Paternal Income a | |||||||
| Lower Middle Income | 312 | 126(40.4%) | 186(59.6%) | 1.21(0.87–1.68) | 1 | 1.419 | 0.23 |
| Expected count | 118.6 | 193.4 | |||||
| Upper Middle- Income | 343 | 123(35.8%) | 220(64.2%) | ||||
| Expected count | 130.4 | 212.6 | |||||
| Maternal education b | |||||||
| Illiterate, read & write | 159 | 104(65.4%) | 55 (34.6%) | 2.31(1.55–3.44) | 1 | 45.983 | <0.001* |
| Expected count | 60.4 | 98.6 | |||||
| High education | 496 | 173(34.9%) | 323(65.1%) | ||||
| Expected count | 188.6 | 307.4 | |||||
| Maternal occupation | |||||||
| House wife | 503 | 203(40.4%) | 300(59.6%) | 1.56(1.04–2.35) | 1 | 5.048 | 0.03* |
| Expected count | 191.2 | 311.8 | |||||
| Working | 152 | 46(30.3%) | 106(69.7%) | ||||
| Expected count | 57.8 | 94.2 |
a Lower middle Income father: Father is unemployed, day by day worker, farmer or laborer; Upper middle Income father: Father is an employee, professional, or dealer
b High Education = High School and University
* significant at p <0.05
Maternal and infant well-being
All recorded maternal medical factors (chronic disease before or during pregnancy, malnourished or anemic mothers) or perinatal factors (gestational age, type of labor, delivery problems) did not seem to affect the probability of having a below average socio-emotional composite score in our sample. Moreover, children's medical conditions as being anemic or having subnormal serum B12 did not convey a risk for socio-emotional development (p>0.05). All infants had normal serum copper and normal serum iron regardless the mode of feeding. Serum zinc level appeared as a significant risk factor for socio-emotional development. The risk of having below average socio-emotional composite score in infants with subnormal serum zinc level was nearly 3 times higher than in infants with normal serum zinc level. (OR = 2.83, 95% CI (0. 97–8.64), P<0.05) (Table 3).
Table 3. Association of the level of infant biochemical parameters with socio-emotional composite scores categories in a group of 655 Egyptian infants and children aged 6–24 months old.
| Parameter | n** | Below average (n = 249) | Average &above (n = 406) | OR (95%CI) | df | t /X2 | P |
|---|---|---|---|---|---|---|---|
| RBCs (mean ± SD) | 193 | 4.6±0.5 | 4.7±0.4 | 1.448 | 0.151 | ||
| (Min.–Max.) | (3.4–5.93) | (2.8–5.6) | |||||
| HCT (mean ± SD) | 193 | 31.6±2.4 | 32.0±3.8 | 0.86 | 0.391 | ||
| (Min—Max.) | (28.0–36.8) | (23.1–42.0) | |||||
| MCV (fL) (mean ± SD) | 193 | 69.0±6.8 | 66.7±6.9 | 2.170 | 0.031* | ||
| (Min.–Max.) | (54.0–89.3) | (47.0–85.0) | |||||
| MCH (pg/cell) (mean ± SD) | 193 | 23.5±2.7 | 23.0±3.5 | 1.123 | 0.263 | ||
| (Min.–Max.) | (18.0–30.0) | (15.0–43.0) | |||||
| MCHC (g/dl) (mean ± SD) | 193 | 33.8±1.8 | 34.2±2.4 | 1.16 | 0.248 | ||
| (Min.–Max.) | (30.0–38.3) | (29.0–51.0) | |||||
| Hb (gm/dl) (mean ± SD) | 193 | 10.6±1.2 | 10.8±1.4 | 0.766@ | |||
| (Min.–Max.) | (8.9–13.7) | (7.2–14.7) | |||||
| Fe (ug/dl)4 (mean ± SD) | 193 | 163.0±47.4 | 157.4±54.2 | 0.701 | 0.484 | ||
| (Min.–Max.) | (54.0–228.0) | (84.0–234.0) | |||||
| Cu (ug/dL)4 (mean ± SD) | 193 | 116.7±41.2 | 130.0±44.6 | 1.969 | 0.056 | ||
| (Min.–Max.) | (70.0–234.0) | (72.0–234.0) | |||||
| Anemia of infant 1 | |||||||
| Yes | 89 | 31(34.8%) | 58(65.2%) | 0.94 (0.49–1.81) | 1 | 0.061 | 0.85 |
| Expected count | 31.8 | 57.2 | |||||
| No | 104 | 38(36.5%) | 66(63.5%) | ||||
| Expected count | 37.2 | 66.8 | |||||
| Zn (ug/dL)2 (mean ± SD) | 83.5±31.8 | 101.7±47.9 | 0.015* | ||||
| (Min.–Max.) | (36.0–162.0) | (36.0–228.0) | |||||
| Subnormal level | 17 | 9(52.9%) | 8(47.1%) | 2.83 (0. 97–8.64)* | 1 | 4.396 | 0.03* |
| Expected count | 5.2 | 11.8 | |||||
| Normal level | 176 | 50(28.4%) | 126(71.6%) | ||||
| Expected count | 53.8 | 122.2 | |||||
| vitamin B12 (pg/mL)3 (mean ± SD) | 981.6±422.0 | 1109.4±433.3 | 0.028*# | ||||
| (Min.–Max.) | (50.0–1500.0) | (50.0–1670.0) | |||||
| Subnormal level | 9 | 5(55.6%) | 4(44.4%) | 3.35 (0.74–15.61) | 1 | 3.392 | 0.06 |
| Expected count | 2.6 | 6.4 | |||||
| Normal level | 184 | 50(27.2%) | 134(72.8%) | ||||
| Expected count | 52.4 | 131.6 |
@ independent t test
# Mann-Whitney test
* significant at p <0.05
** biochemical parameters were done for a subsample of 193 children
1 Hemoglobin concentration < 11 g/dl was used as cutoff point for the diagnosis of anemia
2 cutoff point for zinc deficiency was <65 ug/dL.
3 cutoff point for vitamin B12 deficiency was <203 pg/Ml
4 iron and copper levels were within normal reference range
RBC: red blood cell count, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration. HCT: Hematocrit, Hb: Hemoglobin
Table 4 includes variables that predict below average socioemotional composite score according to logistic regression analysis. It shows that subnormal level of serum zinc, formula feeding and introduction of complementary food before the age of six months were statistically significantly positively associated with the probability of having below average socio-emotional composite score (p<0.001, p = 0.001and p = 0.006 respectively). The table also shows that the likelihood of having below average socioemotional composite score will increase 1.15 folds if the level of serum zinc was below normal and other variables were controlled, 0.62 if complementary food was introduced before six months of age and 0.85 if the infant was formula fed. On the other hand, child's order of birth, mother education, mother occupation and mixed feeding were not significantly associated with the probability of having below average socio-emotional composite score (p>0.05).
Table 4. Logistic regression of variables predicting below average socio-emotional composite score.
| B | S.E. | Wald | df | p | Adjusted odds | 95% Confidence Interval for adjusted odds | ||
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Intercept | .001 | .744 | .000 | 1 | .999 | |||
| Child order | .140 | .255 | .300 | 1 | .584 | 1.150 | .697 | 1.897 |
| Maternal education | .405 | .264 | 2.351 | 1 | .125 | 1.500 | .893 | 2.518 |
| Maternal occupation | -.345 | .301 | 1.316 | 1 | .251 | .708 | .393 | 1.277 |
| Level of serum zinc: subnormal a | 1.154 | .293 | 15.518 | 1 | .000 | .315 | .178 | .560 |
| Introduction of complementary food: before six month b | .620 | .227 | 7.489 | 1 | .006 | .538 | .345 | .839 |
| Feeding type: bottle c | .848 | .247 | 11.745 | 1 | .001 | 2.335 | 1.438 | 3.793 |
| mixed c | -.048 | .320 | .023 | 1 | .880 | .953 | .509 | 1.783 |
a. Reference group: normal serum zinc
b. Reference group: Introduction of complementary food after six months,
c. Reference group: breast feeding.
Discussion
Factors influencing socio-emotional development were grouped into nutritional variables, social elements and maternal and infant well-being elements.
Nutrition
The most important nutritional factors were kind of feeding practices during the initial six months of life and the time of introduction of complementary food. As indicated by Bayley III, the socio-emotional composite score grouped infants into average and below average classes. The risk of having below average socio-emotional composite score in formula-fed infants was nearly two and half times higher than breast fed infants (OR = 2.35, P<0.001). Our outcomes are in concurrence with the findings of past studies that have analyzed the impact of breastfeeding on early indicators of children’s development [48, 49, 50, 51]. Breast milk contains a suite of nutrients, growth factors, and hormones that are important for brain development, including critical building blocks such as docosahexaenoic acid (DHA) and choline. In addition, the physical act of breastfeeding may enhance mother-infant interaction, which are important for cognitive and socioemotional development [52].
However, various research findings clarifying the impact of breast feeding on infant neurodevelopment are not consistent. In a review by Jansen et al [53], they reported that effects seen in development could not be explained by breastfeeding alone. Swain et al [54], also found that brain areas involved in parent-infant bonding and interaction are involved in other social situations such as love and attachment. Moreover, skin-to-skin contact is suggested to play a major role in maternal sensitivity [55], which has been related to better cognitive development of the child [56]. On the other hand, no significant statistical distinction could be recognized between breast fed and mixed fed infants in the likelihood of having average and above average socio-emotional composite score. This is because of the known relationship between breast milk feeding and optimal brain function even if it is not the sole source of infant nutrition, suggesting that any amount of breastfeeding during infancy is associated with points of interest on assessments of neuro-development. This is a potentially important finding as it suggests that breastfeeding is not an ‘all or none response’ and that some benefits of breastfeeding on neuro-development may be presented regardless of the possibility that the mother is not ready to provide exclusive breastfeeding for a time of 6 months [50]. Breastfeeding may enhance mental development owing to the composition of breast milk and to the act of breastfeeding, as it may foster a positive mother-infant relationship, which is important for socio-emotional development [16]. However, the pathways by which breastfeeding affects emotional development are not direct and are always intermingled [57].
The mean socio-emotional composite score was significantly higher in infants whose mothers introduced CF after 6 months of age. Likewise, the percentage of below average infants was significantly less when CF was introduced after 6 months of age. Proper complementary feeding for breastfed infants is crucial for their optimal growth and development. WHO recommended the introduction of complementary foods around the sixth month of life, instead of between the fourth and sixth month, as was previously recommended [58]. Several studies carried out in both developing and industrialized countries showed that early introduction of complementary foods increases infant morbidity and mortality, shortens the duration of breastfeeding [59], interferes with the uptake of important nutrients found in breastmilk such as iron [60], zinc [61], and reduces the efficiency of lactation in preventing new pregnancies [62].
Late introduction of complementary foods is also disadvantageous, because infant growth stops or slows down and the risk of malnutrition and micronutrient deficiency increases [63, 64].
Social factors
Developmental outcomes are influenced to different extents by a number of risk factors such as biological, social, family and medical factors [65, 66, 67]. Children living in poverty are at risk of adverse developmental outcomes because of the aggregate impacts of introduction to hazard components as repeated infections or malnutrition. In addition, living in low financial conditions may diminish open doors for profitable learning and social communication [68]. Children’s cognitive and emotional competencies are observed to be influenced by living in impoverished socio-economic environments [69, 70].
In this study, maternal education was one of the influential social factors. Infants belonging to uneducated mothers had a higher risk of getting below average composite score than those belonging to highly educated mothers. This finding is in concurrence with previous studies, which reported that infants of less-educated mothers are more likely to be exposed to inadequate dietary intake, poorer sanitation [71] and receive less cognitive stimulation, than those of more-educated mothers [72]. In addition, the current results revealed that infants of housewives had a higher risk of getting below average socio-emotional composite score than infants of working mothers. This may be attributed to the sense of financial security and sense of well-being of the employed mother, which provide some sort of mother-child interactions linked to better development outcomes. This finding is similar to the results of a comprehensive meta-analysis of 69 research studies evaluating the impact of maternal employment during infancy/early childhood on child achievement and behavior problems [73]. These studies indicated that when families were at risk because of financial challenges, early maternal employment was associated with higher levels of achievement and lower levels of internalizing behaviors such as anxiety and depression. Maternal employment provided those families with financial security, lower levels of family stress and enhanced learning opportunities for children.
Moreover, child birth order more than the third appeared to have a positive effect on socio-emotional development; this may be because of stimulation and interaction with older siblings.
In this study, logistic regression analysis revealed the interaction between infant feeding variables and socio-economic variables in predicting infant socio-emotional outcome. Subnormal level of serum zinc, formula feeding in the first six months of life, and introduction of complementary food before the age of six months were significantly associated with the probability of having below average socio-emotional composite score. It is believed that zinc is a vital nutrient for the brain. It has an important role in neurogenesis, maturation, migration of neurons and in synapse formation [16, 74]. For each nutrient, there is a limit at which insufficiency can bring about impairment for the child. Evaluation of this level remains a vital inquiry that must be answered for each nutrient independently [16]. Although iron deficiency anemia (which affected 46% of the studied subsample) had no effect on the score, yet three studies in developing countries found altered socio-emotional outcomes in full-term infants with iron deficiency anemia when compared to infants without anemia [75, 76, 77]. These differences may be due to the variations in environmental settings, where enriched environment shields kids from negative impacts of under-nutrition. In addition, the degree of nutritional deficiency is another factor that may have an influence leading to mental deficits [16]. There was scarce evidence for the effect of other micronutrients such as vitamin B12 on the socio-emotional development of young children [78].
Maternal and infant well-being
Various studies suggested that psychosocial risk factors influence socio-emotional development as opposed to biological risk factors of pregnancy and delivery [79, 80]. Usually prenatal and intrapartum factors and preterm labor can adversely influence the outcome for infants causing a broad range of neurodevelopmental impairments [81]. However, in this study many medical factors as maternal chronic diseases before or during pregnancy, delivery associated problems and prematurity did not affect the socio-emotional composite score. The effect of these risk factors especially prematurity could be subtle, and may be detected later on in childhood or adolescence. Symptoms suggestive of Attention Deficit Hyperactivity Disorder (ADHD), which are observed in childhood, occur two to six times more frequently in children who are born preterm [82]. On the other hand, recent advances in early neonatal care may result in improved survival of infants born preterm and ameliorate subsequent developmental and health problems [29].
It is clear that not all children exposed to risk factors will encounter adverse development. Multiple factors as family characteristics, child characteristics, socioeconomic status and sources of support outside the family will interact in a complex fashion [83].
Limitations of the current study
This study was cross-sectional and consequently causality could not be inferred. Feeding information was based on administered questionnaires, which are subject to recall bias. Depending on interview, observation, and lack of comprehensive assessment to exclude mothers with mental health affection may disregard one of the important risk factors affecting infant socio-emotional development.
In conclusion: It is clear that complex interactions among multiple influences will result in great variability in infant socio-emotional development. Clear advantages of breastfeeding even if it is not exclusive are evident in this study. These advantages could be conferred to infants with mixed feeding. Maternal education stands as a strong influence on children’s development. Micronutrient sufficiency shows up as an important determinant of infant psychological well-being. Therefore, our endeavors ought to be directed towards integrated interventions addressing multiple influences of children’s development (e.g., healthy nutrition and stimulation) rather than singular interventions to prevent developmental decline in the developing world. Media should be involved in the promotion of breastfeeding, proper complementary feeding practices and increasing awareness about the importance of social and emotional development. Consideration ought to concentrate on socio-economic empowerment especially education of females. Enhancing antenatal and postnatal care can be achieved through micronutrient supplements, dietary fortification, food distribution and nutrition counseling.
Data Availability
Due to ethical restrictions imposed by Ethical Committee of the National Research Centre Data Access, data may not be made publicly available. Data are available by request from the Department of Community Medicine Research (National Research Centre, Egypt) authors who may be contacted at am.metwally@nrc.sci.eg.
Funding Statement
The authors have no support or funding to report.
References
- 1.Hinshaw-Fuselier S, Zeanah P, Larrieu J. “Training in Infant Mental Health,” Chapter 33, Principles of Infant Mental Health In: Zeanah Charles Jr., ed., Handbook of Infant Mental Health, 3rd Edition New York: Guilford Press; 2009. [Google Scholar]
- 2.Parlakian R. Before the ABCs: Promoting School Readiness in Infants and Toddlers. Washington, D.C.: Zero to Three Press; 2003. [Google Scholar]
- 3.Tomasello M, Carpenter M. Shared intentionality Developmental Science. 2007; 10 (1): 121–125. [DOI] [PubMed] [Google Scholar]
- 4.Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations In Gross J. J. (Ed.), Handbook of emotion regulation New York, NY: Guilford Press; 2007. p. 3–26. [Google Scholar]
- 5.Thompson RA. Feeling and understanding through the prism of relationships In Calkins S D. & Bell MA. (Eds.), Moral personality, identity, and character: Prospects for a new field of study. New York, NY: Cambridge University Press; 2010. [Google Scholar]
- 6.Saarni C, Campos JJ, Camras LA, Witherington D. Emotional development: Action, communication, and understanding In: Einsenberg N, editor. Handbook of child psychology. Vol. 3. Social, emotional, and personality development. 6th Edition New York: John Wiley & Sons; 2006. [Google Scholar]
- 7.Emde RN, Egger H, Fenichel E, Guedeney A, Wise BK, Wright HH. Zero to Three Diagnostic Classification of Mental Health and Developmental Disorders of Infancy and Early Childhood, Revised Edition Washington, DC: Zero to Three Press; 2005. [Google Scholar]
- 8.Egger HL, Angold A. Common Emotional and Behavioral Disorders in Preschool Children: Presentation, Nosology, and Epidemiology.” J. Child Psychology and Psychiatry 2006; 47:3(4): 313–337. [DOI] [PubMed] [Google Scholar]
- 9.Center on the Developing Child at Harvard University. The Foundations of Lifelong Health are Built in Early Childhood. Center on the Developing Child. 2010. Available: www.developingchild.harvard.edu.
- 10.Perry BD, Pollard RA, Blakley TL, Baker WL, Vigilante D. Childhood trauma, the neurobiology of adaptation and use-dependent development of the brain: How states become traits. Infant Mental Health Journal. 1995; 16: 271–291. [Google Scholar]
- 11.WHO-AIMS. Report on Mental Health System in Egypt, WHO and Ministry of Health, Cairo, Egypt, 2006.
- 12.Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, et al. Inequality in early childhood: risk and protective factors for early child development www.thelancet.com Published online September 23, 2011. (http://www.ecdgroup.com/pdfs/Lancet%202011_papers%201%20and%202.pdf) accessed on 23 April 2016. [DOI] [PubMed]
- 13.Chilton M, Chyatte M, Breaux J. The negative effects of poverty and food insecurity on child development. Indian J Med Res. 2007; 126:262–72. [PubMed] [Google Scholar]
- 14.Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, Wright RO, et al. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009; 117: 1607–11. 10.1289/ehp.0900625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posada G, Jacobs A, Richmond MK, Carbonell OA, Alzate G, Bustamante MR, Quiceno J. Maternal caregiving and infant security in two cultures. Dev Psychol. 2002; 38: 67–78. [DOI] [PubMed] [Google Scholar]
- 16.Prado EL, Dewey KG Nutrition and brain development in early life. Nutrition Reviews. 2014; 72(4): 267–284. 10.1111/nure.12102 [DOI] [PubMed] [Google Scholar]
- 17.Richter L. The Importance of Caregiver-Child Interactions for the Survival and Healthy Development of Young Children: A Review. Geneva, Switzerland: Department of Child and Adolescent Health and Development, World Health Organization; 2004. [Google Scholar]
- 18.Prado EL, Dewey KG. Nutrition and brain development in early life. Alive & Thrive Technical Brief January 2012; (Issue 4). [Google Scholar]
- 19.Darling N, Steinberg L. Parenting style as context: An integrative model. Psychological Bulletin. 1993; 113:487–496. [Google Scholar]
- 20.Raver CC. Placing emotional self-regulation in sociocultural and socioeconomic contexts. Child Development. 2004; 75:346–353. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. ‘World Report on Violence and Health’, ed. By Krug Etienne G., et al. , Geneva, 2002. [Google Scholar]
- 22.Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The Role of the Family Context in the Development of Emotion Regulation. Soc Dev. 2007. May 1; 16(2): 361–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman E, McEwen BS, Dolan LM, Schafer-Kalkhoff T, Adler NE. Social disadvantage and adolescent stress. Journal of Adolescent Health. 2005;37:484–492 [DOI] [PubMed] [Google Scholar]
- 24.Saarni C, Mumme D, Campos JJ. Emotional development: Action, communication, and understanding In: Eisenberg N, editor. Handbook of child psychology: Vol. 3 Social, emotional and personality development. 5th ed New York: Wiley; 1998. pp. 237–309. [Google Scholar]
- 25.Thompson RA. Early attachment and later development: Familiar questions, new answers In Cassidy J. & Shaver P. R. (Eds.), Handbook of attachment, (Vol. 2, pp. 348–365). New York, NY: Guilford Press; 2008. [Google Scholar]
- 26.Easterbrooks MA, Bartlett JD, Beeghly M, Thompson RA. Social and Emotional Development in Infancy In Weiner IB. ed. Handbook of Psychology, Second Edition John Wiley & Sons, Inc; 2012. [Google Scholar]
- 27.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol. 2006;46:4–14. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh M, Shah AH, Dhir K. Behavior in children with Down syndrome. Indian J Pediatr. 2008; 75 (7): 685–689. 10.1007/s12098-008-0129-z [DOI] [PubMed] [Google Scholar]
- 29.Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Washington (DC): National Academies Press (US) 2007. [PubMed] [Google Scholar]
- 30.Raina P, O'Donnell M, Rosenbaum P, Brehaut J, Walter SD, Russell D, et al. The health and well-being of caregivers of children with cerebral palsy. Pediatrics. 2005. June; 115(6):e626–36. [DOI] [PubMed] [Google Scholar]
- 31.Williams KR, Wishart JG. The Son-Rise Program intervention for autism: an investigation into family experiences. J Intellect Disabil Res. 2003. May-Jun; 47(Pt 4–5):291–9. [DOI] [PubMed] [Google Scholar]
- 32.Wachs TD. Risk factors and the development of competence in children from low-income countries: The importance of social-emotional outcomes and multiple process models. Child Health and Education, 2009; 1 (2): 107–121. [Google Scholar]
- 33.Demographic Egypt and health Survey (EDHS). El–Zanaty F, Way A. Ministry of Health and population Council, El Zanaty and Associates, and ORC Marco. 2005.
- 34.Kelsey JL, Whittemore AS, Evans AS, Thompson WD. Methods in Observational Epidemiology, 2nd Edition, Table 12–15 Oxford University Press; 1996. Updated February 16, 2007. [Google Scholar]
- 35.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition Psychological Corporation, 2006. [Google Scholar]
- 36.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. J Pediatr. 2012; 160(4): p. 553–8. 10.1016/j.jpeds.2011.09.047 [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman IL, Steiner VG, Pond RE. Preschool language scale (4th ed.). San Antonio, TX: The Psychological Corporation, 2002. [Google Scholar]
- 38.Folio RM, Fewell RR. Peabody developmental motor scales, second edition Austin, TX: Pro-Ed; 2000. [Google Scholar]
- 39.Chou PP. Zinc In: Pesce AJ, Kaplan LA, editors. Methods in clinical chemistry. St. Louis, Missouri: The C.V. Mosby Company; 1987. p. 596–602. [Google Scholar]
- 40.Taylor A, Bryant TN. Comparison of procedures for determination of copper and zinc serum by atomic absorption spectroscopy. Clin Chim Acta. 1981;110:83–90. [DOI] [PubMed] [Google Scholar]
- 41.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011. (WHO/NMH/NHD/MNM/11.1) (http://www.who.int/vmnis/indicators/haemoglobin.pdf, (accessed May 2016). [Google Scholar]
- 42.Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. Geneva: WHO, 2004. [Google Scholar]
- 43.The World Health Organization. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull 2008; 29(2). [DOI] [PubMed] [Google Scholar]
- 44.Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull 2007; 28(3 Suppl): S403–S429. [DOI] [PubMed] [Google Scholar]
- 45.Murray RK, Jacob M, Varghese J. Plasma Proteins & Immunoglobulins Bender DA, Botham KM, Weil PA, Kennelly PJ, Murray RK, Rodwell VW. Harper's Illustrated Biochemistry. 29th New York: McGraw-Hill; 2011. [Google Scholar]
- 46.CIOMS/WHO. International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva: CIOMS; 1993. [PubMed] [Google Scholar]
- 47.Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 3rd ed Boca Raton: Chapman & Hall/CRC; 2004. [Google Scholar]
- 48.Fergusson DM, Woodward LJ. Breast feeding and later psychosocial adjustment. Paediatric and Perinatal Epidemiolog. 1999; 13(2):144–157. [DOI] [PubMed] [Google Scholar]
- 49.Jain A, Concato J, Leventhal JM. How good is the evidence linking breastfeeding and intelligence? Pediatrics. 2002; 109:1044–53. [DOI] [PubMed] [Google Scholar]
- 50.Kramer MS, Fombonne E, Igumnov S, Vanilovich I, Matush L, Mironova E, et al. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: evidence from a large, randomized trial. Pediatrics. 2008. March 1; 121(3):e435–40. 10.1542/peds.2007-1248 [DOI] [PubMed] [Google Scholar]
- 51.McCrory C, Murray A. The effect of breastfeeding on neuro-development in infancy. Maternal and child health journal. 2013; 1680–1688. 10.1007/s10995-012-1182-9 [DOI] [PubMed] [Google Scholar]
- 52.Reynolds A. Breastfeeding and brain development. Pediatr Clin North Am. 2001; 48(1): 159–71. [DOI] [PubMed] [Google Scholar]
- 53.Jansen J, de Weerth C, Riksen-Walraven JM. Breastfeeding and the mother-infant relationship-a review. Developmental Review. 2008; 28(4):503–521. [Google Scholar]
- 54.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007; 48(3–4): 262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bystrova K, Ivanova V, Edhborg M, Matthiesen AS, Ransjö-Arvidson AB, Mukhamedrakhimov R, et al. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009; 36(2): 97–109. 10.1111/j.1523-536X.2009.00307.x [DOI] [PubMed] [Google Scholar]
- 56.Narvaez D, Gleason T, Wang L, Brooks J, Lefever JB, Cheng Y. The evolved development niche: Longitudinal effects of caregiving practices on early childhood psychosocial development. Early Childhood Research Quarterly. 2013; 28(4): 759–773. [Google Scholar]
- 57.Marquis GS. Breastfeeding and its impact on child psychosocial and emotional development: Comments on Woodward and Liberty, Greiner, Pérez-Escamilla, and Lawrence. In: Tremblay RE, Barr RG, Peters RDeV, eds. Encyclopedia on Early Childhood Development [online]. Montreal, Quebec: Centre: of Excellence for Early Childhood Development 2005; 1–4. Available: http://www.child-encyclopedia.com/documents/MarquisANGxp.pdf. [Google Scholar]
- 58.World Health Organization (WHO). World Health Assembly Resolution. Infant and young child nutrition. WHA. 54. 2, 18 May 2001.
- 59.Zeitlin MT, Ahmed NU. Nutritional correlates of frequency and length of breastfeeding in rural Bangladesh. Early Hum Develop. 1995; 41:97–100. [DOI] [PubMed] [Google Scholar]
- 60.Oski FA, Landaw AS. Inhibition of iron absorption from human milk by baby food. Am J Dis Child. 1980; 134:459–60. [DOI] [PubMed] [Google Scholar]
- 61.Bell JG, Keen CL, Lonnerdal B. Effect of infant cereals in zinc and copper absorption during weaning. Am J Clin Dis Child. 1987; 131:1128–32. [DOI] [PubMed] [Google Scholar]
- 62.McNeilly AS, Glasier A, Howie PW. Endocrinology control of lactational infertility. I In: Dobbing J, editor. Maternal and lactation infertility. New York: Raven Press; 1985. p. 1–24. [Google Scholar]
- 63.WHO/UNICEF. Complementary feeding of young children in developing countries: a review of current scientific knowledge. Geneva: World Health Organization, WHO/NUT/98.1,1998. [Google Scholar]
- 64.PAHO/WHO. Guiding principles for complementary feeding of the breastfed child Division of Health Promotion and Protection. Food and Nutrition Program. Pan American Health Organization/ World Health Organization; Washington/Geneva; 2003. [Google Scholar]
- 65.Forns J, Julvez J, García-Esteban R, et al. Maternal intelligence-mental health and child neuropsychological development at age 14 months. Gac Sanit. 2012; 26: 397–404. 10.1016/j.gaceta.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 66.Bornstein MH, Bradley RH. Socioeconomic Status, Parenting, and Child Development. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers, 2003. [Google Scholar]
- 67.Walker SP, Wachs TD, Gardner JM, Lozof B, Wasserman GA, Pollitt E, et al. "Child development: risk factors for adverse outcomes in developing countries." The lancet. 2007; 369: 145–157. [DOI] [PubMed] [Google Scholar]
- 68.Shonkoff JP, Phillips DA. From Neurons to Neighborhoods: The Science of Early Child Development, Washington, D.C.: National Academy Press; 2000. [PubMed] [Google Scholar]
- 69.National Institute of Child Health and Human Development Early Child Care Research Network. Duration and Developmental Timing of Poverty and Children’s Cognitive and Social Development from Birth through Third Grade. Child Development. 2005; 76(4): 795–810. [DOI] [PubMed] [Google Scholar]
- 70.WHO. Risks to Mental Health: An overview of vulnerabilities and risk factors. Background paper by WHO secretariat for the development of a comprehensive mental health action plan. 2012.
- 71.Wachs TD. Linking nutrition and education: a cross-generation model. Food Nutr Bull. 2005; 26: S159–S167. [DOI] [PubMed] [Google Scholar]
- 72.Von der Lippe A. The impact of maternal schooling and occupation on child-rearing attitudes and behaviours in low income neighborhoods in Cairo, Egypt. Int J Behav Dev. 1999; 23: 703–29. [DOI] [PubMed] [Google Scholar]
- 73.Lucas-Thompson R, Goldberg W, Prause J. Maternal work early in the lives of children and its distal associations with achievement and behavior problems: A meta-analysis. Psychological Bulletin. 2010; 136(6): 915–942. 10.1037/a0020875 [DOI] [PubMed] [Google Scholar]
- 74.Gardner JM, Powell CA, Baker-Henningham H, Walker S, Cole TJ, Grantham-McGregor SM, et al. Zinc supplementation and psychosocial stimulation: effects on the development of undernourished Jamaican children. Am J Clin Nutr. 2005; 82: 399–405. [DOI] [PubMed] [Google Scholar]
- 75.Lozoff B, Wolf AW, Jimenez E. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996; 129: 382–89. [DOI] [PubMed] [Google Scholar]
- 76.Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics. 1989; 84: 7–17. [PubMed] [Google Scholar]
- 77.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998; 69: 24–36. [PubMed] [Google Scholar]
- 78.Bryan J, Osendarp S, Hughes D, Calvaresi E, Baghurst K, van Klinken JW. Nutrients for cognitive development in school-aged children. Nutr. Rev. 2004; 62: 295–306. [DOI] [PubMed] [Google Scholar]
- 79.Bendersky M, Lewis M. Environmental risk, biological risk and developmental outcome. Developmental Psychology. 1994:30:484–94. [Google Scholar]
- 80.Lukeman D, Melvin D. Annotation: The preterm infant. Psychological issues in childhood. Journal of Child Psychology and Psychiatry. 1993; 34: 837–849. [DOI] [PubMed] [Google Scholar]
- 81.Allen MC. Risk assessment and neurodevelopmental outcomes In: Taeusch HW, Ballard RA, Gleason CA, editors. Avery’s Diseases of the Newborn. Philadelphia, PA: Elsevier Saunders; 2005. pp. 1026–1042. [Google Scholar]
- 82.Aylward GP. Cognitive and neuropsychological outcomes: More than IQ scores. Mental Retardation and Developmental Disabilities Research Reviews. 2002; 8(4):234–240. [DOI] [PubMed] [Google Scholar]
- 83.Quinn KP, McDougal JL. A Mile Wide and a Mile Deep: Comprehensive Interventions for Children and Youth with Emotional and Behavioral Disorders. Journal of Emotional and Behavioral Disorders. 1998; 7:54–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions imposed by Ethical Committee of the National Research Centre Data Access, data may not be made publicly available. Data are available by request from the Department of Community Medicine Research (National Research Centre, Egypt) authors who may be contacted at am.metwally@nrc.sci.eg.


